Abstract
Background
The role of extracellular vesicles (EVs) in human immunodeficiency virus (HIV) pathogenesis is unknown. We examine the cellular origin of plasma microvesicles (MVs), a type of ectocytosis-derived EV, the presence of mitochondria in MVs, and their relationship to circulating cell-free mitochondrial deoxyribonucleic acid (ccf-mtDNA) in HIV-infected patients and controls.
Methods
Five participant groups were defined: 30 antiretroviral therapy (ART)-naive; 30 ART-treated with nondetectable viremia; 30 elite controllers; 30 viremic controllers; and 30 HIV-uninfected controls. Microvesicles were quantified and characterized from plasma samples by flow cytometry. MitoTrackerDeepRed identified MVs containing mitochondria and ccf-mtDNA was quantified by real-time polymerase chain reaction.
Results
Microvesicle numbers were expanded at least 10-fold in all HIV-infected groups compared with controls. More than 79% were platelet-derived MVs. Proportions of MVs containing mitochondria (22.3% vs 41.6%) and MV mitochondrial density (706 vs 1346) were significantly lower among HIV-infected subjects than controls, lowest levels for those on ART. Microvesicle numbers correlated with ccf-mtDNA levels that were higher among HIV-infected patients.
Conclusions
A massive release of platelet-derived MVs occurs during HIV infection. Some MVs contain mitochondria, but their proportion and mitochondrial densities were lower in HIV infection than in controls. Platelet-derived MVs may be biomarkers of platelet activation, possibly reflecting pathogenesis even in absence of HIV replication.
Keywords: elite controllers, extracellular vesicles, HIV, microvesicles, mitochondria
A massive release of platelet-derived CD9+ microvesicles occurs during HIV infection, regardless of virologic control or antiretroviral therapy administration. Some of these microvesicles contain mitochondria, but their prevalence and mitochondrial density are reduced in HIV infection.
Antiretroviral therapy (ART) has dramatically improved outcomes in human immunodeficiency virus (HIV) infection [1]. Despite suppression of HIV replication with ART, immune activation, heightened coagulation, and inflammation often persist [2]. High levels of inflammatory mediators are linked to acquired immune deficiency syndrome (AIDS) and non-AIDS morbidities and mortality [3–5]. Because the underlying mechanisms of these relationships have not been fully elucidated, better characterization of the proinflammatory and procoagulant environment and its role in disease outcome is warranted.
Increasing evidence supports a role for extracellular vesicles (EVs) in cancer, cardiovascular and neurologic diseases, and viral infections. Extracellular vesicles comprise a heterogeneous population of membrane-bound vesicles secreted by various cells into different body fluids including blood, urine, saliva, semen, and breast milk. Initially described as a waste elimination pathway, EVs facilitate intercellular communication, allowing cells to exchange proteins, lipids, genetic materials, and cytokines [6–10]. Extracellular vesicles also shuttle intact or fragmented mitochondria between cells preserving cellular homeostasis and triggering alert signals [11, 12].
Extracellular vesicles have been implicated in HIV pathogenesis, activating recipient cells, increasing levels of proinflammatory cytokines, viral replication, and carcinogenesis [13–16]. The exact role of EVs in HIV pathogenesis remains poorly understood in part due to the enormous variability in EV phenotypes (eg, size, cellular origins, cargo) and in methodologies for EV isolation and analysis. Extracellular vesicles are generally classified into 2 types: submicron-size microvesicles (MVs) derived from ectocytosis, and nanometer-size (30 to 150 nm) exosomes derived from exocytosis [17].
We evaluated the profile of a subset of approximately 300 to 1000 nm-sized plasma MVs that expressed tetraspanin CD9 in well characterized cohorts of HIV-infected patients and HIV-uninfected controls. We observed a massive increase of circulating MVs among HIV-infected patients irrespective of HIV control. Most were derived from platelets. Some MVs contained mitochondria, yet mitochondrial content was lower in HIV infection than among controls, especially for persons on suppressive ART. Circulating cell-free mitochondrial deoxyribonucleic acid (ccf-mtDNA) levels correlated with total MV numbers and with MV subpopulations containing mitochondria and/or platelet markers.
METHODS
Study Design
Frozen plasma samples were obtained with informed consent from the Spanish AIDS Research Network (RIS) from University Hospital of Ferrol and Hospital Álvaro Cunqueiro, Vigo. There were 30 patients in 5 groups: (1) ART-naive patients; (2) ART-treated patients with nondetectable viremia for at least 1 year; (3) elite controllers (EC) with <50 HIV-ribonucleic acid (RNA) copies/mL in the absence of ART for at least 12 months (from the RIS cohort of HIV Controllers Study Group (ECRIS) [18]); (4) viremic controllers (VC) with HIV-RNA between 50 and 2000 copies/mL without ART for more than 1 year (from ECRIS); and (5) HIV-uninfected controls. The study was approved by the regional Ethic Committee (CAEIG), an external Scientific and Ethic Committee of the HIV Biobank (Spanish HIV/AIDS Research Network).
Plasma Cell-Free Mitochondrial Deoxyribonucleic Acid Quantification
Blood was drawn in ethylenediaminetetraacetic acid (ETDA) tubes, sedimented over Ficoll-Hypaque and plasma collected. Circulating cell-free mitochondrial DNA (ccf-mtDNA) was quantified as described [19]. An equal volume of phosphate-buffered saline (PBS) was added to 400 µL plasma and centrifuged at 16 000 ×g for 15 minutes at 4°C. Deoxyribonucleic acid was isolated using the DNAeasy Blood and Tissue kit (QIAGEN), and 100 µL elution buffer was added to solubilize the DNA.
Th ccf-mtDNA levels were measured in duplicate by SYBR Green dye-based quantitative real-time polymerase chain reaction assay using a LightCycler II (Roche) thermal cycler. Ribosomal 12S RNA (mtDNA) primer sequences were as follows: forward CCACGGGAAACAGCAGTGAT; reverse CTATTGACTTGGGTTAATCGTGTGA. The ccf-mtDNA levels were calculated as described [19] and expressed as copies/µL.
Microvesicle Isolation and Flow Cytometry
To 500 µL plasma, 500 µL PBS was added and then centrifuged at 16 000 ×g for 15 minutes at 4°C. The pellet containing MVs was resuspended gently with a cocktail of MitoTrackerDeepRed (Thermo Fisher) and the monoclonal antibodies: anti-CD3-BUV737 (BD), anti-CD4-BUV395 (BD), anti-CD8-BV605 (BD), anti-CD9-PE-Dazzle 594 (BioLegend), anti-CD11b-PE-Cy7 (BD), anti-CD14-BV711 (BD), anti-CD16-APC-H7 (BD), anti-CD41a-BV650 (BD), anti-CD56-APC-R700 (BD), anti-CD61-BV605 (BD), anti-CD62P-BUV395 (BD), anti-CD66b-PerCP-Cy5.5 (BD), anti-CD146-BV421 (BioLegend). Size reference beads (Thermo Fisher) were used to determine MV size and to exclude residual platelets by gating. Liquid counting beads (BD) were used to enumerate MV number. Samples were run on a LSRFortessa (BD) flow cytometer and analyzed with FlowJo software.
Electron Microscopy
Plasma was pelleted from 100 mL whole blood from 2 uninfected and 2 HIV-infected donors (University Hospitals, Cleveland Medical Center). Microvesicles in pellets were fixed in phosphate-buffered quarter-strength Karnofsky’s fixative as reported [20]. After rinsing in 0.1 M phosphate buffer (pH 7.3), MVs were postfixed in ferrocyanide-reduced osmium tetroxide. Semi-thin sections (0.7 μm) were cut with a diamond knife and stained with Toluidine blue. Thin sections (70 nm) were sequentially stained with acidified uranyl acetate followed by a modification of Sato’s triple lead stain [21] and examined in an FEI Tecnai Spirit (T12) with a Gatan US4000 4k × 4k CCD camera.
NanoSight Measurement of Extracellular Vesicles
Microvesicles in plasma from 5 donors from each group were characterized for size distribution and concentration on a NanoSight NS300 (Malvern, UK) using Nanoparticle Tracking Analysis software (version 3.4). Plasma samples were diluted 1:100–1:1000 in PBS and infused by a syringe pump set at 25. Three video captures of 60 seconds each were collected for each sample, and measurements were recorded at camera level 12 and detection threshold of 5.
Statistical Analysis
Statistical analysis was performed using Statistical Package for the Social Sciences Software (v19.0; SPSS), using descriptive statistics to summarize characteristics of the study population. Continuous variables were expressed as the median and interquartile range (IQR), and qualitative variables were expressed as percentages. For continuous variables, normality was assessed with the Kolmogorov-Smirnov test. Univariate analyses using Student t test or Mann-Whitney U test compared 2 groups, and analysis of variance or Kruskal-Wallis tests compared more than 2. The χ 2 test was used for qualitative variables to compare groups. Correlations were determined using Spearman’s correlation. A P < .05 was considered significant.
To construct the multivariate linear model, we first performed a univariate analysis, then we selected all independent variables having a P value lower than .1. Variables with the highest P value were successively eliminated until the most appropriate model was obtained.
RESULTS
Study Population
Antiretroviral therapy-exposed and EC groups had control of HIV replication, high CD4 cell counts, a CD4/CD8 ratio close to or over 1, and were infected for a median of >13 years. The median time on ART for the treated patients was 9 years (IQR, 6–18), and most were receiving an integrase strand transfer inhibitor-based regimen (n = 15), 9 were on a protease inhibitor (PI)-based regimen, and 6 were on a non-nucleotide transcriptase inhibitor (NRTI)-based regimen. Regarding the NRTI-backbone, 21 were on tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide (TAF) + lamivudine (3TC) or emtricitabine (FTC) or not receiving an NRTI, and the rest (n = 9) were on abacavir (ABC) + 3TC therapy. The ART-naive group had detectable HIV levels, low CD4 cell counts, a CD4/CD8 ratio lower than 1, and most were recently diagnosed (median 7 days after diagnosis of HIV infection) and had not yet begun treatment. The VC group includes chronically infected patients (median >6 years) with relative control of HIV replication with median HIV levels of 361 copies/mL without ART, CD4 counts >500 cells/mm3, and a CD4/CD8 ratio near 1. The control group includes HIV-uninfected persons with high CD4 cell counts and an average CD4/CD8 ratio of 1.6 (Table 1).
Table 1.
Epidemiological and Clinical Characteristics of the Study Populationa
| Characteristics | Control (n = 30) | ART Naive (n = 30) | ART Exposed (n = 30) | EC (n = 30) | VC (n = 30) | P Value |
|---|---|---|---|---|---|---|
| Age | 36.0 [29.0–41.0] | 41.0 [32.0–52.0] | 52.0 [43.0–56.0] | 52.5 [43.5–57.2] | 50.5 [38.5–54.2] | <.001 |
| Sex (male) | 50.0 (15) | 71.0 (22) | 66.7 (20) | 50.0 (15) | 56.7 (17) | n.s. |
| Year of HIV diagnosis | NA | 2017 [2017–2018] | 2004 [1997–2011] | 1996 [1986–2004] | 2003 [1990–2008] | <.001 |
| Days since HIV diagnosis | NA | 7 [0–64] | 4803 [2316–7373] | 5246 [2048–8249] | 2347 [787–7421] | <.001 |
| HIV viral load (copies/mL) | NA | 27 450 [9700–110 000] | ND | ND [35–50] | 361 [85–788] | <.001 |
| CD4 (cells/µL) | 842 [712–1018] | 456 [267–657] | 827 [609–1071] | 830 [519–1077] | 670 [540–1100] | <.001 |
| CD8 (cells/µL) | 474 [404–653] | 983 [730–1284] | 824 [512–933] | 855 [637–1133] | 925 [718–1177] | <.001 |
| CD4/CD8 ratio | 1.6 [1.4–2.1] | 0.4 [0.2–0.7] | 1 [0.8–1.3] | 0.9 [0.6–1] | 0.8 [0.7–1] | <.001 |
| Platelet (cells/µL) | 246 [211–275] | 219 [186–257] | 212 [192–245] | n.a | n.a | n.s |
| Neutrophils (cells/µL) | 3.40 [2.70–4.73] | 2.64 [1.91–3.21] | 3.67 [2.68–4.90] | n.a. | n.a | .002 |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; NA, not applicable; ND, not detectable; n.s., not significant.
aData are expressed as median [interquartile range] and as percentage (n). Statistical significance was determined using χ 2 test and Kruskal-Wallis test for qualitative and continuous variables, respectively.
Microvesicle Numbers, Cellular Source, and Mitochondrial Content
Extracellular vesicles (MVs and exosomes) in plasma were characterized by NanoSight. Total EV concentrations, 300–1000 nm MV concentrations, and particle sizes are reported in Supplemental Table 1. Average size distribution profile and 300–1000 nm EV size distributions are shown in Supplemental Figure 1.
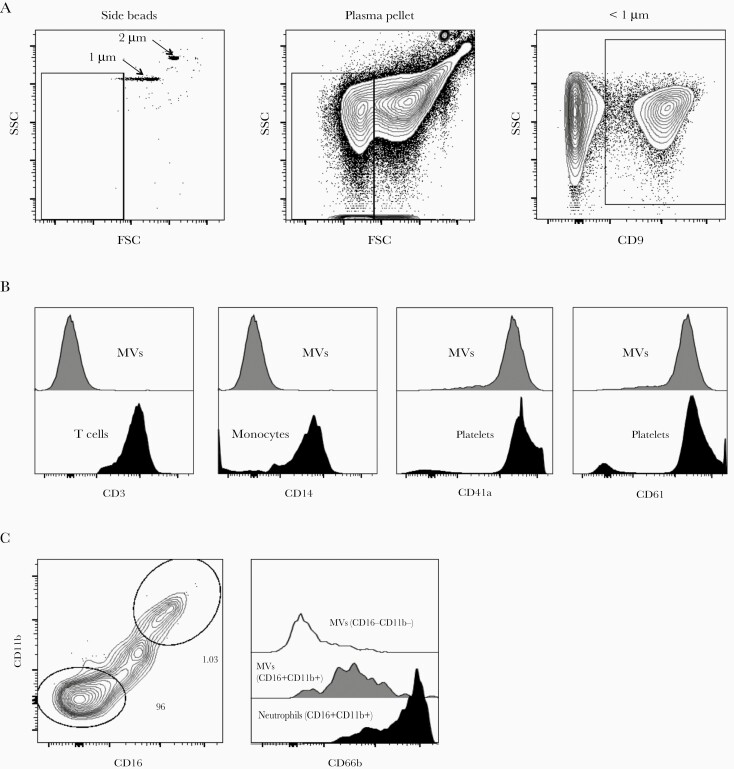
Microvesicles were identified by size less than 1 µm and surface expression of tetraspanin CD9, which is expressed on all major subsets of leukocyte and endothelial cell surfaces (Figure 1). Median MV count was significantly higher among all the HIV-infected groups than in the control group (89 792 vs 6982/mL; P < .001) (Table 2). Among the HIV-infected patients, the ART-naive group had the fewest MVs (68 468 vs 111 116/mL; P < .001).
Figure 1.
Gating strategy to characterize microvesicles (MVs) by flow cytometry. (a) Microvesicles less than 1 μM were gated using size reference beads and CD9 expression. Representative contour plots showing side scatter (SSC) and forward scatter (FSC) or CD9 labeling of MVs from antiretroviral therapy-exposed human immunodeficiency virus-infected donors (n = 30); (b) CD3 staining on plasma-derived MVs or peripheral blood-derived T cells; CD14 staining on plasma-derived MVs or monocytes, and CD41a and CD61 staining on plasma-derived MVs or platelets; (c) coexpression of CD11b and CD16 on MVs and CD66b staining on plasma-derived MVs subsets or peripheral blood-derived neutrophils.
Table 2.
Microvesicles Profile and Mitochondrial Density (MFI) Among the Study Populationa
| Control (n = 30) | ART-Naive (n = 30) | ART-Exposed (n = 30) | EC (n = 30) | VC (n = 30) | P Value | |
|---|---|---|---|---|---|---|
| MVs (count/mL) | 6982 [5185–8971] | 68 468 [27 299–145 591] | 116 385 [66 713–220 466] | 120 009 [29 266–377 811] | 139 602 [81 729–259 127] | <.001 |
| Platelet-derived MVs (%) | 40.6 [25.6–51.1] | 84.8 [71.9–89.9] | 91.8 [85.5–94.5] | 79.7 [64.7–91.4] | 81.9 [72.0–89.6] | <.001 |
| Neutrophil-derived MVs (%) | 6.2 [2.7–24.2] | 1.1 [0.3–2.4] | 0.1 [0.1–0.5] | 1.04 [0.3–6.6] | 1.88 [0.3–4.5] | <.001 |
| mito+ MVs (count/mL) | 2661 [1893–4196] | 20 432 [7687–34 460] | 19 710 [8262–30 118] | 26 451 [7650–51 141] | 29 783 [12 165–45 518] | <.001 |
| mito+ MVs (%) | 41.65 [21.5–56.10] | 24.30 [19.6–31.7] | 17.35 [11.3–24.0] | 24.20 [16.4–38.0] | 23.40 [13.2–31.4] | <.001 |
| Platelet-derived mito+ MVs (%) | 29.4 [19.3–37.2] | 22.1 [16.6–25.1] | 13.4 [11.1–21.7] | 20.9 [10.6–36.7] | 21.3 [10.8–29.6] | <.001 |
| Neutrophil-derived mito+ MVs (%) | 88.9 [72.5–93.1] | 88.6 [57.9–98.1] | 48.2 [34.8–81.7] | 65.9 [31.4–84.3] | 64.1 [30.1–85.9] | <.001 |
| Mitochondrial density (MFI) | 1346 [1101–1799] | 720 [691–1260] | 628 [584–754] | 764 [567–1038] | 711 [632–796] | <.001 |
Abbreviations: ART, antiretroviral therapy; EC, elite controllers; MFI, mitochondria fluorescence intensity; MV, microvesicle; VC, viremic controllers.
aData are expressed as median [interquartile range]. Statistical significance was determined using Kruskal-Wallis test.
We next sought to determine the cellular origin of circulating MVs by staining for markers of platelets, neutrophils, T and B lymphocytes, and monocytes (Figure 1). Most of the MVs from HIV-infected participants had platelet surface markers (85.2% were CD61+/CD41a+ vs 40.6% among controls, P < .001). Few MVs expressed neutrophil markers (CD16+/CD11b+/CD66b+) among the HIV-infected patients, and this proportion was lower than among the controls (0.9% vs 6.2%, P < .001). Within the HIV-infected population, patients on ART showed lower levels of neutrophil-derived MVs than the rest (0.1% vs 1.3%, P < .001). Within the ART group, those patients receiving ABC showed slightly higher proportions of platelet-derived MVs than among those receiving TDF/TAF+3TC/FTC (94.6% vs 89.9%, P = .04), even though the groups had similar platelet numbers (212 vs 212/μL, P = .894).
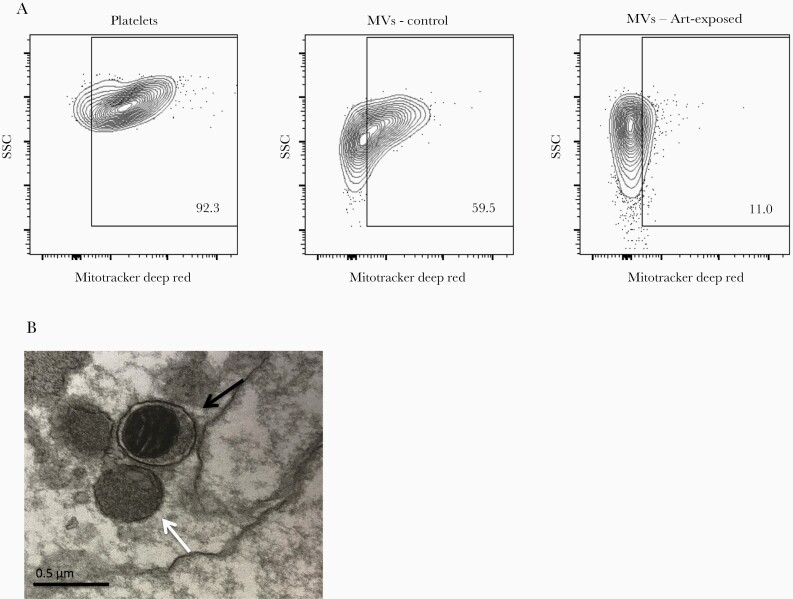
We found some MVs stained with MitoTrackerDeepRed, a mitochondria-labeling dye (Figure 2a). We confirmed the presence of morphologically intact mitochondria within MVs by electron microscopy (Figure 2b). The proportion of MVs containing mitochondria (mito+ MVs) was significantly lower among HIV-infected patients than among uninfected controls (22.3% vs 41.6%, P < .001), and those receiving ART had the lowest proportion of mito+ MVs (17.3% vs 24.0%, P = .003) (Table 2).
Figure 2.
Microvesicles (MVs) contain mitochondria. (a) Representative contour plots showing side scatter (SSC) and MitoTrackerDeepRed labeling of platelets and MVs from human immunodeficiency virus (HIV)-uninfected controls (n = 30) or MVs from antiretroviral therapy (ART)-exposed HIV-infected donors (n = 30); (b) transmission electron microscopy visualization of plasma-derived MVs with (black arrow) and without (white arrow) mitochondria from an ART-exposed HIV-infected donor (representative of 2 donors).
Neutrophil-derived MVs more often contained mitochondria than did platelet-derived MVs in both controls (88.9% vs 29.4%; P < .001) and HIV-infected donors (66.7% vs 19.4%; P < .001). Among HIV-infected donors, the lowest frequencies of mito+ MVs from neutrophils or platelets were found among ART-treated patients (48.2% and 13.4%, respectively), with significant differences for platelet-derived MVs between ART-treated patients and the rest of the HIV-infected population never exposed to ART (13.4% vs 21.3%; P = .019) (Table 2).
Mitochondrial density (as measured by mean fluorescence intensity [MFI] of MitoTrackerDeepRed labeling) was also lower within mito+ MVs in the HIV-infected population than in controls (706 vs 1346; P < .001). Here too, the ART-exposed group showed the lowest mitochondrial density, and this was moderately inversely correlated with the duration of ART exposure (rho = −0.492; P = .012). The proportion of MVs containing mitochondria was lower among patients receiving ABC/3TC than among those receiving TDF/TAF+3TC/FTC (12.8% vs 18.5%; P = .032).
Circulating Cell-Free Mitochondrial Deoxyribonucleic Acid Levels and Correlation With Microvesicle Count
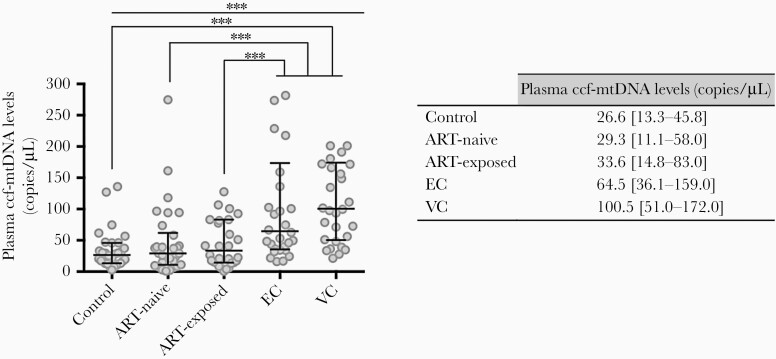
We next quantified plasma ccf-mtDNA and observed higher median levels of ccf-mtDNA among HIV-infected patients than in uninfected controls (50.2 vs 26.6 copies/μL, respectively; P < .001), with significantly higher median levels for the EC and VC groups (64.5 and 100.5 copies/μL, respectively; P < .001) (Figure 3).
Figure 3.
Plasma circulating cell-free mitochondrial deoxyribonucleic acid (ccf-mtDNA) levels among the study groups. Whiskers represent median values ± interquartile range. Statistical significance was determined by Mann-Whitney U test to compare 2 groups, and Kruskal-Wallis test was used to compare more than 2 groups. ***, P > .001. EC, elite controllers; VC, viremic controllers.
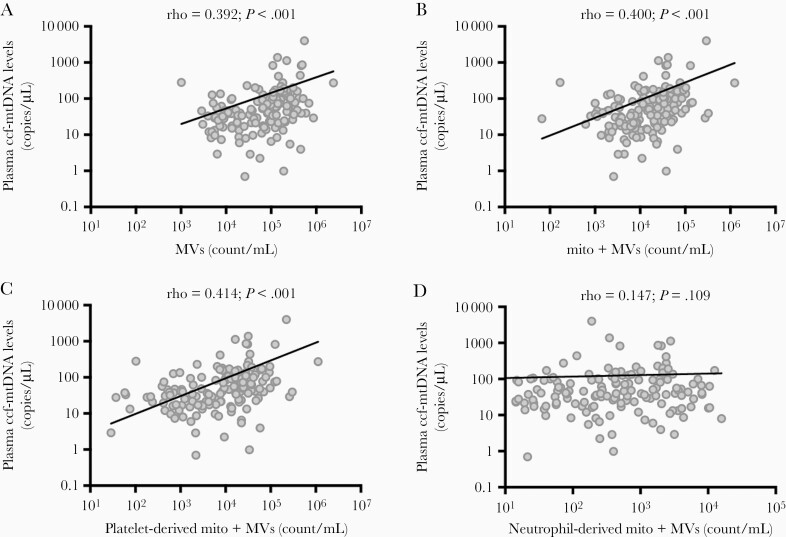
The ccf-mtDNA levels positively correlated with total MV counts (rho = 0.392; P < .001) (Figure 4a). Positive correlations were found between ccf-mtDNA levels and total mito+ MVs (rho = 0.400; P < .001) and platelet-derived mito+ MVs (CD61+/CD41a+, MitoTrackerDeepRed+) (rho = 0.414; P < .001), but not neutrophil-derived mito+ MVs (CD16+/CD11b+, MitoTrackerDeepRed+) (rho = 0.147; P = .109) (Figure 4b–d).
Figure 4.
Correlation between plasma circulating cell-free mitochondrial deoxyribonucleic acid (ccf-mtDNA) levels: (a) microvesicles (MVs) count; (b) mito+ MVs count; (c) platelet-derived mito+ MVs count; (d) neutrophil-derived mito+ MVs count. Statistical correlation was determined using Spearman’s correlation analysis and was expressed as Rho-Spearman’s coefficient; P value.
Clinical Indices, Plasma Microvesicle Count, Circulating Cell-Free Mitochondrial Deoxyribonucleic Acid, and Mitochondrial Density
Within the HIV-infected population, neither age, sex, HIV viral load, nor CD4 or CD8 T-cell counts were related to the plasma MV numbers, nor to the proportions of mito+ MVs, the density of mitochondria within them, or the levels of ccf-mtDNA in plasma. Moreover, neither platelet nor neutrophil counts were related to the total MV counts, mito+ MV counts, or numbers of MVs derived from platelets or neutrophils (these numbers were not available for elite or viremic controllers).
Multivariate linear regression identified as independent correlates of higher MV numbers: presence of HIV infection (B [regression coefficient] = 48.2 × 103; 95% confidence interval [CI], 4.5 × 103 to 91.8 × 103; P = .031), MVs with platelet markers (B = 2.12; 95% CI, 1.97–2.27; P < .001), and lower mitochondrial MFI (B = −57.7; 95% CI, −90.8 to −24.6; P < .001) after proportions of mito+ MVs, ccf-mtDNA levels, and age were excluded from the model.
DISCUSSION
We report a significant increase in the number of circulating platelet-derived MVs during HIV infection; elevated MV numbers were not related to viroimmunological status or to administration of ART, and some MVs contained mitochondria. Overall, we observed lower numbers of mito+ MVs and reduced mitochondrial density within MVs in HIV infection, especially for patients on ART. It is interesting to note that plasma ccf-mtDNA levels correlated with the total number of MVs and with the MV subpopulations containing mitochondria and/or platelet markers. Moreover, higher levels of ccf-mtDNA were observed among HIV-infected patients, especially those with long-term natural control of HIV replication.
Human immunodeficiency virus infection is associated with activated platelets, even with virological suppression by ART, and platelet activation appears related to immune activation and inflammation [22, 23]. Activated platelets release MVs (with or without mitochondria) and release free mitochondria in vitro [24, 25]. Our findings may therefore reflect the consequences of platelet activation during HIV infection—a massive release of platelet-derived MVs—that we show here in a large population (n = 120) of HIV-infected persons with different levels of virologic control, including patients with endogenous control of HIV replication. In concordance with our findings, Hijmans et al [26] recently described elevated numbers of plasma MVs derived from activated platelets in 15 HIV-infected patients on ART. Their MV numbers in both HIV-infected persons and controls were similar to ours (124 [IQR, 86–169] vs 38 [IQR, 30–78] microparticles/µL). They also found that MVs from HIV-infected donors promoted the release of cytokines and other inflammatory molecules by human endothelial cells in vitro, promoting inflammation, oxidative stress, senescence, and apoptosis. Thus, circulating MVs may transport inflammatory cytokines and other “danger signals” to endothelium during HIV infection that could contribute to an increased cardiovascular disease risk observed in the HIV-infected population [27, 28]. These findings warrant further studies to elucidate the specific role of MVs and other danger signals (ie, mtDNA) that they contain in the pathogenesis of HIV infection.
Recent studies have identified immunometabolic defects during HIV infection, suggesting a key role of mitochondrial dysfunction in HIV disease progression [29–32]. Mitochondrial dysfunction is associated with the spontaneous loss of virological control in EC patients [30] and with immune nonresponse on ART [31]. Activated platelets from ART-controlled patients also have evidence of mitochondrial dysfunction [22]. In our study, although platelet-derived MV numbers were increased in HIV infection, their mitochondrial content was proportionally diminished, and whereas mitochondrial density was decreased in MVs in all HIV+ groups, the lowest mitochondrial density was seen in MVs of ART-treated patients—consistent with observations that ART regimens may also contribute to mitochondrial dysfunction [33]. In particular, the proportion of MVs containing mitochondria was lower among patients receiving ABC/3TC than among those receiving TDF/TAF+3TC/FTC. Additional studies evaluating biomarkers directly related with mitochondrial dysfunction to address the impact of current ART drugs on the mitochondria and the mechanisms underlying are warranted.
When mitochondrial damage occurs, mitophagy eliminates them to maintain cell health, inhibiting inflammation [34]. When mitophagy is impaired or inadequate, mitochondrial components leak into the cytosol, triggering danger-associated molecular pattern (DAMP)-mediated innate immune responses [26, 35]. Although we and others have described increased levels of ccf-mtDNA during HIV infection [19, 36–38], its role as a DAMP and its relationship to with inflammation and disease outcome is still controversial [39–42].
We propose that during chronic HIV infection, effective mitophagy is maintained for patients on ART. In contrast, for patients with long-term natural control of HIV replication (EC and VC), mitophagy mechanisms may become exhausted and inefficient, allowing accumulation of damaged mitochondria and mtDNA release, which could explain the higher levels of mtDNA in plasmas of EC and VC. It is plausible that the presence of ccf-mtDNA in EC and VC may activate innate responses and contribute to the higher levels of inflammatory mediators that are linked with increased risk of comorbidities such as cardiovascular disease [43–46]. In this study, we observed a correlation between plasma levels of ccf-mtDNA and the number of mito+ MVs. The release of ccf-mtDNA into the circulation (either free or MV-associated) is related to several pathogenic processes [47], but, to our knowledge, this is the first study that evaluated and correlated mito+ MV numbers with ccf-mtDNA levels during HIV infection.
The lower proportion of mito+ MVs and lower mitochondrial density observed within the ART-treated group might be related to the mitochondrial toxicity of the ART regimens as previously described with older NRTIs (eg, didanosine, stavudine) [48]. Although current HIV medications are far-better tolerated [49], it is possible that components of these regimens also alter mitochondrial function. Accordingly, we found an inverse correlation between ART duration and mitochondrial density, as well as a lower proportion of platelet-derived MVs containing mitochondria for patients who were receiving an ABC/3TC NRTI-based regimen than among those receiving TDF or TAF/3TC or FTC. Higher levels of platelet-derived MVs among patients receiving ABC/3TC might be related to the greater platelet aggregation observed in patients on ABC/3TC than in those on TAF [50]. Moreover, these observations need confirmation in a larger study to better understand the impact of current antiretroviral drugs on mitochondrial homeostasis and to explore the significance of these observations on the occurrence of HIV-related comorbidities.
The use of conventional flow cytometry reveals certain methodological limitations in this study. We gated on MVs using forward scatter to approximate size and antibody labeling of CD9 to positively identify membrane components. However, some MVs were likely smaller than our detection threshold, so we may have underestimated MV numbers, although our numbers are consistent with other reports [26]. Because ccf-mtDNA quantification was performed from plasma samples after a 16 000 ×g centrifugation, we cannot exclude the possibility that some of the ccf-mtDNA we measured was contained within smaller vesicles (ie, exosomes) that require higher speed to pellet. Our preliminary experiments suggest that these mitochondria may be functional because they appear to metabolize dimethyl succinate (data not shown). This functionality of mitochondria should be confirmed and investigated with other methods.
CONCLUSIONS
In conclusion, we describe an increase of circulating platelet-derived MVs during HIV infection irrespective of the level of HIV control. The ccf-mtDNA levels are highest among patients with natural control of HIV infection, and among HIV-infected patients, frequencies of mito+ MVs correlate with ccf-mtDNA levels. We propose a potential dual role for MVs as intercellular messengers that can deliver cytokine packages (and possibly mitochondria) and as products of mitophagy reflecting and/or driving immune activation. High levels of mtDNA released into the circulation either within MVs or free due to inefficient mitophagy in patients with natural or pharmacological control of HIV replication might promote proinflammatory pathways, increasing the risk of cardiovascular and other comorbidities. More importantly, these indices should be examined further as potential surrogate biomarkers of platelet activation and mitochondrial dysfunction in the settings of treated and untreated HIV infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplemental Table 1. Characteristics of total EVs in plasma samples by NanoSight.
Supplemental Figure 1. Nanosight characterization of plasma samples. Average size distributions for plasma EVs (n = 5 per group), red indicates SEM (A, C, E, G), and enlargement of size distribution in 300–1000 nm range (B, D, F, H).
Notes
Acknowledgments. The authors would like to thank to the Biobank of Vigo (SERGAS) for providing us technical, ethical, and legal advice necessary for the development of our research and to the Genomic Platform of the INIBIC (A Coruña, Spain) for the scientific and technical advice for mtDNA quantification.
Financial support. This work was funded by Plan Estatal de I+D+I 2013–2016 and 2017–2020 and cofinanced by Instituto de Salud Carlos III (ISCIII)-Subdirección General de Evaluación y Fomento de la investigación del Fondo Europeo de Desarrollo Regional (FEDER) (E. P.: FI14/00557, PI16/02159, MV16/00054, BA18/00034, PI19/00747, and PI16/00684 and PI19/01127 to E. R. -M). Redes Temáticas de Investigación Cooperativa en Salud, Red de Investigación en SIDA (RD16/0025/0020, RD16/0025/0026); Xunta Galicia-Fondo Social Europeo (IN606A-2016/023); Fundación Biomédica Galicia Sur; HIV Biobank-Spanish HIV/AIDS Network; Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC); Case Western Reserve University Center for AIDS Research (AI 036219, AI 068636); and the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development Intramural Program. E. R. -M. was supported by CSIC and Consejería de Salud y Bienestar Social of Junta de Andalucía through the Nicolás Monardes program (C-0032/17).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: Conference on Retroviruses and Opportunistic Infections (CROI), March 4–7, 2019, Seattle, WA; XI Congreso Nacional GeSIDA and XIII Reunión Docente de la Red de Investigación en SIDA, December 10–13, 2019, Toledo, Spain.
References
- 1. Palella FJ Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338:853–60. [DOI] [PubMed] [Google Scholar]
- 2. French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis 2009; 200:1212–5. [DOI] [PubMed] [Google Scholar]
- 3. Shive CL, Jiang W, Anthony DD, Lederman MM. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS 2015; 29:1263–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Z, Chew GM, Shikuma CM, et al. Red blood cell distribution width as an easily measurable biomarker of persistent inflammation and T cell dysregulation in antiretrovirally treated HIV-infected adults. HIV Clin Trials 2018; 19:172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poveda E, Freeman ML. Exosomes as new players in HIV pathogenesis—new data from the IAS 2017. AIDS Rev 2017; 19:173–5. [PMC free article] [PubMed] [Google Scholar]
- 7. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018; 19:213–28. [DOI] [PubMed] [Google Scholar]
- 8. Fitzgerald W, Freeman ML, Lederman MM, Vasilieva E, Romero R, Margolis L. A system of cytokines encapsulated in extracellular vesicles. Sci Rep 2018; 8:8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Konadu KA, Chu J, Huang MB, et al. Association of cytokines with exosomes in the plasma of HIV-1-seropositive individuals. J Infec Dis 2015; 211:712–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DeMarino C, Pleet ML, Cowen M, et al. Antiretroviral drugs alter the content of extracellular vesicles from HIV-1-infected cells. Sci Rep 2018; 8:7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berridge MV, Neuzil J. The mobility of mitochondria: intercellular trafficking in health and disease. Clin Exp Pharmacol Physiol 2017; 44(Suppl 1):15–20. [DOI] [PubMed] [Google Scholar]
- 12. Miliotis S, Nicolalde B, Ortega M, Yepez J, Caicedo A. Forms of extracellular mitochondria and their impact in health. Mitochondrion 2019; 48:16–30. [DOI] [PubMed] [Google Scholar]
- 13. Torralba D, Baixauli F, Villarroya-Beltri C, et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat Commun 2018; 9:2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chiozzini C, Arenaccio C, Olivetta E, et al. Trans-dissemination of exosomes from HIV-1-infected cells fosters both HIV-1 trans-infection in resting CD4+ T lymphocytes and reactivation of the HIV-1 reservoir. Arch Virol 2017; 162:2565–77. [DOI] [PubMed] [Google Scholar]
- 15. Arakelyan A, Fitzgerald W, Zicari S, Vanpouille C, Margolis L. Extracellular vesicles carry HIV env and facilitate HIV infection of human lymphoid tissue. Sci Rep 2017; 7:1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen L, Feng Z, Yue H, et al. Exosomes derived from HIV-1-infected cells promote growth and progression of cancer via HIV TAR RNA. Nat Commun 2018; 9:4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hartjes T, Mytnyk S, Jenster G, van Steijn V, van Royen ME. Extracellular vesicle quantification and characterization: common methods and emerging approaches. Bioengineering (Basel) 2019; 6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leon A, Perez I, Ruiz-Mateos E, et al. ; EC and Immune Pathogenesis Working group of the Spanish AIDS Research Network . Rate and predictors of progression in elite and viremic HIV-1 controllers. AIDS 2016; 30:1209–20. [DOI] [PubMed] [Google Scholar]
- 19. Pernas B, Rego-Pérez I, Tabernilla A, et al. Plasma mitochondrial DNA levels are inversely associated with HIV-RNA levels and directly with CD4 counts: potential role as a biomarker of HIV replication. J Antimicrob Chemother 2017; 72:3159–62. [DOI] [PubMed] [Google Scholar]
- 20. Fujioka H, Tandler B, Hoppel CL. Mitochondrial division in rat cardiomyocytes: an electron microscope study. Anat Rec (Hoboken) 2012; 295:1455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hanaichi T, Sato T, Iwamoto T, Malavasi-Yamashiro J, Hoshino M, Mizuno N. A stable lead by modification of Sato’s method. J Electron Microsc (Tokyo) 1986; 35:304–6. [PubMed] [Google Scholar]
- 22. Mayne E, Funderburg NT, Sieg SF, et al. Increased platelet and microparticle activation in HIV infection: upregulation of P-selectin and tissue factor expression. J Acquir Immune Defic Syndr 2012; 59:340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mesquita EC, Hottz ED, Amancio RT, et al. Persistent platelet activation and apoptosis in virologically suppressed HIV-infected individuals. Sci Rep 2018; 8:14999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boudreau LH, Duchez AC, Cloutier N, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 2014; 124:2173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Paoli SH, Tegegn TZ, Elhelu OK, et al. Dissecting the biochemical architecture and morphological release pathways of the human platelet extracellular vesiculome. Cell Mol Life Sci 2018; 75:3781–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hijmans JG, Stockelman KA, Garcia V, et al. Circulating microparticles are elevated in treated HIV -1 infection and are deleterious to endothelial cell function. J Am Heart Assoc 2019; 8:e011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zicari S, Sessa L, Cotugno N, et al. Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation 2019; 140:e98–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Valle-Casuso JC, Angin M, Volant S, et al. Cellular metabolism is a major determinant of HIV-1 reservoir seeding in CD4+ T cells and offers an opportunity to tackle infection. Cell Metab 2019; 29:611–26.e5. [DOI] [PubMed] [Google Scholar]
- 30. Tarancon-Diez L, Rodríguez-Gallego E, Rull A, et al. ; ECRIS integrated in the Spanish AIDS Research Network . Immunometabolism is a key factor for the persistent spontaneous elite control of HIV-1 infection. EBioMedicine 2019; 42:86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Younes SA, Talla A, Pereira Ribeiro S, et al. Cycling CD4+ T cells in HIV-infected immune nonresponders have mitochondrial dysfunction. J Clin Invest 2018; 128:5083–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pérez-Matute P, Pérez-Martínez L, Blanco JR, Oteo JA. Role of mitochondria in HIV infection and associated metabolic disorders: focus on nonalcoholic fatty liver disease and lipodystrophy syndrome. Oxid Med Cell Longev 2013; 2013:493413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Apostolova N, Blas-García A, Esplugues JV. Mitochondrial interference by anti-HIV drugs: mechanisms beyond Pol-γ inhibition. Trends Pharmacol Sci 2011; 32:715–25. [DOI] [PubMed] [Google Scholar]
- 34. Ney PA. Mitochondrial autophagy: Origins, significance, and role of BNIP3 and NIX. Biochim Biophys Acta 2015; 1853:2775–83. [DOI] [PubMed] [Google Scholar]
- 35. Oka T, Hikoso S, Yamaguchi O, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 2012; 485:251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee SH, Du J, Stitham J, et al. Inducing mitophagy in diabetic platelets protects against severe oxidative stress. EMBO Mol Med 2016; 8:779–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. West AP, Shadel GS. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol 2017; 17:365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ingelsson B, Söderberg D, Strid T, et al. Lymphocytes eject interferogenic mitochondrial DNA webs in response to CpG and non-CpG oligodeoxynucleotides of class C. Proc Natl Acad Sci U S A 2018; 115:E478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gardner K, Hall PA, Chinnery PF, Payne BA. HIV treatment and associated mitochondrial pathology: review of 25 years of in vitro, animal, and human studies. Toxicol Pathol 2014; 42:811–22. [DOI] [PubMed] [Google Scholar]
- 40. Cossarizza A, Pinti M, Nasi M, et al. Increased plasma levels of extracellular mitochondrial DNA during HIV infection: a new role for mitochondrial damage-associated molecular patterns during inflammation. Mitochondrion 2011; 11:750–5. [DOI] [PubMed] [Google Scholar]
- 41. Lauring AS, Lee TH, Martin JN, Hunt PW, Deeks SG, Busch M. Lack of evidence for mtDNA as a biomarker of innate immune activation in HIV infection. PLoS One 2012; 7:e50486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arshad O, Gadawska I, Sattha B, Côté HCF, Hsieh AYY; Canadian Institutes of Health Research Team on Cellular Aging and HIV Comorbidities in Women and Children (CARMA) . Elevated cell-free mitochondrial dna in filtered plasma is associated with HIV infection and inflammation. J Acquir Immune Defic Syndr 2018; 78:111–8. [DOI] [PubMed] [Google Scholar]
- 43. Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis 2008; 197:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li JZ, Arnold KB, Lo J, et al. Differential levels of soluble inflammatory markers by human immunodeficiency virus controller status and demographics. Open Forum Infect Dis 2015; 2:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krishnan S, Wilson EM, Sheikh V, et al. Evidence for innate immune system activation in HIV type 1-infected elite controllers. J Infect Dis 2014; 209:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Crowell TA, Gebo KA, Blankson JN, et al. ; HIV Research Network . hospitalization rates and reasons among hiv elite controllers and persons with medically controlled HIV infection. J Infect Dis 2015; 211:1692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Picca A, Guerra F, Calvani R, et al. Mitochondria dysfunction and aging: insights from the analysis of extracellular vesicles. Int J Mol Sci 2019; 20:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Margolis AM, Heverling H, Pham PA, Stolbach A. A review of the toxicity of HIV medications. J Med Toxicol 2014; 10:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Venter WDF, Kambugu A, Chersich MF, et al. Efficacy and safety of tenofovir disoproxil fumarate versus low-dose stavudine over 96 weeks: a multicountry randomized, noninferiority trial. J Acquir Immune Defic Syndr 2019; 80:224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mallon PW, Winston A, Post F, et al. Platelet function upon switching to TAF vs continuing ABC: a randomized substudy [abstract 80]. In: Conference of Retroviruses and Opportunistics Infectious (Boston, MA). March 4-7, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.