Abstract
Background
THO complex members (THOCs) are essential for mRNA processing, transcription elongation, mRNA export and genome integrity, which may be associated with tumor. However, the significance of THO complex members in Hepatocellular carcinoma (HCC) has not been clarified.
Methods
In this study, we investigated expression levels, clinicopathological correlation, diagnostic and prognostic value, disease associations, co-expression network, functional enrichment, genetic variation, drug sensitivity, molecular targets, and immune infiltration of THOCs in HCC using UALCAN, ICGC, HPA, Kaplan–Meier Plotter, The Open Targets Platform database, GeneMANIA, WebGestalt, GSCALite, LinkedOmics, TIMER. Meanwhile, the mRNA expression levels of THOCs in HCC were verified by qRT-PCR.
Results
THOCs were significantly up-regulated at the transcription level in HCC, and overexpression of THOCs was correlated with clinicopathological features. THOCs had potential diagnostic and prognostic value for HCC. THOCs could participate in apoptosis, regulate cell cycle, and were also involved in DNA damage response. Important cancer-related signaling pathways such as EMT, RAS/MAPK pathway and RKT pathway were also related to THOCs expression. In addition, the expression level of THOC1/2/6/7 was negatively correlated with drug resistance. And miRNA, kinase, and transcription factor targets associated with THOCs have been identified. Meanwhile, THOCs were associated with immune infiltration in HCC.
Conclusion
Our study revealed that THO complex members have important clinical significance for HCC and have potential clinical utility in diagnosis, treatment and prognosis.
Keywords: HCC, THOC, bioinformatics, prognosis, diagnosis
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common disease worldwide and the fourth most common cause of cancer-related death.1 HCC is most prevalent in East and Southeast Asia, with China alone accounting for approximately 50% of all cases and deaths.2 Currently, The best treatment for HCC patients is radical resection surgery, but the timing of the operation is crucial.3 Metastasis and recurrence after resection are worrisome problems, which affect the prognosis of HCC.4 Evidence suggested that identification of HCC gene markers contributed to predicting prognosis.5 Moreover, the genetic alterations in HCC cells have been well known.1 Therefore, it is very important to identify novel biomarkers closely related to the diagnosis, therapy and prognosis of HCC, which has great potential to improve the prognosis of HCC.
THO complex was initially identified as a four-subunit (Hpr1p, Tho2p, Mft1p and Thp2p) complex in yeast. Tex1p was later founded as an important component of THO subcomplex.6,7 In humans, the corresponding ortholog of Hpr1p, Tho2p, and Tex1p are THOC1, THOC2 and THOC3 respectively. THOC5, THOC6 and THOC7 were also important components of THO, were first discovered in humans and Drosophila, and there had no corresponding ortholog in yeast. THOC4 (also named ALYREF), THO complex and other export proteins together form a larger and conserved multisubunit TRanscription and EXport (TREX) complex which played a key role in the coupling of transcription and mRNA export.8 The main role of THO complex in cells was to be recruited to chromatin and ensure the successful completion of mRNA ribonucleoprotein (mRNP) biogenesis. THO was essential for pre-mRNA processing, transcription elongation, mRNA export and genome integrity during transcription.9 Lack of THO was closely related to transcription impairment and increased genome instability. The specific performance was the production of suboptimal mRNPs, leading to the formation of DNA:: RNA hybrids (R-loops), and the accumulation of R-loops induces DNA damage and eventually blocks the transcription extension.10,11
Abnormal expression of some members of the THO complex had been confirmed in some cancers. THOC1 was overexpressed in prostate and colorectal cancer.12,13 THOC2 was overexpressed in melanoma.14 And THOC4 was overexpressed in bladder cancer.15 At present, some studies have confirmed that some THO complex members were found to be significantly overexpressed in HCC and participated in the progression of HCC, leading to poor prognosis. Reports showed that the inhibition of THOC1 and THOC2 could inhibit the proliferation and migration of HCC cells.16,17 He et al reported that THOC4 regulated 5-methylcytosine (m5C) methylation modifications in HCC and had diagnostic value.18 Saran et al reported that the expression of THOC5 was increased by 78% in HCC cells with cytological differentiation grading G2 and G3 tumor.19 However, THOC3,6,7 in HCC had not been investigated, and we have not seen a comprehensive analysis of the significance of THO complex members in HCC. In this study, we systematically and deeply explored the expression of all THO complex members in HCC by using various comprehensive public databases to determine the potential diagnostic, treatment and prognostic value of THO complex members in the occurrence and development of HCC.
Methods
Analysis of THO Complex Members Expression
In our study, we used UALCAN database to detect the differential expression of THO complex members in normal tissues and liver tumor tissues. UALCAN database is an interactive web-portal for analyzing The Cancer Genome Atlas (TCGA) data, which could easily obtain a variety of functions such as gene differential expression analysis, clinicopathological correlation analysis and survival analysis.20 Verify the expression of THOCs in HCC using International Cancer Genome Consortium (ICGC), which has data on genomic changes present in a variety of cancers. Immunohistochemical (IHC) analyses of THOCs proteins were performed by Human Protein Atlas (HPA). HPA is a Swedish-based program that aims to map all the human proteins in cells, tissues and organs. Immunohistochemical images of proteins could be directly visualized on its public website.21
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Ten paired HCC tissues and adjacent normal tissues were taken from the resected tissues of HCC patients in The Second Hospital of Jilin University, which were immediately frozen after resection and stored in −80°C liquid nitrogen. And the pathological type of each HCC tissues specimen is verified by the pathology department of The Second Hospital of Jilin University. The pathological type of all tumor tissue specimens was hepatocellular carcinoma. Detailed character of HCC patients was shown in Table S1. Our study was approved by the ethics committee of The Second Hospital of Jilin University (2018169), and the informed consent of patients and their families was obtained. Patients who participated in the study had signed informed consent. Total RNA of HCC tissues and normal liver tissues were extracted with TRIzol reagent (Vazyme). cDNA was synthesized using PrimeScript RT-polymerase (Vazyme). SYBRGreen Premix (Vazyme) with specific PCR primers (Sangon Biotech Co., Ltd., Shanghai, China). Primers used were listed as follows: THOC1 forward primer: TGTGGACGGATTCAGCTCTT, THOC1 reverse primer: AGGGTGCTTTCCTGCTCATT; THOC2 forward primer: ATCAGCTAAAGGCGGGCAAA, THOC2 reverse primer: GCTCTCCACCAGTCATAGCC; THOC3 forward primer: GAGAAGGACCGGTTGGTCA, THOC3 reverse primer: ACCACATCATCCTTGTTGCCT; THOC4 forward primer: CGTGGAGACAGGTGGGAAAC, THOC4 reverse primer: TGCGACCAGAGCGATCATAG; THOC5 forward primer: GGAGACCCTCACCAGCAAAC, THOC5 reverse primer: GCTTGTGAGCCTGGTCGAAT; THOC6 forward primer: CGCTGGATTGGATGTTTGGC, THOC6 reverse primer: AAGGTGACGTGCTTCTGTGG; THOC7 forward primer: CCATCCAGACAGGCATGAGAC, THOC7 reverse primer: GCTTGCTTCCTGAGCTTCTTC. Reactions were run 95°C for 30 second, followed by 40 cycles at 95°C, 5 second; 56°C, 15 second; 72°C, 10 second. The 2-ΔΔct method was used to calculate fold-changes.
Clinicopathological Analysis of THO Complex Members in HCC
The relationship between mRNA expression of distinct THOC1-THOC7 and clinicopathological parameters in HCC was analyzed by UALCAN, including tumor stage, grade and nodal metastasis status. We aimed to understand the potential role of THOCs in clinicopathological factors of HCC patients.
Diagnosis and Prognosis Analysis
The accuracy of diagnosis value of THO complex members in HCC patients was determined by receiver operating characteristic (ROC) analysis with TCGA and ICGC data. The ROC curves were plotted by GraphPad prism 8.3. And the cut-off value of sensitivity and specificity was selected according to the maximum value of Youden’s index. P < 0.05 indicates statistical significance. Additionally, the influence of THO complex members on the prognosis of HCC patients is explored through Kaplan-Meier Plotter. And the analysis results of Overall Survival (OS), Recurrence-Free Survival (RFS), Progression-Free Survival (PFS) and Disease-Specific Survival (DSS) could be obtained. Kaplan-Meier Plotter is an online one-stop analysis database, including data from Gene Expression Omnibus (GEO), European Genome-phenome Archive (EGA) and TCGA, from which we can analyze the prognostic value of a particular gene and draw a survival curve. Concurrently, the hazard ratio with 95% confidence intervals and the log-rank P-value is calculated.22
The Relationship of THO Complex Members and Multiple Diseases
The Open Targets Platform is a comprehensive and robust data integration for access to and visualization of potential drug targets associated with disease.23 The association between THO complex members and various diseases is shown by The Open Targets Platform. The association score calculated based on the confidence levels in the evidence between the target gene and the disease ranged from 0 to 1. Data types were screened according to mRNA expression.
Functional Enrichment Analysis
GeneMANIA is a web tool that can explore the potential biological functions of gene lists and generate protein-protein interaction (PPI) networks.24 GeneMANIA was used to construct the PPI network for THO complex members. And the most related genes of THOCs were identified. The Web-based gene set analysis toolkit (WebGestalt) was employed to comprehensively estimate the potential biological functions and pathways of THO complex members. This user-friendly interface supports the overrepresentation enrichment analysis (ORA) approach for enrichment analysis.25 Gene Ontology (GO) analysis consists of biological processes (BP), cellular components (CC) and molecular functions (MF). Pathway analysis was carried out through Kyoto Encyclopedia of Genes and Genomes (KEGG) and Reactome. False discovery rate (FDR)<0.25 and P<0.05 was considered statistically significant.
Genetic Variation, Pathway and Drug Sensitivity Analysis
GSCALite database is a comprehensive database with multiple functions such as gene variation analysis, pathway analysis, drug sensitivity analysis, etc.26 We observed gene variation of THOCs in HCC. And Pearson correlation is performed between gene expression and copy number variation (CNV) of each gene. Pathway analysis defined pathway activation or inhibition based on a pathway activity score (PAS). t-test was used to analyze the difference in PAS. Spearman correlation analysis was performed between THO complex members and drug sensitivity. The drugs or small molecules for investigation are derived from Genomics of Drug Sensitivity in Cancer (GDSC) and Cancer Therapeutics Response Portal (CTRP).
Kinase Target, miRNA Target and Transcription Factor Target Analysis
LinkedOmics is a publicly available portal that includes multi-omics data from all 32 TCGA Cancer types.27 Analysis of kinase target, miRNA target, and transcription factor target of THOCs was constructed in LinkedOmics. The analysis was carried out using TCGA-LIHC datasets, and P < 0.05 represents statistical significance.
Correlation Between THO Complex Members and Immune Infiltration
TIMER is a flexible and friendly web server for the comprehensive analysis of immune infiltration in a variety of tumors. Also, it has gene expression analysis, survival analysis, correlation analysis and other powerful functions.28 In this study, the correlation between THOCs and the abundance of six immune infiltrates (B cells, CD4+ T cells, CD8+ T cells, Neutrophils, Macrophages, and Dendritic cells) was evaluated. And the Cox proportional hazard model was constructed by TIMER.
Statistical Analysis
GraphPad Prism 8.3 was used for the statistical analyses and generate figures. t-test was used to analyze the differential expression of THOCs in HCC tissues and normal tissues. Similarly, correlation analysis of clinicopathological features between multiple groups was also performed using the t-test. Kaplan-Meier and Log rank tests were adopted to analyze the survival time of HCC patients. The ROC curves were plotted by GraphPad prism 8.3. P-value < 0.05 had been regarded as significantly significant in all statistical analyses.
Results
Expression of THO Complex Members in Transcriptional and Protein Levels in Patients with HCC
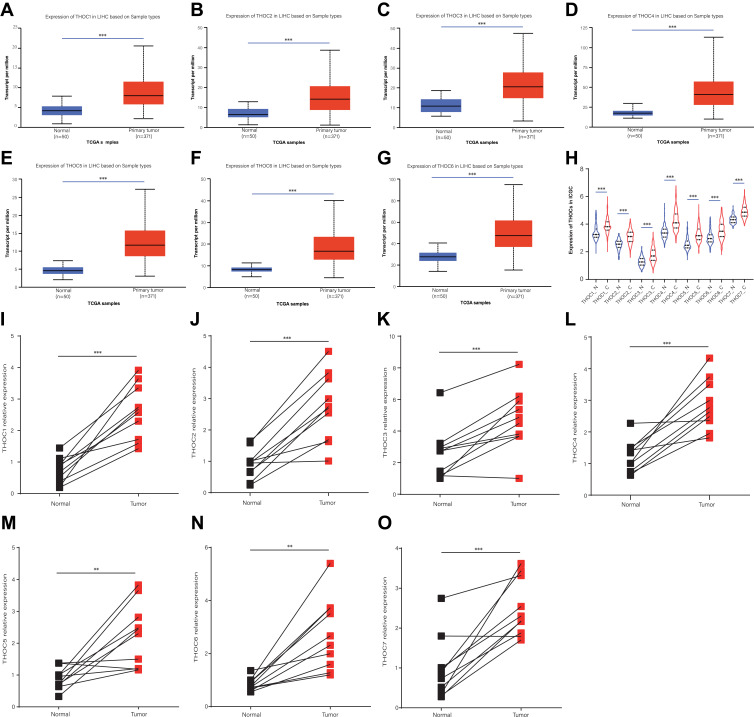
In order to understand the differential expression of THO complex members at the transcriptional level in patients with HCC, we preliminarily explored the expression of THO complex members mRNA in HCC tissues by using UALCAN database. The results showed that the mRNA expression of THOC1/2/3/4/5/6/7 in HCC samples was significantly higher than that in normal liver tissue (P<0.05, Figure 1A–G). We also detected the expression of THOCs with ICGC data. As a result, we still obtained significant overexpression of THOC1/2/3/4/5/6/7 in HCC tissues (P<0.05, Figure 1H). We then verified the expression of THOCs in 10 paired tumor tissues and adjacent normal tissues of HCC patients using qRT-PCR. Consistent with the previous results, THOCs were overexpressed in HCC. (P<0.05, Figure 1I–O).
Figure 1.
Expression of THO complex members in transcriptional levels in patients with HCC. (A–G) The mRNA expression of THO complex members in HCC tissues (UALCAN). Compared with normal liver tissues, the expression of THOC1/2/3/4/5/6/7 was increased in HCC tissues. (H) The level of THOCs in normal and HCC tissues (ICGC). (I–O) The relative expression levels of THOCs in 10 paired normal and HCC tissues (qRT-PCR). **P < 0.01, ***P < 0.001.
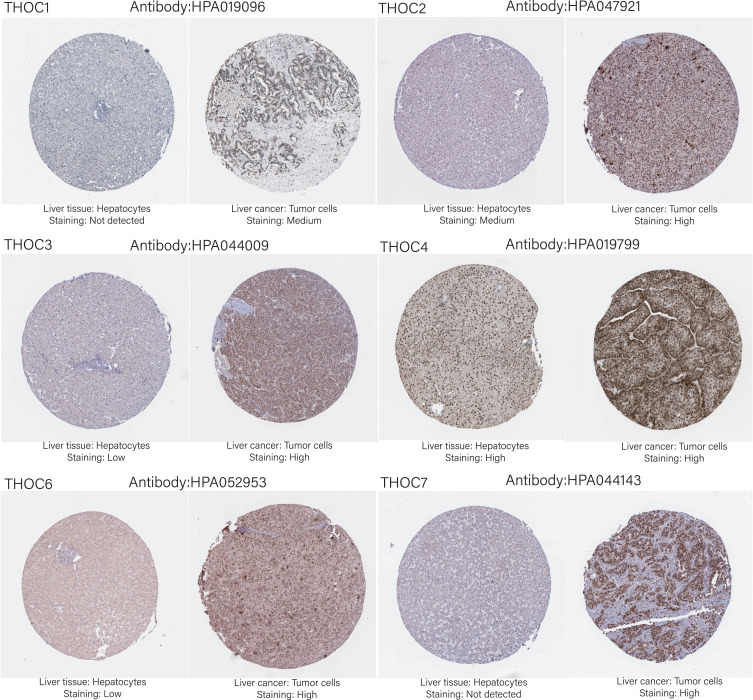
Next, we obtained the immunohistochemical staining images of THOCs proteins in HCC through HPA database (Figure 2). THOC1/7 protein was not expressed in normal liver tissues, whereas was highly expressed in liver tumor cells. The expression of THOC3/6 was low in normal liver tissues, while high or median expression was observed in HCC tissues. THOC2 had a median and high expression in normal and tumor tissues, respectively. Besides, high expression of THOC4 was observed in both normal and tumor tissues. Unfortunately, the expression of THOC5 was not detected.
Figure 2.
Representative immunohistochemical staining images of THOCs proteins in HPA.
Consequently, transcriptional and protein expression of THOCs in HCC were generally increased, which may play an important role in the occurrence and development of HCC.
Association of THO Complex Members mRNA Expression with Clinicopathological Features of HCC Patients
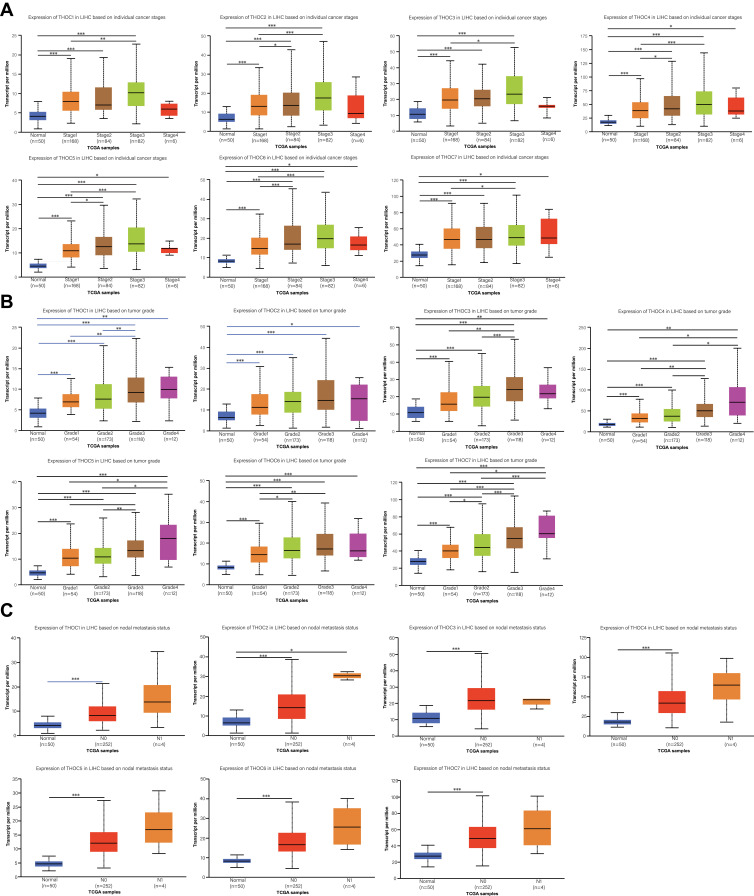
Correlation between mRNA expression of distinct THOC1-THOC7 and clinicopathological features in HCC analyzed using UALCAN, including tumor stage, grade and nodal metastasis status. The results showed that the mRNA expression of THOC1-7 was significantly correlated with clinicopathological features. In terms of HCC stages, the expression of THO complex members was remarkably correlated with different HCC stages (Figure 3A). The expression levels of THOCs increased with the increase of stages. Likewise, we found that THOCs expression was prominently correlated with different HCC grades (Figure 3B). And higher grade of HCC tended to have higher expression levels of THOCs. Regarding nodal metastasis status, we could roughly see that the expression of THOCs increased with node metastasis status (Figure 3C). The overall results indicated that abnormal expression of THOCs is involved in the progression of HCC.
Figure 3.
The relationship between the expression of different THOCs and clinicopathological parameters (UALCAN). (A) The relationship between the expression of different THOCs and individual cancer stages of HCC patients. THOCs mRNA expression levels were higher at the high cancer stage. (B) The relationship between the expression of different THOCs and tumor grades of HCC patients. With the increase of mRNA expression, their tumor grade tended to be higher. (C) The relationship between the expression of different THOCs and nodal metastasis status of HCC patients. N0; No regional lymph node metastasis, N1; Metastases in 1 to 3 axillary lymph nodes. *P < 0.05, **P < 0.01, ***P < 0.001.
Diagnostic and Prognostic Value of THO Complex Members in HCC
To analyze the diagnostic and prognostic value of THOCs in HCC, ROC analysis was first used to detect the diagnostic value of THO complex members in HCC with TCGA data. The results suggested that THOCs had better diagnostic value for HCC. And the area under curve (AUC) values of THOC1, THOC2, THOC3, THOC4, THOC5, THOC6, THOC7 were 0.9059 (sensitivity: 0.8770; specificity: 0.8400),0.8826 (sensitivity:0.7005; specificity: 0.9800),0.9188 (sensitivity: 0.7914; specificity: 0.9600),0.9130 (sensitivity: 0.8182; specificity: 0.8600),0.9583 (sensitivity: 0.8583; specificity: 0.9600),0.9159 (sensitivity: 0.8957; specificity: 0.8600),0.9023 (sensitivity: 0.7914; specificity: 0.9000), respectively (Figure S1A, Table S2). The same analysis with ICGC data also showed that THOCs had quite good prognostic value. All AUC values of THOCs ranged from 0.75 to 0.87. (Figure S1B, Table S2).
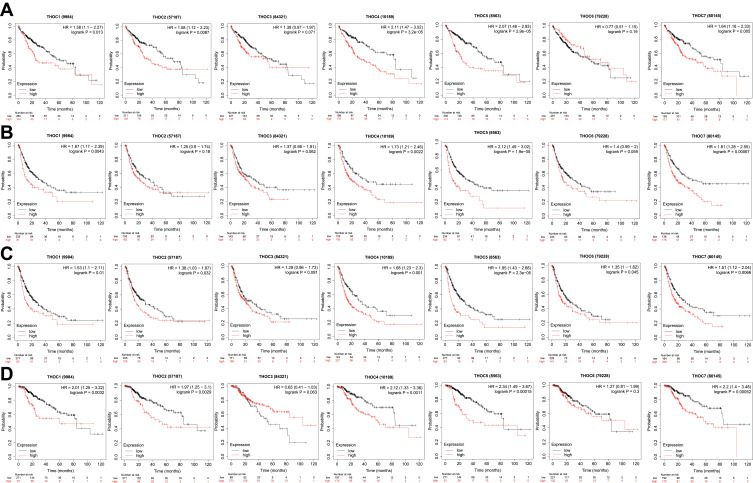
We then examined the prognostic significance of THO complex members in HCC patients with Kaplan-Meier Plotter. And mRNA expressions of all THOCs were significantly associated with the prognosis of HCC patients. Overall survival analysis demonstrated that HCC patients with a high level of THOC1 (HR=1.58, P=0.013)/2(HR=1.58, P=0.0087)/4(HR=2.11, P=3.2e-05)/5(HR=2.7, P=2.9e-05)/7(HR=1.64, P=0.005) had a poor overall survival rate, while the expression level of THOC3 (HR=1.38, P=0.071)/6(HR=0.77, P=0.19) had no remarkable/significant effect on overall survival (Figure 4A). Results of Recurrence-free survival studies have indicated that patients with HCC who have higher THOC1 (HR=1.67, P=0.0043)/4(HR=1.73, P=0.0022)/5(HR=2.12, P=1.9e-05)/7(HR=1.81, P=0.00067) tend to have poorer RFS rates. Conversely, THOC2 (HR=1.25, P=0.18)/3(HR=1.37, P=0.062)/6(HR=1.4, P=0.059) was not significantly associated with RFS in HCC patients (Figure 4B). We also examined the influence of THO complex members on progression-free survival and disease-specific survival in HCC patients. Results showed/revealed that HCC patients with high THOC1 (HR=1.53, P=0.01)/2(HR=1.38, P=0.032)/4(HR=1.68, P=0.01)/5(HR=1.95, P=2.3e-05)/6(HR=1.35, P=0.045)/7(HR=1.51, P=0.0066) expression had shorter PFS (Figure 4C). And patients with higher THOC1 (HR=2.01, P=0.0032)/2(HR=1.97, P=0.0029)/4(HR=2.12, P=0.0011)/5(HR=2.34, P=0.00015)/7(HR=2.2, P=0.00052) had shorter DSS (Figure 4D).
Figure 4.
Prognostic value of THO complex members in HCC. (A) Overall survival curves of THO complex members in patients with HCC. (B) Recurrence-free survival curves of THO complex members in patients with HCC. (C) Progression-free survival curves of THO complex members in patients with HCC. (D) Disease-specific survival curves of THO complex members in patients with HCC.
Abbreviations: HCC; hepatocellular carcinoma, OS; overall survival, RFS; recurrence-free survival, PFS; progression-free survival, DSS; disease-specific survival, ROC; receiver operating characteristic.
In general, these results demonstrated that the mRNA expression levels of THOCs had better diagnostic value for HCC, and could be considered as optional biomarkers for predicting the prognosis of patients with HCC.
The Association Between THO Complex Members and Various Diseases
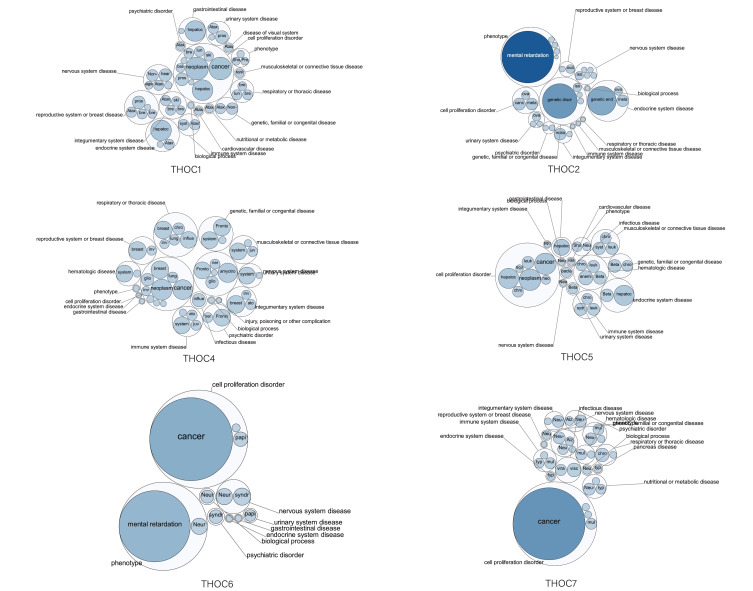
The Open Targets Platform was used to explore the associations between THO complex members and different diseases to assess whether THOCs are likely to become potential drug targets. The results showed that THOC1/2/4/5/6/7 was related to cancer, especially THOC1 and THOC5, which were correlated with HCC (Figure 5, Table 1). Unfortunately, no information about THOC3 was detected. Therefore, THO complex members have the possibility of becoming potential therapeutic targets for HCC.
Figure 5.
Relationship between THOCs with HCC among the different diseases (The Open Targets Platform). The bubbles color and bubbles size represent the association score.
Table 1.
The Top 5 Diseases Associated with THO Complex Members
| Disease | Association Score | |
|---|---|---|
| THOC1 | Neoplasm | 0.098299426 |
| Cancer | 0.096805334 | |
| Hepatocellular carcinoma | 0.0799 | |
| Prostate adenocarcinoma | 0.0308 | |
| Hearing loss | 0.029788889 | |
| THOC2 | Mental retardation | 1 |
| Genetic disorder | 0.446608333 | |
| Genetic endocrine growth disease | 0.3066 | |
| Cancer | 0.0541 | |
| Melanoma | 0.0508 | |
| THOC4 | Neoplasm | 0.047346111 |
| Cancer | 0.0427 | |
| Amyotrophic lateral sclerosis | 0.040566667 | |
| Infectious disease | 0.0362 | |
| Breast carcinoma | 0.034093058 | |
| THOC5 | Neoplasm | 0.070951685 |
| Cancer | 0.070836145 | |
| Hepatocellular carcinoma | 0.0628 | |
| Anemia (disease) | 0.031 | |
| Infectious disease | 0.0296 | |
| THOC6 | Cancer | 0.269289039 |
| Mental retardation | 0.19845713 | |
| Syndromic intellectual disability | 0.0116 | |
| Neurodegeneration | 0.0104 | |
| Neurodevelopmental disorder | 0.0104 | |
| THOC7 | Cancer | 0.474912212 |
| Visceral Leishmaniasis | 0.017316776 | |
| Chronic obstructive pulmonary disease | 0.0168 | |
| Neu-Laxova syndrome | 0.0132 | |
| Viral disease | 0.0116 |
Enrichment Analysis of THO Complex Members in HCC
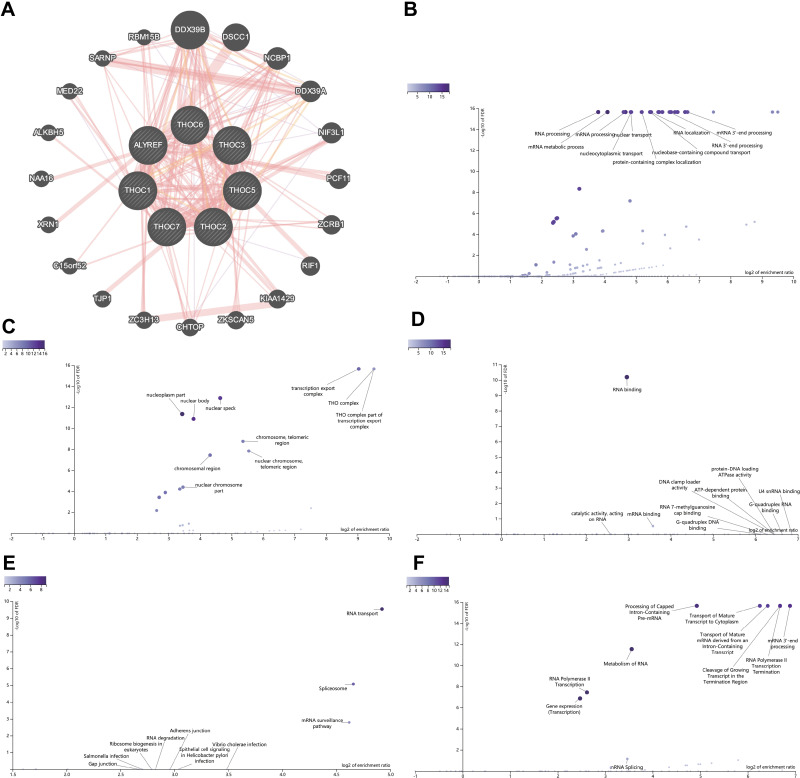
Using GeneMANIA, we established the PPI network of THO complex members and identified the 20 most related genes (Figure 6A). Table S3 showed more specific results. Furthermore, the potential biological functions and involved pathways of THO complex members and 20 interacting genes in HCC were explored in WebGestalt. GO analysis showed that BP significantly related to THO complex members mainly included RNA processing, mRNA metabolic process, mRNA processing, nuclear transport and nucleocytoplasmic transport (Figure 6B, Table 2). CC prominently changed in transcription export complex, THO complex, THO complex part of transcription export complex, nuclear speck and nucleoplasm part (Figure 6C, Table 2). And MF is remarkably involved in RNA binding and mRNA binding (Figure 6D, Table 2). As for the pathway analysis, KEGG analysis uncovered that RNA transport, spliceosome and mRNA surveillance pathway were notably associated with THO complex members in HCC (Figure 6E, Table 3). While Reactome analysis significantly enriched in Processing of Capped Intron-Containing Pre-mRNA, transport of mature transcript to cytoplasm, transport of mature mRNA derived from an intron-containing transcript, cleavage of growing transcript in the termination region, RNA polymerase II transcription termination (Figure 6F, Table 3).
Figure 6.
Enrichment Analysis of THO complex members and 20 related genes in HCC. (A) PPI network of THO complex members in GeneMANIA, different colors of the network edge indicate the bioinformatics methods applied. (B–D) GO functional enrichment analysis predicted three main functions of THOCs and their 20 related genes, including BP, CC, MF. (E and F) KEGG and Reactome pathway analysis on THOCs and their 20 related genes.
Abbreviations: BP; biological process, CC; cellular components, MF; molecular functions.
Table 2.
Mainly GO Terms of the THOCs and Their 20 Related Genes in HCC Patients (WebGestalt)
| Category | GO ID | GO Term | Size | Overlap | Enrichment Ratio | P-value | FDR |
|---|---|---|---|---|---|---|---|
| BP | GO:0006396 | RNA processing | 887 | 17 | 13.88599 | 0 | 0 |
| BP | GO:0016071 | mRNA metabolic process | 765 | 18 | 17.04757 | 0 | 0 |
| BP | GO:0006397 | mRNA processing | 487 | 17 | 25.29131 | 0 | 0 |
| BP | GO:0051169 | Nuclear transport | 356 | 14 | 28.49243 | 0 | 0 |
| BP | GO:0006913 | Nucleocytoplasmic transport | 353 | 14 | 28.73457 | 0 | 0 |
| CC | GO:0000346 | Transcription export complex | 14 | 10 | 523.6012 | 0 | 0 |
| CC | GO:0000347 | THO complex | 6 | 6 | 733.0417 | 0 | 0 |
| CC | GO:0000445 | THO complex part of transcription export complex | 6 | 6 | 733.0417 | 0 | 0 |
| CC | GO:0016607 | Nuclear speck | 383 | 13 | 24.88131 | 4.44E-16 | 1.30E-13 |
| CC | GO:0044451 | Nucleoplasm part | 1087 | 16 | 10.78994 | 1.87E-14 | 4.38E-12 |
| MF | GO:0003723 | RNA binding | 1603 | 18 | 7.798503 | 3.46E-14 | 6.50E-11 |
| MF | GO:0003729 | mRNA binding | 233 | 4 | 11.92275 | 3.17E-04 | 0.297836 |
Table 3.
Mainly Pathway Terms of the THOCs and Their 20 Related Genes in HCC Patients (WebGestalt)
| Category | Pathway ID | Pathway Name | Size | Overlap | Enrichment Ratio | P-value | FDR |
|---|---|---|---|---|---|---|---|
| KEGG | hsa03013 | RNA transport | 166 | 9 | 30.32391 | 8.98E-13 | 2.93E-10 |
| KEGG | hsa03040 | Spliceosome | 133 | 6 | 25.23193 | 5.17E-08 | 8.43E-06 |
| KEGG | hsa03015 | mRNA surveillance pathway | 91 | 4 | 24.58495 | 1.51E-05 | 0.001637 |
| Reactome | R-HSA-72203 | Processing of Capped Intron-Containing Pre-mRNA | 243 | 14 | 30.39959 | 0 | 0 |
| Reactome | R-HSA-72202 | Transport of Mature Transcript to Cytoplasm | 83 | 12 | 76.28675 | 0 | 0 |
| Reactome | R-HSA-159236 | Transport of Mature mRNA derived from an Intron-Containing Transcript | 74 | 12 | 85.56486 | 0 | 0 |
| Reactome | R-HSA-109688 | Cleavage of Growing Transcript in the Termination Region | 67 | 13 | 102.3799 | 0 | 0 |
| Reactome | R-HSA-73856 | RNA Polymerase II Transcription Termination | 67 | 13 | 102.3799 | 0 | 0 |
Genetic Variation, Pathway and Drug Sensitivity Analysis of THO Complex Members in HCC
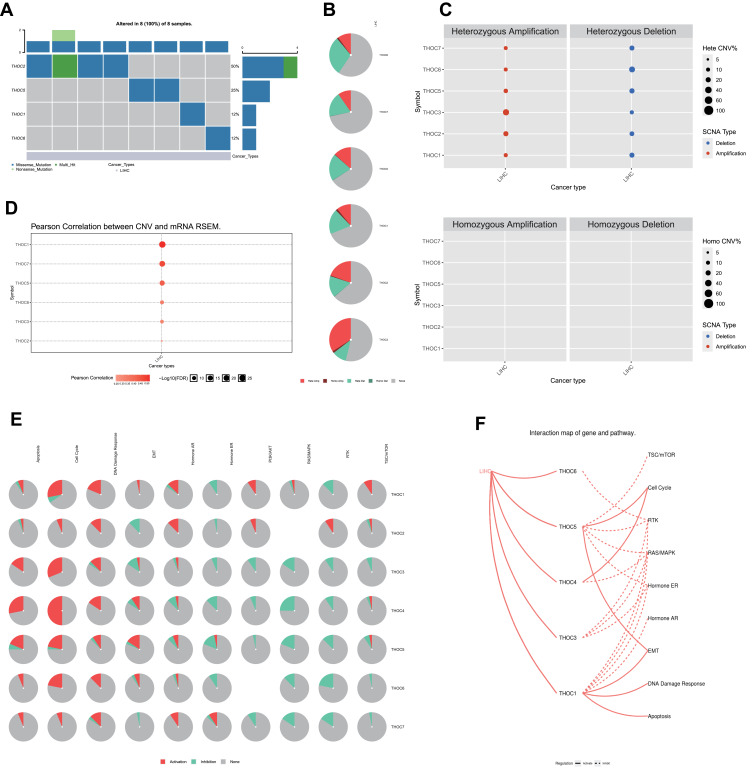
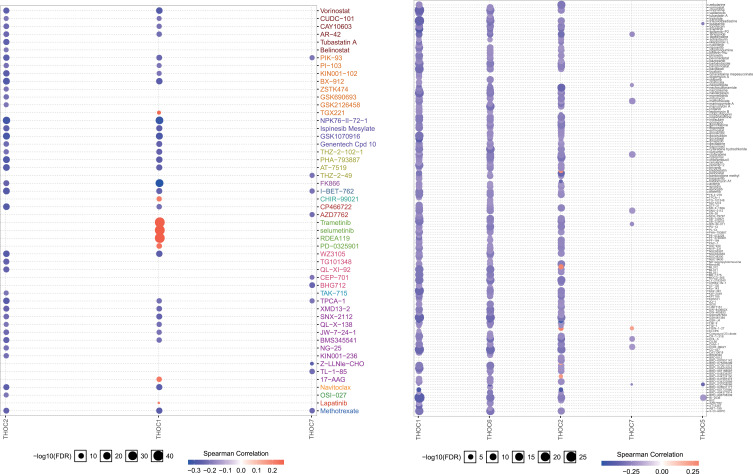
To understand the role of THOCs in HCC, we explored the genetic variations, pathway and drug sensitivity of THOCs in HCC by GSCALite database. We found that THOC1/2/5/6 had single nucleotide variation (SNV), which mainly consisted of missense mutation and nonsense mutation (Figure 7A). Copy number variation (CNV) also existed in THOC1/2/3/5/6/7. Both hetero-zygous CNV and homozygous CNV of THOCs were observed in HCC (Figure 7B). However, only hetero-zygous CNV of THOCs was statistically significant, including amplification and deletion (Figure 7C). Moreover, Pearson correlation analysis indicated that there was a positive correlation between CNV and THOCs expression (Figure 7D). Therefore, the occurrence of CNV may be the possible mechanism for the dysregulation of THOCs expression. The results of pathway analysis revealed that THO complex members were significantly involved in the regulation of several important cancer-related signaling pathways in HCC, such as apoptosis, cell cycle, DNA damage response, EMT, RAS/MAPK pathway and RKT pathway (Figure 7E and F). Correlation analysis between THO complex members and drug sensitivity showed that most THO complex members were negatively correlated with most drug resistance. Low expression of THOC1/2/6/7 could lead to drug sensitivity. (Figure 8). This will provide a basis for the screening of therapeutic drugs for HCC, which is conducive to the more rapid discovery of therapeutic drugs with better efficacy and better matching with patients.
Figure 7.
GSCALite analysis of THO complex members. (A) SNV frequency and variant types of THO complex members in HCC. (B) CNV pie distribution of THO complex members in HCC. (C) Significant results of hetero-zygous CNV of THOCs were observed in HCC. And no Significant results of homozygous CNV of THOCs were observed. Red represents amplification and blue represents deletion. The size of the dot represents the mutation frequency. (D) Pearson correlation analysis between CNV and THOCs expression. Red represents positive correlation, and color depth represents correlation intensity. The size of the dot represents statistical significance. (E) The relationship between pathways and THO complex members. (F) The interaction between pathways and THO complex members.
Figure 8.
The Spearman correlation between THOCs expression and drug sensitivity. The positive correlation means that the gene high expression is resistant to the drug, vise verse.
Kinase Target, miRNA Target and Transcription Factor Target of THO Complex Members in HCC
Aiming to understand the potential molecular regulatory mechanisms of THOCs, we searched LinkedOmics database for kinase target, miRNA target and transcription factor target of THO complex members in HCC. Table S4 showed the top five most significant kinase targets, miRNA targets and transcription factor targets for each THOCs. It is not difficult to see that kinase CDK1, kinase CDK2, kinase ATM are the common targets of most THO complex members. As for miRNA target, (GGGATGC) MIR-324-5P was considered as a miRNA target for THOC3/4/5. And (ATGCAGT) MIR-217 was considered as a miRNA target for THOC4/6. Results of transcription factor target showed (SCGGAAGY) V$ELK1_02 and (RCGCANGCGY) V$NRF1_Q6 was suggested as the target of THOC2/3/4/5/6/7.
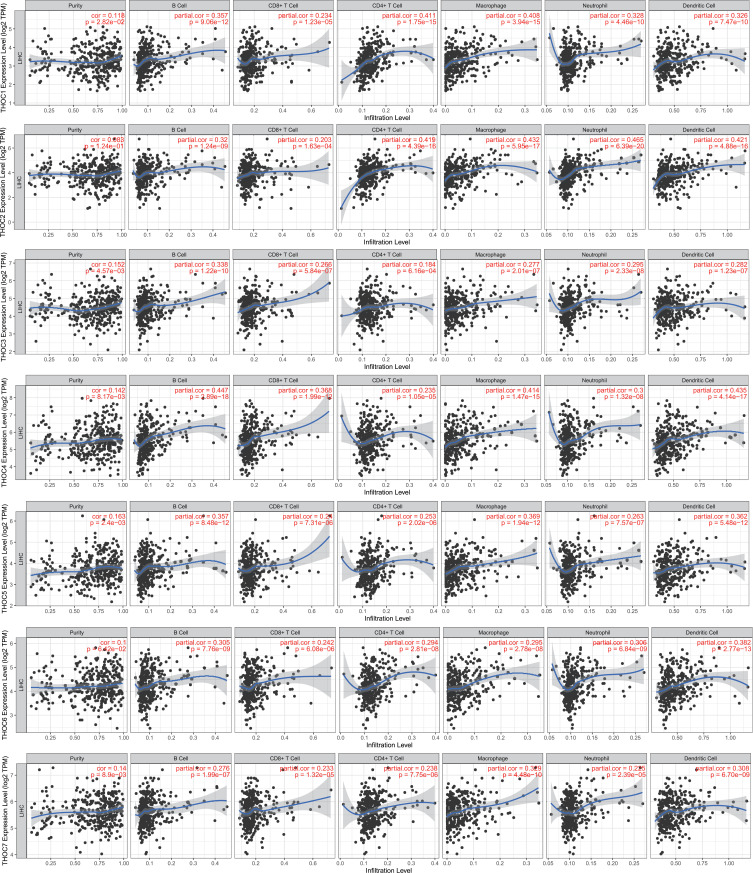
Association of Immune Infiltration with THO Complex Members in HCC
In the next study, we attempted to explore whether there is a relationship between THO complex members and tumor immune infiltration. The results in TIMER illustrated that THOC1/2/3/4/5/6/7 was significantly correlated with the abundance of six immune infiltrates (B cells, CD4+ T cells, CD8+ T cells, Neutrophils, Macrophages, and Dendritic cells) in HCC (Figure 9). Meanwhile, the Cox proportional hazard model of THO complex members was constructed (Table 4). Univariate Cox analysis showed that macrophages, neutrophils and THOC1/2/4/5/6/7 were risk factors for the prognosis of HCC patients. Further Cox multivariate analysis showed that B cells, dendritic cells and THOC4/5 were the independent risk factors for the prognosis of HCC patients. These results uncovered that THOCs are involved in immune cell infiltration in HCC, which affect the clinical outcome of HCC patients. Especially THOC4 and THOC5.
Figure 9.
Association of the abundance of different immune cells with THO complex members in HCC (TIMER).
Table 4.
Cox Proportional Hazard Model of THO Complex Members in HCC Patients
| Variables | HR | Univariate | P-value | HR | Multivariate | P-value | ||
|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||
| Low | High | Low | High | |||||
| B cells | 0.864 | 0.053 | 13.978 | 0.918 | 0 | 0 | 0.108 | 0.01 |
| CD8+ T cells | 0.515 | 0.053 | 5.035 | 0.569 | 0.006 | 0 | 1.15 | 0.056 |
| CD4+ T cells | 11.602 | 0.483 | 278.815 | 0.131 | 0.019 | 0 | 10.948 | 0.222 |
| Macrophages | 22.634 | 1.631 | 314.017 | 0.02 | 33.365 | 0.197 | 5641.398 | 0.18 |
| Neutrophils | 486.294 | 2.269 | 104,217.1 | 0.024 | 0.82 | 0 | 53,227.289 | 0.972 |
| Dendritic cells | 1.74 | 0.54 | 5.612 | 0.354 | 123.586 | 3.136 | 4869.723 | 0.01 |
| THOC1 | 1.566 | 1.2 | 2.044 | 0.001 | 1.115 | 0.76 | 1.636 | 0.576 |
| THOC2 | 1.427 | 1.158 | 1.757 | 0.001 | 1.115 | 0.842 | 1.476 | 0.446 |
| THOC3 | 1.287 | 0.998 | 1.659 | 0.051 | 0.793 | 0.563 | 1.117 | 0.185 |
| THOC4 | 1.545 | 1.258 | 1.899 | 0 | 1.376 | 1.007 | 1.88 | 0.045 |
| THOC5 | 1.922 | 1.459 | 2.53 | 0 | 1.597 | 1.109 | 2.3 | 0.012 |
| THOC6 | 1.35 | 1.028 | 1.774 | 0.031 | 0.782 | 0.54 | 1.132 | 0.193 |
| THOC7 | 1.947 | 1.389 | 2.728 | 0 | 1.311 | 0.859 | 2.003 | 0.21 |
Note: Bold values indicate statistical significance.
Discussion
HCC is a well-known malignant tumor with complex molecular pathogenesis and individual heterogeneity. The discovery of common genetic alterations in HCC is necessary for extensive and effective targeted therapy.29 THOCs were connected to mRNA processing, mRNA nuclear export and genomic stability, ultimately affecting gene expression. THOCs’ abnormal expression was related to oncogenesis and development have been reported.8 In this study, we systematically explored the potential role of THOCs in the development of HCC.
In our study, we first analyzed and evaluated the data from public databases, and the results showed that THOC1/2/3/4/5/6/7 mRNA was significantly overexpressed in HCC. And the expression of THOC1/2/3/6/7 protein in HCC was higher than that in normal tissues. Meanwhile, the overexpression of THOCs in 10 paired tumors and adjacent normal tissues of patients with HCC had been detected by qRT-PCR. Secondly, we observed that high expression of THOCs was positively correlated with higher HCC stage and tumor grade. Then, we found that the expression level of THOC1/2/3/4/5/6/7 had diagnostic value for HCC and THOC1/2/4/5/6/7 was negatively correlated with the prognosis of HCC. Therefore, THOCs may be closely related to the progress of HCC and has potential diagnostic and prognostic value. It may be prognostic biomarkers and potential therapeutic targets for patients with HCC.
Functional enrichment analysis of the PPI network composed of THOCs and their 20 interacting proteins predicted the potential mechanism of THOCs in the progression of HCC. GO analysis was mainly concentrated in RNA processing, nuclear transport, mRNA binding, etc. KEGG pathway and Reactome analysis showed that THOCs were involved in RNA transport, spliceosome, mRNA surveillance pathway, etc. Previous studies had shown that the role of THOCs is to complete the transcription process and ensure the correct processing and transport of mRNA to the cytoplasm,9 which was consistent with functional enrichment analysis. Any abnormality in the process of gene transcription may lead to up-regulation or down-regulation of genes, especially when cancer-related gene transcription abnormality occurs, which was closely related to the occurrence of tumors. As a step of mRNA processing, splicing could regulate gene expression. Abnormal splicing activity often occurs in cancer cells.30 The main role of mRNA surveillance pathway was to degrade aberrant or harmful RNAs in cells. Genetic changes in components of mRNA surveillance pathway could cause diseases, including cancer, which may be an important potential pathogenesis of cancer.31
Pathway analysis in HCC showed that THO complex members were involved in the regulation of several important cancer-related signaling pathways, such as cell cycle, apoptosis, DNA damage response pathways, EMT, RAS/MAPK pathway and RKT pathway. Abnormal expression of THOCs induced abnormal cell cycle and cell proliferation in tumor.32 THOC1 played a role in activating G2/M cell cycle checkpoint.33 THOC4 was involved in the regulation of S and G2 cell cycle, which may be related to the effect of THOC4 on the expression of cyclins.34 THOCs were associated with apoptosis.32,34 Previous evidence suggested that the consumption of THOC1,2,5–7 could induce apoptosis of HCC cells, but THOC3 did not.32 In addition, depletion of THOCs led to the accumulation of R-loops, which could cause DNA damage and was associated with genomic instability, thereby affecting tumorigenesis.8 In HCC, epithelial-to-mesenchymal transition (EMT) was mainly driven by Wnt/β-catenin signaling pathway, which was involved in the shaping of tumor microenvironment and closely related to tumor metastasis.35 There had evidence that the target gene of Wnt/β-catenin signaling pathway depended on the regulation of THOC5, which may have certain significance in the treatment of cancer.36 Meanwhile, THOCs were associated with RTK pathway and RAS/MAPK pathway, which were carcinogenic. These two pathways interact with each other and participated in the abnormal proliferation and differentiation of tumor cells, thus inducing tumor growth and invasion.37–39
Our study also explored the correlation between THOCs and drug sensitivity, and the results showed that THOCs were significantly associated with some drugs or small molecules. Recent research revealed that knockdown of THOC1 inhibited the proliferation of HCC cells and increased the sensitivity to cisplatin, which was related to the increase of R-loops caused by the decrease of THOC1. DNA damage caused by the accumulation of R-loops made cells more susceptible to genotoxic drugs. Luteolin could reduce the expression of THOC1 and then increase the sensitivity of cisplatin to liver cancer, providing a new idea for drug therapy.16 Downregulation of THOC1 or THOC5 did not induce apoptosis in normal cells but induces apoptosis in cancer cells,40–42 which would make it possible to achieve the goal of drugs targeting only tumor cells. Zhu et al reported that the difference in THOC2 expression may lead to different sensitivity of HCC patients to drugs such as methotrexate, erlotinib and vinorelbine. Low-expression THOC2 patients had higher sensitivity to chemotherapy.17 In addition, recent experiments had confirmed that THOC4 is the drug target of neuroblastoma.43 Therefore, the research of THO complex members in the direction of targeted treatment of HCC has great potential and further research is very necessary.
The potential molecular regulatory mechanism of THOCs in HCC was examined. We identified several kinase targets for THOCs. Kinase CDK1, kinase CDK2 and kinase ATM were the common targets of most THO complex members. These kinases played a role in maintaining genomic stability and regulating the cell cycle.44 ATM could induce DNA damage and led to tumor cell death.45 In addition, we found that MiR-324-5p and MiR-217 related to the expression of most THO complex members. These MiRNA targets were involved in the proliferation, invasion and metastasis of HCC cells. Previous studies revealed that MiR-324-5p is associated with the degradation of HCC extracellular matrix.46 And MiR-217 was involved in the regulation of the MAPK pathway in HCC, thus affecting the progression of EMT.47 The transcriptional factor targets of THOCs complex members were mainly related to ELK1 and NRF1. ELK1 was an important participant in EMT and mediated oxaliplatin resistance in HCC.48 ERF1 was associated with cell cycle and cell stability, and was involved in carcinogenic processes (cell proliferation, invasion, metastasis, and EMT) in HCC.49 Therefore, regulation of different targets by THO complex members may mediate tumorigenesis and progression of HCC.
Tumor immune infiltration analysis demonstrated that the expression of THOCs in HCC was significantly correlated with the infiltration of immune cells (B cells, CD4+ T cells, CD8+ T cells, Neutrophils, Macrophages, and Dendritic cells). And Cox multivariate analysis showed that THOC4 and THOC5 still affected the overall survival of HCC under the condition of immune infiltration, which may be independent prognostic biomarkers of HCC. The expression levels of the immune checkpoints including PD1 and CTLA4 were positively correlated with THOC2 expression in HCC, which had a significant function in regulating the activation of T cells.17 THOC4, one of the components of T cell enhanceosome, was involved in T cell maturation and provided the molecular basis for the expression of MHC class I genes.50 As a cytoplasmic substrate for Fms tyrosine kinase activated by macrophage colony-stimulating factor, THOC5 was directly involved in the differentiation of macrophages.51 Consequently, THOCs may be involved in the regulation of tumor immunity in HCC and affect the prognosis of HCC.
Conclusions
In summary, our research indicated that THOCs were prominently up-regulated in HCC and closely related to clinicopathological features, which may play an important role in the occurrence and development of HCC. At the same time, THOCs had great potential diagnostic and prognostic value for HCC and were negatively associated with drug sensitivity, indicating that THOCs had potential targeted therapeutic significance for HCC. Furthermore, THOCs may involve in the regulation of tumor immunity. THOCs could be new potential biomarkers and therapeutic targets for HCC.
Acknowledgments
We would like to acknowledge the databases referred to in this article that provide the genomic data and clinical data of hepatocellular carcinoma.
Funding Statement
This work was supported by Special Health Program of Jilin Province Finance Department, Special Medical and Health Personnel Program of Jilin Province Finance Department (2019SCZT015), and Health Technology Innovative Program of Jilin Province (2018J053).
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Ethics Committee of The Second Hospital of Jilin University (2018169) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients had signed informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors declare no conflicts of interest for this work.
References
- 1.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263 [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25(2):181–200. doi: 10.1055/s-2005-871198 [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853. doi: 10.1053/j.gastro.2015.12.041 [DOI] [PubMed] [Google Scholar]
- 5.Villanueva A, Hoshida Y, Battiston C, et al. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma. Gastroenterology. 2011;140(5):1501–12.e2. doi: 10.1053/j.gastro.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jimeno S, Rondón AG, Luna R, Aguilera A. The yeast THO complex and mRNA export factors link RNA metabolism with transcription and genome instability. EMBO J. 2002;21(13):3526–3535. doi: 10.1093/emboj/cdf335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strässer K, Masuda S, Mason P, et al. TREX is a conserved complex coupling transcription with messenger RNA export. Nature. 2002;417(6886):304–308. doi: 10.1038/nature746 [DOI] [PubMed] [Google Scholar]
- 8.Heath CG, Viphakone N, Wilson SA. The role of TREX in gene expression and disease. Biochem j. 2016;473(19):2911–2935. doi: 10.1042/bcj20160010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domínguez-Sánchez MS, Barroso S, Gómez-González B, Luna R, Aguilera A. Genome instability and transcription elongation impairment in human cells depleted of THO/TREX. PLoS Genet. 2011;7(12):e1002386. doi: 10.1371/journal.pgen.1002386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rondón AG, Jimeno S, Aguilera A. The interface between transcription and mRNP export: from THO to THSC/TREX-2. Biochim Biophys Acta. 2010;1799(8):533–538. doi: 10.1016/j.bbagrm.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 11.Tous C, Aguilera A. Impairment of transcription elongation by R-loops in vitro. Biochem Biophys Res Commun. 2007;360(2):428–432. doi: 10.1016/j.bbrc.2007.06.098 [DOI] [PubMed] [Google Scholar]
- 12.Liu C, Yue B, Yuan C, et al. Elevated expression of Thoc1 is associated with aggressive phenotype and poor prognosis in colorectal cancer. Biochem Biophys Res Commun. 2015;468(1–2):53–58. doi: 10.1016/j.bbrc.2015.10.166 [DOI] [PubMed] [Google Scholar]
- 13.Chinnam M, Wang Y, Zhang X, et al. The Thoc1 ribonucleoprotein and prostate cancer progression. J Natl Cancer Inst. 2014;106(11):dju306–dju306. doi: 10.1093/jnci/dju306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X, Liu X, Zhang G, et al. Knockdown THOC2 suppresses the proliferation and invasion of melanoma. Bioengineered. 2019;10(1):635–645. doi: 10.1080/21655979.2019.1685727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang JZ, Zhu W, Han J, et al. The role of the HIF-1α/ALYREF/PKM2 axis in glycolysis and tumorigenesis of bladder cancer. Cancer Commun. 2021;41(7):560–575. doi: 10.1002/cac2.12158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai S, Bai Y, Wang H, et al. Knockdown of THOC1 reduces the proliferation of hepatocellular carcinoma and increases the sensitivity to cisplatin. J Exp Clinl Cancer Res. 2020;39(1):135. doi: 10.1186/s13046-020-01634-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu J, Tang B, Gao Y, et al. Predictive models for HCC prognosis, recurrence risk, and immune infiltration based on two exosomal genes: MYL6B and THOC2. J Inflamm Res. 2021;14:4089–4109. doi: 10.2147/jir.S315957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Y, Yu X, Li J, Zhang Q, Zheng Q, Guo W. Role of m(5) C-related regulatory genes in the diagnosis and prognosis of hepatocellular carcinoma. Am J Transl Res. 2020;12(3):912–922. [PMC free article] [PubMed] [Google Scholar]
- 19.Saran S, Tran DD, Ewald F, et al. Depletion of three combined THOC5 mRNA export protein target genes synergistically induces human hepatocellular carcinoma cell death. Oncogene. 2016;35(29):3872–3879. doi: 10.1038/onc.2015.433 [DOI] [PubMed] [Google Scholar]
- 20.Chandrashekar DS, Bashel B, Balasubramanya SAH, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649–658. doi: 10.1016/j.neo.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uhlen M, Oksvold P, Fagerberg L, et al. Towards a knowledge-based human protein atlas. Nat Biotechnol. 2010;28(12):1248–1250. doi: 10.1038/nbt1210-1248 [DOI] [PubMed] [Google Scholar]
- 22.Menyhárt O, Nagy Á, Győrffy B. Determining consistent prognostic biomarkers of overall survival and vascular invasion in hepatocellular carcinoma. Royal Soc Open Sci. 2018;5(12):181006. doi: 10.1098/rsos.181006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochoa D, Hercules A, Carmona M, et al. Open targets platform: supporting systematic drug-target identification and prioritisation. Nucleic Acids Res. 2021;49(D1):D1302–d10. doi: 10.1093/nar/gkaa1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warde-Farley D, Donaldson SL, Comes O, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38:W214–W220. doi: 10.1093/nar/gkq537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Duncan D, Shi Z, Zhang B. WEB-based gene set analysis toolkit (WebGestalt): update 2013. Nucleic Acids Res. 2013;41:W77–W783. doi: 10.1093/nar/gkt439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu CJ, Hu FF, Xia MX, Han L, Zhang Q, Guo AY. GSCALite: a web server for gene set cancer analysis. Bioinformatics. 2018;34(21):3771–3772. doi: 10.1093/bioinformatics/bty411 [DOI] [PubMed] [Google Scholar]
- 27.Vasaikar SV, Straub P, Wang J, Zhang B. LinkedOmics: analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018;46(D1):D956–D963. doi: 10.1093/nar/gkx1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li T, Fan J, Wang B, et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77(21):e108–e10. doi: 10.1158/0008-5472.Can-17-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63(5):844–855. doi: 10.1136/gutjnl-2013-306627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coltri PP, Dos Santos MGP, da Silva GHG. Splicing and cancer: challenges and opportunities. Wiley interdisciplinary reviews. RNA. 2019;10(3):e1527. doi: 10.1002/wrna.1527 [DOI] [PubMed] [Google Scholar]
- 31.Wolin SL, Maquat LE. Cellular RNA surveillance in health and disease. Science. 2019;366(6467):822–827. doi: 10.1126/science.aax2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran DD, Saran S, Koch A, Tamura T. mRNA export protein THOC5 as a tool for identification of target genes for cancer therapy. Cancer Lett. 2016;373(2):222–226. doi: 10.1016/j.canlet.2016.01.045 [DOI] [PubMed] [Google Scholar]
- 33.Doostzadeh-Cizeron J, Terry NH, Goodrich DW. The nuclear death domain protein p84N5 activates a G2/M cell cycle checkpoint prior to the onset of apoptosis. J Biol Chem. 2001;276(2):1127–1132. doi: 10.1074/jbc.M006944200 [DOI] [PubMed] [Google Scholar]
- 34.Okada M, Jang SW, Ye K. Akt phosphorylation and nuclear phosphoinositide association mediate mRNA export and cell proliferation activities by ALY. Proc Natl Acad Sci USA. 2008;105(25):8649–8654. doi: 10.1073/pnas.0802533105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurzu S, Kobori L, Fodor D, Jung I. Epithelial mesenchymal and endothelial mesenchymal transitions in hepatocellular carcinoma: a review. Biomed Res Int. 2019;2019:2962580. doi: 10.1155/2019/2962580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saran S, Tran DD, Klebba-Färber S, et al. THOC5, a member of the mRNA export complex, contributes to processing of a subset of wingless/integrated (Wnt) target mRNAs and integrity of the gut epithelial barrier. BMC Cell Biol. 2013;14:51. doi: 10.1186/1471-2121-14-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delire B, Stärkel P. The Ras/MAPK pathway and hepatocarcinoma: pathogenesis and therapeutic implications. Eur J Clin Invest. 2015;45(6):609–623. doi: 10.1111/eci.12441 [DOI] [PubMed] [Google Scholar]
- 38.Heppner DE, Eck MJ. A structural perspective on targeting the RTK/Ras/MAP kinase pathway in cancer. Protein Sci. 2021;30(8):1535–1553. doi: 10.1002/pro.4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tilak M, Holborn J, New LA, Lalonde J, Jones N. Receptor tyrosine kinase signaling and targeting in glioblastoma multiforme. Int J Mol Sci. 2021;22(4):1831. doi: 10.3390/ijms22041831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Lin AW, Zhang X, Wang Y, Wang X, Goodrich DW. Cancer cells and normal cells differ in their requirements for Thoc1. Cancer Res. 2007;67(14):6657–6664. doi: 10.1158/0008-5472.Can-06-3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guria A, Tran DD, Ramachandran S, et al. Identification of mRNAs that are spliced but not exported to the cytoplasm in the absence of THOC5 in mouse embryo fibroblasts. RNA. 2011;17(6):1048–1056. doi: 10.1261/rna.2607011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran DD, Koch A, Tamura T. THOC5, a member of the mRNA export complex: a novel link between mRNA export machinery and signal transduction pathways in cell proliferation and differentiation. Cell commun signaling. 2014;12:3. doi: 10.1186/1478-811x-12-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma P, Yue L, Zhang S, et al. Target RNA modification for epigenetic drug repositioning in neuroblastoma: computational omics proximity between repurposing drug and disease. Aging. 2020;12(19):19022–19044. doi: 10.18632/aging.103671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herrera MC, Chymkowitch P, Robertson JM, et al. Cdk1 gates cell cycle-dependent tRNA synthesis by regulating RNA polymerase III activity. Nucleic Acids Res. 2018;46(22):11698–11711. doi: 10.1093/nar/gky846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drosos Y, Escobar D, Chiang MY, et al. ATM-deficiency increases genomic instability and metastatic potential in a mouse model of pancreatic cancer. Sci Rep. 2017;7(1):11144. doi: 10.1038/s41598-017-11661-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao L, Xie B, Yang X, et al. MiR-324-5p suppresses hepatocellular carcinoma cell invasion by counteracting ECM degradation through post-transcriptionally downregulating ETS1 and SP1. PLoS One. 2015;10(7):e0133074. doi: 10.1371/journal.pone.0133074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H, Ke J, Guo Q, Barnabo Nampoukime KP, Yang P, Ma K. Long non-coding RNA CRNDE promotes the proliferation, migration and invasion of hepatocellular carcinoma cells through miR-217/MAPK1 axis. J Cell Mol Med. 2018;22(12):5862–5876. doi: 10.1111/jcmm.13856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma J, Zeng S, Zhang Y, et al. BMP4 promotes oxaliplatin resistance by an induction of epithelial-mesenchymal transition via MEK1/ERK/ELK1 signaling in hepatocellular carcinoma. Cancer Lett. 2017;411:117–129. doi: 10.1016/j.canlet.2017.09.041 [DOI] [PubMed] [Google Scholar]
- 49.Ren Y, Qiu L, Lü F, et al. TALENs-directed knockout of the full-length transcription factor Nrf1α that represses malignant behaviour of human hepatocellular carcinoma (HepG2) cells. Sci Rep. 2016;6:23775. doi: 10.1038/srep23775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howcroft TK, Weissman JD, Gegonne A, Singer DS. AT lymphocyte-specific transcription complex containing RUNX1 activates MHC class I expression. J Immunol. 2005;174(4):2106–2115. doi: 10.4049/jimmunol.174.4.2106 [DOI] [PubMed] [Google Scholar]
- 51.Mancini A, Koch A, Whetton AD, Tamura T. The M-CSF receptor substrate and interacting protein FMIP is governed in its subcellular localization by protein kinase C-mediated phosphorylation, and thereby potentiates M-CSF-mediated differentiation. Oncogene. 2004;23(39):6581–6589. doi: 10.1038/sj.onc.1207841 [DOI] [PubMed] [Google Scholar]