Abstract
Background
Fecal microbiota transplantation (FMT) is a highly efficacious procedure used most commonly for the treatment of recurrent Clostridioides difficile infection (CDI). Despite the high value of incorporating FMT into practice, there remain concerns about its safety. To the best of our knowledge, there has not been an updated meta-analysis reporting pooled rates of adverse events in FMT for CDI.
Methods
A search for studies of FMT in patients with CDI was performed with the rate of serious adverse events (SAEs) related to FMT evaluated as the primary outcome. Secondary outcomes included SAEs unrelated to FMT and minor adverse events associated with FMT. A pooled analysis was then performed.
Results
Initial search identified 378 reference articles. Data were extracted from the 61 of these studies that met the inclusion criteria, comprising 5099 patients. Pooled analysis showed that SAEs related to FMT developed in less than 1% of patients. The pooled rate of SAEs not related to FMT was higher at 2.9%. The pooled rate of minor adverse events also showed infrequent self-limited gastrointestinal and systemic discomfort.
Conclusions
This meta-analysis supports FMT as a safe option for treating recurrent CDI. Future randomized trials are needed to improve our current understanding of FMT safety and further examine the improvements in the quality of life of patients treated with FMT compared to standard therapy of antibiotics.
Keywords: Fecal microbiota transplantation, Clostridioides difficile, adverse events
Introduction
Clostridioides difficile infection (CDI) has emerged as a significant cause of human morbidity and mortality [1]. It is now estimated that CDI has an incidence up to 32.6 per 100,000 person-years, with a direct care cost of $4.8 billion per year in the USA alone [2]. This gram-positive, spore-forming anaerobe is the most common cause of pseudomembranous colitis—a condition characterized by intractable diarrhea with the formation of intestinal pseudomembranes of cellular material in the colon [3]. As a result of these physical characteristics, as well as the development of multidrug resistance, the challenge of effectively treating CDI continues to grow [1].
Given the prevalence and increasing antibiotic resistance of CDI, fecal microbiota transplantation (FMT) is emerging as an exciting alternative to antibiotic therapies in preventing recurrent and complicated CDI. Since its initial implementation, the frequency of use has grown significantly. Current guidelines recommend FMT for patients with multiple recurrences of antibiotic-treated CDI [4]. Accurate study of FMT is challenging, given the heterogeneity of administration protocols. One issue that arises is the variation in stool preparation—studies have described usage of both fresh and frozen stool, various sources of stool (family, pooled, or standardized preparation), and inconsistent donor and stool screening protocols [5,6]. An additional challenge has been the quality of these studies; many of the randomized controlled trials that have compared FMT to antibiotic therapy have limited follow up as well as antibiotic protocols not within the standard of care [5]. Regardless, FMT has been shown to be of comparable efficacy to standard medical management [7,8]. Evidence regarding its efficacy with various routes of administration shows inconsistent results, but has widely demonstrated significant efficacy [8,9].
These data speak to the exciting role FMT is coming to play in the treatment of CDI. However, many continue to have concerns about the procedure’s safety [5,6,10-14]. Recent studies have shown that many patients are unsure of whether they would accept FMT as a treatment option [15,16]. A major concern expressed by many patients is consequences arising from insufficient donor screening for infectious agents [16]. Some physicians also echo this fear, with many citing the need for further research on the topic, even voicing concerns of harms outweighing benefits [17,18]. With the increasing utilization of antibiotics and chemotherapeutic agents, the incidence of CDI will continue to rise. It is crucial to understand the risks of FMT so that patients may be counseled appropriately before undergoing the procedure. Moreover, awareness of FMT-related complications may drive the development of improved treatment modalities and protocols. There has not, to our knowledge, been a meta-analysis defining the pooled rates of major and minor adverse events for CDI in the general population. This information is vital for ensuring patients and providers are able to make informed decisions regarding their treatment.
Materials and methods
Search methodology
A literature search was conducted using the electronic database engines MEDLINE through PubMed, Ovid, Cochrane Library (Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews) and EMBASE, from January 1st 2015 to January 1st 2021, to identify published articles and reports addressing the use of FMT in patients with CDI. The combinations of keywords used were (“Enterocolitis, Pseudomembranous”[Mesh] OR “CDI”) AND (“Fecal Microbiota Transplantation”[Mesh] OR “FMT”). The reference list of all eligible studies was reviewed to identify additional studies. The retrieved studies were carefully examined to exclude potential duplicates or overlapping data. Titles and abstracts selected from the initial search were scanned, and the full papers of potentially eligible studies were reviewed.
Study eligibility
Published studies were eligible for inclusion if they reported the use of FMT for the management of CDI. Articles were excluded if they were not written in English or did not have English translations, if they included a pediatric population or studied FMT for non-CDI indications, or if no outcomes were reported. In studies using multiple modalities for the management of CDI, data from the cohort of patients who underwent FMT were collected and analyzed. Two reviewers (ER, MB) independently performed study selection according to eligibility criteria. Disagreements were resolved by discussion or a third reviewer. The agreement between reviewers for the collected data gave a Cohen k value of 1.0.
Data extraction
The following data were independently abstracted onto a standardized form: study characteristics (primary author, time period of study, year of publication, and country of the population studied), study design, baseline characteristics of the study population (the numbers of patients enrolled, participant demographics, route of FMT), the intervention details and outcomes (adverse events). Risk of bias was rated for each study by 2 authors independently, using the Cochrane criteria for randomized controlled trials [19].
Outcome definition
The primary outcome of interest was the rate of serious adverse events (SAEs) (NCI Common Terminology Criteria for Adverse Events grade 3-5) related to FMT. The rate of SAEs determined to be unrelated to FMT, minor adverse events (grades 1-2) and the rate of specific SAEs were evaluated as a secondary outcome.
Statistical analysis
This meta-analysis was performed by calculating pooled proportions. First, the individual study proportions were transformed into a quantity using the Freeman-Tukey variant of the arcsine square root transformed proportion. The pooled proportion is calculated as the back-transform of the weighted mean of the transformed proportions, using inverse arcsine variance weights for the fixed effects model and DerSimonian-Laird weights for the random effects model [20]. Forest plots were drawn to show the point estimates in each study in relation to the summary pooled estimate. The width of the point estimates in the Forest plots indicates the weight assigned to that study. The heterogeneity among studies was tested using the I2 statistic and Cochran Q test based upon inverse variance weights [20]. I2 values of 0-39% were considered as nonsignificant heterogeneity, 40-75% as moderate heterogeneity, and 76-100% as considerable heterogeneity. If the P-value is >0.10, it rejects the null hypothesis that the studies are heterogeneous. The effect of publication and selection bias on the summary estimates was tested using the Harbord-Egger bias indicator [21].
Results
A total of 378 studies were found using the above search criteria. After removing duplicated studies, studies that did not describe adverse events, studies that focused primarily on pediatric populations, and studies for non-CDI indications, 61 remained (Table 1) [22-82]. A Preferred Reporting Items for Systematic Reviews and Meta-analysis flow diagram for the review process is shown in Fig. 1 [83]. Of these 61 studies, 16 were randomized control trials. Pooled estimates were calculated by the fixed effect model for better accuracy, based on the nature of individual study characteristics and heterogeneity. Data were collected for a total of 5099 patients receiving 5551 FMTs. An upper gastrointestinal route was specified in 30% of cases of FMTs and a lower gastrointestinal route in 56%. In the overall population of patients, 4.8% of recipients had inflammatory bowel disease and 8.0% were immunosuppressed.
Table 1.
Characteristics of studies reviewed
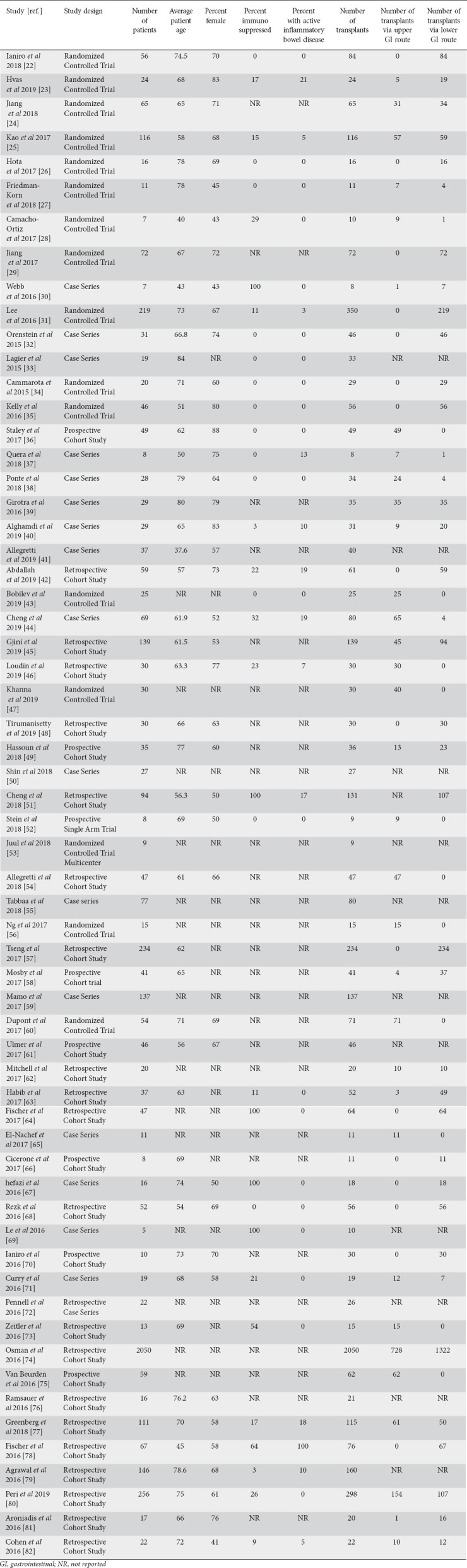
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram detailing the review process
Table 2.
Outcomes of reviewed studies
Primary outcome
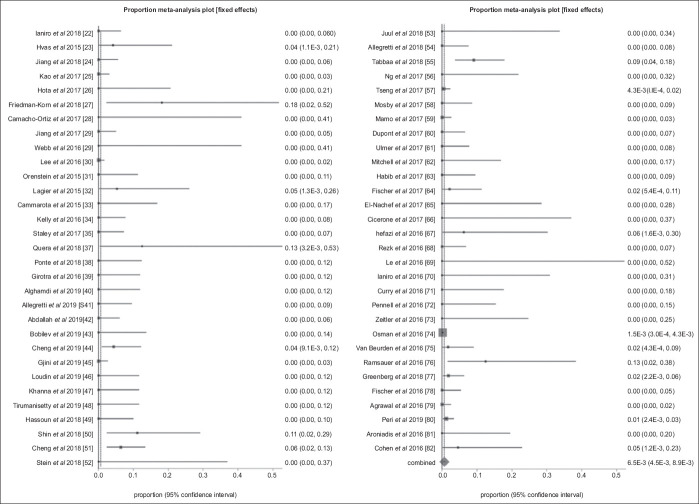
In pooled analysis, the overall rate of SAEs related to FMT was 0.65% (95%CI 0.45-0.89; P<0.001). A forest plot diagram of this pooled analysis is shown in Fig. 2. Publication bias calculated using the Harbord-Egger bias indicator gave a value of 1.10 (95%CI 0.26-1.94; P=0.02), indicating no publication bias. Fig. 3 is a funnel plot assessing the publication bias for the same variable.
Figure 2.
Forest plot. Individual study proportions and the pooled estimate of the rate of serious adverse events related to fecal microbiota transplantation (random effect)
Figure 3.
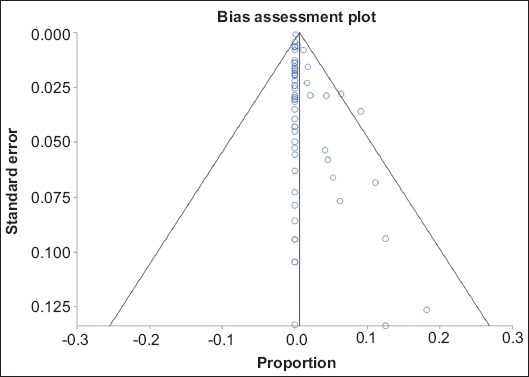
Bias assessment plot of publication bias in reporting serious adverse events in fecal microbiota transplantation
Secondary outcomes
Rate of individual SAEs
Sepsis or sepsis-like conditions were reported in 0.19% (95%CI 0.09-0.31), aspiration pneumonia in 0.27% (95%CI 0.15-0.43), and bowel perforation was noted after 0.20% (95%CI 0.09-0.34) of FMTs. The pooled rate of SAEs not related to FMT was 2.91% (95%CI 2.47-3.39).
Rate of minor adverse events
Among minor adverse events, constipation was reported in 1.03% (95%CI 0.77-1.33), abdominal pain in 1.66% (95%CI 1.33-2.03), nausea in 0.92% (95%CI 0.67-1.20), vomiting in 0.34% (95%CI 0.20-0.52), flatulence in 0.70% (95%CI 0.49-0.94), and febrile episodes were noted after 0.33% (95%CI 0.19-0.50) of FMTs.
Discussion
FMT is rapidly gaining acceptance as a treatment for CDI. In an episode of CDI, major dysbiosis is commonly seen, with suppression of native Bacteroidetes and Firmicutes species and an increase in Proteobacteria [84]. FMT has been shown to restore this balance, with evidence that the composition of an FMT recipient’s microbiome retains similarity to the donor’s for months to years [85]. With the complexity of the microbiota being transplanted, several mechanisms have been observed. The first consists of direct competition of the transplanted microorganisms—through both resource competition and production of antimicrobial peptides [85]. Additionally, FMT restores a normal balance of bile acid metabolization in the gut, a process demonstrated to affect cellular signaling and spore germination [84,85]. Finally, it has been suggested that the protection FMT offers the mucosal barrier of the colon plays a role in favorably altering the immune system’s response to CDI [85].
A wealth of randomized clinical trials supports the effectiveness of FMT for recurrent CDI. This progress is vital, given the heavy disease burden CDI carries and the major risks associated with uncontrolled CDI [4,7,8]. Antimicrobial success rate in recurrent CDI is low, only about 35%, and surgery has very poor outcomes, with mortality up to 50% [86,87]. FMT involves the infusion of stool from a healthy donor to an infected patient with the goal of restoring a healthy microbiome, and exists as an exciting alternate approach for treatment that utilizes a novel and exciting mechanism [85]. However, there is still hesitancy regarding the implementation of FMT in the standard of care [15-18]. Our analysis seeks to further explore the safety of FMT, to ensure patients and physicians have an optimal data-driven approach to considering FMT.
To our knowledge, this is the largest published systematic review with a meta-analysis of adverse events for FMT in CDI, and it offers several advantages compared to the previously published literature. This meta-analysis establishes that FMT is safe when used for CDI, with significant adverse events noted in less than 1% of the patients. This knowledge is invaluable in aiding decision making for patients and physicians and supports FMT as an excellent alternative option to standard therapy with antibiotics—especially for recurrent CDI. The majority of the significant adverse events noted in our review were unrelated to the FMT itself, which is unsurprising given that FMT is often administered in patients with severe, treatment-refractory CDI with multiple baseline medical comorbidities. Additionally, a relatively high percentage of the included patients were immunosuppressed, which could account for exaggeration of negative sequelae. Finally, minor adverse events, including nausea, vomiting, abdominal pain and constipation, were also noted very rarely, with an individual pooled rate of less than 2%, lower than previously reported [10,14].
The primary challenge faced by our review was the determination of SAE causality. The process for determining whether or not to attribute an adverse event to FMT was based on each study’s own standards. An area that highlights this difficulty is the unclear causality of inflammatory bowel disease flares and FMT. While some studies listed this as a sequela of FMT, others ruled it to be unrelated. An additional challenge was the mild inconsistency in several of the measured outcomes. This can probably be attributed to the heterogeneous patient populations and study protocols. Similarly, the average duration of follow up varied widely, as did symptom reporting. Missing data on demographics, method of stool transplantation, volume and amount of stool, and relationships of donor and recipients were also common [6].
This study, despite its limitations, demonstrated that FMT is a largely safe procedure. As the understanding of the effects of the fecal microbiome expands, causal relationships with new adverse events and long-term sequelae of FMT may continue to be discovered. Nevertheless, our current knowledge of both related and unrelated SAEs indicates that FMT should be a therapy strongly considered for patients with recurrent CDI.
This meta-analysis supports FMT as a safe option for treating recurrent CDI. While the short-term safety of fecal microbiota transplantation for treating recurrent CDI is promising from our meta-analysis, the potential long-term consequences of altering a patient’s gut microbiota are not fully known. Future randomized trials are needed to improve our current understanding of FMT safety and further clarify the improvements in the quality of life of patients treated with FMT compared to standard antibiotic therapy.
Summary Box.
What is already known:
● Fecal microbiota transplantation (FMT) is a highly efficacious procedure used in the treatment of recurrent Clostridioides difficile infection
● A residual concern in the integration of FMT is concerns about the safety of the procedure
● Published studies have struggled with heterogeneous protocols that display various durations of follow up
What the new findings are:
● Our analysis shows a very low pooled rate of significant adverse events related to FMT, in total less than 1%, despite a significant portion of patients being immunocompromised or having underlying gastrointestinal conditions
● The pooled rate of minor adverse events was also relatively rare, and were most commonly diarrhea, constipation, abdominal pain, nausea and vomiting
● Further high-quality randomized control trials are necessary to evaluate the longer-term safety of FMT and its impact on quality of life
Acknowledgment
We would like to thank Ms. Carmen Howard, Regional Health Sciences Librarian & Instructor for the University of Illinois College of Medicine in Peoria for her assistance in developing search criteria and her excellent guidance
Biography
University of Illinois College of Medicine at Peoria, Peoria, IL, USA
Footnotes
Conflict of Interest: None
References
- 1.Spigaglia P. Recent advances in the understanding of antibiotic resistance in Clostridium difficile infection. Ther Adv Infect Dis. 2016;3:23–42. doi: 10.1177/2049936115622891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Depestel DD, Aronoff DM. Epidemiology of Clostridium difficile infection. J Pharm Pract. 2013;26:464–475. doi: 10.1177/0897190013499521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czepiel J, Dróżdż M, Pituch H, et al. Clostridium difficile infection:review. Eur J Clin Microbiol Infect Dis. 2019;38:1211–1221. doi: 10.1007/s10096-019-03539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children:2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis. 2018;66:e1–e48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan X, Johnson S. Fecal microbiota transplantation (FMT) for C difficile infection, just say 'No'. Anaerobe. 2019;60:102092. doi: 10.1016/j.anaerobe.2019.102092. [DOI] [PubMed] [Google Scholar]
- 6.Lai CY, Sung J, Cheng F, et al. Systematic review with meta-analysis:review of donor features, procedures and outcomes in 168 clinical studies of faecal microbiota transplantation. Aliment Pharmacol Ther. 2019;49:354–363. doi: 10.1111/apt.15116. [DOI] [PubMed] [Google Scholar]
- 7.Khan MY, Dirweesh A, Khurshid T, Siddiqui WJ. Comparing fecal microbiota transplantation to standard-of-care treatment for recurrent Clostridium difficile infection:a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:1309–1317. doi: 10.1097/MEG.0000000000001243. [DOI] [PubMed] [Google Scholar]
- 8.Quraishi MN, Widlak M, Bhala N, et al. Systematic review with meta-analysis:the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. 2017;46:479–493. doi: 10.1111/apt.14201. [DOI] [PubMed] [Google Scholar]
- 9.Tariq R, Pardi DS, Bartlett MG, Khanna S. Low cure rates in controlled trials of fecal microbiota transplantation for recurrent Clostridium difficile infection:a systematic review and meta-analysis. Clin Infect Dis. 2019;68:1351–1358. doi: 10.1093/cid/ciy721. [DOI] [PubMed] [Google Scholar]
- 10.Wang S, Xu M, Wang W, et al. Systematic review:adverse events of fecal microbiota transplantation. PLoS One. 2016;11:e0161174. doi: 10.1371/journal.pone.0161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li YT, Cai HF, Wang ZH, Xu J, Fang JY. Systematic review with meta-analysis:long-term outcomes of faecal microbiota transplantation for Clostridium difficile infection. Aliment Pharmacol Ther. 2016;43:445–457. doi: 10.1111/apt.13492. [DOI] [PubMed] [Google Scholar]
- 12.Alhifany AA, Almutairi AR, Almangour TA, et al. Comparing the efficacy and safety of faecal microbiota transplantation with bezlotoxumab in reducing the risk of recurrent Clostridium difficile infections:a systematic review and Bayesian network meta-analysis of randomised controlled trials. BMJ Open. 2019;9:e031145. doi: 10.1136/bmjopen-2019-031145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali FS, Soin S, Abu-Sbeih H, Sundararajan N. Mo1959 –a meta-analysis of the safety and efficacy of fecal microbiota transplantation for the treatment of Clostridium difficile infection in solid organ transplant recepients. Gastroenterology. 2019;156:S-901. [Google Scholar]
- 14.Kim S. The safety and effectiveness of fecal microbiota transplantation:systematic review and meta-analysis. Value Health. 2018;21:S41–S42. [Google Scholar]
- 15.Kahn SA, Vachon A, Rodriquez D, et al. Patient perceptions of fecal microbiota transplantation for ulcerative colitis. Inflamm Bowel Dis. 2013;19:1506–1513. doi: 10.1097/MIB.0b013e318281f520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gundling F, Roggenbrod S, Schleifer S, Sohn M, Schepp W. Patient perception and approval of faecal microbiota transplantation (FMT) as an alternative treatment option for obesity. Obes Sci Pract. 2019;5:68–74. doi: 10.1002/osp4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madar PC, Petre O, Baban A, Dumitrascu DL. Medical students'perception on fecal microbiota transplantation. BMC Med Educ. 2019;19:368. doi: 10.1186/s12909-019-1804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill M, Blacketer C, Chitti F, et al. Physician and patient perceptions of fecal microbiota transplant for recurrent or refractory Clostridioides difficile in the first 6 years of a central stool bank. JGH Open. 2020;4:950–957. doi: 10.1002/jgh3.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 [Internet] 2021. [Accessed 28 January 2022]. [updated February;cited Dec 5, 2021] Available from: https://training.cochrane.org/handbook .
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 22.Ianiro G, Masucci L, Quaranta G, et al. Randomised clinical trial:faecal microbiota transplantation by colonoscopy plus vancomycin for the treatment of severe refractory Clostridium difficile infection-single versus multiple infusions. Aliment Pharmacol Ther. 2018;48:152–159. doi: 10.1111/apt.14816. [DOI] [PubMed] [Google Scholar]
- 23.Hvas CL, Dahl Jørgensen SM, Jørgensen SP, et al. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent Clostridium difficile infection. Gastroenterology. 2019;156:1324. doi: 10.1053/j.gastro.2018.12.019. 1332.e3. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Z, Jenq RR, Ajami NJ, et al. Safety and preliminary efficacy of orally administered lyophilized fecal microbiota product compared with frozen product given by enema for recurrent Clostridium difficile infection:A randomized clinical trial. PLoS One. 2018;13:e0205064. doi: 10.1371/journal.pone.0205064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kao D, Roach B, Silva M, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection:a randomized cinical trial. JAMA. 2017;318:1985–1993. doi: 10.1001/jama.2017.17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hota SS, Sales V, Tomlinson G, et al. Oral vancomycin followed by fecal transplantation versus tapering oral vancomycin treatment for recurrent Clostridium difficile infection:an open-label, randomized controlled trial. Clin Infect Dis. 2017;64:265–271. doi: 10.1093/cid/ciw731. [DOI] [PubMed] [Google Scholar]
- 27.Friedman-Korn T, Livovsky DM, Maharshak N, et al. Fecal transplantation for treatment of Clostridium difficile infection in elderly and debilitated patients. Dig Dis Sci. 2018;63:198–203. doi: 10.1007/s10620-017-4833-2. [DOI] [PubMed] [Google Scholar]
- 28.Camacho-Ortiz A, Gutiérrez-Delgado EM, Garcia-Mazcorro JF, et al. Randomized clinical trial to evaluate the effect of fecal microbiota transplant for initial Clostridium difficile infection in intestinal microbiome. PLoS One. 2017;12:e0189768. doi: 10.1371/journal.pone.0189768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang ZD, Ajami NJ, Petrosino JF, et al. Randomised clinical trial:faecal microbiota transplantation for recurrent Clostridum difficile infection - fresh, or frozen, or lyophilised microbiota from a small pool of healthy donors delivered by colonoscopy. Aliment Pharmacol Ther. 2017;45:899–908. doi: 10.1111/apt.13969. [DOI] [PubMed] [Google Scholar]
- 30.Webb BJ, Brunner A, Ford CD, Gazdik MA, Petersen FB, Hoda D. Fecal microbiota transplantation for recurrent Clostridium difficile infection in hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2016;18:628–233. doi: 10.1111/tid.12550. [DOI] [PubMed] [Google Scholar]
- 31.Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection:a randomized clinical trial. JAMA. 2016;315:142–149. doi: 10.1001/jama.2015.18098. [DOI] [PubMed] [Google Scholar]
- 32.Orenstein R, Dubberke E, Hardi R, et al. Safety and durability of RBX2660 (microbiota suspension) for recurrent Clostridium difficile infection:results of the PUNCH CD study. Clin Infect Dis. 2016;62:596–602. doi: 10.1093/cid/civ938. [DOI] [PubMed] [Google Scholar]
- 33.Lagier J-, Delord M, Million M, et al. Dramatic reduction in Clostridium difficile ribotype 027-associated mortality with early fecal transplantation by the nasogastric route:a preliminary report. Eur J Clin Microbiol Infect Dis. 2015;34:1597–6101. doi: 10.1007/s10096-015-2394-x. [DOI] [PubMed] [Google Scholar]
- 34.Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial:faecal microbiota transplantation by colonoscopy vs vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41:835–843. doi: 10.1111/apt.13144. [DOI] [PubMed] [Google Scholar]
- 35.Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection:a randomized trial. Ann Intern Med. 2016;165:609–916. doi: 10.7326/M16-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staley C, Hamilton MJ, Vaughn BP, et al. Successful resolution of recurrent Clostridium difficile infection using freeze-dried, encapsulated fecal microbiota. Gastroenterology. 2017;152:S343–S344. doi: 10.1038/ajg.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quera R, Ibáñez P, Simian D, Rivera D, Acuña G, Espinoza R. Fecal microbiota transplantation through colonoscopy for Clostridium difficile recurrent infection. Report of eight cases. Rev Med Chil. 2018;146:823–830. doi: 10.4067/s0034-98872018000800823. [DOI] [PubMed] [Google Scholar]
- 38.Ponte A, Pinho R, Mota M, et al. Fecal microbiota transplantation in refractory or recurrent Clostridium difficile infection:a real-life experience in a non-academic center. Rev Esp Enferm Dig. 2018;110:311–315. doi: 10.17235/reed.2018.5099/2017. [DOI] [PubMed] [Google Scholar]
- 39.Girotra M, Garg S, Anand R, Song Y, Dutta SK. Fecal microbiota transplantation for recurrent Clostridium difficile infection in the elderly:long-term outcomes and microbiota changes. Dig Dis Sci. 2016;61:3007–3015. doi: 10.1007/s10620-016-4229-8. [DOI] [PubMed] [Google Scholar]
- 40.Alghamdi AA, Tabb D. Fecal microbiota transplantation after oral vancomycin for recurrent Clostridium difficile infection. Infect Dis Clin Pract. 2019;27:356–359. [Google Scholar]
- 41.Allegretti JR, Mullish B, Hurtado J, et al. Short chain fatty acid profiles are altered by fecal microbiota transplantation for the treatment of inflammatory bowel disease and recurrent Clostridioides difficile infection. Am J Gastroenterol. 2019;114:S484–S485. [Google Scholar]
- 42.Abdallah M, Abdalla A, Wiedel N, Baloun B, Gilbert J. Fecal microbiota transplantation for recurrent Clostridioides difficile infection:Efficacy and predictors of success. Am J Gastroenterol. 2019;114:S78. [Google Scholar]
- 43.Bobilev D, Vaickus L, DePetrillo P, et al. VE303, a live biotherapeutic product for prevention of recurrent Clostridioides difficile (C difficile) infection. Preliminary results of a phase 1, open-label healthy volunteers study of oral VE303 after vancomycin. Gastroenterology. 2019;156:S-900. [Google Scholar]
- 44.Cheng Y-, Alhaffar D, Saha S, et al. Fecal microbiota transplantation is safe and effective for the treatment of Clostridium difficile infection in patients with liver cirrhosis. Gastroenterology. 2019;156:S-899. [Google Scholar]
- 45.Gjini P, Rybnicek D, Green P. Constipation is a complication of oral encapsulated fecal microbial transplantation. Gastroenterology. 2019;156:S-900. [Google Scholar]
- 46.Loudin M, Hakki M. Institutional experience with capsule fecal microbiota transplantation for recurrent Clostridium difficile infection. Gastroenterology. 2019;156:S-904. [Google Scholar]
- 47.Khanna S, Pardi DS, Gerding DN, et al. Durable freedom from Clostridium difficile infection recurrence and microbiome restoration during six-month follow-up for a phase 1 clinical trial of RBX7455?An investigational room temperature-stable, oral microbiota-based therapeutic. Gastroenterology. 2019;156:S-1158. [Google Scholar]
- 48.Tirumanisetty P, Disalle M, Chodos A. Volume of fecal filtrate for fecal microbial transplantation does not affect the Clostridium difficile infection cure rate. Gastroenterology. 2019;156:S-910. [Google Scholar]
- 49.Hassoun A, Edwards J. Evaluation of fecal microbiota transplant (FMT) in elderly patients with recurrent Clostridium difficile infection (CDI) Open Forum Infect Dis. 2018;5:S336. [Google Scholar]
- 50.Shin Y, Bang B, Kwon K. Fecal microbiota transplantation for refractory and recurrent C difficile. Helicobacter. 2018;23:101–102. [Google Scholar]
- 51.Cheng Y-, Phelps EL, Ganipini V, et al. Fecal microbiota transplantation for the treatment of Clostridium difficile infection is efficacious and safe in solid organ transplant recipients. Gastroenterology. 2018;154:S-1044. [Google Scholar]
- 52.Stein DJ, Harrington K, Bader H, et al. Oral freeze-dried fecal microbiota capsule treatment is effective for recurrent and refractory C difficile infection. Gastroenterology. 2018;154:S-856. [Google Scholar]
- 53.Juul FE, Skudal H, Øines MN, et al. Fecal microbiota transplant versus antibiotics for primary Clostridium difficile infection –a multicenter, randomized proof-of-concept trial. Gastroenterology. 2018;154:S-10. [Google Scholar]
- 54.Allegretti JR, Fischer M, Shu E, et al. Fecal microbiota transplantation capsules with targerted colonic versus gastric delivery in recurrent C difficile infection:a comparative cohort analysis of high and low doses. Gastroenterology. 2018;154:S-1050. [Google Scholar]
- 55.Tabbaa O, Rastogi P, Alukal J, Laster J, Mattar M. Long-term safety and efficacy of fecal microbiota transplantation in the treatment of Clostridium difficile infection in patients with and without inflammatory bowel disease:A tertiary care center's experience. Gastroenterology. 2018;154:S78–S79. doi: 10.14740/gr1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ng SCC, Wong SH, Lui RN, et al. Vancomycin followed by fecal microbiota transplantation versus vancomycin for initial Clostridium difficile infection:An open-label randomised controlled trial. United Eur Gastroenterol J. 2017;5:A314. [Google Scholar]
- 57.Tseng AS, Crowell M, Orenstein R, Patron RL, DiBaise J. Older patient age is associated with similar safety but higher relapse after fecal microbiota transplantation for recurrent Clostridium difficile infection. Am J Gastroenterol. 2017;112:S51. [Google Scholar]
- 58.Mosby D, Mcgraw P, Duffalo C, et al. Factors affecting effectiveness of fecal microbiota transplant. Open Forum Infect Dis. 2017;4:S386. [Google Scholar]
- 59.Mamo Y, Woodworth M, Sitchenko K, Dhere T, Kraft C. Durability and long-term clinical outcomes of fecal microbiota transplant (FMT) treatment in patients with recurrent C difficile infection. Open Forum Infect Dis. 2017;4:S384–S385. doi: 10.1093/cid/cix1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dupont H, Jiang ZD, Alexander A, et al. Lyophilized fecal microbiota transplantation capsules for recurrent Clostridium difficile infection. Open Forum Infect Dis. 2017;4:S381. [Google Scholar]
- 61.Ulmer L, Verma A, Brock J, Iyer R. Fecal microbiota transplant for C difficile colitis from thawed frozen stool and “real world”experience in a community hospital over two years. Gastroenterology. 2017;152:S341. [Google Scholar]
- 62.Mitchell SW, DeZoysa P, Leis S, et al. Adverse effects of liquid vs encapsulated lyophilized fullspectrum microbiota for the treatment of Clostridium difficile infection. Gastroenterology. 2017;152:S346–S347. [Google Scholar]
- 63.Habib I, Huq N, Muddana V. Standardized openbiome product as a treatment for Clostridium difficile infections:a single center experience. Gastroenterology. 2017;152:S951. [Google Scholar]
- 64.Fischer M, Khan M, Phelps EL, et al. Fecal microbiota transplantation is safe and effective for the treatment of Clostridium difficile infection in solid organ transplant recipients. Gastroenterology. 2017;152:S1005. [Google Scholar]
- 65.El-Nachef N, Piceno YM, Kassam Z, et al. Fecal microbiota transplantation is safe and effective in chronic pouchitis patients. Gastroenterology. 2017;152:S1009. [Google Scholar]
- 66.Cicerone C, Bruno G, Lamonaca L, Trancassini M, Corazziari ES. Fecal microbiota transplantation for recurrent Clostridium difficile infection:Transplant protocol by retention enema and preliminary results. Dig Liver Dis. 2017;49:e175. [Google Scholar]
- 67.Hefazi M, Patnaik MM, Hogan WJ, Litzow MR, Pardi DS, Khanna S. Safety and efficacy of fecal microbiota transplantation for recurrent Clostridium infection in patients with hematologic malignancies. Blood. 2016;128:3599. [Google Scholar]
- 68.Rezk AN, Stewart D, West S, Miao C, Khara HS, Komar M. Outcomes, safety and predictors of failure of fecal microbiota transplantation for refractory Clostridium difficile infection. Am J Gastroenterol. 2016;111:S82–S83. [Google Scholar]
- 69.Le L, El-Nachef N. Fecal microbiota transplantation in solid organ transplant and hematopoietic stem cell transplant patients with recurrent or refractory Clostridium difficile infection:A case series. Am J Gastroenterol. 2016;111:S615. [Google Scholar]
- 70.Ianiro G, Masucci L, Nagel D, et al. Repeat fecal microbiota transplantation by colonoscopy for Clostridium difficile-associated pseudomembranous colitis:results from a prospective, single-centre cohort. United Eur Gastroenterol J. 2016;4:A654. [Google Scholar]
- 71.Curry S, Bogdanovich T, Pakstis D, Schwartz M, Binion D. Fecal microbiota transplantation for treatment of recurrent Clostridium difficile infections using recipient-directed donors sero- matched for latent viruses:The University of Pittsburgh Medical Center (UPMC) experience. Open Forum Infect Dis. 2016;3(suppl_1):1200. [Google Scholar]
- 72.Pennell B, Hussain C, Theodoropoulos N, et al. Safety and efficacy of fecal microbiota transplants for the treatment of Clostridium difficile at a tertiary care academic medical center. Open Forum Infect Dis. 2016;3(Suppl_1):1208. [Google Scholar]
- 73.Zeitler K, Joshi R, Montero J, Alrabaa S. Fecal microbiota transplantation outcomes in immunocompetent and immunocompromised patients:A single center experience. Open Forum Infect Dis. 2016;3(Suppl_1):2111. doi: 10.1111/tid.12726. [DOI] [PubMed] [Google Scholar]
- 74.Osman M, O'Brien K, Stoltzner Z, et al. Safety and efficacy of fecal microbiota transplantation for recurrent Clostridium difficile infection from an international public stool bank:Results from a 2050-patient multicenter cohort. Open Forum Infect Dis. 2016;3(Suppl_1):2120. [Google Scholar]
- 75.Van Beurden YH, De Groot PF, Van Nood E, Nieuwdorp M, Keller JJ, Goorhuis A. Complications and long term follow-up of fecal microbiota transplantation for treatment of recurrent Clostridium difficile infection. Gastroenterology. 2016;150:S544. doi: 10.1177/2050640616678099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramsauer B, König C, Sabelhaus T, Ockenga J, Otte J-M. Fecal microbiota transplantation in relapsing Clostridium difficile colitis. MMW Fortschr Med. 2016;158(Suppl 4):17–20. doi: 10.1007/s15006-016-8305-y. [DOI] [PubMed] [Google Scholar]
- 77.Greenberg SA, Youngster I, Cohen NA, et al. Five years of fecal microbiota transplantation - an update of the Israeli experience. World J Gastroenterol. 2018;24:5403–5414. doi: 10.3748/wjg.v24.i47.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fischer M, Kao D, Kelly C, et al. Fecal microbiota transplantation is safe and efficacious for recurrent or refractory Clostridium difficile infection in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:2402–2409. doi: 10.1097/MIB.0000000000000908. [DOI] [PubMed] [Google Scholar]
- 79.Agrawal M, Aroniadis OC, Brandt LJ, et al. The long-term efficacy and safety of fecal microbiota transplant for recurrent, severe, and complicated Clostridium difficile infection in 146 elderly individuals. J Clin Gastroenterol. 2016;50:403–407. doi: 10.1097/MCG.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 80.Peri R, Aguilar RC, Tüffers K, et al. The impact of technical and clinical factors on fecal microbiota transfer outcomes for the treatment of recurrent Clostridioides difficile infections in Germany. United European Gastroenterol J. 2019;7:716–722. doi: 10.1177/2050640619839918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aroniadis OC, Brandt LJ, Greenberg A, et al. Long-term follow-up study of fecal microbiota transplantation for severe and/or complicated Clostridium difficile infection:a multicenter experience. J Clin Gastroenterol. 2016;50:398–402. doi: 10.1097/MCG.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 82.Cohen NA, Livovsky DM, Yaakobovitch S, et al. A Retrospective comparison of fecal microbial transplantation methods for recurrent Clostridium difficile infection. Isr Med Assoc J. 2016;18:594–599. [PubMed] [Google Scholar]
- 83.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses:the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weingarden AR, Chen C, Bobr A, et al. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am J Physiol Gastrointest Liver Physiol. 2014;306:G310–G319. doi: 10.1152/ajpgi.00282.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol. 2016;13:508–516. doi: 10.1038/nrgastro.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Longo WE, Mazuski JE, Virgo KS, Lee P, Bahadursingh AN, Johnson FE. Outcome after colectomy for Clostridium difficile colitis. Dis Colon Rectum. 2004;47:1620–1626. doi: 10.1007/s10350-004-0672-2. [DOI] [PubMed] [Google Scholar]
- 87.Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection:fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(Suppl 2):S154–S161. doi: 10.1093/cid/cis462. [DOI] [PMC free article] [PubMed] [Google Scholar]