Abstract
The location and environment of the acquired blaIMP gene, which encodes the IMP-1 metallo-β-lactamase, were investigated in a Japanese Pseudomonas aeruginosa clinical isolate (isolate 101/1477) that produced the enzyme. In this isolate, blaIMP was carried on a 36-kb plasmid, and similar to the identical alleles found in Serratia marcescens and Klebsiella pneumoniae clinical isolates, it was located on a mobile gene cassette inserted into an integron. The entire structure of this integron, named In31, was determined. In31 is a class 1 element belonging to the same group of defective transposon derivatives that originated from Tn402-like ancestors such as In0, In2, and In5. The general structure of In31 appeared to be most closely related to that of In5 from pSCH884, suggesting a recent common phylogeny for these two elements. In In31, the blaIMP cassette is the first of an array of five gene cassettes that also includes an aacA4 cassette and three original cassettes that have never been described in other integrons. The novel cassettes carry, respectively, (i) a new chloramphenicol acetyltransferase-encoding allele of the catB family, (ii) a qac allele encoding a new member of the small multidrug resistance family of proteins, and (iii) an open reading frame encoding a protein of unknown function. All the resistance genes carried on cassettes inserted in In31 were found to be functional in decreasing the in vitro susceptibilities of host strains to the corresponding antimicrobial agents.
Metallo-β-lactamases represent new and formidable challenges to antimicrobial chemotherapy owing to their usually broad substrate profiles, which invariably include carbapenems, and to their resistance to conventional β-lactamase inhibitors (40, 47).
Among the various metallo-β-lactamase-encoding genes thus far discovered, blaIMP appears to be the most threatening one, given its ability to spread rapidly among clinically relevant species (21, 22, 47, 51, 52, 60) and to the very broad substrate profile of its product, the IMP-1 enzyme (26, 32, 36). The blaIMP gene was initially discovered in imipenem-resistant Serratia marcescens and Pseudomonas aeruginosa clinical isolates in Japan (36, 60). Identical or very similar alleles have subsequently been identified in additional isolates of S. marcescens, P. aeruginosa, Klebsiella pneumoniae, Citrobacter freundii, Pseudomonas putida, Pseudomonas stutzeri, and Alcaligenes xylosoxidans (21, 22, 51, 52, 61). In all of them blaIMP, which is not endogenous to the respective species, has recently been acquired by horizontal gene transfer.
The genetic background of the acquired blaIMP alleles has been investigated in some isolates and appeared to be heterogeneous. In S. marcescens and P. aeruginosa, the gene was found either on the chromosome or on plasmids of various sizes, only some of which are apparently transferable by conjugation (22, 23, 36, 51, 60). In S. marcescens and P. aeruginosa, blaIMP alleles were found to be carried on mobile elements of the type of gene cassettes but were inserted into integrons of different classes (2, 23, 36, 48, 54). The occurrence of a similar variability was also suggested by the results of PCR mapping experiments performed with blaIMP-positive clinical isolates of various species (52). The blaIMP-carrying integrons found in S. marcescens and P. aeruginosa have been only partially characterized (2, 23, 36).
In this work we have cloned the blaIMP gene from an IMP-1-producing P. aeruginosa clinical isolate (isolate 101/1477) from Japan and analyzed its location and environment. In P. aeruginosa 101/1477, blaIMP was carried on a medium-sized plasmid, named pPAM-101, and was located on a mobile gene cassette inserted into a class 1 integron. Complete characterization of this integron, named In31, showed that it belongs to the group of integrons which are defective transposon derivatives originating from Tn402-like ancestors (5) and is most closely related to In5 from the evolutionary standpoint. In31 contains a unique array of five gene cassettes that, in addition to blaIMP, includes an aacA4 cassette and three original cassettes that have never been described in other integrons.
MATERIALS AND METHODS
Bacterial strains.
P. aeruginosa 101/1477, a clinical isolate from Japan, was kindly provided by David Livermore (Antibiotics Reference Unit, Central Public Health Laboratory, London, United Kingdom). Escherichia coli DH5α [supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1; Gibco BRL, Gaithersburg, Md.) was used as the host for recombinant plasmids.
In vitro susceptibility testing.
In vitro susceptibility to antimicrobial agents was assayed by a broth macrodilution technique with cation-supplemented Mueller-Hinton broth and a bacterial inoculum of approximately 5 × 105 CFU per tube, according to the guidelines of the National Committee for Clinical Laboratory Standards (34). Imipenem was obtained from Merck Sharp & Dohme (Rahway, N.J.); ceftazidime was from Glaxo-Wellcome (Verona, Italy); other antimicrobial agents, quaternary ammonium compounds, and ethidium bromide were from Sigma Chemical Co. (St. Louis, Mo.).
β-Lactamase assays.
Carbapenemase activity in the crude cell extracts was determined by monitoring the hydrolysis of 200 μM imipenem at 300 nm (Δɛ = −9,000 M−1 cm−1) in 50 mM HEPES buffer (HB; pH 7.5) at 25°C. One unit of carbapenemase activity hydrolyzes 1 μmol of substrate per min under these conditions. Crude cell extracts were prepared from early-stationary-phase bacterial cultures grown aerobically in Mueller-Hinton broth at 37°C. The cells were harvested by centrifugation, washed twice with HB, resuspended in HB, and disrupted by sonication (five times for 30 s each time at 60 W). Cell debris was removed by centrifugation at 10,000 × g for 15 min. The cleared supernatant represented the crude extract. Protein concentrations were determined by the method of Bradford (4) with bovine serum albumin as a standard. Susceptibility to EDTA was assayed by measuring the residual imipenem-hydrolyzing activity of the crude extract after incubation in the presence of 5 mM EDTA for 15 min at 25°C.
Genetic vectors.
Plasmids pBC-SK (Stratagene Corp., La Jolla, Calif.) and pK19 (44) were used as vectors for the subcloning of various restriction fragments of pPAM-101.
Recombinant DNA methodology.
The basic recombinant DNA methodology was carried out as described by Sambrook et al. (49). Genomic DNA was extracted from P. aeruginosa as described previously (14). Plasmid DNA was extracted from P. aeruginosa and E. coli with the Nucleobond kit for plasmid purification (Macherey-Nagel GmbH & Co., Düren, Germany). This procedure was found to be satisfactory not only for small plasmids but also for pPAM-101. Electroporation of pPAM-101 into E. coli was done in 0.2-cm cuvettes with a Bio-Rad Gene Pulser apparatus (Bio-Rad, Richmond, Calif.) set at 2.4 kV, 25 μF, and 800 Ω. Electrocompetent E. coli cells were prepared as recommended by the manufacturer. Southern blots were performed with nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). The blaIMP-specific probe used for the Southern blot experiments was a 0.5-kb HindIII restriction fragment internal to the gene (36), purified by agarose gel electrophoresis, and labeled with 32P by the random priming technique with a commercial kit (Boehringer, Mannheim, Germany).
PCR.
PCR amplification of the blaIMP gene was performed by 30 cycles of 94°C for 60 s, 58°C for 60 s, and 72°C for 90 s in a volume of 100 μl with 2.5 U of Taq DNA polymerase (Eurogentec, Liège, Belgium) and the reaction buffer provided by the Taq manufacturer, to which 1.5 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 100 μM, 50 pmol of each primer, and 10 ng of DNA template were added. PCR was performed in a Trio Thermoblock TB1 thermal cycler (Biometra, Göttingen, Germany).
DNA sequencing.
DNA sequences were determined by the dideoxy-chain termination method (50) on denatured double-stranded DNA templates with an ALF DNA sequencer (Pharmacia, Uppsala, Sweden) and fluorescein-labeled primers. The nucleotide sequences of both strands were always determined. The sequences of the cloned fragments were determined either by a random fragmentation strategy (13) or by gene walking with custom sequencing primers. Computer analysis of sequence data was performed with an updated version (version 8.0.1) of the University of Wisconsin Genetic Computer Group package (11). Comparison of experimentally determined nucleotide sequences and of their deduced protein products against sequence databases was performed with updated versions of the BLAST (1) and the FASTA (41) programs. Multiple sequence alignments were performed with the CLUSTAL (version W) program (57). Computer programs were run at the server of the Italian EMBNet node of Bari and at the Belgian EMBNet node of Brussels. Search for promoter sequences was performed with a computer program for promoter prediction (48a).
Nucleotide sequence accession number.
The nucleotide sequence of In31 and its flanking sequences, reported in this paper, will appear in the EMBL/GenBank/DDBJ sequence databases under accession no. AJ223604.
RESULTS
Characterization of the P. aeruginosa 101/1477 carbapenemase as the product of a blaIMP gene.
P. aeruginosa 101/1477 showed high-level resistance to various β-lactams including carbapenems (imipenem MIC, >128 μg/ml). A crude extract prepared from this isolate efficiently hydrolyzed imipenem (specific activity, 0.24 μmol/min/mg of protein), and the carbapenemase activity was inhibited in the presence of EDTA.
PCR performed with genomic DNA from P. aeruginosa 101/1477 as the template and a couple of primers corresponding to regions flanking the blaIMP gene of S. marcescens TN9106 (36) (BLAIMP-f, 5′-GCAGCAAGCGCGTTACGCCGTGGG, located in the 5′ conserved segment (5′-CS) of the integron, and BLAIMP-r, 5′-GTGGAATACTTTGCGACGAACCAC, located in the 59-base element of the blaIMP cassette) yielded the expected 0.93-kb amplimer. Direct sequencing showed that the amplimer contained a blaIMP allele identical to those sequenced previously (2, 23, 36, 61).
The blaIMP gene of P. aeruginosa 101/1477 is located on a 36-kb plasmid.
Since in P. aeruginosa blaIMP has previously been mapped on plasmids (23, 51), a plasmid-enriched preparation was obtained from P. aeruginosa 101/1477. The plasmid DNA present in this preparation was recognized by a blaIMP-specific probe in Southern blot experiments (Fig. 1).
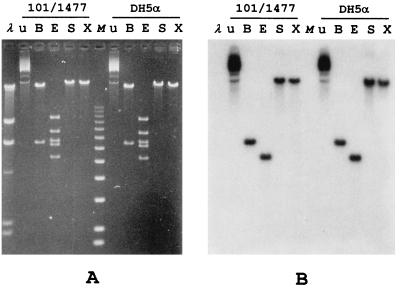
FIG. 1.
(A) Plasmid profiles of P. aeruginosa 101/1477 and of an E. coli DH5α ampicillin-resistant transformant obtained following electroporation of DH5α with the Pseudomonas plasmid preparation. The plasmid profiles were identical for seven additional randomly selected E. coli transformants. Lanes: λ, bacteriophage λ DNA HindIII molecular size markers (the sizes of visible bands, from top to bottom, are 23,130, 9,416, 6,557, 4,361, 2,322, and 2,027 bp); u, uncut; B, after digestion with BamHI; E, after digestion with EcoRI; S, after digestion with SmaI, X, after digestion with XbaI; M, molecular size markers X (Boehringer) (the sizes of visible bands, from top to bottom, are 12,216, 11,198, 10,180, 9,162, 8,144, 7,126, 6,108, 5,090, 4,072, 3,054, 2,036, and 1,636 bp). (B) Results of a Southern blot analysis performed on the same gel with the blaIMP-specific probe.
This plasmid-enriched preparation was used to transfect E. coli DH5α by electroporation, resulting in several ampicillin-resistant transformants. Analysis of eight randomly selected transformants showed that all of them contained a plasmid apparently identical to that harbored by P. aeruginosa 101/1477 and that the plasmids from all of them were similarly recognized by the blaIMP-specific probe in Southern blot experiments (Fig. 1) (data not shown). The plasmid was named pPAM-101, and its size was estimated to be approximately 36 kb by means of restriction analysis and agarose gel electrophoresis (Fig. 1). Unlike the parental strain, DH5α(pPAM-101) was able to produce carbapenemase activity (specific activities of the crude extracts toward imipenem, 0.034 and <0.002 μmol/min/mg of protein for the transformant and the parent, respectively).
The blaIMP gene of pPAM-101 is located in a mobile gene cassette inserted into a class 1 integron.
The blaIMP gene was mapped within a 5.2-kb EcoRI restriction fragment of pPAM-101 by means of Southern blot analysis (Fig. 1). This fragment was subcloned into the pBC-SK plasmid vector, to yield recombinant plasmid pBCAM-52E (Fig. 2), and was sequenced.
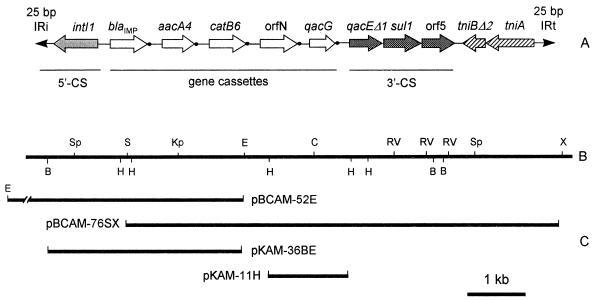
FIG. 2.
(A) Schematic representation of the structure of In31. ORFs are indicated by arrows. •, the 59-base elements of the gene cassettes. (B) Restriction map of the corresponding region. (C) Subclones used for sequencing and functional analysis of resistance genes (see text and Table 1 for further descriptions of subclones). B, BamHI; Sp, SphI; S, SmaI; Kp, KpnI; E, EcoRI; H, HindIII; C, ClaIRV, EcoRV; X, XbaI.
The blaIMP gene cloned from pPAM-101 is identical to those sequenced previously (2, 23, 36, 61). Also in this case, blaIMP appeared to be part of a mobile gene cassette inserted into an integron-like structure. The blaIMP cassette is located immediately downstream of the 5′-CS of a class 1 integron and is followed by an aacA4 cassette (7, 9) and by a new catB cassette (Fig. 2 and 3; see below for integron and cassette descriptions).
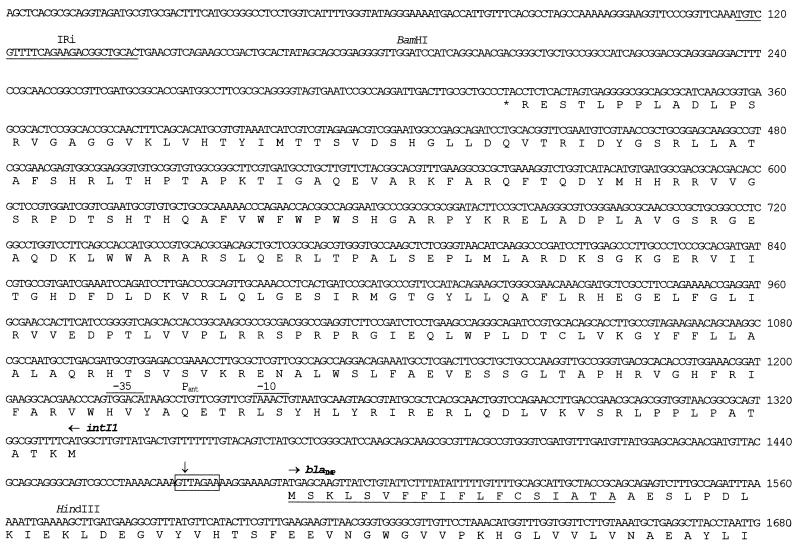
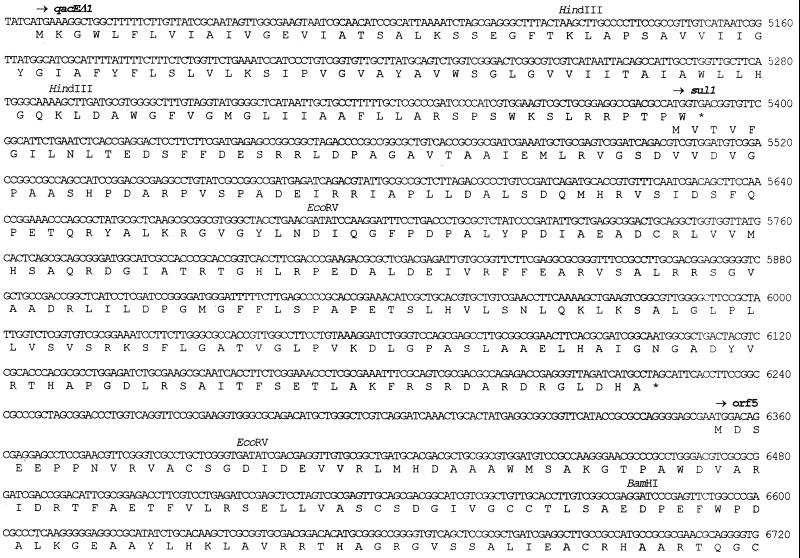
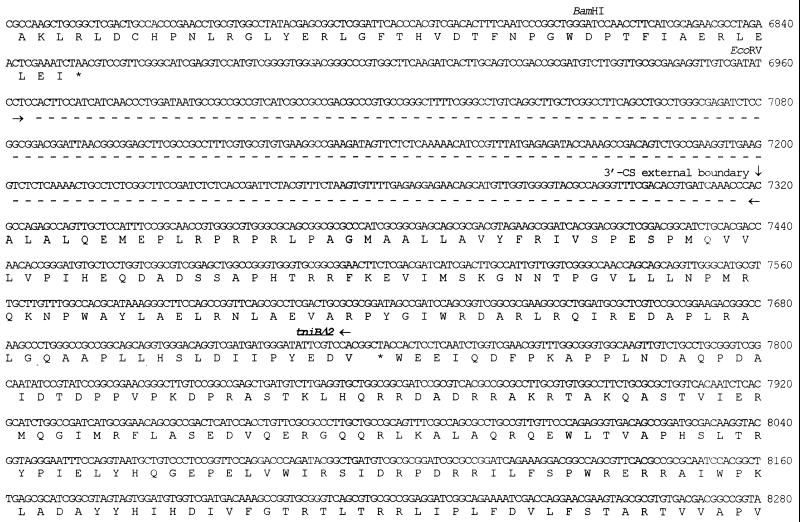
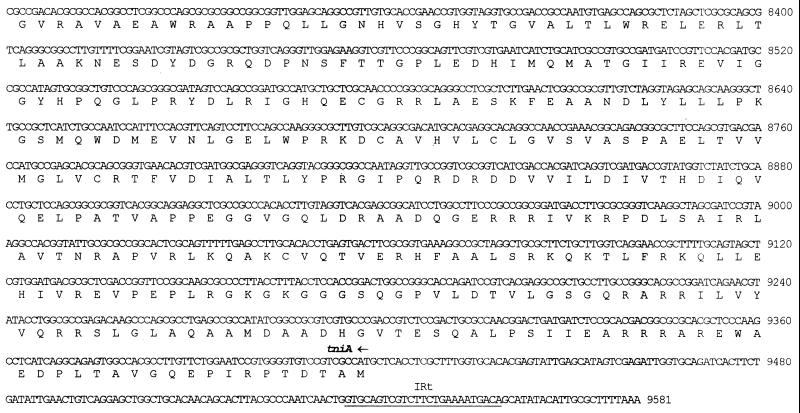
FIG. 3.
Nucleotide sequence of In31 and flanking sequences. The nucleotide number 1 corresponds to the first nucleotide of the AluI site upstream of In31. The 25-bp IRi and IRt sequences located at the integron boundaries are underlined. The start codons of the various ORFs are indicated by horizontal arrows, and the corresponding protein translation is reported below the nucleotide sequence. The signal peptide for secretion of the IMP-1 protein is underlined. The −35 and −10 hexamers of the Pant promoter (10, 54) and of the putative promoter located in the untranslated leader of the qacG cassette are overlined. The conserved 7-bp core sites located at the cassette boundaries and the 7-bp inverse core sites located at the left end of each 59-base element (8, 19) are boxed. The cassette boundaries are indicated by vertical arrows. The internal 2L and 2R core sites (55) of each 59-base element are underlined with arrows, and the conserved A and T residues of 2L and 2R, respectively, are represented in boldface type. The internal and external boundaries of the 3′-CS of the integron are indicated by vertical arrows. The sequence spanning from nucleotide 6962 to nucleotide 7320, underlined with a dashed line bounded by convergent arrows, corresponds to the 359-bp region found in the 3′-CS of In5 but not in In0 and In2 (20), except for the last 2 bp that are missing from In31. The differences observed between the sequence of the 59-base element of the blaIMP cassette inserted in In31 and those of previously sequenced blaIMP cassettes (2, 36) are indicated above (for comparison with data from reference 36) or below (for comparison with data from reference 2) the sequence. ▵, a deletion from that position; ⊥, an insertion at that position. It should be noted that sequence data from reference 36 are available for comparison only until nucleotide 2327.
Structure of the integron carried by pPAM-101.
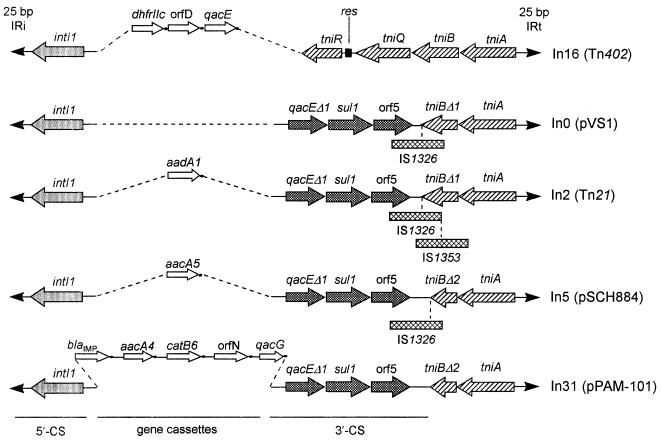
To further analyze the structure of this integron, a 7.6-kb SmaI-XbaI restriction fragment of pPAM-101 that partially overlaps the 5.2-kb EcoRI fragment (Fig. 2) was subcloned into the pBC-SK vector, to yield recombinant plasmid pBCAM-76SX, and was sequenced. This completed the structural analysis of the integron, which appeared to contain two additional gene cassettes followed by a 3′ conserved segment (3′-CS). The 3′-CS is flanked by an incomplete set of tni genes (Fig. 2 and 3) similar to that found in other integrons, such as In0, In2, and In5, which are recognized as defective derivatives of Tn402-like transposable elements (5). The 9,443-bp integron carried by pPAM-101 is different from other known integrons and was named In31.
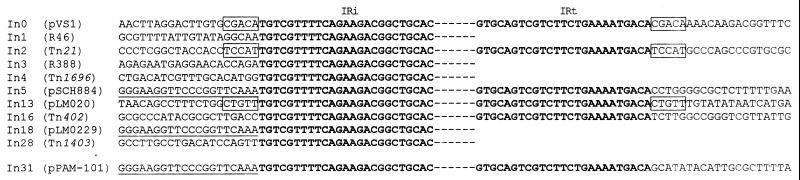
In31 is bounded by two 25-bp inverted repeats (IRs) identical to IRi and IRt sequences identified at the boundaries of In0 from pVS1, In2 from Tn21, In5 from pSCH884, In13 from pLM020, and In16 from Tn402 (also named Tn5090) (Fig. 4). The nucleotide sequence flanking IRi in In31 is identical to that flanking IRi of In5 and of In18, a Tn402-like integron-containing element carried by the E. coli plasmid pLMO229, but different from that flanking IRi of other integrons. The nucleotide sequence flanking IRt of In31 is different from that flanking IRt of other integrons including In5. Unlike In0, In2, and In13, In31 is not flanked by a direct 5-bp duplication (Fig. 4).
FIG. 4.
Comparison of the IRs (boldfaced) and flanks of In31 with those of other integrons of the same family. Identical flanking sequences are underlined. The 5-bp duplications flanking the IRs of In0, In2, and In13 are boxed. References for the various sequences are as follows: In0, In1, In2, In3, In4, and In5, reference 20 and references therein; In13, In16, and In18, reference 46; and In28, reference 59. The integron names are as reported in references 3, 5, and 20.
The 5′-CS of In31 contains an intI1 allele (Fig. 2 and 3) and is identical to that of In1 from R46 (18). In In31 the Pant promoter (10, 54) contains a TGGACA(−35) hexamer and a TAAACT(−10) hexamer spaced by 17 bp (Fig. 3). This hybrid configuration, which is identical to that found in In1 (18) and in the partially characterized blaIMP-containing integron carried by K. pneumoniae plasmid RDK4 (61), is different from that of most other integrons, in which the configurations TGGACA(−35) and TAAGCT(−10) (with weak promoter activity) or TTGACA(−35) and TAAACT(−10) (with strong promoter activity) are found (10), and has been shown to have an intermediate strength (28).
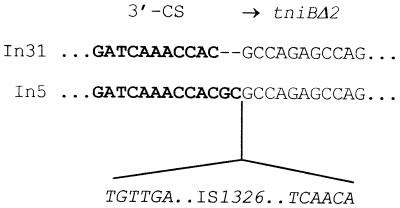
The 3′-CS of In31 contains the series of qacEΔ1, sul1, and orf5 genes (Fig. 2 and 3) typical of sul1-associated integrons (20, 54). Beyond the EcoRV site located downstream of orf5, In31 contains the same sequence found in the 3′-CS of In5 (20) except for a deletion of the last 2 bp. In In31 the 3′-CS merges directly into a truncated tniB allele identical to that (tniBΔ2) found in In5 (5) (Fig. 3 and 5). The length of the 3′-CS of In31 is 2,384 bp, being 2 bp shorter than that of In5, which contains the longest known 3′-CS region (5). The sul1 gene present in the 3′-CS of In31 is functional, since E. coli DH5α(pPAM-101) and E. coli DH5α(pBCAM-76SX) exhibited decreased susceptibilities to sulfonamides (Table 1).
FIG. 5.
Comparison of the boundaries between the 3′-CS and tni regions of In31 and In5. The 3′-CS sequences are represented in boldface type. In In31 the 3′-CS merges directly with the truncated tniBΔ2 gene, while in In5 IS1326 is inserted between the 3′-CS boundary and tniBΔ2. A 2-bp deletion is also present at the 3′-CS boundary of In31 compared with that of In5 (5).
TABLE 1.
In vitro susceptibilities to various antimicrobial agents of E. coli DH5α carrying pPAM-101 or subclones containing some of the resistance determinants of In31a
| Agent | MIC (μg/ml) for strain:
|
|||||
|---|---|---|---|---|---|---|
| DH5α | DH5α(pPAM-101) | DH5α(pBCAM-52R)b | DH5α(pKAM-36BE)c | DH5α(pKAM-11H)d | DH5α(pBCAM-76SX)e | |
| Imipenem | <0.25f | 2 | 4 | —g | — | — |
| Ceftazidime | <0.25f | >100 | >100 | — | — | — |
| Streptomycin | 4f | 4 | 4 | — | — | — |
| Kanamycin | <0.5f | 8 | 8 | — | — | — |
| Gentamycin | 0.5f | 0.5 | 0.5 | — | — | — |
| Tobramycin | <0.5f | 8 | 8 | — | — | — |
| Amikacin | 1f | 2 | 4 | — | — | — |
| Netilmicin | <0.5f | 4 | 4 | — | — | — |
| Chloramphenicol | 4h | 8 | — | 64 | — | — |
| Cetylpyridinium chloride | 10h | — | — | — | 25 | — |
| Benzalkonium chloride | 15h | — | — | — | 20 | — |
| Ethidium bromide | 75h | — | — | — | 200 | — |
| Sulfonamide | 16f | >1,000 | — | — | — | >1,000 |
The susceptibility of DH5α alone or DH5α carrying the corresponding empty vectors is also shown for comparison.
pBCAM-52R is a pBC-SK derivative that contains the 5.2-kb EcoRI fragment of pPAM-101 that spans the 5′-CS of In31 together with the blaIMP, aacA4, and catB6 cassettes (Fig. 2). A 1.6-kb region of pPAM-101 flanking the 5′-CS of In31 is also present in this fragment, but no additional resistance genes are contained within this region (27). In this clone the insert orientation is such that the polarity of the gene cassettes is opposite that of the lac promoter flanking the vector polylinker.
pKAM-36BE is a pK19 derivative that contains a 3.6-kb BamHI-EcoRI fragment spanning most of the 5′-CS of In31 together with the blaIMP, aacA4, and catB6 cassettes (Fig. 2).
pKAM-11H is a pK19 derivative that contains a 1.1-kb HindIII fragment that spans the entire qacG cassette (Fig. 2). The MICs of quaternary ammonium compounds and ethidium bromide were the same for strains with both orientations of the insert.
pBCAM-76SX is a pBC-SK derivative that contains the 7.6-kb SmaI-XbaI fragment of pPAM-101 that spans the 3′-CS of In31 together with part of the cassette array and the incomplete tni module (Fig. 2).
The MIC for DH5α(pBC-SK) was identical.
—, not assayed.
The MIC for DH5α(pK19) was identical.
In In31 the truncated tniBΔ2 allele is preceded by a complete tniA allele, which is in turn preceded by IRt (Fig. 2 and 3). The sequence of the incomplete tni module of In31 is virtually identical to that of the corresponding region of tni modules carried by other elements of this family (5, 46).
The gene cassettes of In31.
Five gene cassettes, identified on the basis of the presence of structural motifs typical of these elements (7–9, 17, 19, 48, 55), are inserted in tandem between the 5′-CS and the 3′-CS of In31 (Fig. 2 and 3).
The blaIMP is the first cassette located downstream of the 5′-CS. It is 878 bp long and is nearly identical (>99% sequence identity) to those previously cloned from S. marcescens TN9106 and AK9373 (2, 36), the only differences being located within the 59-base element (Fig. 3). The function of the blaIMP allele was confirmed by the production of carbapenemase activity by DH5α(pPAM-101) (see above) and DH5α(pBCAM-52R) (specific activity of the crude extract of this strain toward imipenem, 0.04 μmol/min/mg of protein) and by the decreased susceptibilities to β-lactams exhibited by the same strains (Table 1).
The second gene cassette is 639 bp long and contains an aacA4 allele encoding an AAC(6′)-Ib aminoglycoside acetyltransferase (53). This cassette is virtually identical to those previously found in other integrons (12, 30, 33, 35, 43). In the aacA4 cassette carried by In31, translation could start either at the GTG codon located 24-bp downstream the 5′ end or at one of the ATG codons located farther downstream (Fig. 3), as reported for aacA4 cassettes inserted in other integrons (12, 30, 35, 43). The function of the aacA4 allele was confirmed by the decreased susceptibilities of DH5α(pPAM-101) and DH5α(pBCAM-52R) to several aminoglycosides, including kanamycin, tobramycin, netilmicin, and amikacin but not streptomycin or gentamicin (Table 1). The apparent activity against amikacin but not gentamicin was consistent with the production of an AAC(6′)-Ib enzyme (53).
The third gene cassette is 730 bp long and contains an open reading frame (ORF) potentially encoding a protein that exhibits a high degree of sequence similarity to members of the CATB lineage of chloramphenicol acetyltransferases (6, 37). The similarity with the other CATB proteins ranges from 64.6 to 89.1% identical residues, with the strongest similarity being that with the cassette-encoded CATB5 (89.1%) and CATB3 (85.2%) proteins (Fig. 6). The new catB allele appeared to be functional since both DH5α(pPAM-101) and DH5α(pKAM-36BE) showed a decreased chloramphenicol susceptibility (Table 1) and was named catB6. The catB6 cassette was identified by the recognition of features typical of these elements (7–9, 19, 55): (i) the presence at the cassette boundaries of 7-bp core site sequences that fit the consensus sequence and (ii) the presence of a 59-base element downstream of the catB6 ORF with putative IntI1-binding domains at the left and right ends of the 59-base element (Fig. 3). The 59-base element of the catB6 cassette is 77 bp long and is different from those of the other catB cassettes (6, 25, 37, 48).
FIG. 6.
Comparison of the deduced amino acid sequence of the product of the catB6 allele (CATB6) with those of other CATB proteins and homologues. CATB5, CATB5 protein encoded by a gene cassette from the Morganella morganii transposon Tn840 (25); CATB4Δ1, truncated CATB4 protein encoded by a truncated gene cassette from Serratia sp. strain 45 isolate (6, 58); CATB3, CATB3 protein encoded by a gene cassette from the Enterobacter plasmid pBWH301 (6); CATB2, CATB2 protein encoded by a gene cassette from the E. coli transposon Tn2424 (37); CATB1, CATB1 protein from Agrobacterium tumefaciens (56); Ps orf, hypothetical protein from a P. aeruginosa PAO1 ORF (37). Identical residues are indicated by asterisks; conserved amino acid substitutions are indicated by dots.
The fourth gene cassette is 689 bp long and contains a 615-bp ORF that is preceded by a recognizable ribosome-binding site and that potentially encodes a 22.9-kDa protein (Fig. 3). No significant similarity between this hypothetical protein and any other known sequenced protein was detected in a search performed with the BLAST program. The ORF carried by this cassette was named orfN. The orfN cassette and its 59-base element, which are 74 bp long, were identified according to the same criteria described for the catB6 cassette (Fig. 3).
The fifth gene cassette is 532 bp long and contains an ORF potentially encoding a protein that exhibits significant sequence similarity to members of the small multidrug resistance family of efflux proteins (38) (Fig. 7). The strongest similarities were observed with the cassette-encoded QacF (78.2% of identical residues) and QacE (76.4% of identical residues) proteins and with the QacEΔ1 derivative carried by the 3′-CS of sul1-associated integrons, including In31 itself (Fig. 7). This new qac allele was apparently functional since DH5α(pKAM-11H) exhibited decreased susceptibility to quaternary ammonium compounds and ethidium bromide (Table 1) and was named qacG. The qacG cassette and its 59-base element, which is 94 bp long, were identified according to the same criteria described for the catB6 cassette (Fig. 3). It should be noted that qacG, defined on the basis of the homology of its potential product with other proteins of known function, begins with a rather unusual start codon (TTG) and appears to be preceded by a long (103-bp) leader. However, this putative start codon is preceded by a recognizable ribosome binding sequence, while in-frame ATG or GTG codons are not present in the upstream region. A search of the cassette leader with a computer program for promoter prediction indicated the likely presence of promoter sequences within this region (Fig. 3).
FIG. 7.
Comparison of the deduced amino acid sequence of the qacG product (QacG) with that of other proteins of the SMR family (38). QacF, QacF protein encoded by a gene cassette from In40 of Enterobacter aerogenes BM2688 (42); QacE, QacE protein encoded by a gene cassette from In16 (39); QacEΔ1, QacE derivative encoded by the truncated qacEΔ1 allele found in the 3′-CS of several sul1-associated integrons (39, 54); EmrE, EmrE ethidium efflux protein from E. coli (45); QacC, QacC protein from Staphylococcus aureus (29). Identical residues are indicated by asterisks; conserved amino acid substitutions are indicated by dots.
A comparison of the codon usage among the various coding sequences carried by In31 is reported in Table 2.
TABLE 2.
Correspondence analysis of codon usage of the coding sequences carried by In31
|
D2 valuea
|
Element | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| blaIMP | aacA4 | catB6 | orfN | qacG | qacEΔ1 | sul1 | orf5 | tniBΔ2 | tniA | |
| 7.75 | 5.22 | 5.07 | 7.26 | 7.84 | 7.96 | 1.89 | 4.01 | 3.70 | 0.79 | intI1 |
| 3.73 | 3.18 | 2.77 | 4.75 | 5.16 | 7.36 | 8.31 | 10.69 | 8.53 | blaIMP | |
| 2.06 | 4.18 | 4.75 | 6.67 | 4.45 | 4.56 | 7.01 | 5.21 | aacA4 | ||
| 2.67 | 3.40 | 6.40 | 4.05 | 4.46 | 7.27 | 4.83 | catB6 | |||
| 3.83 | 5.14 | 6.25 | 8.19 | 9.80 | 7.64 | orfN | ||||
| 6.02 | 7.08 | 8.80 | 11.57 | 7.96 | qacG | |||||
| 8.20 | 11.37 | 12.07 | 9.36 | qacEΔ1 | ||||||
| 4.11 | 3.00 | 1.25 | sul1 | |||||||
| 3.03 | 3.95 | orf5 | ||||||||
| 2.91 | tniBΔ2 | |||||||||
D2 values of <3.0 (in bold) indicate the possibility of a common origin (15).
DISCUSSION
Investigation of the genetic bases for the production of the IMP-1 metallo-β-lactamase in P. aeruginosa 101/1477 showed that in this isolate (i) the blaIMP gene is identical to those previously cloned from other S. marcescens, K. pneumoniae, and P. aeruginosa isolates (2, 23, 36, 61) and, similarly to them, is located on a mobile gene cassette inserted into an integron; (ii) the blaIMP-containing integron is carried on a medium-sized plasmid (named pPAM-101), similar to the case for P. aeruginosa GN17203 (23) but unlike the situation for S. marcescens TN9106 (in which the integron is on the chromosome) (36) or S. marcescens AK9373 (in which the integron is on a large conjugative plasmid) (2); and (iii) the blaIMP-containing integron (named In31) belongs to class 1, being different from the element partially characterized from S. marcescens AK9373, which is a class 3 element (2, 48), and also from the element partially characterized from P. aeruginosa GN17203, which has a different cassette content (23). These findings confirm the ability of the blaIMP gene to spread among clinically relevant species and highlight the considerable heterogeneity of the genetic environment in which blaIMP alleles can be found in different clinical isolates. A similar condition likely reflects the intervention of various mechanisms, such as conjugational plasmid transfer and cassette excision or integration, in the dissemination of the blaIMP gene among different hosts and different replicons.
Although the location of blaIMP in integron-borne cassettes has been reported previously (2, 23), the structures of the respective integrons have been only partially characterized. In31 of pPAM-101 from P. aeruginosa 101/1477 represents the first blaIMP-containing integron for which the entire structure has been determined. According to the structures of the 5′-CS and the 3′-CS and of the region located downstream of the 3′-CS, which are the landmarks for integron classification (5, 20, 48), In31 appears to belong to the lineage of defective transposon derivatives of the Tn402 family that also includes In0, In2, and In5 (5). In fact, the 5′-CS of In31 is virtually identical to those of In0, In2, In5, In16, and other class 1 integrons that have been partially characterized (20, 46), while the 3′-CS of In31 and the downstream region bounded by IRt have several features in common (the presence of the qacEΔ1-sul1-orf5 gene block and the presence of a truncated tni module) with those found in In0, In2, and In5 (5), although In31 lacks any insertion sequence at the junction between the 3′-CS and the truncated tni module (Fig. 8). Among the elements of this lineage, In31 appears to be the most closely related to In5 on the basis of the similarities of their 3′-CSs and tni modules. In fact, the 3′-CS of In31 retains the same 359-bp sequence (except for the last 2 bp) found in the 3′-CS of In5 but not in those of In0 and In2 (20), while the pattern of truncation of the tni module in In31 is identical to that of the tni module found in In5 (5) (Fig. 3, 5, and 8). Considering the model proposed by Brown et al. (5) for the evolutionary history of this group of integrons, In31 could be derived from the same ancestor as In5 (indicated as InY by Brown et al. [5]) following the excision of IS1326 and the acquisition of its array of gene cassettes. The 2 bp missing from the 3′-CS of In31, compared to the sequence of the 3′-CS of In5, could have been generated following excision of IS1326 with imprecise rejoining and repair of the ends that were generated, as previously hypothesized for the evolution of Tn2608 and its derivatives (5). However, an alternative evolutionary model in which cassettes may have been added directly to an ancestor which never saw IS1326 cannot be ruled out unless and until old culture collections are thoroughly screened with sul1- and IS1326-specific probes. The existence of a close evolutionary relationship between In5 and In31 is supported by the sequence identity of their IRi-flanking regions (Fig. 4). On the other hand, the sequence divergence of their IRt-flanking regions indicates that one or the other is a recombinant or cointegrate which could have originated via homologous recombination between two integrons, following an IntI1-mediated cointegration (31), or following a cointegration mediated by Tni functions (provided in trans) and not resolved owing to the lack of a res site (24).
FIG. 8.
Schematic comparison of the structure of In31 with those of In0, In2, In5, and In16 (5, 46).
The cassette content of In31 is different from the cassette contents of other integrons (3, 42, 48). In addition to blaIMP, which likely represents the most recently acquired cassette, as suggested by its leading position, In31 contains four additional gene cassettes identified on the basis of structural features typical of these mobile elements. Although an integron with five cassettes is known (6), this number exceeds that found in most other integrons (3, 42, 48). Of the five gene cassettes carried by In31, four carried known or putative determinants of resistance to various antimicrobial agents; the functions of these four cassettes were confirmed by the decreased in vitro susceptibility to the corresponding agents exhibited by E. coli DH5α carrying the respective determinants. Three of the In31-borne cassettes are original, being different from any other element of this type described previously. One of them contains a new chloramphenicol acetyltransferase-encoding allele of the catB family, and this allele is most closely related to other cassette-borne catB alleles (6). The higher level of chloramphenicol resistance exhibited by DH5α(pKAM36-BE) compared to that exhibited by DH5α(pPAM-101) (Table 1) is likely due to the fact that, in the former plasmid, catB6 transcription could be enhanced by the lac promoter flanking the cloned In31 fragment. In its original background, therefore, the catB6 cassette is apparently able to confer only a low-level resistance to chloramphenicol, possibly due to its downstream position within the cassette array (10). Another of the original cassettes of In31 carries an ORF encoding a protein of unknown function. Although most of the gene cassettes thus far discovered carry antibiotic resistance genes, there are a few other examples of cassettes that carry genes that are not involved in antimicrobial resistance or whose function remains unknown (48). The orfN product falls in the latter category. The last of the original cassettes carried by In31 contains a new qac allele, named qacG, that is most closely related to qacF found in In40 (42) and to qacE found in In16 (39), and that encodes an exporter protein of the small multidrug resistance family (38) that mediates resistance to quaternary ammonium compounds and ethidium bromide. Similar to the qacE (48) and qacF (42) cassettes, the qacG cassette also contains an unusually long untranslated leader. In the qacE cassette the long leader has been shown to contain promoter sequences (16), and promoter sequences were also putatively identified in the long leader of the qacG cassette (Fig. 3). The fact that the MICs of the quaternary ammonium compounds and ethidium bromide for DH5α(pKAM-11H) were the same for both orientations of the insert in pKAM-11H (Table 1) is consistent with this hypothesis.
A comparison of the codon usage among the various genes carried by In31 (Table 2) showed similarities in the pattern of codon usage among blaIMP, catB6, and orfN, suggesting the possibility of a common origin for these genes.
ACKNOWLEDGMENTS
This work was supported by the European research network on metallo-β-lactamases within the TMR program (contract ERB FMRCX-CT98-0232), by a grant from the Belgian Government in the frame of “Poles d’Attraction Interuniversitaire” (program PAI P4/03), and by a grant (grant 98.00510.CT04) from the Italian National Research Council.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Arakawa Y, Murakami M, Suzuki K, Ito H, Wacharotayankun R, Ohsuka S, Kato N, Ohta M. A novel integron-like element carrying the metallo-β-lactamase gene blaIMP. Antimicrob Agents Chemother. 1995;39:1612–1615. doi: 10.1128/aac.39.7.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bissonnette L, Roy P H. Characterization of In0 of Pseudomonas aeruginosa plasmid pVS1, an ancestor of integrons of multiresistance plasmids and transposons of gram-negative bacteria. J Bacteriol. 1992;174:1248–1257. doi: 10.1128/jb.174.4.1248-1257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Brown H J, Stokes H W, Hall R M. The integrons In0, In2, and In5 are defective transposon derivatives. J Bacteriol. 1996;178:4429–4437. doi: 10.1128/jb.178.15.4429-4437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunny K L, Hall R M, Stokes H W. New mobile gene cassettes containing an aminoglycoside resistance gene, aacA7, and a chloramphenicol resistance gene, catB3, in an integron in pBWH301. Antimicrob Agents Chemother. 1995;39:686–693. doi: 10.1128/AAC.39.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collis C M, Hall R M. Site-specific deletion and rearrangement of integron insert genes catalyzed by the integron DNA integrase. J Bacteriol. 1992;174:1574–1585. doi: 10.1128/jb.174.5.1574-1585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collis C M, Hall R M. Gene cassettes from the insert region of integrons are excised as covalently closed circles. Mol Microbiol. 1992;6:2875–2885. doi: 10.1111/j.1365-2958.1992.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 9.Collis C M, Grammaticopoulos G, Briton J, Stokes H W, Hall R M. Site-specific insertion of gene cassettes into integrons. Mol Microbiol. 1993;9:41–52. doi: 10.1111/j.1365-2958.1993.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 10.Collis C M, Hall R M. Expression of antibiotic resistance genes in the integrated cassettes of integrons. Antimicrob Agents Chemother. 1995;39:155–162. doi: 10.1128/aac.39.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galimand M, Lambert T, Gerbaud G, Couvalin P. Characterization of the aac(6′)-Ib gene encoding an aminoglycoside 6′-N-acetyltransferase in Pseudomonas aeruginosa BM2656. Antimicrob Agents Chemother. 1993;37:1456–1462. doi: 10.1128/aac.37.7.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glaser P, Kunst F, Arnaud M, Coudart M P, Gonzales W, Hullo M F, Ionescu M, Lubochinsky B, Marcelino L, Moszer I, Presecan E, Santana M, Schneider E, Schweizer J, Vertes A, Rapoport G, Danchin A. Bacillus subtilis genome project: cloning and sequencing of the 97 kb region from 325 degrees to 333 degrees. Mol Microbiol. 1993;10:371–384. [PubMed] [Google Scholar]
- 14.Goldberg J B, Ohman D E. Cloning and expression in Pseudomonas aeruginosa of a gene involved in the production of alginate. J Bacteriol. 1984;158:1115–1121. doi: 10.1128/jb.158.3.1115-1121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grantham R, Gautier C, Gouy M, Jacobzone M, Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981;9:43–74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerineau F, Brooks L, Mullineaux P. Expression of sulphonamide resistance gene from plasmid R46. Plasmid. 1990;23:35–41. doi: 10.1016/0147-619x(90)90042-b. [DOI] [PubMed] [Google Scholar]
- 17.Hall R M, Collis C M. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol Microbiol. 1995;15:593–600. doi: 10.1111/j.1365-2958.1995.tb02368.x. [DOI] [PubMed] [Google Scholar]
- 18.Hall R M, Vockler C. The region of the IncN plasmid R46 coding for resistance to β-lactam antibiotics, streptomycin/spectinomycin and sulphonamides is closely related to antibiotic resistance segments found in IncW plasmids and in Tn21-like transposons. Nucleic Acids Res. 1987;15:7491–7501. doi: 10.1093/nar/15.18.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall R M, Brookes D E, Stokes H W. Site-specific insertion of genes into integrons: role of the 59-base element and determination of the recombination cross-over point. Mol Microbiol. 1991;5:1941–1959. doi: 10.1111/j.1365-2958.1991.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 20.Hall R M, Brown H J, Brookes D E, Stokes H W. Integrons found in different locations have identical 5′ ends but variable 3′ ends. J Bacteriol. 1994;176:6286–6294. doi: 10.1128/jb.176.20.6286-6294.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirakata Y, Izumikawa K, Yamaguchi T, Takemura H, Tanaka H, Yoshida R, Matsuda J, Nakano M, Tomono K, Maesaki S, Kaku M, Yamada Y, Kamihira S, Kohno S. Rapid detection and evaluation of clinical characteristics of emerging multiple-drug-resistant gram-negative rods carrying the metallo-β-lactamase gene blaIMP. Antimicrob Agents Chemother. 1998;42:2006–2011. doi: 10.1128/aac.42.8.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito H, Arakawa Y, Ohsuka S, Wacharotayankun R, Kato N, Ohta M. Plasmid-mediated dissemination of the metallo-β-lactamase gene blaIMP among clinically isolated strains of Serratia marcescens. Antimicrob Agents Chemother. 1995;39:824–829. doi: 10.1128/aac.39.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iyobe S, Yamada H, Minami S. Insertion of a carbapenemase gene cassette into an integron of a Pseudomonas aeruginosa plasmid. J Antimicrob Chemother. 1996;38:1114–1115. doi: 10.1093/jac/38.6.1114. [DOI] [PubMed] [Google Scholar]
- 24.Kholodii G Y, Mindlin S Z, Bass I A, Yurieva O V, Minakhina S V, Nikiforov V G. Four genes, two ends, and a res region are involved in transposition on Tn5053: a paradigm for a novel family of transposons carrying either a mer operon or an integron. Mol Microbiol. 1995;17:1189–1200. doi: 10.1111/j.1365-2958.1995.mmi_17061189.x. [DOI] [PubMed] [Google Scholar]
- 25.Kupzig, S., and P. M. Bennett. 1994. EMBL/GenBank database entry X82455.
- 26.Laraki N, Franceschini N, Rossolini G M, Santucci P, Meunier C, de Pauw E, Amicosante G, Frére J-M, Galleni M. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob Agents Chemother. 1999;43:813–817. doi: 10.1128/aac.43.4.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laraki, N., M. Galleni, G. M. Rossolini, and J.-M. Frére. Unpublished results.
- 28.Lévesque C, Brassard S, Lapointe J, Roy P H. Diversity and relative strength of tandem promoters for the antibiotic resistance genes of several integrons. Gene. 1994;142:49–54. doi: 10.1016/0378-1119(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 29.Littlejohn T G, DiBerardino D, Messerotti L J, Spiers S J, Skurray R A. Structure and evolution of a family of genes encoding antiseptic and disinfectant resistance in Staphylococcus aureus. Gene. 1991;101:59–66. doi: 10.1016/0378-1119(91)90224-y. [DOI] [PubMed] [Google Scholar]
- 30.Mabilat C, Lourençao-Vital J, Goussard S, Courvalin P. A new example of physical linkage between Tn1 and Tn21: the antibiotic multiple-resistance region of plasmid pCFF04 encoding extended-spectrum β-lactamase TEM-3. Mol Gen Genet. 1992;235:113–121. doi: 10.1007/BF00286188. [DOI] [PubMed] [Google Scholar]
- 31.Martinez E, de la Cruz F. Genetic elements involved in Tn21 site-specific integration, a novel mechanism for the dissemination of antibiotic resistance genes. EMBO J. 1990;9:1275–1281. doi: 10.1002/j.1460-2075.1990.tb08236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marumo K, Takeda A, Nakamura Y, Nakaya K. Purification and characterization of metallo-β-lactamase from Serratia marcescens. Microbiol Immunol. 1995;39:27–33. doi: 10.1111/j.1348-0421.1995.tb02164.x. [DOI] [PubMed] [Google Scholar]
- 33.Mugnier P, Podglajen I, Goldstein F W, Collatz E. Carbapenems as inhibitors of OXA-13, a novel, integron-encoded β-lactamase in Pseudomonas aeruginosa. Microbiology. 1998;144:1021–1031. doi: 10.1099/00221287-144-4-1021. [DOI] [PubMed] [Google Scholar]
- 34.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard. NCCLS document M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 35.Nobuta K, Tolmasky M E, Crosa L M, Crosa J H. Sequencing and expression of the 6′-N-acetyltransferase gene of transposon Tn1331 from Klebsiella pneumoniae. J Bacteriol. 1988;170:3769–3773. doi: 10.1128/jb.170.8.3769-3773.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osano E, Arakawa Y, Wacharotayankun R, Ohta M, Horii T, Ito H, Yoshimura F, Kato N. Molecular characterization of an enterobacterial metallo-β-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob Agents Chemother. 1994;38:71–78. doi: 10.1128/aac.38.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parent R, Roy P H. The chloramphenicol acetyl-transferase gene of Tn2424: a new breed of cat. J Bacteriol. 1992;174:2891–2897. doi: 10.1128/jb.174.9.2891-2897.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulsen I T, Skurray R A, Tam R, Saier M H, Jr, Turner R J, Weiner J H, Goldberg E B, Grinius L L. The SMR family: a novel family of multidrug efflux proteins involved with the efflux of lipophilic drugs. Mol Microbiol. 1996;19:1167–1175. doi: 10.1111/j.1365-2958.1996.tb02462.x. [DOI] [PubMed] [Google Scholar]
- 39.Paulsen I T, Littlejohn T G, Rådström P, Sundström L, Sköld O, Swedberg G, Skurray R A. The 3′ conserved segment of integrons contains a gene associated with multidrug resistance to antiseptics and disinfectants. Antimicrob Agents Chemother. 1993;37:761–768. doi: 10.1128/aac.37.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Payne D J. Metallo-β-lactamases—a new therapeutic challenge. J Med Microbiol. 1993;39:93–99. doi: 10.1099/00222615-39-2-93. [DOI] [PubMed] [Google Scholar]
- 41.Pearson W R. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 1990;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- 42.Ploy M-C, Courvalin P, Lambert T. Characterization of In40 of Enterobacter aerogenes BM2688, a class 1 integron with two new gene cassettes, cm1A2 and qacF. Antimicrob Agents Chemother. 1998;42:2557–2563. doi: 10.1128/aac.42.10.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Preston K E, Kacica M A, Limberger R J, Archinal W A, Venezia R A. The resistance and integrase genes of pACM1, a conjugative multiple-resistance plasmid, from Klebsiella oxytoca. Plasmid. 1997;37:105–118. doi: 10.1006/plas.1997.1284. [DOI] [PubMed] [Google Scholar]
- 44.Pridmore R D. New and versatile cloning vectors with kanamycin resistance marker. Gene. 1987;56:309–312. doi: 10.1016/0378-1119(87)90149-1. [DOI] [PubMed] [Google Scholar]
- 45.Purewal A S. Nucleotide sequence of the ethidium efflux gene from Escherichia coli. FEMS Microbiol Lett. 1991;66:229–231. doi: 10.1016/0378-1097(91)90338-b. [DOI] [PubMed] [Google Scholar]
- 46.Rådström P, Sköld O, Swedberg G, Flensburg J, Roy P H, Sundström L. Transposon Tn5090 of plasmid R751, which carries an integron, is related to Tn7, Mu, and the retroelements. J Bacteriol. 1994;176:3257–3268. doi: 10.1128/jb.176.11.3257-3268.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rasmussen B A, Bush K. Carbapenem-hydrolyzing β-lactamases. Antimicrob Agents Chemother. 1997;41:223–232. doi: 10.1128/aac.41.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Recchia G D, Hall R M. Gene cassettes: a new class of mobile element. Microbiology. 1995;141:3015–3027. doi: 10.1099/13500872-141-12-3015. [DOI] [PubMed] [Google Scholar]
- 48a.Reese, M. G. 18 December 1998, revision date. Promoter prediction by neural network program. [Online.] Drosophila Genome Center, Lawrence Berkeley National Laboratory, Berkeley, Calif. http://www-hgc.lbl.gov./projects/promoter.html. [20 December 1998, last date accessed.]
- 49.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 50.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Senda K, Arakawa Y, Nakashima K, Ito H, Ichiyama S, Shimokata K, Kato N, Ohta M. Multifocal outbreaks of metallo-β-lactamase-producing Pseudomonas aeruginosa resistant to broad-spectrum β-lactams, including carbapenems. Antimicrob Agents Chemother. 1996;40:349–353. doi: 10.1128/aac.40.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Senda K, Arakawa Y, Ichiyama S, Nakashima K, Ito H, Ohsuka S, Shimokata K, Kato N, Ohta M. PCR detection of metallo-β-lactamase gene (blaIMP) in gram-negative rods resistant to broad-spectrum β-lactams. J Clin Microbiol. 1996;34:2909–2913. doi: 10.1128/jcm.34.12.2909-2913.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaw K J, Rather P N, Hare R S, Miller G H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stokes H W, Hall R M. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol Microbiol. 1989;3:1669–1683. doi: 10.1111/j.1365-2958.1989.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 55.Stokes H W, O’Gorman D B, Recchia G D, Parsekhian M, Hall R M. Structure and function of 59-base element recombination sites associated with mobile gene cassettes. Mol Microbiol. 1997;26:731–745. doi: 10.1046/j.1365-2958.1997.6091980.x. [DOI] [PubMed] [Google Scholar]
- 56.Tennigkeit J, Matzura H. Nucleotide sequence analysis of a chloramphenicol-resistance determinant from Agrobacterium tumefaciens and identification of its gene product. Gene. 1991;98:113–116. doi: 10.1016/0378-1119(91)90112-o. [DOI] [PubMed] [Google Scholar]
- 57.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toriya M, Sakakibara M, Matsushita K, Morohoshi T. Nucleotide sequence of aminoglycoside 6′-N-acetyltransferase [AAC(6′)] determinant from Serratia sp. 45. Chem Pharm Bull. 1992;40:2473–2477. doi: 10.1248/cpb.40.2473. [DOI] [PubMed] [Google Scholar]
- 59.Vézina G, Levesque R C. Molecular characterization of the class II multiresistance transposable element in Tn1043 from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991;35:313–321. doi: 10.1128/aac.35.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991;35:147–151. doi: 10.1128/aac.35.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamaguchi, H., M. Nukaya, and T. Sawai. 1994. EMBL/GenBank database entry D29636.