Abstract
Purpose
Benzoyl peroxide (BPO) is an effective acne treatment and has been used as a cleanser and short contact therapy. However, data on the minimum contact time of BPO needed to kill Cutibacterium acnes are lacking. Thus, the aim of this study was to determine the minimum contact time of commonly used BPO concentrations for bactericidal effects on C. acnes.
Materials and Methods
An in vitro experimental study of clinically isolated C. acnes was performed to determine the minimal inhibitory concentration (MIC) of BPO using the broth microdilution method. Subsequently, the minimum contact times of various concentrations of BPO were evaluated, and their bactericidal effects were assessed by the plate count method.
Results
The median MIC of BPO was 9375 µg/mL, which did not significantly differ between antibiotic-resistant and nonresistant C. acnes. The minimum contact time of BPO with C. acnes was significantly different among the BPO concentrations. For bactericidal activity against all isolates, 1.25%, 2.5%, 5%, and 10% BPO required 60 min, 15 min, 30 sec, and 30 sec, respectively.
Conclusion
BPO demonstrated bactericidal activity against both antibiotic-resistant and antibiotic-susceptible C. acnes. The in vitro contact time needed to kill C. acnes was almost immediate with 5% or more BPO, but ≤ 2.5% BPO required longer contact times for bactericidal effects.
Keywords: acne, cleanser, short contact therapy, C. acnes, P. acnes, time killing
Plain Language Summary
BPO is an effective acne treatment that can be used in leave-on, rinse-off, or cleanser formulas. However, data on the contact time of various concentrations of BPO for bactericidal effects on C. acnes are lacking.
The MIC of BPO did not significantly differ between clinically isolated antibiotic-resistant and antibiotic-susceptible C. acnes.
The median contact time of the bactericidal effect on all C. acnes isolates was at least 60 min, 15 min, 30 sec and 30 sec for concentrations of 1.25%, 2.5%, 5% and 10% BPO, respectively.
Lower BPO concentrations (≤ 2.5%) required more contact time than higher concentrations to produce a bactericidal effect on C. acnes.
BPO concentrations of 5% or higher have rapid bactericidal activity against C. acnes, making it more appropriate for use as a rinse-off formulation.
Introduction
Benzoyl peroxide (BPO) is effective against a wide range of microorganisms and is well known for its ability to reduce the numbers of Cutibacterium acnes, which is important in the pathogenesis of acne.1–3 Unlike antibiotics, BPO is a powerful oxidizing agent that disrupts vital bacterial cell components.4 Therefore, it can be used effectively in treating antibiotic-resistant C. acnes4,5 and can help prevent the emergence of antibiotic-resistant C. acnes when used in combination with topical antibiotics. BPO has an increasingly important role in treating acne when the isolates have high bacterial resistance and has been recommended by many clinical guidelines.3,4,6–8
Despite its extensive use and good efficacy against acne, some patients cannot tolerate BPO’s side effects. BPO can cause skin irritation when applied as a leave-on formulation, especially at the beginning of treatment.4,9 Hence, some physicians advise short contact times for leave-on formulations (such as applying 2.5% BPO for 5–15 min and then rinsing it off) to decrease irritation and increase patient compliance. Many physicians also advise the use of cleansers containing BPOs during treatment as maintenance therapy for acne.10,11 Unfortunately, data are limited regarding the proper contact time of commonly used concentrations. Previous studies by Okamoto et al12 demonstrated that 1 hour of contact time decreased the numbers of C. acnes over 3 log and 5 log after exposure to 1 and 2 times the MIC (256 µg/mL (0.025%) and 512 µg/mL (0.05%)), respectively. However, this 1-hour contact time was much longer than that of the short contact and rinse-off formulation used in clinical practice. Moreover, the concentration in the study was far below the concentration used in clinical practice. Thus, whether higher concentrations have shorter required contact times and the minimum contact times of commonly used BPO concentrations for killing C. acnes remain unknown. The aim of the study was to determine the minimum contact time of BPO for treating C. acnes at varying concentrations to produce a bactericidal effect against C. acnes in vitro. This information can provide further guidelines for minimal BPO application times to achieve a bactericidal effect against C. acnes.
Materials and Methods
Preparation of Bacteria
Clinical isolates of C. acnes were obtained from 95 patients at the Srinakharinwirot skin center by a previous study by Laochunsuwan et al13 that was approved by the clinical research ethical committee of Srinakharinwirot University. All participants voluntarily signed an informed consent form. The mean age of the patients was 21.74 years, ranging from 18–44 years, and patients had experienced acne for an average of 5.83 years. Of these patients, 62.1% had previously been treated with topical or systemic antibiotics and 37.9% had never received antibiotics. Samples were collected from facial closed comedones using the comedone extraction technique and then immediately smeared onto brain-heart infusion agar plates supplemented with horse serum. The inoculated plates were incubated at 37 °C in anaerobic jars under anaerobic conditions (0% oxygen, 5% carbon dioxide, 5% hydrogen, 90% nitrogen). C. acnes were identified by colony morphology and Gram staining. Cultured organisms were identified to the species level using an API 20A kit, a manual identification system for anaerobic bacteria (bioMeriux®, France). Antibiotic susceptibility was tested by using an epsilometer test (E test) for five commonly used antibiotics (doxycycline, tetracycline, amoxicillin, clindamycin and erythromycin). To preserve the organisms that were tested, C. acnes were kept under liquid nitrogen (−180 °C). In this study, we used 70 viable isolates, including 41 isolates defined as antibiotic resistant (resistant to ≥ 1 antibiotic) and 29 isolates defined as antibiotic susceptible. Before the experiment, C. acnes was subcultured into brain-heart infusion (BHI) medium supplemented with 10% horse serum (InvitrogenTM, Thermo Fisher Scientific Inc., MA, USA) and incubated at 37 °C for 72 h under anaerobic conditions. Subsequently, the culture was adjusted to 1.5×108 CFU/mL (0.5 McFarland standard) for experimental use.
Preparation of BPO
BPO (Merck, Darmstadt, Germany) was dissolved in dimethyl sulfoxide (DMSO) (Amresco, Ohio, USA). We determined a suitable DMSO concentration that did not interfere with bactericidal activity against C. acnes and could dissolve up to 100,000 µg/mL BPO. The most suitable DMSO used in our study was 25% DMSO in the culture medium. Different amounts of BPO were dissolved in DMSO and then mixed into the bacterial suspension to obtain final concentrations of 12,500, 25,000, 50,000 and 100,000 µg/mL (1.25%, 2.5%, 5%, and 10% BPO (W/V)). Since the bactericidal activity of BPO is due to its role as a powerful oxidizing agent, sodium hydroxide (NaOH (Merck, Darmstadt, Germany)) was added to stop the bactericidal activity of BPO at the study time limits of 30 sec and 1, 5, 10, 15, 30 and 60 min.
In vitro Antimicrobial Study
Determination of the Minimal Inhibitory Concentration (MIC)
The MIC is defined as the lowest concentration of a drug that inhibits bacterial growth; MICs in the current study were determined using the broth microdilution method as described in the Clinical and Laboratory Standard Institute (CLSI) Methodology for anaerobic bacteria.14 BPO was dissolved in 25% DMSO and subsequently added to the bacterial suspension to obtain a final concentration of 25,000 µg/mL (2.5%) BPO and 1×106 CFU/mL C. acnes. A twofold serial dilution in a 96-well microplate was performed. The plates were incubated for 72 h at 37 °C in an anaerobic jar. Then, resazurin was added as an indicator to help read the MIC results. The lowest concentration of BPO that inhibited the visible growth of bacteria was recorded as the MIC.
Contact Time and Bactericidal Effect of BPO
Thirty representative isolates were selected, including 17 antibiotic-resistant isolates and 13 nonresistant isolates, to evaluate the bactericidal effect of BPO. The bactericidal effect is defined as antibacterial agents either completely preventing bacterial growth or resulting in a 99.9% decrease in the initial inoculum on a subculture. Each isolated C. acnes (1x106 CFU/mL) sample was added to BPO in DMSO to yield final concentrations of 12,500, 25,000, 50,000 and 100,000 µg/mL (1.25%, 2.5%, 5%, and 10%) BPO and was incubated under anaerobic conditions for various times (30 s, 1 min, 5 min, 10 min, 15 min, and 60 min). Then, 10 µL of NaOH was added to stop the bactericidal reaction of BPO. Bacterial growth was examined by the drop plating method,15 and the bacteria were incubated under anaerobic conditions for 72 h to determine the bactericidal activity (Figure 1).
Figure 1.
The bacterial growth plate shows the bactericidal effect of 1.25%, 5% and 10% BPO on C. acnes after 1 min. No bacterial colonies remained after a 1-min incubation with 1.25%, 5% and 10% BPO compared with those in the control area (blue arrow).
Statistical Analysis
The MICs of antibiotic-resistant and antibiotic-susceptible C. acnes are presented as the means ± SD or medians (interquartile range). The Wilcoxon rank-sum test was used to identify the difference in MIC between the C. acnes-resistant and nonresistant groups. The Kaplan–Meier method was used to estimate the median time and rate of the bactericidal effect against C. acnes in the 1.25%, 2.5%, 5% and 10% BPO groups. Cox’s proportional hazard model was used to estimate the HR of bactericidal effects.
Statistical analyses were performed with STATA version 14 (StataCorp, Texas, USA). P values less than 0.05 were considered statistically significant.
Results
MICs of BPO for Antibiotic-Resistant and Antibiotic-Susceptible C. acnes
The final MICs of BPO in all 70 isolates ranged from 780–25,000 μg/mL. The median was 9375 μg/mL (IQR: 6250–12,500). There was no significant difference in the MICs between the antibiotic-resistant and antibiotic-susceptible groups (P = 0.084) (Table 1).
Table 1.
Minimal Inhibitory Concentration of BPO Between Antibiotic-Resistant and Antibiotic-Susceptible C. acnes
| C. acnes Groups (Number of Isolates) | P value* | |||
|---|---|---|---|---|
| Total (N=70) | Antibiotic Resistant (N=41) | Antibiotic Susceptible (N=29) | ||
MIC (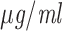 ) † ) †
|
9375 (6250–12,500) | 6250 (6250–12,500) | 12,500 (6250–12,500) | 0.084 |
| Minimum - maximum | 780–25,000 | 780–25,000 | 1560–25,000 | |
Notes: †Data are presented as the median (interquartile range). *P value obtained when comparing the antibiotic-resistant and antibiotic-susceptible groups using the Wilcoxon rank-sum test; <0.05 was considered statistically significant.
Abbreviation: MIC, minimal inhibitory concentration.
Minimum Contact Times of BPO for a Bactericidal Effect on C. acnes Isolates
The results of the BPO contact time profiles on C. acnes (while testing the bactericidal effect as assessed by the survival probability and the percentage of bacterial isolates at each concentration and contact times) are shown in Table 2. The median contact time to the bactericidal effect of all C. acnes isolates at 1.25%, 2.5%, 5% and 10% BPO was at least 60 min, 15 min, 30 sec and 30 sec, respectively. For the 1-minute contact time for the rinse-off formulation, the bactericidal effects of 1.25%, 2.5%, 5% and 10% BPO achieved reductions of 70%, 93.4%, 100% and 100% of the isolates, respectively. There was no difference between 5% and 10% BPO because a rapid bactericidal effect was achieved after 30 sec of contact.
Table 2.
Bactericidal Effect of BPO on C. acnes
| Contact Time (min) | Bactericidal Isolates (n (%)) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.25% | 2.5% | 5% | 10% | |||||||||
| Total (n=30) | R† (n=17) | NR‡ (n=13) | Total (n=30) | R† (n=17) | NR‡ (n=13) | Total (n=30) | R† (n=17) | NR‡ (n=13) | Total (n=30) | R† (n=17) | NR‡ (n=13) | |
| 0.5 | 20 (66.7%) | 9 (53%) | 11 (84.6%) | 28 (93.4%) | 15 (88.2%) | 13 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 13 (100%) |
| 1 | 21 (70%) | 10 (58.8%) | 11 (84.6%) | 28 (93.4%) | 15 (88.2%) | 13 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 13 (100%) |
| 5 | 21 (70%) | 10 (58.8%) | 11 (84.6%) | 29 (96.7%) | 16 (94%) | 13 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 13 (100%) |
| 10 | 22 (73.3%) | 10 (58.8%) | 12 (92.3%) | 29 (96.7%) | 16 (94%) | 13 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 13 (100%) |
| 15 | 24 (80%) | 12 (70%) | 12 (92.3%) | 30 (100%) | 17 (100%) | 13 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 13 (100%) |
| 30 | 26 (86.7%) | 14 (82.3%) | 12 (92.3%) | 30 (100%) | 17 (100%) | 13 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 13 (100%) |
| 60 | 30 (100%) | 17 (100%) | 13 (100%) | 30 (100%) | 17 (100%) | 13 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 30 (100%) | 13 (100%) |
Notes: Bactericidal effect on  ≤ 80% of isolates
≤ 80% of isolates  80–89% of isolates
80–89% of isolates  90–99% of isolates
90–99% of isolates  100% isolates. †Antibiotic-resistant isolates. ‡Antibiotic-susceptible isolates.
100% isolates. †Antibiotic-resistant isolates. ‡Antibiotic-susceptible isolates.
Abbreviations: BPO, benzoyl peroxide; MIC, minimal inhibition concentration; DMSO, dimethyl sulfoxide.
According to comparisons of the effect of BPO concentrations by analyzing the survival data using the Log rank test, the BPO concentration significantly affected the minimum contact time with C. acnes isolates (P < 0.001); lower BPO concentrations required greater contact time than higher concentrations. A multivariate Cox model was used to compare bactericidal effects among the 1.25%, 2.5%, 5% and 10% BPO groups after adjusting for antibiotic resistance. The bactericidal effect of BPO in the antibiotic-resistant group was approximately 10% lower than that in the susceptible C. acnes group, but the difference was not statistically significant. (HR 0.9; 95% CI: 0.632–1.296; P = 0.567).
Discussion
This study emphasizes the importance of the contact time of BPO for treating C. acnes. We studied the influence of the BPO contact time on the bactericidal effects of various concentrations on C. acnes isolates from acne patients, which included both antibiotic-resistant and antibiotic-susceptible C. acnes, thus simulating what is seen in clinical practice.
There is very limited evidence in the literature regarding BPO contact times and its bactericidal effect on C. acnes. A previous study by Cove et al16 demonstrated that 100 µg/mL (0.01%) BPO can decrease the C. acnes count at 59 min with a decimal reduction time of 12.5 min thereafter. However, the very low concentration of BPO in that study is very different from the much higher concentration often used in clinical practice. To obtain a clinical correlation, we chose commonly used BPO concentrations and application times; furthermore, instead of using a specific strain of C. acnes for testing, we used C. acnes isolates from clinical cultures to represent the C. acnes population in acne patients. In this study, all isolates of 1.25% BPO required 60 min of contact time to achieve bactericidal effects, and 15 min of contact time was required for 2.5% BPO. However, 5% and 10% BPO demonstrated rapid bactericidal activity within 30 sec. At these commonly used concentrations, our study revealed that the contact time of BPO significantly affected its bactericidal effect on C. acnes isolates. Therefore, in practice, 1.25% or 2.5% BPO cleansing formulations are likely ineffective for killing C. acnes. Additionally, short BPO contact times or wash-off formulations with concentrations greater than 5% do not provide added benefits in terms of its bactericidal effect on C. acnes. Moreover, regarding the short contact use of BPO in patients who have irritated skin problems, 2.5% BPO should be applied at least 15 minutes before rinsing off.
In this study, the MIC of BPO was 9375 μg/mL. In the literature, the MIC of BPO against C. acnes widely varied (from 64 to 2048 μg/mL)12,17–22 due to factors including different of C. acnes isolates, solvents, and media. The clinical isolates from our acne patients demonstrated higher MIC values than those from the previous in vitro study, which could be due to prior exposure of C. acnes to acne treatment and the higher number of C. acnes isolates used in this study. Prior research has shown that BPO is effective in suppressing existing antibiotic-resistant C. acnes isolates as well as preventing resistance during antibiotic therapy, both in laboratory and clinical studies.18,23 In our study, there was also no significant difference in the MIC of BPO in the resistant and nonresistant C. acnes isolates; this evidence supports the efficacy of BPO for treating acne with antibiotic-resistant C. acnes. Moreover, when the BPO contact time was analyzed after adjusting for antibiotic resistance, it seemed to have less of an effect on antibiotic-resistant C. acnes, but this difference did not reach statistical significance (HR: 0.9; 95% CI: 0.632–1.296; P value = 0.567).
Our study is the first to explore the minimum contact time of BPO for a bactericidal effect on C. acnes in vitro. When translated into clinical practice, additional factors may affect the efficacy of BPO in acne treatment. C. acnes is a bacterium that is found deep in sebaceous follicles.2 When BPO is applied to the skin, it might take more time to penetrate follicles to reach C. acnes than the minimum contact times in vitro. Moreover, when BPO is used as a cleanser, there might be a diluting effect from the water used to rinse. Together, these factors both suggest longer contact times and higher concentrations of BPO to be applied to the skin to kill C. acnes in vivo. However, our in vitro study demonstrates the minimum contact time required for a bactericidal effect of BPO on C. acnes. Further studies need to be conducted to explore the clinical relevance of our findings.
Conclusion
BPO has excellent and rapid bactericidal effects on both antibiotic-resistant and antibiotic-susceptible C. acnes. Lower BPO concentrations (≤ 2.5%) require more contact time than higher concentrations for bactericidal effects on C. acnes. Almost immediate bactericidal effects occur when using 5% or higher concentrations of BPO, making these concentrations more appropriate for use as a rinse-off formulation.
Acknowledgments
The authors thank all the staff of the Microbiology Department of Srinakharinwirot University for helping with this study.
Funding Statement
This work was supported by the Faculty of Medicine, Srinakharinwirot University under grant number 144/2564.
Ethics Approval
This study complies with the Declaration of Helsinki and was reviewed and approved by the Srinakharinwirot University IRB; approval number SWUEC-002/2564.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: focus on antibiotic resistance. Cutis. 2007;79(6 Suppl):9–25. [PubMed] [Google Scholar]
- 2.Dréno B, Pécastaings S, Corvec S, et al. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. J Eur Acad Dermatol Venereol. 2018;32:5–14. doi: 10.1111/jdv.15043 [DOI] [PubMed] [Google Scholar]
- 3.Cong T-X, Hao D, Wen X, et al. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311(5):337–349. doi: 10.1007/s00403-019-01908-x [DOI] [PubMed] [Google Scholar]
- 4.Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10(15):2555–2562. doi: 10.1517/14656560903277228 [DOI] [PubMed] [Google Scholar]
- 5.Leyden JJ, Preston N, Osborn C, et al. In-vivo effectiveness of Adapalene 0.1%/benzoyl peroxide 2.5% gel on antibiotic-sensitive and resistant Propionibacterium acnes. J Clin Aesthet Dermatol. 2011;4(5):22. [PMC free article] [PubMed] [Google Scholar]
- 6.Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973. doi: 10.1016/j.jaad.2015.12.037 [DOI] [PubMed] [Google Scholar]
- 7.Thiboutot DM, Dréno B, Abanmi A, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78(2):S1–S23. doi: 10.1016/j.jaad.2017.09.078 [DOI] [PubMed] [Google Scholar]
- 8.Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80(2):538–549. doi: 10.1016/j.jaad.2018.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman SR, Chen DM. How patients experience and manage dryness and irritation from acne treatment. J Drugs Dermatol. 2011;10(6):605–608. [PubMed] [Google Scholar]
- 10.Del Rosso JQ, Leyden JJ, Thiboutot D, et al. Antibiotic use in acne vulgaris and rosacea: clinical considerations and resistance issues of significance to dermatologists. Cutis. 2008;82(Suppl 2):5–12. [PubMed] [Google Scholar]
- 11.Del Rosso JQ. Benzoyl peroxide cleansers for the treatment of acne vulgaris: status report on available data. Cutis. 2008;82(5):336–342. [PubMed] [Google Scholar]
- 12.Okamoto K, Ikeda F, Kanayama S, et al. In vitro antimicrobial activity of benzoyl peroxide against Propionibacterium acnes assessed by a novel susceptibility testing method. J Infect Chemother. 2016;22(6):426–429. doi: 10.1016/j.jiac.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 13.Laochunsuwan A, Taweechotipatr M, Udompataikul M, Karnjanawanichkul W. In vitro study of antibiotic susceptibility of Propionibacterium acnes strains isolated from acne vulgaris patients. J Med Assoc Thai. 2017;100(10):24–31.29911376 [Google Scholar]
- 14.Brook I, Wexler HM, Goldstein EJ. Antianaerobic antimicrobials: spectrum and susceptibility testing. Clin Microbiol Rev. 2013;26(3):526–546. doi: 10.1128/CMR.00086-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoben HJ, Somasegaran P. Comparison of the pour, spread, and drop plate methods for enumeration of Rhizobium spp. in inoculants made from presterilized peat. Appl Environ Microbiol. 1982;44(5):1246–1247. doi: 10.1128/aem.44.5.1246-1247.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cove J, Holland K. The effect of benzoyl peroxide on cutaneous micro‐organisms in vitro. J Appl Bacteriol. 1983;54(3):379–382. doi: 10.1111/j.1365-2672.1983.tb02631.x [DOI] [PubMed] [Google Scholar]
- 17.Decker LC, Deuel DM, Sedlock DM. Role of lipids in augmenting the antibacterial activity of benzoyl peroxide against Propionibacterium acnes. Antimicrob Agents Chemother. 1989;33(3):326–330. doi: 10.1128/AAC.33.3.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eady E, Farmery M, Ross J, et al. Effects of benzoyl peroxide and erythromycin alone and in combination against antibiotic‐sensitive and‐resistant skin bacteria from acne patients. Br J Dermatol. 1994;131(3):331–336. doi: 10.1111/j.1365-2133.1994.tb08519.x [DOI] [PubMed] [Google Scholar]
- 19.Farmery M, Jones’ C, Eady E, et al. In vitro activity of azelaic acid, benzoyl peroxide and zinc acetate against antibiotic-resistant propionibacteria from acne patients. J Dermatolog Treat. 1994;5(2):63–65. doi: 10.3109/09546639409084531 [DOI] [Google Scholar]
- 20.Nakatsuji T, Kao MC, Fang JY, et al. Antimicrobial property of lauric acid against Propionibacterium acnes: its therapeutic potential for inflammatory acne vulgaris. J Invest Dermatol. 2009;129(10):2480–2488. doi: 10.1038/jid.2009.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pannu J, McCarthy A, Martin A, et al. In vitro antibacterial activity of NB-003 against Propionibacterium acnes. Antimicrob Agents Chemother. 2011;55(9):4211–4217. doi: 10.1128/AAC.00561-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blaskovich MAT, Elliott AG, Kavanagh AM, et al. In vitro antimicrobial activity of acne drugs against skin-associated bacteria. Sci Rep. 2019;9(1):14658. doi: 10.1038/s41598-019-50746-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bojar R, Cunliffe W, Holland K. The short‐term treatment of acne vulgaris with benzoyl peroxide: effects on the surface and follicular cutaneous microflora. Br J Dermatol. 1995;132(2):204–208. doi: 10.1111/j.1365-2133.1995.tb05014.x [DOI] [PubMed] [Google Scholar]



