Abstract
The bacterial pathogen Listeria monocytogenes invades host cells, ruptures the internalization vacuole, and reaches the cytosol for replication. A high-content small interfering RNA (siRNA) microscopy screen allowed us to identify epithelial cell factors involved in L. monocytogenes vacuolar rupture, including the serine/threonine kinase Taok2. Kinase activity inhibition using a specific drug validated a role for Taok2 in favoring L. monocytogenes cytoplasmic access. Furthermore, we showed that Taok2 recruitment to L. monocytogenes vacuoles requires the presence of pore-forming toxin listeriolysin O. Overall, our study identified the first set of host factors modulating L. monocytogenes vacuolar rupture and cytoplasmic access in epithelial cells.
Keywords: Listeria monocytogenes, Taok2, STE1-like kinase, vacuolar escape, siRNA screen
The gram-positive pathogen Listeria monocytogenes is a major model microorganism to understand bacterial subversion of host functions in the context of disease. L. monocytogenes is a facultative intracellular pathogen able to invade mammalian cells. Upon interaction of bacterial surface molecules (InlA, InlB) with host receptors (E-cadherin, Met), signaling cascades are triggered to promote actin rearrangements and bacterial engulfment. Rupture of the internalization vacuole allows L. monocytogenes to reach the cytosol, where it replicates [1].
Three L. monocytogenes exotoxins control vacuolar escape: a phosphatidylinositol-specific phospholipase C (PlcA), a broad-range phospholipase C (PlcB), and the cholesterol-dependent cytotoxin listeriolysin O (LLO) [1]. In phagocytic cells, host factors have also been recognized as modulators of L. monocytogenes vacuolar rupture, including γ-interferon–inducible lysosomal thiol reductase (GILT), cystic fibrosis transmembrane conductance regulator (CFTR), and calpain [2]. Whether host factors in nonphagocytic cells control L. monocytogenes vacuolar escape has not been explored so far.
In the present study, we used a high-content microscopy approach to identify epithelial host cell components specifically required for efficient rupture of the L. monocytogenes-containing vacuole.
METHODS
Bacterial and Mammalian Growth Conditions, Plasmids, Drugs, and Infections
Bacterial strains were: L. monocytogenes EGDe PrfA* (BUG 3057), EGDe PrfA* β-lact (BUG 3358), EGDe PrfA* ΔplcAΔplcB (BUG3620), EGDe PrfA* ΔhlyΔplcAΔplcB (BUG 3648), L. innocuaβ-lact/InlB (BUG 3614) [3], Shigella flexneri wild-type strain M90T expressing the adhesin AfaI [4], Francisella tularensis subsp. novicida strain U112 (or F. novicida wild type), and an isogenic strain with the Francisella pathogenicity island (ΔFPI) deleted [5]. The plasmid encoding YFP-CBD, a yellow fluorescent protein (YFP) chimera protein of the cell wall binding domain (CBD) from the Listeria phage endolysin Ply118, has been described [6]. The plasmids encoding actin-mOrange and galectin-3-enhanced green fluorescent protein (EGFP) were used as a markers for ruffle formation and vacuole lysis by S. flexneri [4]. Human HeLa cells (American Type Culture Collection CCL-2) were transfected with 1.5 μg of the plasmids encoding YFP-CBD, actin-mOrange, or galectin-3-EGFP using Lipofectamine LTX for 24 hours. Bacterial strains and Hela cells were grown as described [3]. The specific Taok2 inhibitor SW172006 (Chembridge) [7] was added to HeLa cells at 50 μM for 24 hours before infection.
L. monocytogenes infections were performed as described [8, 9] to: (1) assess the efficiency of L. monocytogenes EGDe PrfA* invasion into HeLa cells treated with dimethyl sulfoxide (DMSO) or SW172006 at 1 hour post infection (hpi); (2) identify cytosolic bacteria labeled with CBD following vacuolar escape after Taok2 knockdown using small interfering RNA (siRNA) or after Taok2 kinase activity inhibition using SW172006 at 1 hpi (100 cells were counted in 3 representative fields to estimate the number of CBD-labeled bacteria); (3) perform immunofluorescence studies to analyze the distribution of Taok2 in HeLa cells at 30 minutes post infection (mpi); and (4) quantify the number of intracellular bacteria that escape from vacuoles and are associated with actin at 5 hpi after Taok2 siRNA knockdown. The multiplicity of infection (MOI) used was 25 (EGDe PrfA*), 50 (EGDe PrfA* ΔplcAΔplcB), or 125 (EGDe PrfA* ΔhlyΔplcAΔplcB). S. flexneri infections were performed as described [4]. HeLa cells transfected with scrambled or Taok2 siRNAs were infected (37°C, MOI 50), and live imaging of S. flexneri vacuolar rupture was monitored and quantified as the time interval between ruffle formation and appearance of a galectin-3 signal around the bacteria. Images were recorded in a Nikon Ti-E microscope (× 20 objective) with a time lapse of 2 minutes for 2 hours. F. novicida infections were performed using a MOI of 1000. Bacterial entry synchronization was performed by a 5-minute centrifugation at 1000 rpm and plates incubated at 37°C. After 1 hour plates were incubated at 37°C in fresh medium supplemented with gentamycin for 24 hours. Bacterial multiplication was monitored by plating cell lysates on chocolate agar plates. Each experiment was conducted at least twice in triplicates.
siRNA Library Screen
Four distinct oligos were used for each target gene. The screen was performed as described [4]. Each 96-well screen plate contained: 8 positive control wells transfected with scrambled siRNA and challenged with L. monocytogenes EGDe PrfA* β-lact [3]; 8 negative control wells transfected with scrambled siRNA and challenged with invasive L. innocuaβ-lact/InlB (which invades host cells but remains trapped in vacuoles); and 4 replicates for each siRNA target infected with L. monocytogenes EGDe PrfA* β-lact. Reverse siRNA transfection of HeLa cells was performed using Lipofectamine RNAi Max at a 10-nM siRNA concentration for 72 hours. The CCF4 assay for L. monocytogenes vacuole rupture quantification and differential staining of extracellular versus total L. monocytogenes for cell entry quantification at 1 hpi was performed as described [3, 7]. Images were acquired on a confocal microscope Opera QEHS (PerkinElmer) as described [3, 7]. The strictly standardized mean difference (SSMD) statistical test was used for quality control analysis and for hit scoring [3, 7].
Image Acquisition
Bacterial cytoplasmic access was determined by actin comet tail formation using phalloidin [9]. Briefly, extracellular L. monocytogenes were labeled with a primary goat anti-L. monocytogenes serum and with a secondary chicken anti-goat Alexa 647. Cells were permeabilized using 0.1% Triton X-100 and total L. monocytogenes were labeled with the same primary antibody and a secondary chicken anti-goat Alexa 488. Actin was labeled with phalloidin Alexa 546. At 5 hpi, cytosolic bacteria were those stained with Alexa 546 and Alexa 488 but lacking Alexa 647. In 3 representative fields 100 cells were counted to estimate the number of cytosolic L. monocytogenes cells.
For Taok2 labeling, 96-well tissue culture plates were seeded with HeLa cells to reach 80% confluence on the infection day. Cell infection with indicated strains was performed as reported [9]. At 1 hpi cells were washed and fixed with a paraformaldehyde (4% in phosphate-buffered saline) for 15 minutes. Extracellular L. monocytogenes were labeled with a primary rabbit anti-L. monocytogenes serum and with a secondary goat anti-rabbit Alexa 647. Next, cells were permeabilized using 0.1% Triton X-100 for 4 minutes. Taok2 was labeled overnight at 4°C with a mouse monoclonal antibody (Santa Cruz 514268) and a secondary goat anti-mouse Alexa 488. Actin was labeled with phalloidin Alexa 546. DNA was stained with Hoechst 33342.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism v6. Differences between control and experimental groups were evaluated by 2-tailed unpaired t tests or 1-way ANOVA followed by a Dunnett multiple comparison test. P < .05 was considered as significant.
RESULTS
Identification of Host Factors Controlling L. monocytogenes Vacuolar Escape in Epithelial Cells
To identify epithelial host cell factors specifically involved in L. monocytogenes vacuolar escape, we exploited a previous genome-wide siRNA screen that distinguished host genes modulating the cytoplasmic accumulation of the L. monocytogenes secreted molecule InlC (an event requiring L. monocytogenes cell invasion, vacuolar escape, and cytoplasmic proliferation) [10]. We siRNA-inactivated the 165 most significant hits identified in this previous screen and performed a novel high-content microscopy screen in which we combined: (1) a CCF4 fluorescence resonance energy transfer (FRET)-based assay to measure L. monocytogenes vacuolar rupture and cytoplasmic access [3, 7], and (2) a differential immunofluorescence staining to distinguish extracellular versus total L. monocytogenes [3, 7].
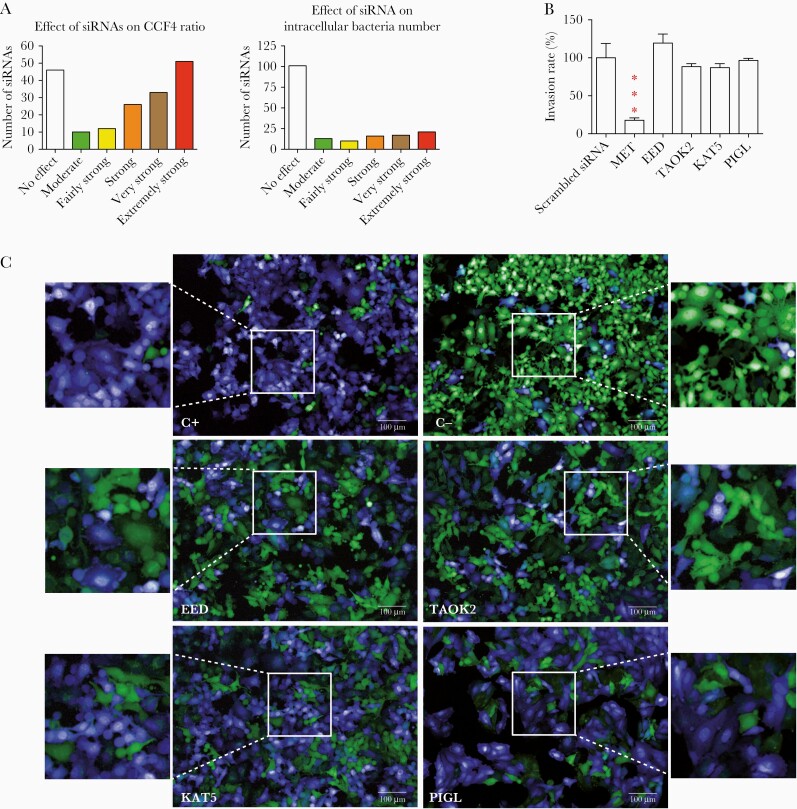
Fifty siRNA targeted genes were identified as extremely strong hits affecting L. monocytogenes vacuolar escape (Figure 1A–C and Supplementary Table 1). Some of these genes displayed either inhibition of bacterial entry into host cells (PODXL, SLC12A4, USE1, and TTC19) or induced strong cytotoxicity (PPP3R2) (Supplementary Table 1). However, we also identified genes that had a bona fide effect on L. monocytogenes cytoplasmic access and did not affect bacterial entry or cell integrity (EED, KAT5, MTOR, SYK, TAOK2, DPEP1, and PIGL) (Figure 1A–C and Supplementary Table 1).
Figure 1.
High-content microscopy siRNA screen reveals host factors required by Listeria monocytogenes for vacuolar escape in epithelial cells. A, Overview of the screen results. After data normalization based on the strictly standardized mean difference method, 165 targeted genes were classified into 6 categories based on their effect on the FRET probe CCF4 green to blue ratio (vacuolar escape step, left) and in the intracellular bacteria number (entry step, right): no effect, moderate, fairly strong, strong, very strong, and extremely strong effects. B, Contributions of MET (control for invasion), EED, TAOK2, KAT5, and PIGL to L. monocytogenes invasion. Target genes were knocked down by siRNA transfection of HeLa cells. One hour after infection cells were washed and L. monocytogenes entry was measured by counting colony forming units. Bars represent the mean and SD from 3 experiments. Significant differences from the scramble treatment *** P < .001. C, Examples of images from the siRNA screen. HeLa cells were grown in 96-well plates and transfected by siRNAs. Host cells were loaded with CCF4-AM and infected with L. monocytogenes EGDe PrfA* β-lact (C+) or L. innocuaβ-lact/InlB (C−) for 1 hour. C+ and C− negative wells were transfected with scrambled siRNA. After paraformaldehyde fixation, nuclei were stained with Draq5 and cells were imaged using an Opera QEHS confocal microscope, as previously described [3]. Images were obtained with the following merged channels: the intact CCF4 probe peaks at 535 nm (green), and the cleaved CCF4 probe peaks at 450 nm (blue). L. monocytogenes EGDe PrfA* β-lact (C+) can escape its vacuolar compartments and induce the cleavage of the CCF4 probe (top left). Scale bar 100µm. Abbreviations: FRET, fluorescence resonance energy transfer; siRNA, small interfering RNA.
Taok2 Controls L. monocytogenes Vacuolar Escape
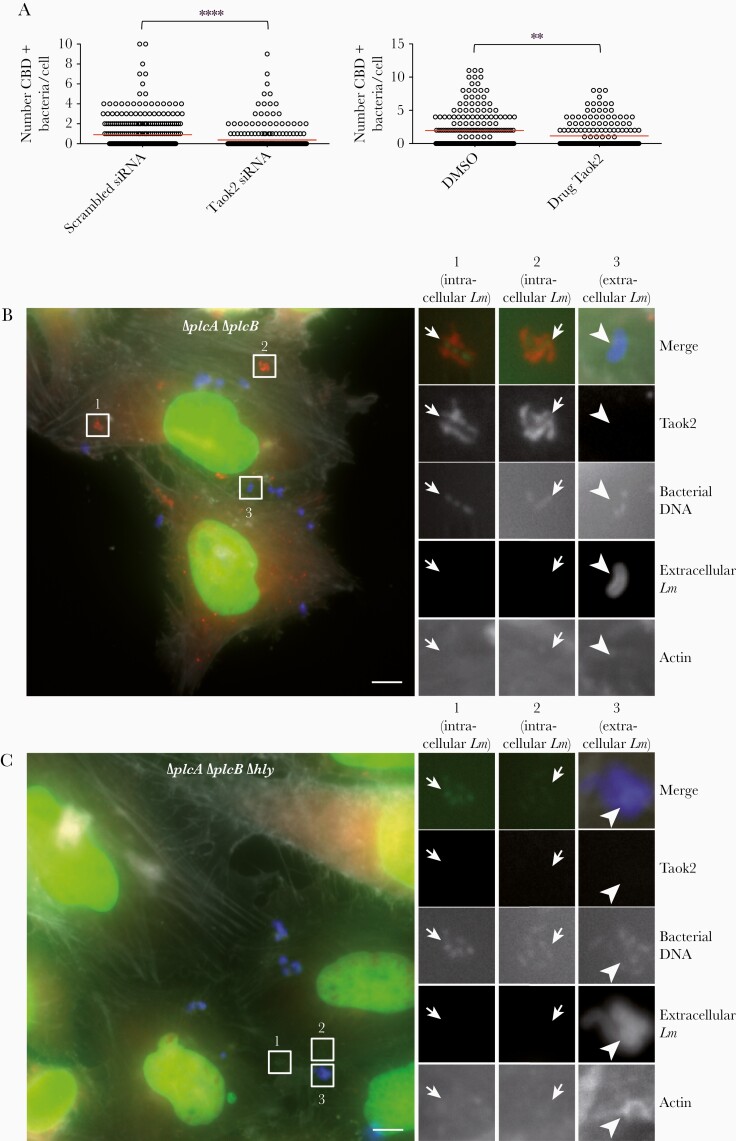
We selected Taok2 for further validation based on its strong phenotype and on reports showing its localization to vesicular compartments and its implication in surface receptor endocytosis [11, 12]. Taok2 depletion efficiency was verified by quantitative reverse transcription polymerase chain reaction (qRT-PCR; Supplementary Figure 1A). We investigated the impact of Taok2 depletion on L. monocytogenes cytoplasmic access using as readout the recruitment of the fluorescent chimera of the cell wall-binding domain (YFP-CBD) of the L. monocytogenes phage endolysin Ply118 [6], which labels cytosolic bacteria shortly after vacuolar escape [6]. After 1 hpi, Taok2 knockdown significantly reduced the number of YFP-CBD–positive L. monocytogenes per infected HeLa cell, in comparison to cells treated with control siRNA (0.90 for scrambled siRNA vs 0.36 for Taok2 siRNA; P < .0001; Figure 2A).
Figure 2.
Taok2 siRNA knockdown or inhibition of its kinase activity by the chemical compound SW172006 impairs efficient rupture of Listeria monocytogenes-containing vacuoles. A, Taok2 knockdown by siRNA (left) or inhibition of its kinase activity (right) results in reduced cytoplasmic access. HeLa cells were transfected with the YFP-CBD of the L. monocytogenes phage endolysin Ply118, which identifies cytosolic bacteria shortly after escape. The red line indicates the mean. Results were from 100 cells counted in 3 representative fields to estimate the number of CBD-labeled bacteria. Statistical analysis: 2-tailed unpaired t tests. **P < .01, ****P < .0001. B and C, Taok2 accumulation in a compartment resembling a L. monocytogenes-containing vacuole. B, HeLa cells infected with L. monocytogenes EGDe PrfA*ΔplcAΔplcB for 30 minutes and imaged. Taok2 (labeled with anti-Taok2 antibodies) is shown in red, DNA (labeled with Hoechst) is shown in green, extracellular bacteria (labeled with anti-L. monocytogenes antibodies) are shown in blue, and actin (labeled with phalloidin) is shown in white. Left image: overview. Right images: enlargement of 3 ROIs shown in the overview. In ROIs 1 and 2, Taok2 decorates intracellular bacteria (small arrows, revealed by the bacterial DNA labeling and by the absence of extracellular bacterial labeling), which are present in a vacuolar compartment and do not polymerize cytoplasmic actin (absence of actin labeling). In ROI 3, extracellular bacteria (large arrowhead, revealed by the presence of both DNA and extracellular L. monocytogenes labeling) are not decorated by Taok2. Scale bar, 5 μm. C, HeLa cells infected as in (B) using the strain L. monocytogenes EGDe PrfA* ΔplcAΔplcBΔhly. Left image: overview. Right images: enlargement of 3 ROIs shown in the overview. In all the ROIs showing either intracellular or extracellular L. monocytogenes Taok2 does not decorate the bacteria. Scale bar, 5 μm. Abbreviations: CBD, cell wall binding domain; DMSO, dimethyl sulfoxide; LM, Listeria monocytogenes; ROI, regions of interest; siRNA, small interfering RNA; YFP, yellow fluorescent protein.
We next used a small molecule (SW172006) that specifically inhibits the kinase activity of Taok2 [13]. We demonstrated by gentamicin assays that L. monocytogenes entry is not affected in cells treated with SW172006 (Supplementary Figure 1B). Importantly, Taok2 kinase activity inhibition caused a decrease in the number of YFP-CBD–positive L. monocytogenes per infected HeLa cell (1.99 for DMSO vs 1.27 for Taok2 SW172006; P < .01; Figure 2A), confirming our previous siRNA results (Figure 1). We then quantified in Taok2-depleted cells the number of cytoplasmic L. monocytogenes decorated with actin at 5 hpi. Taok2 depletion significantly increased the percentage of actin-negative (vacuole-trapped) L. monocytogenes in comparison to cells treated with control siRNA (13.5% for scrambled siRNA vs 38.5% for Taok2 siRNA; P < .05; Supplementary Figure 1C). Altogether, these results demonstrate that Taok2 enhances L. monocytogenes vacuolar rupture in HeLa cells.
Taok2 Is Recruited to L. monocytogenes Vacuoles
We next analyzed the distribution of Taok2 in L. monocytogenes-infected HeLa cells. Using anti-Taok2 antibodies, we observed rare events of Taok2 association with the L. monocytogenes EGDe PrfA* strain (not shown), suggesting that Taok2 recruitment to L. monocytogenes vacuoles is transient. We hypothesized that by delaying L. monocytogenes vacuolar escape, we might increase the probability of detecting Taok2 recruitment. Indeed, when using an L. monocytogenes EGDe PrfA*ΔplcAΔplcB strain, which lacks both phospolipases C and displays delayed vacuolar escape [14], we were able to observe a clearer association of Taok2 with L. monocytogenes in a compartment resembling a bacteria-containing vacuole (Figure 2B). Taok2 recruitment was abolished when using an EGDe PrfA*ΔplcAΔplcBΔhly strain lacking the phospholipases and LLO, demonstrating that Taok2 recruitment is dependent on LLO production (Figure 2C).
Taok2 Does Not Affect S. flexneri or F. novicida Cytoplasmic Translocation
We evaluated whether Taok2 displays a general role on vacuolar rupture for bacterial pathogens other than L. monocytogenes by analyzing its contribution to the cytoplasmic access of S. flexneri and F. novicida, 2 gram-negative pathogens that rupture their internalization vacuole as L. monocytogenes. Using a galectin-3 readout that follows the same principle of the YFP-CBD readout described above, we did not observe any role for Taok2 in S. flexneri cytoplasmic access (Supplementary Figure 1D). Furthermore, using gentamicin assays we did not detect any contribution of Taok2 to the late replication (24 hpi) of F. novicida (Supplementary Figure 1E), indirectly suggesting that Taok2 does not affect its cytoplasmic translocation.
DISCUSSION
Our results identify Taok2 as the first host cell factor specifically affecting vacuolar rupture and cytoplasmic translocation of L. monocytogenes in epithelial cells. Taok2 is a poorly studied serine/threonine kinase that belongs to the MAP3K family, which has been associated with neurological disorders [11, 15]. Taok2 has been localized to vesicular compartments, has been implicated in the endocytosis of N-cadherin at the synapse, and phosphorylates members of the septin family in neurons [11, 12, 15]. Our results therefore suggest a broader role for Taok2 in controlling integrity of phagosomal vacuoles. As our microscopy study highlights that Taok2 can be directly recruited by intracellular L. monocytogenes, and the use of the SW172006 inhibitor indicates that the Taok2 kinase activity is required for its role in L. monocytogenes vacuolar escape, our hypothesis is that Taok2 phosphorylates an unknown factor that is probably also recruited to the L. monocytogenes vacuole and which plays a more direct role on facilitating vacuolar rupture. Our results indicating that Taok2 association with L. monocytogenes vacuoles is dependent on LLO production suggest either that specific membrane damages produced by LLO are the signal triggering Taok2 vacuolar recruitment or, alternatively, that Taok2 recruitment is driven by other L. monocytogenes components being exposed to the cytosolic environment upon effective vacuolar rupture mediated by LLO. The molecular cascade of events downstream of Taok2 that leads to facilitated L. monocytogenes vacuolar rupture remains to be identified.
Apart from Taok2, we also found other host genes with a strong effect on L. monocytogenes vacuolar rupture and no effect on entry. These results propose a multifaceted mechanism where several host pathways are hijacked by L. monocytogenes to control vacuolar rupture.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by Institut Pasteur, Université Paris Diderot, Agence Nationale de la Recherche (grant numbers ANR-15-CE15-0017 StopBugEntry to J. P. C., J. E., and A. C.; and ANR-10-INSB-04-01, France-BioImaging to N. A.). J. J. Q. is supported by theSpanish Ministry of Science, Innovation and Universities (grant number RYC-2018-024985-I Ramón y Cajal contract). J. E. was supported by the European Research Council (consolidator grant EndoSubvert). P. B. I. is supported by the Région Ile de France (DIM1Health) and Groupement d’Intérêt Scientifique Infrastructures en Biologie Santé et Agronomie. The teams of P. C., J. P. C., N. A., and J. E. are members of Laboratory of Excellence Integrative Biology of Emerging Infectious Diseases.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Pizarro-Cerdá J, Cossart P. Microbe profile: Listeria monocytogenes: a paradigm among intracellular bacterial pathogens. Microbiology 2019; 165:719–21. [DOI] [PubMed] [Google Scholar]
- 2. Pizarro-Cerda J, Cossart P. Listeria monocytogenes: cell biology of invasion and intracellular growth. Microbiol Spectr 2018; 6: 10.1128/microbiolspec.GPP3-0013-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Quereda JJ, Pizarro-Cerdá J, Balestrino D, et al. A dual microscopy-based assay to assess Listeria monocytogenes cellular entry and vacuolar escape. Appl Environ Microbiol 2016; 82:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mellouk N, Weiner A, Aulner N, et al. Shigella subverts the host recycling compartment to rupture its vacuole. Cell Host Microbe 2014; 16:517–30. [DOI] [PubMed] [Google Scholar]
- 5. Weiss DS, Brotcke A, Henry T, Margolis JJ, Chan K, Monack DM. In vivo negative selection screen identifies genes required for Francisella virulence. Proc Natl Acad Sci U S A 2007; 104:6037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Henry R, Shaughnessy L, Loessner MJ, Alberti-Segui C, Higgins DE, Swanson JA. Cytolysin-dependent delay of vacuole maturation in macrophages infected with Listeria monocytogenes. Cell Microbiol 2006; 8:107–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Quereda JJ, Sachse M, Balestrino D, et al. Assessing vacuolar escape of Listeria monocytogenes. Methods Mol Biol 2017; 1535:173–95. [DOI] [PubMed] [Google Scholar]
- 8. Mostowy S, Danckaert A, Tham TN, et al. Septin 11 restricts InlB-mediated invasion by Listeria. J Biol Chem 2009; 284:11613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quereda JJ, Nahori MA, Meza-Torres J, et al. Listeriolysin S is a streptolysin S-like virulence factor that targets exclusively prokaryotic cells in vivo. MBio 2017; 8:e00259-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kühbacher A, Emmenlauer M, Rämo P, et al. Genome-wide siRNA screen identifies complementary signaling pathways involved in Listeria infection and reveals different actin nucleation mechanisms during Listeria cell invasion and actin comet tail formation. mBio 2015; 6:e00598-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yasuda S, Tanaka H, Sugiura H, et al. Activity-induced protocadherin arcadlin regulates dendritic spine number by triggering N-cadherin endocytosis via TAO2beta and p38 MAP kinases. Neuron 2007; 56:456–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moore TM, Garg R, Johnson C, Coptcoat MJ, Ridley AJ, Morris JD. PSK, a novel STE20-like kinase derived from prostatic carcinoma that activates the c-Jun N-terminal kinase mitogen-activated protein kinase pathway and regulates actin cytoskeletal organization. J Biol Chem 2000; 275:4311–22. [DOI] [PubMed] [Google Scholar]
- 13. Piala AT, Akella R, Potts MB, et al. Discovery of novel TAOK2 inhibitor scaffolds from high-throughput screening. Bioorg Med Chem Lett 2016; 26:3923–7. [DOI] [PubMed] [Google Scholar]
- 14. Smith GA, Marquis H, Jones S, Johnston NC, Portnoy DA, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun 1995; 63:4231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yadav S, Oses-Prieto JA, Peters CJ, et al. TAOK2 kinase mediates PSD95 stability and dendritic spine maturation through Septin7 phosphorylation. Neuron 2017; 93:379–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.