Abstract
The blaIMP gene coding for the IMP-1 metallo-β-lactamase produced by a Pseudomonas aeruginosa clinical isolate (isolate 101/1477) was overexpressed via a T7 expression system in Escherichia coli BL21(DE3), and its product was purified to homogeneity with a final yield of 35 mg/liter of culture. The structural and functional properties of the enzyme purified from E. coli were identical to those of the enzyme produced by P. aeruginosa. The IMP-1 metallo-β-lactamase exhibits a broad-spectrum activity profile that includes activity against penicillins, cephalosporins, cephamycins, oxacephamycins, and carbapenems. Only monobactams escape its action. The enzyme activity was inhibited by metal chelators, of which 1,10-o-phenanthroline and dipicolinic acid were the most efficient. Two zinc-binding sites were found. The zinc content of the P. aeruginosa 101/1477 metallo-β-lactamase was not pH dependent.
The ingenuity of chemists has produced a large number of new potent antimicrobial agents, but resistant bacterial strains have consistently emerged. The case of the β-lactam compounds is a good example. Bacteria have developed several strategies to escape the activities of these lethal molecules: synthesis of β-lactamases, enzymes which hydrolyze the β-lactam antibiotics, decreased target sensitivity, the development of efflux systems, and/or modification of the diffusion barrier (14). The production of β-lactamase is the most prevalent mechanism of resistance to β-lactams. To date, more than 200 different β-lactamases have been described and characterized (3). Their catalytic mechanisms involve either an active-site serine residue (serine β-lactamase) or a divalent transition metal ion (metallo-β-lactamase) (27).
Metallo-β-lactamases are endogenously produced by some gram-positive and gram-negative species including Bacillus cereus (17), a cluster of Bacteroides fragilis (9), Stenotrophomonas maltophilia (25, 28), some Aeromonas spp. (20), and Chryseobacterium meningosepticum (24). Recently, a metallo-β-lactamase (IMP-1), encoded by a mobile gene (blaIMP) located on an integron-like element, was found in some clinical isolates of Serratia marcescens, Klebsiella pneumoniae, Pseudomonas aeruginosa, Pseudomonas putida, and Alcaligenes xylosoxidans that acquired the gene by horizontal transfer (18, 19, 29, 30). From the clinical point of view and due to the ability of the blaIMP gene to rapidly spread among such major pathogens, the IMP-1 enzyme currently represents the most dangerous metallo-β-lactamase.
Metallo-β-lactamases constitute a heterogeneous family. Although their primary structures exhibit a relatively low degree of sequence isology (23) (most often less than 40% and sometimes less than 11% identical residues), their three-dimensional structures show a high degree of similarity (4–6) and they share four main characteristics: (i) inactivation of the carbapenem antibiotics, (ii) no interaction with monobactams, (iii) susceptibility to chelating agents such as EDTA and dipicolinic acid, and (iv) the presence of Zn2+ ions as the naturally occurring cations, although the Cd2+ and Co2+ derivatives are enzymatically active in vitro (27). Two zinc-binding sites are present in the molecule (4, 6, 8). The three-dimensional structure of the B. cereus 569H metallo-β-lactamase shows that the side chains of residues His86, His88, and His149 and a water molecule provide the metal ligands in the first binding site. The second involves the side chains of residues Asp90, Cys168, and His210 (4, 6). By one possible catalytic mechanism, the zinc ion acts as a Lewis acid to stabilize the transient tetrahedral intermediate formed by the nucleophilic attack of a hydroxide ion on the carbonyl group of the β-lactam ring. The hydroxide ion is provided by the water molecule present in the active site which interacts with Asp90. This residue might act as a general base by subtracting a proton from the water molecule (2).
Metallo-β-lactamases are not sensitive to the conventional β-lactamase inhibitors of their active-site serine counterparts and, except for the enzymes produced by Aeromonas species, exhibit a broad-spectrum activity profile against β-lactams (11, 12, 13). This also appears to be the case for the IMP-1 enzyme, according to a previous study performed with a limited number of substrates (21, 22). However, a detailed analysis of the IMP-1 properties has not yet been performed.
In this study, the enzymatic activity of the IMP-1 metallo-β-lactamase was determined on a large number of substrates. A system for the overproduction of the enzyme in Escherichia coli and a simplified purification protocol were developed. The properties of the recombinant IMP-1 enzyme produced in E. coli were shown to be identical to those of the original enzyme produced by the P. aeruginosa clinical isolate. The effects of various chelating agents on the enzyme activity and the pH dependence of the zinc content were also measured.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
P. aeruginosa 101/1477 (a clinical isolate from Japan producing IMP-1) was a gift of D. Livermore (Antibiotic Reference Unit, Central Public Health Laboratory, London, United Kingdom). E. coli DH5α [supE4 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1] was used as the host for recombinant plasmids, and E. coli BL21(DE3) [hsdS gal(λcIts857 ind1 Sam7 nin5 lacUV5-T7 gene 1)] was used for the overexpression of the IMP-1 enzyme via the T7 expression system (Novagen Inc., Madison, Wis.). The bacteria were grown aerobically at 37°C in Luria-Bertani medium supplemented, when necessary, with the appropriate antibiotics (kanamycin at 50 μg/ml or ampicillin at 100 μg/ml) for plasmid selection. Plasmid PCR II (Invitrogen BV, NV Leek, The Netherlands) was used as the vector for the cloning and sequencing of a PCR-amplified fragment of the blaIMP gene. Plasmid pET9a (Novagen Inc.) was used as a T7-based expression vector for the overexpression of the blaIMP gene in E. coli.
Antibiotics and other chemicals.
Benzylpenicillin, ampicillin, cephalothin, and cephaloridine were purchased from Sigma Chemical Co. (St. Louis, Mo.). Nitrocefin was purchased from Unipath (Milan, Italy). Cefoxitin and imipenem were gifts of Merck Sharp & Dohme Research Laboratories (Rahway, N.J.). Piperacillin was a gift of Lederle Wyeth (Catania, Italy). Cefpirome, cefotaxime, and desacetyl-cefotaxime were gifts of Hoechst AG (Frankfurt, Germany). Aztreonam was a gift of the Squibb Institute for Medical Research (Princeton, N.J.). Cefuroxime and ceftazidime were gifts of Glaxo Wellcome (Verona, Italy). Carumonam was a gift of Hoffmann-La Roche (Basel, Switzerland). Temocillin, carbenicillin, and ticarcillin were gifts of SmithKline Beecham Pharmaceuticals (Brentford, United Kingdom). Moxalactam and loracarbef were gifts of Ely Lilly & Co. (Indianapolis, Ind.). Cefepime was a gift of Bristol Meyers (Wallingford, Conn.). Meropenem was a gift of Zeneca Pharmaceuticals (Cheshire, United Kingdom). Biapenem was a gift of Cyanamid (Catania, Italy). Panipenem was a gift of Sankyo Co. Ltd., Biological Research Laboratories (Tokyo, Japan). EDTA, EGTA, pyridine-2,6-dicarboxylic acid (dipicolinic acid), and 1,10-o-phenanthroline were purchased from Sigma, kanamycin was purchased from Merck (Darmstad, Germany), and isopropyl-β-d-thiogalactopyranoside (IPTG) was purchased from Eurogentech (Liège, Belgium).
Recombinant DNA methodology.
Plasmid pBCAM-52R, a pBCSK vector containing a cloned copy of the blaIMP gene of P. aeruginosa 101/1477 (19), was used as a source of the gene for PCR amplification of the moiety of the blaIMP gene corresponding to the NH2-terminal sequence of the protein. A new restriction site (NdeI) was introduced directly before the blaIMP gene by PCR. The oligonucleotide primers used for the PCR were imp-NdeI (5′-CATATGAGCAAGTTATCTGTATTCTTTATA-3′) and imp-SmaI (5′-AGTGTGTCCCGGGCCTGG-3′) (the underscores indicate the NdeI and SmaI restriction sites, respectively).
The PCR was performed with 25 ng of pBCAM-52R and 5 U of Taq DNA polymerase, which were incubated in the reaction buffer proposed by the Taq manufacturer. After 4 min at 95°C, 30 cycles of amplification were performed under the following conditions: denaturation for 30 s at 95°C, annealing for 1 min at 58°C, and extension for 1 min at 72°C. The PCR product was cloned into plasmid PCR II to yield pCIP2 and was sequenced by the Sanger method with a fluorescent universal primer on an ALF DNA sequencer (Pharmacia, Uppsala, Sweden) (1). A SmaI-BamHI fragment was isolated from pBCAM-52R and was subcloned into pCIP2 to yield pCIP3. The NdeI-BamHI fragment of pCIP3 containing the entire blaIMP open reading frame was subcloned into pET 9a, which had been digested with the same enzymes, to obtain pCIP4, and pCIP4 was transformed into E. coli BL21(DE3).
Expression and purification of the IMP-1 β-lactamase.
The zinc β-lactamase was produced by E. coli BL21(DE3) carrying pCIP4 in Luria-Bertani medium at 37°C under orbital agitation. Kanamycin (50 μg/ml) was used as the selecting agent during the growth of the bacteria. At an A600 of 0.6, IPTG (final concentration, 1 mM) was added, and the culture was further incubated for 2 h and centrifuged at 5,000 × g for 10 min. The pellet was resuspended in 50 mM HEPES (pH 7.5) containing 50 μM ZnSO4 (buffer H), and the cells were disrupted with the help of cell disrupter equipment (Basic Z Model; Constant Systems Ltd., Warwick, United Kingdom). The lysate was clarified by centrifugation at 30,000 × g for 20 min in order to eliminate the cell debris. The solution was loaded onto an S-Sepharose FF column (2 by 30 cm; Pharmacia) equilibrated in 50 mM HEPES (pH 7.5)–50 μM ZnSO4 (buffer H). The column was washed with the same buffer, and the enzyme was eluted with the help of a linear NaCl gradient (0 to 0.5 M). The active fractions were pooled and concentrated by ultrafiltration with a YM-10 membrane (Amicon, Beverly, Mass.). The protein solution was dialyzed against buffer H and was loaded onto a MonoS column (0.5 by 5 cm; Pharmacia) that had been prequilibrated in buffer H. The enzyme was eluted with the help of a linear salt gradient (0 to 0.5 M). The active fractions were collected, pooled, and concentrated to 1 mg/ml by ultrafiltration as described above. The enzymatic solution was stored at −70°C.
Purification of the IMP-1 enzyme from P. aeruginosa 101/1477 was done as described by Watanabe et al. (29).
Protein analysis techniques.
The N-terminal sequence was determined with the help of a gas-phase sequencer (Prosite; 492 protein sequencer; Applied Biosystems, Foster City, Calif.). The Mr value of the enzyme was determined on an electrospray mass spectrometer (VG Platform; Micromass, Fisons, United Kingdom) in acetonitrile-water (50:50 [vol/vol]; pH 6.5). The source temperature was kept at 80°C. The sample was directly introduced into the ionization chamber (at atmospheric pressure) through a steel capillary at a flow rate of 40 μl/min. The sampling cone voltage was maintained at 40 V.
Kinetic parameters.
The hydrolysis of the antibiotics by the IMP-1 metallo-β-lactamase was monitored by monitoring the variation in absorbance resulting from the opening of the β-lactam ring under the experimental conditions reported in Table 1. All the measurements were made on an Uvikon 80 spectrophotometer connected to a personal computer via an RS232C interface. All the reactions were performed in a total volume of 500 μl. All the solutions were made in 50 mM HEPES (pH 7.5)–50 μM ZnSO4. Bovine serum albumin (final concentration, 20 μg/ml) was added to the diluted solutions of β-lactamase to prevent enzyme denaturation. The steady-state kinetic parameters (Km and kcat) were determined by analyzing the complete hydrolysis time courses as described by De Meester et al. (10). When the Km value was too small (Km ≤ 20 μM), it was measured as a Ki in a competition experiment with 100 μM nitrocefin as the reporter substrate (15). The initial rate of hydrolysis of the reporter substrate was measured in the presence of different concentrations of the compounds that were studied. The kcat values were then obtained by monitoring the hydrolysis of the antibiotic at a concentration ≥10 times the Km.
TABLE 1.
Experimental conditions for kinetic studies
| Antibiotic | Concn range (μM) | Δɛ (M−1 cm−1) | λ (nm) | Enzyme (μg) |
|---|---|---|---|---|
| Benzylpenicillin | 500–1,000 | −775 | 235 | 0.5–1 |
| Ampicillin | 250–1,000 | −820 | 235 | 1–2 |
| Carbenicillin | 800–1,500 | −780 | 235 | 1–2 |
| Piperacillin | 500–2,000 | −820 | 235 | 1–10 |
| Temocillin | 100–1,200 | −660 | 235 | 5–10 |
| Ticarcillin | 100–1,200 | −660 | 235 | <10 |
| Imipenem | 50–120 | −9,000 | 300 | 0.02–0.05 |
| Meropenem | 100–200 | −6,500 | 300 | 0.5–2 |
| Panipenem | 50–100 | −7,400 | 300 | 0.02–0.5 |
| Biapenem | 100–200 | −7,600 | 293 | 1–2 |
| Nitrocefin | 50–150 | +15,000 | 482 | 0.05–0.5 |
| Cephaloridine | 25–150 | −10,000 | 260 | 0.5–1 |
| Cephalothin | 25–150 | −6,500 | 260 | 0.5–1 |
| Cefuroxime | 50–150 | −7,600 | 260 | 0.5–1 |
| Ceftazidime | 50–120 | −9,000 | 260 | 5–10 |
| Cefotaxime | 50–100 | −7,500 | 260 | 1–5 |
| Desacetyl-cefotaxime | 50–100 | −7,250 | 260 | 0.05–1 |
| Cefoxitin | 25–100 | −7,700 | 260 | 0.2–0.5 |
| Cefpirome | 50–100 | −4,500 | 260 | 0.5–2 |
| Cefepime | 25–75 | −10,000 | 260 | 1–3 |
| Loracarbef | 50–100 | −10,600 | 260 | 0.05–0.5 |
| Moxalactam | 50–100 | −4,000 | 260 | 0.5–1 |
| Aztreonam | 1,000 | −700 | 320 | 5 |
| Carumonam | 1,000 | −810 | 310 | 5 |
Inactivation of the metallo-β-lactamase by chelating agents.
The loss of β-lactamase activity was monitored in the presence of EDTA, EGTA, dipicolinic acid, and 1,10-o-phenanthroline. The progressive inactivation of the enzyme (2.5 nM) by EDTA, EGTA, 1,10-o-phenanthroline, and dipicolinic acid was monitored in the presence of 100 μM nitrocefin (reporter substrate) in a total volume of 500 μl. All the solutions were prepared in 50 mM HEPES (pH 7.5). The rate constants characterizing the inactivation of the enzyme were derived from the dependence of the pseudo-first-order constant ki upon the chelating agent concentration, on the basis of scheme 1, in which E.Zn, C, E.Zn.C, E, and Zn.C are the metalloenzyme, the chelator, a ternary metalloenzyme-chelator complex, the apoenzyme, and the metal-chelator complex, respectively, as follows:
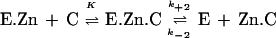 |
K represents the dissociation constant of the E.Zn.C ternary complex, k+2 is the individual rate constant for the dissociation of E.Zn.C, and k−2 is the second-order rate constant for the formation of the ternary complex. Because [Zn.C] was higher than [E], the formation of the ternary complex from the apoenzyme and the Zn-chelator complex was characterized by the pseudo-first-order rate constant k′−2 = k−2 [C]. The values of the individual rate constants were obtained with the help of the following equation, in which Km,S and [S] are the Km of the reporter substrate and the concentration of the reporter substrate, respectively:
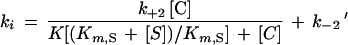 |
1 |
When the K value is much larger than the chelator concentration, this equation simplifies to the following:
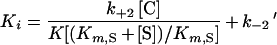 |
2 |
Determination of the metal pH dependence of the metal content of the IMP-1 metallo-β-lactamase.
Inductively coupled plasma mass spectrometry (ICPMS) was used to determine the pH dependence of the metal content of the metallo-β-lactamase. All the determinations were done after an overnight dialysis at 4°C against 300 volumes of buffer. The enzyme concentration was 38 μM. The following buffers were used: citric acid-sodium citrate (pH 5), acetic acid-sodium acetate (pH 5), sodium cacodylate-HCl (pH 6, 6.5, and 7), and HEPES-HCl (pH 7.5 and 8). The concentration of the buffering component was 50 mM in all cases. The catalytic efficiencies of the various dialyzed enzyme solutions were determined against imipenem.
RESULTS AND DISCUSSION
Overexpression and purification of the IMP-1 β-lactamase.
The protocol originally used for the purification of the IMP-1 metallo-β-lactamase involved an ammonium sulfate precipitation step followed by two column chromatographies (23, 29). In our hands, with P. aeruginosa 101/1477 as the enzyme source, the yield of the procedure was low (<40%). In order to facilitate the study of the enzyme, the blaIMP gene was cloned into a T7 expression vector (pET9a) and was overexpressed in E. coli BL21(DE3). In this strain, under the conditions described in the Materials and Methods section, high levels of enzyme accumulated in the periplasmic space. As a consequence, the purification protocol was simplified and avoided the ammonium sulfate precipitation step. Moreover, the purification yield was increased to >93%, as reported in Table 2. By using this system and the simplified purification protocol the average yield of pure enzyme was 35 mg per liter of culture. The specific activity of the purified enzyme toward nitrocefin was 19.5 μmol s−1 mg−1. The pI value was 9 ± 0.2, which was not significantly different from that observed with the enzyme purified from the original P. aeruginosa strain.
TABLE 2.
Summary of purification of metal-dependent β-lactamase of P. aeruginosa 101/1477 produced in E. coli BL21(DE3)a
| Product or purification step | Vol (ml) | Amt of protein (mg) | Total activity (μmol min−1) | Yield (%) |
|---|---|---|---|---|
| Crude extract | 150 | 380 | 41,800 | 100 |
| S-Sepharose FF column elution | 80 | 49 | 40,800 | 97 |
| MonoS column elution | 20 | 35 | 39,000 | 93 |
Standard deviations were ±10%.
The N-terminal sequence of the IMP-1 β-lactamase produced by E. coli was NH2-AESLP, showing that the processing of the proenzyme was identical in E. coli and P. aeruginosa 101/1477 (19). Moreover, the N-terminal sequence was identical to that reported by Muramo et al. (21) and Osano et al. (22). These data suggested that the enzymes produced by different S. marcescens strains and P. aeruginosa were closely related.
Mass spectrometry confirmed the homogeneity of the preparation (data not shown). The measured Mr was estimated to be 25,103 ± 10, which is in good agreement with that deduced from the amino acid sequence (Mr, 25,111).
Kinetic parameters of the IMP-1 enzyme.
The values of kcat and Km for a representative set of antibiotics are presented in Table 3. The enzyme exhibited a broad-spectrum activity profile but did not significantly hydrolyze the monobactam compounds aztreonam and carumonam (kcat/Km < 0.0001 μM−1 s−1). A high concentration (1 mM) of the various monobactams did not affect the rate of hydrolysis of 100 μM nitrocefin. A prolonged incubation (3 h) of the enzyme in the presence of 1 mM monobactams did not modify its activity.
TABLE 3.
Kinetic parameters of the P. aeruginosa metallo-β-lactamase at pH 7 and 30°C
| Antibiotics | kcat (s−1) | Km (μM) | kcat/Km (μM−1 s−1) |
|---|---|---|---|
| Benzylpenicillin | 320 ± 30 | 520 ± 30 | 0.62 |
| Ampicillin | 950 ± 50 | 200 ± 25 | 4.8 |
| Carbenicillin | NDa | ND | 0.02 |
| Piperacillin | ND | ND | 0.72 |
| Temocillin | ND | >2,000 | <0.00010 |
| Ticarcillin | 1.1 | 740 | 0.0015 |
| Imipenem | 46 ± 3 | 39 ± 4 | 1.2 |
| Meropenem | 50 ± 5 | 10 ± 2 | 0.12 |
| Panipenem | 44 ± 6 | 30 ± 3 | 1.5 |
| Biapenem | 160 ± 20 | 28 ± 2 | 6 |
| Nitrocefin | 63 ± 10 | 27 ± 3 | 2.3 |
| Cephaloridine | 53 ± 2 | 22 ± 2 | 2.4 |
| Cephalothin | 48 ± 4 | 21 ± 2 | 2.4 |
| Cefuroxime | 8 ± 1 | 37 ± 3 | 0.22 |
| Ceftazidime | 8 ± 1 | 44 ± 3 | 0.18 |
| Cefotaxime | 1.3 ± 0.5 | 4 ± 0.5b | 0.35 |
| Desacetyl-cefotaxime | ND | ND | 0.72 |
| Cefpirome | 9 ± 1 | 14 ± 3b | 0.64 |
| Cefepime | 7 ± 0.5 | 11 ± 1b | 0.66 |
| Cefoxitin | 16 ± 1 | 8 ± 1b | 2 |
| Moxalactam | 88 ± 10 | 10 ± 2b | 8.8 |
| Loracarbef | ND | ND | 0.9 |
| Aztreonam | >0.01 | >1,000 | <0.0001 |
| Carumonam | >0.01 | >1,000 | <0.0001 |
ND, not determined.
Km was obtained as the Ki value.
For penicillins, the structure of the C-6 side chain played an important function. Ampicillin behaved as a good substrate (kcat/Km = 5 μM−1 s−1). Substitution of the amino group by a bulky group (for piperacillin, kcat/Km = 0.72 μM−1 s−1) or a carboxylic function (for carbenicillin, kcat/Km = 0.02 μM−1 s−1) decreased the catalytic efficiency. For carbenicillin, the large Km value did not allow the determination of the individual kinetic parameters. Temocillin was neither recognized nor hydrolyzed by the IMP-1 β-lactamase, as observed for the Zn β-lactamase produced by B. cereus 569H (11), while ticarcillin was poorly hydrolyzed (kcat/Km = 0.0015 μM−1 s−1). This difference may be explained by the presence of an α-methoxy group at position C-6 of temocillin. A 3-h incubation of the enzyme in a 2 mM solution of temocillin did not affect the activity of IMP-1.
All the cephalosporin derivatives tested were well recognized and hydrolyzed by the IMP-1 enzyme. The catalytic efficiency varied from 0.2 to 3 μM−1 s−1. Except for the Km and kcat values for desacetyl-cefotaxime, the values of Km and kcat were small compared to those for the best penicillins. Interestingly, the modification of the C-3 side chain affected the Km value, which was larger for desacetyl-cefotaxime than for cefotaxime. The comparison of the kinetic constant for the hydrolysis of cephalothin and cefoxitin indicated that the presence of an α-methoxy group at position C-7 did not affect the activity of the metallo-β-lactamase. Both compounds are good substrates for IMP-1, and their kinetic parameters are similar. Moxalactam (an oxacephem compound) is also a good substrate. Oximinocephalosporins (cefotaxime, ceftazidime) were efficiently recognized and relatively well hydrolyzed by the β-lactamase, with catalytic efficiencies of >0.1 μM−1 s−1. Loracarbef was well hydrolyzed (kcat/Km = 0.9 μM−1 s−1).
All the carbapenem compounds tested (imipenem, biapenem, meropenem, and panipenem) behaved as good substrates (kcat/Km = 0.12 to 6 μM−1 s−1).
The kinetic parameters of the enzymes produced by P. aeruginosa 101/1477 and E. coli BL21(DE3) against a panel of selected substrates were quite similar in our laboratory. For the enzyme purified from P. aeruginosa 101/1477, the indicated kcat, Km, and kcat/Km values were found for benzylpenicillin (kcat = 280 ± 50 s−1, Km = 600 ± 30 μM, kcat/Km = 0.47 μM−1 s−1), cefuroxime (kcat = 10 ± 2 s−1, Km = 45 ± 9 μM, kcat/Km = 0.22 μM−1 s−1), ceftazidime (kcat = 8 ± 2 s−1, Km = 35 ± 5 μM, kcat/Km = 0.22 μM−1 s−1), cefotaxime (kcat = 2 ± 0.5 s−1, Km = 4 ± 0.5 μM, kcat/Km = 0.5 μM−1 s−1), cephaloridine (kcat = 50 ± 10 s−1, Km = 20 ± 10 μM, kcat/Km = 2.5 μM−1 s−1), and imipenem (kcat = 40 ± 4 s−1, Km = 40 ± 5 μM, kcat/Km = 1 μM−1 s−1).
Our kinetic data are in good agreement with the data reported by Muramo et al. (21), confirming that our enzyme was similar to the carbapenemase from S. marcescens FHSM4055. Surprisingly, we found major discrepancies with the kinetic data obtained by Osano et al. (22) with the metallo-β-lactamase of S. marcescens TN9106. The differences observed for the Km values might be due to different assay conditions.
The catalytic properties of IMP-1 toward β-lactam antibiotics were similar to those exhibited by two other clinically relevant metallo-β-lactamases, those of B. fragilis CcrA (31) and S. maltophilia (7, 11, 12, 13). IMP-1 is the most active against moxalactam and cefoxitin. These compounds are poorly hydrolyzed by the B. cereus enzyme and are poor inactivators of the Aeromonas β-lactamase. Among all the studied Zn β-lactamases, the Aeromonas enzyme (CphA) can be considered a strict carbapenemase since it is significantly active only against carbapenems. Cephaloridine and cefoxitin are poorly hydrolyzed or behave as inactivators, respectively. All the other known metalloenzymes exhibit broad spectra of activity. For example, the oximinocephalosporins cefotaxime and ceftazidime are well hydrolyzed by these enzymes. By contrast, none of the Zn β-lactamases could hydrolyze monobactams such as aztreonam.
Influence of the zinc ion concentration on the enzyme activity.
At pH 7.5, when increasing Zn2+ ion concentrations were added to the enzyme, the initial rate of nitrocefin hydrolysis was not affected. The kcat and Km values in the absence (Km = 63 ± 10 μM, kcat = 27 ± 3 s−1, kcat/Km = 0.4 μM−1 s−1) and in the presence (Km = 60 ± 15 μM, kcat = 30 ± 5 s−1, kcat/Km = 0.5 μM−1 s−1) of 100 μM Zn2+ were not significantly different.
Inactivation by Zn-chelating agents.
Of the four chelating agents tested, EDTA and EGTA behaved as poor inactivators (Table 4). Even at final concentrations of 10 mM and after 1 h, the activity was reduced by only 10%. For 1,10-o-phenanthroline and dipicolinic acid, a time-dependent pseudo-first-order inactivation was observed. The value of the pseudo-first-order rate constant ki increased with the concentration of the chelating agent in a hyperbolic manner with dipicolinic acid and linearly with 1,10-o-phenanthroline. These data indicate that the chelators do not act by scavenging the free metal but act via the formation of a transient enzyme-metal-chelator ternary complex. In the case of dipicolinic acid, the curve was analyzed on the basis of equation 1 assuming that k2′ was negligible. With 1,10-o-phenanthroline, on the basis of equation 2, k−2′ was obtained by extrapolation of the line to the ordinate.
TABLE 4.
Individual parameters for inactivation of P. aeruginosa 101/1477 metallo-β-lactamase by chelating agents
| Chelating agent | k+2 (s−1) | k−2′ (s−1) | K (mM) | k+2/K (M−1 s−1) |
|---|---|---|---|---|
| EDTA | NDa | ND | >10 | ND |
| EGTA | ND | ND | >10 | ND |
| 1,10-o-Phenanthroline | ND | 0.032 ± 0.004 | ND | 120 ± 20 |
| Dipicolinic acid | 0.024 ± 0.004 | ND | 0.3 ± 0.03 | 380 ± 50 |
ND, not determined.
1,10-o-Phenanthroline and dipicolinic acid inactivated IMP-1 with a rather good efficiency (k+2/K > 100 μM−1 s−1). The Zn enzyme produced by A. hydrophila was more efficiently inhibited by the chelating agents than IMP-1 (16). For example, the K values for dipicolinic acid were 5 and 300 μM for CphA and IMP-1, respectively.
pH dependence of activity and zinc content of IMP-1.
The pH dependence of the Zn content of the metallo-β-lactamase of P. aeruginosa was measured by ICPMS (Table 5). Cd(II), Co(II), Ca(II), Cu(II), Mn(II), and Ni(II) were not found. In the absence of excess of metal and between pH 6 and pH 8, the enzyme contained almost two zinc ions per molecule. At pH 5 the nature of the buffer influenced the [Zn]/[enzyme] molar ratio. In citrate buffer, the ratio decreased to a value of 0.3, while in acetate buffer, the ratio was close to 2. Finally, the pH dependence of the catalytic efficiency of the enzyme against imipenem in the absence of added Zn2+ was determined. The IMP-1 metallo-β-lactamase exhibited a maximum activity against imipenem between pH 6.5 and pH 7.5. All metallo-β-lactamases studied exhibit two Zn-binding sites. However, the occupancy of the second binding site has a different effect on the catalytic activities of the enzymes. For the B. cereus 569H enzyme, the catalytic efficiency is not strongly modified by the presence of a second zinc ion (2). The A. hydrophila AE036 enzyme is fully active with one zinc, but its activity is inhibited by the binding of a second zinc ion (16). The B. fragilis metallo-β-lactamase tightly binds to two zinc ions at pH 7 (7), but the activity of the mono zinc form is unknown at present, and the same situation appears to prevail for the IMP-1 enzyme.
TABLE 5.
Metal/enzyme ratios and catalytic efficiencies of the metallo-β-lactamase at different pH valuesa
| pH | Buffer | [Zn]/[enzyme] | kcat/Km (μM−1 s−1) |
|---|---|---|---|
| 5 | Acetate | 1.8 ± 0.2 | 0.8 |
| 5 | Citrate | 0.3 ± 0.1 | <0.01 |
| 6 | Cacodylate | 1.9 ± 0.2 | 1 |
| 6.5 | Cacodylate | 1.8 ± 0.2 | 1.2 |
| 7 | Cacodylate | 1.9 ± 0.2 | 1.2 |
| 7.5 | HEPES | 1.7 ± 0.2 | 1.2 |
| 8 | HEPES | 1.8 ± 0.2 | 0.5 |
The substrate was imipenem.
Conclusions.
The spread of the IMP-1 metallo-β-lactamase in major nosocomial strains is one of the most powerful responses toward the anti-infective strategies widely used in several hospitals in Japan. In the study described in this report, a recombinant E. coli strain was used to produce large amount of IMP-1. The enzyme produced in E. coli and P. aeruginosa exhibited the same properties. The metal content of the protein was not significantly affected by the pH. Under all conditions tested except when citrate was used as the buffer, the metallo-β-lactamase contained two zinc ions. As expected, the enzyme was inactivated by metal chelating agents. These compounds did not act by scavenging the free metal, and therefore, the transient formation of a ternary enzyme-metal-chelator complex is proposed. Finally, at pH 7.5, the enzymatic activity was not influenced by the presence of increasing concentration of free zinc ions.
The di-zinc form of the enzyme exhibited a broad-spectrum activity profile. Only the monobactam compounds and temocillin seemed to escape the hydrolytic action of the metallo-β-lactamase. A comparison of the activities of the mono-zinc and di-zinc forms of IMP-1 is being performed. Furthermore, detailed structural and mechanistic studies of IMP-1 are in progress.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the European Union (grant ERBFMRCX-CT98-0232) as part of the Training and Mobility of Researchers Programme, by the Belgian Programme Poles d’Attraction Interuniversitaire initiated by the Belgian State, Prime Minister’s Office, Services Fédéraux des Affaires Scientifiques, Techniques et Culturelles (PAI no. P4/03), and by a grant from MURST ex-40%, project Structural Biology. During his stay at the University of l’Aquila (Department STB), M.G. was supported by a grant from MURST and CNR.
M.G. thanks P.S. for patience and work in front of the Perkin-Elmer spectrophotometer. The ICPMS measurements were performed by the Laboratoire de la Santé et de l’Environnement, Institut Malvoz de la Province de Liège, Liège, Belgium.
REFERENCES
- 1.Ansorge W, Sproat B, Stegemann J, Schwager C, Zenke M. Automated DNA sequencing: ultrasensitive detection of fluorescent bands during electrophoresis. Nucleic Acids Res. 1987;15:4593–4602. doi: 10.1093/nar/15.11.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bounaga S, Laws A P, Galleni M, Page M I. Unusual pH dependence of the class B β-lactamase catalysed hydrolysis of substrates and their inhibition by thiols. Biochem J. 1998;331:703–711. doi: 10.1042/bj3310703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush K, Jacoby G. A functional classification for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carfi A, Pares S, Duée E, Galleni M, Duez C, Frère J M, Dideberg O. The 3D structure of a zinc metallo-β-lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J. 1995;14:4914–4921. doi: 10.1002/j.1460-2075.1995.tb00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carfi A, Duée E, Soto R P, Galleni M, Duez C, Frère J M, Dideberg O. X-ray structure of the Zn II β-lactamase from Bacteroides fragilis in an orthorhombic crystal form. Acta Crystallogr. 1998;D54:47–57. doi: 10.1107/s090744499700927x. [DOI] [PubMed] [Google Scholar]
- 6.Concha N O, Rasmussen B A, Bush K, Herzberg O. Crystal structure of the wide-spectrum binuclear zinc β-lactamase from Bacteroides fragilis. Structure. 1996;4:823–836. doi: 10.1016/s0969-2126(96)00089-5. [DOI] [PubMed] [Google Scholar]
- 7.Crowder M W, Walsh T R, Banovic L, Pettit M, Spencer J. Overexpression, purification, and characterization of the cloned metallo-β-lactamase L1 from Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 1998;42:921–926. doi: 10.1128/aac.42.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowder M W, Wang Z, Franklin S L, Zovinka E P, Benkovic S J. Characterisation of the metal binding sites of the β-lactamase from Bacteroides fragilis. Biochemistry. 1996;35:12126–12132. doi: 10.1021/bi960976h. [DOI] [PubMed] [Google Scholar]
- 9.Cuchural G J, Jr, Malamy M H, Tally F P. β-Lactamase-mediated imipenem resistance in Bacteroides fragilis. Antimicrob Agents Chemother. 1986;30:645–648. doi: 10.1128/aac.30.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Meester F, Joris B, Reckinger G, Bellefroid-Bourguignon C, Frère J M, Waley S G. Automated analysis of enzyme inactivator phenomena. Biochem Pharmacol. 1987;36:2393–2403. doi: 10.1016/0006-2952(87)90609-5. [DOI] [PubMed] [Google Scholar]
- 11.Felici A, Amicosante G. Kinetic analysis of extension of substrate specificity with Xanthomonas maltophilia, Aeromonas hydrophila, and Bacillus cereus metallo-β-lactamases. Antimicrob Agents Chemother. 1995;39:192–199. doi: 10.1128/aac.39.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felici A, Amicosante G, Oratore A, Strom R, Ledent P, Joris B, Fanuel L, Frère J M. An overview of the kinetic parameters of class B β-lactamase. Biochem J. 1993;291:151–155. doi: 10.1042/bj2910151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felici A, Perilli M, Segatore B, Franceschini N, Setacci D, Oratore A, Stefani S, Galleni M, Amicosante G. Interactions of biapenem with active-site serine and metallo-β-lactamases. Antimicrob Agents Chemother. 1995;39:1300–1305. doi: 10.1128/aac.39.6.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frère J M. Beta-lactamases and bacterial resistance to antibiotics. Mol Microbiol. 1995;16:385–395. doi: 10.1111/j.1365-2958.1995.tb02404.x. [DOI] [PubMed] [Google Scholar]
- 15.Galleni M, Franceschini N, Quinting B, Fattorini L, Orefici G, Oratore A, Frère J M, Amicosante G. Use of the chromosomal class A β-lactamase of Mycobacterium fortuitum D316 to study potentially poor substrates and inhibitory β-lactam compounds. Antimicrob Agents Chemother. 1994;38:1608–1614. doi: 10.1128/aac.38.7.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez Valladares M, Felici A, Weber G, Adolph H W, Zeppezauer M, Rossolini G M, Amicosante G, Frère J M, Galleni M. Zn (II) dependence of the Aeromonas hydrophila AEO36 metallo-β-lactamase activity and stability. Biochemistry. 1997;36:11534–11541. doi: 10.1021/bi971056h. [DOI] [PubMed] [Google Scholar]
- 17.Hussain M, Carlino A, Madonna M J, Lampen O. Cloning and sequencing of the metallothioprotein β-lactamase II gene of Bacillus cereus 569H in Escherichia coli. J Bacteriol. 1985;164:223–229. doi: 10.1128/jb.164.1.223-229.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito H, Arakawa Y, Ohsuka S, Wachorotayankun R, Kato N, Ohta M. Plasmid-mediated dissemination of the metallo-β-lactamase gene blaIMP among clinically isolated strains of Serratia marcescens. Antimicrob Agents Chemother. 1995;39:824–829. doi: 10.1128/aac.39.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laraki N, Galleni M, Thamm I, Riccio M L, Amicosante G, Frere J-M, Rossolini G M. Structure of In101, a blaIMP-containing Pseudomonas aeruginosa integron phyletically related to In5, which carries an unusual array of gene cassettes. Antimicrob Agents Chemother. 1999;43:818–829. doi: 10.1128/aac.43.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massida O, Rossolini G M, Satta G. The Aeromonas hydrophila cphA gene: molecular heterogeneity among class B metallo-β-lactamases. J Bacteriol. 1991;173:4611–4617. doi: 10.1128/jb.173.15.4611-4617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muramo K, Takeda A, Nakamura Y, Nakaya K. Purification and characterization of metallo-β-lactamase from Serratia marcescens. Microbiol Immunol. 1995;39:27–33. doi: 10.1111/j.1348-0421.1995.tb02164.x. [DOI] [PubMed] [Google Scholar]
- 22.Osano E, Arakawa Y, Wacharotayankun R, Ohta M, Horii T, Ito H, Yoshimura F, Kato N. Molecular characterization of an enterobacterial metallo β-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob Agents Chemother. 1994;38:71–78. doi: 10.1128/aac.38.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen B A, Bush K. Carbapenem-hydrolyzing beta-lactamases. Antimicrob Agents Chemother. 1997;41:223–232. doi: 10.1128/aac.41.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossolini G M, Franceschini N, Riccio M L, Mercuri P S, Perilli M G, Galleni M, Frère J M, Amicosante G. Characterisation and sequence of the Chryseobacterium (Flavobacterium) meningosepticum carbapenemase: a new molecular class B β-lactamase showing a broad substrate profile. Biochem J. 1998;332:145–152. doi: 10.1042/bj3320145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanschagrin F, Dufresne J, Levesque R C. Molecular heterogeneity of the L-1 metallo-beta-lactamase family from Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 1998;42:1245–1248. doi: 10.1128/aac.42.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sendo H, Arakawa Y, Nakeshima K, Ito H, Ichiyama S, Shimakotu K, Kato N, Ohta M. Multifocal outbreak of metallo-β-lactamase producing Pseudomonas aeruginosa resistant to broad spectrum β-lactam including carbapenems. Antimicrob Agents Chemother. 1986;40:349–353. doi: 10.1128/aac.40.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waley S G. β-Lactamase: mechanism of action. In: Page M I, editor. The chemistry of β-lactam. London, United Kingdom: Blackie A. et P.; 1992. pp. 198–228. [Google Scholar]
- 28.Walsh T R, Hall L, Assinda S J, Nichols W W, Cartwright S J, MacGowan A P, Bennett P M. Sequence and analysis of the L1 metallo-β-lactamase from Xanthomonas maltophilia. Biochem Biophys Acta. 1994;1218:199–201. doi: 10.1016/0167-4781(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991;35:147–151. doi: 10.1128/aac.35.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi H, Nukaya M, Sawaï T. Sequence of Klebsiella pneumoniae RDK4 metallo-β-lactamase. EMBO database accession no. D29636. 1994. EMBO, Heidelberg, Germany. [Google Scholar]
- 31.Yang Y, Rasmussen B A, Bush K. Biochemical characterization of an imipenem-hydrolyzing metallo-β-lactamase from Bacteroides fragilis TAL3636. Antimicrob Agents Chemother. 1992;36:1155–1157. doi: 10.1128/aac.36.5.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]


