Abstract
Background
Long-term nucleos(t)ide analogue (NA) treatment can reverse liver fibrosis in chronic hepatitis B (CHB), but its effect on fibrosis regression remains limited. Biejia-Ruangan (BR) has been approved in China as an antifibrotic traditional Chinese medicine drug in patients with chronic liver diseases. A multicenter randomized controlled trial aims to evaluate the effect of BR on fibrosis regression in CHB patients treated with NAs.
Methods
CHB patients with histologically confirmed advanced fibrosis or cirrhosis were randomly assigned to receive entecavir (ETV) (0.5 mg per day) plus BR (2 g 3 times a day) or placebo for 72 weeks. Liver fibrosis regression was defined as a reduction of ≥ 1 point by the Ishak fibrosis stage (IFS).
Results
Overall, 500 patients were enrolled in each group as the intention-to-treat population. The rate of fibrosis regression after 72 weeks of treatment was significantly higher in the ETV + BR group (40% vs 31.8%; P = .0069). Among 388 patients with cirrhosis (ie, IFS ≥ 5) at baseline, the rate of cirrhosis reversal (ie, IFS ≤ 4) was significantly higher in the ETV + BR group (41.5% vs 30.7%; P = .0103).
Conclusions
Addition of BR to the current standard treatment with NAs in CHB patients with advanced fibrosis or cirrhosis can improve liver fibrosis regression.
Clinical Trials Registration
Keywords: hepatitis B, liver fibrosis, liver cirrhosis, entecavir, traditional Chinese medicine
In this multicenter randomized controlled trial of 1000 patients with chronic hepatitis B and advanced fibrosis or cirrhosis, Biejia-Ruangan as an add-on therapy to entecavir significantly increased rates of fibrosis regression and cirrhosis reversal.
Chronic hepatitis B (CHB) can progress to liver cirrhosis, thereby resulting in the development of hepatocellular carcinoma (HCC), liver failure, and liver-related mortality [1, 2]. Historically, liver cirrhosis was considered an irreversible state [3]. Recently, however, it has been reported that long-term treatment of hepatitis B virus (HBV) with nucleoside or nucleotide analogues (NAs) can lead to histological improvement and even regression of liver fibrosis/cirrhosis [4–6]. However, the effect of NAs alone on reversing fibrosis/cirrhosis is limited [7]. Moreover, it remains unknown whether the additional use of an antifibrotic agent can further enhance the effect of NAs on reversing fibrosis/cirrhosis in CHB patients.
Traditional Chinese medicine (TCM) is one of the most ancient medical systems worldwide with its well-known unique principles and comprehensive theory [8]. According to the TCM theory, liver cirrhosis is a condition secondary to the weakened qi and blood (where “qi” refers to the biological substance or energy that preserves life and “blood” refers to the transporter for qi) and blockage of meridians, which are the circulation channels for qi, by “blood stasis” [9]. Biejia-Ruangan (BR) tablet, a quintessence complex of TCM, has been used in China for the treatment of liver cirrhosis since at least 1967. With the use of modern pharmacological and toxicological analysis, the composition of BR has been identified as a formula of 10 different Chinese herbal medicines, the quality and quantity of each component being strictly maintained via national standardization. In 1999, based on the results of a randomized, double-blind, clinical trial on 420 CHB patients, the China Food and Drug Administration approved BR as the first TCM antifibrotic regimen for treating liver fibrosis/cirrhosis caused by CHB [10]. However, NAs, the standard therapy for CHB in the contemporary era, were not employed in this early trial.
According to chromatographic analysis, BR contains at least 32 antifibrotic compounds [11]. Unlike NAs, BR exerts its antifibrotic effect in a direct fashion via several different mechanisms [12]. BR is able to reduce the expression of the tissue inhibitor of metalloproteinase and type I and III procollagen produced by fibroblast cells and also has a strong inhibition of transforming growth factor–β/Smad-induced fibrogenesis and suppression of hepatic stellate cell proliferation [13–15]. Aside from TCM theory, it is possible that there may be a bona fide scientific basis for BR’s purported benefit in liver disease that merits formal clinical study utilizing the accepted methodology of modern medical science. Considering that the potential mechanism of action is different between NAs and BR in CHB patients, we have hypothesized that an add-on therapy with BR in CHB patients treated with NAs can result in a synergistic antifibrotic effect. Thus, we conducted a multicenter randomized controlled trial (RCT) to compare the rate of fibrosis regression in CHB patients treated with NAs alone vs in combination with BR.
PATIENTS AND METHODS
Study Design
This is a multicenter, randomized, double-blind, placebo-controlled trial conducted in 14 institutions in China. The study was approved by the institutional review board at all study sites, and written informed consent was obtained from all study subjects. The data were collected and reported as recommended by the Consolidated Standards of Reporting Trials (CONSORT) guidelines and the CONSORT extension for Chinese herbal medicine formulas [16, 17]. The study protocol has been published [18]. Herein we report the first interim analysis of this 5-year follow-up study, which remains ongoing.
Endpoints
The primary endpoint was regression of liver fibrosis, which is defined as a reduction of ≥ 1 point by the Ishak fibrosis stage (IFS) system at treatment week 72 [19]. Secondary endpoints included histological improvement, which is defined as a reduction of ≥ 2 points by the Knodell necroinflammatory score (KNS) system with no worsening of fibrosis [20], undetectable serum HBV DNA (< 40 IU/mL), normalization of serum alanine aminotransferase (ALT) level (< 40 IU/mL), hepatitis B e antigen (HBeAg) seroconversion in HBeAg-positive CHB patients, and hepatitis B surface antigen (HBsAg) loss and seroconversion.
Study Population
Patients who met the following inclusion criteria and agreed to participate were enrolled: (1) men or women aged 18–65 years with positive HBsAg for at least 6 months; (2) for HBeAg-positive CHB, HBV DNA ≥ 20 000 IU/mL and ALT ≥ 2 times the upper limit of normal (ULN); for HBeAg-negative CHB, HBV DNA ≥ 2000 IU/mL and ALT ≥ 2 times the ULN; or clinically compensated cirrhosis, regardless of HBeAg status, with detectable serum HBV DNA at screening before liver biopsy, with the following criteria: (a) normal ALT and liver stiffness > 9 kPa or elevated ALT < 5 times the ULN and liver stiffness > 12 kPa; (b) platelet counts of < 100 000/mL and ultrasonography findings suggestive of cirrhosis, including a blunted, nodular liver edge accompanied by splenomegaly (length of spleen > 12 cm); and (c) clinical signs of portal hypertension, such as esophageal or gastric varices, in the absence of ascites, variceal hemorrhage, and hepatic encephalopathy; (3) liver histology showing an IFS of ≥ 3; and (4) naive to treatment with NAs or no HBV antiviral or antifibrotic therapy for at least 6 months.
The exclusion criteria were as follows: (1) decompensated cirrhosis, which is defined by the presence of overt clinical complications of cirrhosis, such as variceal hemorrhage, ascites, or hepatic encephalopathy; (2) other viral hepatitis, human immunodeficiency virus infection, or other chronic liver diseases; (3) IFS < 3; (4) serious diseases of other organs or mental illness; (5) pregnancy or lactation; or (6) history of any concurrent malignancy, including HCC.
Sample Size Calculation
In patients who received entecavir (ETV) monotherapy, the rates of fibrosis regression were 32% at 48 weeks [4] and 48% at 72 weeks (our unpublished data). The rate of fibrosis regression was estimated to be 60% at 72 weeks in patients who received ETV + BR treatment. Using PASS statistical software (version 11.0, NCSS), 186 cases would be needed in each group to achieve a power of 0.80 and a significance level of .05. Considering a dropout rate of approximately 15%, 223 cases would be needed in each group. Additionally, based on a prestudy survey in the main study center, we assume that approximately 45% of the study patients would agree to have a follow-up liver biopsy. Thus, 500 cases would be needed in each group for the present study.
Interventions and Follow-up
After enrollment and pretreatment liver biopsy, patients were randomized blindly by unsealing opaque envelopes with random numbers in a 1:1 ratio to the BR or control group. All patients received ETV 0.5 mg/day + BR 2.0 g 3 times/day (BR group) or placebo tablets, 2.0 g 3 times/day (control group). The placebo tablets were made of barley powder and similar to BR in taste, shape, and color. All agents were administered orally.
All participants were followed every 12 weeks with clinical and safety evaluation, serum ALT, HBV DNA (the Roche Amplicor COBAS polymerase chain reaction assay), HBV serologic tests (by enzyme immunoassay), and abdominal sonography for HCC screening. For patients with new ultrasound finding of hepatic lesion or elevated alpha-feta protein, the diagnosis of HCC was based on standard histological and/or compatible radiological findings [21].
All patients were offered a follow-up liver biopsy at treatment week 72. Ultrasound-guided liver biopsy was performed according to a standard protocol. A quick-cut needle or Menghini needle, 16 G (Allegiance Corporation) was used for biopsy. A minimum (20 mm) length of the liver tissue and at least 2 pieces of liver tissue were collected to ensure that there were at least 11 portal tracts for pathological evaluation [22]. All liver biopsies were reviewed in a central pathology by 2 liver pathologists blinded to treatment assignment and time for biopsy. Biopsies with inconsistent reports were re-reviewed by both pathologists together to reach a consensus. Histological assessment included 2 major parts: (1) fibrosis stage evaluated by the IFS system (ie, 0 indicates no fibrosis and ≥ 5 indicates cirrhosis) [19]; and (2) the inflammation activity assessed by the KNS system (ie, a score of ≤ 3 indicates mild or no necroinflammation and a score of ≥ 10 indicates severe necroinflammation) [20].
The data and safety monitoring board (DSMB) of the present study consisted of 3 independent hepatologists and a statistician. The DSMB protected the ethical interests and safety of each participant by reviewing the safety results every 12 weeks. Additionally, the DSMB was empowered to recommend study termination based on safety concerns or as soon as sufficient evidence indicated that ETV + BR was statistically superior to ETV alone or that ETV + BR did not provide a significant advantage over ETV alone. During the study, patients were free to choose discontinuing the study medicines, but it was advised that they should be followed for additional 24 weeks to assess the safety and outcomes.
Safety Analyses
The safety analysis was performed on all participants who received even a single dose of study medication. All events related to the disease itself or study medications were recorded. Adverse events (AEs) were considered to be serious or life-threatening in cases of hospitalization, disability, or death by the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 4.0). Analysis included the incidence of AEs, serious AEs (SAEs), laboratory abnormalities, and discontinuations due to AEs.
Statistical Analysis
Data analyses were performed using SAS version 9.4 software (SAS Institute). Categorical data were expressed as frequencies and continuous variables as either mean or median. Comparisons between groups were conducted by the Student t test (variance homogeneity), the Satterthwaite test (variance not homogeneity), or the Wilcoxon rank-sum test (variance not symmetric) for continuous variables and the χ 2 test for categorical variables. Statistical significance was defined as a 2-sided P < .05.
RESULTS
Study Population
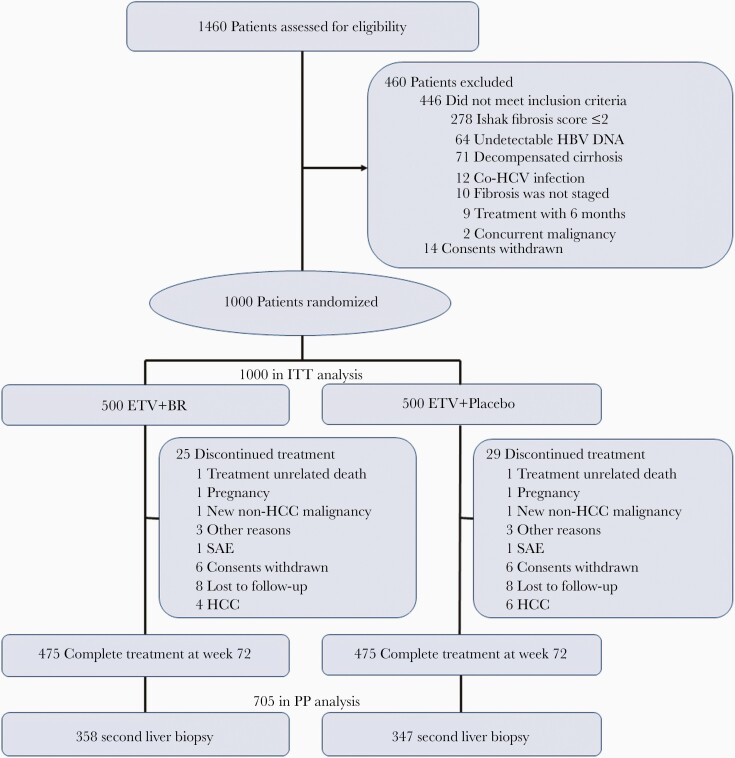
From October 2013 to October 2014, 1460 CHB patients were screened for eligibility. In total, 1000 patients were eligible and randomized (BR: 500; control: 500), and constituted the intention-to-treat (ITT) population (Figure 1). A total of 946 patients completed the 72-week posttreatment follow-up (BR: 475; control: 471). Among them, 705 patients underwent the second liver biopsy (BR: 358; control: 347) and constituted the per-protocol (PP) population. Of the 295 patients who did not undergo the second liver biopsy, 241 (81.69%) refused the procedure (BR: 117; control: 124) and 54 (18.31%) discontinued treatment (BR: 25; control: 29). Reasons for the treatment discontinuation included treatment-unrelated death (BR: 1; control: 3), occurrence of HCC (BR: 4; control: 6), SAE (BR: 1; control: 1), pregnancy (BR: 1; control: 1), occurrence of non-HCC cancer (BR: 1; control: 2), other reasons (BR: 3; control: 3), withdrawal of consent (BR: 6; control: 5), and loss to follow-up (BR: 8; control: 8) (Figure 1).
Figure 1.
Flowchart of patient enrollment. Abbreviations: BR, Biejia-Ruangan; ETV, entecavir; HBV, hepatitis B virus; HCV, hepatitis C virus; ITT, intention-to-treat; PP, per protocol; SAE, serious adverse events.
The characteristics of the ITT and PP populations are shown in Tables 1 and 2, respectively. Regardless of the ITT or PP population, all variables were well comparable between the 2 groups.
Table 1.
Characteristics of the Intention-to-Treat Population
| Characteristic | ETV + BR (n = 500) | ETV + Placebo (n = 500) | P Value |
|---|---|---|---|
| Age, y, mean ± SD | 41.9 ± 9.7 | 41.7 ± 10.0 | .75 |
| Male sex, No. (%) | 348 (69.6) | 351 (70.2) | .84 |
| WBC count, × 109/L, mean ± SD | 5.5 ± 1.7 | 5.5 ± 1.7 | .99 |
| Hemoglobin, g/L, mean ± SD | 142.7 ± 16.7 | 144.2 ± 16.1 | .15 |
| Prothrombin time, sec, mean ± SD | 12.6 ± 1.7 | 12.7 ± 1.7 | .63 |
| ALT, U/L, median (P25, P75) | 54 (32, 89.5) | 54 (33, 107.5) | .16 |
| Bilirubin, μmol/L, median (P25, P75) | 13.6 (10.45, 18.75) | 14.0 (11.00, 19.00) | .48 |
| Albumin, g/L, median (P25, P75) | 42.05 (39, 45) | 42 (39, 45) | .64 |
| Platelet count, × 109/L, mean ± SD | 161.7 ± 59.8 | 162.3 ± 60.1 | .89 |
| HBV DNA, 1og10 copies/mL, mean ± SD | 6.9 ± 1.7 | 6.9 ± 1.6 | .87 |
| HBeAg positive, No. (%) | 290 (58) | 291 (58.2) | .95 |
| Knodell necroinflammatory score, No. (%) | .77 | ||
| 3–6 | 225 (45) | 214 (42.8) | |
| 7–10 | 238 (47) | 249 (49.8) | |
| 11–14 | 37 (7) | 37 (7.4) | |
| Ishak fibrosis score, No. (%) | .32 | ||
| 3 | 118 (23.6) | 142 (28.4) | |
| 4 | 117 (23.4) | 104 (20.8) | |
| 5 | 106 (21.2) | 96 (19.2) | |
| 6 | 159 (31.8) | 158 (31.6) |
Abbreviations: ALT, alanine aminotransferase; BR, Biejia-Ruangan; ETV, entecavir; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; P25, 25th percentile; P75, 75th percentile; SD, standard deviation; WBC, white blood cell.
Table 2.
Characteristics of the Per Protocol Population
| Characteristic | ETV + BR (n = 358) | ETV + Placebo (n = 347) | P Value |
|---|---|---|---|
| Age, y, mean ± SD | 42.2 ± 9.8 | 42.4 ± 10.1 | .74 |
| Male sex, No. (%) | 243 (67.9) | 241 (69.5) | .65 |
| WBC count, × 109/L, mean ± SD | 5.4 ± 1.7 | 5.5 ± 1.8 | .59 |
| Hemoglobin, g/L, mean ± SD | 142.0 ± 16.9 | 143.5 ± 17.0 | .24 |
| Prothrombin time, sec, mean ± SD | 12.6 ± 1.7 | 12.5 ± 1.6 | .97 |
| ALT, U/L, median (P25, P75) | 53 (30, 87) | 53 (33, 117) | .16 |
| Bilirubin, μmol/L, median (P25, P75) | 13.4 (10.4, 18.0) | 14.2 (11.0, 19.0) | .15 |
| Albumin, g/L, median (P25, P75) | 42.0 (39.0, 44.8) | 42.0 (39.0, 45.0) | .34 |
| Platelet count × 109/L, mean ± SD | 161.7 ± 58.9 | 161.0 ± 58.3 | .87 |
| HBV DNA, 1og10 copies/mL, mean ± SD | 6.2 ± 1.6 | 6.1 ± 1.6 | .72 |
| HBeAg positive, No. (%) | 202 (56.4) | 200 (57.6) | .74 |
| Knodell necroinflammatory score | .64 | ||
| 3–6 | 158 (44.1) | 145 (41.8) | |
| 7–10 | 177 (49.5) | 174 (50.1) | |
| 11–14 | 23 (6.4) | 28 (8.1) | |
| Ishak fibrosis score | .59 | ||
| 3 | 86 (24.0) | 99 (28.5) | |
| 4 | 68 (19.0) | 64 (18.4) | |
| 5 | 77 (21.5) | 70 (20.2) | |
| 6 | 127 (35.5) | 114 (32.9) |
Abbreviations: ALT, alanine aminotransferase; BR, Biejia-Ruangan; ETV, entecavir; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; P25, 25th percentile; P75, 75th percentile; SD, standard deviation; WBC, white blood cell.
Primary Endpoint
The primary endpoint was met in 200 and 159 patients in the BR and control groups, respectively.
In the ITT analysis of 1000 patients, the BR group had a significantly higher rate of fibrosis regression than the control group (40% [200/500] vs 31.8% [159/500]; P = .0069). In the ITT analysis of 519 patients with liver cirrhosis at baseline, the BR group had a significantly higher rate of cirrhosis regression than the control group (41.5% [110/265] vs 30.7% [78/254]; P = .0103).
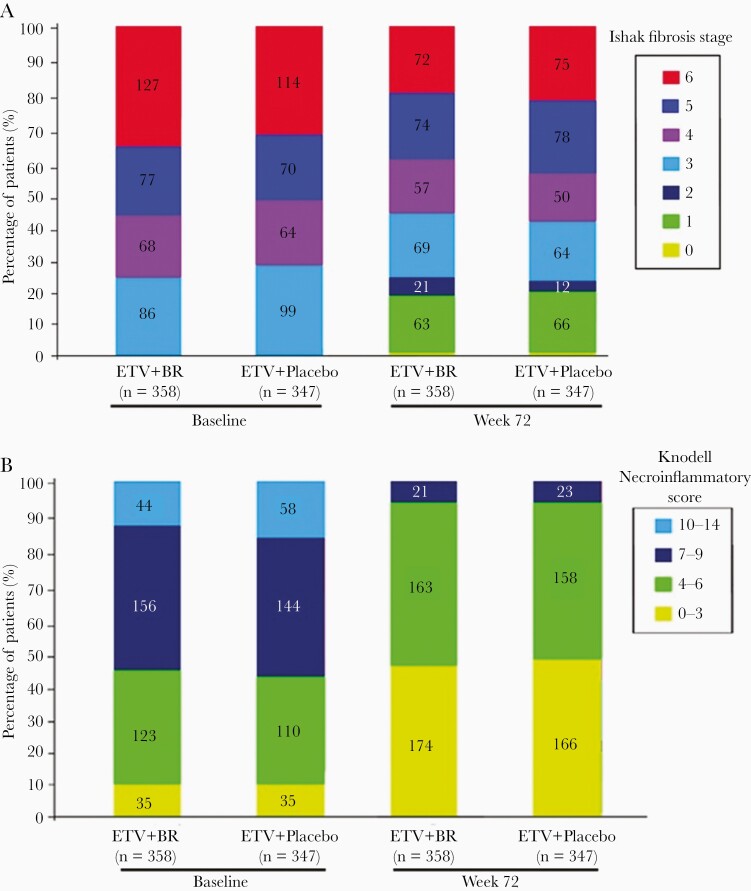
In the PP analysis of 705 patients, the distribution of IFS at baseline and treatment week 72 for both groups is shown in Figure 2A. The BR group had a significantly higher rate of fibrosis regression than the control group (55.9% [200/358] vs 45.8% [159/347]; P = .0076). The fibrosis progression rate was statistically similar between the 2 groups (10.3% [37/358] vs 11.8% [41/347]; P = .531). In the PP analysis of 388 patients with liver cirrhosis at baseline, the BR group had a significantly higher rate of cirrhosis regression than the control group (53.9% [110/204] vs 42.4% [78/184]; P = .0233). The fibrosis progression rate was statistically similar between the 2 groups (6.4% [13/204] vs 4.9% [9/184]; P = .0.529).
Figure 2.
Distribution of Ishak fibrosis stage (A) and Knodell necroinflammatory score (B) in the entecavir + Biejia-Ruangan and entecavir + placebo groups with baseline and 72-week treatment data. Abbreviations: BR, Biejia-Ruangan; ETV, entecavir.
Secondary Endpoints
In the PP analysis of 705 patients, the distribution of KNS at baseline and treatment week 72 for both groups is shown in Figure 2B. The rate of histologic necroinflammatory improvement was not significantly different between the BR and control groups (69.27% [248/358] vs 67.15% [233/347]; P = .5443).
In the PP analysis of 946 patients who completed the treatment, the rate of undetectable serum HBV DNA at week 72 was not significantly different between the BR and control groups (85.89% [408/475] vs 85.14% [401/471]; P = .6620).
In the PP analysis of 628 patients with baseline ALT level of > 40 U/L who completed the treatment, the rate of ALT normalization at week 72 was not significantly different between the BR and control groups (84.38% [254/301] vs 82.26% [269/327]; P = .4763).
In the PP analysis of 543 HBeAg-positive patients who completed the treatment, the rate of serum HBeAg seroconversion was significantly higher in the BR group than the control group (13.87% [38/274] vs 8.18% [22/269]; P = .0341).
No patient developed HBsAg seroconversion in either group.
Safety
All grade AEs occurred in 751 (75.1%) patients during the treatment and were not significantly different between the BR and control groups (77.0% [385/500] vs 73.2% [366/500]; P = .32) (Table 3). Among them, SAEs occurred in 87 patients and were not significantly different between the BR and control groups (8.8% [44/500] vs 8.6% [43/500]; P = .88).
Table 3.
Safety of the Intention-to-Treat Population
| Events | ETV + BR (n = 500) | ETV + Placebo (n = 500) | P Value |
|---|---|---|---|
| Adverse events | 385 (77.0) | 366 (73.2) | .32 |
| Serious adverse events | 44 (8.8) | 43 (8.6) | .88 |
| Discontinuation due to adverse events | 1 (0.2) | 1 (0.2) | 1.00 |
| ALT > 2 times baseline and > 10 times ULN | 17 (3.4) | 19 (3.8) | .52 |
| Death | 1 (0.2) | 3 (0.6) | .23 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ALT, alanine aminotransferase; BR, Biejia-Ruangan; ETV, entecavir; ULN, upper limit of normal.
AEs with a frequency of > 5% included headache, diarrhea, and nausea. Two patients discontinued treatment, including 1 patient in the BR group due to esophageal variceal bleeding and another in the control group due to acute renal injury. Thirty-six patients experienced on-treatment ALT flares and all of them developed spontaneous resolution with continued treatment. Four patients died from treatment-unrelated causes during the study, including 3 patients due to cardiovascular events (BR: 1; control: 2) and 1 patient due to motor vehicle accident in the control group.
DISCUSSION
This is the first large-scale, multicenter, double-blind RCT with paired liver biopsy and central pathology to assess the potential synergistic antifibrotic effects of combining ETV with BR for 72 weeks in patients with CHB- or HBV-related compensated cirrhosis. The present study demonstrated that a combination of ETV and BR resulted in a significantly higher rate of regression of fibrosis/cirrhosis than ETV monotherapy in overall patients with CHB and those with liver cirrhosis at baseline. This combination therapy also showed an excellent safety profile, even in compensated cirrhosis.
Numerous clinical studies, including RCTs, have been performed to explore the potential effectiveness of TCM for CHB patients [23, 24]. However, the quality of many published RCTs regarding the effectiveness of TCM was poor, including missing methods of randomization or blinding and unavailable AEs [25]. By comparison, the present study has many strengths. First, the study design is rigorous, because we employed the placebo tablets as the control group and had independent persons responsible for data monitoring. Second, a large number of HBV-infected patients from multiple Chinese institutions were enrolled, which made the study population more representative and the statistical results more stable. Third, liver biopsy, the gold standard for determining histologic outcomes, not alternative noninvasive makers, such as elastography [26] or biomarkers [27], was employed for follow-up evaluation of changes in liver histology. Fourth, paired liver biopsy specimens were available in a large subset of our patients (n = 705). By comparison, among the previous studies regarding regression of fibrosis or cirrhosis by NAs, paired liver biopsy specimens were available in 348 patients [5], 57 patients [4], and 21 patients [28]. Fifth, central pathologists, blinded to the treatment assignment, were employed to achieve a consistent interpretation of histological results. Sixth, patients with advanced fibrosis or cirrhosis accounted for 74% of the study population. Thus, our conclusions may be more generalizable in CHB patients with or without liver cirrhosis.
Several extended follow-up studies based on the large-scale phase 2 and 3 trials suggested the probability of regressing liver fibrosis and cirrhosis in CHB patients treated with NAs [4, 6, 28]. The rate of regression of liver fibrosis and cirrhosis seemed to be higher in these previous studies than the present study. This can be explained by the following points. First, these previous studies had a longer follow-up duration. Follow-up biopsy was performed at week 240 [5], at a median follow-up of 6 years [4], and at either week 100 or week 148 [28]. Duration of NAs should be positively associated with treatment efficacy. Second, potential selection bias should not be neglected, because only survivors can undergo a second liver biopsy and the patients who progressed into decompensated cirrhosis during follow-up are less likely to have received a second liver biopsy. Third, the number of patients with liver cirrhosis was small among the previous studies, suggesting that the statistical results might not be stable.
The present study found no significant difference in HBV DNA suppression, ALT normalization, or improvement of histological necroinflammation between patients who received ETV + BR and ETV monotherapy. These findings suggest that BR may not provide additional benefits to the antiviral effects of ETV and do not have an appreciable modulatory effect on hepatic inflammation at the histologic level. On the other hand, it supports the hypothesis that the improved likelihood of fibrosis regression of this combination therapy should be attributed to direct antifibrotic effects of BR. Interestingly, the present study also found that the combination of ETV with BR resulted in a significantly higher rate of serum HBeAg seroconversion in HBeAg-positive patients, as compared to ETV monotherapy. It has been reported that BR can enhance host immune response to HBV infection [23, 29]. An increased probability of HBeAg seroconversion by this combination therapy may be due to BR-enhanced host immune response.
A major limitation of the present study is that the outcomes of interest are restricted to observations at treatment week 72. A longer follow-up of observation will allow us to explore the long-term safety and efficacy and the risk of HCC development. In addition, the underlying mechanisms for the benefit of this combination therapy have not yet been explored, and the specific medicinal components of BR responsible for the antifibrotic effects remain unknown. From our experience, however, it is clear that BR in liver disease has potential benefits, without significant toxicity, and is worthy of future clinical study.
In conclusion, adding BR to current standard antiviral treatment may increase the regression of liver fibrosis in CHB patients and challenges the conventional perspective that regression of liver fibrosis in CHB can only be achieved by long-term antiviral treatment with NAs. This study suggests that an antifibrotic agent as a supplement to the current etiology-targeted treatment can further improve the regression of liver fibrosis and overall outcomes in various chronic liver diseases. Further pharmacokinetic and pharmacodynamic studies may be necessary to elucidate the effect of BR on increasing blood or intrahepatic levels of entecavir.
Notes
Author contributions. Study concept and design: G. R., Y. C., Z. Y., Q. L., Y. C., J. B., K.-Q. H., and Y. Y. Acquisition of data: L. T., D. X., Q. S., C. L., L. C., X. H., J. W., H. L., W. L., Z. D., and W. B. Analysis and interpretation of data: G. R., J. B., E. M. Y., N. M.-S., K.-Q. H., X. Q., and Y. Y. Drafting of the manuscript: G. R., J. B., K.-Q. H., X. Q., and Y. Y. Critical revisions to the manuscript: E. M. Y., N. M.-S., X. Q., and Y. Y. Statistical analysis: J. B. Technical support: D. Z. and W. B.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported in part by the State Key Projects Specialized on Infectious Disease, Chinese Ministry of Science and Technology (grant number 2013ZX10005002) and the Beijing Key Research Project of Special Clinical Application (grant number Z151100004015221) to Y. Y.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Tang LSY, Covert E, Wilson E, Kottilil S. Chronic hepatitis B Infection: a review. JAMA 2018; 319:1802–13. [DOI] [PubMed] [Google Scholar]
- 2. Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet 2018; 392:2313–24. [DOI] [PubMed] [Google Scholar]
- 3. Schuppan D, Afdhal NH. Liver cirrhosis. Lancet 2008; 371:838–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang TT, Liaw YF, Wu SS, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 2010; 52:886–93. [DOI] [PubMed] [Google Scholar]
- 5. Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013; 381:468–75. [DOI] [PubMed] [Google Scholar]
- 6. Schiff ER, Lee SS, Chao YC, et al. Long-term treatment with entecavir induces reversal of advanced fibrosis or cirrhosis in patients with chronic hepatitis B. Clin Gastroenterol Hepatol 2011; 9:274–6. [DOI] [PubMed] [Google Scholar]
- 7. Lok AS. Hepatitis: Long-term therapy of chronic hepatitis B reverses cirrhosis. Nat Rev Gastroenterol Hepatol 2013; 10:199–200. [DOI] [PubMed] [Google Scholar]
- 8. Lu AP, Jia HW, Xiao C, Lu QP. Theory of traditional Chinese medicine and therapeutic method of diseases. World J Gastroenterol 2004; 10:1854–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang L, Schuppan D. Traditional Chinese medicine (TCM) for fibrotic liver disease: hope and hype. J Hepatol 2014; 61:166–8. [DOI] [PubMed] [Google Scholar]
- 10. Chen JM, Yang YP, Chen DY, et al. Efficacy and safety of Fufang Biejia Ruangan tablet in patients with chronic hepatitis B complicated with hepatic fibrosis [in Chinese]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2007; 21:358–60. [PubMed] [Google Scholar]
- 11. Dong Q, Qiu LL, Zhang CE, et al. Identification of compounds in an anti-fibrosis Chinese medicine (Fufang Biejia Ruangan pill) and its absorbed components in rat biofluids and liver by UPLC-MS. J Chromatogr B Analyt Technol Biomed Life Sci 2016; 1026:145–51. [DOI] [PubMed] [Google Scholar]
- 12. Luk JM, Wang X, Liu P, et al. Traditional Chinese herbal medicines for treatment of liver fibrosis and cancer: from laboratory discovery to clinical evaluation. Liver Int 2007; 27:879–90. [DOI] [PubMed] [Google Scholar]
- 13. Zhou J, Chen XM, Liu SW, Fu B, Hong Q, Wang SJ. Effects of Biejia Ruangan tablet-containing serum on matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 expression in cultured renal interstitial fibroblasts. Chin J Integr Med 2015; 21:152–6. [DOI] [PubMed] [Google Scholar]
- 14. Yang FR, Fang BW, Lou JS. Effects of Fufang Biejia Ruangan pills on hepatic fibrosis in vivo and in vitro. World J Gastroenterol 2013; 19:5326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo SG, Zhang W, Jiang T, et al. Influence of serum collected from rat perfused with compound Biejiaruangan drug on hepatic stellate cells. World J Gastroenterol 2004; 10:1487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schulz KF, Altman DG, Moher D; CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010; 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng CW, Wu TX, Shang HC, et al. CONSORT-CHM Formulas 2017 Group . CONSORT extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration. Ann Intern Med 2017; 167:112–21. [DOI] [PubMed] [Google Scholar]
- 18. Qu J, Yu Z, Li Q, et al. Blocking and reversing hepatic fibrosis in patients with chronic hepatitis B treated by traditional Chinese medicine (tablets of Biejia Ruangan or RGT): study protocol for a randomized controlled trial. Trials 2014; 15:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22:696–9. [DOI] [PubMed] [Google Scholar]
- 20. Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981; 1:431–5. [DOI] [PubMed] [Google Scholar]
- 21. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56:908–43. [DOI] [PubMed] [Google Scholar]
- 22. Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases . Liver biopsy. Hepatology 2009; 49:1017–44. [DOI] [PubMed] [Google Scholar]
- 23. Cui X, Wang Y, Kokudo N, Fang D, Tang W. Traditional Chinese medicine and related active compounds against hepatitis B virus infection. Biosci Trends 2010; 4:39–47. [PubMed] [Google Scholar]
- 24. Qi FH, Wang ZX, Cai PP, et al. Traditional Chinese medicine and related active compounds: a review of their role on hepatitis B virus infection. Drug Discov Ther 2013; 7:212–24. [DOI] [PubMed] [Google Scholar]
- 25. He J, Du L, Liu G, et al. Quality assessment of reporting of randomization, allocation concealment, and blinding in traditional Chinese medicine RCTs: a review of 3159 RCTs identified from 260 systematic reviews. Trials 2011; 12:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chon YE, Park JY, Myoung SM, et al. Improvement of liver fibrosis after long-term antiviral therapy assessed by Fibroscan in chronic hepatitis B patients with advanced fibrosis. Am J Gastroenterol 2017; 112:882–91. [DOI] [PubMed] [Google Scholar]
- 27. Liu R, Guo J, Lu Y, et al. Changes in APRI and FIB-4 in HBeAg-negative treatment-naive chronic hepatitis B patients with significant liver histological lesions receiving 5-year entecavir therapy. Clin Exp Med 2019; 19:309–20. [DOI] [PubMed] [Google Scholar]
- 28. Yokosuka O, Takaguchi K, Fujioka S, et al. Long-term use of entecavir in nucleoside-naïve Japanese patients with chronic hepatitis B infection. J Hepatol 2010; 52:791–9. [DOI] [PubMed] [Google Scholar]
- 29. Poon PM, Wong CK, Fung KP, et al. Immunomodulatory effects of a traditional Chinese medicine with potential antiviral activity: a self-control study. Am J Chin Med 2006; 34:13–21. [DOI] [PubMed] [Google Scholar]