Abstract
Objective
Health condition and outcome registry systems (registries) are used to collect data related to diseases and other health-related outcomes in specific populations. The implementation of these programs encounters various barriers and facilitators. Therefore, the present review aimed to identify and classify these barriers and facilitators.
Materials and Methods
Some databases, including PubMed, Embase, ISI Web of Sciences, Cochrane Library, Scopus, Ovid, ProQuest, and Google Scholar, were searched using related keywords. Thereafter, based on the inclusion and exclusion criteria, the required data were collected using a data extraction form and then analyzed by the content analysis method. The obtained data were analyzed separately for research and review studies, and the developed and developing countries were compared.
Results
Forty-five studies were reviewed and 175 unique codes were identified, among which 93 barriers and 82 facilitators were identified. Afterward, these factors were classified into the following 7 categories: barriers/facilitators to management and data management, poor/improved collaborations, technological constraints/appropriateness, barriers/facilitators to legal and regulatory factors, considerations/facilitators related to diseases, and poor/improved patients’ participation. Although many of these factors have been more cited in the literature related to the developing countries, they were found to be common in both developed and developing countries.
Conclusion
Lack of budget, poor performance of managers, low data quality, and low stakeholders’ interest/motivation on one hand, and financing, providing adequate training, ensuring data quality, and appropriate data collection on the other hand were found as the most common barriers or facilitators for the success of the registry implementation.
Keywords: registries, health registry, disease registry, barrier, facilitator, implementation, development
INTRODUCTION
A health condition and outcome registry system (registry) is an organized system in which uniformed data (including both clinical and nonclinical ones) are systematically collected and then analyzed in order to assess the trends and health outcomes related to a specific disease, health condition, intervention, or health outcomes in predefined populations.1
Some registries are provided to patients with a particular disease or condition, and some of them register patients receiving a product, service, or intervention. As well, some other registries include both diseases and interventions.2 The epidemiological registries collect data on specific disorders in order to provide epidemiological data, so many potential patients should be covered by these systems. The clinical registries collect data on clinical care and outcomes to support physicians with one or some specialties. Accordingly, these systems play an important role in monitoring diseases and care delivery patterns and are used with an increasing trend in improving health-care processes, developing clinical guidelines and standards, and reducing care costs.1 Nowadays, research registries are developed for data collection that can be used for specific research programs, developing research infrastructure, and helping researchers to identify and recruit eligible cases for their studies.3 Moreover, registries are classified according to the covered population4 and the geographical coverage, which includes various types of registries as population-based, hospital-based, regional, national, or international registries.2
Some registries, such as cancer, trauma, or surgery ones, are implemented more widely. The purpose of cancer registries is to record all the cancer cases diagnosed in a clearly defined population, in order to report incidence and mortality rates of different types of cancers. The needed data are collected from 3 major data sources, including pathology departments, hospitals, and death certifications.5 The purposes of trauma registries mostly are as follows: improving the quality of care offered to patients and improving patient outcomes. Additionally, these registries are known as powerful tools for the collection of data on trauma for comparative and cost-effectiveness investigations. Accordingly, these data may also be used for the development of standardized trauma care guidelines.6 Surgery registries are used to collect the data to improve patient (surgery) care, monitoring devices, comparison of standards, and evaluating both interventions and performance. These registries help to obtain a better understanding of surgery epidemiology and to promote future studies in this field.7 In all of these systems, functions such as data entry, data analysis, and developing reports should be well managed and evaluated.8 Generally, the objectives of registries include clinical, scientific, and health policy-related programs. Of note, well-designed registries can provide a real-world vision of patient outcomes, safety, clinical practice, and comparative effectiveness.9 Furthermore, these systems provide epidemiological data (on mortality, prevalence, and incidence) related to diseases and diseases’ control programs,10 as well as presenting an important source consisting of real-world evidence to improve health decision-making.11
Developing and implementing registries are complex processes that require some fundamental efforts.10 In this regard, to develop and implement registries, various inputs (such as financial resources and guidelines), processes (such as case finding, data collection, and analysis), and outputs (such as output information and reports) should be well designed and evaluated8 in order to ensure the success of the programs. The process of the implementation of registries is always encountered with various barriers and facilitators.12 Therefore, finding some solutions for a successful implementation of registries is important.13 Some previous studies in the United States have reported a number of barriers such as cost constraints, poor quality of information, and technology problems.14 Moreover, some reviews performed previously have specifically focused on a specific disease or condition.7,15–17
Therefore, due to the lack of systematic reviews on identifying these barriers and facilitators, regardless of the type of disease or registry, and lack of studies on comparing these factors between developing and developed countries, we conducted the current review to identify these factors and also to provide suggestions for improving the implementation of registries.
METHODS
This review was conducted in terms of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).18
Definition
A registry is defined as an organized system in which uniformed data are systematically collected and then analyzed for a predefined population and for a specific disease, condition, or health outcome.1 In this review, any type of registry system related to a specific group of patients, regardless of the type of diseases, procedures, or health outcomes, was considered as a registry.
A barrier is a condition, person, or thing preventing other people, systems, or entities from doing their work, communication, or progressing.19 Conversely, a facilitator is defined as a person or thing that either helps another person, system, or organization to do something more easily or provides a solution for a problem.20 In this review, barriers and facilitators were included regardless of their type.
Information sources and search strategy
The Electronic search was performed on some databases including PubMed (MEDLINE), Embase, ISI Web of Sciences, Cochrane Library, Scopus, Ovid, and ProQuest. The main keywords were the following: registries, health registry, barriers, and facilitators. Moreover, we developed our search strategy (Supplement File 1, Table S1) with the combination of the used main keywords, synonymous, and related keywords in MeSH and Emtree, as well as other keywords obtained from the literature review. In addition, to check the gray literature and to ensure that the relevant studies were not omitted, the Google and Google Scholar search engines were searched. Furthermore, the reference lists of the related studies were reviewed to identify further relevant articles.
Inclusion and exclusion criteria
In the present study, research and review studies published in conference proceedings and peer-reviewed journals (in full text) from 2000 to June 2021 were included. As well, the studies related to “any type” of diseases, procedures, and health outcome registry systems were included. Studies that specifically identified barriers or challenges or both of them were also included. Moreover, those studies that identified facilitators or barriers after the implementation of a registry or reviewed other registries were selected. However, short communication, letters to the editor, commentaries, perspectives, books, book reviews, conference abstracts, and clinical trial registry systems were excluded. We also excluded articles published in non-English languages.
Selection of studies
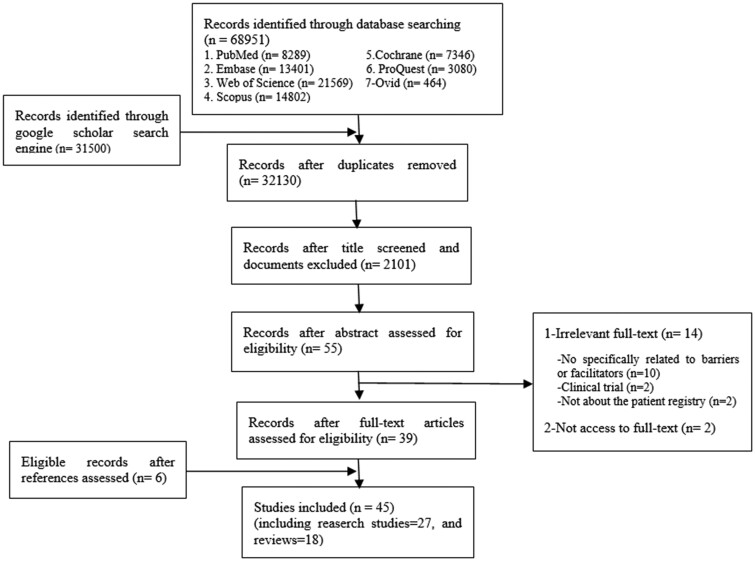
At first, all duplicate titles were removed using Endnote software. In the next step, unrelated studies were removed based on their title and the abstracts. Thereafter, the full text of the remained studies was reviewed. The references of the included studies were also checked to find relevant articles (Figure 1). In all these steps, the 2 researchers independently reviewed the titles, abstracts, and full text of the studies, respectively. In case of any disagreement, the agreement was reached through consensus.
Figure 1.
Selection of studies based on the PRISMA flowchart.
Data extraction and analysis
To collect the required data, we developed a data extraction form and then extracted the author, year of publication, the living country of the first author, the country/countries in which those registries were evaluated, geographic coverage and type of registry, research methodology, and summary of the findings (barriers and facilitators). To do this, 2 researchers, after reviewing the articles, independently extracted the data and concepts related to barriers and facilitators of registries. If there was any difference between the findings of these 2 researchers, these cases would be reviewed in a joint meeting and agreement would be reached finally.
Data analysis was done using the content analysis method. The concepts obtained from each included study were discussed in various meetings. Finally, these concepts were organized in the form of the main themes and subthemes. The frequency of each one of the extracted concepts was reported as well. Besides, the findings were classified based on publishing in the developed and developing countries.21 To this end, we considered the countries in which registries were evaluated. Some articles did not focus on a specific group of countries,10,22–27 so their reported factors were considered for both developed and developing countries. Finally, the facilitators and barriers reported in reviews and research studies were analyzed separately. To calculate the percentage of these reported barriers and facilitators, the number of studies in each category (review or research studies, and studies related to the developing or developed countries) was considered as the denominator. Additionally, the frequency of barriers and facilitators was reported based on the condition, disease, or intervention. In this subanalysis, those registries with higher number of included publications, including trauma, cancer, stroke, surgery, implant, and vaccination registries, were considered.
Ethical consideration
This study was approved by the ethics committee of Iran University of Medical Sciences, Tehran, Iran (IR.IUMS.REC.1397.374).
RESULTS
Selection of the studies
From 100,451 studies retrieved initially, 45 studies were included in this review. Figure 1 shows the processes of searching and screening the selected studies based on the PRISMA flowchart.18 Based on our comprehensive search strategies, there were many initial results; however, many of them were related to the description or analysis of registry data, so they were considered as unrelated to our purpose and excluded thereafter.
Description of the included studies
The specifications of the registries mentioned in the included studies are reported in Table 1. The description of the included studies is presented in Supplement File 2, Tables S2 to S4.
Table 1.
Specifications of health condition and outcome registry systems in the included studies
| Specifications | Number of studies | References | |
|---|---|---|---|
| 1. Type of registry | Hospital-based | 14 | 6 , 16 , 23 , 27–30 , 36 , 38 , 40 , 41 , 54 , 57 , 58 |
| Population-based | 10 | 5 , 22 , 31 , 32 , 34 , 47 –49,55,56 | |
| Clinical/research-based | 6 | 14 , 17 , 24 , 26 , 37 , 52 | |
| Clinical | 4 | 33 , 35 , 45 , 53 | |
| Clinical/hospital-based | 3 | 7 , 39 , 42 | |
| Research-based | 2 | 44 , 51 | |
| Not mentioned | 6 | 10 , 15 , 25 , 43 , 46 , 50 | |
| 2. Registry geographical coverage | National | 15 | 5–7 , 30 , 33 , 34 , 36 , 39 , 40 , 42 , 47 , 49 , 50 , 54 , 55 |
| Local | 9 | 35 , 38 , 41 , 43 , 44 , 48 , 52 , 53 , 57 | |
| International | 4 | 22 , 37 , 45 , 58 | |
| National/local | 4 | 14 , 23 , 24 , 29 | |
| Not mentioned | 12 | 10 , 15–17 , 25–28 , 31 , 32 , 46 , 51 , 56 | |
| 3. Condition, disease, or intervention | Trauma | 9 | 6 , 15 , 16 , 25 , 27–29 , 41 , 57 |
| Cancer | 3 | 5 , 43 , 55 | |
| Stroke | 3 | 40 , 42 , 54 | |
| Surgery | 3 | 17 , 45 , 58 | |
| Implant | 3 | 7 , 36 , 39 | |
| Vaccination | 3 | 47–49 | |
| Diabetes | 2 | 53 , 56 | |
| Primary care | 2 | 38 , 44 | |
| No limited to a health condition (outcome) or intervention | 2 | 10 , 50 | |
| Spinal cord injury | 1 | 30 | |
| Herpes virus | 1 | 31 | |
| Emergency care | 1 | 32 | |
| Bone infection | 1 | 22 | |
| Cardiac rehabilitation | 1 | 33 | |
| Rare diseases | 1 | 34 | |
| Familial hypercholesterolemia | 1 | 35 | |
| Acupuncture in premature ovarian insufficiency | 1 | 37 | |
| Urology | 1 | 14 | |
| Neurological diseases | 1 | 46 | |
| Lupus | 1 | 26 | |
| Human embryonic stem cells | 1 | 51 | |
| Psychological problems during pregnancy and after childbirth | 1 | 52 | |
| Cystic fibrosis | 1 | 24 |
As shown in Table 1, the highest frequency of registries belonged to hospital-based (14 studies), followed by population-based registries (10 studies). Most registries were national (15 studies) and local registries (9 studies). Furthermore, the highest number of studies belonged to trauma registry programs (9 studies).
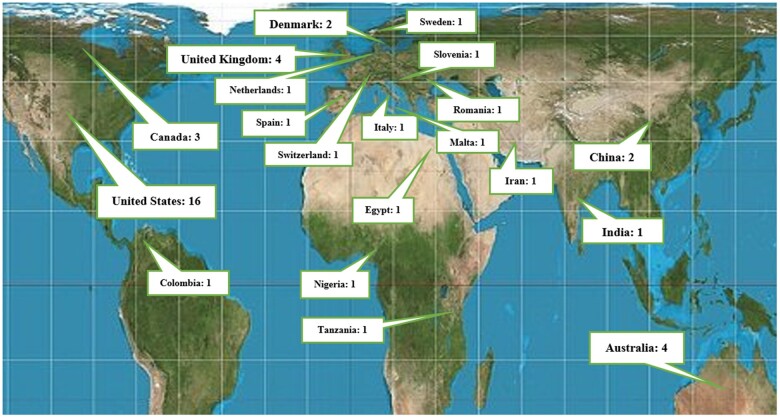
Figure 2 shows the geographical distribution of studies based on the living countries of the first authors. The United States with 16 studies (35.5%) and United Kingdom and Australia each one with 4 studies (8.8%) had the highest number of investigations.
Figure 2.
Geographical location and number of included studies.
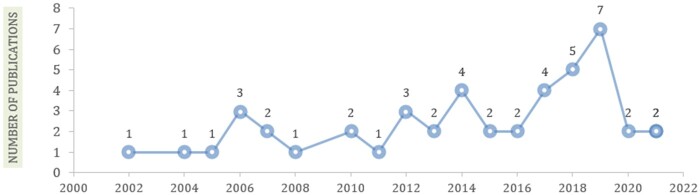
Figure 3 shows the most studies were published in 2019 (n = 7, 15.5%).
Figure 3.
Distribution of studies by publication year.
Of 45 studies included, 26 (19 research studies and 7 reviews), 12 (6 research studies and 6 reviews), and 7 (2 research studies and 5 reviews) discussed the barriers or facilitators related to the registries of developed and developing countries, or both of them, respectively.
Barriers and facilitators
The detailed findings related to these barriers and facilitators are presented in Supplement File 2, Tables S2 to S4. Overall, 607 concepts were extracted from these included studies, which were then organized into 175 unique codes by integrating similar concepts. Of these, 93 codes (Supplement File 3, Table S5) and 82 codes (Supplement File 3, Table S6) were related to the barriers and facilitators, respectively. Thereafter, these codes were classified into 14 themes (including 7 barriers and 7 facilitators), namely management, data management, stakeholder cooperation, technology, ethics/confidentiality/data security, patient’s participation, and disease-related factors (Tables 2 and 3).
Table 2.
Barriers to implementation of health condition and outcome registries
| Themes and subthemes (References) | |
|---|---|
| Theme1: Management barriers | |
| 1. Lack of or insufficient resources | |
| Lack of financial resources5–7,10,14–17,22,23,27–29,31,34,36,37,39,41,43,50,52–54,57 | |
| Inadequate human resources6,15,23,28–31,36,41,43,50,53,55,57 | |
| Inadequate training5,10,15,16,22,27,29–31,33,35,43,53,57 | |
| Insufficient time6,35,36,42,52,53,57 | |
| Insufficient working space52 | |
| 2. Poor performance of managers5,15,16,22,26,27,31–33,35,36,50,52,55,56 | |
| 3. Inappropriate instructions and guidelines for registries10,14–16,22,23,26,27,31,32,34,44,50,54 | |
| 4. Use of inappropriate processes10,15,25,32–34,36,43,47,50,58 | |
| 5. Inappropriate formulation of registry purposes10,24,27,34,43,50,51 | |
| 6. Inappropriate steering committee10,16,33,50 | |
| Theme 2: Data management barriers | |
| 1. Poor data quality5,7,14–16,22–25,28,30–33,36,37,41,43,45,47,48,51,54,56–58 | |
| 2. Inappropriate data collection methods6,7,15,17,24,25,27,28,30–33,41–43,45,51,54–57 | |
| 3. Lack of data standardization10,15,23–25,27,33,34,36,39,48,49,54,55,57,58 | |
| Theme 3: Barriers to collaboration | |
| 1. Low interest and motivation7,28,29,31,33,46,47,49,50,52,55,56 | |
| 2. Limited participation of stakeholders7,10,28,31,34,43,46,49 | |
| 3. Limited coordination between stakeholders23,33,49,51,55,58 | |
| Theme 4: Technological constraints | |
| 1. Lack of integration and interoperability with other information systems5,26,27,29,31,33,34,37,47,49,57 | |
| 2. Poor design or acquiring inappropriate software26,31,33,46,47,50,51 | |
| 3. Deficiencies and limitations of infrastructure, software, hardware, and maintenance6,10,14,23,27,31,41,54 | |
| 4. Failure to determine the technical architecture of the registry system50 | |
| Theme 5: Legal and regulatory barriers | |
| 1. Inappropriate guidelines for confidentiality and data security10,15,24,26,31,34,36,39,40,46,47,51,52,57 | |
| 2. Non considering ethical, legal, and regulatory principles6,10,26,28,36,37,40,50,51,57 | |
| Theme 6: Considerations related to diseases | |
| 1. Uncertain and various consequences of a disease16,31,33,34,37,56 | |
| 2. Complexity, diversity, and extent of disease27,40 | |
| 3. Inappropriate tools for diagnosing disease and evaluating patients16,51 | |
| 4. Unclear geographical patterns of a disease in a population27,48 | |
| 5. Difficulty and limitations of biological sample collection22 | |
| Theme 7: Poor patients’ participation | |
| 1. Patients’ nonparticipation due to privacy concerns35,46,52 | |
| 2. Lack of knowledge of patients with a registry program35,52 | |
| 3. Lack of a tracking and follow-up system or lack of patients’ referral27,43 | |
| 4. Difficulty in long-term participation of a large number of patients6,26,32 | |
| 5. Difficulty in the administrative process for negotiating and contracting with patients22 | |
| 6. Nonparticipation of patients due to the possibility of additional visits and costs46 | |
Note: Some themes do not have subthemes; therefore, only the relevant codes were reported in this table, and the details related to the codes of other themes were reported in Supplement File 3 (Table S5).
Table 3.
Facilitators to implementation of health condition and outcome registries
| Themes and subthemes(References) | |
|---|---|
| Theme 1: Management facilitators | |
| 1. Securing the required resources | |
| Providing adequate training7,22,23,25,28,31,33,35,41,43,46,51–53,56,57 | |
| Financing5–7,15,16,22,23,28,31,38,39,41,43,46,56,57 | |
| Supplying human resources15,22,23,33,38,41,42,57 | |
| Allocating enough time33,38 | |
| Allocating enough working space38 | |
| 2. Managers’ performance5–7,22,23,31–33,38,41,43,50,52,53,56 | |
| 3. Formulating appropriate purposes6,7,22–24,26,32,33,44,46,50,51,57 | |
| 4. Developing registry guidelines and protocols5,7,16,22–26,29,44,46,50 | |
| 5. Developing an appropriate steering committee7,22,23,32,33,36,50,52,56,57 | |
| 6. Implementation of appropriate and well-defined processes36,42,50,53 | |
| Theme 2: Improving collaborations | |
| 1. Creating interest and motivation6,7,23,25,28,33,38,43,44,46,52–54,56,57 | |
| 2. Increasing stakeholders’ participation6,7,22–25,29,30,35,42,46,51,54,57 | |
| 3. Better coordination between stakeholders5,43 | |
| Theme 3: Data management facilitators | |
| 1. Data quality assurance7,16,23–26,28,31–33,36,41–43,45,52,57 | |
| 2. Appropriate data collection methods6,7,16,23–26,28,31–33,41,42,56–58 | |
| 3. Data standardization7,15,16,22–25,33,36,39 | |
| Theme 4: Implementing appropriate technologies | |
| 1. Compatibility and integration of the registry software with other information systems23,30,31,38,45,47,51 | |
| 2. Appropriateness, simplicity of the software, and support and maintenance services5,7,23–26,31,33,56 | |
| 3. Developing a general architectural document to determine all technical specifications of appropriate software50 | |
| 4. Establishment of Internet-based disease registry network51,58 | |
| 5. Use of Electronic Medical Records/Electronic Health Records (EMRs/EHRs) as an infrastructure for a registry16 | |
| 6. Developing a comprehensive questionnaire to identify and evaluate registry software58 | |
| Theme 5: Increasing patients’ participation | |
| 1. Trying to attract the informed participation of patients with trust22,24,31,33,35,52 | |
| 2. Development of automatic patient tracking or follow-up systems16,56 | |
| 3. Considering the needs and interests of patients31 | |
| 4. Patient’s involvement in the management of a registry7 | |
| 5. Setting a separate goal for patient follow-up53 | |
| Theme 6: Legal and regulatory facilitators | |
| 1. Developing appropriate guidelines and mechanisms for data confidentiality and security22,24,31,39,40,57 | |
| 2. Appropriate setting of ethical, legal guidelines23,39,40,50 | |
| Theme 7: Facilitators related to disease conditions | |
| 1. Existence of standard disease screening tools52 | |
| 2. Use of standard biological sample collection methods42 | |
| 3. Use of evidence-based solutions for patient evaluation16 | |
Note: Some themes do not have subthemes; therefore, only the relevant codes were reported in this table, and the details related to the codes of other themes were reported in Supplement File 3 (Table S6).
Comparison of the developing and developed countries
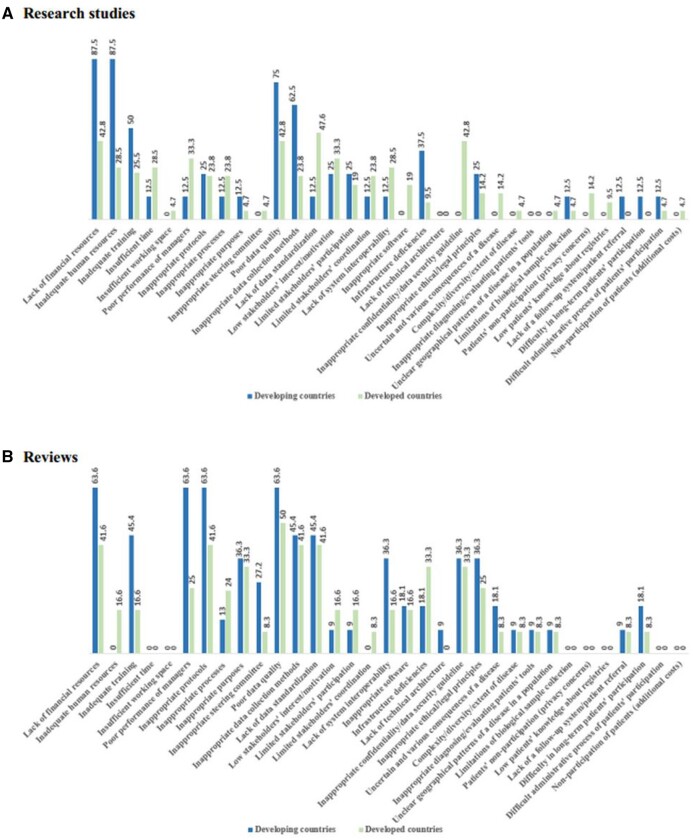
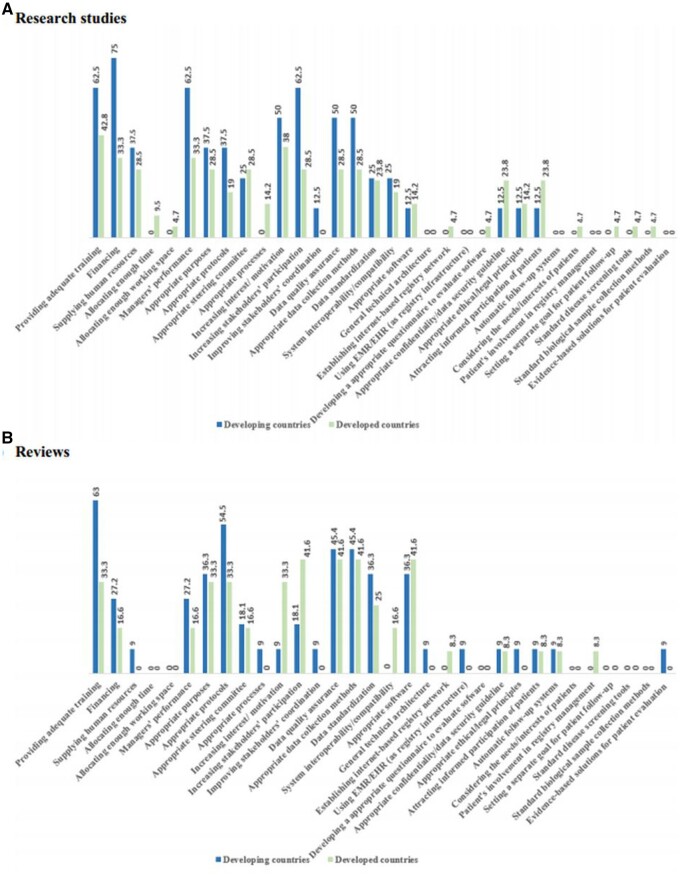
As shown in Figure 4, it was indicated that some barriers, especially registry management barriers (including inadequate financial, human, and training resources, poor-performance managers, and inappropriate guidelines) and data-related problems (including poor data quality and inappropriate data collection methods), as well as some technological constraints (including the lack of system interoperability and infrastructure deficiencies) are reported more in the developing countries than in the developed ones.
Figure 4.
The reported barriers in developed and developing countries in research studies (A) and reviews (B).
The most of the other reported barriers were mentioned relatively equally in these 2 types of countries; however, some barriers such as insufficient working space, patients’ nonparticipation due to privacy concerns, their low knowledge on registries, and the possibility of additional costs were not reported in those studies published in the developing countries at all. As well, lack of technical architecture was not reported in the studies published in the developed countries.
Furthermore, insufficient time and working space, limitations of biological sample collection, patients’ nonparticipation due to privacy concerns/additional visits/costs, difficult administrative process of patients’ participation, and lack of patients’ knowledge about registries were not reported in previous reviews.
According to Figure 5, all 7 facilitator themes were indicated in both types of countries. However, management facilitators (including providing adequate training, financing, managers’ good performance, and developing registry guidelines), increasing stakeholders’ participation, and data-related solutions (including data quality assurance and appropriate data collection methods) were more reported in the developing countries compared to the developed ones.
Figure 5.
The reported facilitators in developed and developing countries in research studies (A) and reviews (B).
Most of the other facilitators were cited relatively equal in studies related to both developing and developed countries. However, some facilitators, such as allocating enough time and workspace, developing questionnaires to evaluate registry software, patient’s involvement in registry management, setting a separate goal for patient’s follow-up, considering the patients’ needs/interests, and existence of standard disease screening and biological sample collection tools/methods, were not reported in the studies related to the developing countries.
Moreover, research studies did not mention some facilitators such as developing a general technical architectural document of software, using Electronic Medical Record (EMR)/Electronic Health Record (EHR) as the infrastructure of registries, developing automatic patient’s follow-up systems, patient’s involvement in registry management, and using evidence-based solutions for patient’s evaluation. Furthermore, allocating enough time and workspace, developing a comprehensive questionnaire to evaluate registry software, considering the patients’ needs/interests, setting a separate goal for patient’s follow-up, and using the standard disease screening and biological sample collection tools/methods were not reported in previous reviews.
The subanalysis performed based on the common reported registries (Supplement File 4, Tables S7 and S8) showed that lack of financial/human resources and other management problems, poor data quality, inadequate data collection, and limited cooperation of stakeholders as well as some facilitators such as financing and supplying human resources, data quality assurance, appropriate data collection methods, and increasing stakeholders’ cooperation were the most frequently cited barriers in trauma, cancer, stroke, surgery, implant, and vaccination registries.
It is noteworthy that some specific barriers such as the complexity, diversity, and extent of disease and unclear geographical patterns of a disease in a population were reported only for trauma/stroke and trauma/vaccination registries, respectively. On the other hand, facilitators such as the patient’s involvement in registry management and developing a comprehensive questionnaire to evaluate registry software were respectively mentioned only for implant and surgery registries. Coordinating among stakeholders as well as using EMR and evidence-based patient evaluation tools were only mentioned in cancer and trauma registries-related publications, respectively.
DISCUSSION
In the present review, 607 barriers and facilitators were identified and then classified into the following 7 categories: (1) management, (2) data management, (3) collaboration, (4) technology, (5) legal and regulatory, (6) considerations related to diseases, and (7) poor/improved patient’s participation. Stanimirovic et al.34 in their study have previously identified barriers to registry development and classified them in the following 7 themes: inability in anticipating clinical benefits, lack of registry prospects, weakness of infrastructure, weakness of legal and regulatory frameworks, weakness of conceptualization of registries, insufficient budget, and nonparticipation of stakeholders. Moreover, Mandavia and Knight36 in their study have categorized barriers into the following 9 themes: lack of completion of data, lack of agreement on a data set, insufficient resources, weakness in managing the registry, weak data management, inadequate legal factors, lack of paying attention to information protection, weakness in information governance and data security/ownership, and uncertainty regarding data quality. They have also categorized the facilitators into 7 classes, including determining the leadership committee; stakeholders’ cooperation; simple registry design; creating a minimum data set as well as maximizing data completeness; hiring legal, administrative, and technology consultants; establishing a pilot program; and user feedback. Although the classifications of the barriers and facilitators in these studies are different from that of our study, the details of these categories are somewhat similar to ours. In the current study, we also compared these factors between the developing and developed countries, and as a result, we found that problems and subsequent solutions for implementing registries are almost similar in both the developed and developing countries. However, most of them such as insufficient resources (especially financial issues) and problems related to the management of registry setup, data management, poor patients’ participation, and technological constraints have been more frequently reported in the developing countries. Notably, remaining issues in this regard are common to all registries in both the developing and developed countries. In the following, the most reported barriers and facilitators are discussed.
Management
Some barriers such as insufficient funding and budget6,7,15,16,28 and high costs related to infrastructure and equipment10,14–16 are among the most frequently cited problems. Correspondingly, these barriers could delay the progress or result in a failure of the implementation of registries, because registries heavily depend on resources and manpower. Therefore, having a plan to secure sustainable funds22,31,43 should be considered as one of the concerns of the registry managers from the start of the implementation of these programs.
Inadequate staff training5,10,22,33,43 is known as another most reported barrier leading to a lack of knowledge and skills, and doing unscientific and unprincipled activities. Therefore, providing adequate training and continuous feedback to registrars7,23,28,41,52 may possibly increase the scientific mastery of employees and guarantee their skills consequently. Having limited dedicated staff6,15,28,29 results in continuous rotation of the staff, rework, the increased training costs, and inconsistent activities. Therefore, one of the solutions in this regard is having the initial planning for hiring or employing more dedicated staff,22,33,38 which is known as one of the most basic facilitators to provide a permanent and informed staff in registries.
Poor performance of managers15,16,32,36,52 along with the lack of complete and accurate registry guidelines5,10,14,26 consequently lead to ambiguity or inconsistency in the registry operations. In this regard, registry managers should carefully develop the registry protocol, inclusion and exclusion criteria7,22,24 and registry guidelines.16,29
Data management
Incomplete and incorrect medical records14–16,28,32 and invalid reports in patients’ records22,31,33 were the most cited problems for data collection in disease registry programs. Registries usually collect data retrospectively from patients’ records. Hence, data deficiencies are difficult to be corrected, and it is not easy to obtain high-quality data from these data sources, due to the reason that each of which is documented in the health-care facilities based on different perspectives and definitions. Correspondingly, continuous data validation and auditing with ongoing feedback7,28,33,42 by data quality managers in registry centers as well as holding periodic data quality control workshops can help to identify data quality issues and also to improve data quality in registries.
Inappropriate data collection may possibly occur due to several reasons such as the large amount of required data items24,41,42 and the lack of appropriate guideline or method for data collection. A large number of data elements make the process of data collection difficult, time-consuming, and costly, and finally result in incomplete data collection. In determining the required data elements, registry managers should always pay enough attention to its appropriateness for the purposes and feasibility of data collection in terms of providing appropriate data format and the available methods and tools for data collection in participant centers, and they should also make sure of data availability23,36; otherwise, the registry will face a lack of resources, unnecessary data, or the increased missing data. Integrating data collection into daily clinical and managerial activities6,33 and automating data entry into registry software, for example, using EHRs6,28,32 with a standard data format, are some of the solutions proposed for facilitating data collection.
It was found that lack of data standardization such as uncertain minimum data set [15, 23, 34] and nonuniform definitions of data items10,25,33 cause differences in the volume, type, and structure of data, which consequently result in data incomparability, poor data quality, and nonuniformed data collection. Therefore, developing a common and agreed-upon data set7,15,16,22,33,36 and a uniform method used to collect data31,42 from different data sources should be considered by the managers from the very initial steps of the development of registries to the final stages.
Collaboration
Insufficient interest and motivation, poor participation, and coordination of stakeholders can lead to a failure of a registry. As well, ignoring financial or nonfinancial incentives7,28 leads to loss of interest and motivation, as well as the increased discouragement and resistance in registry staff. In this regard, some measures such as creating financial or nonfinancial incentives or credits6,33,38,46 are highly suggested for improving the collaboration. Poor stakeholders’ participation and involvement,7,10,28,31,34 such as their disagreement in the characteristics of a registry,51,58 lead to a lack of cooperation. Hence, promoting an active and sustainable participation among stakeholders7,29,30 in doing various activities of registries by holding regular meetings of the steering committee, and collaborative design and formulation of purposes, activities, and protocols from the very beginning of the pilot program can increase trust in the program and improve agreement and collaboration among stakeholders.
Technology
Lack of integration and interoperability with other information systems29,31,33,34 limits the possibility of the use of the available data once again in other information systems in registries. Consequently, this can lead to the increased costs due to duplicate data collection. Interoperability and integration of registry software with other systems and automatic data importing from available systems23,30,45 were the most cited measures that can be used by registry managers to enable data exchange between systems, and even to develop registry networks51,58 for taking the advantages of shared facilities. Inappropriate design and acquiring inappropriate software,31,33,46 usually due to the lack of initial needs assessment and nonstandard technical requirements, along with the lack of infrastructure and technical support,6,10,14 were the other most frequently reported barriers. In this regard, the appropriateness and a standard development of registry software, appropriate support and maintenance services,7,23,33 and identifying technical requirements58 are highly recommended.
Legal and regulatory factors
The inappropriate formulation of data confidentiality and security principles as well as noncompliance with the ethical and legal principles can cause a serious challenge to registries. Many studies have previously addressed privacy and security concerns of registries as well.15,31,36,52 Accordingly, the lack of appropriate plans for the privacy and security of patients’ data subsequently threatens the patients’ sensitivity and also leads to identifying information with unauthorized access and then the misuse of this information. Therefore, developing legal and principled measures to keep the patient anonymous,24,31,39 such as separating identity data from clinical data and not disclosing information without any permission,40 is an example of the strategies that should be implemented in this regard. On the other hand, the uncertain intellectual property of data10,36,50 causes ambiguity in data ownership, concerns and legal disputes, and reluctance in sharing patients’ data. It is suggested that registry managers should transparently formulate and agree on data ownership23,50 as well as on accurate data access and disclosure policies,40,50 especially in multicenter registry programs.
Disease’s considerations
Limitations and considerations related to a specific disease or the subject of the registry such as uncertainty and various outcomes of a disease,16,31,34 or on the extent of a disease,27,40 may result in both ambiguity and complexity for the development of a registry. For example, having difficulty in agreeing on the minimum data set, case finding criteria, or the covered population of a registry are some affected areas in this regard. Hence, involving specialized clinical and epidemiological teams along with considering evidence-based guidelines16 can consequently decrease ambiguities and increase the body of knowledge of a registry for patients’ evaluation and treatment methods. Therefore, it can lead to the appropriate design of a registry.
Patients’ participation and involvement
Patients’ participation in registries is considered as an important process in ensuring patient’s follow-up and assessment of disease’s outcomes; however, concerns related to privacy,35,46,52 poor patients’ awareness,35,52 or the possibility of additional visits and costs46 may reduce this rate of participation. Increasing patients’ trust and awareness levels,22,24,35 considering patients’ interests,31 and involving patients’ representative in the management of a registry7 are some of the strategies effective on increasing patients’ participation in registries or on improving patients’ follow-up.
CONCLUSION
The present review showed the barriers and challenges of implementing and continuing the registry programs as well as proposing some common strategies to eliminate or reduce these barriers. Overall, 93 and 82 unique barriers and facilitators were identified, respectively, which were organized into 7 themes. The barriers and facilitators related to the management and data management were the most reported registry success factors. Moreover, 4 common barriers were the lack of budget, the poor performance of managers, poor data quality, and low stakeholders’ interest/motivation, and 4 common solutions were providing adequate training, financing, data quality assurance, and appropriate data collection methods. Although many of these factors have been more cited in the literature published in the developing countries, they were common in both the developed and developing countries. Considering these barriers and facilitators, disease registry managers and policymakers can play more effective roles in the success of the development and implementation of registries. As an operational solution, registry managers can use the results of this review to develop a guideline or a roadmap for the evaluation or setting up their registry programs, in order to improve the chance of the registry success.
LIMITATIONS OF THE STUDY
In the current review, we aimed to identify and classify all the possible barriers and facilitators; however, the quality of the included studies was not investigated, and all types of registries were considered as well. Despite searching and reviewing a large number of studies, still there may be some studies that have not been reviewed. Finally, it should be noted that the reported frequency of the studies can only indicate which barriers/facilitators have been more or less reported in the literature, and it should not be considered as an indicator for the relative importance of these factors. Most studies included in this review did not report the importance of the barriers or facilitators. Therefore, prioritization of these factors can be considered in further studies.
FUNDING
This study was supported by the Iran University of Medical Sciences, Tehran, Iran (97.01.136.33278).
AUTHOR CONTRIBUTIONS
The authors contributed to the study as follows: ML: Conceptualization, methodology, data curation, formal analysis, writing—original draft, investigation, and visualization. AS: Conceptualization, methodology, validation, formal analysis, writing—review and editing, supervision, project administration, and funding acquisition.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY
The data underlying this article are available in the article and in its online supplementary material.
Supplementary Material
REFERENCES
- 1. Gliklich RE, Dreyer NA, Leavy MB.. Registries for Evaluating Patient Outcomes: A User’s Guide. Washington: Agency for Healthcare Research and Quality; 2014. [PubMed]
- 2. Zaletel M, Kralj M, Magajne M, Doupi P.. Methodological Guidelines and Recommendations for Efficient and Rational Governance of Patient Registries. Ljubljana: National Institute of Public Health; 2015. [Google Scholar]
- 3. Workman T. Defining Patient Registries and Research Networks. Engaging Patients in Information Sharing and Data Collection: The Role of Patient-Powered Registries and Research Networks [Internet]. Rockville: Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 4. Aljurf M, Rizzo J, Mohty M, et al. Challenges and opportunities for HSCT outcome registries: perspective from international HSCT registries experts. Bone Marrow Transplant 2014; 49 (8): 1016–21. [DOI] [PubMed] [Google Scholar]
- 5. Behera P, Patro BK.. Population based cancer registry of India – the challenges and opportunities. Asian Pac J Cancer Prev 2018; 19 (10): 2885–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson ED, Oak S, Griswold DP, Olaya S, Puyana JC, Rubiano AM.. Neurotrauma registry implementation in Colombia: a qualitative assessment. J Neurosci Rural Pract 2021; 12 (3): 518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mandavia R, Knight A, Phillips J, Mossialos E, Littlejohns P, Schilder A.. What are the essential features of a successful surgical registry? A systematic review. BMJ Open 2017; 7 (9): e017373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sheikhtaheri A, Nahvijou A, Sedighi Z, et al. Development of a tool for comprehensive evaluation of population-based cancer registries. Int J Med Inform 2018; 117(9): 26–32. [DOI] [PubMed] [Google Scholar]
- 9. Gliklich R, Leavy M, Dreyer N.. Patient Registries. Registries for Evaluating Patient Outcomes: A User’s Guide [Internet]. Rockville: Agency for Healthcare Research and Quality; 2020. [PubMed]
- 10. Pop B, Fetica B, Blaga ML, et al. The role of medical registries, potential applications and limitations. Med Pharm Rep 2019; 92 (1): 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gliklich RE, Leavy MB.. Assessing real-world data quality: the application of patient registry quality criteria to real-world data and real-world evidence. Ther Innov Regul Sci 2020; 54 (2): 303–7. [DOI] [PubMed] [Google Scholar]
- 12. Bianconi F, Brunori V, Valigi P, Rosa LF, Stracci F.. Information technology as tools for cancer registry and regional cancer network integration. IEEE Trans Syst, Man, Cybern A 2012; 42 (6): 1410–24. [Google Scholar]
- 13. da Silva KR, Costa R, Crevelari ES, et al. Correction: glocal clinical registries: pacemaker registry design and implementation for global and local integration–methodology and case study. PLoS One 2013; 8 (7): e71090. doi: 10.1371/journal.pone.0071090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tyson MD, Barocas DA.. Improving quality through clinical registries in urology. Curr Opin Urol 2017; 27 (4): 375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. St-Louis E, Paradis T, Landry T, Poenaru D.. Factors contributing to successful trauma registry implementation in low- and middle-income countries: a systematic review. Injury 2018; 49 (12): 2100–10. [DOI] [PubMed] [Google Scholar]
- 16. Bommakanti K, Feldhaus I, Motwani G, Dicker RA, Juillard C.. Trauma registry implementation in low- and middle-income countries: challenges and opportunities. J Surg Res 2018; 223(6): 72–86. doi: 10.1016/j.jss.2017.09.039. [DOI] [PubMed] [Google Scholar]
- 17. Stey AM, Russell MM, Ko CY, Sacks GD, Dawes AJ, Gibbons MM.. Clinical registries and quality measurement in surgery: a systematic review. Surgery 2015; 157 (2): 381–95. [DOI] [PubMed] [Google Scholar]
- 18. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62 (10): e1–e34. [DOI] [PubMed] [Google Scholar]
- 19. Oxford Dictionary [Internet]. UK: University Press; 2018. Barrier. [Google Scholar]
- 20. Cambridge Dictionaries [Internet]. Cambridge: Cambridge University Press; 2015. Facilitator. [Google Scholar]
- 21.United Nations. Country Classification: Data Sources, Country Classifications and Aggregation Methodology. New York: United Nations New York; 2014: 143–50. [Google Scholar]
- 22. Kates SL, Hurni S, Chen MS. Development and challenges in setting up an international bone infection registry. Arch Orthop Trauma Surg 2020; 140 (6): 741–9. [DOI] [PMC free article] [PubMed]
- 23. O'Reilly GM, Gabbe B, Braaf S, Cameron PA.. An interview of trauma registry custodians to determine lessons learnt. Injury 2016; 47 (1): 116–24. [DOI] [PubMed] [Google Scholar]
- 24. Viviani L, Zolin A, Mehta A, Olesen HV.. The European cystic fibrosis society patient registry: valuable lessons learned on how to sustain a disease registry. Orphanet J Rare Dis 2014; 9 (1): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zehtabchi S, Nishijima DK, McKay MP, Mann NC.. Trauma registries: history, logistics, limitations, and contributions to emergency medicine research. Acad Emerg Med 2011; 18 (6): 637–43. [DOI] [PubMed] [Google Scholar]
- 26. Lu LJ, Wallace DJ, Navarra SV, Weisman MH.. Lupus registries: evolution and challenges. Semin Arthritis Rheum 2010; 39 (4): 224–45. [DOI] [PubMed] [Google Scholar]
- 27. Nwomeh BC, Lowell W, Kable R, Haley K, Ameh EA.. History and development of trauma registry: lessons from developed to developing countries. World J Emerg Surg 2006; 1: 32. doi: 10.1186/1749-7922-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosenkrantz L, Schuurman N, Arenas C, Jimenez MF, Hameed MS.. Understanding the barriers and facilitators to trauma registry development in resource-constrained settings: a survey of trauma registry stewards and researchers. Injury 2021; 52 (8): 2215–24. [DOI] [PubMed] [Google Scholar]
- 29. Sawe HR, Sirili N, Weber E, Coats TJ, Wallis LA, Reynolds TA.. Barriers and facilitators to implementing trauma registries in low-and middle-income countries: qualitative experiences from Tanzania. Afr J Emerg Med 2020; 10 (Suppl 1): S23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Azadmanjir Z, Mohtasham-Amiri Z, Ziabari SM, Kochakinejad L, Haidari H, Mohseni M.. Sustaining the national spinal cord injury registry of Iran (NSCIR-IR) in a regional center: challenges and solutions. Iran J Public Health 2020; 49 (4): 736–43. [PMC free article] [PubMed] [Google Scholar]
- 31. Surodina S, Lam C, de Cock C, van Velthoven M, Milne-Ives M, Meinert E.. Engineering requirements of a herpes simplex virus patient registry: discovery phase of a real-world evidence platform to advance pharmacogenomics and personalized medicine. Biomedicines 2019; 7 (4): 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mowafi H, Ngaruiya C, O'Reilly G, et al. Emergency care surveillance and emergency care registries in low-income and middle-income countries: conceptual challenges and future directions for research. BMJ Global Health 2019; 4 (Suppl 6): e001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Egholm CL, Helmark C, Doherty P, Nilsen P, Zwisler AD, Bunkenborg G. “Struggling with practices” – a qualitative study of factors influencing the implementation of clinical quality registries for cardiac rehabilitation in England and Denmark. BMC Health Serv Res 2019; 19 (1): 102. doi: 10.1186/s12913-019-3940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stanimirovic D, Dadlez NM, Marshall AC.. Development of a pilot rare disease registry: a focus group study of initial steps towards the establishment of a rare disease ecosystem in Slovenia. Congenit Heart Dis 2019; 14 (1): 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tilney M. Establishing a familial hypercholesterolaemia register - the first year. Atheroscler Suppl 2019; 36(5): 24–7. doi: 10.1016/j.atherosclerosissup.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 36. Mandavia R, Knight A.. What are the requirements for developing a successful national registry of auditory implants? A qualitative study. Adv Ther 2018; 8 (9): e021720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gao D, He L, Fang Y, et al. International patient registry on acupuncture therapy for premature ovarian insufficiency: challenges and opportunities. World J Acupunct Moxibustion 2018; 28 (1): 1–3. [Google Scholar]
- 38. Holtrop JS, Hall TL, Rubinson C, Dickinson LM, Glasgow RE.. What makes for successful registry implementation: a qualitative comparative analysis. J Am Board Fam Med 2017; 30 (5): 657–65. [DOI] [PubMed] [Google Scholar]
- 39. Rakhorst HA, Mureau MAM, Cooter RD, et al. The new opt-out Dutch national breast implant registry – lessons learnt from the road to implementation. J Plast Reconstr Aesthet Surg 2017; 70 (10): 1354–60. [DOI] [PubMed] [Google Scholar]
- 40. Andrew NE, Sundararajan V, Thrift AG, et al. Addressing the challenges of cross-jurisdictional data linkage between a national clinical quality registry and government-held health data. Aust N Z J Public Health 2016; 40 (5): 436–42. [DOI] [PubMed] [Google Scholar]
- 41. Ozoilo KN, Ali M, Peter S, Chirdan L, Mock C.. Trauma registry development for Jos University Teaching Hospital: report of the first year experience. Indian J Surg 2015; 77 (4): 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eldh AC, Fredriksson M, Halford C, et al. Facilitators and barriers to applying a national quality registry for quality improvement in stroke care. BMC Health Serv Res 2014; 14: 354. doi: 10.1186/1472-6963-14-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zullig LL, Vanderburg SB, Muiruri C, Abernethy A, Weiner BJ, Bartlett J.. Sustainability of cancer registration in the Kilimanjaro region of Tanzania – a qualitative assessment. World Health Popul 2014; 15 (1): 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McBride D, Dohan D, Handley MA, Powe NR, Tuot DS.. Developing a CKD registry in primary care: provider attitudes and input. Am J Kidney Dis 2014; 63 (4): 577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hume K, Crotty C, Simmons C, Neumeister M, Chung K.. Medical specialty society-sponsored data registries: opportunities in plastic surgery. Plast Reconstr Surg 2013; 132 (1): 159e–67e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Korngut L, MacKean G, Casselman L, et al. Perspectives on neurological patient registries: a literature review and focus group study. BMC Med Res Methodol 2013; 13: 135. doi: 10.1186/1471-2288-13-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chin LK, Crawford NW, Rowles G, Buttery JP.. Australian immunisation registers: established foundations and opportunities for improvement. Eurosurveillance 2012; 17 (16): 20148. doi.org/10.2807/ese.17.16.20148-en. [PubMed] [Google Scholar]
- 48. Bernal-González PJ, Navarro-Alonso JA, Pérez-Martín J.. Computerised vaccination register for the Murcia region, Spain, 1991 to 2011. Eurosurveillance 2012; 17 (16): 20150. https://doi.org/10.2807/ese.17.16.20150-en. [PubMed] [Google Scholar]
- 49. Grove Krause T, Jakobsen S, Haarh M, Mølbak K.. The Danish vaccination register. Eurosurveillance 2012; 17 (17): 2. [DOI] [PubMed] [Google Scholar]
- 50. Morsy A, Lim TO, Varatharajan S, Lim JY. IEEE. National registries in developing countries: understanding construction challenges and implementing steps. In: 2010 5th Cairo International Biomedical Engineering Conference; December 16–18, 2010; Cairo, Egypt. New York: IEEE: 75–8.
- 51. Luong MX, Smith KP, Stein GS.. Human embryonic stem cell registries: value, challenges and opportunities. J Cell Biochem 2008; 105 (3): 625–32. [DOI] [PubMed] [Google Scholar]
- 52. Bentley SM, Melville JL, Berry BD, Katon WJ.. Implementing a clinical and research registry in obstetrics: overcoming the barriers. Gen Hosp Psychiatry 2007; 29 (3): 192–8. [DOI] [PubMed] [Google Scholar]
- 53. Helfrich CD, Savitz LA, Swiger KD, Weiner BJ.. Adoption and implementation of mandated diabetes registries by community health centers. Am J Prev Med 2007; 33 (1 Suppl): S50–65. [DOI] [PubMed] [Google Scholar]
- 54. Schwamm L, Reeves MJ, Frankel M.. Designing a sustainable national registry for stroke quality improvement. Am J Prevent Med 2006; 31 (6): S251–7. [DOI] [PubMed] [Google Scholar]
- 55. Sehgal A, Davies E.. Lessons from developing and running a clinical database for colorectal cancer. J Eval Clin Pract 2006; 12 (1): 94–101. [DOI] [PubMed] [Google Scholar]
- 56. Gabbay RA, Khan L, Peterson KL.. Critical features for a successful implementation of a diabetes registry. Diabetes Technol Ther 2005; 7 (6): 958–67. [DOI] [PubMed] [Google Scholar]
- 57. Cameron PA, Finch CF, Gabbe BJ, Collins LJ, Smith KL, McNeil JJ.. Developing Australia’s first statewide trauma registry: what are the lessons? ANZ J Surg 2004; 74 (6): 424–8. [DOI] [PubMed] [Google Scholar]
- 58. Roder C, El-Kerdi A, Grob D, Aebi M.. A European spine registry. Eur Spine J 2002; 11 (4): 303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.