Abstract
Background:
There is known practice variation in the treatment of frequently relapsing, steroid-dependent, and steroid-resistant nephrotic syndrome in children. Rituximab is an emerging therapy for difficult-to-treat nephrotic syndrome; however, there are no clear treatment guidelines. We therefore hypothesized that a wide variety of approaches to this therapy exist.
Objective:
To evaluate when and how rituximab is used for the treatment of childhood nephrotic syndrome in Canada.
Design and setting:
An online survey was used.
Participants:
Canadian pediatric nephrologists.
Methods:
A cross-sectional survey was distributed across Canada through the Canadian Association of Pediatric Nephrologists (CAPN) to evaluate rituximab treatment practices.
Results:
Of a total of 20 responses, 19 (95%) use rituximab in the treatment of nephrotic syndrome, usually as a third or fourth agent. For the number of rituximab doses, the majority (68%) uses 2 doses each time they use it. Eighteen respondents (90%) measure B cells when using this medication, mostly monthly (50%) or every 3 months (39%). Respondents were administered additional doses of rituximab prophylactically (74%) or at first relapse (47%). Long-term drug safety and drug funding were identified as the main barriers to rituximab use.
Limitations:
This survey represents the practice styles of physicians in a single country, and there is a nonresponse bias of 63%. Also, associations were not calculated.
Conclusions:
Among Canadian pediatric nephrologists, rituximab use for nephrotic syndrome appears to be increasing, but significant practice variations remain, including approaches to B-cell monitoring. It is reserved mostly for second-line and third-line use due to cost, funding issues, and residual uncertainty regarding long-term safety. Understanding these critical areas of practice uncertainty is a first step to optimize treatment of nephrotic syndrome in children.
Keywords: children, nephrotic syndrome, rituximab, practice variation, survey
Abrégé
Contexte:
Des variations sont connues dans les pratiques liées au traitement du syndrome néphrotique infantile, dépendant ou résistant aux stéroïdes, à récidives fréquentes. Le rituximab constitue une nouvelle approche thérapeutique pour soigner le syndrome néphrotique difficile à traiter. Il n’existe cependant aucune directive de traitement claire dans ce contexte. Nous avons donc émis l’hypothèse qu’il existait une grande variété d’approches dans l’utilisation de ce médicament.
Objectifs:
Examiner les pratiques d’utilisation (quand et comment) du rituximab dans le traitement du syndrome néphrotique infantile au Canada.
Cadre et type d’étude:
Étude menée par sondage en ligne.
Participants:
Des néphrologues pédiatriques canadiens.
Méthodologie:
Un sondage transversal a été distribué partout au Canada par l’entremise de l’Association canadienne des néphrologues pédiatriques (ACPN) afin d’examiner les pratiques de traitement au rituximab.
Résultats:
Parmi les 20 néphrologues ayant répondu au sondage, 19 (95 %) utilisent le rituximab dans le traitement du syndrome néphrotique, généralement comme 3e ou 4e agent, et la majorité d’entre eux (68 %) administre deux doses à chaque utilisation. Dix-huit répondants (90 %) mesurent les lymphocytes B lorsqu’ils emploient ce médicament, principalement tous les mois (50 %) ou tous les trois mois (39 %). Certains répondants avaient administré des doses additionnelles de rituximab à des fins prophylactiques (74 %) ou lors de la première rechute (47 %). Le recours au rituximab serait principalement freiné par des enjeux liés à son innocuité à long terme et au financement des médicaments.
Limitations:
Ce sondage représente les pratiques des médecins d’un seul pays et comporte un biais de non-réponse de 63 %. De plus, les associations n’ont pas été calculées.
Conclusion:
Chez les néphrologues pédiatriques canadiens, l’utilisation du rituximab dans le traitement du syndrome néphrotique semble augmenter; bien que des variations significatives demeurent dans la pratique, notamment en ce qui concerne les approches de surveillance des lymphocytes B. Actuellement, le rituximab est surtout utilisé comme thérapie de deuxième et de troisième ligne en raison de son coût, d’enjeux liés au financement et d’une incertitude résiduelle en ce qui concerne son innocuité à long terme. La compréhension de ces zones critiques d’incertitude dans les pratiques est une première étape pour optimiser le traitement du syndrome néphrotique chez les enfants.
Introduction
Childhood nephrotic syndrome is the most common glomerular pediatric kidney disease. 1 Glucocorticoids are the first-line therapy for the initial episode and for successive relapses of the disease. Treatment with glucocorticoids alone is effective and sufficient in treating children with steroid-sensitive nephrotic syndrome with infrequent relapses. However, a significant proportion of patients will require nonsteroidal agents to manage a steroid-dependent course, frequent-relapsing course (>50%), or steroid-resistant course (~10%). 2
Regarding the use of nonsteroidal agents for the management of nephrotic syndrome, there remain areas of uncertainty and there is significant practice variation worldwide, even within countries or centers. 3 Various nonsteroidal agents are commonly used in the treatment of frequent-relapsing nephrotic syndrome (FRNS), steroid-dependent nephrotic syndrome (SDNS), and steroid-resistant nephrotic syndrome (SRNS). These agents include calcineurin inhibitors (cyclosporine, tacrolimus), mycophenolate mofetil, alkylating agents (cyclophosphamide), or the newer monoclonal antibody rituximab.4-6
Rituximab is a newer agent with emerging evidence demonstrating positive treatment outcomes7-10 by inhibiting B-cell function and promoting B-cell apoptosis. Although the exact pathogenesis of nephrotic syndrome is not well understood, it is widely believed that immune-mediated B-cell and T-cell interactions play a role. 1 With B-cell depletion after rituximab, B-cell and T-cell interactions are suppressed, and a treatment effect observed. 9 Rituximab is also thought to affect direct regulation of glomerular podocyte function, which would provide a positive treatment effect in conditions where B cells may not play a main pathogenic role. 11 Emerging evidence for rituximab use in the treatment of childhood nephrotic syndrome has been obtained with different protocols from patients with various clinical courses and disease severity. To the best of our knowledge, there are no standardized practices or protocols for the use of rituximab in childhood nephrotic syndrome. Not surprisingly, the literature reports significant variation in the indication and timing of rituximab initiation, number of doses given, frequency of doses, B-cell monitoring, and duration of treatment. 12
Establishing the differences in management decisions is the first step to create future research studies evaluating the impact of practice variation on treatment outcomes. We therefore set out to evaluate when and how rituximab is used for the treatment of childhood nephrotic syndrome in Canada using an online survey. We hypothesized that there would be substantial practice variations in the key areas of rituximab use among Canadian pediatric nephrologists.
Methods
Nephrotic Syndrome Definitions
We defined the different courses of nephrotic syndrome according to the 2021 Kidney Disease Improving Global Outcomes (KDIGO) guidelines 2 : FRNS as 2 or more relapses within 6 months of disease onset or 4 or more relapses per 12 months in any subsequent 12-month period; SDNS as 2 consecutive relapses during therapy with prednisone or prednisolone (either at full dose or during tapering) or within 15 days of prednisone or prednisolone discontinuation; and SRNS when there is lack of complete remission at 4 weeks of therapy with daily prednisone or prednisolone at standard dose.
Survey Creation
We developed a detailed survey to evaluate practice patterns in the use of rituximab in childhood nephrotic syndrome. The checklist from the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) for cross-sectional studies was used to guide the analysis and preparation of this work. 13 The survey questions were reviewed in detail by 3 pediatric nephrologists at the University of Alberta. The final version of the survey was administered using a Web-based survey program (REDCap). The creation, distribution, and data collection period for this survey occurred in 2020. See Supplemental Appendix A for survey questions.
Survey Distribution
All pediatric nephrologists currently listed with the Canadian Association of Pediatric Nephrologists (CAPN) were eligible for completing the survey which was distributed electronically through the CAPN email. A reminder email was sent after 4 weeks, and a second reminder email was sent after an additional 2 weeks.
Analysis
Surveys that were not fully completed were excluded from the final analysis.
All variables were categorical due to the nature of the survey design. These were reported using the proportion of total respondents and groups compared to evaluate variability.
Ethics approval was obtained through the Health Ethics Research Board (Pro00089826)—Health Panel at the University of Alberta prior to survey distribution.
Results
Survey Responses
The survey was distributed to a total of 54 Canadian pediatric nephrologists. A total of 20 survey submissions were completed and included in the study for a survey response rate of 37%. The respondent demographics are outlined in Table 1. All 10 provinces, the Northwest Territories, and the Yukon were represented as areas of practice for responding pediatric nephrologists.
Table 1.
Respondent Demographics.
| Demographic | Number | % |
|---|---|---|
| Sex | ||
| Male | 11 | 55 |
| Female | 9 | 45 |
| Age, y | ||
| <40 | 3 | 15 |
| 40-50 | 10 | 50 |
| 51-60 | 5 | 25 |
| >60 | 2 | 10 |
| Year of fellowship completion | ||
| 1990-1999 | 5 | 25 |
| 2000-2009 | 10 | 50 |
| >2010 | 5 | 25 |
| Training program/University | ||
| Canadian | 14 | 70 |
| Non-Canadian | 6 | 30 |
| Current practice | ||
| ≥50% clinical | 16 | 80 |
| <50% clinical | 4 | 20 |
Rituximab Indications
Nineteen (95%) of 20 participants reported using rituximab for the treatment of nephrotic syndrome. Of these 19 providers, 17 (89%), 19 (100%), and 15 (79%) use rituximab for FRNS, SDNS, and SRNS, respectively. Six (32%) providers have used rituximab for 2 to 5 years in their practice and the remaining 13 (68%) for more than 5 years. No physician stated that they would use rituximab for the treatment of nephrotic syndrome with a known structural mutation. Participants described a wide variety of criteria for when they would consider initiating rituximab therapy, including when previous medications failed (58%), other therapies contribute side effects (32%), compliance is a concern (26%), or if drug funding is available (10%). Most participants do not use rituximab as the first option when starting a steroid-sparing agent for nephrotic syndrome (Table 2). Instead, it was considered a third-line or fourth-line therapy for treatment of FRNS, SDNS, and SRNS. Most (84%) participants described using a standardized protocol for the use of rituximab in the treatment of nephrotic syndrome.
Table 2.
Initial Second-Line Agent (Following Steroids).
| Medication | FRNS (%) | SDNS (%) | SRNS (%) |
|---|---|---|---|
| Tacrolimus | 4 (20) | 10 (50) | 15 (75) |
| Cyclophosphamide | 7 (35) | 5 (25) | — |
| Mycophenolate mofetil | 6 (30) | 3 (15) | — |
| Cyclosporine | 1 (5) | 1 (5) | 3 (15) |
| Rituximab | — | — | 1 (5) |
| Other | 2 (10) | 1 (5) | 1 (5) |
Note. FRNS = frequent-relapsing nephrotic syndrome; SDNS = steroid-dependent nephrotic syndrome; SRNS = steroid-resistant nephrotic syndrome.
Prescriptions and B-cell Monitoring
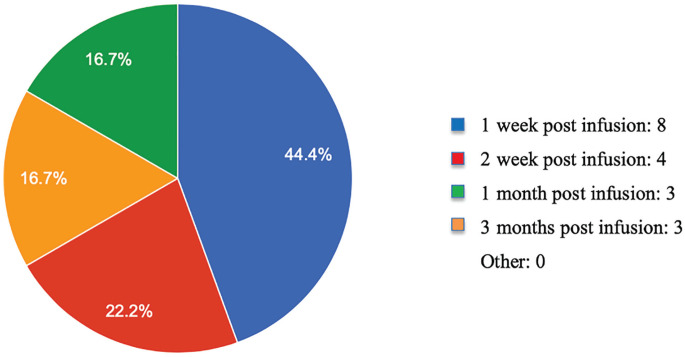
Most commonly, participants administer rituximab at a dose of 375 mg/m2 twice, 2 weeks apart (Figure 1). One participant does not measure B-cell levels after initiating rituximab treatment, and the other respondents most commonly measure B-cell levels 1 week after rituximab administration (Figure 2). If B cells levels were not depleted after initial rituximab infusion, 5 (28%) of 18 respondents would not administer another dose of rituximab. The other 13 (72%) of 18 would administer a subsequent dose of rituximab. The majority (67%) measure B-cell levels until they recover (Table 3). When asked about further rituximab administration after the initial doses of rituximab if it resulted in complete recovery, 9 (47%) respondents would readminister rituximab at first relapse, whereas 7 others report using the drug prophylactically (37%). When asked about guiding factors physicians use to make rituximab dosing decisions in their own practice, 7 (37%) responded, “I give the same dose and frequency each time I prescribe rituximab”; 6 (31%) responded, “I use clinical presentation to guide further dosing and/or frequency (eg, relapses); 4 (21%) responded, “I use B-cell levels to guide dosing and/or frequency”; and 2 (11%) responded “Other.”
Figure 1.
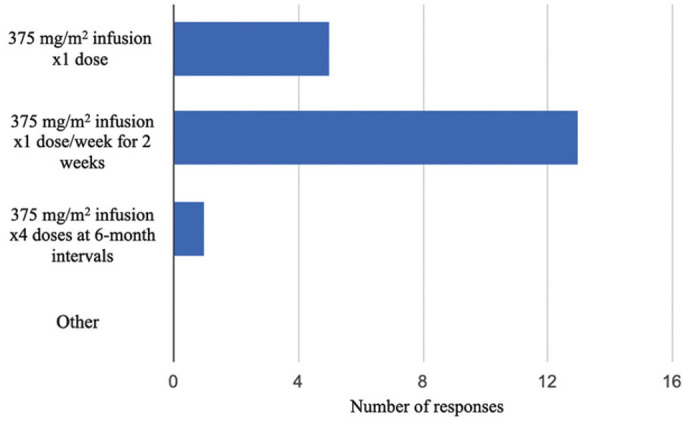
Different rituximab prescriptions used by Canadian pediatric nephrologists for difficult-to-treat nephrotic syndrome.
Figure 2.
Time of initial B-cell measurement following the first dose of rituximab.
Table 3.
B-cell Measurement Frequency and Duration.
| Number | % | |
|---|---|---|
| Interval | ||
| Weekly | 1 | 5.5 |
| Monthly | 7 | 39 |
| Every 3 months | 9 | 50 |
| Every 6 months | 1 | 5.5 |
| Duration | ||
| 6 months | 3 | 16.5 |
| 1 year | 1 | 5.5 |
| 2 years | 1 | 5.5 |
| Until B-cell recovery | 12 | 67 |
| Until off rituximab | 1 | 5.5 |
Rituximab and Other Agents
Participants were asked when they would consider weaning other medications if patients were being treated with other agents (eg, calcineurin inhibitors) at the initiation of rituximab therapy. Eleven (58%) of 19 stated that they would wean the other agent only when the patient is at full remission, 2 (11%) responded that they would wean at a set time of 3 months, 1 (5%) responded they would wean between 2 and 4 weeks, 1 (5%) responded that they would never wean the other agent, and 4 (21%) stated they would wean on an individual basis that varies between patients.
Funding and Barriers
When asked how rituximab is funded in each institution, specifically for the treatment of nephrotic syndrome, 15 (75%) described rituximab as being provincially funded, 3 (15%) identified Federal/Treaty funding, and 2 (10%) described known local/private insurance companies that can assist with rituximab funding. Many factors were identified as barriers to rituximab use, and these are summarized in Table 4.
Table 4.
Barriers to Rituximab Use.
| Barrier | Number | % |
|---|---|---|
| None perceived | 7 | 32 |
| Complicated drug application/funding requirements | 9 | 40 |
| Side effects of rituximab | 2 | 9 |
| Unknown long-term effects | 3 | 14 |
| Lack of treatment response | 1 | 5 |
Discussion
In this online survey to evaluate practice patterns in the use of rituximab in childhood nephrotic syndrome among Canadian pediatric nephrologists, there was uniformity in some aspects of the use of rituximab and variation in others. These aspects include the decision to initiate rituximab therapy, its use in different types of nephrotic syndrome, prescriptions, B-cell monitoring, B-cell use in treatment decision-making, and barriers to rituximab use.
Comparing our results with the Canadian survey completed by Samuel et al, 3 95% of our respondents reported the use of rituximab for this condition (95% confidence interval, 75%-99%), in contrast to the 60% reported in the previous survey (95% confidence interval, 42%-76%). Perhaps due to the growing evidence of its effectiveness and safety, prescribers have become more familiar with this medication, resulting in an increased use of rituximab compared with 8 years ago.
For FRNS and SDNS, rituximab was not the first steroid-sparing agent used by any physician and was considered as a third-line or fourth-line agent by most respondents. This matches with current recommendations 2 that suggest only considering rituximab in children with SDNS who have frequent relapses despite optimal combinations of prednisone and steroid-sparing agents and/or who have serious adverse effects of therapy. Despite various studies supporting the efficacy of rituximab for the treatment of childhood nephrotic syndrome6,14-18 and the majority of survey responders having experience with rituximab for more than 5 years, there were still perceived “unknowns” about this medication that created hesitancy to its use, including potential side effects, and unknown long-term consequences. For SRNS, the current KDIGO guidelines 2 state that there may be a limited role for treatment with rituximab. However, 79% of our participants would consider using rituximab for SRNS if other therapies have failed. There is emerging literature reporting clinical utility in SRNS for children without structural mutations that affect the structure and function of the glomerular filtration barrier,5,14,19 but more research is clearly needed in this area.
When administering rituximab, all respondents reported using the same dose; however variation occurred in number of infusions and timing of subsequent doses. We observed a range with some providers starting with a single dose and others with 4 doses planned and at 6-month intervals. Each of these dose schedules has been reported in the literature,4,8,9 but no specific dose schedule is considered the gold standard. 2 This finding is unchanged from the Samuel et al study 3 where 1 to 4 doses were used with no significant differences. Interestingly, there are new data emerging that challenge the standard dose of 375 mg/m2 of rituximab in the treatment of childhood nephrotic syndrome. A recent study by Chan et al 20 showed that low dose (375 mg/m2) of rituximab had a shorter relapse-free period and a higher relapse risk when compared with medium-dose (750 mg/m2) and high-dose (1125-1500 mg/m2) therapy, although this difference was mitigated when maintenance immunosuppression was added to therapy. As our data collection occurred prior to this publication, there was no impact of these findings on the prescribed dose of rituximab among Canadian pediatric nephrologists; however, the “standard” prescription of rituximab for nephrotic syndrome may change in the coming years.
Controversies exist in the use of B-cell levels to guide rituximab treatment decisions. Varying B-cell depletion effects after rituximab have been reported, from partial depletion at 1-week post administration to complete depletion without B-cell recovery at 2 years. 21 The average recovery time of B cells is approximately 6 to12 months post rituximab administration,7,22 and it has been suggested that nephrotic syndrome relapses are related to B-cell recovery. 21 However, other work 8 observed patients who remained relapse-free despite B-cell recovery. The 2021 KDIGO guidelines 2 state that when comparing rituximab dosing on a fixed schedule versus dosing guided by the reappearance of B cells, there are insufficient data to make a recommendation; however, B-cell levels should still be monitored. More investigation into the utility of B-cell levels guiding rituximab therapy is needed to optimize the treatment of childhood nephrotic syndrome.
Finally, there exist significant barriers to rituximab use. Almost half of the participants described that funding dictates treatment decisions. With the estimated cost of around $5/mg, this is not an insignificant amount for patients to pay personally; thus, alternative funding sources are required. The majority of rituximab drug funding is provided provincially unless a patient qualifies for federal funding through Crown-Indigenous Relations Canada (CIRNAC) and/or Indigenous Services Canada (ISC). Sometimes the provincial funding for rituximab requires at least one other second-line agent to be used prior to the application. Therefore, funding is an impediment to using rituximab as the primary second-line agent in those jurisdictions. Other barriers include the fear of adverse reactions, chronic immunosuppression including persistent hypogammaglobulinemia, and unknown long-term side effects. We anticipate more literature detailing the effectiveness and safety of rituximab, and perhaps when this medication is off patent, the costs will be lower, thus eliminating this barrier.
There are limitations to this study. This survey was only conducted in Canada and therefore only represents the practice styles of physicians in a single country. The total response rate among all physicians eligible was 37%, which introduces a nonresponse bias of 63%. Despite our participation from different physicians in different provinces, and our balanced respondent demographics, there may be a lack of generalizability with our survey response rate. Finally, we did not calculate associations, which may limit some of the conclusions that could have been drawn from our data set.
The main strength of this study is that we have started to identify common and divergent aspects on the use of rituximab at a national level, which is the first step to generate effective studies on practice variation and its association with outcomes.
Conclusions
Among Canadian pediatric nephrologists, rituximab use for nephrotic syndrome appears to be increasing but is still largely reserved for second-line and third-line use due to cost, difficult access to funding, and residual uncertainty about long-term safety. Approaches to B-cell monitoring were also highly variable. These data represent a first step in understanding critical areas of practice uncertainty that will need to be addressed to optimize treatment of nephrotic syndrome in children.
Supplemental Material
Supplemental material, sj-docx-1-cjk-10.1177_20543581221079959 for Rituximab Use for the Treatment of Childhood Nephrotic Syndrome by Canadian Pediatric Nephrologists: A National Survey by Cory Meeuwisse, Catherine J. Morgan, Susan Samuel, R Todd Alexander and Sara Rodriguez-Lopez in Canadian Journal of Kidney Health and Disease
Footnotes
Ethics Approval and Consent to Participate: Ethics approval was obtained through the Health Ethics Research Board (Pro00089826)—Health Panel at the University of Alberta. Patients were not involved in this study.
Consent for Publication: The final version of the manuscript was reviewed and approved by all authors.
Availability of Data and Materials: The materials analyzed during the study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this project was granted by the Women and Children’s Health Research Institute at the University of Alberta, Edmonton, Alberta, Canada
ORCID iDs: R Todd Alexander  https://orcid.org/0000-0001-7396-7894
https://orcid.org/0000-0001-7396-7894
Sara Rodriguez-Lopez  https://orcid.org/0000-0003-0543-4855
https://orcid.org/0000-0003-0543-4855
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Colucci M, Corpetti G, Emma F, Vivarelli M. Immunology of idiopathic nephrotic syndrome. Pediatr Nephrol. 2018;33(4):573-584. [DOI] [PubMed] [Google Scholar]
- 2. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1-S276. [DOI] [PubMed] [Google Scholar]
- 3. Samuel S, Morgan CJ, Bitzan M, et al. Substantial practice variation exists in the management of childhood nephrotic syndrome. Pediatr Nephrol. 2013;28(12):2289-2298. [DOI] [PubMed] [Google Scholar]
- 4. Iwabuchi Y, Miyabe Y, Makabe S, et al. Comparison of the response of frequently relapsing steroid-dependent minimal change nephrotic syndrome to rituximab therapy between childhood-onset and adult-onset disease. Medicine (Baltimore). 2018;97(42):e12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jellouli M, Charfi R, Maalej B, Mahfoud A, Trabelsi S, Gargah T. Rituximab in the management of pediatric steroid-resistant nephrotic syndrome: a systematic review. J Pediatr. 2018;197:191-197. [DOI] [PubMed] [Google Scholar]
- 6. Maratea D, Bettio M, Corti MG, Montini G, Venturini F. The efficacy and safety of rituximab in treating childhood nephrotic syndrome: an Italian perspective. Ital J Pediatr. 2016;42(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ravani P, Rossi R, Bonanni A, et al. Rituximab in children with steroid-dependent nephrotic syndrome: a multicenter, open-label, noninferiority, randomized controlled trial. J Am Soc Nephrol. 2015;26(9):2259-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Niu XL, Hao S, Wang P, et al. Single dose of rituximab in children with steroid-dependent minimal change nephrotic syndrome. Biomed Rep. 2016;5(2):237-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iijima K, Sako M, Nozu K. Rituximab treatment for nephrotic syndrome in children. Curr Pediatr Rep. 2015;3(1):71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamei K, Ogura M, Sato M, Sako M, Iijima K, Ito S. Risk factors for relapse and long-term outcome in steroid-dependent nephrotic syndrome treated with rituximab. Pediatr Nephrol. 2016;31(1):89-95. [DOI] [PubMed] [Google Scholar]
- 11. Fornoni A, Sageshima J, Wei C, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3(85):85ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ravani P, Magnasco A, Edefonti A, et al. Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol. 2011;6(6):1308-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Plos Med. 2007;4(10):e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoseini R, Sabzian K, Otukesh H, et al. Efficacy and safety of rituximab in children with steroid- and cyclosporine-resistant and steroid- and cyclosporine-dependent nephrotic syndrome. Iran J Kidney Dis. 2018;12(1):27-32. [PubMed] [Google Scholar]
- 15. Gulati A, Sinha A, Jordan SC, et al. Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: multicentric report. Clin J Am Soc Nephrol. 2010;5(12):2207-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ravani P, Ponticelli A, Siciliano C, et al. Rituximab is a safe and effective long-term treatment for children with steroid and calcineurin inhibitor-dependent idiopathic nephrotic syndrome. Kidney Int. 2013;84(5):1025-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iijima K, Sako M, Nozu K, et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2014;384(9950):1273-1281. [DOI] [PubMed] [Google Scholar]
- 18. Ruggenenti P, Ruggiero B, Cravedi P, et al. Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol. 2014;25(4):850-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Magnasco A, Ravani P, Edefonti A, et al. Rituximab in children with resistant idiopathic nephrotic syndrome. J Am Soc Nephrol. 2012;23(6):1117-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chan EY, Webb H, Yu E, et al. Both the rituximab dose and maintenance immunosuppression in steroid-dependent/frequently-relapsing nephrotic syndrome have important effects on outcomes. Kidney Int. 2020;97(2):393-401. [DOI] [PubMed] [Google Scholar]
- 21. Kim JH, Park E, Hyun HS, et al. Long-term repeated rituximab treatment for childhood steroid-dependent nephrotic syndrome. Kidney Res Clin Pract. 2017;36(3):257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Papakrivopoulou E, Shendi AM, Salama AD, Khosravi M, Connolly JO, Trompeter R. Effective treatment with rituximab for the maintenance of remission in frequently relapsing minimal change disease. Nephrology (Carlton). 2016;21(10):893-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cjk-10.1177_20543581221079959 for Rituximab Use for the Treatment of Childhood Nephrotic Syndrome by Canadian Pediatric Nephrologists: A National Survey by Cory Meeuwisse, Catherine J. Morgan, Susan Samuel, R Todd Alexander and Sara Rodriguez-Lopez in Canadian Journal of Kidney Health and Disease