Abstract
Background
Primary antibiotic resistance in Helicobacter pylori (H. pylori) strains is increasing worldwide, affecting therapy success. The use of therapies tailored on susceptibility pre-testing at culture has been proposed, but data are still conflicting.
Method
We performed a systematic review to evaluate the role of a culture-based therapeutic approach for H. pylori treatment, taking into account the sensitivity of culture and the success rates achieved with tailored therapies in different therapeutic steps.
Results
We analyzed data from 51 studies. Overall, H. pylori strains were isolated in 80.7% of 7889 patients, the success rates being 78.1%, 77.5%, 86.3% and 86.6%, before first-, second-, third-line or more therapies, respectively. In comparative studies, the infection was cured in 89.9% of 2052 patients treated with tailored therapies, and in 77.6% of 2516 patients receiving empiric therapy (P<0.001). However, in the subanalysis, the tailored approach achieved optimal eradication rates (>90%) only when it was applied before first- and second-line therapies, but not before third-line or more attempts (<80%). Moreover, no significant difference emerged between the 2 approaches when data from only the most recent (last 5 years) studies were considered, as well as in those performed in Western populations.
Conclusions
The attempt to achieve antibiotic susceptibility testing before treatment failed in 20% of infected patients managed in dedicated laboratories. Culture-tailored therapies administered after 2 or more therapies achieved suboptimal eradication rates. The role of bacterial culture in patients whose therapeutic management failed to eradicate H. pylori probably needs to be corroborated by further data.
Keywords: Helicobacter pylori, culture, therapy, resistance
Introduction
Helicobacter pylori (H. pylori) infection plays a major role in the pathogenesis of different gastroduodenal disorders. These comprise benign diseases, such as non-ulcer dyspepsia, peptic ulcer, idiopathic thrombocytopenic purpura, and idiopathic iron deficiency anemia, as well as tumors, including gastric mucosa-associated lymphoid tissue lymphoma and adenocarcinoma [1-5]. Indeed, H. pylori eradication definitely changes the natural history of both peptic ulcer disease and gastric lymphoma, and significantly improves dyspeptic symptoms and platelet count. Moreover, the risk of gastric cancer development is reduced by 44% following the cure of H. pylori infection [5]. Unfortunately, curing this infection remains challenging for clinicians, as no available empiric therapy regimen is able to achieve bacterial eradication in all treated patients. Therefore, a definite portion of patients requires 2 or more treatments in clinical practice. To overcome the impact of antibiotic resistance, administration of therapies tailored on susceptibility pre-testing at culture has been proposed as a more rational approach than empirical use of standard treatment regimens. According to the Kyoto consensus, H. pylori gastritis is fundamentally an infectious disease, and principles of antimicrobial stewardship focusing on optimization of therapy to reliably achieve high cure rates and prevent resistance to the antimicrobials should be used [6]. As for other infectious diseases, this approach is expected to achieved bacterial eradication in virtually all patients. Moreover, the diffusion of bacterial resistance toward different antibiotics in other bacterial strains might be limited by reducing the use of empiric therapies. Current Italian and European guidelines suggest that susceptibility pretesting should be performed following 2 or more consecutive therapy failures [7,8]. Some studies have even proposed the use of bacterial culture to choose the first-line therapy according to antibiotic susceptibility results [9,10]. Nevertheless, clear evidence favoring the tailored over the empiric treatment is still lacking. In addition, isolating H. pylori from gastric biopsies is challenging, and dedicated laboratories for culture, with skilled personnel and specific equipment, are not widespread, limiting the implementation of this approach in clinical practice. Based on these observations, we aimed to evaluate the role of a culture-based therapeutic approach for H. pylori treatment by considering the sensitivity of culture and the success rate achieved with tailored therapies in different therapeutic steps.
Materials and methods
Literature review
A systematic literature review was performed to find studies reporting data on: a) H. pylori culture sensitivity and/or b) the cure rates with successive tailored treatments. Only those studies that provided these data separately according to different therapeutic steps − from before first-line to 3 or more attempts − were considered. Studies that included pediatric patients and those in languages other than English were excluded, and case series with less than 5 patients were not considered. The search was performed in PubMed for publications from January 1, 2004, through March 5, 2021, using the following algorithm (all fields): (Helicobacter pylori OR H. pylori) AND culture. Following title and abstract evaluation, the full text of all relevant studies was retrieved, and the reference lists from the selected articles were reviewed to identify additional studies of potential interest. For each study, the variables extracted were as follows: author, year of publication, country, setting (pre- or post-treatment step), number of patients, the type of culture (E-test or agar dilution) as well as the rate of both successful culture and bacterial eradication. Authors were not contacted in case of absent/incomplete data; those studies were excluded from the analysis. Two investigators (VDF and AZ) extracted data according to a specifically designed database and conflicting data were eventually resolved by another investigator (DV).
Statistical analysis
Eradication rates and their 95% confidence intervals (CI) on intention-to-treat (ITT) analysis were computed for each subanalysis. Comparison of cure rates was performed using the chi-squared test. Differences were considered significant at a 5% probability level. Analyses were performed using Statsoft version 7.1 (StatSoft Europe GmbH, 22301 Hamburg, Germany) for Windows 10.
Results
Descriptive analysis
A total of 3015 citations were found, of which 51 met the inclusion criteria on evaluation (Fig. 1) [11-61]. Eleven studies originated from Japan, 10 from China, 7 from each of Italy and Taiwan, 5 from Spain, 4 from Korea, 2 studies from each Australia and Poland, and 1 study from each of Portugal, USA and Israel. The main characteristics of the studies are shown in Table 1.
Figure 1.
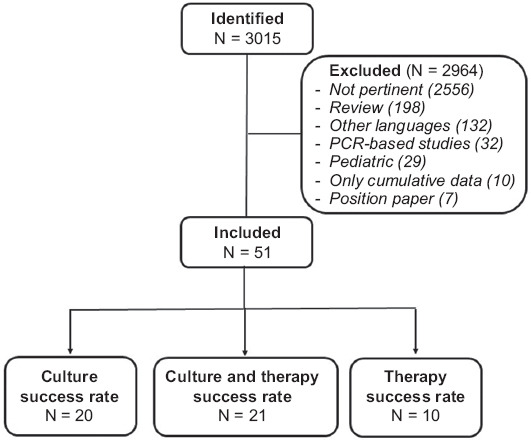
The PRISMA flow diagram
PCR, polymerase chain reaction
Table 1.
Characteristics of studies

Culture sensitivity
Data on culture sensitivity separately reported for different lines of treatment were available in 41 studies [11-51]. Overall, H. pylori strains were isolated in 6371 (80.7%, 95%CI 79.8-81.6) of 7889 infected patients. More specifically, culture was performed using a single antral specimen in 5053 patients and it was positive in 4052 of them (80.1%, 95%CI 79-81.2), and by antral plus gastric body mucosa specimens in 2836 patients, being positive in 2319 cases (81.7%, 95%CI 80.3-83.1), without a significant difference between groups. Bacterial growth was achieved in 2055 (78.1%, 95%CI 76.5-79.7) of 2538 patients before first-line therapy, in 2691 (77.5%, 95%CI 76.1-78.9) of 3470 patients before second-line, in 1313 (86.3%, 95%CI 84.6-88) of 1521 patients before third-line, and in 312 (86.6%, 95%CI 83.1-90.1) of 360 patients following more therapeutic failures. The success rates of culture performed following either 2 (86.3%) or more (86.6%) treatments were significantly higher than those achieved before (78.1%) and after (77.5%) the first-line therapy (P<0.001).
Eradication rates
A total of 31 studies reported data on therapeutic outcomes [31-61]. Of these, 19 studies compared the cure rates between culture-tailored and empiric therapies [31-42,52-58], whilst only the cure rates of tailored therapies were reported in the remaining 12 studies [43-51,59-61].
In comparative studies, the overall cure rate following tailored therapies (1842 of 2052 patients; 89.7%, 95%CI 88.4-91.2) was higher (P<0.001) than that of empiric treatments (1954 of 2516 patients; 77.6%, 95%CI 76-79.2). Specifically, the eradication rates were 91.6% (914/997) and 78.2% (1006/1285), respectively, before first-line therapy (P<0.001), 91.2% (702/769) and 79% (753/953) (P<0.001) before second-line, and 79% (226/286) and 70.1% (195/278) (P=0.03) before third-line (or more) approaches (Table 2). When subanalyses of these data were performed according to either different periods or diverse geographic areas, some differences emerged. Indeed, a comparison of the results of studies published in the last 5 years with earlier studies showed that tailored therapies administered before third-line treatments were significantly more effective than empiric therapy only in the older, but not in the more recent studies (Table 3). Considering the different populations, the overall H. pylori cure rates following tailored and empiric therapy were, respectively, 84.3% (2115/2491) and 79.1% (563/711) (P<0.001) in Western and 89.2% (1632/1828) and 77% (1391/1805) (P<0.001) in Asian countries. The success rate of tailored therapies in Western populations was lower than that of Asian patients (84.3% vs. 89.2%; P<0.001), whilst no difference emerged between empiric therapies (79.1% vs. 77%; P=0.25).
Table 2.
Cure rates at intention to treat analysis following culture-tailored and empiric therapies

Table 3.
Eradication rates following tailored and empiric therapy in different periods

Data from 12 non-comparative studies showed a cumulative eradication rate of 83.1% (1885/2268), with values of 88.6% in 220 patients, 82.4% in 490, and 82.5% in 1558 cases, when the culture was performed before first-, second- and third-line (or more) therapies, respectively (Table 4). The cure rate achieved before first-line (88.6%) therapy was significantly higher than that following other steps (82.4% and 83.8%; P=0.04). By totaling all available (comparative and non-comparative) studies, the susceptibility-based approach achieved a cure rate of 91.1% (1109/1217), 87.8% (1106/1259) and 83% (1532/1844) before first-, second- or third-line (or more) therapies, respectively, with a statistically significant (P=0.03) trend in the reduction of cure rates among the 3 steps.
Table 4.
Overall eradication rates at intention to treat following culture-tailored therapies

Susceptibility-based method
As far as the method for antibiotic susceptibility testing used in the considered studies is concerned, the E-test was utilized in 19 series with a total of 2935 patients, and the agar dilution method in 12 studies with 1259 cases. In comparative studies, a similar eradication rate was achieved by therapies tailored on agar dilution testing (811 of 905; 89.6%, 95%CI 87.6-91.6) and that based on the E-test (918 of 1021; 89.9%, 95%CI 88-91.7). In non-comparative studies, the overall cure rate achieved in the 4 studies (321 of 354 patients) with therapies tailored on agar dilution was higher than that of 8 studies (1584 of 1914 patients) based on the E-test results (90.6% vs. 82.7%, P<0.001). By totalling the data of all studies, the success rate was significantly (P<0.001) higher when therapies were tailored on agar dilution (1132 of 1254; 89.9%, 95%CI 88.2-91.6) as compared to the E-test (2502 of 2935; 85.2%, 95%CI 84-86.5).
Discussion
Although several factors are involved, primary and secondary bacterial resistance towards the few available antibiotics are the most relevant for H. pylori therapy failure. More specifically, resistance to clarithromycin, metronidazole and levofloxacin is increasing worldwide and affects the success rate of therapeutic regimens containing these drugs [10]. In contrast, the prevalence of primary resistance towards amoxicillin, tetracycline and rifabutin remains distinctly low [62,63]. Therefore, tailoring eradication therapy on the antibiotic susceptibility testing is expected to be successful. We performed this study in order to assess the probability of bacterial recovery at culture and to estimate the success rate of antibiotic susceptibility-tailored therapies in different steps of the therapeutic sequence for H. pylori eradication.
Overall, our pooled-data analysis found that the bacterial growth failed in 20% of infected patients managed in dedicated microbiology laboratories. The data showed that the success rate of culture was significantly higher when performed following 2 or more therapeutic attempts, compared to that performed in naïve patients or just following first-line therapy, when the value was even lower than 80%. Therefore, the attempt to guide first-line therapy for H. pylori failed in 1 of every 5 infected patients. On the other hand, when the culture was attempted in unselected naïve patients who underwent upper endoscopy, the antibiotic susceptibility testing was achieved in only 247 (13.3%) of 1862 cases [59]. Based on all these observations, tailoring first-line therapy seems to be impractical in clinical practice.
By considering data from more than 4500 patients, we compared the cure rates achieved by therapies tailored on antibiotic susceptibility and those empirically administered. As expected, the culture-tailored therapies were more successful overall than empiric regimens. Moreover, another advantage of the tailored strategy is to achieve bacterial eradication, in keeping with the rules of antibiotic stewardship, whilst empiric therapies inevitably lead to the misuse of antibiotics. Nevertheless, optimal (>90%) eradication rates were achieved only when the tailored strategy was adopted before first- and second-line regimens, whilst suboptimal (<80%) cure rates were observed following 2 or more therapy failures, namely when current European guidelines suggest that culture-tailored therapies should be administered [8]. In addition, the therapeutic advantage of culture-tailored first-line therapy was mainly due to the low efficacy of the empiric therapies used as comparator in the studies we evaluated. Indeed, despite the use of first-line regimens including only those antibiotics with a proven susceptibility at culture, the eradication rate was not distinctly higher than 90%. This success rate would appear to be no different to that achieved by the more effective therapies currently available, such as sequential, concomitant and quadruple first-line regimens administered empirically [64]. In addition, the lack of any therapeutic advantage of culture-based therapies clearly emerged in more recent studies, and in Western populations. Some host factors, especially those affecting the proton pump inhibitor metabolism (e.g., CYP 2C19 polymorphisms, etc.) may be involved in the difference between Asian and Caucasian studies.
The failure of an antibiotic-based therapy may depend on several factors, such as drug doses, therapy duration, and potency of the anti-secretory drug used. Moreover, tailored therapy may fail to achieve a 100% eradication rate because of the impossibility of assembling in this phase a successful therapy regimen that includes only the still available antibiotics to which H. pylori is susceptible. Indeed, the synergism among different antibiotics, chosen because of the absence of bacterial resistance, could be lacking. For instance, following the administration of a 14-day bismuth–tetracycline–amoxicillin combination, 3 drugs with no or a very low (<5%) primary resistance rate, the eradication rate was as low as 43% [65]. Therefore, apart from antibiotic susceptibility, drug combinations are a matter for concern in H. pylori. On other hand, the possibility of a hetero-resistant status—i.e., the coexistence of susceptible and resistant H. pylori strains at different gastric sites in the same patient—may undermine the correct classification of antibiotic susceptibility in vitro [66]. The same result is expected when antibiotic susceptibility is genetically assessed using a culture-free, polymerase chain reaction-based tool, which overcomes the culture limitation of bacterial growth.
Notably, data from a recent systematic review showed that by adopting an empiric therapeutic sequence with bismuth-based quadruple therapy, levofloxacin- and rifabutin-based regimens following first-line therapy failure, H. pylori infection might be cured in virtually all patients, with only 1 in every 170 patients eventually remaining infected [67]. Therefore, the real advantage of resorting to bacterial susceptibility testing before third-line therapy, as suggested in the current guidelines [7,8], probably needs to be corroborated by further data. Finally, our analysis found that the tool used for antibiotic susceptibility testing seem to plays a role in predicting the success rate. Specifically, higher eradication rates were achieved when the therapy was tailored on agar dilution as compared to the E-test. Therefore, the agar dilution method should be preferred when deciding on culture-based antibiotic susceptibility testing, but this method is impractical as it is very laborious and time-consuming.
Some limitations of our study should be acknowledged, such as inclusion of case series with heterogeneous samples (from 20 to 813), the use of an arbitrary timeline, and the absence of any search for publications in other databases. However, we identified as many as 51 studies with more than 4500 patients, so it is highly improbable there are many other studies not present in PubMed able to significantly change the results of this systematic review.
In conclusion, our study found that the probability of isolating H. pylori in infected patients by culture in dedicated laboratories is 80%. The overall success rate of tailored regimens was higher than that of empiric therapies, but data from more recent studies and those performed in Western population failed to find a statistically significant difference, particularly following 2 or more therapy failures. Even when only antibiotics to which the infection is susceptible were used, the eradication rate was not higher than 90%.
Summary Box.
What is already known:
Helicobacter pylori (H. pylori) eradication remains challenging for clinicians, as no available empiric therapy regimen is able to achieve bacterial eradication in all treated patients
Primary and secondary bacterial resistance towards antibiotics is the most relevant factor for therapy failure
Culture-based therapeutic approaches are expected to cure the infection in virtually all treated patients
What the new findings are:
Data found that H. pylori growth failed in 20% of infected patients managed in dedicated microbiology laboratories, particularly when performed in naive patients
Culture-tailored therapies were more successful than empiric regimens, but optimal (>90%) eradication rates were achieved only when the tailored strategy was adopted before first- and second-line therapies, whilst suboptimal (<80%) cure rates were observed following 2 or more therapy failures
The lack of advantage of culture-based therapies over empiric approaches emerged in more recent studies, and in the Western populations
Biography
Riuniti Hospital, Foggia; Nuovo Regina Margherita Hospital, Rome; Generale Hospital, Perugia; Villa Aurora Clinic, Foligno, Perugia; University of Bologne; Sant’Orsola Hospital, Bologna, Italy
Footnotes
Conflict of Interest: None
References
- 1.Alakkari A, Zullo A, O'Connor HJ. Helicobacter pylori and nonmalignant diseases. Helicobacter. 2011;16(Suppl 1):33–37. doi: 10.1111/j.1523-5378.2011.00878.x. [DOI] [PubMed] [Google Scholar]
- 2.Rokkas T, Rokka A, Portincasa P. A systematic review and meta-analysis of the role of Helicobacter pylori eradication in preventing gastric cancer. Ann Gastroenterol. 2017;30:414–423. doi: 10.20524/aog.2017.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zullo A, Rago A, Felici S, Licci S, Ridola L, Caravita di Toritto T. Onset and progression of precancerous lesions on gastric mucosa of patients treated for gastric lymphoma. J Gastrointestin Liver Dis. 2020;29:27–31. doi: 10.15403/jgld-516. [DOI] [PubMed] [Google Scholar]
- 4.Abrignani MG, Gatta L, Gabrielli D, et al. Gastroprotection in patients on antiplatelet and/or anticoagulant therapy:a position paper of National Association of Hospital Cardiologists (ANMCO) and the Italian Association of Hospital Gastroenterologists and Endoscopists (AIGO) Eur J Intern Med. 2021;85:1–13. doi: 10.1016/j.ejim.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Elli L, Norsa L, Zullo A, et al. Diagnosis of chronic anaemia in gastrointestinal disorders:A guideline by the Italian Association of Hospital Gastroenterologists and Endoscopists (AIGO) and the Italian Society of Paediatric Gastroenterology Hepatology and Nutrition (SIGENP) Dig Liver Dis. 2019;51:471–483. doi: 10.1016/j.dld.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Sugano K, Tack J, Kuipers EJ, et al. faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zagari RM, Romano M, Ojetti V, et al. Guidelines for the management of Helicobacter pylori infection in Italy:The III Working Group Consensus Report 2015. Dig Liver Dis. 2015;47:903–912. doi: 10.1016/j.dld.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Malfertheiner P, Megraud F, O'Morain CA, et al. European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 9.López-Góngora S, Puig I, Calvet X, et al. Systematic review and meta-analysis:susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother. 2015;70:2447–2455. doi: 10.1093/jac/dkv155. [DOI] [PubMed] [Google Scholar]
- 10.Megraud F, Bruyndonckx R, Coenen S, et al. European Helicobacter pylori Antimicrobial Susceptibility Testing Working Group. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut. 2021;70:1815–1822. doi: 10.1136/gutjnl-2021-324032. [DOI] [PubMed] [Google Scholar]
- 11.Ueki N, Miyake K, Kusunoki M, et al. Impact of quadruple regimen of clarithromycin added to metronidazole-containing triple therapy against Helicobacter pylori infection following clarithromycin-containing triple-therapy failure. Helicobacter. 2009;14:91–99. doi: 10.1111/j.1523-5378.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- 12.Borody TJ, Pang G, Wettstein AR, et al. Efficacy and safety of rifabutin-containing 'rescue therapy'for resistant Helicobacter pylori infection. Aliment Pharmacol Ther. 2006;23:481–488. doi: 10.1111/j.1365-2036.2006.02793.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuo CH, Hsu PI, Kuo FC, et al. Comparison of 10 day bismuth quadruple therapy with high-dose metronidazole or levofloxacin for second-line Helicobacter pylori therapy:a randomized controlled trial. J Antimicrob Chemother. 2013;68:222–228. doi: 10.1093/jac/dks361. [DOI] [PubMed] [Google Scholar]
- 14.Kuo CH, Hu HM, Kuo FC, et al. Efficacy of levofloxacin-based rescue therapy for Helicobacter pylori infection after standard triple therapy:a randomized controlled trial. J Antimicrob Chemother. 2009;63:1017–1024. doi: 10.1093/jac/dkp034. [DOI] [PubMed] [Google Scholar]
- 15.Liang CM, Tai WC, Hsu PI, et al. Trend of changes in antibiotic resistance in Helicobacter pylori from 2013 to 2019:a multicentre report from Taiwan. Therap Adv Gastroenterol. 2020;13:1756284820976990. doi: 10.1177/1756284820976990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuah SK, Tai WC, Hsu PI, et al. The efficacy of second-line anti-Helicobacter pylori therapy using an extended 14-day levofloxacin/amoxicillin/proton-pump inhibitor treatment—a pilot study. Helicobacter. 2012;17:374–381. doi: 10.1111/j.1523-5378.2012.00960.x. [DOI] [PubMed] [Google Scholar]
- 17.Dore MP, Tadeu V, Are B, et al. Efficacy of a rescue ciprofloxacin-based regimen for eradication of Helicobacter pylori infection after treatment failures. Gastroenterol Res Pract. 2012;2012:484591. doi: 10.1155/2012/484591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuta T, Kato M, Sugimoto M, et al. Japan Gast Study Group. Triple therapy with ecabet sodium, amoxicillin and lansoprazole for 2 weeks as the rescue regimen for Hpylori infection. Intern Med. 2011;50:369–374. doi: 10.2169/internalmedicine.50.4305. [DOI] [PubMed] [Google Scholar]
- 19.Hori K, Miwa H, Matsumoto T. Efficacy of 2-week, second-line Helicobacter pylori eradication therapy using rabeprazole, amoxicillin, and metronidazole for the Japanese population. Helicobacter. 2011;16:234–240. doi: 10.1111/j.1523-5378.2011.00842.x. [DOI] [PubMed] [Google Scholar]
- 20.Liou JM, Chen CC, Chen MJ, et al. Empirical modified sequential therapy containing levofloxacin and high-dose esomeprazole in second-line therapy for Helicobacter pylori infection:a multicentre clinical trial. J Antimicrob Chemother. 2011;66:1847–1852. doi: 10.1093/jac/dkr217. [DOI] [PubMed] [Google Scholar]
- 21.Murakami K, Okimoto T, Kodama M, Sato R, Watanabe K, Fujioka T. Evaluation of three different proton pump inhibitors with amoxicillin and metronidazole in retreatment for Helicobacter pylori infection. J Clin Gastroenterol. 2008;42:139–142. doi: 10.1097/MCG.0b013e31802cbc1a. [DOI] [PubMed] [Google Scholar]
- 22.Murakami K, Okimoto T, Kodama M, et al. Comparison of amoxicillin-metronidazole plus famotidine or lansoprazole for amoxicillin-clarithromycin-proton pump inhibitor treatment failures for Helicobacter pylori infection. Helicobacter. 2006;11:436–440. doi: 10.1111/j.1523-5378.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 23.Murakami K, Sato R, Okimoto T, et al. Maintenance therapy with H2-receptor antagonist until assessment of Helicobacter pylori eradication can reduce recurrence of peptic ulcer after successful eradication of the organism:prospective randomized controlled trial. J Gastroenterol Hepatol. 2006;21:1048–1053. doi: 10.1111/j.1440-1746.2005.04038.x. [DOI] [PubMed] [Google Scholar]
- 24.Nishizawa T, Suzuki H, Masaoka T, Iwasaki E, Hibi T. A new eradication resistance index as a predictor of metronidazole-containing second-line treatment of Helicobacter pylori. Digestion. 2007;76:215–220. doi: 10.1159/000112649. [DOI] [PubMed] [Google Scholar]
- 25.Perna F, Zullo A, Ricci C, Hassan C, Morini S, Vaira D. Levofloxacin-based triple therapy for Helicobacter pylori re-treatment:role of bacterial resistance. Dig Liver Dis. 2007;39:1001–1005. doi: 10.1016/j.dld.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Shirai N, Sugimoto M, Kodaira C, et al. Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after the standard triple therapy. Eur J Clin Pharmacol. 2007;63:743–749. doi: 10.1007/s00228-007-0302-8. [DOI] [PubMed] [Google Scholar]
- 27.Kudo T, Fujinami H, Ando T, et al. Comparison of lafutidine and rabeprazole in 7-day second-line amoxicillin- and metronidazole-containing triple therapy for Helicobacter pylori:a pilot study. Helicobacter. 2012;17:277–281. doi: 10.1111/j.1523-5378.2012.00943.x. [DOI] [PubMed] [Google Scholar]
- 28.Togawa J, Inamori M, Fujisawa N, et al. Efficacy of a triple therapy with rabeprazole, amoxicillin, and faropenem as second-line treatment after failure of initial Helicobacter pylori eradication therapy. Hepatogastroenterology. 2005;52:645–648. [PubMed] [Google Scholar]
- 29.Tai WC, Lee CH, Chiou SS, et al. The clinical and bacteriological factors for optimal levofloxacin-containing triple therapy in second-line Helicobacter pylori eradication. PLoS One. 2014;9:e105822. doi: 10.1371/journal.pone.0105822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu DC, Hsu PI, Chen A, et al. Randomized comparison of two rescue therapies for Helicobacter pylori infection. Eur J Clin Invest. 2006;36:803–809. doi: 10.1111/j.1365-2362.2006.01725.x. [DOI] [PubMed] [Google Scholar]
- 31.Huang HT, Wang HM, Yang SC, et al. Efficacy of a 14-day quadruple-therapy regimen for third-line Helicobacter pylori eradication. Infect Drug Resist. 2018;11:2073–2080. doi: 10.2147/IDR.S185511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mascellino MT, Oliva A, De Angelis M, Pontone S, Porowska B. Helicobacter pylori infection:antibiotic resistance and eradication rate in patients with gastritis showing previous treatment failures. New Microbiol. 2018;41:306–309. [PubMed] [Google Scholar]
- 33.Dong F, Ji D, Huang R, et al. Multiple genetic analysis system-based antibiotic susceptibility testing in Helicobacter pylori and high eradication rate with phenotypic resistance-guided quadruple therapy. Medicine (Baltimore) 2015;94:e2056. doi: 10.1097/MD.0000000000002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon YH, Kim N, Lee JY, et al. Comparison of the efficacy of culture-based tailored therapy for Helicobacter pylori eradication with that of the traditional second-line rescue therapy in Korean patients:a prospective single tertiary center study. Scand J Gastroenterol. 2016;51:270–276. doi: 10.3109/00365521.2015.1095352. [DOI] [PubMed] [Google Scholar]
- 35.Martos M, Bujanda L, Salicio Y, et al. Clarithromycin for first-line treatment of Helicobacter pylori infection after culture in high-resistance regions. Eur J Gastroenterol Hepatol. 2014;26:1380–1384. doi: 10.1097/MEG.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 36.Cosme A, Montes M, Martos M, et al. Usefulness of antimicrobial susceptibility in the eradication of Helicobacter pylori. Clin Microbiol Infect. 2013;19:379–383. doi: 10.1111/j.1469-0691.2012.03844.x. [DOI] [PubMed] [Google Scholar]
- 37.Molina-Infante J, Pazos-Pacheco C, Vinagre-Rodriguez G, et al. Nonbismuth quadruple (concomitant) therapy:empirical and tailored efficacy versus standard triple therapy for clarithromycin-susceptible Helicobacter pylori and versus sequential therapy for clarithromycin-resistant strains. Helicobacter. 2012;17:269–276. doi: 10.1111/j.1523-5378.2012.00947.x. [DOI] [PubMed] [Google Scholar]
- 38.Marzio L, Coraggio D, Capodicasa S, Grossi L, Cappello G. Role of the preliminary susceptibility testing for initial and after failed therapy of Helicobacter pylori infection with levofloxacin, amoxicillin, and esomeprazole. Helicobacter. 2006;11:237–242. doi: 10.1111/j.1523-5378.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 39.Park CS, Lee SM, Park CH, et al. Pretreatment antimicrobial susceptibility-guided vs clarithromycin-based triple therapy for Helicobacter pylori eradication in a region with high rates of multiple drug resistance. Am J Gastroenterol. 2014;109:1595–1602. doi: 10.1038/ajg.2014.222. [DOI] [PubMed] [Google Scholar]
- 40.Zhou J, Huo H, Wu M, Jiang X. Role of drug sensitivity test in the triple therapy for eradication of Helicobacter pylori. Chin J Gastroenterol. 2010;15:358–360. [Google Scholar]
- 41.Zhuo RP, Chen XP, Wu SZ, Xie JL, Hu SK. Clinical effects of quadruple therapy based on antimicrobial susceptibility testing in treatment of Helicobacter pylori associated upper digestive tract diseases. Shijie Huaren Xiaohua Zazhi. 2015;23:196–201. [Google Scholar]
- 42.Kong S, Huang KM, Wang J, et al. Efficacy of tailored second-line therapy of Helicobacter pylori eradication in patients with clarithromycin-based treatment failure:a multicenter prospective study. Gut Pathog. 2020;12:39. doi: 10.1186/s13099-020-00378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saracino IM, Pavoni M, Zullo A, et al. Antibiotic resistance and therapy outcome in Hpylori eradication failure patients. Antibiotics (Basel) 2020;9:121. doi: 10.3390/antibiotics9030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu L, Luo L, Long X, et al. Susceptibility-guided therapy for Helicobacter pylori infection treatment failures. Therap Adv Gastroenterol. 2019;12:1756284819874922. doi: 10.1177/1756284819874922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szadkowski A, Zemlak M, Muszyński J. Effectiveness of Helicobacter pylori eradication established on the basis of examination of antibiotic resistance of the bacteria. Prz Gastroenterol. 2018;13:93–98. doi: 10.5114/pg.2018.75821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan B, Yang JC, Young CL, et al. Helicobacter pylori antimicrobial susceptibility testing-guided salvage therapy in the USA:a real life experience. Dig Dis Sci. 2018;63:437–445. doi: 10.1007/s10620-017-4880-8. [DOI] [PubMed] [Google Scholar]
- 47.Cammarota G, Martino A, Pirozzi G, et al. High efficacy of 1-week doxycycline- and amoxicillin-based quadruple regimen in a culture-guided, third-line treatment approach for Helicobacter pylori infection. Aliment Pharmacol Ther. 2004;19:789–795. doi: 10.1111/j.1365-2036.2004.01910.x. [DOI] [PubMed] [Google Scholar]
- 48.Fiorini G, Vakil N, Zullo A, et al. Culture-based selection therapy for patients who did not respond to previous treatment for Helicobacter pylori infection. Clin Gastroenterol Hepatol. 2013;11:507–510. doi: 10.1016/j.cgh.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Tay CY, Windsor HM, Thirriot F, et al. Helicobacter pylori eradication in Western Australia using novel quadruple therapy combinations. Aliment Pharmacol Ther. 2012;36:1076–1083. doi: 10.1111/apt.12089. [DOI] [PubMed] [Google Scholar]
- 50.Matsumoto Y, Miki I, Aoyama N, et al. Levofloxacin- versus metronidazole-based rescue therapy for Hpylori infection in Japan. Dig Liver Dis. 2005;37:821–825. doi: 10.1016/j.dld.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 51.Shimoyama T, Fukuda S, Mikami T, Fukushi M, Munakata A. Efficacy of metronidazole for the treatment of clarithromycin-resistant Helicobacter pylori infection in a Japanese population. J Gastroenterol. 2004;39:927–930. doi: 10.1007/s00535-004-1424-8. [DOI] [PubMed] [Google Scholar]
- 52.Yahav J, Samra Z, Niv Y, et al. Susceptibility-guided vs empiric retreatment of Helicobacter pylori infection after treatment failure. Dig Dis Sci. 2006;51:2316–2321. doi: 10.1007/s10620-006-9302-2. [DOI] [PubMed] [Google Scholar]
- 53.Cosme A, Lizasoan J, Montes M, et al. Antimicrobial susceptibility-guided therapy versus empirical concomitant therapy for eradication of Helicobacter pylori in a region with high rate of clarithromycin resistance. Helicobacter. 2016;21:29–34. doi: 10.1111/hel.12231. [DOI] [PubMed] [Google Scholar]
- 54.Criado RB, Pérez Citores L, Pérez-Millán AG, et al. Prospective comparative study of the treatment of Helicobacter pylori with antibiotic susceptibility testing-guided triple therapy compared to quadruple therapy with Pylera®. Rev Esp Enferm Dig. 2021;113:597–601. doi: 10.17235/reed.2020.7395/2020. [DOI] [PubMed] [Google Scholar]
- 55.Ji CR, Liu J, Li YY, et al. Susceptibility-guided quadruple therapy is not superior to medication history-guided therapy for the rescue treatment of Helicobacter pylori infection:A randomized controlled trial. J Dig Dis. 2020;21:549–557. doi: 10.1111/1751-2980.12934. [DOI] [PubMed] [Google Scholar]
- 56.Byambajav TO, Bira N, Choijamts G, et al. Initial trials with susceptibility-based and empiric anti-Hpylori therapies in Mongolia. Front Pharmacol. 2019;10:394. doi: 10.3389/fphar.2019.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferenc S, Gnus J, Kościelna M, et al. High antibiotic resistance of Helicobacter pylori and its effect on tailored and empiric eradication of the organism in Lower Silesia, Poland. Helicobacter. 2017:22. doi: 10.1111/hel.12365. [DOI] [PubMed] [Google Scholar]
- 58.Zhou LY, Zhang JZ, Song Z, et al. Tailored versus triple plus bismuth or concomitant therapy as initial Helicobacter pylori treatment:a randomized trial. Helicobacter. 2016;21:91–99. doi: 10.1111/hel.12242. [DOI] [PubMed] [Google Scholar]
- 59.Kim YM, Lee KH, Kim JH, et al. Is only clarithromycin susceptibility important for the successful eradication of Helicobacter pylori? Antibiotics (Basel) 2020;9:589. doi: 10.3390/antibiotics9090589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee JW, Kim N, Nam RH, et al. Favorable outcomes of culture-based Helicobacter pylori eradication therapy in a region with high antimicrobial resistance. Helicobacter. 2019;24:e12561. doi: 10.1111/hel.12561. [DOI] [PubMed] [Google Scholar]
- 61.Costa S, Soares JB, Gonçalves R. Efficacy and tolerability of culture-guided treatment for Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2017;29:1258–1263. doi: 10.1097/MEG.0000000000000960. [DOI] [PubMed] [Google Scholar]
- 62.Gisbert JP. Rifabutin for the treatment of Helicobacter pylori infection:a review. Pathogens. 2020;10:15. doi: 10.3390/pathogens10010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baylina M, Muñoz N, Sánchez-Delgado J, López-Góngora S, Calvet X, Puig I. Systematic review:would susceptibility-guided treatment achieve acceptable cure rates for second-line Helicobacter pylori therapy as currently practiced? Helicobacter. 2019;24:e12584. doi: 10.1111/hel.12584. [DOI] [PubMed] [Google Scholar]
- 64.Gisbert JP. Empirical or susceptibility-guided treatment for Helicobacter pylori infection?A comprehensive review. Therap Adv Gastroenterol. 2020;13:1756284820968736. doi: 10.1177/1756284820968736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Graham DY, Lew GM, Ramirez FC, Genta RM, Klein PD, Malaty HM. Short report:a non-metronidazole triple therapy for eradication of Helicobacter pylori infection—tetracycline, amoxicillin, bismuth. Aliment Pharmacol Ther. 1993;7:111–113. doi: 10.1111/j.1365-2036.1993.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 66.De Francesco V, Giorgio F, Ierardi E, D'Ercole C, Hassan C, Zullo A. Helicobacter pylori clarithromycin resistance assessment:are gastric antral biopsies sufficient? Gastroenterol Insights. 2012;4:7–8. [Google Scholar]
- 67.De Francesco V, Zullo A, Manta R, et al. Helicobacter pylori eradication following first-line treatment failure in Europe:what, how and when chose among different standard regimens?A systematic review. Eur J Gastroenterol Hepatol. 2021;33(1S Suppl 1):e66–e70. doi: 10.1097/MEG.0000000000002100. [DOI] [PubMed] [Google Scholar]


