Abstract
Because antimicrobial usage (AMU) data are crucial in understanding and dealing with the threat that antimicrobial resistance (AMR) poses to global health, data of the sale of antimicrobials from 2016 to 2019 of CDMV Inc. — a major distributor of veterinary products in Canada — were collected and analyzed for the province of Quebec. The primary objective was to describe the evolution of AMU sales data for dairy cattle, small animals, and horses; a secondary objective was to determine effects of a new provincial regulation on antimicrobials of very high importance (in Quebec) on sales for dairy cattle. Results are described in milligrams of antimicrobials per kilogram of animal biomass (mg/PCU) for dairy cattle, small animals, and horses; intramammary products were analyzed for number of treatments per 100 cow-years; and results for dairy cattle were compared in Canadian-defined course doses for cattle (DCDbovCA) per 100 cow-years to a recent study for this species in Quebec. Between 2016 and 2019, there were decreased sales of Category 1 antimicrobials for all species included in the study (Category 1-VI for small animals). This reduction was even more apparent for dairy cattle, for which a 76% decrease occurred from 2018 to 2019 (1.7 to 0.4 mg/PCU). This marked reduction was attributed to the new regulation implemented in February 2019. Since a farm- and clinic-level AMU monitoring system has not yet been implemented in Quebec, analysis of CDMV Inc. sales enabled observations of temporal trends in AMU for dairy cattle, horses, and small animals. These temporal trends based on CDMV Inc. sales will be useful for making comparisons and validating trends derived from farm- and clinic-level data generated by a monitoring system.
Résumé
Analyse des données de ventes d’antibiotiques du principal distributeur de médicaments au Québec de 2016 à 2019 : une estimation de l’utilisation des antibiotiques chez les bovins laitiers, les chevaux et les animaux de compagnie. Étant donné que les données sur l’utilisation des antimicrobiens (UAM) sont cruciales pour comprendre et faire face à la menace que la résistance aux antimicrobiens (RAM) fait peser sur la santé mondiale, les données sur les ventes d’antibiotiques de 2016 à 2019 de CDMV Inc. – un important distributeur de produits vétérinaires au Canada – ont été recueillies et analysées pour la province de Québec. L’objectif principal du projet était de décrire l’évolution des données de ventes d’antibiotiques pour les bovins laitiers, les animaux de compagnie et les chevaux; un objectif secondaire était de déterminer les effets d’une nouvelle réglementation provinciale relative à l’usage des antimicrobiens de catégorie 1 – très haute importance – (au Québec) sur les ventes d’antibiotiques pour les bovins laitiers. Les résultats sont décrits en milligrammes d’antibiotiques par kilogramme de biomasse animale (mg/PCU) pour les bovins laitiers, les animaux de compagnie et les chevaux. Les produits intramammaires sont analysés selon le nombre de traitements par 100 vaches-années. Les résultats pour les bovins laitiers ont aussi été comparés à une étude récente réalisée au Québec pour cette espèce en « traitement type pour les bovins au Canada » (DCDbovCA) par 100 vaches-années. Entre 2016 et 2019, les ventes d’antimicrobiens de catégorie 1 ont diminué pour toutes les espèces de l’étude (catégorie 1-VI pour les animaux de compagnie). Cette réduction a été encore plus apparente pour les bovins laitiers, pour lesquels une diminution de 76 % s’est produite de 2018 à 2019 (1,7 à 0,4 mg/PCU). Cette baisse marquée a été attribuée à la nouvelle réglementation mise en place en février 2019. Puisqu’un système de surveillance sur l’utilisation des antibiotiques à l’échelle des fermes et des cliniques vétérinaires n’a pas encore été mis en place au Québec, l’analyse des ventes de CDMV Inc. a permis d’observer les tendances temporelles de l’UAM pour les bovins laitiers, les chevaux et les animaux de compagnie. Ces tendances temporelles, basées sur les ventes de CDMV Inc., seront utiles pour effectuer des comparaisons et valider les tendances dérivées des données au niveau des fermes et des cliniques vétérinaires générées par un futur système de surveillance sur l’utilisation des antibiotiques.
(Traduit par les auteurs)
Introduction
Antimicrobial resistance (AMR) poses a threat to global human and animal health; therefore, obtaining data about antimicrobial usage (AMU) is crucial. These data are needed for studying the relationships between AMU and AMR, tracking modifications of usage over time, and supporting stewardship programs (1). In animal health, many countries have already established monitoring systems to collect national antimicrobial sales data, and a growing number of countries are implementing farm-level monitoring systems (2).
From 2018 to 2021, a feasibility study was conducted in Quebec to determine the best way to establish a multispecies AMU monitoring system in the province. This study was requested and financed by the Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ). In the context of this feasibility study, antimicrobial sales data from CDMV Inc. (2016 to 2019) were collected and analyzed for the province. CDMV Inc. is a major distributor of veterinary products in Quebec and Canada; it is responsible for most anti-microbial sales for dairy cattle and small animals in Quebec and most antimicrobial sales for horses in the province (Marie-Josée Bayard, Vice-President of Sales and Business Development, CDMV Inc., personal communication, 2021). As establishing a farm- and clinic-level (for small animals and horses) AMU monitoring system presents many challenges and requires time, analysis of global antimicrobial sales data of CDMV Inc. represented a convenient and inexpensive way to conduct yearly AMU trend analysis. In addition, a new provincial regulation regarding AMU came into effect on February 25, 2019, providing an opportunity to determine if this policy change could be detected in the antimicrobial sales data of CDMV Inc. Antimicrobials of very high importance for humans were banned by this regulation for use in production animals (e.g., 3rd and 4th generation cephalosporins, fluoroquinolones, and polymyxins) and their curative uses were delimited (3).
In the province of Quebec, CDMV Inc. is the main distributor of commercially available antimicrobials (for dairy cattle, small animals, and horses) but not of medicated feed or compounded products, which CDMV Inc. does not supply. For the dairy sector, this exclusive right to sell antimicrobials has historical roots reaching back to 1971 with establishment of Quebec’s animal health improvement program [Amélioration de santé animale au Québec (ASAQ)] and creation of CDMV Inc. in 1972 (4,5). To improve access to veterinary services across all of Quebec’s regions, the ASAQ program covers part of the costs of a veterinary visit and professional fees for producers included in the program (6). Moreover, it regulates the prices at which veterinary products are sold, in part by making it mandatory for veterinarians participating in this government program to purchase from CDMV Inc. (7). The ASAQ program is designed to improve the health of food-producing animal species (e.g., beef cattle, dairy cattle, small ruminants, swine). However, its resources are mainly used by the dairy sector. Between 2016 and 2019, at least 86% of visits and 88% of hours covered by the program were used by this sector (Hugo Plante, MAPAQ; written communication, 2021). This was followed by the beef cattle sector (which accounted for at least 7% of visits and 5% of hours covered); horses destined for production (at least 3% of visits and 2% of hours); and the swine sector (at least 1% of visits and 2% of hours). Since CDMV Inc. was already the sole distributor of veterinary products for the dairy sector, it quickly became a major supplier for veterinarians working with small animals and horses in the province.
The primary objective of this project was to describe trends of AMU from 2016 to 2019 for dairy cattle, small animals, and horses in the province of Quebec using CDMV Inc. data; a secondary objective was to determine what effects the new policy that banned preventive usage of antimicrobials of very high importance for humans had on sales of antimicrobials for dairy cattle.
Materials and methods
Data collection
A letter of support for the project was signed by the presidents of all 3 veterinary associations in Quebec that this initiative involved [Association des médecins vétérinaires du Québec (AMVQ), Associations des médecins vétérinaires practiciens du Québec (AMVPQ), and Association des vétérinaires équins du Québec (AVEQ)], and all customers of CDMV Inc. were notified. For this project, 2 databases were used. First, a database containing all of CDMV Inc.’s antimicrobial sales data for the province of Quebec between 2016 and 2019 was obtained. This file was acquired after a data-sharing agreement was signed between CDMV Inc. and the Faculté de médecine vétérinaire de l’Université de Montréal. This database contained the commercial name of each antimicrobial product merged with its format, CDMV Inc. internal codes, information regarding packaging (individual units, boxes of 12, 24, etc.), and the quantities sold, the year, and the drug identification number (DIN) of each product.
To carry out the analyses, this database was merged with Health Canada’s Drug Product Database (DPD). Data extracted from the DPD are available on the Government of Canada’s website which contains “complete product information for all approved, marketed, cancelled and dormant products, for human, veterinary, disinfectant and radiopharmaceutical use” (8). As data extracted from the DPD were contained in a series of files, they had to be merged prior to usage. In addition, some modifications of the DPD were made to add information regarding the antimicrobial class of each antimicrobial, to add the category of importance of each product based on Health Canada’s categorization of antimicrobials, and to harmonize dosage units.
Calculation of mg per kg of biomass (mg/PCU)
Data were analyzed in mg of antimicrobials per kg of animal biomass (mg/PCU) using the biomass distribution method described by Carmo et al (9). In this method, the proportion of product sales attributed to a specific species is equal to the proportion of the biomass of that species relative to the total biomass of all species for which the product is licensed. Calculations, therefore, are made individually for each product and results are summarized to determine the total quantity of antimicrobials attributed to each species.
Information concerning quantities of antimicrobials sold and the species for which each product is licensed came from CDMV Inc.’s database and Health Canada’s DPD, respectively. To calculate biomass, Canadian average weight at treatment for dairy cows, horses, dogs, and cats, as defined by the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) (10), were used. Animal population data for the province of Quebec came from the Canadian Dairy Information Center for dairy cows (11), Cheval Québec for horses (Renée Lévesque, Cheval Québec; written communication, 2019), and from surveys requested by the AMVQ for small animals (12,13). Information related to biomass calculations are summarized (Table 1). All analyses were performed with SAS Version 9.4 (SAS Institute, Cary, North Carolina, USA).
Table 1.
Biomass calculations.
| Dairy cows | Horses | Cats | Dogs | |
|---|---|---|---|---|
| Animal population (11–13) | 344 100 to 361 600 | 119 669 to 125 154 | 1 980 000 | 1 020 000 |
| Canadian average weight at treatment (kg) (10) | 635 | 500 | 4 | 15 |
| Biomass 2016 (kg) | 220 281 500 | 60 865 000 | 7 920 000 | 15 300 000 |
| Biomass 2017 (kg) | 218 503 500 | 59 834 500 | 7 920 000 | 15 300 000 |
| Biomass 2018 (kg) | 224 917 000 | 62 577 000 | 7 920 000 | 15 300 000 |
| Biomass 2019 (kg) | 229 616 000 | 61 092 000 | 7 920 000 | 15 300 000 |
| Mean percentage of animal biomass (%) | 72.6 | 19.9 | 2.6 | 5.0 |
Since the dairy sector was the main industry benefiting from the ASAQ program between 2016 and 2019, the biomass of animals from other production sectors in Quebec (e.g., beef cattle, veal cattle, and small ruminants) was not included in the calculations. Moreover, most antimicrobials sold to the swine sector, poultry sector, and for aquaculture in Quebec are provided by sources other than CDMV Inc. In fact, antimicrobial products only licensed for these species represented only 1.2% of the total amount of antimicrobials sold by CDMV Inc. between 2016 and 2019; therefore, they were excluded from our analyses. Lastly, all antimicrobial products licensed for humans and sold by CDMV Inc. were attributed to small animals (dogs and cats), as 93% of these antimicrobials were capsules and tablets and were most likely consumed by small animals. In summary, we assumed that all dairy cows, horses, dogs, and cats in Quebec were at risk of being treated with antimicrobials dispensed by CDMV Inc., and no other source of antimicrobials was present. Since we formulated the hypothesis that our results reflect AMU in the province of Quebec for the populations of the 3 groups under study, confidence intervals are not shown when variations of sales data are presented.
In order to evaluate the types of antimicrobials used in Quebec between 2016 and 2019, quantities of antimicrobials sold in mg/PCU are presented according to Health Canada’s categorization of importance (14). In the case of products containing active ingredients belonging to more than 1 category, the category of highest importance was assigned to the product.
Comparison of Canadian-defined course doses for cattle (DCDbovCA) per 100 cow-years
To compare results obtained for dairy cattle in this project with those presented in Lardé et al (15), analyses were performed with an average weight of 650 kg for dairy cows — which was used to establish Canadian-defined course doses for cattle (DCDbovCA) — instead of 635 kg (16). Quantities of antimicrobials attributed to this species were then converted to DCDbovCA by following the assignment of DCDbovCA values and the exact method described in Lardé et al (16). Finally, we divided the DCDbovCA obtained by the number of cows for each year in Quebec and multiplied the results by 100. Results from CDMV Inc. data for 2017 and 2018 were compared with those obtained using veterinary invoices (VET Method) in Lardé et al (15) to match study periods. We observed if our results, which encompassed all sales of antimicrobials in Quebec for dairy cattle, were within the confidence intervals described by Lardé et al for each route of administration.
Number of intramammary treatments per 100 cow-years
Intramammary treatments were defined according to quantities of active ingredients in mg/animal/course (DCDbovCA), as described by Lardé et al (16). However, in contrast with the method presented by these authors, when intramammary products contained more than 1 active ingredient, treatments were counted according to the total quantity of antimicrobials in mg/animal/course (1 treatment per combination of agents) and not individually for each active ingredient. This was applied to only 2 products with combinations of active ingredients (1 for lactating cows and 1 for dry cow therapy). This was done to avoid overestimating the number of treatments for products containing more than 1 active ingredient.
Results
Calculation of mg per kg of biomass (mg/PCU)
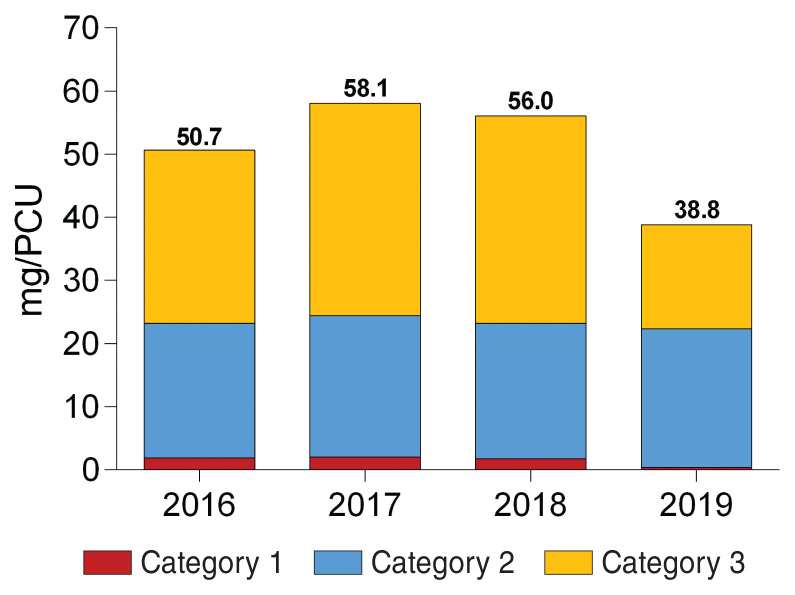
Quantities of antimicrobials sold for dairy cattle in mg/PCU between 2016 and 2019 are presented according to Health Canada’s categorization of importance (Figure 1). There were 2 main trends: i) there was a 76 to 80% reduction of sales of Category 1 antimicrobials (very high importance) in 2019 compared to previous years, which were 1.9, 2.0, 1.7, and 0.4 mg/PCU from 2016 to 2019, respectively; and ii) there was a 50% decrease (from 32.8 to 16.5 mg/PCU) in sales of Category 3 antimicrobials between 2018 and 2019. However, sales of antimicrobials in Category 2 were stable in the observed period, ranging from 21.3 to 22.4 mg/PCU.
Figure 1.
Antimicrobials (mg/PCU) sold for dairy cattle between 2016 and 2019 according to Health Canada’s categorization of product importance. Antimicrobials in category 4 (low importance) are not shown in this figure.
For horses, sales of antimicrobials between 2016 to 2019 were relatively stable (17.7, 17.8, 16.6, 17.0 mg/PCU, respectively). Most antimicrobials sold for horses (between 97.1 and 98.7%) were from Category 2 (high importance). Sales of antimicrobials in Category 1 decreased by 75% for horses in 3 to 4 y (0.4, 0.5, 0.3, and 0.1 mg/PCU from 2016 to 2019).
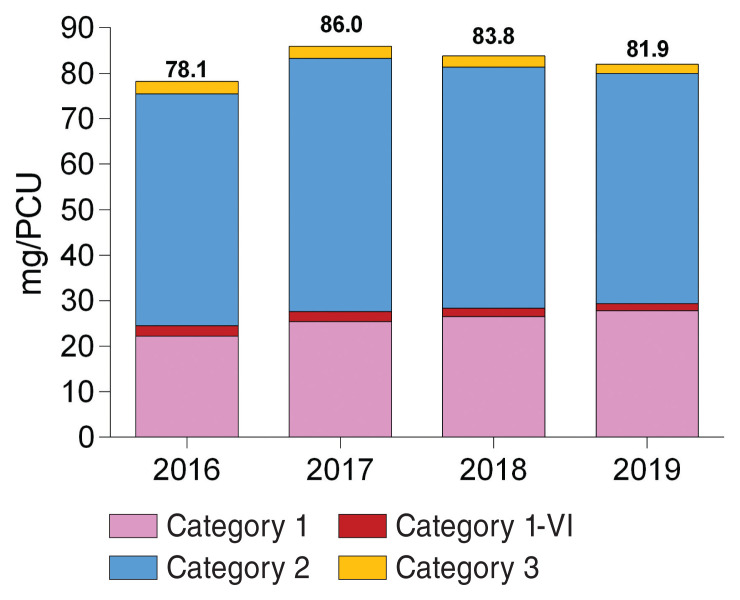
Antimicrobial use in small animals during the studied period is shown (Figure 2); note that antimicrobials in Category 1 were divided into 2 categories (Category 1 and Category 1-VI — for Very Important). The amoxicillin-clavulanic acid combination and metronidazole were classified as Category 1, whereas 3rd and 4th generation cephalosporins, fluoroquinolones, and products containing polymyxins were classified as Category 1-VI. This was done because the amoxicillin-clavulanic acid combination and metronidazole are not classified in the highest priority category in more recent international classifications (17–18). From 2016 to 2019, there was a 25% increase in the sales of antimicrobials in Category 1 for small animals (from 22.2 to 27.8 mg/PCU). On the contrary, sales of antimicrobials in Category 1-VI diminished by 30% during this same period, moving from 2.3 to 1.6 mg/PCU. Sales of antimicrobials in Category 2 represented most antimicrobials used for the treatment of small animals and quantities sold fluctuated (51.0, 55.7, 53.0, and 50.5 from 2016 to 2019, respectively). Lastly, there was a 26% reduction in the quantities of antimicrobials sold in Category 3 (from 2.7 to 2.0 mg/PCU in the studied period).
Figure 2.
Antimicrobials (mg/PCU) sold for small animals between 2016 and 2019 according to Health Canada’s categorization of product importance. Category 1 includes products containing the amoxicillin-clavulanic acid combination and metronidazole. Category 1-VI includes products containing third and fourth generation cephalosporins, fluoroquinolones, and polymyxins.
Number of intramammary treatments per 100 cow-years
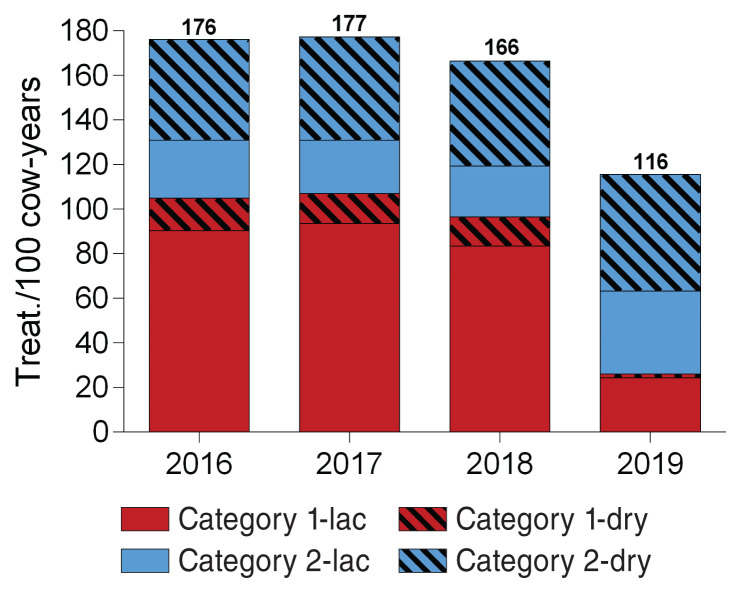
During the studied period, the number of intramammary treatments per 100 cow-years decreased by 34%, from 177 treatments per 100 cow-years in 2017 to 116 treatments in 2019. Specifically, sales of lactation and drying-off products in Category 1 greatly diminished in 2019 compared to previous years, whereas sales of both types of products in Category 2 increased in the same interval (Figure 3). The most notable change was a 71% reduction of sales of lactation products in Category 1, which varied from 83 treatments per 100 cow-years in 2018 to 24 treatments per 100 cow-years in 2019.
Figure 3.
Number of intramammary treatments per 100 cow-years between 2016 and 2019 by Health Canada’s categorization of product importance and by type of intramammary product (lactation or drying off).
Comparison of DCDbovCA per 100 cow-years
The DCDbovCA/100 cow-years by administration route obtained from CDMV Inc. data and from the VET Method described by Lardé et al (15) are compared (Table 2).
Table 2.
DCDbovCA/100 cow-years from CDMV Inc. data compared with those from the VET Method (15).
| Route of administration | DCDbovCA per 100 cow-years | ||
|---|---|---|---|
|
| |||
| 2017 | 2018 | VET Methoda (95% CI) | |
| Intramammary for lactating cows | 317 | 279 | 275 (220, 344) |
| Intramammary at dry-off | 79 | 78 | 73 (65, 83) |
| Injectable | 123 | 114 | 77 (65, 92) |
| Oralb | 152 | 137 | 78 (58, 105) |
| Intrauterine | 18 | 16 | 17 (12, 23) |
| Topical | 0.4 | 0.4 | 1 (0, 2) |
Data from Lardé et al (15) were collected between March 2017 and May 2018.
Oral products other than in the feed.
CI — Confidence interval.
Discussion
Sales reduction of antimicrobials of very high importance
Between 2016 and 2019, there were decreased sales of Category 1 antimicrobials for all species included in the study (called Category 1-VI for small animals). This reduction was even more apparent for dairy cattle, for which a 76% decrease occurred from 2018 to 2019 (1.7 to 0.4 mg/PCU). For these animals, the sharp decrease in sales of Category 1 injectable antimicrobials was mainly caused by a diminution of 3rd generation cephalosporins. A reduction was also observed for intramammary products. In this route of administration, Category 1 lactation and drying-off products were responsible for the important decrease in the number of treatments per 100 cow-years from 2018 to 2019.
We inferred that the February 2019 provincial regulation, banning preventive usage of antimicrobials of very high importance and delimiting their curative uses on production animals, markedly reduced antimicrobial usage for dairy cattle. Influences of regulations on AMU have also been documented in other countries. For example, in France, a target was imposed in 2014 to reduce antimicrobials sales of fluoroquinolones and 3rd and 4th generation cephalosporins by 25% by December 2016. In addition, in March 2016, a similar regulation to Quebec’s was implemented for antimicrobials of very high importance (no preventive usage and a requirement to have an antibiogram before authorizing a prescription for curative usage) (19). With implementation of these additional measures in France, animal exposure to fluoroquinolone and 3rd and 4th generation cephalosporins decreased by 75% between 2013 (reference year) and 2016 (20). It is noteworthy that, unlike in France, no reduction targets were enforced in Quebec prior to the implementation of the regulation in February 2019.
Other factors may also have reduced the use of antimicrobials of very high importance for all species, including awareness and education of veterinarians regarding responsible use of antimicrobials (3,21), more publications on antimicrobial resistance, and the perception of greater public pressure for the reduction of antimicrobial usage (22,23).
Reduction of sales for dairy cattle
In association with the sales decrease of Category 1 antimicrobials for dairy cattle, there was also a major sales reduction of Category 3 antimicrobials from 2018 to 2019. When dairy cattle antimicrobial sales were observed by commercial product, this reduction (from 32.8 to 16.5 mg/PCU) was explained by the withdrawal of Aureo S 700 G from the market in 2018, whereas sales of other products remained stable. Aureo S 700 G contained chlortetracycline and sulfamethazine and was licensed for cattle as an aid to maintain weight gain in the presence of respiratory disease.
Comparison of results
When DCDbovCA/100 cow-years from CDMV Inc. data were compared to those from the VET Method described by Lardé et al (15) (Table 2), there were similarities for routes of administration that are more specific to dairy cattle (i.e., intramammary for lactating cows, intramammary at dry-off, and intrauterine). In fact, for these routes, results were either very close to those reported by Lardé et al (15) or they were within the 95% confidence interval (CI) described. On the contrary, results for routes of administration that are less specific to this species (i.e., injectable and oral) were more different, and are not included in the 95% CI. Differences in results obtained for injectable and oral routes of administration could be explained by some antimicrobials sold by CDMV Inc. to beef cattle, veal cattle, and small ruminants in Quebec. Indeed, exclusion of the biomass of these species from the analyses may have led to an overestimation of the quantities of antimicrobials sold for dairy cattle. In the case of oral antimicrobials, another possible source of overestimation is the frequent use of oral tetracyclines for topical treatment of digital dermatitis in dairy cattle. In the United Kingdom VARSS 2019 report, total results in mg/PCU obtained from 3410 dairy farms (34% of UK dairy cattle) were 21.9 for 2017, 22.8 for 2018, and 22.5 for 2019 (24). In comparison, results from CDMV Inc. data (50.7, 58.1, 56.0, and 38.8 mg/PCU from 2016 to 2019, respectively) were much higher. In addition, the UK-VARSS report used ESVAC methodology to calculate mg/PCU, which uses an average weight at treatment of 425 kg for dairy cattle; therefore, the UK-VARSS results would be even lower if the Canadian average weight at treatment for dairy cattle (635 kg) was used. Perhaps dairy cattle antimicrobial consumption in the UK is lower than in Quebec, although antimicrobials sold for beef cattle, veal cattle, and small ruminants may have caused an overestimation of the quantities sold for dairy cattle in this study. Moreover, when comparing estimates between countries, other differences in methodologies and in data representability should be considered.
For small animals, results obtained for Quebec using CDMV Inc. data (78.1, 86.0, 83.8 and 81.9 mg/PCU from 2016 to 2019, respectively) appeared to be slightly lower than those observed in Canada. In fact, when quantities of antimicrobials sold for small animals described in CIPARS reports (including sales in Quebec) were divided by their biomass, results were 89.0, 92.2, and 84.9 mg/PCU from 2016 to 2018 (10,25–26). In France, using the same average weights at treatment (4 kg for cats and 15 kg for dogs), results obtained (95.4, 98.1, 94.9, and 96.1 mg/PCU from 2016 to 2019, respectively) were also higher than those observed in this study (27). On the contrary, quantities of antimicrobials sold for small animals presented in the UK VARSS report (76.3, 74.7, 66.5, and 63.0 mg/PCU from 2016 to 2019) were lower than those from CDMV Inc. data (24). However, average weights at treatment used for small animals in the UK VARSS reports are adjusted annually and they are higher than those used in this study (~4.5 kg for cats and 18.5 kg for dogs between 2016 and 2019) (28). Furthermore, unlike this study, quantities of antimicrobials licensed for humans and used by veterinarians are not included in reports for France and the UK, which may have caused underestimates in reported results.
Few results are available to compare values in mg/PCU for horses in this study (17.7, 17.8, 16.6, and 17.0 from 2016 to 2019). In France, quantities of antimicrobials sold for horses were 29.3, 30.7, 17.2, and 16.7 mg/PCU for the studied period (20,27,29–30). Nevertheless, 4 average weights were used for horses (300, 350, 550, and 850 kg, depending on breed), which limits comparisons. It is also likely that the quantities of antimicrobials sold for horses reported in this study were underestimated (see Limits).
In summary, analysis of CDMV Inc.’s antimicrobials sales data facilitated assessment of trends in AMU for dairy cattle, small animals and horses in Quebec. Since these data were easily accessible, provided interesting insights and were simple to analyze, we recommend conducting these analyses on an annual basis to follow future AMU trends.
Limitations of the study
There were certain limits in the methods used or in the analyses performed. First, it was assumed that CDMV Inc. had the entire market share for distribution of antimicrobials for the species examined (dairy cattle, horses, cats, and dogs) and that this was constant over the studied years. Corrections for the exact market share held by species were therefore not made. Moreover, as mentioned earlier, antimicrobials for beef cattle, veal cattle, and small ruminants sold by CDMV Inc. may have affected results for dairy cattle by overestimating them. In addition, for the species of interest, the possibility of direct sales between pharmaceutical companies and some veterinary clinics without these sales going through CDMV Inc. was not considered.
The number of animals recorded in each year had a great impact on the calculation of biomass by species. The resulting biomasses had a decisive role in the method of weighting the quantities of antimicrobials sold by biomass and thus on the results. For horses, numbers used ranged from ~120 000 to 125 000 and seemed high according to various experts consulted. To the best of our knowledge, more accurate numbers for horses in Quebec are not yet available, but Cheval Québec is currently working on a new method to improve this estimation. For small animals, numbers used (1.98 million cats and 1.02 million dogs) lacked precision and more accurate results could be achieved for these species if more population data were available in Quebec. Dairy cattle numbers from the Canadian Dairy Information Centre website seemed adequate.
Furthermore, the use of compounded products for the species of interest were not included in these analyses. Since CDMV Inc. does not distribute compounded products, sales related to these products could not be included. Antimicrobials sold in the form of compounded products apparently represent an important part of sales for horses (Karen Rodier, President of AVEQ; personal communication, 2020) because compounding increases palatability and facilitates administration. A considerable quantity of antimicrobials for this species sold as compounded products could explain limited antimicrobial use based on CDMV Inc. data.
In addition, for products licensed for multiple species, the biomass distribution method assigned the quantities of antimicrobials according to the proportions of biomass of each species and not according to the real usage practices of these products. In this study, the mean proportions of biomass were 72.6, 19.9, 2.6, and 5 for dairy cattle, horses, cats, and dogs, respectively. This could have influenced results reported for horses, as many products licensed for this species are also licensed for dairy cattle. However, since only a few products are licensed for dairy cattle and small animals, it is unlikely that this markedly affected results for small animals.
Finally, data analysis of a distributor such as CDMV Inc. cannot support modification of individual practices. Indeed, the resolution of these analyses does not allow tracking of individual practices at the veterinarian or producer’s level. More granular data would be required to support personalized feedback to these stakeholders.
In conclusion, the analysis of sales data of CDMV Inc. — which supplies antimicrobials to most of the community for specific species — was a simple and inexpensive way to observe temporal trends in AMU for dairy cattle, horses, and small animals in Quebec. It permitted, among other things, detection of decreased usage of Category 1 antimicrobials in dairy cattle after implementation of a new regulation regarding such products in production animals. The analyses also gave a much-needed assessment of antimicrobial usage in small animals, including sales of products licensed for humans. Results described for horses should, however, be interpreted with caution, as the population data used was approximate for this species and because compounded products were not taken into consideration in this study.
As demonstrated in this study, analysis of CDMV Inc. data enabled comparisons between AMU of 3 groups of species in the province of Quebec to the AMU of these species at the national and international levels. Although international comparisons of AMU can be challenging due to differences in methodologies, they can offer relevant information regarding potential progress in responsible use of antimicrobials. Lastly, since a farm- and clinic-level multispecies AMU monitoring system has not yet been implemented in Quebec, CDMV Inc. data can offer baseline information about AMU of 3 groups of species in the province. When farm- and clinic-level data are made available through a monitoring system, temporal trends observed with CDMV Inc. data will be useful for conducting comparisons and validating trends detected with this new source of information.
Acknowledgments
We sincerely thank CDMV Inc. for granting us access to their data and for their collaboration throughout this project, which was financed by the Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ) in the context of the Politique gouvernementale de prévention en santé. CVJ
Footnotes
This project was financed by the Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec in the context of the Politique gouvernementale de prévention en santé.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Collineau L, Belloc C, Stärk KDC, et al. Guidance on the selection of appropriate indicators for quantification of antimicrobial usage in humans and animals. Zoonoses Public Health. 2017;64:165–184. doi: 10.1111/zph.12298. [DOI] [PubMed] [Google Scholar]
- 2.Sanders P, Vanderhaeghen W, Fertner M, et al. Monitoring farm-level antimicrobial use to guide stewardship: Overview of existing systems and analysis of key components and processes. Front Vet Sci. 2020;7:540. doi: 10.3389/fvets.2020.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy JP, Archambault M, Desrochers A, et al. New Quebec regulation on the use of antimicrobials of very high importance in food animals: Implementation and impacts in dairy cattle practice. Can Vet J. 2020;61:193–196. [PMC free article] [PubMed] [Google Scholar]
- 4.Association des Médecins Vétérinaires Praticiens du Québec. Historique et mission. 2021. [Last accessed February 1, 2022]. Available from: https://www.amvpq.org/article/historique-et-mission/#:~:text=Fond%C3%A9e%20en%201969%2C%20l'AssociationV%C3%A9t%C3%A9rinaires%20Praticiens%20du%20Qu%C3%A9bec%20(A.M.V.P.Q.)&text=%C3%80%20l'origine%2C%20ce%20regroupement,animale%20au%20Qu%C3%A9bec%20(ASAQ)
- 5.CDMV Inc. The company. 2021. [Last accessed February 1, 2022]. Available from: https://www.cdmv.com/en/the-company/
- 6.Ministère de l’Agriculture. des Pêcheries et de l’Alimentation du Québec. Amélioration de la santé animale au Québec (ASAQ) 2021. [Last accessed February 1, 2022]. Available from: https://www.mapaq.gouv.qc.ca/fr/Productions/md/programmesliste/santeanimale/Pages/ASAQ.aspx#:~:text=Am%C3%A9lioration%20de%20la%20sant%C3%A9%20animale%20au%20Qu%C3%A9bec%20(ASAQ),-Contenu1&text=Par%20l'entremise%20de%20cequ'%C3%A0%20des%20m%C3%A9dicaments%20v%C3%A9t%C3%A9rinaires.
- 7.Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec. Entente relative à la transition du programme d’amélioration de la santé animale au Québec (ASAQ) vers le programme intégré de santé animale du Québec (PISAQ) 2017 [Google Scholar]
- 8.Government of Canada. What is the DPD Data Extract? — Drug Product Database (DPD) Data Extract — Health Canada. 2021. [Last accessed February 1, 2022]. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/drug-product-database/what-data-extract-drug-product-database.html.
- 9.Carmo LP, Schüpbach-Regula G, Müntener C, Chevance A, Moulin G, Magouras I. Approaches for quantifying antimicrobial consumption per animal species based on national sales data: A Swiss example, 2006 to 2013. Euro Surveill. 2017;22:30458. doi: 10.2807/1560-7917.ES.2017.22.6.30458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2018: Figures and Tables. Guelph, Ontario: Public Health Agency of Canada; 2020. [Last accessed February 1, 2022]. Available from: http://publications.gc.ca/collections/collection_2020/aspc-phac/HP2-4-2018-eng-4.pdf. [Google Scholar]
- 11.Government of Canada. Canadian Dairy Information Centre: Number of Dairy Cows and Heifers by Province. 2021. [Last accessed February 1, 2022]. Available from: https://aimis-simia-cdic-ccil.agr.gc.ca/rp/index-eng.cfm?action=pR&r=219&pdctc=
- 12.Association des médecins vétérinaires du Québec en pratique des petits animaux. Communiqué : Il y a désormais 1 million de chiens au Québec! 2016 [Google Scholar]
- 13.Association des médecins vétérinaires du Québec en pratique des petits animaux. Communiqué : Les chats plus populaires que jamais au Québec! Ils sont maintenant près de 2 millions! 2017 [Google Scholar]
- 14.Government of Canada. Categorization of Antimicrobial Drugs Based on Importance in Human Medicine. 2009. [Last accessed February 1, 2022]. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/antimicrobial-resistance/categorization-antimicrobial-drugs-based-importance-human-medicine.html.
- 15.Lardé H, Francoz D, Roy JP, et al. Comparison of quantification methods to estimate farm-level usage of antimicrobials other than in medicated feed in dairy farms from Québec, Canada. Microorganisms. 2021;9:1106. doi: 10.3390/microorganisms9091834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lardé H, Dufour S, Archambault M, et al. Assignment of Canadian defined daily doses and Canadian defined course doses for quantification of antimicrobial usage in cattle. Front Vet Sci. 2020;7:10. doi: 10.3389/fvets.2020.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. WHO list of Critically Important Antimicrobials for Human Medicine (WHO CIA list) 2019. [Last accessed February 1, 2022]. Available from: https://apps.who.int/iris/bitstream/handle/10665/325036/WHO-NMH-FOS-FZD-19.1-eng.pdf?ua=1.
- 18.European Medicines Agency. Categorisation of antibiotics for use in animals for prudent and responsible use. 2019. [Last accessed February 1, 2022]. Available from: https://www.ema.europa.eu/en/documents/report/infographic-categorisation-antibiotics-use-animals-prudent-responsible-use_en.pdf.
- 19.Anses. Suivi des ventes de médicaments vétérinaires contenant des antibiotiques en France en 2020. 2021. [Last accessed February 1, 2022]. Available from: https://www.anses.fr/fr/system/files/ANMV-Ra-Antibiotiques2020.pdf.
- 20.Anses. Suivi des ventes de médicaments vétérinaires contenant des antibiotiques en France en 2016. 2017. [Last accessed February 1, 2022]. Available from: https://www.anses.fr/fr/system/files/ANMV-Ra-Antibiotiques2016.pdf.
- 21.Carmo LP, Nielsen LR, Alban L, da Costa PM, Schüpbach-Regula G, Magouras I. Veterinary expert opinion on potential drivers and opportunities for changing antimicrobial usage practices in livestock in Denmark, Portugal, and Switzerland. Front Vet Sci. 2018;5:29. doi: 10.3389/fvets.2018.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Speksnijder DC, Wagenaar JA. Reducing antimicrobial use in farm animals: How to support behavioral change of veterinarians and farmers. Anim Front. 2018;8:4–9. doi: 10.1093/af/vfy006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Speksnijder DC, Mevius DJ, Bruschke CJM, Wagenaar JA. Reduction of veterinary antimicrobial use in the Netherlands. The Dutch Success Model. Zoonoses Public Health. 2015;62:79–87. doi: 10.1111/zph.12167. [DOI] [PubMed] [Google Scholar]
- 24.Veterinary Medicines Directorate. UK Veterinary Antibiotic Resistance and Sales Surveillance Report (UK-VARSS 2019 Report) 2020. [Last accessed February 1, 2022]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/950126/UK-VARSS_2019_Report2020-TPaccessible.pdf.
- 25.Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2016 Annual Report. Guelph, Ontario: Public Health Agency of Canada; 2018. [Last accessed February 1, 2022]. Available from: https://publications.gc.ca/collections/collection_2018/aspc-phac/HP2-4-2016-eng.pdf. [Google Scholar]
- 26.Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2017 Figures and tables. Guelph, Ontario: Public Health Agency of Canada; 2019. [Last accessed February 1, 2022]. Available from: https://publications.gc.ca/collections/collection_2019/aspc-phac/HP2-4-2017-4-eng.pdf. [Google Scholar]
- 27.Anses. Suivi des ventes de médicaments vétérinaires contenant des antibiotiques en France en 2019. 2020. [Last accessed February 1, 2022]. Available from: https://www.anses.fr/fr/system/files/ANMV-Ra-Antibiotiques2019.pdf.
- 28.Veterinary Medicines Directorate. Supplementary Material (UK-VARSS 2019) 2020. [Last accessed February 1, 2022]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/950128/UK-VARSS_2019_Supplementary_Material__2020-TPaccessible.pdf.
- 29.Anses. Suivi des ventes de médicaments vétérinaires contenant des antibiotiques en France en 2017. 2018. [Last accessed February 1, 2022]. Available from: https://www.anses.fr/fr/system/files/ANMV-Ra-Antibiotiques2017.pdf.
- 30.Anses. Suivi des ventes de médicaments vétérinaires contenant des antibiotiques en France en 2018. 2019. [Last accessed February 1, 2022]. Available from: https://www.anses.fr/fr/system/files/ANMV-Ra-Antibiotiques2018.pdf.