Abstract
Although previous studies have indicated that clavulanate may induce AmpC expression in isolates of Pseudomonas aeruginosa, the impact of this inducer activity on the antibacterial activity of ticarcillin at clinically relevant concentrations has not been investigated. Therefore, a study was designed to determine if the inducer activity of clavulanate was associated with in vitro antagonism of ticarcillin at pharmacokinetically relevant concentrations. By the disk approximation methodology, clavulanate induction of AmpC expression was observed with 8 of 10 clinical isolates of P. aeruginosa. Quantitative studies demonstrated a significant induction of AmpC when clavulanate-inducible strains were exposed to the peak concentrations of clavulanate achieved in human serum with the 3.2- and 3.1-g doses of ticarcillin-clavulanate. In studies with three clavulanate-inducible strains in an in vitro pharmacodynamic model, antagonism of the bactericidal effect of ticarcillin was observed in some tests with regimens simulating a 3.1-g dose of ticarcillin-clavulanate and in all tests with regimens simulating a 3.2-g dose of ticarcillin-clavulanate. No antagonism was observed in studies with two clavulanate-noninducible strains. In contrast to clavulanate, tazobactam failed to induce AmpC expression in any strains, and the pharmacodynamics of piperacillin-tazobactam were somewhat enhanced over those of piperacillin alone against all strains studied. Overall, the data collected from the pharmacodynamic model suggested that induction per se was not always associated with reduced killing but that a certain minimal level of induction by clavulanate was required before antagonism of the antibacterial activity of its companion drug occurred. Nevertheless, since clinically relevant concentrations of clavulanate can antagonize the bactericidal activity of ticarcillin, the combination of ticarcillin-clavulanate should be avoided when selecting an antipseudomonal β-lactam for the treatment of P. aeruginosa infections, particularly in immunocompromised patients. For piperacillin-tazobactam, induction is not an issue in the context of treating this pathogen.
Pseudomonas aeruginosa is a serious threat to immunocompromised patients, and infections with this bacterium are increasingly more difficult to treat due to both intrinsic and acquired resistance to multiple antimicrobial agents. Thus, the selection of an appropriate drug regimen is essential for the successful treatment of serious P. aeruginosa infections (21). A combination of an aminoglycoside with an antipseudomonal β-lactam, often an antipseudomonal penicillin, is considered the regimen of choice for the treatment of such infections. Since combinations of antipseudomonal penicillins with β-lactamase inhibitors have become available clinically, there has been a tendency to use these in lieu of the penicillin alone. For piperacillin-tazobactam, this switch should not present any problems. However, for ticarcillin-clavulanate, the ability of clavulanate to induce expression of the P. aeruginosa AmpC cephalosporinase (8, 20, 22) could antagonize the antibacterial activity of ticarcillin in the combination. In immunocompetent mice and humans infected with P. aeruginosa, antagonism between clavulanate and ticarcillin has not been observed (4, 15). However, the potential for antagonism in the absence of adequate host defenses, i.e., neutropenic patients, has not been systematically studied.
A study was designed to determine if clinically achievable concentrations of clavulanate could indeed induce AmpC expression in P. aeruginosa and if this induction resulted in antagonism of the antibacterial effect of ticarcillin. In this study the frequency of AmpC induction by clavulanate among clinical isolates of P. aeruginosa was evaluated by the disk approximation methodology. From among these clinical isolates, a panel of organisms was selected to represent both clavulanate-inducible and -noninducible populations, and the quantitative induction of AmpC expression by clavulanate at pharmacokinetically relevant concentrations was evaluated. Finally, to determine if clavulanate’s induction of AmpC would antagonize the antibacterial activity of ticarcillin against P. aeruginosa in the absence of host defenses, an in vitro pharmacokinetic model (IVPM) was used to simulate the pharmacokinetics of ticarcillin (3.0-g dose), ticarcillin-clavulanate (3.1-g dose), and ticarcillin-clavulanate (3.2-g dose) and to study their pharmacodynamic activities. For comparative purposes, similar studies were performed with tazobactam and piperacillin and the 3.0- and 3.375-g doses of piperacillin and piperacillin-tazobactam.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Ten clinical isolates of P. aeruginosa were selected for this study. P. aeruginosa 1, P. aeruginosa 3, P. aeruginosa 13, P. aeruginosa 27, P. aeruginosa 31, P. aeruginosa 105, P. aeruginosa 164, P. aeruginosa 239, P. aeruginosa 242, and P. aeruginosa 246 were all wild-type clinical isolates with respect to their basal (uninduced) levels of AmpC production, susceptibilities to ticarcillin and piperacillin, and the absence of any detectable plasmid-mediated β-lactamases in sonic extracts (Table 1). Stocks of all strains were frozen at −70°C in 50% brain heart infusion broth (Becton Dickinson, Cockeysville, Md.) and 50% sterile horse serum (Colorado Serum Company, Denver, Colo.). Prior to use in experiments, frozen cultures were subcultured onto Trypticase soy agar supplemented with 5% sheep blood (blood agar plates [BAPs]; BBL Prepared Media, Becton-Dickinson Microbiology Systems) and incubated overnight at 37°C to ensure strain purity.
TABLE 1.
Susceptibilities of P. aeruginosa to piperacillin, piperacillin-tazobactam, ticarcillin, and ticarcillin-clavulanate
| Strain | MIC (μg/ml)a
|
|||
|---|---|---|---|---|
| Piperacillin | Piperacillin-tazobactam | Ticarcillin | Ticarcillin-clavulanate | |
| P. aeruginosa 1 | 8 | 4 | 32 | 32 |
| P. aeruginosa 13 | 8 | 8 | 32 | 64 |
| P. aeruginosa 246 | 16 | 8 | 32 | 32 |
| P. aeruginosa 242 | 8 | 8 | 32 | 32 |
| P. aeruginosa 164 | 4 | 8 | 16 | 32 |
| P. aeruginosa 3 | 2 | 4 | 16 | 32 |
| P. aeruginosa 27 | 4 | 4 | 32 | 32 |
| P. aeruginosa 31 | 8 | 8 | 32 | 32 |
| P. aeruginosa 105 | 4 | 8 | 32 | 32 |
| P. aeruginosa 239 | 4 | 4 | 32 | 32 |
MICs were measured by broth macrodilution methodology by the procedure recommended by the National Committee for Clinical Laboratory Standards (14).
For pharmacodynamic experiments, logarithmic-phase cultures were prepared by inoculating colonies from overnight BAP cultures into 70 ml of Mueller-Hinton broth (MHB; Oxoid, Unipath Ltd., Basingstoke, England) to equal an optical density at 540 nm of 0.1. The broth cultures were then incubated at 37°C with shaking for approximately 2 h until the optical density at 540 nm increased to 0.4. Logarithmic-phase cultures were diluted 10-fold in fresh MHB at 37°C to give a final inoculum of 107 to 108 CFU/ml.
Antibiotics.
Standard diagnostic powders of each of the following antibiotics were obtained from the indicated sources: piperacillin sodium, Lederle Piperacillin, Inc. (Carolina, Puerto Rico); tazobactam sodium, Lederle Parenterals, Inc.; ticarcillin disodium, SmithKline Beecham Pharmaceuticals (Philadelphia, Pa.); lithium clavulanate, SmithKline Beecham Pharmaceuticals; cefoxitin, Merck Sharp & Dohme (West Point, Pa.); and cephalothin, Eli Lilly & Co. (Indianapolis, Ind.). Antibiotic solutions were prepared by reconstituting the diagnostic powders in sterile distilled water or 0.1 M phosphate buffer (4 g of potassium phosphate, monobasic, per liter and 13.6 g of potassium phosphate, dibasic, per liter). Each antibiotic solution was then sterilized via filtration through 0.22-μm-pore-size filters (Poretics Corporation, Livermore, Calif.) fitted to clean syringes.
Disk approximation screen for AmpC induction.
The induction of P. aeruginosa AmpC by clavulanate, tazobactam, and cefoxitin was initially evaluated by the disk approximation methodology (17). With a sterile cotton swab, P. aeruginosa colonies from overnight cultures on BAPs were suspended in 1 ml of sterile normal saline until a turbidity of a 0.5 McFarland standard was obtained. This suspension was then used to create a lawn culture on Mueller-Hinton agar (MHA; Oxoid). Sterile paper disks impregnated with 30 μg of clavulanate, tazobactam, or cefoxitin were placed onto the lawn culture at distances of 13, 15, and 17 mm from commercial disks containing 75 μg of ticarcillin per disk or 100 μg of piperacillin per disk. The plates were then incubated for 18 to 24 h at 37°C in air. Induction of AmpC by cefoxitin, clavulanate, or tazobactam was evaluated visually as a flattening of the zone of inhibition between the disks containing cefoxitin, clavulanate, or tazobactam and the disks containing piperacillin or ticarcillin.
Antimicrobial susceptibility testing.
Susceptibility testing with piperacillin, ticarcillin, piperacillin-tazobactam, and ticarcillin-clavulanate was performed by the broth macrodilution method by the procedure recommended by the National Committee for Clinical Laboratory Standards (14).
Analysis of β-lactamases.
For β-lactamase analysis, 5-ml aliquots from overnight MHB cultures were transferred to centrifuge bottles containing 95 ml of sterile MHB, and the bottles were incubated at 37°C with shaking for 1.5 h to achieve logarithmic-phase growth. After 1.5 h of incubation, 1 ml of either sterile normal saline, 5,000 μg of cefoxitin per ml (final concentration, 50 μg/ml), 5,000 μg of clavulanate per ml (final concentration, 50 μg/ml), 1,600 μg of clavulanate per ml (final concentration, 16 μg/ml), 800 μg of clavulanate per ml (final concentration, 8 μg/ml), 200 μg of clavulanate per ml (final concentration, 2 μg/ml), or 3,000 μg of tazobactam per ml (final concentration, 30 μg/ml) was added to the cultures. Cefoxitin at 50 μg/ml was selected as a positive control due to its previously described ability to induce chromosomal β-lactamases (6). Clavulanate at 50 μg/ml was selected as an equal concentration for comparison to the positive control. Clavulanate at concentrations of 16 and 8 μg/ml was selected to correspond to peak concentrations achieved in human serum following the administration of intravenous doses of 3.2 and 3.1 g of ticarcillin-clavulanate, respectively (13, 18). Tazobactam at 30 μg/ml was selected to correlate with the peak concentration of tazobactam achieved in human serum following the intravenous administration of the 3.375-g dose of piperacillin-tazobactam (19). Clavulanate at 2 μg/ml represented the constant concentration of clavulanate used in dilution susceptibility testing (14). After the additional 2 h of incubation with each potential inducer or normal saline, protein synthesis was halted by the addition of 1 ml of 1 mM 8-hydroxyquinoline. Cells were then collected by centrifugation at 5,858 × g for 20 min and washed once in 0.1 M phosphate buffer. The supernatants were discarded and the bacterial pellets were frozen overnight at −20°C. On the following day the bacterial pellets were resuspended in 4 ml of 0.1 M phosphate buffer and were lysed by sonication with an ultrasonic disintegrator (Bronwill Scientific, Rochester, N.Y.). The cells were disrupted by 10 cycles of 10-s sonications at 10 to 12 mA with 10-s rest intervals between cycles. Bacterial suspensions were maintained in an ice-water bath to protect the enzymes from excessive heat during sonication. Cellular debris was removed from each sonicate by centrifugation at 5,858 × g for 1 h at 4°C. Immediately after removal of the cellular debris, sonicates were assayed for protein content (3), and β-lactamase activity was measured spectrophotometrically, with cephalothin serving as the hydrolysis substrate (17). Broth induction studies were performed in duplicate on separate days, and data were expressed as the mean ± 2 standard deviations (SDs). Differences in means were considered significant if there was no overlapping of the 2-SD ranges. In addition to quantitative induction analysis, sonicates were also evaluated for the presence of plasmid-encoded β-lactamases by isoelectric focusing and a nitrocefin overlay (16).
IVPM.
The basics of the IVPM used in these studies have previously been described in detail (2, 12). The specific parameters of the model are as follows. The hollow-fiber cartridges (model BR130; Unisyn Fibertech, San Diego, Calif.) used in these studies consisted of 2,250 cellulose acetate hollow fibers contained within a polycarbonate housing, with each fiber having within its wall pores that allowed the passage of compounds with molecular weights of 30,000 or less. The surface area of exchange between the hollow fibers and the extracapillary space (peripheral compartment) was 1.5 ft2. Medium containing antibiotic was pumped through the lumens of the fibers at a flow rate of 20 ml/min with Masterflex computerized peristaltic pumps (model 7550-90; Cole-Parmer Instrument Company, Vernon Hills, Ill.) and Easy-Load pump heads (model 7518-00; Cole-Parmer). In addition, the bacterial culture within the peripheral compartment was continuously circulated with similar peristaltic pumps at a rate of 20 ml/min through a loop of silicone tubing attached to two ports entering and exiting the peripheral compartment. The initial volume of culture that circulated through the peripheral compartment and loop of silicone tubing was 35 to 40 ml. When samples from the peripheral compartment were required, 0.5-ml volumes were removed through a four-way sterile stopcock (Medex, Hilliard, Ohio) positioned within the loop of silicone tubing. The volume of antibiotic-containing medium in the central reservoir was 150 ml. Dilution and elimination of the antibiotic-containing medium in the central compartment were set at 1.7 ml/min on the basis of the 1-h elimination half-life for each compound (18, 19).
Pharmacokinetic studies in the IVPM.
For pharmacodynamic studies in the IVPM, the peak concentrations achieved in human serum after the administration of intravenous doses of 3.375 g of piperacillin-tazobactam, 3.2 g of ticarcillin-clavulanate, and 3.1 g of ticarcillin-clavulanate were dosed into the central reservoir (13, 18, 19). For studies in which the penicillins were dosed alone, the pharmacokinetic profiles of the penicillins when they are dosed with the β-lactamase inhibitors were simulated. To evaluate the pharmacokinetics of each compound in the IVPM, samples were removed from the peripheral compartment at 0, 0.5, 1, 2, 4, and 6 h after the doses had been introduced into the central reservoir. The concentrations of piperacillin and ticarcillin were measured by the disk diffusion microbiological assay (5) with a β-lactamase-negative strain of Staphylococcus aureus. Tazobactam and clavulanate concentrations were measured by microbiological assay with a susceptible strain of Acinetobacter calcoaceticus. Samples assayed for clavulanate and tazobactam were each treated for 15 min with a Bush group 1 cephalosporinase (type III from Enterobacter cloacae; Sigma Chemical Co.) to inactivate the piperacillin or ticarcillin. Preliminary tests indicated that this treatment did not significantly alter the concentrations of tazobactam (10) or clavulanate (11).
Pharmacodynamics against P. aeruginosa in the IVPM.
Logarithmic-phase cultures (107 to 108 CFU/ml) of each strain were introduced into the peripheral compartment of the IVPM and were exposed to piperacillin, piperacillin-tazobactam, ticarcillin, or ticarcillin-clavulanate. For drug-free control cultures, 105 to 106 CFU/ml was inoculated into the peripheral compartment. Antibiotic regimens were dosed at 0, 6, 12, and 18 h. At 0.5 h after the introduction of each dose, samples were removed from the peripheral compartment, filter sterilized, and assayed for antibiotic or β-lactamase inhibitor concentrations to ensure that the desired peak concentrations were achieved. At 0, 1, 2, 4, 6, 8, 12, 18, and 24 h, 400-μl samples removed from the peripheral compartment of the IVPM were treated for 15 min at 37°C with 100 μl of penicillinase from culture supernatants of Bacillus cereus (BBL) to inactivate residual piperacillin or ticarcillin. Total viable bacterial counts were measured by plating serial 10-fold dilutions of each sample into MHA. The least-diluted sample that was plated was 0.1 ml of undiluted sample from the peripheral compartment. Since 30 colonies is the lower limit of accurate quantitation by the pour plate methodology, the smallest number of bacteria that could be accurately counted was 300 CFU/ml. The lowest level of detection, although actual counts were inaccurate, was 10 CFU/ml. Additionally, samples taken at the 24-h time point were plated into MHA supplemented with antibiotic at a concentration eightfold above the MIC to detect mutants with significantly decreased susceptibilities.
RESULTS
Initial studies.
The susceptibilities of the 10 clinical isolates to piperacillin, piperacillin-tazobactam, ticarcillin, and ticarcillin-clavulanate are shown in Table 1. Piperacillin was two- to eightfold more potent than ticarcillin against these strains, with MICs ranging from 2 to 16 μg/ml. Ticarcillin MICs ranged from 16 to 32 μg/ml. The MICs of piperacillin-tazobactam were similar to those of piperacillin, ranging from 4 to 8 μg/ml. Similarly, the MICs of ticarcillin and ticarcillin-clavulanate were consistently within 1 twofold dilution of each other. No plasmid-mediated β-lactamases were observed in any of the strains.
The 10 clinical isolates of P. aeruginosa were screened for induction of AmpC cephalosporinase by clavulanate, tazobactam, and cefoxitin by the disk approximation methodology. Cefoxitin, the positive control, induced AmpC cephalosporinase expression in all 10 clinical isolates, as indicated by a flattening of the zone of inhibition between the disks containing cefoxitin and those containing ticarcillin or piperacillin. Clavulanate induced AmpC cephalosporinase expression in 8 of 10 isolates, with flattening of both the ticarcillin and piperacillin zones. In contrast, tazobactam did not appear to induce AmpC expression in any of the isolates. On the basis of disk approximation data, five strains were selected for further evaluation. P. aeruginosa 1, P. aeruginosa 13, and P. aeruginosa 246 represented three strains that were clavulanate inducible, and P. aeruginosa 164 and P. aeruginosa 242 were selected as representative strains that were clavulanate noninducible.
Cephalosporinase induction.
To determine if clavulanate’s induction of AmpC cephalosporinase observed in the disk approximation studies would occur at clinically relevant concentrations, quantitative induction studies were performed in broth. The results of those experiments are shown in Table 2. Sonic extracts from uninduced cultures of the five P. aeruginosa strains contained relatively low levels of cephalosporinase activity (2 to 9 nmol/min/mg). Expression of AmpC cephalosporinase in P. aeruginosa 1 increased significantly when the strain was induced with cefoxitin or with 8, 16, or 50 μg of clavulanate per ml. No induction occurred following exposure of this strain to 2 μg of clavulanate per ml or 30 μg of tazobactam per ml. Similar results were observed in tests with P. aeruginosa 13 and P. aeruginosa 246 (Table 2). In studies with P. aeruginosa 164 and P. aeruginosa 242, induction of AmpC expression was observed only when cultures were treated with 50 μg of either cefoxitin or clavulanate per ml. No induction occurred with other concentrations of clavulanate or with tazobactam.
TABLE 2.
Induction of AmpC expression in P. aeruginosa by cefoxitin, clavulanate, and tazobactam
| Strain | Disk approximation induction results for clavulanic acid | Cephalosporinase activity in cell-free sonic extractsa
|
||||||
|---|---|---|---|---|---|---|---|---|
| Uninduced control | Clavulanate at 2 μg/ml | Clavulanate at 8 μg/ml | Clavulanate at 16 μg/ml | Clavulanate at 50 μg/ml | Cefoxitin at 50 μg/ml | Tazobactam at 30 μg/ml | ||
| P. aeruginosa 1 | Positive | 2 ± 0 | 2 ± 2 | 22 ± 4bc | 36 ± 6b | 230 ± 80b | 146 ± 6b | 3 ± 4 |
| P. aeruginosa 13 | Positive | 8 ± 6 | 8 ± 2 | 20 ± 2bc | 36 ± 8b | 128 ± 8b | 147 ± 26b | 7 ± 2 |
| P. aeruginosa 246 | Positive | 9 ± 4 | 7 ± 2 | 143 ± 22b | 177 ± 16b | 285 ± 24b | 310 ± 46b | 5 ± 4 |
| P. aeruginosa 242 | Negative | 2 ± 2 | 1 ± 0 | 2 ± 2 | 1 ± 2 | 98 ± 14b | 98 ± 14b | 2 ± 2 |
| P. aeruginosa 164 | Negative | 4 ± 6 | 2 ± 2 | 3 ± 2 | 2 ± 0 | 25 ± 2b | 90 ± 18b | 1 ± 2 |
Cephalosporinase activity (nanomoles of cephalothin hydrolyzed per minute per milligram of protein) in sonic extracts of logarithmic-phase cultures treated for 2 h with normal saline (uninduced control), clavulanate (2, 8, 16, or 50 μg/ml), cefoxitin (50 μg/ml), or tazobactam (30 μg/ml). Data represent the mean ± 2 SDs for duplicate experiments.
Cephalosporinase activity was significantly increased compared to that for uninduced controls.
Cephalosporinase activity was significantly decreased compared to that for cultures treated with 16 μg of clavulanate per ml.
Pharmacokinetic and pharmacodynamic studies in the IVPM.
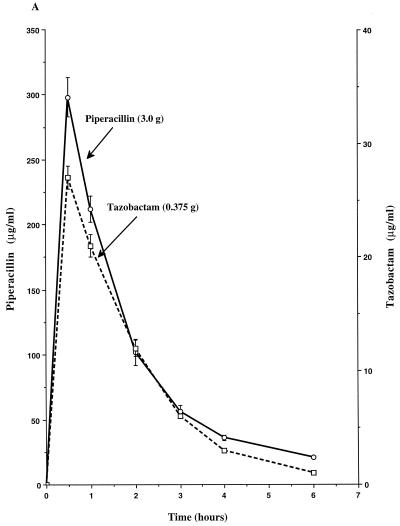
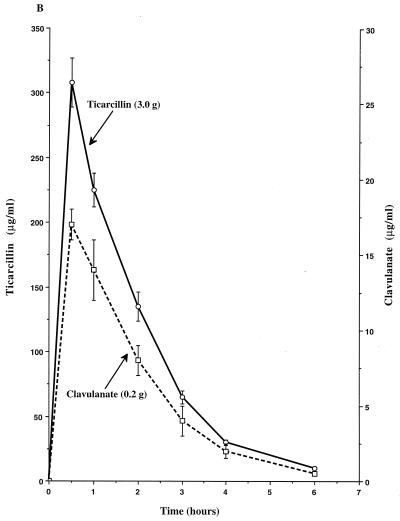
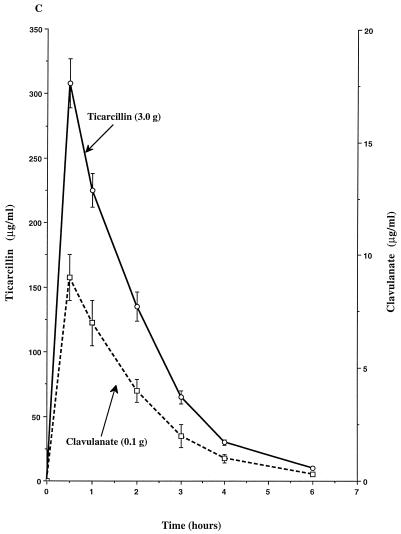
The single-dose pharmacokinetics of piperacillin, tazobactam, ticarcillin, and clavulanate within the peripheral compartment of the IVPM are shown in Fig. 1. Peak concentrations (mean ± SD) of each compound were achieved in the peripheral compartment 0.5 h after introduction of the dose into the central reservoir and were 298 ± 13 μg/ml for piperacillin, 27 ± 1 μg/ml for tazobactam, 308 ± 19 μg/ml for ticarcillin, 17 ± 1 μg/ml for the 0.2-g dose of clavulanate, and 9 ± 1 μg/ml for the 0.1-g dose of clavulanate. The calculated elimination half-lives ranged from 0.9 to 1.1 h.
FIG. 1.
Single-dose pharmacokinetic profiles of piperacillin-tazobactam (3.375-g dose) (A), ticarcillin-clavulanate (3.2-g dose) (B), and ticarcillin-clavulanate (3.1-g dose) (C) in the peripheral compartment of the IVPM after introducing peak concentrations into the central reservoir. Drug levels were measured by bioassay. Each datum point represents the mean drug level in the peripheral compartment (in micrograms per milliliter) for duplicate experimental runs. Error bars show SDs.
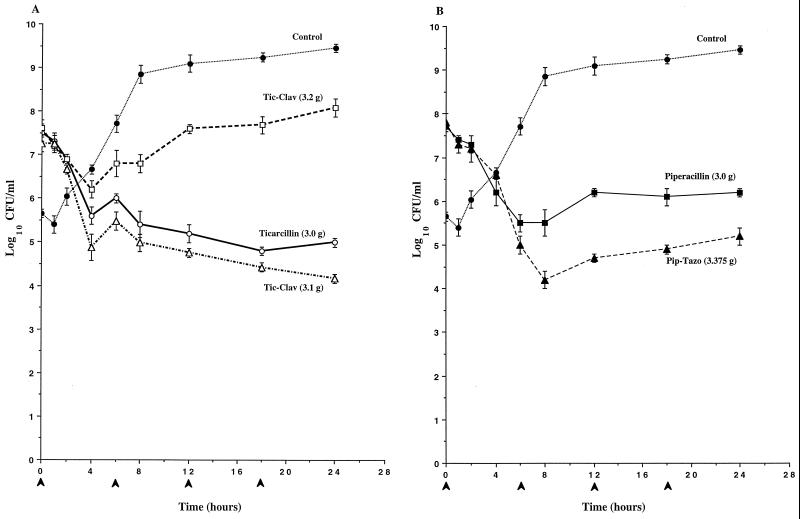
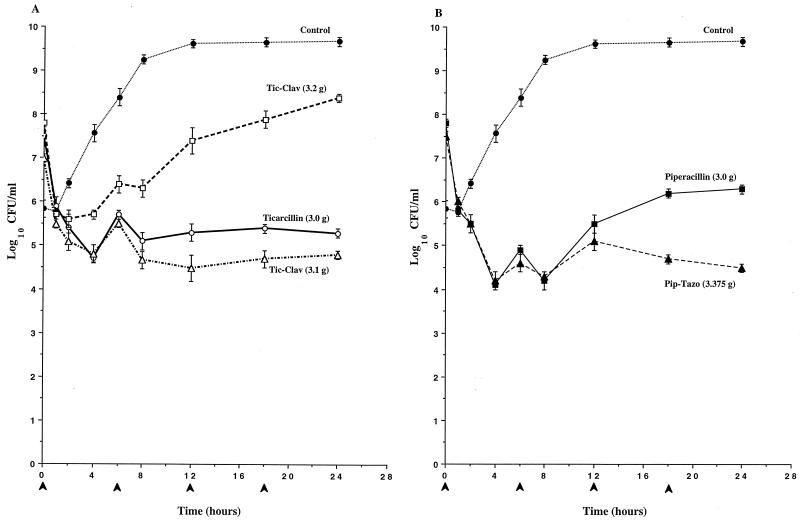
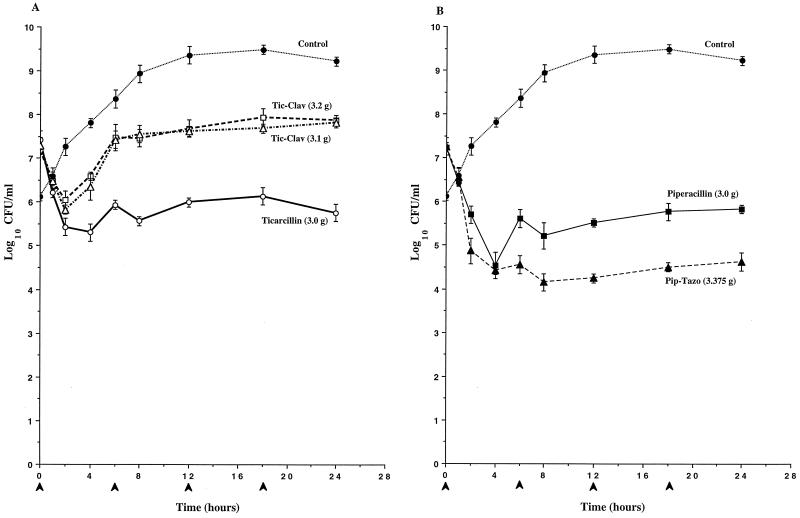
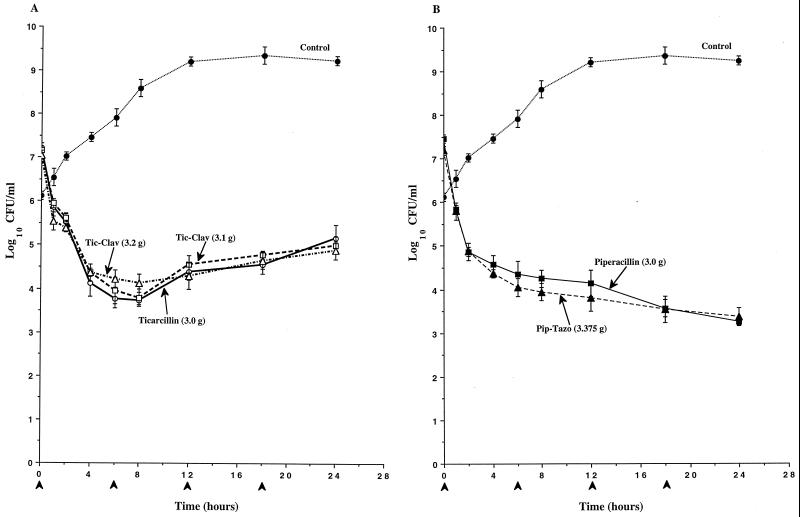
To determine if clavulanate’s induction of AmpC expression observed in broth induction studies would result in antagonism of the antibacterial activity of ticarcillin against P. aeruginosa, the time-kill pharmacodynamics of ticarcillin and ticarcillin-clavulanate were evaluated in an IVPM. As a comparison, the pharmacodynamics of piperacillin and piperacillin-tazobactam were also evaluated. In studies with P. aeruginosa 1 (Fig. 2) and P. aeruginosa 13 (Fig. 3), the antibacterial activity of the simulated 3.2-g regimen of ticarcillin-clavulanate was significantly diminished compared to that of ticarcillin alone, and the divergence of the pharmacodynamics of the two regimens became apparent as early as 2 h (Fig. 2A and 3A). Differences in viable counts between cultures treated with ticarcillin and the 3.2-g regimen of ticarcillin-clavulanate exceeded 3 logs by 24 h. In contrast, similar pharmacodynamics were observed with the simulated 3.1-g regimen of ticarcillin-clavulanate and ticarcillin alone. In studies with both of these strains, the killing produced by piperacillin-tazobactam was somewhat greater than that produced by piperacillin alone (Fig. 2B and 3B). In studies with P. aeruginosa 246, the antibacterial activities of both regimens of ticarcillin-clavulanate were diminished compared to that of ticarcillin alone, and the divergence of the pharmacodynamics of the regimens became apparent as early as 2 h (Fig. 4A). Differences in viable counts between cultures treated with ticarcillin and the 3.2-g regimen of ticarcillin-clavulanate exceeded 2 logs by 24 h. Again, the killing produced by piperacillin-tazobactam was somewhat greater than that produced by piperacillin alone (Fig. 4B).
FIG. 2.
Time-kill pharmacodynamics of ticarcillin (A), ticarcillin-clavulanate (Tic-Clav) (A), piperacillin (B), and piperacillin-tazobactam (Pip-Tazo) (B) against P. aeruginosa 1. The arrowheads at 0, 6, 12, and 18 h on the x axis represent antibiotic dosing times. Each datum point represents the mean numbers of CFU per milliliter of MHB from the peripheral compartment for duplicate experiments. Error bars show SDs.
FIG. 3.
Time-kill pharmacodynamics of ticarcillin (A), ticarcillin-clavulanate (Tic-Clav) (A), piperacillin (B), and piperacillin-tazobactam (Pip-Tazo) (B) against P. aeruginosa 13. The arrowheads at 0, 6, 12, and 18 h on the x axis represent antibiotic dosing times. Each datum point represents the mean numbers of CFU per milliliter of MHB from the peripheral compartment for duplicate experiments. Error bars show SDs.
FIG. 4.
Time-kill pharmacodynamics of ticarcillin (A), ticarcillin-clavulanate (Tic-Clav) (A), piperacillin (B), and piperacillin-tazobactam (Pip-Tazo) (B) against P. aeruginosa 246. The arrowheads at 0, 6, 12, and 18 h on the x axis represent antibiotic dosing times. Each datum point represents the mean numbers of CFU per milliliter of MHB from the peripheral compartment for duplicate experiments. Error bars show SDs.
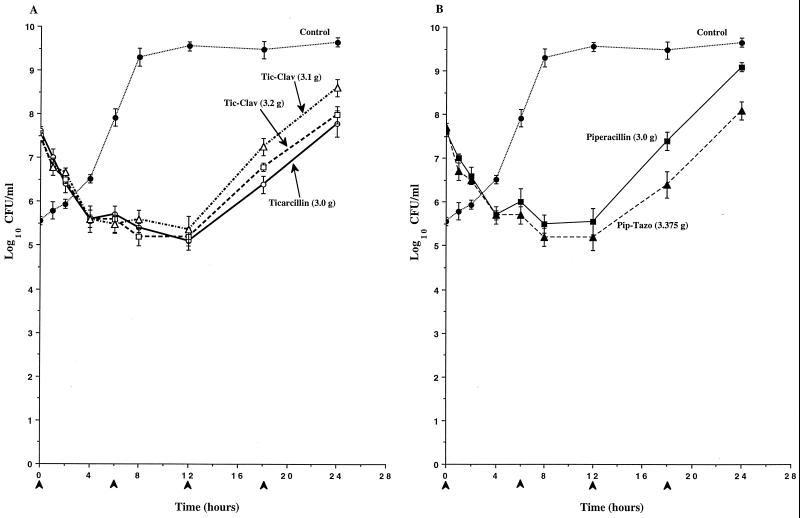
In studies with P. aeruginosa 242 and P. aeruginosa 164, the killing produced by inhibitor-drug combinations was similar to that produced by the respective drug alone (Fig. 5 and 6). In studies with P. aeruginosa 164, substantial increases in viable bacterial counts were observed over the third and fourth dose intervals with all drug regimens, and these increases were associated with the outgrowth of resistant mutant populations in all cultures (Fig. 6). These mutants were found to be 8- to 32-fold less susceptible than the parent strain P. aeruginosa 164 in broth dilution susceptibility tests and were partially derepressed for AmpC expression, as determined by analysis of cephalosporinase activity in the presence and absence of cefoxitin (9). No resistant mutant populations were selected in studies with any of the other four isolates of P. aeruginosa.
FIG. 5.
Time-kill pharmacodynamics of ticarcillin (A), ticarcillin-clavulanate (Tic-Clav) (A), piperacillin (B), and piperacillin-tazobactam (Pip-Tazo) (B) against P. aeruginosa 242. The arrowheads at 0, 6, 12, and 18 h on the x axis represent antibiotic dosing times. Each datum point represents the mean numbers of CFU per milliliter of MHB from the peripheral compartment for duplicate experiments. Error bars show SDs.
FIG. 6.
Time-kill pharmacodynamics of ticarcillin (A), ticarcillin-clavulanate (Tic-Clav) (A), piperacillin (B), and piperacillin-tazobactam (Pip-Tazo) (B) against P. aeruginosa 164. The arrowheads at 0, 6, 12, and 18 h on the x axis represent antibiotic dosing times. Each datum point represents the mean numbers of CFU per milliliter of MHB from the peripheral compartment for duplicate experiments. Error bars show SDs.
DISCUSSION
The results of this study indicate that clavulanate induces AmpC expression in P. aeruginosa at clinically relevant concentrations. They further show that such induction correlates with antagonism of the killing of P. aeruginosa by ticarcillin. No induction of AmpC β-lactamase was observed in similar tests with tazobactam, and no antagonism of piperacillin’s bactericidal activity was observed in pharmacodynamic studies.
By a disk approximation screening assay, induction of the AmpC cephalosporinase by clavulanate was observed with 8 of 10 clinical isolates of P. aeruginosa. These data confirm those of Weber and Sanders (22) who demonstrated antagonism between clavulanate and ticarcillin with 7 of 10 clinical isolates by the same methodology. Although data from both of these studies suggest that 70 to 80% of P. aeruginosa isolates increase their levels of expression of their AmpC cephalosporinases when they are exposed to clavulanate, these data do not provide any indication of how much AmpC was being produced or whether induction would occur if these strains were exposed to pharmacokinetically relevant concentrations of clavulanate. To answer these questions, five strains were selected for further evaluation.
In broth induction studies, P. aeruginosa 1, P. aeruginosa 13, and P. aeruginosa 246 significantly increased their levels of production of AmpC when they were exposed to the peak concentrations of clavulanate achieved in human serum with the 3.2- and 3.1-g doses of ticarcillin-clavulanate. In contrast, no induction of AmpC occurred when P. aeruginosa 164 and P. aeruginosa 242 were exposed to these concentrations of clavulanate or when any of the strains were exposed to pharmacokinetically relevant concentrations of tazobactam. In studies with P. aeruginosa 1 and P. aeruginosa 13, a dose-response relationship was observed in clavulanate’s induction of AmpC, with the levels of induction achieved with 8 μg of clavulanate per ml being significantly diminished compared to those achieved with 16 μg of clavulanate per ml and the levels of induction achieved with 50 μg/ml being significantly increased compared to those achieved with 16 μg of clavulanate per ml. This dose-response relationship has been observed in previous studies (20, 22). Although the levels of induction observed with 8 and 16 μg of clavulanate per ml were not significantly different in studies with P. aeruginosa 246, the lack of a dose-response effect with this strain may have been a reflection of its apparent hyperinducible nature compared to the inducible natures of P. aeruginosa 1 and P. aeruginosa 13. Among all three inducible strains, the level of induction observed with 50 μg of clavulanate per ml suggests that clavulanate may be as potent if not more potent an inducer than cefoxitin against inducible strains.
Despite the potential of clavulanate to induce AmpC expression in P. aeruginosa 1, P. aeruginosa 13, and P. aeruginosa 246, no difference was observed between the MICs of ticarcillin and ticarcillin-clavulanate against these strains. The lack of antagonism between clavulanate and ticarcillin in susceptibility tests has been reported previously (1, 20). In the current study, the lack of antagonism in susceptibility tests was directly related to the lack of AmpC induction in the presence of 2 μg of clavulanate per ml, the concentration used in dilution MIC tests. Without a significant increase in AmpC induction upon exposure to 2 μg of clavulanate per ml, antagonism would not be expected and the MICs of ticarcillin and ticarcillin-clavulanate should have been comparable.
The ability of clavulanate to induce AmpC expression was associated with antagonism or diminished killing of P. aeruginosa 1, P. aeruginosa 13, and P. aeruginosa 246 by the ticarcillin-clavulanate combinations in a pharmacodynamic model. Conversely, the inability of clavulanate to induce AmpC expression in P. aeruginosa 164 and P. aeruginosa 242 was associated with similar ticarcillin-clavulanate and ticarcillin pharmacodynamics. These data suggest that the antagonism observed in the pharmacodynamic model was in fact due to induction of the AmpC β-lactamase. The failure of the simulated 3.1-g dose of ticarcillin-clavulanate to reduce the level of killing of P. aeruginosa 1 and P. aeruginosa 13 was probably related to the lower levels of AmpC expression induced by 8 μg of clavulanate per ml in these strains in comparison to the levels induced by 16 μg of clavulanate per ml. These data suggest that induction per se is not always associated with reduced killing but that some minimal level of induction must be achieved before antagonism of ticarcillin is observed. This conclusion is supported by the observation that pharmacodynamic differences between ticarcillin and the 3.2-g dose of ticarcillin-clavulanate against P. aeruginosa 1 and P. aeruginosa 13 did not become apparent until after 2 h. Thus, it appeared that the antibacterial activity of ticarcillin in the 3.2-g dose was not compromised until levels of AmpC increased to some critical level.
The antagonism observed between clavulanate and ticarcillin in this study contradicts what has been observed clinically or in animal models of infection (4, 15). This discrepancy most likely relates to the presence or absence of host defenses. In the presence of adequate host defenses, the levels of antagonism observed in these pharmacodynamic studies may not be relevant, because bactericidal activity may not be essential in immunocompetent patients. In neutropenic patients, however, bactericidal activity is important to ensure clinical success and the antagonism observed in these studies may be more relevant.
In contrast to the antagonism observed with ticarcillin-clavulanate, the pharmacodynamics of piperacillin-tazobactam against all five P. aeruginosa strains were somewhat enhanced compared to those of piperacillin alone. The lack of antagonism between tazobactam and piperacillin against these strains can be related directly to the lack of induction of AmpC by tazobactam, while the enhanced pharmacodynamics of piperacillin-tazobactam may be the result of tazobactam’s inhibition of the low basal levels of AmpC produced by the wild-type strains (7).
In summary, data from this study demonstrated that clavulanate may induce AmpC expression in clinical isolates of P. aeruginosa. Not only was significant induction shown to occur with pharmacokinetically relevant concentrations of clavulanate, but the induction of AmpC by clavulanate was shown to significantly antagonize or substantially diminish the antibacterial activity of ticarcillin. Of further importance was the observation that antagonism was not predicted from MIC data due to the low concentration of clavulanate used in these tests. In contrast to clavulanate, no induction of AmpC expression was observed with tazobactam and no negative interactions were observed when tazobactam and piperacillin were dosed in combination against these P. aeruginosa strains. Therefore, these data suggest that in the selection of an antipseudomonal β-lactam for the treatment of P. aeruginosa infections, the combination of ticarcillin-clavulanate should be avoided, especially with immunocompromised patients, for whom bacterial killing is required to ensure clinical success.
ACKNOWLEDGMENTS
This research project was supported by a grant from Wyeth-Ayerst Laboratories.
We thank Stacey Edward and Brian Fletcher for excellent technical assistance.
REFERENCES
- 1.Akova M, Yang Y, Livermore D M. Interactions of tazobactam and clavulanate with inducibly- and constitutively-expressed class I β-lactamase. J Antimicrob Chemother. 1990;25:199–208. doi: 10.1093/jac/25.2.199. [DOI] [PubMed] [Google Scholar]
- 2.Blaser J, Stone B B, Zinner S H. Two compartment kinetic model with multiple artificial capillary units. J Antimicrob Chemother. 1985;15(Suppl. A):131–137. doi: 10.1093/jac/15.suppl_a.131. [DOI] [PubMed] [Google Scholar]
- 3.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Cavalieri S J, Sanders C C, New C. Influence of β-lactamase inhibitors on the potency of their companion drug with organisms possessing class I enzymes. Antimicrob Agents Chemother. 1991;35:1343–1347. doi: 10.1128/aac.35.7.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edberg S C. The measurement of antibiotics in human fluids: techniques and significance. In: Lorian V, editor. Antibiotics in laboratory medicine. 2nd ed. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 381–476. [Google Scholar]
- 6.Gates M L, Sanders C C, Goering R V, Sanders W E., Jr Evidence of multiple forms of type I chromosomal β-lactamase in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1986;30:453–457. doi: 10.1128/aac.30.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gwicerman B, Lambert P A, Rosdahl V T, Shand G H, Hoiby N. Rapid emergence of resistance in Pseudomonas aeruginosa in cystic fibrosis patients due to in vivo selection of stable partially-derepressed β-lactamase-producing strains. Antimicrob Agents Chemother. 1990;26:247–259. doi: 10.1093/jac/26.2.247. [DOI] [PubMed] [Google Scholar]
- 8.Labia R, Morand A, Peduzzi J. Timentin and β-lactamases. J Antimicrob Chemother. 1986;17(Suppl. C):17–26. doi: 10.1093/jac/17.suppl_c.17. [DOI] [PubMed] [Google Scholar]
- 9.Lister, P. D., V. M. Gardner, and C. C. Sanders. 1998. Unpublished data.
- 10.Lister P D, Prevan A M, Sanders C C. Importance of β-lactamase inhibitor pharmacokinetics in the pharmacodynamics of inhibitor-drug combinations: studies with piperacillin-tazobactam and piperacillin-sulbactam. Antimicrob Agents Chemother. 1997;41:721–727. doi: 10.1128/aac.41.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lister, P. D., and C. C. Sanders. 1997. Unpublished data.
- 12.Lister P D, Sanders W E, Jr, Sanders C C. Cefepime-aztreonam: a unique double β-lactam combination for Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:1610–1619. doi: 10.1128/aac.42.7.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medical Economics Data. Physicians desk reference. Montvale, N.J: Medical Economics Data; 1998. [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 15.Roselle G A, Bode R, Hamilton B, Bibler M, Sullivan R, Douce R, Staneck J L, Bullock W E. Clinical trial of efficacy and safety of ticarcillin and clavulanic acid. Antimicrob Agents Chemother. 1985;27:291–296. doi: 10.1128/aac.27.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders C C, Sanders W E, Jr, Moland E S. Characterization of β-lactamases in situ on polyacrylamide gels. Antimicrob Agents Chemother. 1986;30:951–952. doi: 10.1128/aac.30.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanders C C, Sanders W E., Jr Emergence of resistance to cefamandole: possible role of cefoxitin-inducible β-lactamases. Antimicrob Agents Chemother. 1979;15:792–797. doi: 10.1128/aac.15.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scully B E, Chin N-X, Neu H C. Pharmacology of ticarcillin combined with clavulanic acid in humans. Am J Med. 1985;79(Suppl. 5B):39–43. doi: 10.1016/0002-9343(85)90127-5. [DOI] [PubMed] [Google Scholar]
- 19.Sorgel F, Kinzig M. The chemistry, pharmacokinetics, and tissue distribution of piperacillin/tazobactam. J Antimicrob Chemother. 1993;31(Suppl. A):39–60. doi: 10.1093/jac/31.suppl_a.39. [DOI] [PubMed] [Google Scholar]
- 20.Strobberingh E E. Induction of chromosomal β-lactamase by different concentrations of clavulanic acid in combination with ticarcillin. J Antimicrob Chemother. 1988;21:9–16. doi: 10.1093/jac/21.1.9. [DOI] [PubMed] [Google Scholar]
- 21.Vidal F, Mensa J, Almela M, Martinez J, Marco F, Casals C, Gatell J, Soriano E, de Anta M J. Epidemiology and outcome of Pseudomonas aeruginosa bacteremia, with special emphasis on the influence of antibiotic treatment. Arch Intern Med. 1996;154:2121–2126. [PubMed] [Google Scholar]
- 22.Weber D A, Sanders C C. Diverse potential of β-lactamase inhibitors to induce class 1 enzymes. Antimicrob Agents Chemother. 1990;34:156–158. doi: 10.1128/aac.34.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]