Abstract
With the emergence of β-lactam antibiotic resistance among strains of Streptococcus pneumoniae, vancomycin has assumed an important role in the treatment of bacterial meningitis. Using the rabbit meningitis model, we evaluated the pharmacokinetics and pharmacodynamics of vancomycin in this setting. Animals were given 80 mg/kg of body weight daily in two or four divided doses to determine the penetration and activity of vancomycin in cerebrospinal fluid (CSF); each regimen was administered with and without dexamethasone. Mean peak (2 h) concentrations in CSF that were four- to eightfold higher than the minimum bactericidal concentration (MBC; 0.5 μg/ml) for the pathogen were adequate for bacterial clearance. In both groups concentrations in CSF remained higher than the MBC for greater than 80% of the respective dosing intervals, and the penetration of vancomycin into CSF was 20%. Mean concentrations in CSF at 24 to 36 h of therapy were lower than those achieved during the first 12 h, consistent with a decline in the level of antibiotic entry into CSF as inflammation wanes. Rates of bacterial clearance were similar for the two regimens, and for all animals cultures of CSF were sterile by 36 h. The coadministration of dexamethasone significantly reduced the penetration of vancomycin into CSF by 29% and significantly lowered the rate of bacterial clearance during the first 6 h in animals receiving 20-mg/kg doses of vancomycin. For animals receiving 40-mg/kg doses, therapeutic peak concentrations in CSF were obtained even with steroid use, suggesting that the effect of steroids may be circumvented by the use of larger daily doses of vancomycin.
The recent emergence of penicillin and cephalosporin resistance among strains of Streptococcus pneumoniae has resulted in an increased reliance on vancomycin for the treatment of bacterial meningitis. Although vancomycin has been available for more than 30 years, published experience with this agent for the treatment of central nervous system (CNS) infections is sparse. Few studies have prospectively addressed the penetration of vancomycin into cerebrospinal fluid (CSF) or its efficacy in pneumococcal meningitis (6, 11, 17). Given the anticipated increase in the use of this agent, further investigation of its pharmacokinetics and pharmacodynamics in bacterial meningitis is warranted.
It has traditionally been believed that the penetration of vancomycin into the CNS is erratic and that therapeutic concentrations may not be consistently achieved at the site of infection (8). More recently, experimental meningitis studies demonstrated that the level of penetration of vancomycin was approximately 10% of the concurrent levels in serum (7, 10, 17). The concentrations achieved in CSF were adequate for bacterial killing, but because therapy was limited to less than 24 h, it was unclear whether bactericidal activity could be maintained (7). Subsequent investigations with the rabbit meningitis model demonstrated that adjunctive use of corticosteroids may compromise the penetration and thereby the activity of vancomycin in CSF (2, 17). Although these observations have reinforced traditional concerns about the suitability of vancomycin for the treatment of meningitis, the data are limited, and neither the penetration of vancomycin into CSF nor its resultant activity is well defined.
Prior experimental meningitis studies suggested that, for β-lactam antibiotics, peak concentrations in CSF correlate with bactericidal activity and that concentrations in CSF at least 10-fold greater than the minimum bactericidal concentration (MBC) for the pathogen are required for optimal bacterial killing (24, 25). For vancomycin, the range of concentrations in CSF considered therapeutic and the pharmacodynamic index that best predicts outcome are not known. It has been suggested that higher CSF vancomycin concentrations enhance bacterial clearance (1, 11). To achieve this, Schaad et al. (19) recommended that the dosages used to treat CNS infections in children be larger (60 mg/kg of body weight in four divided doses) than those used for systemic infections (40 mg/kg in four divided doses). The empiric use of larger dosages for CNS infections is also recommended for adults (12). However, the impact of larger dosages on the penetration and effectiveness of vancomycin is unknown; neither dosages nor dosing intervals have been compared in previous studies. With better definition of the pharmacodynamics of vancomycin, dosing regimens can be tailored to optimize treatment for CNS infections.
The purpose of our study was to evaluate the pharmacodynamics and pharmacokinetics of vancomycin in the treatment of highly cephalosporin-resistant pneumococcal meningitis. By using the rabbit meningitis model (4), two dosing regimens were compared to elucidate the effect of dosage and dosing interval on the penetration and activity of vancomycin in CSF. Given the concern for diminished penetration into CSF with the adjunctive use of steroids (2, 17), both regimens were administered with and without concomitant dexamethasone therapy.
MATERIALS AND METHODS
Bacterial strain.
A type 6B strain of S. pneumoniae originally isolated from an infant with meningitis was used for all experiments. After intrathecal passage in rabbits, the strain was grown overnight on blood agar plates. The plates were washed with phosphate-buffered saline, and aliquots of the resultant suspension were frozen at −70°C. For preparation of the inoculum, aliquots were diluted in phosphate-buffered saline to a concentration of approximately 5 × 105 CFU/ml, of which 250 μl was injected intracisternally into each rabbit. The inoculum size was confirmed by quantitative cultures with each experiment.
Susceptibility tests.
The MICs and MBCs of different antibiotics were measured in Mueller-Hinton broth supplemented with 3 to 5% lysed horse blood by the standard microdilution method (15).
Meningitis model.
The rabbit meningitis model, modified from the original description by Dacey and Sande (4), was used. Male New Zealand White rabbits weighing 2 to 2.5 kg were anesthetized with intramuscular ketamine (50 mg/kg of body weight) and acepromazine (4 mg/kg) before every procedure. Flunixin meglumine (1.1 mg/kg) was administered intramuscularly every 12 h for analgesia. Animals were immobilized in stereotactic frames, and a spinal needle was introduced into the cisterna magna to withdraw 250 μl of CSF and inject an equal volume of inoculum. Treatment was initiated 16 to 18 h after inoculation (0 h) once the CSF was withdrawn for quantitation of initial bacterial titers. The animals were euthanized with pentobarbital (120 mg/kg) at the end of each experiment or earlier if they appeared to be severely lethargic or were unable to maintain recumbency.
Treatment.
Vancomycin hydrochloride (Abbott Laboratories, Chicago, Ill.) was administered intravenously over 3 to 5 min at a dosage of 20 mg/kg every 6 h or 40 mg/kg every 12 h. The smaller dosage was chosen to achieve peak concentrations in serum that simulate those observed in humans. We doubled the dosage of vancomycin to determine the influence of a higher peak concentration in serum on CSF penetration. The larger dosage was administered every 12 h to maintain the same daily dose (80 mg/kg) for the two regimens. To evaluate the pharmacodynamics of vancomycin in both the first and second 24 h of therapy, animals were treated for either 12 h (one or two doses) or 36 h (three or six doses). The effect of steroids on the penetration and bacteriologic effectiveness of vancomycin was evaluated during the first 12 h of treatment. For these studies, half the animals in each treatment group received 1 mg of dexamethasone (Elkins-Sinn, Cherry Hill, N.J.) per kg intravenously approximately 10 min before the initiation of therapy (14, 17).
For evaluation of the first 12 h of treatment, 22 animals received two doses of the 20-mg/kg regimen and 18 animals received one dose of the 40-mg/kg regimen. To address the effect of coadministration of steroids, an additional 14 animals received two doses (20 mg/kg) and 10 animals received one dose (40 mg/kg) plus one dose of dexamethasone. In the period of treatment from 24 to 36 h, 11 animals received six doses (20 mg/kg) of vancomycin, whereas 12 animals received three doses (40 mg/kg).
Sample collection and processing.
For purposes of sampling, the animals were immobilized in stereotactic frames with intracisternal needles in place for 6-h intervals. Animals treated for 12 h were immobilized from 0 to 6 h (group 1) or 6 to 12 h (group 2), and those treated for 36 h were immobilized from 24 to 30 h (group 3) or 30 to 36 h (group 4). The experiments were designed to limit the number of interventions per animal and to minimize any effect that removal of CSF, in terms of either frequency or volume, may have on the dynamics of CSF flow and on antibiotic concentrations. Samples of CSF (100 μl) and blood (0.5 to 1.0 ml) were collected subsequent to administration of the initial dose (0 h) as follows: (i) group 1: 1, 2, 3, 4, and 6 h; (ii) group 2: 6, 9, and 12 h; (iii) group 3: 24, 25, 26, 27, 28, and 30 h; and (iv) group 4: 30, 33, and 36 h. CSF and blood samples were centrifuged at 5,000 × g for 5 and 10 min, respectively, and the supernatants were stored at −70°C for determination of antibiotic concentrations. Specimens contaminated with blood were not analyzed.
An additional 100 to 150 μl of CSF was collected for quantification of bacterial titers before the initiation of therapy and at 6 and 12 h (groups 1 and 2) or 24, 30, and 36 h (groups 3 and 4) after the initiation of therapy. Bacterial concentrations were quantified by plating undiluted and serial dilutions of CSF (100 μl) on sheep blood agar and incubating the plates in 5% CO2 at 35°C for 24 h. The lowest bacterial concentration detectable by this method was 10 CFU/ml (7). For purposes of analysis, specimens with <10 CFU/ml were assigned a value of 1 (0 log10) CFU/ml. Testing for antibiotic carryover with 4 μg of vancomycin per ml demonstrated no interference with bacterial counts.
Anesthesia was administered frequently to animals while they were immobilized in the frames. Animals in groups 1 and 2 were killed at 12 h after the initiation of therapy, and those in groups 3 and 4 were killed 36 h after the initiation of therapy.
Antibiotic assays.
Vancomycin concentrations were determined by a disk diffusion microbioassay with Bacillus subtilis ATCC 6633 (11, 19, 23). The lower limit of detection was 0.7 μg/ml for CSF and serum. Interassay and intra-assay coefficients of variation were 4.9 and 5.7%, respectively, at 0.7 μg/ml for both assays. Some samples demonstrated zones of inhibition that could be extrapolated to calculate concentrations lower than 0.7 μg/ml; samples for which there was no zone of inhibition were reported at <0.7 μg/ml. For purposes of analysis, extrapolated concentrations were assigned their own values, whereas samples reported at <0.7 μg/ml were assigned a value of 0.3 μg/ml, which was the lowest extrapolated value. No values of <0.7 μg/ml were used for pharmacokinetic calculations.
Pharmacokinetic indices.
All available concentrations in serum and CSF from the first 12 h of treatment were used to perform a population pharmacokinetic analysis by a nonparametric expectation maximization approach (NPEM program of Schumizky and Jelliffe). Mean penetration was determined by simulation of the plasma concentration-time profile and the CSF concentration-time profile. The extrapolated area under the concentration-time curve (AUC) was determined for each profile, and the ratio of these was used as the estimate of the penetration.
Statistical analysis.
Mean serum and CSF vancomycin concentrations and mean changes in bacterial concentrations for all treatment groups (vancomycin administered with and without steroids) were compared by one-way analysis of variance by the Newman-Keuls multiple comparisons test. For differences in penetration between dexamethasone-treated and nontreated animals, the maximum A-posteriori probability Bayesian estimate of the pharmacokinetic parameters was determined for each animal, and the penetration was determined on an individual basis, as described above. Because previous investigations have demonstrated that the administration of steroids decreased the penetration of antibiotics in meningitis (2, 17), a one-tailed t test was used to test for significance. A P value of <0.05 was considered significant. The relationship between the bactericidal ratio (peak concentration in CSF/MBC) and the rate of bacterial killing during the first 6 h of therapy for animals treated with and without dexamethasone was fitted to a sigmoid Emax model with the computer program WinNonlin, version 1.5. The following formula was used: E = (Emax × Cλ)/(Cλ + EC50λ), where E is the estimated bacterial killing rate (BKR), Emax is the maximum BKR, C is the mean peak concentration/MBC ratio, EC50 is the concentration producing half-maximal BKR, and λ is the Hill coefficient indicating the slope of the sigmoid curve.
RESULTS
In vitro susceptibility.
For the pneumococcal strain used, the MICs and MBCs of penicillin were 4.0 and 4.0 μg/ml, respectively, those of ceftriaxone were 4.0 and 4.0 μg/ml, respectively, and those of vancomycin were 0.5 and 0.5 μg/ml, respectively.
Antibiotic concentrations.
For animals evaluated in the first 12 h of treatment, the mean serum and CSF vancomycin concentrations measured either 1 to 6 h (group 1) or 6 to 12 h (group 2) after the initial dose are presented in Table 1. Maximum concentrations in the CSF of individual animals occurred 2 or 3 h after administration of the initial dose. As predicted by the linear pharmacokinetics of vancomycin, the mean concentrations in serum achieved with the 40-mg/kg dose were twice those achieved with the 20-mg/kg dose. The mean concentrations in the CSF of animals receiving 40 mg/kg followed the same linearity and were significantly higher (P < 0.05) than those in the CSF of animals receiving 20 mg/kg throughout the first 6 h. An additional dose administered at 6 h to animals receiving 20 mg/kg resulted in mean concentrations in CSF at 9 or 12 h that exceeded those measured in the CSF of the group receiving 40 mg/kg, but the differences were not significant.
TABLE 1.
Mean vancomycin concentrations in serum and CSF of animals during the first 12 h of treatment
| Dosage | Groupa | Compartment | Mean ± SD concn (μg/ml) at the following time interval (h) after administration of initial doseb:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | 9 | 12 | |||
| 20 mg/kg every 6 h | 1 (11) | Serum | 25.2 ± 5.6 | 13.3 ± 4.2 | 8.4 ± 3.0 | 6.1 ± 2.9 | 3.3 ± 1.6 | ||
| CSF | 1.2 ± 0.7 | 1.8 ± 1.4 | 1.7 ± 1.4 | 1.4 ± 1.1 | 1.2 ± 0.8 | ||||
| 2 (11) | Serum | 2.1 ± 1.0 | 15.2 ± 7.9 | 8.0 ± 6.0 | |||||
| CSF | 1.0 ± 0.9 | 1.9 ± 0.8 | 2.1 ± 1.3 | ||||||
| 40 mg/kg every 12 h | 1 (12) | Serum | 48.1 ± 6.6 | 29.5 ± 7.2 | 18.3 ± 6.8 | 10.7 ± 2.5 | 6.4 ± 1.3 | ||
| CSF | 2.5 ± 1.4 | 3.8 ± 2.2 | 3.6 ± 2.0 | 3.0 ± 1.2 | 2.7 ± 1.4 | ||||
| 2 (6) | Serum | 5.6 ± 4.2 | 4.5 ± 6.9 | 3.5 ± 5.9 | |||||
| CSF | 2.3 ± 0.9 | 1.4 ± 1.4 | 1.3 ± 1.2 | ||||||
Animals received either one (40-mg/kg) or two (20-mg/kg) doses of vancomycin. CSF and blood were obtained at 1, 2, 3, 4, and 6 h after administration of the initial dose for group 1 and at 6, 9, and 12 h after administration of the initial dose for group 2. Numbers in parentheses represent the number of animals per group for each treatment regimen.
See Materials and Methods for methods of calculation of mean values.
For all but two animals receiving 20 mg/kg at 6-h intervals, concentrations in CSF remained greater than the MBC at all time points, which together represent more than 80% of a 6-h dosing interval (concentrations were not determined at between 0 and 1 h). Because of the higher concentrations in CSF that were attained and the prolonged elimination of drug from the CSF (21, 22), the concentrations in the CSF of animals administered 40 mg/kg every 12 h also exceeded the MBC for more than 80% of the interval.
The mean serum and CSF vancomycin concentrations for animals concomitantly receiving dexamethasone are presented in Table 2. The concentrations in serum were similar to those in animals not given steroids, but the mean concentrations in CSF were consistently lower for both dosing regimens. For animals in both treatment groups not receiving dexamethasone, the penetration of vancomycin into CSF was 20.1%. With concomitant administration of the steroid, the penetration was significantly reduced to 14.3% (P = 0.035).
TABLE 2.
Mean vancomycin concentrations in serum and CSF during the first 12 h of treatment in rabbits receiving a single dose of dexamethasone
| Regimen | Groupa | Compartment | Mean ± SD concn (μg/ml) at the following time intervals (h) after administration of initial doseb:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | 9 | 12 | |||
| Vancomycin at 20 mg/kg every 6 h + DXMc at 1 mg/kg | 1 (7) | Serum | 22.8 ± 3.3 | 14.7 ± 7.1 | 7.4 ± 2.2 | 5.9 ± 3.3 | 3.9 ± 3.5 | ||
| CSF | 0.8 ± 0.5 | 1.0 ± 0.6 | 1.1 ± 0.7 | 1.1 ± 0.8 | 1.2 ± 1.0 | ||||
| 2 (7) | Serum | 1.4 ± 1.2 | 9.2 ± 6.4 | 4.1 ± 4.8 | |||||
| CSF | 0.7 ± 0.6 | 1.1 ± 0.8 | 1.1 ± 0.8 | ||||||
| Vancomycin at 40 mg/kg every 12 h + DXM at 1 mg/kg | 1 (4) | Serum | 47.4 ± 9.5 | 26.5 ± 3.3 | 16.4 ± 5.8 | 13.2 ± 5.2 | 7.2 ± 3.1 | ||
| CSF | 1.8 ± 1.6 | 2.2 ± 2.1 | 2.0 ± 1.7 | 1.8 ± 1.3 | 1.4 ± 1.3 | ||||
| 2 (6) | Serum | 3.6 ± 4.2 | 1.2 ± 1.7 | 0.7 ± 0.9 | |||||
| CSF | 1.0 ± 0.6 | 0.5 ± 0.1 | 0.5 ± 0.2 | ||||||
Animals received either one (40-mg/kg) or two (20-mg/kg) doses of vancomycin and received one (1-mg/kg) dose of dexamethasone at the initiation of therapy. CSF and blood were obtained at 1, 2, 3, 4, and 6 h after administration of the initial dose for group 1 and at 6, 9, and 12 h after the administration of the initial dose for group 2. Numbers in parentheses represent the numbers of animals per group for each treatment regimen.
See Materials and Methods for methods of calculation of mean values.
DXM, dexamethasone.
For experiments evaluating the penetration of vancomycin beyond 24 h of therapy, mean concentrations in serum and CSF measured either 24 to 30 h (group 3) or 30 to 36 h (group 4) after administration of the initial dose are presented in Table 3. Concentrations in serum were similar to those measured in the first 24 h of treatment. Mean concentrations in CSF were lower than those measured for the respective treatment groups during the first 24 h, but the differences were not significant.
TABLE 3.
Mean vancomycin concentrations in serum and cerebrospinal fluid of animals during the second 24 h of treatment
| Dosage | Groupa | Compartment | Mean concn ± SD (μg/ml) at the following time intervals (h) after administration of initial doseb:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 25 | 26 | 27 | 28 | 30 | 33 | 36 | |||
| 20 mg/kg every 6 h | 3 (4) | Serum | 31.8 ± 8.2 | 16.1 ± 5.4 | 11.4 ± 5.4 | 8.1 ± 3.4 | 5.0 ± 2.0 | ||
| CSF | 1.3 ± 0.4 | 1.5 ± 0.6 | 1.5 ± 0.7 | 1.2 ± 0.6 | 1.1 ± 0.6 | ||||
| 4 (7) | Serum | 3.2 ± 2.3 | 15.1 ± 9.8 | 4.0 ± 1.1 | |||||
| CSF | 1.2 ± 0.5 | 1.5 ± 0.6 | 1.2 ± 0.4 | ||||||
| 40 mg/kg every 12 h | 3 (6) | Serum | 60.0 ± 7.8 | 30.9 ± 4.6 | 21.7 ± 6.6 | 14.9 ± 3.7 | 8.7 ± 3.2 | ||
| CSF | 2.4 ± 1.0 | 2.3 ± 0.7 | 2.2 ± 0.6 | 2.0 ± 0.3 | 1.6 ± 0.5 | ||||
| 4 (6) | Serum | 5.0 ± 0.5 | 1.6 ± 0.5 | 0.8 ± 0.4 | |||||
| CSF | 1.6 ± 0.4 | 1.0 ± 0.2 | 0.5 ± 0.3 | ||||||
Animals received either three (40 mg/kg) or six (20 mg/kg) doses of vancomycin. CSF and blood were obtained at 25, 26, 27, 28, and 30 h after administration of the initial dose for group 3 and at 30, 33, and 36 h after administration of the initial dose for group 4. Numbers in parentheses represent the number of animals per group for each treatment regimen.
See Materials and Methods for methods of calculation of mean values.
Bacteriologic effect.
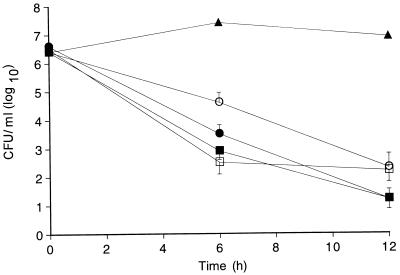
Mean ± standard error of the mean concentrations of S. pneumoniae measured in the CSF of animals treated for 12 h (groups 1 and 2) with either regimen and with or without dexamethasone are shown in Fig. 1 and compared with those in the CSF of untreated (control) animals. Initial mean bacterial titers were similar for all treated and untreated groups. No differences in the bacterial clearance rates were noted between animals treated with a single dose of 40 mg/kg and those treated with two doses of 20 mg/kg without concomitant dexamethasone treatment. Mean reductions in bacterial titers were −5.4 ± 1.8 and −5.3 ± 1.2 log10 CFU/ml at 12 h for animals receiving 20 and 40 mg/kg, respectively; the CSF of 10 of 22 animals in the 20-mg/kg group and 8 of 18 animals in the 40-mg/kg treatment group was sterile by culture at 12 h.
FIG. 1.
Bacterial concentrations in CSF (mean ± standard error of the mean) in rabbits with experimental pneumococcal meningitis. Animals were treated with either one 40-mg/kg dose (■ and □) or two 20-mg/kg doses (● and ○) of vancomycin, with (open symbols) or without (closed symbols) one 1-mg/kg dose of dexamethasone. Compared with four untreated (▴; control) animals, all treated animals had significantly greater reductions in bacterial counts at 6 and 12 h. No differences in bacterial clearance rates were noted between animals receiving 20 mg/kg (−0.45 ± 0.2 log10CFU/ml/h) and those receiving 40 mg/kg (−0.44 ± 0.1 log10CFU/ml/h) without dexamethasone. For animals treated with 20 mg/kg with dexamethasone, the mean reduction in bacterial concentrations between 0 and 6 h was significantly lower (P < 0.05) than that for other treatment groups. At the end of therapy, the CSF of 10 of 22 animals in the 20-mg/kg group, 8 of 18 animals in the 40-mg/kg group, 3 of 14 animals in the 20-mg/kg with dexamethasone group, and 1 of 10 animals in the 40-mg/kg with dexamethasone group was sterile by culture.
In animals treated with dexamethasone, bacterial clearance was reduced compared with that in animals receiving the same regimen without steroids. Mean reductions in bacterial titers at 12 h were −4.2 ± 2.3 and −4.3 ± 1.6 log10 CFU/ml for animals receiving 20 and 40 mg/kg, respectively. The mean reduction in bacterial concentration at 6 h for animals receiving 20 mg/kg with steroids (−1.8 log10CFU/ml) was significantly lower than that for all other treatment groups (P < 0.05). At 12 h, the reduction in bacterial titers for this group continued to be lower than that for animals treated with 20 mg/kg without steroids (P = 0.05). For animals receiving 40 mg/kg with dexamethasone, the mean reduction in bacterial concentrations between 6 and 12 h was significantly less (P < 0.05) than that for all other treatment groups; this was not the case for the overall reduction from 0 to 12 h. Sterilization of CSF occurred in only 3 of 14 animals receiving two 20-mg/kg doses and 1 of 10 animals receiving a single 40-mg/kg dose, both with dexamethasone.
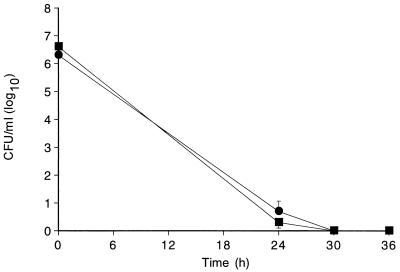
Mean ± standard error of the mean bacterial concentrations in CSF measured after 24 h of treatment (groups 3 and 4) are shown in Fig. 2. Mean reductions in bacterial titers at 36 h were −6.4 ± 0.7 and −6.6 ± 0.8 log10CFU/ml for animals receiving 20 and 40 mg/kg, respectively. At 24 h, the CSF of 7 of 11 animals receiving 20 mg/kg and 10 of 12 animals receiving 40 mg/kg was sterile by culture; by 36 h the CSF of all animals was sterile by culture.
FIG. 2.
Bacterial concentrations in CSF (mean ± standard error of the mean) in rabbits with experimental pneumococcal meningitis. Animals were treated with vancomycin at a dosage of 20 mg/kg every 6 h (●) or 40 mg/kg every 12 h (■). Sampling of CSF for quantitation of bacterial titers was conducted at 0, 24, 30, and 36 h. The rates of bacterial clearance in the first 24 h were −0.24 ± 0.1 and −0.26 ± 0.1 log10CFU/ml/h for the 20- and 40-mg/kg groups, respectively. At 24 h, the CSF of 7 of 11 animals in the 20-mg/kg group and 10 of 12 animals in the 40 mg/kg group was sterile by culture. The CSF of all animals was sterile by culture by the end of therapy.
Pharmacodynamics.
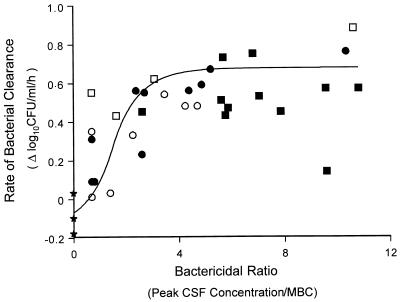
The relationship between bactericidal ratio (peak CSF concentration/MBC) and bacterial clearance during the first 6 h of therapy (group 1) for all four treatment regimens as described by the sigmoid Emax model is depicted in Fig. 3 and demonstrates a maximal killing rate at concentrations fourfold greater than the MBC (r2 = 0.78).
FIG. 3.
Relationship between bactericidal ratio of vancomycin in CSF (peak concentration/MBC [0.5 μg/ml] for the pathogen) and rate of bacterial clearance in rabbits with experimental cephalosporin-resistant pneumococcal meningitis during the first 6 h of therapy. Datum points are derived from animals given vancomycin at doses of 20 mg/kg (● and ○) and 40 mg/kg (■ and □) with (open symbols) and without (closed symbols) a single dose of dexamethasone. The correlation was fit to a sigmoid Emax model as described in Materials and Methods (r2 = 0.78). ★, controls.
DISCUSSION
Historically, vancomycin has not been considered an appropriate antimicrobial agent for the treatment of bacterial meningitis (8). Early clinical experience suggested that therapeutic concentrations could not be achieved in CSF (8). Subsequent reports documented microbiological and clinical success with the use of vancomycin for the treatment of meningitis but also emphasized the interpatient variability of penetration into the CSF (6, 18). Erratic penetration through the blood-brain barrier (BBB), even in the setting of inflammation, applies to many antimicrobial agents and is not unique to vancomycin (21). However, because serum vancomycin concentrations are lower than those of many β-lactams, CSF vancomycin concentrations relative to the susceptibilities of meningeal pathogens are also lower and may not exceed the MBCs for some organisms. Only recently have studies attempted to systematically evaluate the penetration and activity of vancomycin in CSF. In those investigations, administration of vancomycin was limited to two or three doses, and measurement of concentrations in CSF and serum was infrequent (7, 17). Thus, the pharmacokinetics and pharmacodynamics of vancomycin in CNS infections remain poorly defined.
In the present study, we administered multiple doses of vancomycin during a 36-h period. Two dosing regimens were compared to evaluate the impact of dosage and dosing interval on penetration and bacterial clearance. Administration of vancomycin at a dosage that produced concentrations in serum similar to those observed in the serum of humans resulted in a mean peak concentration in CSF that was approximately fourfold higher than the MBC for the pathogen (Table 1). Administration of a twofold higher dose produced proportionately higher peak (1 h) concentrations in serum and, despite variable permeation through the BBB, proportionately higher concentrations in CSF. The penetration into CSF for both regimens was 20.1%. Although this value is higher than the point penetration values reported previously (1, 10, 17), its calculation as a ratio of CSF AUC to serum AUC more accurately represents the true penetration of vancomycin into CSF.
For β-lactam agents, peak concentrations in CSF at least 10-fold greater than the MBC are required for therapeutic effectiveness (24, 25). In the present study, the rate of bacterial clearance was similar for the two treatment regimens, and peak CSF vancomycin concentrations that were four- to eightfold greater than the MBC were adequate for bactericidal activity. The relationship between bactericidal ratio and bacterial killing in CSF (Fig. 3) demonstrated that concentrations greater than fourfold the MBC did not result in more rapid killing, suggesting concentration-independent bactericidal activity. However, this cannot be verified because the time that the concentrations in CSF remained higher than the MBC was similar for the two regimens. As suggested previously for other antimicrobial agents (21), the pharmacokinetics of vancomycin in CSF allowed a higher peak concentration to serve as a surrogate for a more prolonged time over the MBC. Evaluation of additional dosing regimens and dosages is necessary to distinguish the relative importance of concentration versus time over the MBC for vancomycin in meningitis, but our findings suggest that the two factors are interrelated.
For most antimicrobial agents, alteration of the integrity of the BBB induced by meningeal inflammation is critical for penetration into CSF; as inflammation subsides, the level of antibiotic entry and concentrations in CSF decline (21). To what degree this affects vancomycin concentrations has not been addressed. In previous experimental meningitis studies, vancomycin therapy was limited to less than 24 h, and regrowth of bacteria after cessation of therapy suggested that bactericidal activity may be difficult to maintain (7, 17). In our study, mean concentrations in CSF were lower in the second 24 h than in the first 12 h (Table 3), but the differences were not significant. Maximum leukocyte counts in the CSF of animals were noted at 12 or 24 h after the initiation of treatment and declined during the ensuing 12 h. Lower concentrations in CSF thus paralleled the resolution of inflammation, but bactericidal activity was maintained, and by 36 h the CSF of all animals was sterile by culture.
The adjunctive use of corticosteroids has been shown to modulate the inflammation in experimental meningitis and to reduce the incidence of neurologic sequelae in pediatric patients with meningitis (15, 16). By reducing the inflammation, steroids may compromise drug entry into the CSF and thereby potentially diminish the effectiveness of an antibiotic. Although two recent studies suggested that the adjunctive use of dexamethasone reduces the penetration of vancomycin into CSF, the findings were inconsistent. Paris et al. (17) showed that mean peak concentrations measured in the CSF of animals given two 20-mg/kg doses of vancomycin and a single dose of dexamethasone were lower than those in the CSF of animals not given the steroid; differences were significant only after administration of the second dose. Cabellos et al. (2) demonstrated similar findings in animals, but differences in the concentrations in CSF noted after administration of the initial antibiotic dose were no longer present after administration of the third dose. In both studies, the use of dexamethasone delayed bacterial clearance and the sterilization of CSF cultures.
We further analyzed the effects of steroids on the penetration of vancomycin by comparing concentrations in the CSF of steroid-treated and non-steroid-treated animals at multiple times during a 12-h period. For both dosing regimens and throughout the dosing intervals, mean concentrations in CSF were reduced by the coadministration of a single dose of dexamethasone (Table 2). The resultant penetration into the CSF was 29% lower than that into the CSF of animals not treated with steroids. Mean reductions in bacterial titers were also lower for both steroid-treated groups, but significant differences were noted only for animals receiving the lower dose (Fig. 1). During the first 6 h of treatment, mean concentrations in the CSF of animals receiving 40 mg of vancomycin per kg with dexamethasone were fourfold greater than the MBC, and the rate of bacterial clearance was similar to that for animals not receiving steroids. During the same period, mean concentrations in the CSF of animals given vancomycin at 20 mg/kg with dexamethasone were only twofold greater than the MBC, and bacterial clearance was significantly reduced compared with that for animals not receiving steroids. From 6 to 12 h of treatment, mean concentrations in CSF for animals receiving 40 mg/kg were barely twofold higher than the MBC, accounting for the reduced bacterial clearance during this period. In the setting of adjunctive corticosteroid therapy, it is likely that larger dosages must be used to achieve and maintain therapeutic concentrations in CSF.
Viladrich et al. (26) postulated that the adjunctive use of dexamethasone may have diminished the level of antibiotic penetration and thereby contributed to clinical failure in adults with pneumococcal meningitis who were treated with vancomycin (30 mg/kg/day). In comparison, concentrations in the CSF of children treated with larger daily dosages (60 mg/kg/day) of vancomycin and dexamethasone were higher and more consistent (9). Differences in concentrations in the CSF in the two studies may reflect differences in the timing of CSF collection. It is equally likely that, as suggested in the present study, the use of a larger daily dose for the children produced concentrations in CSF that were adequate, despite concomitant steroid use.
The rabbit model has been useful for evaluating the efficacies of antibiotic regimens for the treatment of meningitis, but caution must be exercised in the extrapolation of these findings to humans because of obvious differences in the mode of drug administration, pharmacokinetics, and CSF dynamics. In the present study, we demonstrated that the penetration of vancomycin into CSF is approximately 20% and that concentrations in CSF that are at least fourfold greater than the MBC are required for adequate bacterial clearance, with the caveat that the concentrations be maintained above the MBC for the majority of the dosing interval. The concomitant administration of dexamethasone significantly reduces the penetration of vancomycin into CSF, with a consequent reduction in bactericidal activity. On the basis of similar observations, investigators have advised against the use of vancomycin for the treatment of pneumococcal meningitis in the setting of adjunctive corticosteroid use. On the basis of our findings and clinical experience, larger daily dosages, as already recommended for CNS infections, should be used to achieve therapeutic concentrations in CSF, in the setting of adjunctive steroid therapy. Comparison of other dosages and dosing regimens, with and without steroids, is necessary to further define the pharmacodynamics of vancomycin and optimize its use for the treatment of meningitis.
ACKNOWLEDGMENTS
We are indebted to Roger Jelliffe for help with the pharmacokinetic analysis, which was performed with support from a grant from the National Institutes of Health (grant RO1-RR-11526).
REFERENCES
- 1.Beam T R. Vancomycin therapy of experimental pneumococcal meningitis caused by penicillin-sensitive and resistant strains. J Antimicrob Chemother. 1981;7:89–99. doi: 10.1093/jac/7.1.89. [DOI] [PubMed] [Google Scholar]
- 2.Cabellos C, Martinez-Lacasa J, Martos A, Tubau F, Fernandez A, Viladrich P F, Gudiol F. Influence of dexamethasone on efficacy of ceftriaxone and vancomycin therapy in experimental pneumococcal meningitis. Antimicrob Agents Chemother. 1995;39:2158–2160. doi: 10.1128/aac.39.9.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chesney P J, Davis Y, English B K, Wang W C. Occurrence of Streptococcus pneumoniae meningitis during vancomycin and cefotaxime therapy of septicemia in a patient with sickle cell disease. Pediatr Infect Dis J. 1995;14:1013–1015. [PubMed] [Google Scholar]
- 4.Dacey R G, Sande M A. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974;6:437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everett E D, Strausbaugh L J. Antimicrobial agents and the central nervous system. Neurosurgery. 1980;6:691–714. doi: 10.1227/00006123-198006000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Friedland I R, Shelton S, Paris M, Rinderknecht S, Ehrett S, Krisher K, McCracken G H., Jr Dilemmas in diagnosis and management of cephalosporin-resistant Streptococcus pneumoniae meningitis. Pediatr Infect Dis J. 1993;12:196–200. doi: 10.1097/00006454-199303000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Friedland I R, Paris M, Ehrett S, Hickey S, Olsen K, McCracken G H., Jr Evaluation of antimicrobial regimens for treatment of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1993;37:1630–1636. doi: 10.1128/aac.37.8.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gump D W. Vancomycin for treatment of bacterial meningitis. Rev Infect Dis. 1981;3:S289–S292. [PubMed] [Google Scholar]
- 9.Klugman K P, Friedland I R, Bradley J S. Bactericidal activity against cephalosporin-resistant Streptococcus pneumoniae in cerebrospinal fluid of children with acute bacterial meningitis. Antimicrob Agents Chemother. 1995;39:1988–1992. doi: 10.1128/aac.39.9.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krontz D P, Strausbaugh L J. Effect of meningitis and probenecid on the penetration of vancomycin into cerebrospinal fluid in rabbits. Antimicrob Agents Chemother. 1980;18:882–886. doi: 10.1128/aac.18.6.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCracken G H, Jr, Sakata Y. Antimicrobial therapy of experimental meningitis caused by Streptococcus pneumoniae strains with different susceptibilities to penicillin. Antimicrob Agents Chemother. 1985;27:141–145. doi: 10.1128/aac.27.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moellering R C., Jr Pharmacokinetics of vancomycin. J Antimicrob Chemother. 1984;14:S43–S52. doi: 10.1093/jac/14.suppl_d.43. [DOI] [PubMed] [Google Scholar]
- 13.Moellering R C., Jr Monitoring serum vancomycin levels: climbing the mountain because it is there? Clin Infect Dis. 1994;18:544–546. doi: 10.1093/clinids/18.4.544. [DOI] [PubMed] [Google Scholar]
- 14.Mustafa M M, Ramilo O, Mertsola J, Risser R C, Beutler B, Hansen E J, McCracken G H., Jr Modulation of inflammation and cachectin activity in relation to treatment of experimental Haemophilus influenzae type b meningitis. J Infect Dis. 1989;160:818–825. doi: 10.1093/infdis/160.5.818. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical and Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacterial that grow aerobically, document M7-A3. Wayne, Pa: National Committee for Clinical and Laboratory Standards; 1993. [Google Scholar]
- 16.Odio C M, Faingezicht I, Paris M, Nassar M, Baltodano A, Rogers J, Saez-Llorens X, Olsen K D, McCracken G H., Jr The beneficial effects of early dexamethasone administration in infants and children with bacterial meningitis. N Engl J Med. 1991;324:1525–1531. doi: 10.1056/NEJM199105303242201. [DOI] [PubMed] [Google Scholar]
- 17.Paris M M, Hickey S M, Uscher M I, Shelton S, Olsen K D, McCracken G H., Jr Effect of dexamethasone on therapy of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1994;38:1320–1324. doi: 10.1128/aac.38.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redfield D C, Underman A, Norman D, Overturf G D. Cerebrospinal fluid penetration of vancomycin in bacterial meningitis. In: Nelson J D, Geraci C, editors. Current chemotherapy and infectious disease. Program and abstracts of the 11th International Congress of Chemotherapy and the 19th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1980. pp. 638–640. [Google Scholar]
- 19.Schaad U B, McCracken G H, Jr, Nelson J D. Clinical pharmacology and efficacy of vancomycin in pediatric patients. J Pediatr. 1980;96:119–126. doi: 10.1016/s0022-3476(80)80347-7. [DOI] [PubMed] [Google Scholar]
- 20.Schaad U B, Nelson J D, McCracken G H., Jr Pharmacology and efficacy of vancomycin for staphylococcal infections in children. Rev Infect Dis. 1981;3:S282–S287. [PubMed] [Google Scholar]
- 21.Scheld W M. Theoretical and practical considerations of antibiotic therapy for bacterial meningitis. Pediatr Infect Dis J. 1985;4:74–83. doi: 10.1097/00006454-198501000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Scheld W M. Drug delivery to the central nervous system: general principles and relevance to therapy for infections of the central nervous system. Rev Infect Dis. 1989;11(Suppl. 7):S1669–S1690. doi: 10.1093/clinids/11.supplement_7.s1669. [DOI] [PubMed] [Google Scholar]
- 23.Simon H J, Yin E J. Microbioassay of antimicrobial agents. Appl Microbiol. 1970;19:573–579. doi: 10.1128/am.19.4.573-579.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tauber M G, Zak O, Scheld W M, Hengstler B, Sande M A. The postantibiotic effect in the treatment of experimental meningitis caused by Streptococcus pneumoniae in rabbits. J Infect Dis. 1984;149:575–583. doi: 10.1093/infdis/149.4.575. [DOI] [PubMed] [Google Scholar]
- 25.Tauber M G, Kunz S, Zak O, Sande M A. Influence of antibiotic dose, dosing interval and duration of therapy on outcome in experimental pneumococcal meningitis in rabbits. Antimicrob Agents Chemother. 1989;33:418–423. doi: 10.1128/aac.33.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viladrich P F, Gudiol F, Linares J, Pallares R, Sabate I, Rufi G, Ariza J. Evaluation of vancomycin for therapy of adult pneumococcal meningitis. Antimicrob Agents Chemother. 1991;35:2467–2472. doi: 10.1128/aac.35.12.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]