Abstract
Background:
Recent examination of the STEADY-PD III isradipine clinical trial data concluded that early-stage Parkinson’s disease (PD) participants who had longer exposure to isradipine had a significant delay in their need for symptomatic medication, as well as a lower medication burden at the end of the trial. These findings suggest that greater exposure to isradipine might slow disease progression.
Objectives:
To test this hypothesis, the data from the STEADY-PD II isradipine clinical trial, in which an extended-release (ER) formulation of the drug was used, was re-examined.
Methods:
The re-analysis of the STEADY-PD II data was restricted to participants assigned placebo or tolerable isradipine treatment (10 mg isradipine/day or less). The effect of isradipine treatment was assessed by Unified Parkinson’s Disease Rating Scale (UPDRS) at the end of the 52-week trial, rather than by last observation carried forward at the beginning of symptomatic therapy.
Results:
Participant cohorts were well-matched for baseline disability, initial disease progression, and time to initiation of symptomatic therapy. Participants given 10 mg/day ER isradipine had significantly smaller total and part 3 UPDRS scores at the end of the trial than did the placebo cohort. Post hoc adjustment for symptomatic therapy diminished the statistical significance of these differences. In those participants not taking a monoamine oxidase B inhibitor, the progression in UPDRS scores also was significantly reduced.
Conclusions:
These results are consistent with the recent secondary analysis of the STEADY-PD III clinical trial—suggesting that clinically attainable brain exposure to isradipine may slow early-stage PD progression.
Keywords: Parkinson’s disease, disease-modification, calcium, L-type channel, dihydropyridine, DynaCirc
Parkinson’s disease (PD) is the second most common neurodegenerative disease in the world.1 Despite repeated clinical efforts,2 there are no proven strategies for slowing disease progression.3 Most recently, an National Institutes of Health (NIH)-sponsored phase 3 clinical trial that tested the ability of an immediate-release (IR) formulation of isradipine given twice daily in 5 mg capsules for 36 months to slow disease progression was completed (ClinicalTrials.gov: NCT02168842). Despite extensive preclinical and epidemiological support,4,5 this trial—referred to as the STEADY-PD III trial—failed to find a change in primary or secondary outcome measures with isradipine treatment.6 However, an exploratory pharmacokinetic (PK) analysis of plasma isradipine concentration uncovered a relationship between drug exposure and the need for symptomatic medication.7 In this analysis, participants were stratified into tertiles based on the plasma clearance rate of isradipine, which is inversely proportional to drug exposure. As plasma clearance decreased and isradipine exposure increased, the need for initiating antiparkinson medication was delayed. Additionally, with slower plasma clearance of isradipine, the cumulative dosing of antiparkinson medication was lower and non-tremor motor disability measured by the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) was less severe at the end of the trial. However, there was no impact on other outcome measures.
These findings suggest that sustained target engagement by isradipine may be critical to slowing of disease progression. Why might this be the case? Because substantia nigra pars compacta (SNc) dopaminergic neurons are active throughout the day and night,8 the duration of effective target engagement is a key determinant of protection. With a reversible negative allosteric modulator (NAM), the only way to sustain target engagement is to maintain drug exposures within a range that effectively inhibits Cav1 Ca2+ channels in the brain. An extended-release (ER) formulation is a common strategy for dealing with PK limitations like those of isradipine. In fact, in the initial phase 2 trial with isradipine, an ER formulation was used.9 Although the primary goal of the STEADY-PD II trial was to determine isradipine safety/tolerability, UPDRS scores were measured over the 52-week period of the trial and an exploratory assessment of disability progression was performed. The study did not find that isradipine treatment significantly altered UPDRS scores, although there was a trend in improvement in UPDRS score changes with increasing doses. However, this analysis made two assumptions that are worth re-evaluating in light of the recent STEADY-PD III analysis. One assumption was that the best way to deal with the initiation of symptomatic therapy in the course of the trial was to carry-forward the last UPDRS score before onset of treatment. This “last observation carried-forward” (LOCF) protocol is not uncommon; but it is of questionable use in an assessment of disease-modification because it truncates estimates of disease progression.10 The other assumption was that participants given more than the maximum tolerated dose of isradipine should be included in the efficacy analysis; given potential problems with compliance, this assumption merits re-examination.
Methods
Study Population and Design
The dataset used in this study is from the Safety, Tolerability and Efficacy Assessment of DynaCirc ER in Parkinson Disease (STEADY-PD II) multicenter clinical trial (ClinicalTrials.gov: NCT00909545). The study population, study design, and procedures have been described previously.9 Participants were randomized to 5, 10, or 20 mg of isradipine ER or matching placebo administered once daily and followed for 52 weeks. UPDRS and other clinical assessments were performed at baseline, weeks 2, 4, 6, 8, 12, and every 3 months thereafter. When participants required initiation of symptomatic therapy, an additional study visit was performed before start of the symptomatic medication with UPDRS and other clinical assessments conducted. Because of tolerability issues, some participants assigned to the 20 mg/day isradipine group were titrated to 15 mg/day isradipine. The proportion with 52-week follow up was significantly lower in these groups (75% in 15 mg/day group, 80% in 20 mg/day group, and 96% in other groups).
Outcome Measures
UPDRS subscales 1, 2, and 3 were administered at each study visit. After initiation of symptomatic therapy UPDRS was obtained when patients were in the ON-state. The individual items from each of the three subscales were summed to get total UPDRS score.11 The primary outcomes for this analysis were change in total UPDRS score from the baseline to the week 52 visit and change in part 3 UPDRS subscale from baseline to the week 52 visit. As described in the STEADY-PD II report and its appendices, ~ 40% of the participants started symptomatic medications during the trial (see below). For the purposes of our study, symptomatic medication use was defined as any dopaminergic or non-dopaminergic medication other than rasagiline or selegiline documented during the study period.12 Total daily levodopa-equivalent dose (LED) dose was computed by converting each symptomatic medication to LED using a conversion table and multiplying by strength, number of pills, and frequency taken per day.12 If multiple symptomatic medications were used, LED doses of all medications were summed for total daily LED dose. OFF-state UPDRS scores were not obtained in the STEADY-PD II study.
Statistical Analysis
Descriptive statistics were computed including the number and percent of participants who were ever on rasagiline or selegiline, who started symptomatic medication during the study period and who completed the week 52 visit stratified by treatment group. Differences in mean baseline UPDRS score by treatment group was assessed using analysis of variance (ANOVA) test.
To examine the change in the UPDRS score from baseline to 52 weeks as the outcome analysis of covariance (ANCOVA) was used with assigned treatment group as the primary predictor of interest, and baseline UPDRS score included as a covariate.13,14 ANCOVA assumptions were checked and confirmed: independence of treatment group and baseline UPDRS was checked by the ANOVA of baseline UPDRS by treatment group; homogeneity of slopes was checked by evaluating addition of an interaction term for treatment times baseline; and normality assumptions were checked by examining residuals by predicted distributions and Q-Q plots of residuals.
Adjustments to week 52 UPDRS scores for symptomatic medication use during the trial were made using the method described in the analysis of the STEADY-PD III trial.6 In brief, the total daily LED dose at that visit was multiplied by 100; the reciprocal of this LED value was multiplied by the LED effect coefficient estimated from STEADY-PD III data set (1.5288).6 This adjustment (1.5288/[100 LED]) was then added to the observed UPDRS score at the week 52 visit to get the adjusted UPDRS, therefore representing an estimated UPDRS score for an individual if they would not have received symptomatic medication. A similar approach was used to generate an adjustment term for the part 3 UPDRS scores.
Participant-specific progression rates in the STEADY-PD II population were estimated using longitudinal study follow-up data from the first 15 weeks of the trial. This allowed an estimate to be generated of the rate at which each participant was progressing before symptomatic medication or any significant potential disease-modifying effect of isradipine treatment. Linear mixed models for UPDRS score were fit and included a fixed effect for study week (continuous), random effects on intercept and slope for participant, and an unstructured covariance to model the within-person covariance of the measurements. All UPDRS scores recorded in the first 15 weeks of the trial were included (number of study visits per participant: mean [SD] = 5.9 [0.4], median = 6, range 3–7). The overall regression coefficient for study week (fixed effect coefficient) was estimated as well as the deviation in the regression coefficients from the overall coefficients for each participant (random effect coefficient). To obtain the participant-specific regression coefficients for study week, the estimated fixed effect coefficient was added to the estimated random effect coefficient for each person. This estimated participant-level regression coefficient (slope) represents the estimated change in UPDRS score per week. The participant-specific intercept was also derived using this method.
For sensitivity analyses of LED adjustment, analysis of covariance was used to examine the change in the UPDRS score from baseline to week 52 using the adjusted week 52 UPDRS score as the outcome, with assigned treatment group as the primary predictor of interest, and baseline UPDRS score included as a covariate.
Differences in time to start of symptomatic medication by treatment group were assessed using Kaplan–Meier curves and Cox proportional hazards models stratified by clinical trial site, with low enrolling sites (n < 4) grouped together. For all analyses, two-sided P < 0.05 was used for evaluating statistical significance. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Population Characteristics of Treatment Groups Were Similar
The STEADY-PD II study recruited 100 participants who were randomly assigned to equally sized placebo, 5, 10, and 20 mg/day treatment groups. The study concluded that 10 mg/day was the maximum tolerated dose. The most common reasons given for intolerability were peripheral edema and dizziness, which were reported primarily in the 15 to 20 mg/day treatment group (dose reductions were allowed in the 20 mg/day cohort). Examination of the Drug Dispensing/ Compliance Log for the trial revealed that participants assigned to the 15 to 20 mg/day group returned a significantly greater proportion of capsules than participants in any of the other groups (median proportion returned: placebo = 12%, 5 mg/day = 13%; 10 mg/day = 13%, 15–20 mg/day = 21%; P = 0.006 for 15–20 mg/day vs. placebo). Hence, drug compliance in the group taking more than 10 mg/day is uncertain. As a consequence, our secondary efficacy analysis focused on participants that received doses of ER isradipine that were considered tolerable and where compliance was less of an issue (72/100 participants).
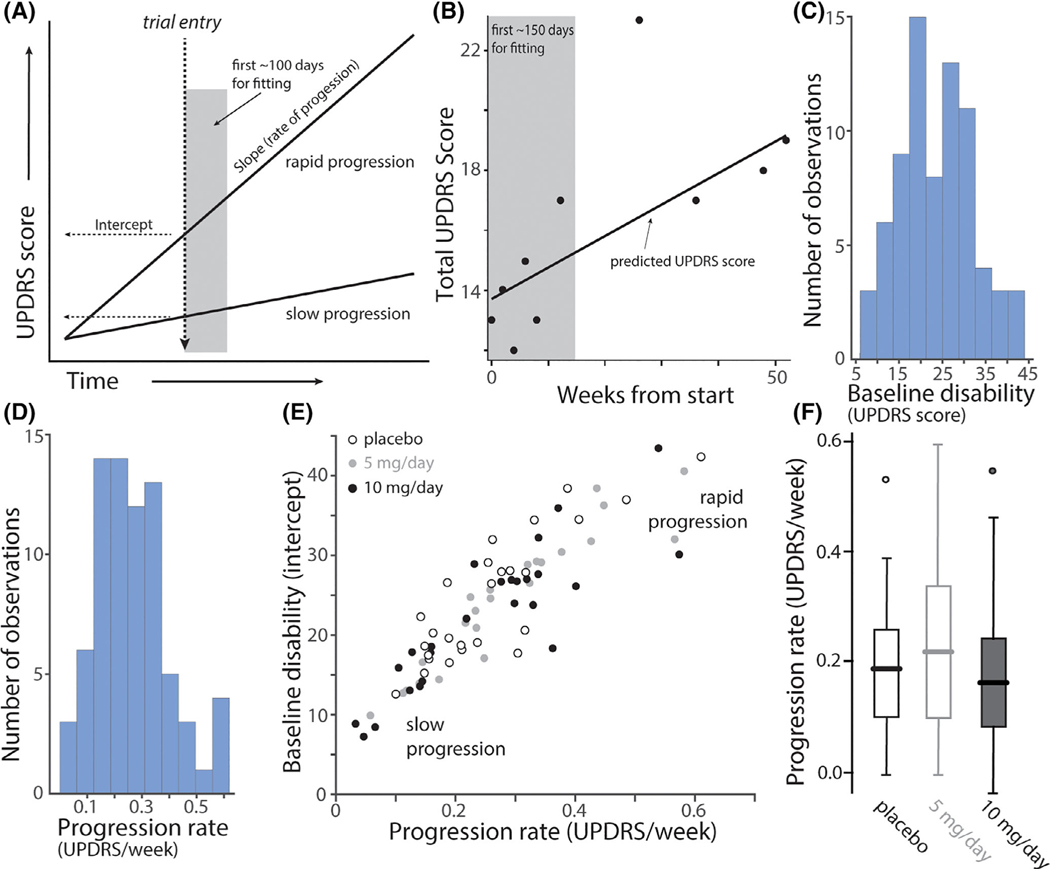
Although the initial characterization of participants was thorough, one clinical feature that was not considered before treatment assignment was one’s individual rate of disease progression. In the early stages of PD, disability (as measured with the UPDRS) typically increases linearly, but with a variable slope.15 In principle, if participants were enrolled at the same time after diagnosis, the baseline disability UPDRS score could be used to estimate progression rate (Fig. 1A). However, this assessment was not part of the stated protocol. To estimate progression rates, UPDRS scores in the first 15 weeks of the trial were subjected to linear regression analysis on a patient-by-patient basis (see Methods, Fig. 1B). This allowed a progression rate estimate to be generated before symptomatic medication (other than monoamine oxidase B [MAO-B] inhibitors) or before there being any likely disease-modifying effect of isradipine treatment. Histograms of the baseline disability (total UPDRS score at enrollment) and the progression rate as estimated from the slope of the regression line fit (UPDRS change/week) revealed that the patient pool was diverse (Fig. 1C,D). Moreover, both parameters had unimodal distributions, suggesting that the cohort could not be readily sub-grouped. A scatter plot of the slope and intercept of the linear regression fits for participants reinforced this inference (Fig. 1E). This plot also revealed a clear correlation between the slope and the intercept (which approximates the baseline UPDRS score at enrollment) of the pooled data from placebo and 5/10 mg/day isradipine treatment groups (Pearson’s correlation coefficient = 0.79, P < 0.0001, n = 75). Last, it was evident that participants assigned to placebo, 5 mg and 10 mg treatment groups had a similar, wide distribution of slopes (Fig. 1F), revealing that they were equally matched for rate of disease progression and, presumably, underlying biology.
FIG. 1.
Progression rates and baseline disabilities as measured by UPDRS rating in treatment cohorts. (A) Hypothetical linear regression plots of UPDRS disability scores as a function of time after PD diagnosis for a rapidly and a slowly progressing patient. Trial entry was assumed to be at the same time after diagnosis for both patients (dashed vertical line). The estimate of UPDRS score at enrollment (baseline disability) is shown as the intercept with trial entry line; the slope of the progression line was estimated from repeated measures during the first 150 days of the trial (grey box), during which time it was assumed that patients were not receiving symptomatic therapy and, during which the treatment intervention may not had a significant effect. (B) An example of data from a patient during the fitting interval and afterward with a linear regression fit. (C) Histograms of the slope (progression rate measured as change in total UPDRS score per week; left panel) and the intercept (baseline UPDRS score; right panel) of the linear regression analysis. (n = 75). Note the wide range of slopes and baseline UPDRS scores. (D) Scatter plot of the progression rate (slope) and baseline disability (intercept) for placebo (n = 26; open circles), low dose isradipine (5 mg/day, grey circles, n = 23) and high dose isradipine (10 mg/day, black circles n = 26). Note that all three groups included a similar distribution of patients that were rapidly and slowly progressing at trial enrollment. (F) Boxplots of the slopes for placebo (n = 26), 5 mg/day (n = 23) and 10 mg/day (n = 26) treatment groups. Outliers are shown as circles. There was no difference between groups in the initial slopes (P = 0.42, ANOVA).
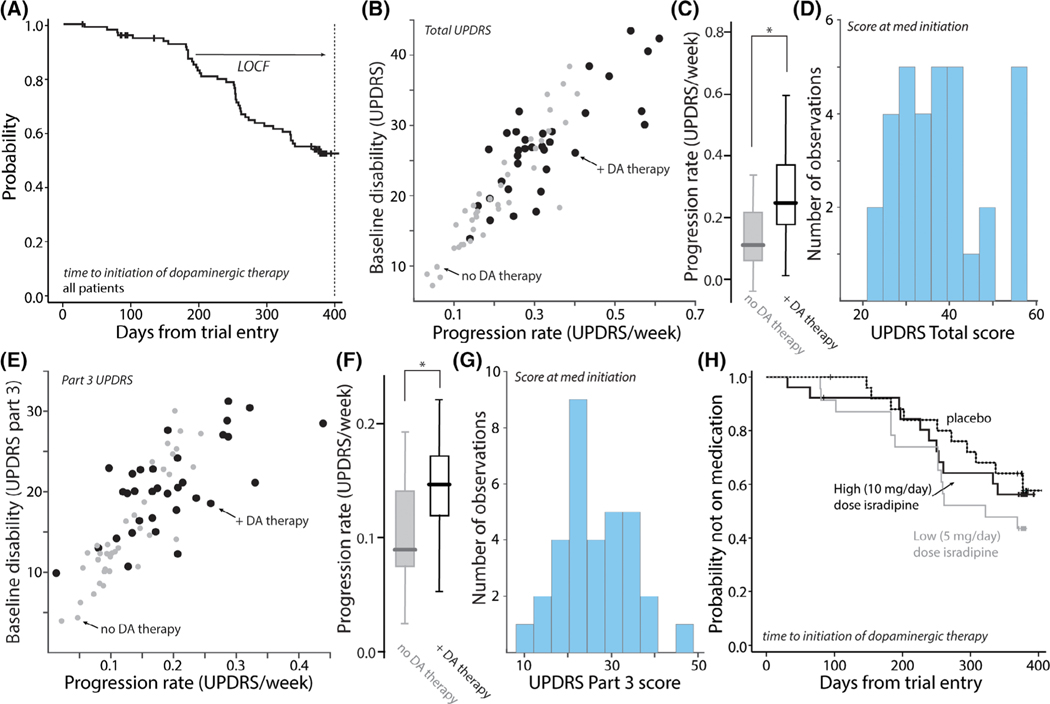
In the course of the 52-week trial, ~ 40% of the participants went on some form of symptomatic therapy.9 A Kaplan–Meier plot of times to initiation of symptomatic therapy revealed that many participants began treatment early in the trial (Fig. 2A). In an attempt to determine why participants initiated symptomatic therapy, the scatter plot of baseline disability and progression rate was re-coded to show those participants who initiated therapy and those who did not (Fig. 2B). This plot revealed that participants initiating symptomatic therapy generally had more rapid disease progression in the early stages of the trial. Indeed, the progression rate of those initiating symptomatic therapy was significantly greater than those who did not (Fig. 2C). However, there was considerable variability in UPDRS-determined disability at the time of therapy initiation (Fig. 2D), suggesting that the determinants of this transition were multi-factorial.
FIG. 2.
Initiation of symptomatic therapy was weakly correlated with total and part 3 UPDRS scores. (A) Kaplan–Meier survival plot of probability of initiating symptomatic therapy as a function of time after trial enrollment. In the initial analysis, the UPDRS scores at conversion were carried forward for the efficacy analysis. This last observation carried forward (LOCF) analysis was not used in our analysis. (B) Scatter plot of progression rate and baseline disability as measured by total UPDRS score. Patients who did not initiate symptomatic therapy during the trial are shown as grey circles (n = 40) and those who did as black circles (n = 35). Although there was overlap in the scores of the two cohorts, patients who initiated symptomatic therapy tended to have a more rapid progression phenotype. (C) Box plots showing the distribution of slopes for the two groups. The slopes of patients initiating symptomatic therapy were significantly greater than those who did not (P < 0.05, t test, n = 40 [no therapy], n = 35 [+DA therapy]); in the box plot the center line is the median, the edges of the box are the interquartiles and the lines mark the ranges of the distributions. (D) Histogram of the total UPDRS disability at the initiation of symptomatic therapy (n = 35). Note the wide range of disability. (E) Scatter plot of baseline disability measured by part 3 UPDRS scores and progression rates for part 3 UPDRS disability. Patients who did not initiate symptomatic therapy during the trial are shown as grey circles (n = 40) and those who did as black circles (n = 35). Although there was overlap in the scores of the two cohorts, patients who initiated symptomatic therapy tended to have a more rapid part 3 progression phenotype. (F) Box plots showing the distribution of slopes for the two groups. The part 3 UPDRS slopes of patients initiating symptomatic therapy were significantly greater than those who did not (P < 0.05, t test, n = 40 [no therapy], n = 35 [+dopaminergic (DA) therapy]). (G) Histogram of the part 3 UPDRS disability at the initiation of symptomatic therapy (n = 35). Note the wide range of disability. (H) Kaplan–Meier survival plots showing that placebo, low and high isradipine treatment groups had similar times to initiate symptomatic therapy (log-rank P = 0.3459).
Given that symptomatic therapy is most effective in treating PD motor disability, part 3 UPDRS scores were examined. As with the total UPDRS, the scatter plot of the parameters derived from the regression fits to individual participant data revealed a positive association with the initiation of symptomatic therapy (Fig. 2E). As with the total UPDRS scores, there was a significant correlation between baseline disability and estimated progression rate (Pearson’s correlation coefficient = 0.98, P < 0.0001, n = 75). Indeed, the progression rate in part 3 scores was significantly greater in participants initiating symptomatic therapy (Fig. 2F). That said, the distribution of disabilities assessed by part 3 scores at treatment initiation was wide (Fig. 2G). Kaplan–Meier plots of initiation of symptomatic therapy for placebo and isradipine treatment groups were overlapping and not significantly different (Fig. 2H).
Isradipine Treatment Was Associated with Diminished UPDRS Progression
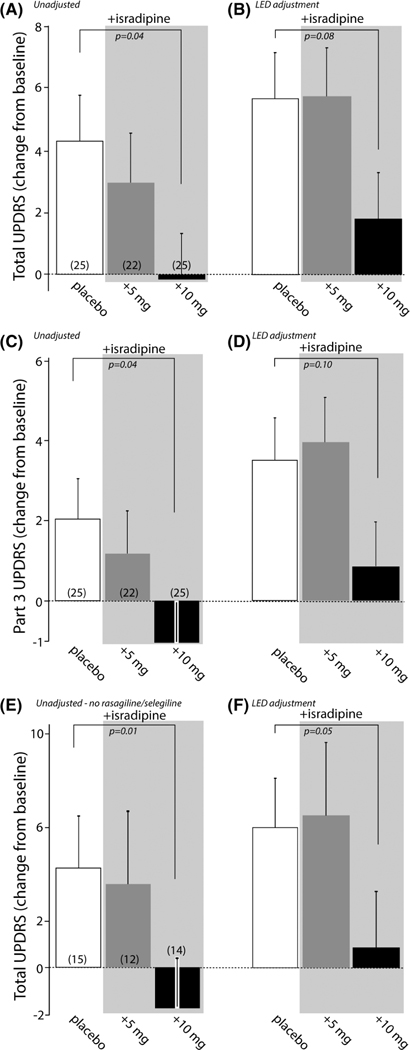
Having established that the placebo and isradipine treatment groups were matched for baseline characteristics and estimates of disease progression in the early portion of the trial, UPDRS scores at the conclusion of the trial (week 52) were examined, rather than using the LOCF procedure used in the initial efficacy analysis because it truncates estimates of disease progression in a large fraction of trial participants.9 For participants receiving the placebo treatment, the change in total UPDRS scores (from baseline at trial initiation to trial end at 52 weeks) increased on average ~4 points. In contrast, participants taking 10 mg/day of ER isradipine had almost no net change in total UPDRS score, with participants taking 5 mg/day having intermediate levels of progression (Fig. 3A).
FIG. 3.
Relationship between isradipine treatment and total and part 3 UPDRS scores. (A) Total UPDRS scores at the end of the STEADY-PD II trial for placebo (n = 25), 5 mg/day isradipine (n = 22) and 10 mg/day isradipine (n = 25) treatment groups. There was no adjustment for symptomatic therapy. Placebo and 10 mg/day isradipine groups were significantly different (P = 0.04). Data are shown as means/SEMs. (B) The difference in total UPDRS scores between placebo and 10 mg/day isradipine treatment groups remained after adjustment for total daily dose of symptomatic medication (P = 0.08); the adjustment was based on placebo group data in the STEADY-PD III trial and the methodology used previously6; see Methods for details. (C) Part 3 UPDRS scores at the end of the STEADY-PD II trial for placebo (n = 25), 5 mg/day isradipine (n = 22) and 10 mg/day isradipine (n = 25) treatment groups. There was no adjustment for symptomatic therapy. Placebo and 10 mg/day isradipine groups were significantly different (P = 0.04). Data are shown as means/SEMs. (D) The difference in part 3 UPDRS scores between placebo and 10 mg/day isradipine treatment groups remained after adjustment for total daily dose of symptomatic medication but was not statistically significant (P = 0.1); the adjustment was based on part 3 UPDRS placebo group data in the STEADY-PD III trial and the methodology used previously6; see Methods for details. (E) Total unadjusted UPDRS scores for patients not taking rasagiline or selegiline in the placebo (n = 15), 5 mg/day isradipine (n = 12) and 10 mg/day isradipine (n = 14) treatment groups. Placebo and 10 mg/day isradipine groups were significantly different (P = 0.01). (F) Adjustment for total daily dose of symptomatic medication did not change the pattern of the results. Placebo and 10 mg/day isradipine groups were significantly different (P = 0.05).
One caveat of this data is ~40% of participants initiated symptomatic therapy in the course of the trial. Although similar numbers of participants in each group received symptomatic medication and progression rates were similar across groups, this intervention is still a concern. In the STEADY-PD III trial, an adjustment equation was developed to estimate the effect of symptomatic medication on UPDRS scores.6,10 Although the LED adjustments increased projected UPDRS scores for all three treatment groups, they did not change the overall relationship between them. With adjustment for daily LED, the differences between groups narrowed, but the 10 mg/day isradipine treatment group had an average total UPDRS score that was smaller than both placebo and 5 mg/day treatment groups, although the P value rose to 0.08 after adjustment (Fig. 3B).
To determine if the part 3 motor UPDRS scores followed a similar pattern, they were analyzed separately. As with the total UPDRS scores, 10 mg/day isradipine treatment was associated with no progression in the part 3 scores (Fig. 3C). Again, adjustment for total daily symptomatic treatment shifted the part 3 scores up, increasing the P values associated with the differences to 0.1 (Fig. 3D).
MAO-B inhibitors also were commonly used by participants in the STEADY-PD II trial. To determine if participants not taking MAO-B inhibitors (rasagiline or selegiline) had a more robust response to isradipine treatment, total UPDRS scores were compiled for these participants. As in the larger cohort, participants taking 10 mg/day ER isradipine had essentially no progression in total UPDRS scores over the course of the trial (Fig. 3E). The pattern of part 3 scores (not shown) was similar (mean ± SEM, N: placebo = 1.93 ± 1.19, n = 15; 5 mg/day isradipine = 1.00 ± 2.22, n = 12; 10 mg/day isradipine = −2.29 ± 1.58, n = 14; P = 0.02 for placebo vs. 10 mg/day isradipine). With adjustments for total daily symptomatic medication, the differences between placebo and 10 mg/day isradipine treatment remained robust and statistically significant (Fig. 3F). For adjusted part 3 UPDRS scores, the pattern remained (placebo = 3.31 ± 1.30, n = 15;±5 mg/ day isradipine = 3.33 ±2.18, n = 12; 10 mg/day isradipine = −0.18 ± 1.67, n = 14; P= 0.07 for placebo vs. 10 mg/day isradipine). In contrast, although sample sizes were small, in the cohort of participants taking rasagiline or selegiline, there were not any significant differences between placebo and isradipine treatment groups for total unadjusted UPDRS scores (mean ± SEM, N: placebo = 3.90 ± 2.63, n = 10; 5 mg/day isradipine = 1.50 + 1.67, n = 10; 10 mg/day isradipine = 2.72 ± 2.26, n = 11; P = 0.65 for placebo vs. 10 mg/day isradipine) or part 3 unadjusted UPDRS scores (mean ± SEM, N: placebo = 2.00 + 1.78, n = 10; 5 mg/day isradipine = 1.00 + 1.07, n = 10; 10 mg/day isradipine = 1.09 + 1.41, n = 11; P = 0.57 for placebo vs. 10 mg/day isradipine).
Discussion
The main conclusion of our re-examination of the STEADY-PD II clinical trial with ER isradipine is that participants taking 10 mg/day had significantly smaller increases in total and part 3 UPDRS scores over the course of the 52-week trial when not adjusted for symptomatic medication. Use of an adjustment equation to correct for symptomatic medication increased the P value of the differences between groups above the 0.05 value set for rejection of the null hypothesis. The conclusion that ER isradipine treatment may be associated with slowing progression differs from that drawn in the initial analysis for two potential reasons. First, our analysis was restricted to the two groups receiving daily doses of isradipine that were tolerable (5 and 10 mg/day) and, for which there was good compliance. Second, rather than using an LOCF protocol, in which UPDRS scores at the time symptomatic therapy was initiated were carried forward for the efficacy analysis, the observed UPDRS scores at the end of the trial were used instead to better estimate disease progression.
Because a large proportion of participants (more than 40%) initiated symptomatic therapy during the trial, this group constituted a significant portion of the patient pool. Although the placebo and isradipine treatment groups were similar in baseline disability, initial rate of disease progression and the time at which symptomatic therapy was initiated, it is possible that there were differences in the type or amount of medication that could account for the apparent slowing of disease progression with isradipine treatment. Although there is no widely accepted statistical methodology for undertaking a post hoc adjustment of UPDRS scores for these aspects of symptomatic treatment,15 an adjustment to the total and part 3 UPDRS scores was performed using an approach similar to that developed for the STEADY-PD III trial data.6 The adjustment for total daily dose of symptomatic medication increased projected UPDRS scores (as expected), but again did not change the ordinal relationship between total and part 3 UPDRS scores in the placebo and 10 mg/day isradipine treatment groups. Nevertheless, the P values for the differences rose above the 0.05 threshold for rejecting the null hypothesis (P = 0.08–0.1).
Does this mean that the null hypothesis should be accepted? We think not for three reasons. First, the linear adjustment procedure itself is of uncertain validity in early-stage PD patients.15 Second, inspection of the linear regression data, on which LED adjustment was based, revealed that the goodness of the fits varied considerably between participants in our cohort, essentially adding a random variation term to the adjusted UPDRS scores. This added variance will increase the likelihood of making a Type II error. Third, the relatively small cohorts in the STEADY-PD II trial limit the power of the analysis. In our view, like that of many statisticians in the field, P values modestly above (or below) the common 0.05 level need to be interpreted cautiously.16
Another potential complication in the interpretation of these results is that there might be a biological interaction between isradipine and MAO-B inhibitors. Recent work in animal models by our group has shown that MAO-B inhibition with rasagiline decreases mitochondrial oxidant stress by diminishing dopamine metabolism in dopaminergic neurons.17 Isradipine also lowers mitochondrial oxidant stress, but by decreasing stimulation of the tricarboxylic acid cycle in mitochondria.4 Because of this interaction, MAO-B inhibitors may mask isradipine mediated neuroprotection. Indeed, although the sample size was small, participants given 10 mg/day isradipine, but not rasagiline or selegiline, had significantly less progression, regardless of LED-adjustment.
Our observations are broadly consistent with a recently published secondary pharmacokinetic analysis of the STEADY-PD III trial data.7 STEADY-PD III tested isradipine IR because ER preparation ceased being commercially available. This modeling work revealed that there was considerable variability between participants in IR pharmacokinetics and in those participants that cleared isradipine the slowest and had the greatest drug exposure, there was separation in some outcomes. In particular, there was a significant reduction in non-tremor motor disability, total symptomatic dosing by the end of the trial, and a delay in the need for symptomatic medication.7
Although the inferences from our analysis and the secondary analysis of the STEADY-PD III data are conceptually consistent, there are two apparent discrepancies. First, in the STEADY-PD II trial there was not a significant delay in the initiation of symptomatic therapy with isradipine treatment. Given the multifactorial nature of the time to symptomatic medication,18–20 the STEADY-PD II trial may not have been adequately powered to reliably detect a change. In fact, the STEADY-PD III trial had approximately twice as many participants in the critical isradipine-treatment cohorts. Another possibility is that the longer duration of the STEADY-PD III trial may have allowed differences in the transition to symptomatic therapy to be resolved.
The second apparent discrepancy between the two studies is that our analysis of the STEADY-PD II trial data suggests that isradipine treatment altered disease course assessed by UPDRS scoring, but this was not the case for the STEADY-PD III trial. Although the sample size in the STEADY-PD II trial was relatively small, one potential explanation for this difference is that the ER formulation isradipine afforded better target engagement, better protection of dopaminergic neurons and slower progression in disability as measured by the UPDRS. Another factor to consider is the difficulty in assessing disease progression using the UPDRS in a longer clinical trial where participants are well-managed clinically. Although mathematical modeling can in principle separate the effects of symptomatic drug treatment and those attributable to a disease-modifying intervention,10,15 in practice this has proven difficult to accomplish with confidence in the absence of reliable biomarkers of disease progression. In the shorter STEADY-PD II trial, more than half of the participants had not begun symptomatic treatment (other than an MAO-B inhibitor), hence making this complication less of a factor and potentially increasing the power to resolve alterations in disease progression with the UPDRS. Additional studies will be necessary to resolve this issue.
In conclusion, our re-examination of the STEADY-PD II phase 2 clinical trial with ER isradipine suggests that 10 mg/day, but not 5 mg/day, may slow the progression of disability in early-stage PD patients. This dose-dependence is consistent with preclinical studies and recent epidemiological work.4,5 The greater drug exposure and target engagement achieved by the ER formulation is a plausible explanation for the difference in outcomes of the STEADY-PD II and III trials. Confirmation of this inference will require additional clinical trials with formulations that maximize the duration and concentration of isradipine in the plasma and brain. Although our analysis provides a useful guide for future clinical trials, having robust biomarkers of disease progression, target engagement, and biological efficacy would be of obvious use. In addition, given that correcting clinical evaluations for symptomatic medication is problematic, the identification of objective biomarkers of disease progression will be critical to the success of disease modification trials, particularly when their duration precludes withholding symptomatic treatment.
Acknowledgments
Full Financial Disclosure
D.J.S. received funding for research from JPB Foundation, National Institutes of Health (NIH), The Michael J. Fox Foundation, William N. and Bernice E. Bumpus Foundation, CHDI, DoD; T.S. received funding for research from NIH; C.S.V. received funding for research from NIH.
Relevant conflicts of interests/financial disclosures: D.J.S. and J.N. are co-founders of Cavalon Therapeutics, which is pursuing disease-modifying therapies for Parkinson’s disease, including the development of Ca2+ channel inhibitors. T.S. is a consultant for Acadia, Denali, General Electric (GE), Neuroderm, Sanofi, Sinopia, Sunovion, Roche, Takeda, Michael J. Fox Foundation, and Voyager. T.S. served on the advisory boards of Acadia, Denali, General Electric (GE), Sunovian, Roche; and Scientific advisory boards of Neuroderm and Sanofi.
Footnotes
Data Availability Statement
All data analysis used in the manuscript is available upon request.
References
- 1.Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Primers 2017; 23(3):17013 [DOI] [PubMed] [Google Scholar]
- 2.McFarthing K, Buff S, Rafaloff G, Dominey T, Wyse RK, Stott SRW. Parkinson’s disease drug therapies in the clinical trial pipeline: 2020. J Parkinsons Dis 2020;10(3):757–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obeso JA, Stamelou M, Goetz CG, Poewe W, Lang AE, Weintraub D, et al. Past, present, and future of Parkinson’s disease: a special essay on the 200th anniversary of the shaking palsy. Mov Disord 2017;32(9):1264–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Surmeier DJ, Halliday GM, Simuni T. Calcium, mitochondrial dysfunction and slowing the progression of Parkinson’s disease. Exp Neurol 2017. Dec;298(Pt B):202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng Y-F, Lin H-C, Chao J C-J, Hsu C-Y, Lin H-L. Calcium Channel blockers are associated with reduced risk of Parkinson’s disease in patients with hypertension: a population-based retrospective cohort study. J Neurol Sci 2021;424:117412 [DOI] [PubMed] [Google Scholar]
- 6.Investigators PSGS-PI. Isradipine versus placebo in early Parkinson disease: a randomized trial. Ann Intern Med 2020;172(9):591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venuto CS, Yang L, Javidnia M, Oakes D, Surmeier DJ, Simuni T. Isradipine plasma pharmacokinetics and exposure–response in early Parkinson’s disease. Ann Clin Transl Neur 2021;8(3):603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller JD, Farber J, Gatz P, Roffwarg H, German DC. Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and waking in the rat. Brain Res 1983;273(1):133–141. [DOI] [PubMed] [Google Scholar]
- 9.Group PS. Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson’s disease (STEADY-PD). Mov Disord. 2013;28(13):1823–1831. [DOI] [PubMed] [Google Scholar]
- 10.Holford NHG, Nutt JG. Interpreting the results of Parkinson’s disease clinical trials: time for a change. Mov Disord. 2011;26(4): 569–577. [DOI] [PubMed] [Google Scholar]
- 11.Ebersbach G, Baas H, Csoti I, Müngersdorf M, Deuschl G. Scales in Parkinson’s disease. J Neurol. 2006;253(Suppl 4):iv32–iv35. [DOI] [PubMed] [Google Scholar]
- 12.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 2010;25(15):2649–2653. [DOI] [PubMed] [Google Scholar]
- 13.Ciolino JD, Martin RH, Zhao W, Hill MD, Jauch EC, Palesch YY. Measuring continuous baseline covariate imbalances in clinical trial data. Stat Methods Med Res 2015;24(2):255–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egbewale BE, Lewis M, Sim J. Bias, precision and statistical power of analysis of covariance in the analysis of randomized trials with baseline imbalance: a simulation study. BMC Med Res Methodol 2014;14(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venuto CS, Potter NB, Dorsey ER, Kieburtz K. A review of disease progression models of Parkinson’s disease and applications in clinical trials. Mov Disord. 2016;31(7):947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature 2019;567(7748):305–307. [DOI] [PubMed] [Google Scholar]
- 17.Graves SM, Xie Z, Stout KA, Zampese E, Burbulla LF, Shih JC, et al. Dopamine metabolism by a monoamine oxidase mitochondrial shuttle activates the electron transport chain. Nat Neurosci 2019; 23(1):15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simuni T, Long JD, Caspell-Garcia C, Coffey CS, Lasch S, Tanner CM, et al. Predictors of time to initiation of symptomatic therapy in early Parkinson’s disease. Ann Clin Transl Neurol 2016; 3(7):482–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marras C, McDermott MP, Marek K, Rochon P, Naglie G, Tanner CM, et al. Predictors of time to requiring dopaminergic treatment in 2 Parkinson’s disease cohorts. Mov Disord. 2011; 26(4):608–613. [DOI] [PubMed] [Google Scholar]
- 20.McDermott MP, Jankovic J, Carter J, Fahn S, Gauthier S, Goetz CG, et al. Factors predictive of the need for levodopa therapy in early, untreated Parkinson’s disease. Arch Neurol 1995;52(6): 565–570. [DOI] [PubMed] [Google Scholar]