Abstract
Macrolide antibiotics such as erythromycin have been reported to be effective for asthma. However, the precise mechanisms of this effect remain unclear. We studied the effect of erythromycin, clarithromycin, josamycin, and other antibiotics on the release by eosinophils of interleukin-8 (IL-8), a potent chemokine for inflammatory cells, including eosinophils themselves. Human eosinophils were isolated from atopic patients, and the effects of the drugs on IL-8 release were evaluated. Only 14-member macrolides (erythromycin and clarithromycin) showed a concentration-dependent suppressive effect on IL-8 release (control, 100%; erythromycin at 1 μg/ml, 67.82% ± 3.45% [P < 0.01]; clarithromycin at 5 μg/ml, 56.81% ± 9.61% [P < 0.01]). The effect was found at therapeutic concentrations and appeared to occur at the posttranscriprtional level. In contrast, a 16-member macrolide (josamycin) had no significant effect. We suggest that 14-member macrolides inhibit IL-8 release by eosinophils and may thereby prevent the autocrine cycle necessary for the recruitment of these cells into the airways.
Erythromycin, a macrolide antibiotic, has been reported to be effective for the treatment of asthma, especially severe, intractable, or steroid-dependent asthma (8, 9). Erythromycin reduces the severity of bronchial hyperresponsiveness in asthmatic patients (15). Recently, low-dose, long-term erythromycin therapy resulted in a decrease in neutrophil chemotactic activity and interleukin-8 (IL-8), as well as neutrophil numbers, in bronchoalveolar lavage fluids from patients with chronic inflammatory airway diseases (20). Therefore, it is likely that erythromycin attenuates airway inflammatory responses via its inhibitory effect on cytokine release.
Eosinophils have been considered to be one of the most important effector cells in the pathogenesis of asthma. Recent studies have shown that eosinophils generate IL-8 (3), and it has recently been proved to be a chemotactic factor and transmigration mediator for eosinophils (6). Therefore, IL-8-mediated eosinophil accumulation in the airways may be an important process in the pathogenesis of bronchial asthma.
Here, we evaluated the effects of macrolides such as erythromycin, clarithromycin, and josamycin on IL-8 production by human eosinophils.
MATERIALS AND METHODS
Reagents.
Calcium ionophore A23187 and EDTA were purchased from Sigma Chemical Co. (St. Louis, Mo.). Percoll solution was obtained from Pharmacia Fine Chemicals (Uppsala, Sweden). Hanks’ balanced salt solution without calcium and magnesium, phosphate-buffered saline (PBS), heat-inactivated fetal calf serum, and RPMI 1640 medium were purchased from GIBCO (Grand Island, N.Y.). Dextran 70 was obtained from Green Cross (Osaka, Japan). Magnetic cell sorting and anti-CD16-bound micromagnetic beads were purchased from Miltenyi Biotec Inc. (Sunnyvale, Calif.). Nonidet P-40 was purchased from IWAI Chemicals Co. (Tokyo, Japan). Enzyme-linked immunosorbent assay (ELISA) kits for IL-8 detection (Compact ELISA kit) were obtained from the Central Laboratory of the Netherlands Red Cross Blood Transfusion Service (Amsterdam, The Netherlands).
Macrolide antibiotics (kind gifts from S. Ohmura, Kitasato Institute, Tokyo, Japan) and tetracycline (Takeda Chemicals Industries, Tokyo, Japan) were dissolved in ethanol as stock solutions. Cefazolin (Fujisawa, Tokyo, Japan) was dissolved in distilled water as stock solutions. They were diluted further in media for experiments. Preliminary experiments demonstrated that the concentration of ethanol used in this study did not show any significant cytotoxicity to eosinophils as assessed by the trypan blue dye exclusion technique (data not shown).
Purification of blood eosinophils.
Eosinophils were isolated from peripheral blood of consenting patients who had allergic disease such as bronchial asthma and atopic dermatitis as reported previously (11). These patients (n = 27; 11 males and 16 females; average age, 60.30 ± 2.0 years) received neither systemic nor inhaled corticosteroid treatment. One hundred millimolar EDTA-anticoagulated blood was obtained from the patients and sedimented with Dextran 70 for 90 min at room temperature. The granulocyte-rich plasma was collected and centrifuged at 400 × g for 10 min at 20°C. After the washing procedure, the cells were suspended in 3 ml of Percoll solution at a density of 1.088 g/ml. The Percoll osmolality was adjusted from 290 to 316 mosmol/kg by addition of Hanks’ balanced salt solution. The preparation was centrifuged at 400 × g for 30 min. at 4°C. After centrifugation, the pellet was collected and washed. Residual erythrocytes were removed by hypotonic lysis. After washing, the pellet of highly purified granulocytes was then incubated with anti-CD16-bound micromagnetic beads for 30 min at 4°C. Magnetic labeled neutrophils were then depleted by passage through a magnetically activated cell sorter column (Miltenyi) (11). The eosinophil-rich suspension was then separated. Eosinophils were counted with a standard hemocytometer after staining with Randorph’s stain (Muto Pure Chemicals, Tokyo, Japan). Differential cell counts were made from cytocentrifuged slides stained with May-Giemsa stain. Eosinophils were shown to be 97.4% ± 1.2% pure, with a few contaminating neutrophils (mean, 1.2%) or mononuclear cells (mean, 0.8%). Eosinophil viability, assessed by the trypan blue dye exclusion technique, was 95% ± 3.2%.
Culture of eosinophils.
Purified eosinophils (2.5 × 105/ml) were cultured in 48-well flat-bottom plates with RPMI 1640 medium supplemented with 10% fetal calf serum. To determine the effects of macrolides on IL-8 release from human blood eosinophils, duplicate aliquots (2.5 × 105/ml, 0.5 ml) were incubated with erythromycin, clarithromycin, and josamycin at different concentrations in a humidified atmosphere at 37°C with 5% CO2. The culture supernatants were harvested by centrifugation after different time periods and subjected to a cytokine assay. Preliminary experiments demonstrated that the ethanol dilution of controls had no significant effect on IL-8 release (data not shown), and therefore, the data were recorded as percentages and the values obtained with medium alone were defined as 100%. We examined the effects of cefazolin (1 to 25 μg/ml) and tetracycline (1 to 25 μg/ml) on IL-8 release by eosinophils in a similar manner. In some experiments, pellets were washed once with PBS and cell lysates were obtained by addition of 0.5 ml of 0.5% Nonidet P-40 (5.0 × 105/ml). Samples were stored at −70°C until assay.
Measurement of IL-8 by ELISA.
The amounts of IL-8 in the supernatants and cell lysates were measured by ELISA kits. The principle of these kits is the quantitative sandwich enzyme immunoassay technique, in which a monoclonal antibody (MAb) is bound to polystyrene microtiter wells. Briefly, IL-8 in the sample was captured by the antibody on the microtiter plate. Subsequently, a biotinylated antibody to the cytokine was added, followed by horseradish peroxidase-conjugated streptavidin. After the reaction was terminated by the addition of a stop solution, A405 was measured in a microtiter plate reader. The lower detection limit was 5 pg/ml. The inter- and intra-assay variations were less than 5%. The assay was specific for IL-8 and did not show cross-reactivity with other cytokines, including IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-11, macrophage colony-stimulating factor, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, leukemia inhibitory factor, RANTES (regulated upon activation in normal T cells, expressed and secreted), stem cell factor, mast cell factor, transforming growth factor β1, tumor necrosis factor alpha; tumor necrosis factor beta, and gamma interferon. Each sample was assayed in duplicate as recommended by the manufacturer (11).
Detection of IL-8 mRNA in human eosinophils.
To determine the effect of erythromycin on IL-8 mRNA expression, a semiquantitative assay utilizing reverse transcription (RT)-PCR) that was previously reported (4) was performed. After removal of the supernatant, total RNA was isolated from eosinophils by the guanidinium thiocyanate-phenol-chloroform extraction method as described by Chomczynski and Sacchi (5). Briefly, 5.0 × 105 eosinophils were lysed in solution D (4 M guanidinium thiocyanate, 25 mM sodium citrate, [pH 7]; 0.5% sarcosyl, 0.1 M 2-mercaptoethanol) and RNA was extracted from the solution by chloroform extraction. After that, the isopropanol-precipitated RNA was washed twice with 70% ethanol, dried, and solubilized in diethylpyrocarbonate-treated water. Extracted RNA was reverse transcribed to cDNA by using a Takara RNA-PCR kit (Takara Shuzo, Tokyo, Japan) in accordance with the manufacturer’s recommendations for PCR amplification as previously described (11). Briefly, total eosinophil RNA, random hexadeoxynucleotides (as a primer), and avian myeloblastosis virus reverse transcriptase were used for cDNA synthesis. Human IL-8-specific primer pairs used for PCR amplification were 5′-ATGACTTCCAAGCTGGCCGTGCT-3′ (5′ primer) and 5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′ (3′ primer). Primers for beta actin were 5′-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3′ (5′ primer) and 5′-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3′ (3′ primer) (Clontech, Palo Alto, Calif.). PCR was performed in a Progene thermal cycler (Techne, Cambridge, Mass.) at 94°C for 2 min, followed by 25 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 1.5 min. The amplified-DNA sizes were 289 bp for IL-8 and 1,126 bp for beta actin. The PCR cycle was determined by preliminary experiments showing a linear relationship between PCR cycles and the intensity of signals on ethidium bromide-stained agarose gels. For semiquantitative evaluation of IL-8 and beta actin, 25 and 25 cycles were chosen, respectively. The PCR product was run on a 1.0% agarose gel. The density of each product was evaluated by standard densitometry.
Detection of IL-8 protein assessed by immunocytochemistry.
Incubated eosinophils were washed twice with PBS, and cytocentrifuged samples were prepared. Slides were air dried for 1 min, treated with a 0.1% Triton X-100 solution (50 mM Tris-HCl, 0.15 M NaCl, 0.1% Triton X-100 [pH 7.6]) for 30 min, and then fixed in methanol for 10 min. The samples were stained by an immunohistochemical technique using an anti-IL-8 MAb (10) (a generous gift from K. Matsushima, Tokyo University, Tokyo, Japan). Signals were visualized by using a commercially available Dako labeled streptavidin-biotin kit (Dako Japan Co., Ltd., Tokyo, Japan). Briefly, cytospins were pretreated with 3% hydrogen peroxide to inhibit endogenous peroxidase. Nonspecific staining was blocked by a 5-min incubation with blocking reagent including bovine serum albumin. The specimens were then incubated with the anti-IL-8 MAb for 120 min and then subjected to sequential 10-min incubations with a biotinylated goat anti-mouse immunoglobulin antibody at room temperature. The samples were extensively washed and reacted with a streptavidin-biotin-peroxidase complex, followed by visualization with aminoethylcarbazole, and the preparations were counterstained with a hematoxylin solution. The number of positive cells per 200 cells was determined in three randomized high-power fields by two independent examiners without knowledge of the experimental groups.
Statistical analysis.
The data were analyzed for significance by single- or two-factor analysis of variance (ANOVA). The data were expressed as the mean ± the standard error of the mean (SEM).
RESULTS
The 14-member macrolides erythromycin and clarithromycin uniquely inhibited IL-8 release from human eosinophils.
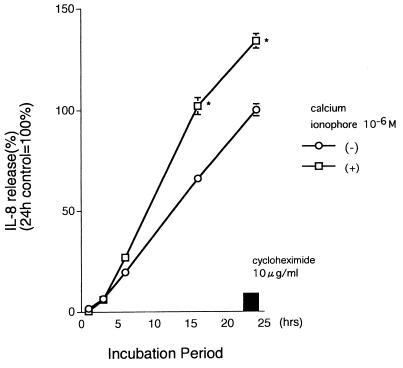
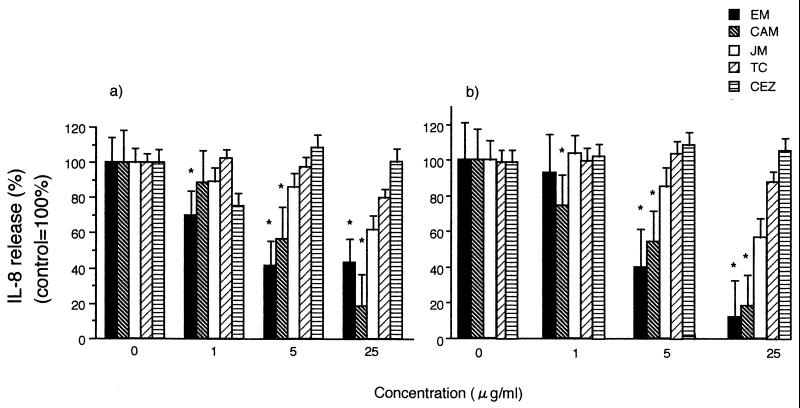
Human peripheral blood eosinophils spontaneously released IL-8 as reported previously, and calcium ionophore (10−6 M) increased the release of IL-8 by eosinophils significantly (11). To examine the influence of macrolides and the other antibiotics on IL-8 release, we cultured eosinophils of atopic patients with various concentrations of them. There was a time-dependent accumulation of IL-8, and the protein synthesis inhibitor cycloheximide (10 μg/ml) clearly blocked this release, suggesting that this process requires de novo protein synthesis (Fig. 1). As shown in Fig. 2a, erythromycin had a concentration-dependent suppressive effect on constitutive IL-8 release from the eosinophils of atopic patients. Clarithromycin (5 and 25 μg/ml) also showed a significant suppressive effect when studied after 24 h. However, the 16-member macrolide josamycin had no significant effect on IL-8 release (control, 100%, at 5 μg/ml, 86.0% ± 5.50%; at 25 μg/ml, 61.8% ± 13.8% [P > 0.05; ANOVA and the Bonferroni test]). Cefazolin or tetracycline had no effect on IL-8 release. Erythromycin at 5 and 25 μg/ml, but not at 1 μg/ml (Fig. 2b), further showed a suppressive effect on IL-8 release by eosinophils stimulated with 10−6 M calcium ionophore when added to the cells 15 min after the calcium ionophore treatment. Clarithromycin, but not josamycin, cefazolin, or tetracycline, inhibited IL-8 release from activated eosinophils (Fig. 2b). We analyzed the effects of macrolides on intracellular IL-8 within eosinophils. The amount of IL-8 extractable from control eosinophil lysate was 69.48 ± 14.58 pg/5.0 × 105 cells/ml. Erythromycin significantly elevated intracellular concentrations of IL-8 at 5 μg/ml (115.89 ± 66.14 pg/5.0 × 105 cells/ml [P < 0.01]).
FIG. 1.
Time course of IL-8 release by human eosinophils. Eosinophils (2.5 × 105/ml, 0.5 ml) were incubated with or without 10−6 M calcium ionophore in a humidified atmosphere at 37°C with 5% CO2. IL-8 release was expressed as a percentage of the spontaneous IL-8 release in 24 h, which was defined as 100%. There was a spontaneous release of IL-8 which was inhibited by the addition of 10-μg/ml cycloheximide. Each point represents the mean ± the SEM of four separate experiments. ∗, P < 0.001 compared to the spontaneous group at each time point.
FIG. 2.
Effects of macrolides and other antibiotics on IL-8 release by human eosinophils. Purified eosinophils (2.5 × 105/ml, 0.5 ml) were cultured with macrolides and other antibiotics at different concentrations (0, 1, 5, and 25 μg/ml) for 24 h without (a) or with (b) 10−6 M calcium ionophore in a humidified atmosphere at 37°C with 5% CO2. Fourteen-member macrolides, but not the 16-member macrolide josamycin (JM), cefazolin (CEZ), or tetracycline (TC), had a concentration-dependent suppressive effect on IL-8 release by eosinophils. The data shown are means plus the SEM (n = 3). ∗, P < 0.01 (ANOVA). EM, erythromycin; CAM, clarithromycin.
Determination of the effect of macrolides on IL-8 mRNA levels in human eosinophils by the RT-PCR technique.
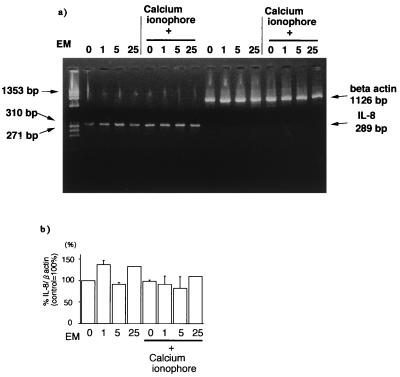
To further study the mechanism of the inhibitory action of erythromycin on IL-8 release, we evaluated IL-8 mRNA levels by a semiquantitative RT-PCR technique. As shown in Fig. 3a, IL-8 mRNA was detected in eosinophils after 2 h of incubation without any stimuli. Calcium ionophore, which increased IL-8 release, did not change IL-8 mRNA expression in eosinophils. Erythromycin showed no significant effect on IL-8 mRNA levels corrected by beta actin transcripts at 1 to 25 μg/ml with or without calcium ionophore treatment (Fig. 3b).
FIG. 3.
Effect of erythromycin (EM) on IL-8 mRNA levels in human eosinophils as evaluated by RT-PCR. (a) Erythromycin had no significant effect on IL-8 mRNA levels corrected by beta actin transcripts at 1 to 25 μg/ml with or without calcium ionophore treatment. (b) Densitometric quantification of IL-8 mRNA levels corrected by beta actin transcripts was performed. Erythromycin (1 to 25 μg/ml) had no significant effect on IL-8 mRNA levels in eosinophils with and without calcium ionophore treatment. The data shown are means plus the SEM from three representative experiments.
Detection of IL-8 protein in eosinophils by immunocytochemistry.
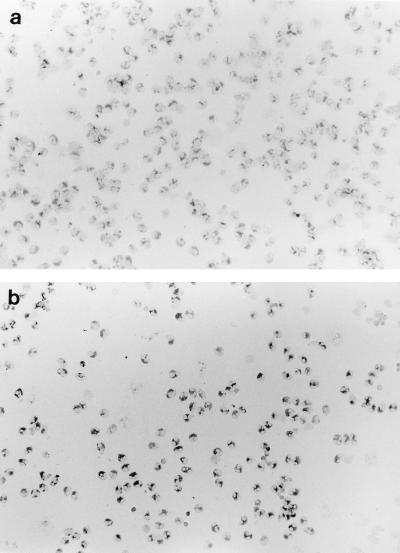
Human peripheral blood eosinophils from patients with atopic diseases constitutively expressed IL-8 protein in their cytoplasm, as reported previously (26) (Fig. 4a and b). The percentage of cells positive for IL-8 among untreated eosinophils was 81.10% ± 5.64% in three patients (Fig. 4a). Although the percentage of cells positive for IL-8 staining did not significantly change (93.24% ± 2.27%, n = 3 P = 0.177) after treatment with 5-μg/ml erythromycin for 24 h (Fig. 4b), a number of cells stained darker than those without erythromycin treatment.
FIG. 4.
Immunocytochemistry of IL-8 protein in eosinophils. (a) Spontaneous expression of IL-8 protein in the cytoplasm as detected by using a mouse anti-IL-8 MAb. (b) Eosinophils incubated with 5-μg/ml erythromycin showed distinct positive staining for IL-8 protein. Original magnification, ×200.
DISCUSSION
The present report shows that erythromycin and clarithromycin, which have a 14-atomic-member macrolide ring structure, suppressed IL-8 release from eosinophils, whereas the other antibiotics, including the 16-member macrolide josamycin, showed no effect. Since the expected concentration of erythromycin in sputum ranges from 0.5 to 5 μg/ml (19), our present data demonstrated that erythromycin and clarithromycin have the potential to suppress eosinophil functions at therapeutic concentrations. Intracellular concentrations of IL-8 were elevated with erythromycin at 5 μg/ml. IL-8 mRNA levels, as assessed by the RT-PCR technique, showed no changes at noncytotoxic and therapeutic concentrations of erythromycin (1 to 5 μg/ml), suggesting that the effect occurred at the posttranscriptional stages.
IL-8, a potent chemokine involved in cell recruitment into the airways, has been reported to be elevated in bronchoalveolar lavage fluids from patients with asthma (18). Kurashima et al. reported that there was an increased level of IL-8 in sputa from asthmatic patients with exacerbation (13). In animal experiments, IL-8 induced eosinophil infiltration of the airways with airway hyperresponsiveness, one of the most characteristic findings in asthma (14). Therefore, it is quite probable that IL-8 plays an important role in the pathogenesis of asthma.
Several reports have suggested that macrolide antibiotics such as erythromycin and troleandomycin favorably affect the clinical status of patients with asthma. They had steroid-sparing effects on steroid-dependent asthma of adults (8, 21) and children (9). This activity seemed unrelated to an antimicrobial effect, because it was effective even in cases with no infection. Erythromycin in combination with theophylline is known to elevate theophylline concentrations in blood through inhibition of its clearance; however, the beneficial effect of erythromycin was not due to increased levels of theophylline (9). Erythromycin alone was capable of reducing the severity of bronchial hyperresponsiveness in patients with asthma (15). Attempts have been made to clarify the mechanisms of its effectiveness at the site of allergic inflammation in asthma. In addition to its inhibitory effects on respiratory glycoconjugate secretion (7) and water secretion (24), recent studies have also shown that erythromycin and related 14-member macrolide compounds have unique effects on cytokine production. Konno et al. (12) showed that another 14-member macrolide, roxithromycin, suppressed IL-2 and tumor necrosis factor alpha production in human peripheral blood mononuclear cells. We have reported that erythromycin significantly blocked the production of IL-6 and IL-8 by the bronchial epithelium at clinical concentrations (0.5 to 2 μg/ml) (22, 23). In contrast, to the best of our knowledge, there have been no reports suggesting the efficacy of josamycin for bronchial asthma or its anti-inflammatory action. Therefore, the inhibitory effect of erythromycin and clarithromycin on IL-8 release by eosinophils shown in the present report may be one of the mechanisms of decreased airway hyperresponsiveness and resulting amelioration of disease severity.
The regulatory mechanism by which eosinophils produce and release IL-8 has not been elucidated in detail. We evaluated steady-state levels of IL-8 mRNA with erythromycin treatment and found no effect at therapeutic concentrations. Intracellular IL-8 levels in eosinophils were elevated with erythromycin. This might be paralleled by the immunohistochemical study findings showing relatively dense cytoplasmic staining after erythromycin treatment. Erythromycin might inhibit IL-8 production at posttranscriptional stages or inhibit the processes of IL-8 secretion from eosinophils. Recently, it was shown that other cytokines, such as IL-6 and IL-4, are associated with eosinophil granules (16, 17, 25). Abdelghaffar and coworkers (1, 2) showed that erythromycin and other macrolides induced degranulation of neutrophils to release lysozyme and other enzymes in vitro. Oishi and associates (19) reported that erythromycin inhibited IL-8 release from human activated neutrophils. Taken together, degranulation pathways of selected granular contents still require further investigation to identify the exact mechanisms of therapeutic agents such as erythromycin.
In conclusions, 14-member macrolides inhibit IL-8 release from human eosinophils. As IL-8 is one of the chemokines for eosinophils, we suggest that 14-member macrolides inhibited the autocrine cycle of eosinophil recruitment to allergic sites. These results may give new insight into the mechanism of action of these macrolide antibiotics in the treatment of airway disorders. The effect of 14-member macrolides on other cytokines or chemokines, such as eotaxin, is another important issue for future research.
ACKNOWLEDGMENTS
We are grateful to Kouji Matsushima, University of Tokyo, for kindly supplying the anti-human IL-8 antibody.
This work was supported in part by a grant from the Japanese Ministry of Education, Science and Culture and by the Manabe Medical Foundation.
REFERENCES
- 1.Abdelghaffar H, Mtairag E M, Labro M T. Effects of dirithromycin and erythromycylamine on human neutrophil degranulation. Antimicrob Agents Chemother. 1994;38:1548–1554. doi: 10.1128/aac.38.7.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelghaffar H, Vazifeh D, Labro M T. Comparison of various macrolides on stimulation of human neutrophil degranulation in vitro. J Antimicrob Chemother. 1996;38:81–93. doi: 10.1093/jac/38.1.81. [DOI] [PubMed] [Google Scholar]
- 3.Braun R K, Franchini M, Erard F, Rihs S, De V I, Blaser K, Hansel T T, Walker C. Human peripheral blood eosinophils produce and release interleukin-8 on stimulation with calcium ionophore. Eur J Immunol. 1993;23:956–960. doi: 10.1002/eji.1830230429. [DOI] [PubMed] [Google Scholar]
- 4.Chelly J, Montarras D, Pinset C, Berwald-Netter Y, Kaplan J C, Kahn A. Quantitative estimation of minor mRNAs by cDNA-polymerase chain reaction. Application to dystrophin mRNA in cultured myogenic and brain cells. Eur J Biochem. 1990;187:691–698. doi: 10.1111/j.1432-1033.1990.tb15355.x. [DOI] [PubMed] [Google Scholar]
- 5.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 6.Erger R A, Casale T B. Interleukin-8 is a potent mediator of eosinophil chemotaxis through endothelium and epithelium. Am J Physiol. 1995;268:L117–L122. doi: 10.1152/ajplung.1995.268.1.L117. [DOI] [PubMed] [Google Scholar]
- 7.Goswami S K, Kivity S, Marom Z. Erythromycin inhibits respiratory glycoconjugate secretion from human airways in vitro. Am Rev Respir Dis. 1990;141:72–78. doi: 10.1164/ajrccm/141.1.72. [DOI] [PubMed] [Google Scholar]
- 8.Itkin I H, Menzel M L, Denver C. The use of macrolide antibiotic substances in the treatment of asthma. J Allergy. 1970;45:146–162. doi: 10.1016/0021-8707(70)90124-3. [DOI] [PubMed] [Google Scholar]
- 9.Kamada A K, Hill M R, Ikle D N, Brenner A M, Szefler S J. Efficacy and safety of low-concentration troleandomycin therapy in children with severe, steroid-requiring asthma. J Allergy Clin Immunol. 1993;91:873–882. doi: 10.1016/0091-6749(93)90345-g. [DOI] [PubMed] [Google Scholar]
- 10.Ko Y, Mukaida N, Panyutich A, Voitenok N N, Matsushima K, Kawai T, Kasahara T. A sensitive enzyme-linked immunosorbent assay for human interleukin-8. J Immunol Methods. 1992;149:227–235. doi: 10.1016/0022-1759(92)90254-q. [DOI] [PubMed] [Google Scholar]
- 11.Kohyama T, Takizawa H, Akiyama N, Sato M, Kawasaki S, Ito K. A novel antiallergic drug epinastine inhibits IL-8 release from human eosinophils. Biochem Biophys Res Commun. 1997;230:125–128. doi: 10.1006/bbrc.1996.5904. [DOI] [PubMed] [Google Scholar]
- 12.Konno S, Asano K, Kurokawa M, Ikeda K, Okamoto K, Adachi M. Antiasthmatic activity of a macrolide antibiotic, roxithromycin: analysis of possible mechanisms in vitro and in vivo. Int Arch Allergy Immunol. 1994;105:308–316. doi: 10.1159/000236773. [DOI] [PubMed] [Google Scholar]
- 13.Kurashima K, Mukaida N, Fujimura M, Schroder J M, Matsuda T, Matsushima K. Increase of chemokine levels in sputum precedes exacerbation of acute asthma attacks. J Leukocyte Biol. 1996;59:313–316. doi: 10.1002/jlb.59.3.313. [DOI] [PubMed] [Google Scholar]
- 14.Medhurst A D, Westwick J, Piper P J. Human recombinant IL-8-induced hyperresponsiveness in guinea pig perfused lungs. Ann N Y Acad Sci. 1991;629:419–421. doi: 10.1111/j.1749-6632.1991.tb38003.x. [DOI] [PubMed] [Google Scholar]
- 15.Miyatake H, Taki F, Taniguchi H, Suzuki R, Takagi K, Satake T. Erythromycin reduces the severity of bronchial hyperresponsiveness in asthma. Chest. 1991;99:670–673. doi: 10.1378/chest.99.3.670. [DOI] [PubMed] [Google Scholar]
- 16.Moller G M, de Jong T A, van der Kwast T H, Overbeek S E, Wierenga-Wolf A F, Thepen T, Hoogsteden H C. Immunolocalization of interleukin-4 in eosinophils in the bronchial mucosa of atopic asthmatics. Am J Respir Cell Mol Biol. 1996;14:439–443. doi: 10.1165/ajrcmb.14.5.8624248. [DOI] [PubMed] [Google Scholar]
- 17.Moqbel R, Lacy P, Levi-Schaffer F, Mannan M M, North J, Gomperts B, Kay A B. Interleukin-6 is a granule-associated pre-formed mediator in eosinophils from asthmatic subjects. Am Rev Respir Dis. 1994;149:A836. . (Abstract.) [Google Scholar]
- 18.Nocker R E, Schoonbrood D F, van de Graaf E A, Hack C E, Lutter R, Jansen H M, Out T A. Interleukin-8 in airway inflammation in patients with asthma and chronic obstructive pulmonary disease. Int Arch Allergy Immunol. 1996;109:183–191. doi: 10.1159/000237218. [DOI] [PubMed] [Google Scholar]
- 19.Oishi K, Sonoda F, Kobayashi S, Iwagaki A, Nagatake T, Matsushima K, Matsumoto K. Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airways of patients with chronic airway diseases. Infect Immun. 1994;62:4145–4152. doi: 10.1128/iai.62.10.4145-4152.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakito O, Kadota J, Kohno S, Abe K, Shirai R, Hara K. Interleukin 1 beta, tumor necrosis factor alpha, and interleukin 8 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis: a potential mechanism of macrolide therapy. Respiration. 1996;63:42–48. doi: 10.1159/000196514. [DOI] [PubMed] [Google Scholar]
- 21.Spector S L, Katz F H, Farr R S. Troleandomycin: effectiveness in steroid-dependent asthma and bronchitis. J Allergy Clin Immunol. 1974;54:367–379. [Google Scholar]
- 22.Takizawa H, Desaki M, Ohtoshi T, Kawasaki S, Kohyama T, Sato M, Tanaka M, Tsuyoshi K, Kobayashi K, Nakajima J, Ito K. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am J Respir Crit Care Med. 1997;156:266–271. doi: 10.1164/ajrccm.156.1.9612065. [DOI] [PubMed] [Google Scholar]
- 23.Takizawa H, Desaki M, Ohtoshi T, Kikutani T, Okazaki H, Sato M, Akiyama N, Shoji S, Hiramastu K, Ito K. Erythromycin suppresses interleukin-6 expression by human bronchial epithelial cells: a potential mechanism of its anti-inflammatory action. Biochem Biophys Res Commun. 1995;210:781–786. doi: 10.1006/bbrc.1995.1727. [DOI] [PubMed] [Google Scholar]
- 24.Tamaoki J, Isono K, Sakai N, Kanemura T, Konno K. Erythromycin inhibits C1 secretion across canine tracheal epithelial cells. Eur Respir J. 1992;5:234–238. [PubMed] [Google Scholar]
- 25.Weller P F. Human eosinophils. J Allergy Clin Immunol. 1997;100:283–287. doi: 10.1016/s0091-6749(97)70237-9. . (Review.) [DOI] [PubMed] [Google Scholar]
- 26.Yousefi S, Hemmann S, Weber M, Holzer C, Hartung K, Blaser K, Simon H U. IL-8 is expressed by human peripheral blood eosinophils. J Immunol. 1995;154:5481–5490. [PubMed] [Google Scholar]