Abstract
Angiosarcoma of the breast is an unusual malignancy and carries a poor prognosis, with a 5-year overall survival rate ranging from 27 to 48%. Radiotherapy-induced angiosarcoma (RIAS) of the breast is very uncommon, with an estimated incidence of 1 in 1,000 cases of breasts treated with radiotherapy for breast cancer. The increase in radiotherapy usage may lead to an increased incidence of RIAS. A case presentation of a 67-year-old patient with tubular adenocarcinoma of the left breast who developed c-MYC-positive RIAS of the breast is presented. The patient was successfully treated with surgery. We presented a classic case of c-MYC RIAS. c-MYC was reported to be positive in RIAS and other types of angiosarcomas. Clinical examination and early detection of RIAS breast angiosarcoma is vital to improving outcomes in these patients.
Keywords: Breast angiosarcoma, MYC positive, Post-radiotherapy
Introduction
Angiosarcoma of the breast is an uncommon and heterogeneous malignancy. It behaves aggressively and carries a poor prognosis, with a 5-year overall survival rate of 27–48% [1, 2]. Due to its scarcity, most of the information regarding the diagnosis and management of this condition is from case reports and retrospective series analyses with relatively small patient numbers [1, 2, 3]. Angiosarcoma of the breast accounts for 3–5% of all soft tissue sarcomas and less than 1% of all breast malignancies. Radiotherapy-induced angiosarcoma of the breast (RIAS) is even less common. The estimated incidence of RIAS (also referred to as secondary breast angiosarcoma) is 1 in 1,000 cases of breasts treated with radiotherapy for breast cancer. The definition of radiation-induced sarcoma (RIS) has changed over the years. Currently, it is recommended that the diagnosis of RIS should meet specific criteria [4]. These criteria are shown in Table 1. In recent years, there has been an increase in radiotherapy treatment for breast cancer management following surgery. The main reason for this increase is the result of women choosing lumpectomy and radiotherapy instead of mastectomy. The increase in radiotherapy usage may lead to an increased incidence of RIAS. Salminen et al. [5] have reported an increased incidence in RIS, including angiosarcoma of the breast. These authors showed a slightly higher incidence than the usually reported rates of 0.03–0.2%. Among RIS, angiosarcoma is the most common.
Table 1.
Criteria for diagnosis of radiation-induced sarcoma
| Criteria for diagnosis of radiation-induced sarcoma |
| The histology of the sarcoma must be proven histologically and different from the histology of the primary tumor |
| The sarcoma should be located within the treatment field or in the tissue volume included in the 5% isodose line |
| Patients with cancer syndromes should be excluded |
| The latent period to the development of RIAS should be at least 4 years from treatment |
Clinical Case Presentation
Mrs. D.K. is a 67-year-old patient with adenocarcinoma of the left breast. The patient had no family history of cancer, no chronic diseases, and was treated with hormone replacement therapy for 5–6 years.
Diagnostic mammogram and sonar of the breast showed a 3.4 mm × 2.4 mm density at 2 o'clock, midway between nipple and axilla in the left breast. MRI of the breasts after biopsy failed to show any evidence of malignancy in both breasts. The patient had a left lumpectomy and left sentinel node biopsy.
The pathology report showed a tubular carcinoma. The lesion was classified as a pT1a, pN0 (0/5), ER negative, PR positive, Her2++, FISH negative, and a Ki67 of less than 5%. She had 3D planned radiotherapy to the left breast, 1.8 Gy/X28#, plus a boost to the tumor bed, guided by the clips placed in the tumor bed, delivering 1.8 Gy daily for seven fractions, with 6-MV photons. At completion of radiotherapy, adjuvant endocrine therapy with tamoxifen was started. The patient received tamoxifen for 5 years. Follow-up visits were done every 6 months and mammograms every year.
Four years after completing radiotherapy, the patient had a left breast biopsy due to a BIRADS IVB lesion in the left inner quadrant. Pathological report showed fat necrosis.
Ten years following the completion of radiotherapy, the patient developed two nodules in the left medial breast skin (Fig. 1). A biopsy of the nodules showed a hyperkeratotic epidermis. Within the dermis, there was a well-circumscribed neoplasm extending to the deep margins of the biopsy. The neoplasm comprised thin-walled vascular channels lined by hyperchromatic, atypical plump endothelial cells with irregular nuclei. There were 12 mitoses per 10 per high-power field. HHV8 immunohistochemical stain was negative. The cells were negative for AE1/AE3 and positive for CD31. A high-grade radiation-associated angiosarcoma was diagnosed. There was no evidence of recurrent tubular breast cancer in this specimen.
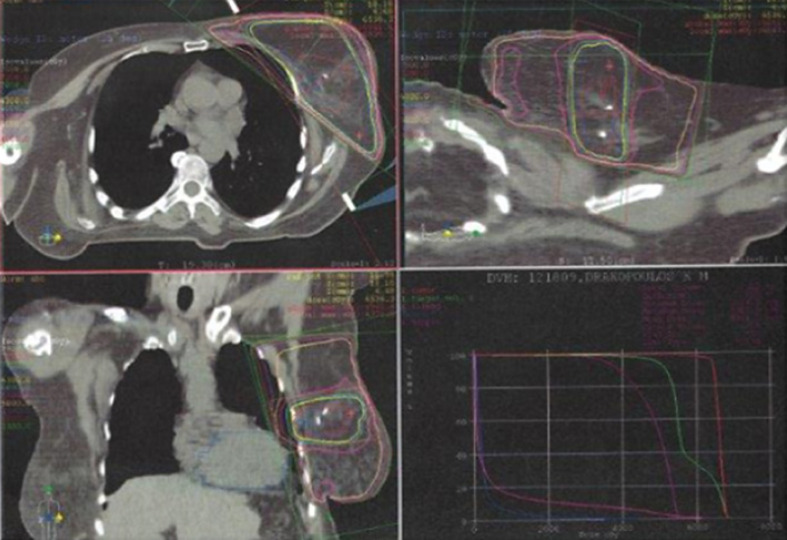
Fig. 1.
CT scan planning for radiation to the breast.
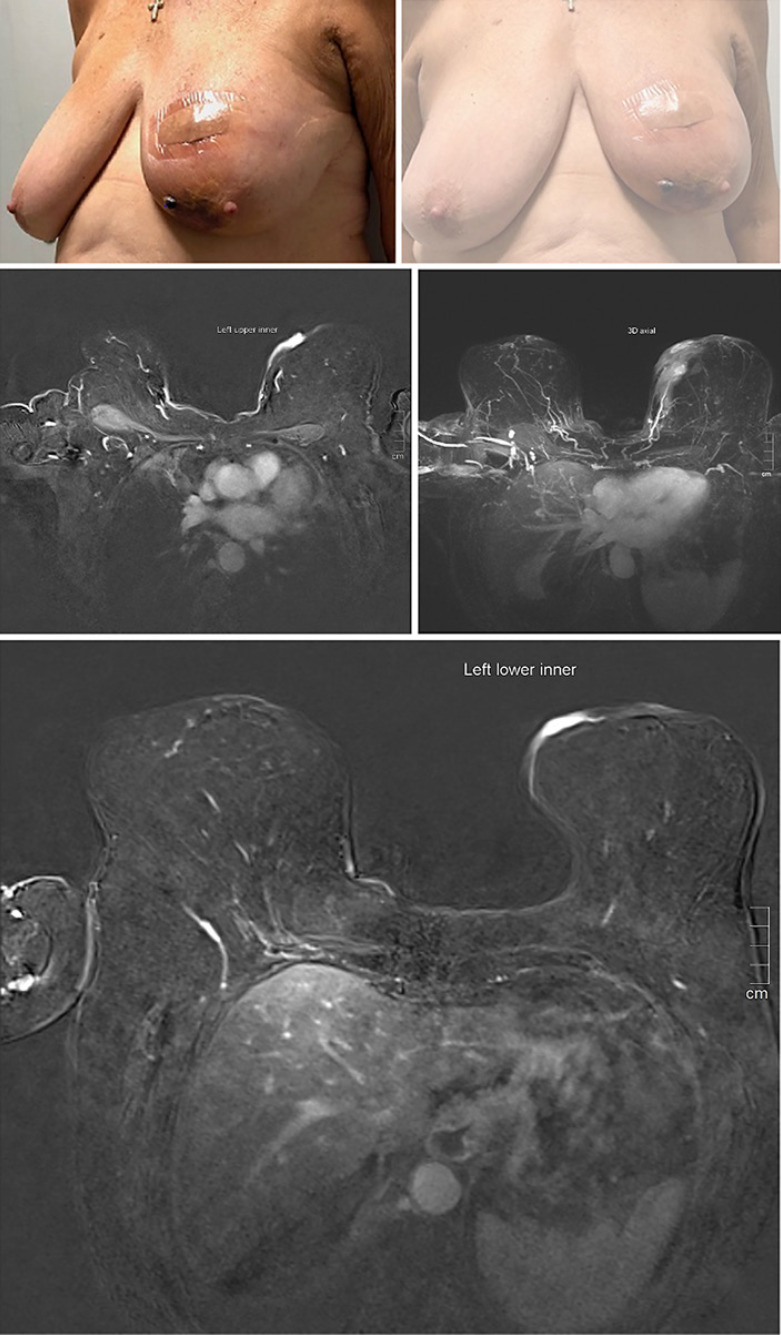
MRI of the breasts showed two lesions. The first was described as an oval focus of enhancement in the left lower inner quadrant in the skin, measuring 10 × 8 mm in size. The second one was a 3-mm, BIRADS II lesion in the right, with bilateral reactive lymphadenopathy (Fig. 2). CT chest, abdomen, and pelvis did not reveal any evidence of systemic disease.
Fig. 2.
Clinical and MRI radiological features of breast angiosarcoma.
A left simple mastectomy and right breast reduction were performed. The pathological report showed two foci of tumor cells, one of 18 mm, in the upper inner quadrant, and an additional 12-mm lesion in the lower inner quadrant (Fig. 3). Histopathology showed a high-grade angiosarcoma with a mitotic count of 30 per ten high-power fields. c-MYC gene amplification was confirmed in fluorescent in situ hybridization studies (Abbott VYSIS probes for CEP8 and C-MYC). The mean absolute copy number of C-MYC was 13.7 copies per nucleus, and the mean copy number of CEP8, the centromeric region of chromosome 8 used to normalize for the number of chromosome 8 copies in the nucleus, was 2.24. The lesion was staged a pT1, T1NxG3, stage II according to AJCC sarcoma staging [6].
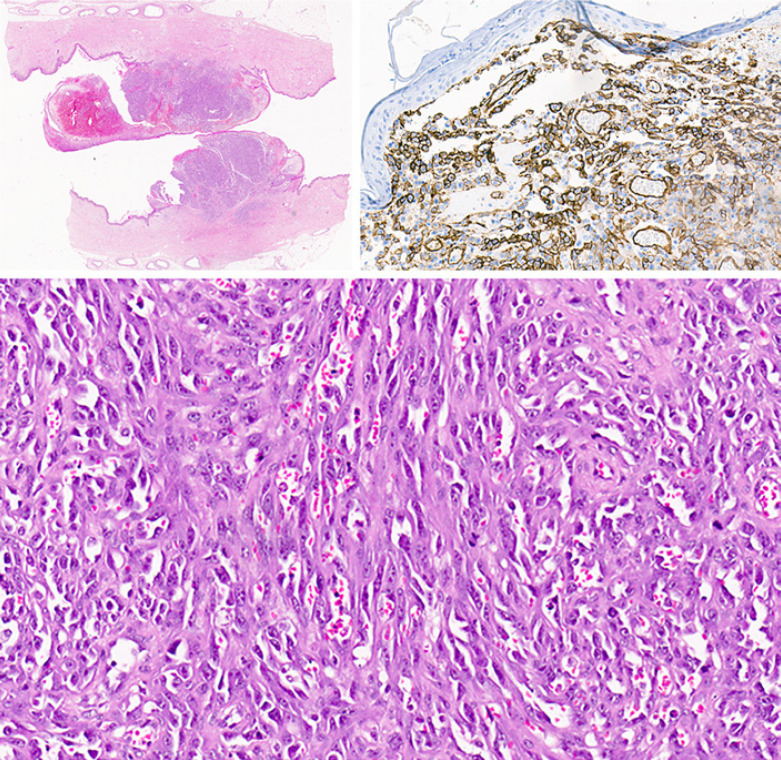
Fig. 3.
Pathology description. Macroscopic examination of the breast showed a polypoid lesion on the skin with ulceration. The low magnification image shows the lesion involving the dermis of the skin and no extension or multifocal foci within the deeper tissues. The tumor morphology shows a vasoformative spindle cell proliferation with red blood cells within the vascular spaces formed by the spindle cells. The tumor cells are positive with CD34 and CD31. In situ hybridization for the MYC gene showed amplification of MYC with a mean copy number of 13.7 copies per nucleus (CEP8 aqua; MYC red). Together the features are indicative of post-radiation angiosarcoma of the breast.
At surgery, clear margins were achieved, in all directions, with 15-mm inferior, 25-mm superior, 30-mm medial, and 60-mm deep margins. The tumor was limited to the skin, with no involvement of breast tissue. No further adjuvant therapy was given.
It was difficult to accurately assess the radiation dose given to the sarcoma area due to changes in the breast shape in 10 years. From a rough estimation, it seems that the volume was in the 40–45 Gy isodose line. Following surgery, the patient was followed up clinically every 3–6 months, and she remained in remission without local or systemic recurrence for 2 years.
Discussion
RIAS is a rare type of secondary angiosarcoma. Recent data from SEER summarized by Snow et al. [7] showed that breast cancer patients had the highest incidence of secondary sarcomas (0.03–0.2%). According to the same authors, considering all post-radiation sarcomas, breast cancer patients have the highest RIS number and account for 52.1% of all radiation-induced sarcomas. The relative risk of RIS in breast cancer patients compared to 17 other primary cancer sites was 1.21 (CI: 1.01–1.46, p < 0.03, adjusted for age at primary diagnosis, gender, and latency).
In recent years, radiotherapy for breast cancer following surgery is often used due to women choosing lumpectomy and radiotherapy instead of mastectomy. The increase in radiotherapy usage may lead to an increased incidence of RIS.
The MYC genes are a family of transcription factors involved in many cell functions, including cell growth, maturation, and death, including c-MYC, l-MYC, and n-MYC. Genetic changes in the MYC genes are well described within malignancies, and these include translocations commonly seen in hematolymphoid malignancies where MYC gene expression is driven off a highly active promoter, for example, in Burkitt lymphoma, where MYC gene expression is driven off the IgH promoter as a result of the T8:14 translocation that essentially defines these tumors. In RIAS, the oncogenic driver stimulation from increased MYC activity is produced by gene amplification of the MYC locus resulting in increased gene dosage and consequent c-MYC oncogene expression. A similar mechanism of the oncogenic driver is seen with n-MYC in neuroblastoma and amplification of other genes in malignancies, including HER2 in breast cancer. The presence of MYC as an oncogenic driver is associated with a poorer prognosis and unfavorable survival [8].
The MYC oncoprotein is a transcription factor and has a role in cell proliferation, cellular differentiation, and apoptosis. MYC also stimulates angiogenesis and promotes invasion and metastasis. MYC amplification can be observed in high-grade chondrosarcomas, epithelioid sarcomas of the proximal type, and high-grade myxoid liposarcoma [8].
In our case, the MYC analysis was performed to differentiate between primary angiosarcoma and RIAS. Although MYC overexpression is often reported to be more common in patients with RIAS, the evidence is neither obvious nor consistent. Our patient's indication for evaluation of c-MYC was to distinguish between radiotherapy-induced angiosarcoma of the breast and primary angiosarcoma of the breast.
Mentzel et al. [9] showed that 24/25 patients (96%) diagnosed with RIAS were positive for MYC gene amplification by FISH analysis. Guo et al. [10] found a high MYC amplification level in 100% of secondary angiosarcoma but none in the radiation-associated atypical vascular lesions. Manner et al. [11] found MYC amplification in 55% of secondary angiosarcoma (radiation, chronic lymphedema) but not in primary angiosarcoma.
Guo et al. [10] analyzed ten RIAS, and all of them had MYC amplification compared to none of the eleven atypical vascular lesions. Laé et al. [12] conducted a study on 47 patients with breast angiosarcomas. Amplification of the c-MYC oncogene (5- to 20-fold) was demonstrated in all 32 breast RIAS and only 1 of the 15 patients (7%) with primary angiosarcomas. The authors concluded that fundamental pathogenetic differences exist between primary breast angiosarcomas and RIAS which are impossible to differentiate morphologically [12].
In contrast, a study conducted at the University of Michigan demonstrated that MYC overexpression could be seen in primary angiosarcoma. In this study, MYC immunohistochemistry was carried out on representative whole tissue sections of a large retrospective cohort of patients with primary angiosarcoma, RIAS, Stewart-Treves syndrome, and AVL and evaluated MYC using a semiquantitative scoring method [13].
In this study, MYC was strongly expressed in most RIAS and Stewart-Treves syndrome, while primary angiosarcoma demonstrated inconsistent MYC expression, and high-grade tumors showed significantly higher MYC expression than low-grade tumors. In contrast, MYC expression in AVL is predominantly negative but may occasionally show focal staining [13].
Additionally, the authors suggested that increased MYC expression in high-grade primary angiosarcoma may play a role in the pathogenesis of angiosarcomas, and MYC IHC may be a prognostic and potentially a predictive biomarker in a subset of these tumors [13]. The Stewart-Treves syndrome is an unusual condition associated with high mortality, defined as angiosarcoma in patients with chronic lymphedema. It is classically seen in women who develop lymphedema in the upper extremity following axillary lymph node dissection for breast cancer surgery [14].
In addition, Maillard et al. [15] reported a 16-year-old patient diagnosed with primary MYC-positive angiosarcoma. Shiraki et al. [16] reported an MYC-positive angiosarcoma in an 80-year-old female. The patient was treated with BCS and did not receive radiotherapy due to her advanced age but was treated with endocrine therapy [16]. Moreover, the diagnosis of RIAS was also described in a patient who had partial breast radiotherapy [17]. Recently, RIAS was also described in a male patient [18]. In this study, the MYC was not evaluated [18].
A high MYC level was also associated with chronic lymphedema associated with secondary angiosarcoma in 55% of cases [11]. RIAS of the breast is a rare and devastating event. With the extended indications for adjuvant radiation therapy in breast cancer to improve local control and survival, more cases may be diagnosed in the future. Even in cases where the volume irradiated is reduced, as practiced in partial breast radiation therapy, secondary angiosarcoma is described [19]. Kirova et al. [20] reported a 0.07% cumulative incidence at 5 years, 0.27% at 10 years, and 0.48% at 15 years.
RIAS mainly presents as a purple nodule in the skin that may be limited to one area or diffuse. The diagnosis is made by core biopsy as the clinical presentation may be confused with other endothelial vascular pathologies or benign conditions. In the past, the presence of MYC amplification in the tumor could differentiate between primary angiosarcoma and RIAS. Recent data suggest that MYC overexpression might not be as valuable as initially described as MYC has been described in other forms of angiosarcomas.
Several gene mutations have been reported in RIAS, including inactivation of p53 and the amplification of the 8q24 region containing the MYC oncogene, as discussed above. Amplification of FLT4 has also been described [9]. A recent report indicated that a mutational pattern associated with genes involved in DNA repair might be important in the pathogenesis of RIAS [21].
The presence of MYC in the tumor can assess the resection margins and ascertain R0 resection, which is a crucial factor in local recurrence. In the future, it might be possible to use these characteristics as a predictive or prognostic biomarker; however, large prospective studies are needed to confirm these observations. Despite the poor prognosis and different adjuvant and neoadjuvant treatment modalities, there is consensus that the most efficient treatment is a mastectomy, with R0 resection, with wide margins, up to 3 cm, where feasible [1].
The prognosis of RIAS is poor. The 5-year survival rates vary from 27% to 48%. Recurrence is not limited to local recurrence, and distant metastases to the lung, bones, liver, nodes, and contralateral breast have been reported. There are no accepted criteria to prognosticate the behavior of this malignancy [1]. The role of adjuvant radiotherapy in RIAS is very controversial and usually not indicated, bearing in mind that this condition is a radiation-induced malignancy, and previous radiation has by definition been received to the dose area.
Radiotherapy alone or combined with chemotherapy has been used for recurrence post-surgery or when initial surgery was not feasible. Chemotherapy as neoadjuvant, adjuvant, and in metastatic or recurrent disease has modest activity [1]. There are no chemotherapy regimens that are regarded as the standard of care. Several reports describe the use of taxanes, anthracycline, ifosfamide, vincristine, cyclophosphamide, and even bevacizumab [1]. The response to these agents alone or in combination is partial and lasts a few months. The possibility of targeted therapy should be explored. Future directions in this field include the usage of gene expression analysis and next-generation sequencing [22]. Hadj-Mamou et al. [23] detected a gene expression signature to differentiate between RIAS and sporadic sarcomas. Gene analysis revealed a signature of 135 genes that differentiated between the two groups. As a result, 96% of RIAS and 62% of sporadic sarcomas were accurately assessed using this signature [23]. Additionally, a more recent RNA sequencing study by Lesluyes et al. [24] reported indistinguishable transcriptomic profiles between radiation-associated sarcomas and sporadic sarcomas. It is possible that genomic testing will help differentiate between the two clinical entities in the future. Due to this tumor aggressiveness, locally and systemically, the follow-up should include local and systemic evaluation, 3 to 6 monthly, with clinical examination and CT scans.
Conclusion
We presented a classic case of RIAS. Our case falls in line with the reported cases in the literature and fulfills all criteria for diagnosing RIAS or secondary angiosarcoma. Our case was MYC positive. Although MYC was considered an important test to distinguish between primary angiosarcoma and RIAS, some studies demonstrated the contrary. Clinical examination and early detection of breast angiosarcoma are essential to improve outcomes and cure patients with this aggressive condition.
Statement of Ethics
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. Ethics approval was not required for the publication of this case report.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Sources
None.
Author Contributions
All of the authors contributed equally to the conceptualization of the manuscript; E.R., C.B., and B.L.R. provided clinical input, C.M. provided pathology input, E.R. and B.L.R. provided editorial oversight. All of the authors provided a critical appraisal of the manuscript and approved its submission.
Data Availability Statement
The data of this case report are available from the medical records of the patient.
Acknowledgments
Professor B.L. Rapoport is supported by the Cancer Association of South Africa (CANSA) and the National Research Foundation (NRF) of South Africa.
References
- 1.Cohen-Hallaleh RB, Smith HG, Smith RC, Stamp GF, Al-Muderis O, Thway K, et al. Radiation induced angiosarcoma of the breast: outcomes from a retrospective case series. Clin Sarcoma Res. 2017 Aug 7;7:15. doi: 10.1186/s13569-017-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonito FJP, de Almeida Cerejeira D, Dahlstedt-Ferreira C, Oliveira Coelho H, Rosas R. Radiation-induced angiosarcoma of the breast: a review. Breast J. 2020 Mar;26((3)):458–463. doi: 10.1111/tbj.13504. [DOI] [PubMed] [Google Scholar]
- 3.Verdin V, Mattart L, Cusumano PG, De Hertogh O, De Meester C, Francart D, et al. Angiosarcoma associated with radiation therapy after treatment of breast cancer. Retrospective study on ten years. Cancer Radiother. 2021 Apr;25((2)):114–118. doi: 10.1016/j.canrad.2020.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Rao N, Wong S, Panikkar V, Shidham VB, Hackbarth DA. Postradiation sarcoma. 2020. Available from: https://emedicine.medscape.com/article/1253714-overview Accessed 2021 Mar 7.
- 5.Salminen SH, Sampo MM, Böhling TO, Tuomikoski L, Tarkkanen M, Blomqvist CP. Radiation-associated sarcoma after breast cancer in a nationwide population: increasing risk of angiosarcoma. Cancer Med. 2018 Sep;7((9)):4825–4835. doi: 10.1002/cam4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cates JMM. The AJCC 8th edition staging system for soft tissue sarcoma of the extremities or trunk: a cohort study of the SEER database. J Natl Compr Canc Netw. 2018 Feb;16((2)):144–152. doi: 10.6004/jnccn.2017.7042. [DOI] [PubMed] [Google Scholar]
- 7.Snow A, Ring A, Struycken L, Mack W, Koç M, Lang JE. Incidence of radiation induced sarcoma attributable to radiotherapy in adults: a retrospective cohort study in the SEER cancer registries across 17 primary tumor sites. Cancer Epidemiol. 2021 Feb;70:101857. doi: 10.1016/j.canep.2020.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dang CV. MYC on the path to cancer. Cell. 2012 Mar 30;149((1)):22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mentzel T, Schildhaus HU, Palmedo G, Büttner R, Kutzner H. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological, immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012 Jan;25((1)):75–85. doi: 10.1038/modpathol.2011.134. [DOI] [PubMed] [Google Scholar]
- 10.Guo T, Zhang L, Chang NE, Singer S, Maki RG, Antonescu CR. Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes Chromosomes Cancer. 2011 Jan;50((1)):25–33. doi: 10.1002/gcc.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manner J, Radlwimmer B, Hohenberger P, Mössinger K, Küffer S, Sauer C, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010 Jan;176((1)):34–39. doi: 10.2353/ajpath.2010.090637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laé M, Lebel A, Hamel-Viard F, Asselain B, Trassard M, Sastre X, et al. Can c-myc amplification reliably discriminate postradiation from primary angiosarcoma of the breast? Cancer Radiother. 2015 May;19((3)):168–174. doi: 10.1016/j.canrad.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Udager AM, Ishikawa MK, Lucas DR, McHugh JB, Patel RM. MYC immunohistochemistry in angiosarcoma and atypical vascular lesions: practical considerations based on a single institutional experience. Pathology. 2016 Dec;48((7)):697–704. doi: 10.1016/j.pathol.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Berebichez-Fridman R, Deutsch YE, Joyal TM, Olvera PM, Benedetto PW, Rosenberg AE, et al. Stewart-Treves syndrome: a case report and review of the literature. Case Rep Oncol. 2016 Apr 1;9((1)):205–211. doi: 10.1159/000445427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maillard C, Duhoux FP, Galant C, Libbrecht L, Lengelé B, Coyette M, et al. High-grade primary angiosarcoma of the breast with MYC amplification: case-report of a 16-year-old patient and review of the literature. Austin J Surg. 2019;6((23)):id1225. [Google Scholar]
- 16.Shiraki E, Kang Y, Shibayama T, Tsuyuki S. Two cases of breast angiosarcoma after breast conserving surgery. Surg Case Rep. 2020 Apr 26;6((1)):81. doi: 10.1186/s40792-020-00841-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansfield SA, Zynger DL, Agnese DM. Angiosarcoma and breast cancer recurrence eight years following mammosite therapy. Breast J. 2014 Nov–Dec;20((6)):658–660. doi: 10.1111/tbj.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsapralis N, Vlachogiorgos A, Pham H, Mowatt D. Nothing is impossible: radiation induced angiosarcoma of breast in a male patient. J Surg Case Rep. 2019 May 23;2019((5)):rjz158. doi: 10.1093/jscr/rjz158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Disharoon M, Kozlowski KF, Kaniowski JM. Case 242: radiation-induced angiosarcoma. Radiology. 2017 Jun;283((3)):909–916. doi: 10.1148/radiol.2017150456. [DOI] [PubMed] [Google Scholar]
- 20.Kirova YM, Vilcoq JR, Asselain B, Sastre-Garau X, Fourquet A. Radiation-induced sarcomas after radiotherapy for breast carcinoma: a large-scale single-institution review. Cancer. 2005 Aug 15;104((4)):856–863. doi: 10.1002/cncr.21223. [DOI] [PubMed] [Google Scholar]
- 21.Thibodeau BJ, Lavergne V, Dekhne N, Benitez P, Amin M, Ahmed S, et al. Mutational landscape of radiation-associated angiosarcoma of the breast. Oncotarget. 2018 Jan 19;9((11)):10042–53. doi: 10.18632/oncotarget.24273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen TW, Burns J, Jones RL, Huang PH. Optimal clinical management and the molecular biology of angiosarcomas. Cancers. 2020 Nov 10;12((11)):3321. doi: 10.3390/cancers12113321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadj-Hamou NS, Ugolin N, Ory C, Britzen-Laurent N, Sastre-Garau X, Chevillard S, et al. A transcriptome signature distinguished sporadic from postradiotherapy radiation-induced sarcomas. Carcinogenesis. 2011 Jun;32((6)):929–934. doi: 10.1093/carcin/bgr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lesluyes T, Baud J, Pérot G, Charon-Barra C, You A, Valo I, et al. Genomic and transcriptomic comparison of post-radiation versus sporadic sarcomas. Mod Pathol. 2019 Dec;32((12)):1786–1794. doi: 10.1038/s41379-019-0300-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this case report are available from the medical records of the patient.