Abstract
Brachyspira hyodysenteriae (B. hyodysenteriae) was identified among the most relevant antimicrobial‐resistant (AMR) bacteria in the EU for swine in a previous scientific opinion. Thus, it has been assessed according to the criteria of the Animal Health Law (AHL), in particular criteria of Article 7 on disease profile and impacts, Article 5 on its eligibility to be listed, Annex IV for its categorisation according to disease prevention and control rules as in Article 9, and Article 8 for listing animal species related to the bacterium. The assessment has been performed following a methodology previously published. The outcome is the median of the probability ranges provided by the experts, which indicates whether each criterion is fulfilled (lower bound ≥ 66%) or not (upper bound ≤ 33%), or whether there is uncertainty about fulfilment. Reasoning points are reported for criteria with uncertain outcome. According to the assessment here performed, it is uncertain whether AMR B. hyodysenteriae can be considered eligible to be listed for Union intervention according to Article 5 of the AHL (33–66% probability). According to the criteria in Annex IV, for the purpose of categorisation related to the level of prevention and control as in Article 9 of the AHL, the AHAW Panel concluded that the bacterium does not meet the criteria in Sections 1, 2 and 3 (Categories A, B and C; 1–10%, 10–33% and 10–33% probability of meeting the criteria, respectively) and the AHAW Panel was uncertain whether it meets the criteria in Sections 4 and 5 (Categories D and E, 50–90% and 33–66% probability of meeting the criteria, respectively). The main animal species to be listed for AMR B. hyodysenteriae according to Article 8 criteria are pigs and some species of birds, such as chickens and ducks.
Keywords: antimicrobial resistance, Brachyspira hyodysenteriae, Animal Health Law, listing, categorisation, impact
1. Introduction
The European Food Safety Authority (EFSA) received a mandate from the European Commission to investigate the global state of play as regards antimicrobial‐resistant (AMR) animal pathogens that cause transmissible animal diseases (Term of Reference (ToR) 1), to identify the most relevant AMR bacteria in the European Union (EU) (first part of ToR 2), to summarise the existing or potential animal health impact of those identified bacteria in the EU (second part of ToR 2) and to perform the assessment of those bacteria to be listed and categorised according to the criteria in Article 5, Annex IV according to Article 9 and Article 8 within the Regulation (EU) No 2016/4291 on transmissible animal diseases (‘Animal Health Law’) (ToR 3).
The global state of play for AMR animal pathogens that cause transmissible animal diseases (ToR 1) and the results of the assessment of the most relevant AMR bacteria in the EU (first part of ToR 2) for swine were published in a separate EFSA scientific opinion (EFSA AHAW Panel, 2021a).
According to the results of the assessment already conducted, Brachyspira hyodysenteriae (B. hyodysenteriae) was identified among the most relevant AMR bacteria in the EU for swine due to the perceived importance of use of antimicrobials to control B. hyodysenteriae infections (no vaccines currently available in spite of its importance), coupled with the limited number of antimicrobials licensed for use to control Brachyspira infections in pigs (EFSA AHAW Panel, 2021a).
This scientific opinion presents the results of the assessment on AMR B. hyodysenteriae in swine on its eligibility to be listed and categorised within the AHL framework. Special focus is placed on the animal health impact of AMR B. hyodysenteriae in swine in the EU, which is also summarised here as part of the assessment conducted according to the profile of the infection and its impact on animal welfare (Article 7).
1.1. Background and Terms of Reference as provided by the requestor
The background and ToRs as provided by the European Commission for the present document are reported in Sections 1.1 and 1.2 of the scientific opinion on the ad hoc method to be followed for the assessment of animal diseases caused by bacteria resistant to antimicrobials within the AHL framework (EFSA AHAW Panel, 2021b).
1.2. Interpretation of the Terms of Reference
The interpretation of the ToRs is as in Sections 1.2.3 and 1.3.3 of the scientific opinion on the ad hoc method to be followed for the assessment of animal diseases caused by bacteria resistant to antimicrobials within the AHL framework (EFSA AHAW Panel, 2021b).
The present document reports the results of the assessment on AMR B. hyodysenteriae in swine according to the criteria of the AHL articles as follows:
Article 7: AMR B. hyodysenteriae infection profile and impacts;
Article 5: eligibility of AMR B. hyodysenteriae infection to be listed;
Article 9: categorisation of AMR B. hyodysenteriae infection according to disease prevention and control rules as in Annex IV;
Article 8: list of animal species (also apart from swine) related to AMR B. hyodysenteriae infection.
2. Data and methodologies
The methodology applied in this opinion is described in detail in a dedicated document about the ad hoc method developed for assessing any animal disease for listing and categorisation of animal diseases within the AHL framework (EFSA AHAW Panel, 2017).
In order to take into account the specifics related to animal diseases caused by bacteria resistant to antimicrobials, the term ‘disease’ as in the AHL was interpreted in a broader sense, referring also to colonisation by commensal and potentially opportunistic bacteria, and the general presence of the identified AMR bacteria in the EU, depending on each criterion.
The following assessment was performed by the EFSA Panel on Animal Health and Welfare (AHAW) based on the information collected and compiled in form of a fact sheet as in Section 3.1 of the present document. The outcome is the median of the probability ranges provided by the experts, which are accompanied by verbal interpretations as spelled out in Table 1.
Table 1.
Approximate probability scale recommended for harmonised use in EFSA (EFSA Scientific Committee, 2018)
| Probability term | Subjective probability range |
|---|---|
| Almost certain | 99–100% |
| Extremely likely | 95–99% |
| Very likely | 90–95% |
| Likely | 66–90% |
| About as likely as not | 33–66% |
| Unlikely | 10–33% |
| Very unlikely | 5–10% |
| Extremely unlikely | 1–5% |
| Almost impossible | 0–1% |
3. Assessment
3.1. Assessment of AMR Brachyspira hyodysenteriae according to Article 7 criteria of the AHL
3.1.1. Article 7(a) Disease profile
This fact sheet concerns Brachyspira hyodysenteriae, the main cause of swine dysentery (SD), and when available and relevant, the data presented include also information on resistance to macrolides, pleuromutilins (such as tiamulin and valnemulin) and tetracyclines in this species. The reason for choosing these drugs is that they are the main recommended antimicrobial classes for treatment of swine dysentery caused by this bacterium, which can only be treated with a limited number of antimicrobials. Apart from the antimicrobial resistance data in this fact sheet, systematically collected antimicrobial resistance data on clinical B. hyodysenteriae isolates can be retrieved from a recent EFSA scientific opinion (EFSA AHAW Panel, 2021a).
SD is most commonly caused by the Gram‐negative anaerobic spirochete B. hyodysenteriae, previously known as Treponema hyodysenteriae and Serpulina hyodysenteriae. Alternatively, SD may be caused by the related bacteria Brachyspira hampsonii and Brachyspira suanatina, but these species have not been described further in this fact sheet. SD is a disease of the large intestine in pigs, most commonly occurring during the growth and finishing periods, hence between 8 and 26 weeks of age (Burrough, 2017). B. hyodysenteriae is transmitted to pigs by ingestion. Clinical signs can develop 5–21 days after infection (Burrough, 2017). The disease is characterised by various degrees of mucoid and haemorrhagic diarrhoea, fever, dehydration, loss of body condition, depression and apathy, and sometimes disease is followed by death (Bellgard et al., 2009). Once a pig herd is infected with B. hyodysenteriae, elimination of the pathogen is extremely difficult therefore often resulting in long‐term losses and continued use of antimicrobials (Neirynck et al., 2020). As described in the following sections, several other animal species have been reported to be subclinical carriers of B. hyodysenteriae.
3.1.1.1. Article 7(a)(i) Animal species concerned by the disease
Susceptible animal species
Parameter 1 – Naturally susceptible wildlife species (or family/order)
Compared to pigs, relatively scarce information is available on occurrence, prevalence and quantity of B. hyodysenteriae in wildlife species. Most studies have been concerned with wildlife species as a reservoir for B. hyodysenteriae in relation to transmission to domesticated pigs (Desrosiers, 2011; Alvarez‐Ordóñez et al., 2013; Zeeh et al., 2018). From these studies, it is not always evident whether the wildlife species are susceptible to disease caused by the organism or are merely to be considered as non‐affected carriers of potential risk to pig production. Feral pig (Sus scrofa), rat (Rattus rattus and Rattus norvegicus), mouse (Mus musculus), common/European sterling (Sturnus vulgaris), crow (Corvus corone) and mallard (Anas platyrhynchos) have all been identified as potential carriers of B. hyodysenteriae (Hampson et al., 1991; Jansson et al., 2001, 2004, 2011; Phillips et al., 2009; Backhans et al., 2010; Pearson et al., 2016; Zeeh et al., 2018). B. hyodysenteriae has also been isolated from cockroaches (Blattodea) and flies (Musca domestica) (Blunt and McOrist, 2009; Gallie et al., 2009).
Clinical manifestations, likely associated with the presence of B. hyodysenteriae in intestinal content, have been reported in common rheas (Rhea americana) (Buckles et al., 1997; McFadzean et al., 2021).
Parameter 2 – Naturally susceptible domestic species (or family/order)
Swine is by far the domestic species most commonly clinically affected by B. hyodysenteriae. Despite the fact that B. hyodysenteriae is frequently isolated from domestic birds, the clinical significance of the bacterium in birds and other non‐porcine domestic animals remains somewhat unclear (Alvarez‐Ordóñez et al., 2013). Nevertheless, clinical manifestations likely associated with the presence of B. hyodysenteriae in intestinal content have been reported in commercial laying hens (Feberwee et al., 2008).
Dogs (Canis lupus familiaris) might also become clinically affected, since one report associated the clinical manifestation of B. hyodysenteriae diarrhoea with the dog’s consumption of faeces from pigs with dysentery (Songer et al., 1978b).
Parameter 3 – Experimentally susceptible wildlife species (or family/order)
Experimental challenge of mallards (Anas platyrhynchos) with B. hyodysenteriae has been attempted (Jansson et al., 2009). Results showed a colonisation rather than infection of birds, and colonisation only occurred with a strain of mallard origin, not with a strain of porcine origin. Joens (1980) inoculated mice intragastrically with B. hyodysenteriae. Mice became colonised with the bacterium but failed to develop disease. Healthy pigs exposed to faeces of these mice became colonised with B. hyodysenteriae within 5–17 days and developed SD within 11–13 days, thus suggesting that rodents in the field may be a reservoir host and may also be involved in spreading swine dysentery.
Parameter 4 – Experimentally susceptible domestic species (or family/order)
Pigs are the primary susceptible domestic species, and experimental infections with B. hyodysenteriae have been induced on multiple occasions in different age groups including only 2‐week‐old piglets (e.g. Eriksen and Andersen, 1970; Kinyon et al., 1977; Leser et al., 2000). However, chicks have also been used as models of disease for this bacterium. Trott and Hampson (1998) in fact induced histological lesions in the intestine and reduced growth rate following oral inoculation of 1‐day‐old specific pathogen‐free chicks.
Reservoir animal species
Parameter 5 – Wild reservoir species (or family/order)
Apart from pigs, farmed mallards appear to constitute the most important domestic reservoir (with no clinical affection) (Jansson et al., 2004).
Parameter 6 – Domestic reservoir species (or family/order)
This information is included under Parameter 1 of this section.
3.1.1.2. Article 7(a)(ii) The morbidity and mortality rates of the disease in animal populations
Morbidity
Parameter 1 – Prevalence/incidence
Although SD occurs globally, relatively few studies have investigated prevalence and epidemiology of the disease (Alvarez‐Ordóñez et al., 2013). The lack of data may also be influenced by the difficulty of the laboratory diagnosis of the bacterium. The reported prevalence of B. hyodysenteriae has ranged from 0% to near 40% (Fellström et al., 1996; Møller et al., 1998; Stege et al., 2000; Suh and Song, 2005; Carvajal et al., 2006), but it should be noted that reported prevalence in the different studies is not always comparable. For example, Stege et al. (2000) found 2.8% of 79 randomly selected farms (with no information on occurrence of diarrhoea) positive for B. hyodysenteriae when culturing 20 faecal samples per farm. Carvajal et al. (2006) found 32.1% of 421 pig farms positive for the agent, but samples were taken from pigs (5–20 per farm) with clinical signs such as diarrhoea or from pigs with decreased growth rate. A US study by Duff et al. (2014) estimated in one endemically SD‐infected farm the prevalence of carriage in sows and suckling pigs at different time points. The proportion of sows positive for B. hyodysenteriae ranged from 0% to 5% with an overall prevalence of 1.04%. In suckling piglets, the proportion ranged from 0% to 5% with an overall prevalence of 1.88%. The same study found 0–1.33% of sows from five endemically infected breeding farms to be positive for B. hyodysenteriae. An earlier study by Høgh and Knox (1976) reported that B. hyodysenteriae could be isolated from 12 of 543 sows (2.2%) and 136 of 680 weaned pigs (20.0%) from non‐clinical faecal samples in 26 Danish herds.
Apart from variations in study design, prevalence can be influenced by the use of different diagnostic methods, feeding regimes, housing, management, etc. (Johnston et al., 2001; Jacobson et al., 2005). The incidence of SD within farms likely varies a lot, but has not been systematically assessed.
Parameter 2 – Case‐morbidity rate (% clinically diseased animals out of infected ones)
It has been reported that the morbidity can approach 90% (Gelberg, 2017). However, case‐morbidity rates for B. hyodysenteriae may vary considerably between farms, as clinical disease manifestations in infected individuals depend on several other factors, including stress (Moreng et al., 1980), the animals’ age (Olson, 1974), acid secretion in the stomach (Savage, 1980), differences in the dose of the infectious agent, diet and B. hyodysenteriae virulence properties (Kinyon et al., 1980; Hampson et al., 1992). Likely, immunity following previous exposure to the bacterium also influences case morbidity.
Mortality
Parameter 3 – Case‐fatality rate
Severity of disease can vary substantially between farms. Case‐fatality rates of up to 50–90% can be seen among pigs with severe haemorrhagic diarrhoea (Alvarez‐Ordóñez et al., 2013). The highest mortality is observed in naïve flocks following exposure to infective animals. It is unknown if antimicrobial resistance contributes to increase case‐fatality rate or not.
In infected rheas, mortality rates during disease outbreaks have been reported to range from 25% to 80% (Sagartz et al., 1992).
3.1.1.3. Article 7(a)(iii) The zoonotic character of the disease
Parameter 1 – Report of zoonotic human cases (anywhere)
Whereas zoonotic transfer of Brachyspira pilosicoli isolates has been reported to be likely to occur after exposure to infected animals, their faeces or contaminated water (Hampson et al., 2006), reports on zoonotic human cases of B. hyodysenteriae appear not to exist (Norris, 2019).
3.1.1.4. Article 7(a)(iv) The resistance to treatments, including antimicrobial resistance
Parameter 1 – Resistant strain to any treatment, even at laboratory level
Antimicrobial treatment constitutes an important tool to control outbreaks of SD. Pleuromutilins like tiamulin and valnemulin are the most widely used antibiotics for treatment of SD, due to efficacy towards B. hyodysenteriae and relatively short withdrawal periods (van Duijkeren et al., 2014). Treatment with antimicrobials other than pleuromutilins is generally limited to the macrolides tylosin and tylvalosin, and lincomycin (a lincosamide), although off‐label use of other antibiotics (e.g. doxycycline) may take place under the cascade system, which allows veterinarians to treat with an alternative product when there is no other appropriate authorised veterinary medicine available (Card et al., 2018).
Resistance to pleuromutilins in B. hyodysenteriae can be due to different point mutations in the 23S rRNA gene and on the ribosomal protein L3 coding sequence (Hillen et al., 2014). More recently, a tiamulin‐valnemulin resistance gene named tva(A) has been identified (Card et al., 2018). Cross‐resistance to the lincosamide lincomycin and the macrolides tylosin and tylvalosin can be attributed to mutations in the V domain of the 23S rRNA gene (Karlsson et al., 1999). In addition, a specific transposon‐associated lincosamide resistance gene, lnu(C) has been detected recently (De Luca et al., 2018). Resistance to doxycycline can be linked to a mutation in the 16S rRNA gene (Pringle et al., 2007).
It is generally agreed that levels of antimicrobial resistance are on the rise in B. hyodysenteriae. A thorough evaluation of antimicrobial resistance in B. hyodysenteriae at a global scale was published recently by Hampson et al. (2019). In the following, a subset of European studies with antimicrobial resistance data are described – when possible with the focus of highlighting temporal trends of antimicrobial resistance. It should be noted that proportions of resistance reported here are not necessarily comparable between studies, as different breakpoints for data interpretation may have been used. This is due to the lack of internationally recognised clinical breakpoints for B. hyodysenteriae.
In a recent German study, the proportion of resistance to pleuromutilins in 116 porcine clinical B. hyodysenteriae isolates obtained between 1990 and 2016 was 40.9%, but ranged from 0% to 85.7% depending on the sequence type assessed (Joerling et al., 2018). The study confirmed trends of increasing resistance to pleuromutilins reported in previous German studies on B. hyodysenteriae (Rohde et al., 2004; Herbst et al., 2014).
In a Belgian study, proportions of resistance in 30 clinical isolates collected between 2010 and 2015 to tiamulin, valnemulin, tylosin, tylvalosin, lincomycin and doxycycline were 53%, 57%, 80%, 70%, 83% and 87%, respectively (Mahu et al., 2017). Tiamulin resistance was observed in 54.3% of 108 Italian isolates obtained from SD between 2002 and 2012, and the authors reported a significant trend in the reduction of susceptibility over time (Rugna et al., 2015). In Sweden, Pringle et al. (2012) reported that minimum inhibitory concentration (MIC) values for tiamulin had gradually increased from 1990 to 2003, whereas levels had ceased afterwards. The authors explained that this cease was likely due to a reduction in tiamulin use following the launch of a national control programme in the year 2000, during which nucleus and multiplying herds were certified as free of SD by going through a sampling and observation period of 6 months. A targeted eradication programme would then be launched if SD were detected. More recent data from the Swedish national surveillance programme on antimicrobial resistance indicates that, for the first time, isolates with tiamulin MICs above the clinical breakpoint (> 2 ug/mL) were detected during 2016, and that therapeutic failure also was observed in that year (SVARM‐Swedres, 2019). However, it was not clearly stated whether treatment failure was linked to those resistant isolates. A Polish study with 21 clinical B. hyodysenteriae isolates collected between 2006 and 2010 did not find resistance to pleuromutilins, whereas all isolates were resistant to tylosin, and 91% of isolates displayed reduced susceptibility to doxycycline (Zmudzki et al., 2012). In the Czech Republic, 202 B. hyodysenteriae isolates from the period 1997 to 2006 were tested for susceptibility to tylosin, lincomycin, tylvalosin, chlortetracycline, tiamulin and valnemulin. The authors reported increasing MICs to all tested antibiotics, and for tiamulin, the proportion of resistance increased from 22.2% in 2000 to 42.8% in 2005–2006 (Prášek et al., 2014). Hidalgo et al. (2011) reported substantially increasing levels of resistance to pleuromutilins, as MIC50 values for tiamulin and valnemulin had increased at least fourfold when comparing isolates from 2008 to 2009 to isolates from 2000 to 2007. In the same period, levels of resistance to lincomycin and tylosin had remained stable.
Importantly, two studies provided typing‐based evidence that B. hyodysenteriae clones, including tiamulin‐resistant variants, appear to have spread within and between countries in Europe, possibly due to subclinically infected pigs being transported from breeding to production herds (Hidalgo et al., 2010; Rugna et al., 2015).
As mentioned above, further details on antimicrobial resistance in porcine B. hyodysenteriae isolates can be found in in Hampson et al. (2019), and antimicrobial resistance data are also available in the recent EFSA scientific opinion (EFSA AHAW Panel, 2021a).
3.1.1.5. Article 7(a)(v) The persistence of the disease in an animal population or the environment
Animal population
Parameter 1 – Duration of infectious period in animals
Asymptomatic carriers can be considered as persistent sources for transfer of the bacteria and potentially SD to other animals. Duration of the infectious period in animals affected by SD may exceed the duration of disease. For example, in a study of pigs convalescent from experimentally induced SD, B. hyodysenteriae was shed for up to 82 days after the last clinical evidence of disease (Fisher and Olander, 1981).
Parameter 2 – Presence and duration of latent infection period
Jacobson et al. (2004) inoculated weaners experimentally with B. hyodysenteriae. All pigs started shedding the bacterium 2 days post‐inoculation and clinical signs developed 5–10 days post‐inoculation. In other words, pigs may start shedding B. hyodysenteriae already before they develop clinical signs of SD, at least in experimental settings. As this study represents limited data under experimental conditions, it is likely that the latent infection period would be differ from this study under different field conditions.
Parameter 3 – Presence and duration of the pathogen in healthy carriers
It is unknown exactly how long pigs may be healthy carriers of B. hyodysenteriae, but asymptomatic carriage appears to be common. For example, a recent study showed that six out of 18 pig herds in Australia with no clinical signs of SD were culture‐positive for the bacterium (Hampson et al., 2016). A similar proportion of asymptomatic carriage (35 of 102 farms) was suspected in an earlier Australian study (Mhoma et al., 1992), but that study was based on serology, hence it could not be excluded that farms had been previously exposed to B. hyodysenteriae and therefore not subclinically infected at the time of sampling. Another study used a B. hyodysenteriae isolate obtained from healthy pigs in an experimental pig model resulting in development of SD (Hampson et al., 1992). This result suggests that not only strain virulence but also factors related to pigs or farms may be determining factors for when clinical signs of SD develop. In that regard, it has been shown that feed composition seems an important determining factor for development of SD (Burrough, 2017), likely due to an influence on gut bacteria, which may act synergistically or antagonistically with B. hyodysenteriae.
Environment
Parameter 4 – Length of survival of the agent and/or detection of DNA in selected matrices (soil, water, air) from the environment
Boye et al. (2001) tested survival of B. hyodysenteriae at 10°C in soil and faeces. The bacterium survived in soil for 10 days, whereas survival increased to 112 days in pure faeces. Another study showed that the bacterium may survive for up to 48 days in dysenteric faeces kept between 0°C and 10°C, but survival was much shorter when the same experiment was carried out at 25°C (7 days) and 37°C (< 24 h) (Chia and Taylor, 1978). Olson (1995) showed that after removal of B. hyodysenteriae‐infected pigs from a contaminated sewage lagoon, the lagoon effluent remained for 5–6 days a source of B. hyodysenteriae infection in healthy pigs when the lagoon was used as the sole source of drinking water.
3.1.1.6. Article 7(a)(vi) The routes and speed of transmission of the disease between animals, and, when relevant, between animals and humans
Routes of transmission
Parameter 1 – Types of routes of transmission from animal to animal (horizontal, vertical)
Carrier animals without clinical signs are epidemiologically important, as shown by Songer (1978a) who demonstrated that a small number of asymptomatic carrier pigs (sows or piglets) can transmit B. hyodysenteriae to susceptible animals, thereby maintaining the infection within herds or recipient herds. This risk is particularly important when introducing new animals into previously uninfected herds (Desrosiers, 2011), and even more so if carrier animals shed pleuromutilin‐resistant clones (Hidalgo et al., 2010; Hampson et al., 2015; Rugna et al., 2015).
B. hyodysenteriae is transmitted horizontally by uptake of contaminated faeces. Furthermore, transmission via vectors appears to be possible, since typing studies using pulsed‐field gel electrophoresis have linked strains detected in infected pigs with those isolated from mice and rats from the same farm (Trott et al., 1996; Backhans et al., 2011). Other wild animal species (e.g. cockroach, feral pigs, mallards) can also be carriers of B. hyodysenteriae and may thus potentially act as vectors for introducing the bacterium into pig farms.
Once a pig herd is infected with B. hyodysenteriae, elimination of the pathogen is extremely difficult therefore often resulting in long‐term losses and continued use of antimicrobials (Neirynck et al., 2020). Transmission from carrier sows to their offspring appears to be possible, most likely by faecal shedding followed by piglets ingesting faeces of the sow or fomites contaminated with faeces.
Parameter 2 – Types of routes of transmission between animals and humans (direct, indirect, including food‐borne)
Not applicable, since zoonotic transmission has not been demonstrated.
Speed of transmission
Parameter 3 – Incidence between animals and, when relevant, between animals and humans
There is no evidence of transmission of B. hyodysenteriae between animals and humans. The incidence between animals is unknown.
Parameter 4 – Transmission rate (β) (from R0 and infectious period) between animals and, when relevant, between animals and humans
No information is available on the transmission rate.
3.1.1.7. Article 7(a)(vii) The absence or presence and distribution of the disease in the Union and, where the disease is not present in the Union, the risk of its introduction into the Union
Presence and distribution
Parameter 2 – Type of epidemiological occurrence (sporadic, epidemic, endemic) at MS level
SD is considered endemic in most parts of the world (Burrough, 2017); hence, it can be assumed that the disease is endemic in all Member States.
Risk of introduction
B. hyodysenteriae has a ubiquitous occurrence. Nevertheless, it should be noted that new clones may be introduced into countries following trade and transport of pigs. As stated above (Section 3.1.1.4), Rugna et al. (2015) showed that this was likely the case for certain clones, e.g. ST52, which is present in both Italy, Belgium, Germany and Spain. Both this and other internationally disseminated clones are known to sometimes display reduced susceptibility to pleuromutilins (Hidalgo et al., 2010; Rugna et al., 2015). Also, it cannot be excluded that certain clones (including clones resistant to antibiotics) are transported across borders with migratory birds or other wild animals.
3.1.1.8. Article 7(a)(viii) The existence of diagnostic and disease control tools
Diagnostic tools
Parameter 1 – Existence of diagnostic tools
Diagnosis of SD relies on a combination of approaches, including evaluation of clinical signs, evaluation of gross lesions in the gut (haemorrhagic typhlocolitis with thickened intestinal wall is characteristic of SD) and detection of the infectious agent (Burrough, 2017). Detection of B. hyodysenteriae can be done in various ways, e.g. by culture of faecal or colonic tissue. The bacterium is slow‐growing; hence, 6–9 days of anaerobic culture is typically required before characteristic haemolytic colonies appear. These can be identified using either biochemical testing, polymerase chain reaction (PCR) or matrix‐assisted laser desorption ionisation–time‐of‐flight mass spectrometry (MALDI‐TOF MS). PCR may also be applied directly on faecal material instead of colonies appearing from culture. The bacterium can also be detected by other means, e.g. by fluorescent in situ hybridisation (FISH) of colonic tissue using probes specific for B. hyodysenteriae (Wilberts et al., 2015), or by the less specific silver staining of tissue, thereby visualising the characteristic spiral‐shaped bacterium in intestinal tissue.
Serology, e.g. using enzyme‐linked immunosorbent assays (ELISAs) can be used to identify if pigs have been exposed to the bacterium (Hampson et al., 2016). Although this is not useful during SD outbreaks, it may be implemented in abattoirs for monitoring of prior infection or asymptomatic carriage. Especially asymptomatic carriage may otherwise go undetected due to (i) reluctance to screen healthy animals in farms or (ii) false‐negative results using methods less likely than serology to capture low‐level shedding of the bacterium (e.g. PCR on faeces or colonic tissue).
Susceptibility to antibiotics can be tested by determination of the MIC using broth or agar dilution. In the lack of internationally recognised breakpoints for interpretation of results, it seems that most recent studies have adapted the epidemiological cut‐offs (ECOFFs) proposed by Pringle et al. (2012).
Parameter 2 – Existence of control tools
There are several tools available to control SD. Yet none of them are fully effective and eradicating an SD infection from a pig farm requires a long and expensive effort, often with disappointing results (Neirynck et al., 2020). Antimicrobial treatment using pleuromutilins or alternative antimicrobial agents may be used once the infection is diagnosed, and treated animals should be moved to a segregated clean environment when possible. In addition, there are several ways to prevent the infection from entering farms or from spreading within farms. These include biosecurity and husbandry measures such as rodent control and isolation from waterfowl, thorough cleaning and disinfection procedures, restricting access of vehicles and persons, hygiene measures for farm personnel and visitors (e.g. proper changing facilities), use of quarantine for newly purchased animals, avoiding mixing of age groups, reduction of stress in the environment (e.g. by ensuring adequate temperature and ventilation, reducing stocking density and minimising movement and handling of pigs), screening to detect subclinically infected breeding stock, use of all‐in–all‐out procedures and potentially the use of autogenous vaccines. A partial or total depopulation strategy is also possible as described later under Parameter 1 in Section 3.1.4.4.
3.1.2. Article 7(b) The impact of diseases
3.1.2.1. Article 7(b)(i) The impact of the disease on agricultural and aquaculture production and other parts of the economy
The level of presence of the disease in the Union
Parameter 1 – Number of MSs where the disease is present
The actual extent or level of SD in individual Member States is unknown, since no systematic surveillance takes place in Member States. Also, levels of antimicrobial resistance are to a large extent unknown on a country‐wide level. Instead, sporadic reports with antimicrobial resistance data are available as elaborated in Section 3.1.1.4.
The loss of production due to the disease
Parameter 2 – Proportion of production losses (%) by epidemic/endemic situation
It is difficult to give precise estimates for production and economic losses associated with SD, since (i) data are scarce with most studies only referring to ‘major losses’, (ii) the few studies available are not necessarily representative across countries, e.g. due to different production costs and different ways of producing pigs and (iii) studies calculate production losses in different ways.
According to Dufresne (2006), a previous study determined that SD resulted in up to 17% decrease in average daily gain (ADG), and dramatically decreased (3–10%) feed efficiency. A study from Hungary estimated that effective metaphylactic antibiotic treatment of farms with SD would result in 3.1% increase in ADG, 28.5% decrease in mortality rate and 0.33% reduction in feed conversion rate in the finishing phase. These estimates are averages compared to herds without SD control (Ózsvári, 2017).
Other studies do not refer specifically to production losses or refer only to the economy of production losses. These studies are referred to and elaborated under Parameter 1 in Section 3.1.5.1.
3.1.2.2. Article 7(b)(ii) The impact of the disease on human health
B. hyodysenteriae is not reported to colonise or cause disease in humans (Norris, 2019).
3.1.2.3. Article 7(b)(iii) The impact of the disease on animal welfare
Parameter 1 – Severity of clinical signs at case level and related level, and duration of impairment
Mostly, pigs in the growing and finishing period (8–26 weeks old) are affected by the disease. The severity of disease may vary from mild diarrhoea to more severe clinical signs. Initially, pigs typically exhibit anorexia and passage of soft faeces. If the disease progresses, diarrhoea becomes more pronounced with watery stools containing blood and mucufibrinous exudate. Depending on the severity of the infection, SD can be accompanied by dehydration, weight loss, depression, fever and sometimes death. The disease progresses through groups of pigs, sometimes with high morbidity rates (see Parameter 2 in Section 3.1.1.2) and may occur in a cyclic manner within farms (3‐ to 4‐week intervals) if left untreated (Alvarez‐Ordóñez et al., 2013). Clinical signs can develop 5–21 days after infection (Burrough, 2017).
In theory, antimicrobial resistance may contribute to treatment failure, but to the authors’ knowledge, no studies have proven a link between microbiological antimicrobial resistance in B. hyodysenteriae and reduced efficacy of SD treatment.
3.1.2.4. Article 7(b)(iv) The impact of the disease on biodiversity and the environment
Biodiversity
Parameter 1 – Endangered wild species affected: listed species as in CITES and/or IUCN list
The greater (also referred to as ‘common’) rhea (Rhea americana) is listed as a near threatened species on the IUCN list.
Parameter 2 – Mortality in wild species
In infected rheas, mortality rates during disease outbreaks have been reported to range from 25% to 80% (Sagartz et al., 1992). Mortality for other wild species has not been reported.
Environment
Parameter 3 – Capacity of the pathogen to persist in the environment and cause mortality in wildlife
As stated above (Section 3.1.1.5), B. hyodysenteriae is able to survive in farm environments for considerable periods of time, depending on the presence of organic matter and the environmental temperature. For example, the bacterium can survive in soil held at 10°C for 10 days, and in faeces for 112 days at the same temperature.
It is possible that the length of survival in the environment impacts transmission to wildlife, but as stated earlier (Parameter 3 in Section 3.1.1.2), it does not appear as if wildlife is particularly susceptible to severe disease, with the exception of the common rhea.
3.1.3. Article 7(c) Its potential to generate a crisis situation and its potential use in bioterrorism
Parameter 1 – Listed in OIE/CFSPH classification of pathogens
Not listed.
Parameter 2 – Listed in the Encyclopaedia of Bioterrorism Defence of Australia Group
Not listed.
Parameter 3 – Included in any other list of potential bio‐agro‐terrorism agents
Not listed.
3.1.4. Article 7(d) The feasibility, availability and effectiveness of the following disease prevention and control measures
3.1.4.1. Article 7(d)(i) Diagnostic tools and capacities
Availability
Parameter 1 – Officially/internationally recognised diagnostic tools, OIE‐certified
Despite the importance of SD, a gold standard diagnostic test is not available (Hartnack et al., 2014). Diagnostic approaches for SD should be sufficiently broad to detect all potentially pathogenic spirochaete species causing the disease, even at low counts.
Effectiveness
Parameter 2 – Sensitivity and specificity of diagnostic tests
A large study comprising 239 samples from 103 pig herds, and using Bayesian latent class modelling (a statistical method applicable for situations of test evaluation where no gold standard test is readily available), estimated diagnostic sensitivity and specificity of a B. hyodysenteriae PCR on faecal material (95% CI) to be 73.2% (62.3; 82.9) and 96.2% (90.9; 99.8), respectively. In that study, bacterial culture was superior in terms of sensitivity (96.2% (90.9; 99.8)), whereas specificity of culture was assumed to be 100% (Hartnack et al., 2014). An earlier study compared sensitivity of six different agar media to recover B. hyodysenteriae from pigs experimentally inoculated with the bacterium. The best medium had 89.9% sensitivity, meaning B. hyodysenteriae grew on this medium nine out of 10 times the bacterium was detected in any of the six media tested (Achacha and Messier, 1992). Positive predictive value and negative predictive value were not assessed in any of these studies.
It has been stated that selective anaerobic culture remains important, as it allows growth of not only B. hyodysenteriae but also other spirochaete species that may cause SD, and it has higher sensitivity than most molecular tests (Hampson and Burrough, 2019). Indeed, culture has on multiple occasions proven superior in terms of ability to detect low concentrations of B. hyodysenteriae when compared to qPCR (Burrough et al., 2012; Patterson et al., 2013; Wilberts et al., 2015) and FISH (Wilberts et al., 2015). This property is particularly important when it comes to surveillance of subclinically infected animals, which tend to shed low concentrations of the bacterium intermittently (Duff et al., 2014). Importantly, the time from sampling to culture should be limited as much as possible to prevent bacteria from dying. As stated earlier (Parameter 1 in Section 3.1.1.8), serological testing by ELISA may also be useful for surveillance purposes, e.g. for screening B. hyodysenteriae carriers at abattoirs.
Feasibility
Parameter 3 – Type of sample matrix to be tested (blood, tissue, etc.)
Faecal samples or colonic mucosa are the most appropriate samples for detection of B. hyodysenteriae, although oral fluids may also be considered for penwise screening of active disease (Hampson and Burrough, 2019). Serum or meat juice (at abattoirs) may be used for serological tests.
3.1.4.2. Article 7(d)(ii) Vaccination
No commercial vaccines exist for control of SD.
3.1.4.3. Article 7(d)(iii) Medical treatments
Availability
Parameter 1 – Types of drugs available on the market
Antimicrobial treatment constitutes an important tool to control outbreaks of SD. Pleuromutilins like tiamulin and valnemulin are the most widely used antibiotics for treatment of SD, due to efficacy towards B. hyodysenteriae and relatively short withdrawal periods (van Duijkeren et al., 2014). Treatment with antimicrobials other than pleuromutilins is generally limited to the macrolides tylosin and tylvalosin, and lincomycin (a lincosamide), although off‐label use of other antibiotics (e.g. doxycycline) may take place under the cascade system, which allows veterinarians to treat with an alternative product when there is no other appropriate authorised veterinary medicine available (Card et al., 2018).
Parameter 2 – Availability/production capacity (per year)
Antimicrobial drugs for treatment of pig infections are widely available on the market.
Effectiveness
Parameter 3 – Therapeutic effects in the field (effectiveness)
Based on susceptibility data and pharmacokinetic properties, pleuromutilins (tiamulin, valnemulin) are the most suitable antibiotics for treatment of SD, and they are also the most widely recommended and used antibiotics for this disease (Hampson and Burrough, 2019). In an experimental study where pigs were infected with susceptible isolates of B. hyodysenteriae or B. hampsonii, water medication with tiamulin resolved clinical signs of SD within 24 h, and the inoculated strain was eradicated from faecal samples within 72 h (Wilberts et al., 2014). Macrolides (e.g. tylosin) and lincosamides (e.g. lincomycin) are suitable alternatives for treatment, whereas other potential drugs generally have pharmacokinetic properties resulting in low concentrations in the porcine gut.
Based on the emergence of pleuromutilin resistance in some countries and the indication that tiamulin‐resistant B. hyodysenteriae clones may easily spread within and across countries, future treatment recommendations may have to be adjusted. This, and the fact that there are not many suitable antimicrobial alternatives for SD are reasons why prevention of infection is key to control the disease.
Feasibility
Parameter 4 – Way of administration
The preferred route of administration of tiamulin is intramuscular or as feed or water adjective for flock treatment (Walczak et al., 2017). The intramuscular route is preferred for severely sick animals. Water medication may be preferable to feed medication, since pigs affected by SD may suffer from anorexia, whereas water consumption typically remains at normal levels.
When eradication of SD through medication is aimed for in a pig farm, large numbers of animals (due to the frequent subclinical carrier stage) are to be treated for a long duration (multiple weeks) resulting in excessively high antimicrobial usage with often disappointing results, as the infection may reappear after cessation of the treatments.
3.1.4.4. Article 7(d)(iv) Biosecurity measures
Availability
Parameter 1 – Available biosecurity measures
It is generally recommended to practice all‐in–all‐out systems to disrupt infection transmission among production stages and from consecutive reared batches. When stables of all‐in–all‐out systems are emptied, they should be properly cleaned before inserting new batches. Cleaning should be done with hot water using high pressure, followed by disinfection and proper drying of premises.
Newly acquired pigs, e.g. breeding gilts, should always originate from the same farm, and the health status of that farm should be high, at least at the level of the destination farm and without indications or evidence of SD. Upon arrival, new pigs should undergo quarantine for at least 28 days in facilities isolated from other pigs. Whenever newly introduced pigs are diagnosed positive for SD in the quarantine stable, they should not be introduced in the farm. Vehicles entering farms should be cleaned and disinfected to minimise the risk of acting as fomites for transmission of SD agents (Giacomini et al., 2018). Farm personnel and visitors should be instructed to respect good hygiene practices, e.g. to avoid bringing boots or clothes from other premises and to wash their hands before entering. Also, hygiene precautions should be taken when moving between batches within a farm.
Biosecurity measures also include proper rodent control, as mice and rats may be carriers of B. hyodysenteriae. At the same time, wild birds should be prevented from entering buildings, and any water supplies should be protected from faeces of birds and rodents. Dogs and cats should not be allowed to enter stables, and fly and cockroach control is also a potential biosecurity measure.
Apart from the above‐mentioned preventative biosecurity measures, more active eradication strategies may be implemented in farms with SD. A recent study by Neirynck et al. (2020) evaluated different, farm‐specific eradication strategies in 10 pig farms. Eradication of SD was possible in only four of these farms indicating the difficulty of this process. Two of the successful farms had applied a total depopulation strategy followed by a 3‐week stand‐empty period. A third farm applied partial depopulation with rooms being empty for 28 days, and the farmer switched to another supplier of breeding gilts. The last successful farm also applied partial depopulation, kept stables empty for 3 weeks and subjected remaining pigs to antimicrobial treatment. All of these successful strategies required long‐term efforts and huge investments by the farmer in terms of time and financial resources. In the remaining six farms, programmes based on antimicrobial treatment with depopulation or partial depopulation, but without strictly adhering to all suggested biosecurity measures, were not successful.
Effectiveness
Parameter 2 – Effectiveness of biosecurity measures in preventing the pathogen introduction
The effectiveness of single biosecurity measures can be difficult to evaluate, as most measures represent ‘common sense’ recommendations. It was, however, clear from the study by Neirynck et al. (2020) that eradication of SD from infected farms is only possible using a multifaceted strategy involving complete or partial depopulation, whereas failure to stick to biosecurity measures (cleaning and disinfection procedures, rodent control, stand‐empty period, etc.) is likely to result in failure to eradicate SD.
Feasibility
Parameter 3 – Feasibility of biosecurity measures
All biosecurity measures mentioned above are feasible, although some may be easier and cheaper to implement than others. For example, handwashing and change of boots and clothes are easy measures, whereas switching to an all‐in–all‐out system requires a new management procedure and sufficient space to be able to keep stables empty for some days between batches of pigs.
3.1.4.5. Article 7(d)(v) Restrictions on the movement of animals and products
Availability
Parameter 1 – Available movement restriction measures
In general, avoiding the purchase of breeding animals is the safest option to avoid buying in diseases. Whenever animals are bought, avoiding mixing of animals in farms is a good way to reduce stress and to limit transfer of any infectious agent including B. hyodysenteriae. This can be practiced in different ways, e.g. by using an all‐in–all‐out production system with appropriate cleaning, disinfection and drying of stables between batches. Keeping newly acquired animals in quarantine, as described under Parameter 1 in Section 3.1.4.5 is another way to restrict movement. Finally, pigs with clinical signs of SD should be moved to separate clean pens where the risk of onwards transmission is minimal. Ideally an SD‐‘free’ certification should become available to assure that animals acquired from other farms are free of SD.
Effectiveness
Parameter 2 – Effectiveness of restriction of animal movement in preventing the between‐farm spread
Controlling SD is very difficult, even when following the above‐mentioned restriction movement measures. This is illustrated by Neirynck et al. (2020) describing that even using a multifaceted strategy involving partial or complete depopulation, a temporary stop for animal purchase as well as all‐in–all‐out production often fails, especially if biosecurity measures are not followed properly.
Feasibility
Parameter 3 – Feasibility of restriction of animal movement
A recent questionnaire‐based survey described that only 10% of 68 surveyed pig herds in Belgium used optimal introduction procedures for newly acquired gilts when considering purchase, quarantine and acclimation (Bernaerdt et al., 2021). Accordingly, such measures may not always be feasible, or at least they would require proper training of staff. All‐in–all‐out procedures are used already by most farms in Member States, but for remaining farms implementing all‐in–all‐out may be costly, as it requires additional space and new managerial procedures. The depopulation procedures are extremely costly and therefore not always feasible, especially as they cannot stand alone in attempts to control SD.
3.1.4.6. Article 7(d)(vi) Killing of animals
Availability
Parameter 1 – Available methods for killing animals
Since B. hyodysenteriae is not regarded a zoonotic and highly contagious agent, infected pigs can be killed in slaughterhouses and killed animals can enter human consumption if they do not show clinical signs or lesions.
Treatment of severely affected animals is pointless, as there is little or no chance of recovery. Hence, such animals should be euthanised on farm (stunning and bleeding, or lethal injection).
Effectiveness
Parameter 2 – Effectiveness of killing animals (at farm level or within the farm) for reducing/stopping spread of the disease
Killing individual animals with clinical signs may be justified for animal welfare reasons, but it would not prevent the infection from spreading from subclinically infected animals. Instead, complete or partial depopulation (together with biosecurity measures) is the only option for reducing/stopping spread of disease (with variable success as described above), but in most cases animals would not need to be killed within farms or at farm level, since the infective agent is not regarded a zoonotic and highly contagious agent. Instead, pigs would be sent for slaughter, and then – over time – stables would be emptied.
Feasibility
Parameter 3 – Feasibility of killing animals
Killing animals within farms or at farm level is feasible.
3.1.4.7. Article 7(d)(vii) Disposal of carcasses and other relevant animal by‐products
No special precautions should be taken concerning disposal of carcasses and animal by‐products, as B. hyodysenteriae is not regarded a zoonotic and highly contagious agent.
3.1.5. Article 7(e) The impact of disease prevention and control measures
3.1.5.1. Article 7(e)(i) The direct and indirect costs for the affected sectors and the economy as a whole
It is difficult to give precise estimates for production and economic losses associated with SD, since (i) data are scarce with most studies only referring to ‘major losses’, (ii) the few studies available are not necessarily representative across countries, e.g. due to different production costs and different ways of producing pigs and (iii) studies calculate production losses in different ways.
Parameter 1 – Cost of control (e.g. treatment/vaccine, biosecurity)
In the USA, it was estimated in 2013 that the total annual financial cost of SD approximates USD168–236 millions. For each pig produced, the cost of medication was estimated at USD11.70–15.50, and in addition to that are losses due to slower growth, decreased feed efficiency and increased mortality (McKean et al., 2013). In the UK, SD has been estimated in 2012 to cost farmers £4–10 per infected pig due to decreased growth rate (Alderton, 2012). Dufresne (2006) quoted three earlier studies (from 1983 to 1990) reporting economic costs from USD2.6–15 per pig produced, but it was not specified exactly what these costs covered. In Sweden, assuming an average of 22 pigs produced per sow per year, SD has been estimated to lead to annual losses of about €133 per sow for fattening pigs affected by clinical SD (Sjölund et al., 2014). That amount comprised of 5‐day prolonged rearing time (€55) and 3% mortality (78€). In an early study, Windsor and Simmons (1981) estimated that, ‘the hidden costs of swine dysentery in terms of an increased food conversion ratio may be more than four times the cost of medication’.
Costs related to biosecurity are already budgeted in many farms; hence, additional costs to minimise the impact of B. hyodysenteriae on animals may not be needed depending on the farm. The actual costs for implementing specific parts of biosecurity (e.g. rodent control) may vary substantially depending on the type and size of a farm and country.
In general, costs for antimicrobial treatment vary depending on the drug used and the length of treatment. Yet, as mentioned before, antimicrobial treatments often have to be maintained for a long period (several weeks) in large groups of animals, which results in an increase of the costs.
Parameter 2 – Cost of eradication (culling, compensation)
As stated under Parameter 1 in Section 3.1.4.4, Neirynck et al. (2020) evaluated the effect of partial and total depopulation in farms infected with SD. The authors drew the conclusion that total or partial depopulation combined with implementing strict biosecurity measures allowed eradication of B. hyodysenteriae. The actual costs of these eradication strategies were not provided, but would necessarily correspond to the cost of depopulation, medical treatment and (if not already present at the farm) continuous implementation of a high‐level biosecurity management system. A questionnaire‐based survey from Switzerland concluded, based on responses from 68 pig farmers having experience with SD eradication, that costs of eradication varied considerably within the different production types (Cadetg et al., 2019). Likely, this variation was largely due to great differences in duration of partial depopulation (0–519 days) and total depopulation (97–768 days). Even without exact figures for costs, it is clear that full or partial depopulation and repopulation requires substantial efforts from the farmer in terms of time and finances.
Parameter 3 – Cost of surveillance and monitoring
The bacterium/infection is not subject to regular surveillance or monitoring, with the exception of occasional reporting of antimicrobial resistance in national surveillance reports, e.g. SVARM‐Swedres (2019). The costs for such surveillance are unknown, but likely very low, as data represent passive surveillance of samples submitted to a diagnostic laboratory.
Parameter 4 – Trade loss (bans, embargoes, sanctions) by animal product
Bans, embargoes or sanctions have not been enforced for this bacterium/disease.
Parameter 5 – Importance of the disease for the affected sector (% loss or € lost compared to business amount of the sector)
Available information on economic losses to individual farms and the pig sector has been provided above under Parameter 1 of this section.
3.1.5.2. Article 7(e)(ii) The societal acceptance of disease prevention and control measures
The control measures for B. hyodysenteriae are all likely to have a societal acceptance. This appears also to be the perception of farmers, since almost half (43%) of 68 surveyed pig farmers having experience with an SD eradication programme, claimed that external pressure (from marketers and the national pig health service association assigning health status of farms) was a driving force to try and eradicate SD (Cadetg et al., 2019). Nevertheless, farmers may be reluctant to devote the resources required for implementation of management practices. Failure to follow biosecurity recommendations concerning acquisition of breeding gilts was recently found to be frequent (Bernaerdt et al., 2021), and another study showed that failure to follow recommendations concerning biosecurity likely attributed to failure to eradicate SD in pig farms (Neirynck et al., 2020).
3.1.5.3. Article 7(e)(iii) The welfare of affected subpopulations of kept and wild animals
Parameter 1 – Welfare impact of control measures on domestic animals
Available control measures, if properly implemented, are likely to have positive impact on the welfare of domestic pigs if SD is reduced or eradicated. Antimicrobial resistance in B. hyodysenteriae may potentially cause treatment failure in pigs undergoing antimicrobial treatment, but research is needed to confirm this theory.
Parameter 2 – Wildlife depopulation as control measure
With the exception of rodent control, wildlife depopulation has not so far been considered as a control measure for SD.
3.1.5.4. Article 7(e)(iv) The environment and biodiversity
Environment
Parameter 1 – Use and potential residuals of biocides or medical drugs in environmental compartments (soil, water, feed, manure)
The extent of antimicrobial treatment for SD in pigs (and consequently spill‐over to the environment) is unknown.
Jensen et al. (2003) assessed the toxicity of different antibacterial agents (including tiamulin) to the invertebrate species Folsomia fimetaria (Collembola) and Enchrytraeus crypticus (Enchytraeidae). There was no toxic effect of the antibacterial agents to adults, and effects on their reproduction occurred above the concentrations normally detected in soil or dung. These results suggest a minor impact on tiamulin residuals on the fauna.
Biodiversity
Parameter 1 – Mortality in wild species
Control measures like antimicrobial treatment and keeping biosecurity appropriate are not expected to result in mortality in wild species.
3.2. Assessment of AMR Brachyspira hyodysenteriae according to Article 5 criteria of the AHL on its eligibility to be listed
3.2.1. Detailed outcome on Article 5 criteria
In Table 2 and Figure 1, the results of the expert judgement on the Article 5 criteria of the AHL for AMR B. hyodysenteriae in swine are presented.
Table 2.
Outcome of the expert judgement on Article 5 criteria
|
Criteria to be met by the disease:According to the AHL, a disease shall be included in the list referred to in point (b) of paragraph 1 of Article 5 if it has been assessed in accordance with Article 7 and meets all of the following criteria |
Outcome | ||||
|---|---|---|---|---|---|
|
Median range (%) |
Criterion fulfilment | Number of na | Number of experts | ||
| A(i) | The disease is transmissible | 95–100 | Fulfilled | 0 | 14 |
| A(ii) | Animal species are either susceptible to the disease or vectors and reservoirs thereof exist in the Union | 99–100 | Fulfilled | 0 | 14 |
| A(iii) | The disease causes negative effects on animal health or poses a risk to public health due to its zoonotic character | 90–99 | Fulfilled | 0 | 12 |
| A(iv) | Diagnostic tools are available for the disease | 90–99 | Fulfilled | 0 | 13 |
| A(v) | Risk‐mitigating measures and, where relevant, surveillance of the disease are effective and proportionate to the risks posed by the disease in the Union | 33–66 | Uncertain | 0 | 13 |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at point A(i)–A(v), the disease needs to fulfil at least one of the following criteria | |||||
| B(i) | The disease causes or could cause significant negative effects in the Union on animal health, or poses or could pose a significant risk to public health due to its zoonotic character | 66–90 | Fulfilled | 0 | 13 |
| B(ii) | The disease agent has developed resistance to treatments which poses a significant danger to public and/or animal health in the Union | 66–90 | Fulfilled | 0 | 13 |
| B(iii) | The disease causes or could cause a significant negative economic impact affecting agriculture or aquaculture production in the Union | 66–90 | Fulfilled | 0 | 13 |
| B(iv) | The disease has the potential to generate a crisis or the disease agent could be used for the purpose of bioterrorism | 1–5 | Not fulfilled | 0 | 13 |
| B(v) | The disease has or could have a significant negative impact on the environment, including biodiversity, of the Union | 10–33 | Not fulfilled | 0 | 13 |
na: not applicable.
Figure 1.

- Listing: The probability of the disease to be listed according to Article 5 criteria of the AHL (overall outcome).
The distribution of the individual answers (probability ranges) provided by each expert for each criterion is reported in Sections A.1 and A.2 of Appendix A.
In Figure 1, the outcome of the expert judgement is graphically shown together with the estimated overall probability of the AMR bacterium meeting the criteria of Article 5 on its eligibility to be listed.
3.2.1.1. Reasoning for uncertain outcome on Article 5 criteria
Criterion A(v) (risk‐mitigating measures and, where relevant, surveillance of the disease are effective and proportionate to the risks posed by the disease in the Union)
Several risk‐mitigating measures (e.g. antimicrobial treatment, biosecurity, all‐in–all‐out, control of entries, depopulation) are available and relatively effective at farm level.
Risk‐mitigating measures may be difficult to implement in certain farms (depending on the farming system – easier in industrialised pig farming) and hence not fully effective.
In some farms in the EU, B. hyodysenteriae may have been eradicated and nowadays only occurs occasionally, but the situation might be different for others. In cases of disease, it can be effectively controlled via depopulation followed by cleaning and disinfection of the affected premises (Neirynck et al., 2020).
Eradication from pig farms requires long and expensive effort, as persistence of B. hyodysenteriae is described as common in infected farms.
Detection of latent carriers can be a challenge.
Antimicrobial resistance is widespread in most EU countries and increasing. Without effective antimicrobials, risk mitigation is difficult.
No surveillance is in place.
3.2.2. Overall outcome on Article 5 criteria
As from the legal text of the AHL, a disease is considered eligible to be listed as laid down in Article 5 if it fulfils all criteria of the first set from A(i) to A(v) and at least one of the second set of criteria from B(i) to B(v). According to the assessment methodology, a criterion is considered fulfilled when the lower bound of the median range lays above 66%.
According to the results shown in Table 2, AMR B. hyodysenteriae complies with four criteria of the first set (A(i)–A(iv)), but there is uncertainty (33–66% probability) on the assessment on compliance with Criterion A(v). Therefore, it is uncertain whether AMR B. hyodysenteriae can be considered eligible to be listed for Union intervention as laid down in Article 5 of the AHL. The estimated overall probability range for the AMR bacterium being eligible to be listed is 33–66% (Figure 1).
3.3. Assessment of AMR Brachyspira hyodysenteriae according to criteria in Annex IV for the purpose of categorisation as in Article 9 of the AHL
In Tables 3, 4, 5, 6, 7–7 and related graphs (Figures 2, 3, 4–4), the results of the expert judgement on AMR B. hyodysenteriae in swine according to the criteria in Annex IV of the AHL, for the purpose of categorisation as in Article 9, are presented.
Table 3.
Outcome of the expert judgement related to the criteria of Section 1 of Annex IV (Category A of Article 9)
|
Criteria to be met by the disease:The disease needs to fulfil all of the following criteria |
Outcome | ||||
|---|---|---|---|---|---|
|
Median range (%) |
Criterion fulfilment | Number of na | Number of experts | ||
| 1 | The disease is not present in the territory of the Union or present only in exceptional cases (irregular introductions) or present in only in a very limited part of the territory of the Union | 1–10 | Not fulfilled | 0 | 13 |
| 2.1 | The disease is highly transmissible | 10–33 | Not fulfilled | 0 | 13 |
| 2.2 | There are possibilities of airborne or waterborne or vector‐borne spread | 33–66 | Uncertain | 0 | 13 |
| 2.3 | The disease affects multiple species of kept and wild animals or single species of kept animals of economic importance | 90–99 | Fulfilled | 0 | 13 |
| 2.4 | The disease may result in high morbidity and significant mortality rates | 33–90 | Uncertain | 0 | 13 |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at point 1–2.4, the disease needs to fulfil at least one of the following criteria | |||||
| 3 | The disease has a zoonotic potential with significant consequences for public health, including epidemic or pandemic potential or possible significant threats to food safety | 0–5 | Not fulfilled | 0 | 14 |
| 4 | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | 10–66 | Uncertain | 0 | 13 |
| 5(a) | The disease has a significant impact on society, with in particular an impact on labour markets | 5–33 | Not fulfilled | 0 | 13 |
| 5(b) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | 66–90 | Fulfilled | 0 | 13 |
| 5(c) | the disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it | 5–33 | Not fulfilled | 0 | 13 |
| 5(d) | The disease has a significant impact in the long term on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | 10–33 | Not fulfilled | 0 | 13 |
na: not applicable.
Table 4.
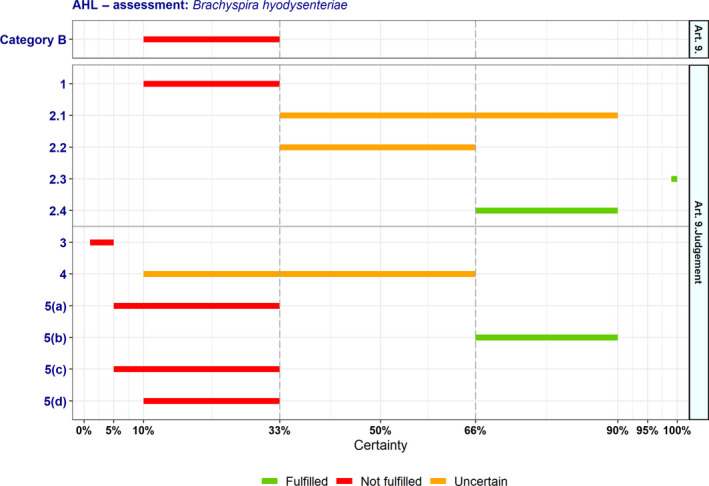
Outcome of the expert judgement related to the criteria of Section 2 of Annex IV (Category B of Article 9)
|
Criteria to be met by the disease: The disease needs to fulfil all of the following criteria |
Outcome | ||||
|---|---|---|---|---|---|
|
Median range (%) |
Criterion fulfilment | Number of na | Number of experts | ||
| 1 | The disease is present in the whole or part of the Union territory with an endemic character and (at the same time) several Member States or zones of the Union are free of the disease | 10–33 | Not fulfilled | 0 | 13 |
| 2.1 | The disease is moderately to highly transmissible | 33–90 | Uncertain | 0 | 13 |
| 2.2 | There are possibilities of airborne or waterborne or vector‐borne spread | 33–66 | Uncertain | 0 | 13 |
| 2.3 | The disease affects single or multiple species | – | Fulfilled | 0 | 13 |
| 2.4 | The disease may result in high morbidity with in general low mortality | 66–90 | Fulfilled | 0 | 12 |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at point 1–2.4, the disease needs to fulfil at least one of the following criteria | |||||
| 3 | The disease has a zoonotic potential with significant consequences for public health, including epidemic potential or possible significant threats to food safety | 1–5 | Not fulfilled | 0 | 14 |
| 4 | The disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals | 10–66 | Uncertain | 0 | 13 |
| 5(a) | The disease has a significant impact on society, with in particular an impact on labour markets | 5–33 | Not fulfilled | 0 | 13 |
| 5(b) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | 66–90 | Fulfilled | 0 | 13 |
| 5(c) | The disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it | 5–33 | Not fulfilled | 0 | 13 |
| 5(d) | The disease has a significant impact in the long term on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long‐term damage to those species or breeds | 10–33 | Not fulfilled | 0 | 13 |
na: not applicable.
Table 5.
Outcome of the expert judgement related to the criteria of Section 3 of Annex IV (Category C of Article 9)
|
Criteria to be met by the disease: The disease needs to fulfil all of the following criteria |
Outcome | ||||
|---|---|---|---|---|---|
|
Median range (%) |
Criterion fulfilment | Number of na | Number of experts | ||
| 1 | The disease is present in the whole or part of the Union territory with an endemic character | 90–95 | Fulfilled | 0 | 14 |
| 2.1 | The disease is moderately to highly transmissible | 33–90 | Uncertain | 0 | 13 |
| 2.2 | The disease is transmitted mainly by direct or indirect transmission | – | Fulfilled | 0 | 13 |
| 2.3 | The disease affects single or multiple species | – | Fulfilled | 0 | 13 |
| 2.4 | The disease usually does not result in high morbidity and has negligible or no mortality and often the most observed effect of the disease is production loss | 10–33 | Not fulfilled | 0 | 12 |
|
At least one criterion to be met by the disease: In addition to the criteria set out above at point 1–2.4, the disease needs to fulfil at least one of the following criteria | |||||
| 3 | The disease has a zoonotic potential with significant consequences for public health or possible significant threats to food safety | 1–5 | Not fulfilled | 0 | 14 |
| 4 | The disease has a significant impact on the economy of the Union, mainly related to its direct impact on certain types of animal production systems | 33–90 | Uncertain | 0 | 13 |
| 5(a) | The disease has a significant impact on society, with in particular an impact on labour markets | 5–33 | Not fulfilled | 0 | 13 |
| 5(b) | The disease has a significant impact on animal welfare, by causing suffering of large numbers of animals | 66–90 | Fulfilled | 0 | 13 |
| 5(c) | The disease has a significant impact on the environment, due to the direct impact of the disease or due to the measures taken to control it | 5–33 | Not fulfilled | 0 | 13 |
| 5(d) | The disease has a significant impact in the long term on biodiversity or the protection of endangered species or breeds, including the possible disappearance or long–term damage to those species or breeds | 10–33 | Not fulfilled | 0 | 13 |
na: not applicable.
Figure 2.

- Category A: the probability of the disease to be categorised according to Section 1 of Annex IV of the AHL (overall outcome).
Figure 3.
- Category B: the probability of the disease to be categorised according to Section 2 of Annex IV of the AHL (overall outcome).
Figure 4.

- Category C: the probability of the disease to be categorised according to Section 3 of Annex IV of the AHL (overall outcome).
The distribution of the individual answers (probability ranges) provided by each expert for each criterion is reported in Sections B.1 and B.2 of Appendix B.
3.3.1. Detailed outcome on Category A criteria
3.3.1.1. Reasoning for uncertain outcome on Category A criteria
Criterion 2.2 (there are possibilities of airborne or waterborne or vector‐borne spread)
There is no evidence of airborne or waterborne spread.
There is a possible role of vectors (e.g. mice, rats, cockroaches, mallards, feral pigs), but more information is required to differentiate those from reservoirs and to establish their epidemiological importance.
Transmission of B. hyodysenteriae occurs mainly horizontally via fomites.
The bacterium survives in water and can be transmitted oro‐faecally, but it is not truly waterborne because between‐farm spread via water is unlikely.
Criterion 2.4 (the disease may result in high morbidity and significant mortality rates)
Under certain circumstances, but not generally, high morbidity and significant mortality have been reported in individual farms.
Morbidity can approach 90%.
Case fatality may reach 50–90% with severe haemorrhagic diarrhoea in naïve herds.
There is no information about the specific role of antimicrobial resistance in increasing mortality compared with non‐resistant B. hyodysenteriae, but this is likely.
Prevalence, morbidity and mortality are variable, as presence, transmission and disease occurrence are multifactorial.
Criterion 4 (the disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals):
Data are scarce for EU countries.
B. hyodysenteriae has been described to cause major economic impact in the USA.
The disease may have significant impact at farm level, but its role at Union level is unclear.
The bacterium can be managed and even eradicated.
Currently there is no significant impact, as the description of challenges regarding the treatment of certain strains is relatively new.
If antimicrobial resistance is increasing, there will potentially be higher costs due to treatment failure. The impact may also be more significant due to increasing morbidity and mortality rates.
3.3.2. Detailed outcome on Category B criteria
3.3.2.1. Reasoning for uncertain outcome on Category B criteria
Criterion 2.1 (the disease is moderately to highly transmissible)
B. hyodysenteriae can be present with high prevalence in some farms, but it does not always result in high prevalence despite a potential ubiquitous presence.
Carriers may be involved in transmission, and their existence can contribute to within‐farm spread.
There are possibilities of vector‐borne transmission (e.g. mice, rats, cockroaches, mallards, feral pigs), but more information is required to differentiate those from reservoirs and to establish their epidemiological importance.
There are not enough data to assess between‐herd transmission.
The bacterium is highly transmissible, but the disease caused by it is multifactorial.
Criterion 2.2 (the disease may result in high morbidity and significant mortality rates): See above in Section 3.3.1.1.
Criterion 4 (the disease has a significant impact on the economy of the Union, causing substantial costs, mainly related to its direct impact on the health and productivity of animals): See above in Section 3.3.1.1.
3.3.3. Detailed outcome on Category C criteria
3.3.3.1. Reasoning for uncertain outcome on Category C criteria
Criterion 2.1 (the disease is moderately to highly transmissible): See above in Section 3.3.2.1.
3.3.4. Detailed outcome on Category D criteria
Table 6.
Outcome of the expert judgement related to the criteria of Section 4 of Annex IV (Category D of Article 9)
| Diseases in Category D need to fulfil criteria of Section 1, 2, 3 or 5 of Annex IV of the AHL and the following: | Outcome | ||||
|---|---|---|---|---|---|
|
Median range (%) |
Criterion fulfilment | Number of na | Number of experts | ||
| D | The risk posed by the disease can be effectively and proportionately mitigated by measures concerning movements of animals and products in order to prevent or limit its occurrence and spread | 50–90 | Uncertain | 0 | 13 |
na: not applicable.
3.3.5. Detailed outcome on Category E criteria
Table 7.
Outcome of the expert judgement related to the criteria of Section 5 of Annex IV (Category E of Article 9)
| Diseases in Category E need to fulfil criteria of Section 1, 2 or 3 of Annex IV of the AHL and/or the following: | Outcome | ||
|---|---|---|---|
|
Median range (%) |
Fulfilment | ||
| E |
Surveillance of the disease is necessary for reasons related to animal health, animal welfare, human health, the economy, society or the environment (If a disease fulfils the criteria as in Article 5, thus being eligible to be listed, consequently Category E would apply.) |
33–66 | Uncertain |
3.3.6. Overall outcome on criteria in Annex IV for the purpose of categorisation as in Article 9
As from the legal text of the AHL, a disease is considered fitting in a certain category (A, B, C, D or E – corresponding to points (a) to (e) of Article 9(1) of the AHL) if it fulfils all criteria of the first set from 1 to 2.4 and at least one of the second set of criteria from 3 to 5(d), as shown in Tables 3, 4, 5, 6–7. According to the assessment methodology, a criterion is considered fulfilled when the lower bound of the median range lays above 66%.
The overall outcome of the assessment on criteria in Annex IV of the AHL, for the purpose of categorisation of AMR B. hyodysenteriae as in Article 9, is presented in Table 8 and Figure 5.
Table 8.
Outcome of the assessment on criteria in Annex IV of the AHL for the purpose of categorisation as in Article 9
| Category | Article 9 criteria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1° set of criteria | 2° set of criteria | ||||||||||
| 1 | 2.1 | 2.2 | 2.3 | 2.4 | 3 | 4 | 5(a) | 5(b) | 5(c) | 5(d) | |
| Geographical distribution | Transmissibility | Routes of transmission | Multiple species | Morbidity and mortality | Zoonotic potential | Impact on economy | Impact on society | Impact on animal welfare | Impact on environment | Impact on biodiversity | |
| A | 1–10 | 10–33 | 33–66 | 90–99 | 33–90 | 0–5 | 10–66 | 5–33 | 66–90 | 5–33 | 10–33 |
| B | 10–33 | 33–90 | 33–66 | – | 66–90 | 1–5 | 10–66 | 5–33 | 66–90 | 5–33 | 10–33 |
| C | 90–95 | 33–90 | – | – | 10–33 | 1–5 | 33–90 | 5–33 | 66–90 | 5–33 | 10–33 |
| D | 50–90 | ||||||||||
| E | 33–66 | ||||||||||
Probability ranges (% certainty; –: criterion is fulfilled by default) and fulfilment of criteria (green: fulfilled; red: not fulfilled; orange: uncertain) (EFSA AHAW Panel, 2017).
Figure 5.
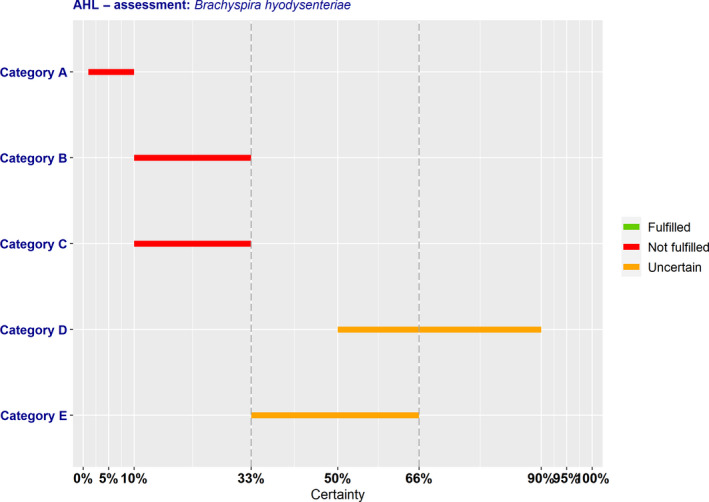
Outcome of the expert judgement on criteria in Annex IV and overall probabilities for categorisation of the AMR bacterium in accordance with Article 9
According to the assessment here performed, AMR B. hyodysenteriae complies with the following criteria of Sections 1–5 of Annex IV of the AHL for the application of the disease prevention and control rules referred to in points (a) to (e) of Article 9(1):
To be assigned to Category A, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and, according to the assessment, AMR B. hyodysenteriae complies only with Criterion 2.3 (90–99% probability). The assessment was inconclusive on compliance with Criteria 2.2 (33–66% probability) and 2.4 (33–90% probability). To be eligible for Category A, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5(a)–(d)) and AMR B. hyodysenteriae complies with Criterion 5(b) (66–90% probability). The assessment was inconclusive on compliance with Criterion 4 (10–66% probability). Overall, it was assessed with 1–10% probability that AMR B. hyodysenteriae may be assigned to Category A according to criteria in Section 1 of Annex IV for the purpose of categorisation as in Article 9 of the AHL.
To be assigned to Category B, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and, according to the assessment, AMR B. hyodysenteriae complies only with Criteria 2.3 (fulfilled by default) and 2.4 (66–90% probability). The assessment was inconclusive on compliance with Criteria 2.1 (33–90% probability) and 2.2 (33–66% probability). To be eligible for Category B, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5(a)–(d)) and AMR B. hyodysenteriae complies with Criterion 5(b) (66–90% probability). The assessment was inconclusive on compliance with Criterion 4 (10–66% probability). Overall, it was assessed with 10–33% probability that AMR B. hyodysenteriae may be assigned to Category B according to criteria in Section 2 of Annex IV for the purpose of categorisation as in Article 9 of the AHL.
To be assigned to Category C, a disease needs to comply with all criteria of the first set (1, 2.1–2.4) and, according to the assessment, AMR B. hyodysenteriae complies with Criteria 1 (90–95% probability), 2.2 and 2.3, which are fulfilled by default. The assessment was inconclusive on compliance with Criterion 2.1 (33–90% probability). To be eligible for Category C, a disease needs to comply additionally with one of the criteria of the second set (3, 4, 5(a)–(d)) and AMR B. hyodysenteriae complies with Criterion 5(b) (66–90% probability). The assessment was inconclusive on compliance with Criterion 4 (33–90% probability). Overall, it was assessed with 10–33% probability that AMR B. hyodysenteriae may be assigned to Category C according to criteria in Section 3 of Annex IV for the purpose of categorisation as in Article 9 of the AHL.
To be assigned to Category D, a disease needs to comply with criteria of Section 1, 2 or 5 of Annex IV of the AHL and with the specific Criterion D of Section 4, for which the assessment for AMR B. hyodysenteriae is inconclusive (50–90% probability).
To be assigned to Category E, a disease needs to comply with criteria of Section 1, 2 or 3 of Annex IV of the AHL, and/or the surveillance of the disease is necessary for reasons related to animal health, animal welfare, human health, the economy, society or the environment. The latter is applicable if a disease fulfils the criteria as in Article 5, for which the assessment is inconclusive (33–66% probability of fulfilling the criteria).
3.4. Assessment of AMR Brachyspira hyodysenteriae according to Article 8 criteria of the AHL
In this section, the results of the assessment on the criteria of Article 8(3) of the AHL for AMR B. hyodysenteriae are presented. The Article 8(3) criteria are about animal species to be listed, as it reads below:
‘3. Animal species or groups of animal species shall be added to the list if they are affected or if they pose a risk for the spread of a specific listed disease because:
they are susceptible to a specific listed disease, or scientific evidence indicates that such susceptibility is likely; or
they are vector species or reservoirs for that disease, or scientific evidence indicates that such role is likely’.
For this reason, the assessment on Article 8 criteria is based on the evidence as extrapolated from the relevant criteria of Article 7, i.e. the ones related to susceptible and reservoir species or routes of transmission, which cover also the possible role of biological or mechanical vectors.2
According to the mapping, as presented in Table 5, Section 3.2, of the scientific opinion on the ad hoc methodology (EFSA AHAW Panel, 2017), the animal species to be listed for AMR B. hyodysenteriae according to the criteria of Article 8(3) of the AHL are as displayed in Table 9 (elaborated from information reported in Section 3.1.1.1 of the present document).
Table 9.
Animal species to be listed for AMR B. hyodysenteriae according to the criteria of Article 8
| Class | Order | Family | Genus/Species | |
|---|---|---|---|---|
| Susceptible | Mammalia | Artiodactyla | Suidae | Pig (Sus scrofa) |
| Carnivora | Canidae | Dog (Canis lupus familiaris) | ||
| Rodentia | Muridae | Mouse (Mus musculus) | ||
| Rat (Rattus rattus and Rattus norvegicus) | ||||
| Aves | Anseriformes | Anatidae | Mallard (Anas platyrhynchos) | |
| Galliformes | Phasianidae | Chicken (Gallus gallus domesticus) | ||
| Passeriformes | Corvidae | Carrion crow (Corvus corone) | ||
| Sturnidae | Common starling (Sturnus vulgaris) | |||
| Struthioniformes | Rheidae | Greater rhea (Rhea americana) | ||
| Reservoir | Mammalia | Artiodactyla | Suidae | Pig (Sus scrofa) |
| Aves | Anseriformes | Anatidae | Mallard (Anas platyrhynchos) | |
| Vector | None | |||
The table contains all animal species in which AMR B. hyodysenteriae has been described, but also those animal species from which only the bacterium itself has been isolated. The latter makes susceptibility to AMR clones likely.
4. Conclusions
The AHAW Panel emphasises that the assessment of impacts, as well as prevention and control measures, related to AMR bacteria using the criteria as laid down in Articles 5 and 9 of the AHL is particularly challenging for opportunistic pathogens that can also be found as commensal bacteria in healthy animals. Furthermore, for AMR B. hyodysenteriae, assessment of the clinical significance of antimicrobial resistance is difficult due to the lack of specific clinical breakpoints.
TOR 1: for each of those identified AMR bacteria considered most relevant in the EU, following the criteria laid down in Article 7 of the AHL, an assessment on its eligibility to be listed for Union intervention as laid down in Article 5(3) of the AHL;
It is uncertain (33–66% probability, ‘as likely as not’) whether AMR B. hyodysenteriae can be considered eligible to be listed for Union intervention as laid down in Article 5 of the AHL.
TOR 2: for each of the AMR bacteria which was found eligible to be listed for Union intervention, an assessment on its compliance with the criteria in Annex IV for the purpose of categorisation in accordance with Article 9 of the AHL;
The AHAW Panel considered with 1–10% probability (from ‘extremely unlikely’ to ‘very unlikely’) that AMR B. hyodysenteriae meets the criteria as in Section 1 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in point (a) of Article 9(1) of the AHL.
The AHAW Panel considered with 10–33% probability (‘unlikely’) that AMR B. hyodysenteriae meets the criteria as in Section 2 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in point (b) of Article 9(1) of the AHL.
The AHAW Panel considered with 10–33% probability (‘unlikely’) that AMR B. hyodysenteriae meets the criteria as in Section 3 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in point (c) of Article 9(1) of the AHL.
The AHAW Panel was uncertain (50–90% probability, from ‘as likely as not’ to ‘likely’) whether AMR B. hyodysenteriae meets the criteria as in Section 4 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in point (d) of Article 9(1) of the AHL.
The AHAW Panel was uncertain (33–66% probability, ‘as likely as not’) whether AMR B. hyodysenteriae meets the criteria as in Section 5 of Annex IV of the AHL, for the application of the disease prevention and control rules referred to in point (e) of Article 9(1) of the AHL.
TOR 3: for each of the AMR bacteria which was found eligible to be listed for Union intervention, a list of animal species that should be considered candidates for listing in accordance with Article 8 of the AHL;
The main animal species that can be considered to be listed for AMR B. hyodysenteriae according to Article 8(3) of the AHL are pigs and birds (chickens and ducks), as reported in Table 9 in Section 3.4 of the present document.
The AHAW Panel highlights that monitoring of antimicrobial resistance in opportunistic pathogens could help to assess their impacts. Therefore, even though the assessment on AMR B. hyodysenteriae is inconclusive on its eligibility to be listed for Union intervention, specific initiatives (e.g. monitoring or applied research) into various aspects of AMR B. hyodysenteriae can be useful to better understand its distribution and to assess its impact on animal health and welfare in the EU.
Abbreviations
- ADG
Average daily gain
- AHAW
Animal Health and Welfare
- AHL
Animal Health Law
- AMR
Antimicrobial‐resistant
- CFSPH
Center for Food Security and Public Health
- CI
Current impact
- CITES
Convention on International Trade in Endangered Species
- DALY
Disability‐adjusted life year
- DIVA
Differentiation of infected from vaccinated animals
- ECOFF
Epidemiological cut‐off
- FISH
Fluorescent in situ hybridisation
- ELISA
Enzyme‐linked immunosorbent assay
- IUCN
International Union for Conservation of Nature
- MALDI‐TOF MS
Matrix‐assisted laser desorption ionisation–time‐of‐flight mass spectrometry
- MIC
Minimum inhibitory concentration
- MS
Member State
- OIE
Office International des Épizooties (World Organisation for Animal Health)
- PCR
Polymerase chain reaction
- PI
Potential impact
- SD
Swine dysentery
- ToR
Term of Reference
Appendix A – Criteria with certain outcome
A.1 Article 5 criteria

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.
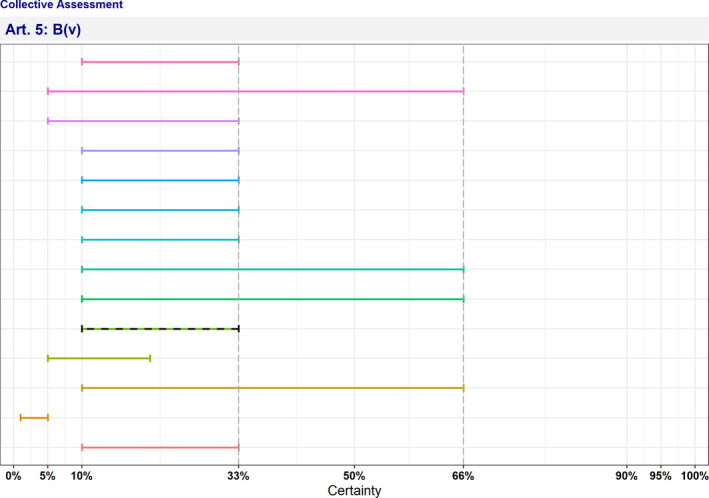
- The median range is displayed as a dashed line.
A.2 Article 9 criteria

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.
Appendix B – Criteria with uncertain outcome
B.1 Article 5 criteria
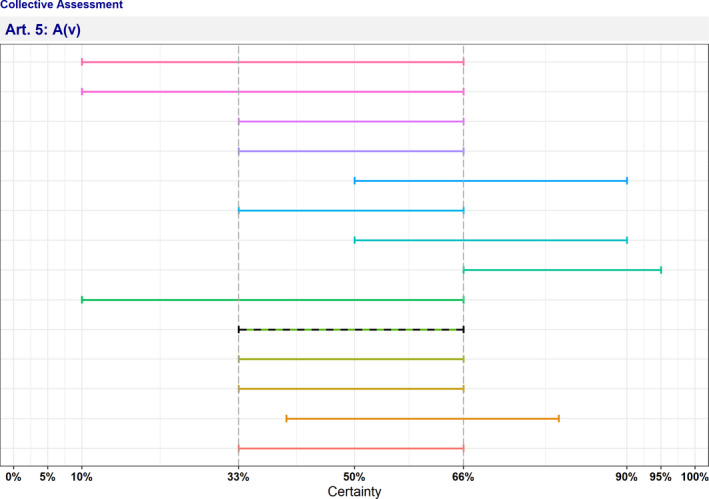
- The median range is displayed as a dashed line.
B.2 Article 9 criteria

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.
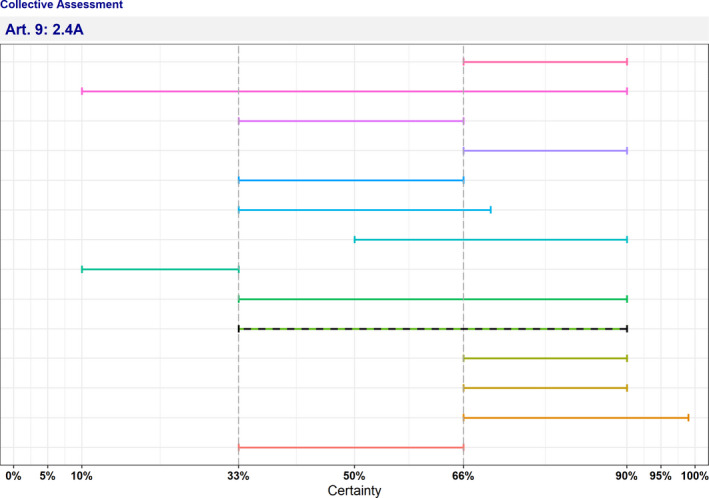
- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.

- The median range is displayed as a dashed line.
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar C, Herskin M, Michel V, Miranda Chueca MÁ, Padalino B, Pasquali P, Roberts HC, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Baldinelli F, Broglia A, Kohnle L, Van der Stede Y and Alvarez J, 2022. Scientific opinion on the assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): antimicrobial‐resistant Brachyspira hyodysenteriae in swine. EFSA Journal 2022;20(3):7124, 71 pp. 10.2903/j.efsa.2022.7124
Requestor: European Commission
Question number: EFSA‐Q‐2022‐00091
Panel members: Søren Saxmose Nielsen, Julio Alvarez, Dominique Joseph Bicout, Paolo Calistri, Elisabetta Canali, Julian Ashley Drewe, Bruno Garin‐Bastuji, José Luis Gonzales Rojas, Christian Gortázar, Mette Herskin, Virginie Michel, Miguel Ángel Miranda Chueca, Barbara Padalino, Paolo Pasquali, Helen Clare Roberts, Hans Spoolder, Karl Ståhl, Antonio Velarde, Arvo Viltrop and Christoph Winckler.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: The AHAW Panel wishes to thank Rikke Heidemann Olsen and Peter Panduro Damborg from the University of Copenhagen for conducting the extensive literature review under the contract OC/EFSA/ALPHA/2020/02 – LOT 1. The AHAW Panel also wishes to thank Jeroen Dewulf from Ghent University and Verena Oswaldi from EFSA for the support provided for this scientific output.
Adopted: 16 February 2022
Notes
Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’). OJ L 84, 31.3.2016, p. 1–208.
A vector is a living organism that transmits an infectious agent from an infected animal to a human or another animal. Vectors are frequently arthropods. Biological vectors may carry pathogens that can multiply within their bodies and be delivered to new hosts, usually by biting. In mechanical vectors, the pathogens do not multiply within the vector, which usually remains infected for shorter time than in biological vectors.
References
- Achacha M and Messier S, 1992. Comparison of six different culture media for isolation of Treponema hyodysenteriae . Journal of Clinical Microbiology, 30, 249–251. 10.1128/jcm.30.1.249-251.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderton S, 2012. Swine dysentery could cost producers £4‐10 a pig. Farmer’s Weekly 6. Available online: https://www.fwi.co.uk/events/swine‐dysentery‐could‐cost‐producers‐4‐10‐a‐pig [Google Scholar]
- Alvarez‐Ordóñez A, Martínez‐Lobo FJ, Arguello H, Carvajal A and Rubio P, 2013. Swine dysentery: aetiology, pathogenicity, determinants of transmission and the fight against the disease. International Journal of Environmental Research and Public Health, 10, 1927–1947. 10.3390/ijerph10051927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhans A, Johansson K‐E and Fellström C, 2010. Phenotypic and molecular characterization of Brachyspira spp. isolated from wild rodents. Environmental Microbiology Reports, 2, 720–727. 10.1111/j.1758-2229.2010.00165.x [DOI] [PubMed] [Google Scholar]
- Backhans A, Jansson DS, Aspán A and Fellström C, 2011. Typing of Brachyspira spp. from rodents, pigs and chickens on Swedish farms. Veterinary Microbiology, 153, 156–162. 10.1016/j.vetmic.2011.03.023 [DOI] [PubMed] [Google Scholar]
- Bellgard MI, Wanchanthuek P, La T, Ryan K, Moolhuijzen P, Albertyn Z, Shaban B, Motro Y, Dunn DS, Schibeci D, Hunter A, Barrero R, Phillips ND and Hampson DJ, 2009. Genome sequence of the pathogenic intestinal spirochete Brachyspira hyodysenteriae reveals adaptations to its lifestyle in the porcine large intestine. PLoS One, 4, e4641. 10.1371/journal.pone.0004641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernaerdt E, Dewulf J, Verhulst R, Bonckaert C and Maes D, 2021. Purchasing policy, quarantine and acclimation practices of breeding gilts in Belgian pig farms. Porcine Health Management, 7, 25. 10.1186/s40813-021-00205-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunt R and McOrist S, 2009. The Potential Transmission of Swine Dysentery by Cockroach Vectors. Proceedings of the 5th Spiroconference, León, Spain, 14 pp. [Google Scholar]
- Boye M, Baloda SB, Leser TD and Møller K, 2001. Survival of Brachyspira hyodysenteriae and B. pilosicoli in terrestrial microcosms. Veterinary Microbiology, 81, 33–40. 10.1016/s0378-1135(01)00328-5 [DOI] [PubMed] [Google Scholar]
- Buckles EL, Eaton KA and Swayne DE, 1997. Cases of spirochete‐associated necrotizing typhlitis in captive common rheas (Rhea americana). Avian Diseases, 41, 144–148. 10.2307/1592454 [DOI] [PubMed] [Google Scholar]
- Burrough ER, 2017. Swine dysentery: etiopathogenesis and diagnosis of a reemerging disease. Veterinary Pathology, 54, 22–31. 10.1177/0300985816653795 [DOI] [PubMed] [Google Scholar]
- Burrough ER, Strait EL, Kinyon JM, Bower LP, Madson DM, Wilberts BL, Schwartz KJ, Frana TS and Songer JG, 2012. Comparative virulence of clinical Brachyspira spp. isolates in inoculated pigs. Journal of Veterinary Diagnostic Investigation, 24, 1025–1034. 10.1177/1040638712457927 [DOI] [PubMed] [Google Scholar]
- Cadetg R, Vidondo B, Nathues H, Schüpbach‐Regula G and Zeeh F, 2019. Retrospektive Studie zur Sanierung von Beständen mit Schweinedysenterie (Brachyspira hyodysenteriae) in der Schweiz. Schweizer Archiv Für Tierheilkunde, 161, 217–230. 10.17236/sat00202 [DOI] [PubMed] [Google Scholar]
- Card RM, Stubberfield E, Rogers J, Nunez‐Garcia J, Ellis RJ, AbuOun M, Strugnell B, Teale C, Williamson S and Anjum MF, 2018. Identification of a new antimicrobial resistance gene provides fresh insights into pleuromutilin resistance in Brachyspira hyodysenteriae, Aetiological Agent of Swine Dysentery. Frontiers in Microbiology, 9, 1183. 10.3389/fmicb.2018.01183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal A, de Arriba ML, Rodríguez H, Vidal AB, Duhamel GE and Rubio P, 2006. Prevalence of Brachyspira species in pigs with diarrhoea in Spain. The Veterinary Record, 158, 700–701. 10.1136/vr.158.20.700 [DOI] [PubMed] [Google Scholar]
- Chia SP and Taylor DJ, 1978. Factors affecting the survival of Treponema hyodysenteriae in dysenteric pig faeces. The Veterinary Record, 103, 68–70. 10.1136/vr.103.4.68 [DOI] [PubMed] [Google Scholar]
- De Luca S, Nicholson P, Magistrali CF, García‐Martín AB, Rychener L, Zeeh F, Frey J and Perreten V, 2018. Transposon‐associated lincosamide resistance lnu(C) gene identified in Brachyspira hyodysenteriae ST83. Veterinary Microbiology, 214, 51–55. 10.1016/j.vetmic.2017.12.003 [DOI] [PubMed] [Google Scholar]
- Desrosiers R, 2011. Transmission of swine pathogens: different means, different needs. Animal Health Research Reviews, 12, 1–13. 10.1017/S1466252310000204 [DOI] [PubMed] [Google Scholar]
- Duff JW, Pittman JS, Hammer JM and Kinyon JM, 2014. Prevalence of Brachyspira hyodysenteriae in sows and suckling piglets. Journal of Swine Health and Production, 22, 71–77. [Google Scholar]
- Dufresne L, 2006. Economics of pig health improvement. Available online: https://porkgateway.org/resource/economics‐of‐pig‐health‐improvement/
- van Duijkeren E, Greko C, Pringle M, Baptiste KE, Catry B, Jukes H, Moreno MA, Pomba MCMF, Pyörälä S, Rantala M, Ružauskas M, Sanders P, Teale C, Threlfall EJ, Torren‐Edo J and Törneke K, 2014. Pleuromutilins: use in food‐producing animals in the European Union, development of resistance and impact on human and animal health. Journal of Antimicrobial Chemotherapy, 69, 2022–2031. 10.1093/jac/dku123 [DOI] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , More S, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortázar Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Stegeman JA, Thulke H‐H, Velarde A, Willeberg P, Winckler C, Baldinelli F, Broglia A, Candiani D, Gervelmeyer A, Zancanaro G, Kohnle L, Morgado J and Bicout D, 2017. Scientific opinion on an ad hoc method for the assessment on listing and categorisation of animal diseases within the framework of the Animal Health Law. EFSA Journal 2017;15(7):4783, 42 pp. 10.2903/j.efsa.2017.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Sihvonen LH, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Dewulf J, Guardabassi L, Hilbert F, Mader R, Baldinelli F and Alvarez J, 2021a. Scientific Opinion on the assessment of animal diseases caused by bacteria resistant to antimicrobials: Swine. EFSA Journal 2021;19(12):7113, 66 pp. 10.2903/j.efsa.2021.7113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Roberts HC, Sihvonen LH, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Guardabassi L, Hilbert F, Mader R, Smith P, Aznar I, Muñoz Guajardo I, Baldinelli F and Alvarez J, 2021b. Scientific Opinion on the ad hoc method for the assessment of animal diseases caused by bacteria resistant to antimicrobials. EFSA Journal 2021;19(6):6645, 29 pp. 10.2903/j.efsa.2021.6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed]
- Eriksen L and Andersen S, 1970. Experimental Swine Dysentery. Nordisk Veterinaermedicin, 22, 161–173. [Google Scholar]
- Feberwee A, Hampson DJ, Phillips ND, La T, van der Heijden HMJF, Wellenberg GJ, Dwars RM and Landman WJM, 2008. Identification of Brachyspira hyodysenteriae and other pathogenic Brachyspira species in chickens from laying flocks with diarrhea or reduced production or both. Journal of Clinical Microbiology, 46, 593–600. 10.1128/JCM.01829-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellström C, Pettersson B, Johansson KE, Lundeheim N and Gunnarsson A, 1996. Prevalence of Serpulina species in relation to diarrhea and feed medication in pig‐rearing herds in Sweden. American Journal of Veterinary Research, 57, 807–811. [PubMed] [Google Scholar]
- Fisher LF and Olander HJ, 1981. Shedding of Treponema hyodysenteriae, transmission of disease, and agglutinin response to pigs convalescent from swine dysentery. American Journal of Veterinary Research, 42, 450–455. [PubMed] [Google Scholar]
- Gallie A, Chesworth M, Blunt R and McOrist S, 2009. Identification of Harmful Dipteroid Communities on Pig Farms. Proceedings of the American Association of Swine Veterinarians, Dallas, Texas, USA. 323 pp. [Google Scholar]
- Gelberg HB, 2017. Alimentary system and the peritoneum, omentum, mesentery, and peritoneal cavity. In: Zachary JF (ed.). Pathologic Basis of Veterinary Disease, Mosby, Maryland Heights, Missouri, USA. pp. 324–411. [Google Scholar]
- Giacomini E, Gasparrini S, Lazzaro M, Scali F, Boniotti MB, Corradi A, Pasquali P and Alborali G, 2018. The role of transportation in the spread of Brachyspira hyodysenteriae in fattening farms. BMC Veterinary Research, 14, 10. 10.1186/s12917-017-1328-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson DJ and Burrough ER, 2019. Swine dysentery and brachyspiral colitis. In: Zimmermann JF, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW and Zhang J (eds.). Diseases of Swine. Wiley, New York, New York, USA. pp. 951–970. [Google Scholar]
- Hampson DJ, Combs BG, Harders SJ, Connaughton ID and Fahy VA, 1991. Isolation of Treponema hyodysenteriae from a wild rat living on a piggery. Australian Veterinary Journal, 68, 308. 10.1111/j.1751-0813.1991.tb03268.x [DOI] [PubMed] [Google Scholar]
- Hampson DJ, Cutler R and Lee BJ, 1992. Virulent Serpulina hyodysenteriae from a pig in a herd free of clinical swine dysentery. The Veterinary Record, 131, 318–319. 10.1136/vr.131.14.318 [DOI] [PubMed] [Google Scholar]
- Hampson DJ, La T and Phillips ND, 2015. Emergence of Brachyspira species and strains: reinforcing the need for surveillance. Porcine Health Management, 1, 8. 10.1186/s40813-015-0002-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson DJ, La T, Phillips ND and Holyoake PK, 2016. Brachyspira hyodysenteriae isolated from apparently healthy pig herds following an evaluation of a prototype commercial serological ELISA. Veterinary Microbiology, 191, 15–19. 10.1016/j.vetmic.2016.05.016 [DOI] [PubMed] [Google Scholar]
- Hampson DJ, Lugsomya K, La T, Phillips ND, Trott DJ and Abraham S, 2019. Antimicrobial resistance in Brachyspira ‐ an increasing problem for disease control. Veterinary Microbiology, 229, 59–71. 10.1016/j.vetmic.2018.12.019 [DOI] [PubMed] [Google Scholar]
- Hampson DJ, Oxberry SL and La T, 2006. Potential for zoonotic transmission of Brachyspira pilosicoli . Emerging Infectious Diseases, 12, 869–870. 10.3201/eid1205.051180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartnack S, Nathues C, Nathues H, Grosse Beilage E and Lewis FI, 2014. Estimating diagnostic test accuracies for Brachyspira hyodysenteriae accounting for the complexities of population structure in food animals. PLoS One, 9, e98534. 10.1371/journal.pone.0098534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst W, Schlez K, Heuser J and Baljer G, 2014. Antimicrobial susceptibility of Brachyspira hyodysenteriae determined by a broth microdilution method. The Veterinary Record, 174, 382. 10.1136/vr.102169 [DOI] [PubMed] [Google Scholar]
- Hidalgo Á, Carvajal A, Pringle M, Rubio P and Fellström C, 2010. Characterization and epidemiological relationships of Spanish Brachyspira hyodysenteriae field isolates. Epidemiology and Infection, 138, 76–85. 10.1017/S0950268809002817 [DOI] [PubMed] [Google Scholar]
- Hidalgo Á, Carvajal A, Vester B, Pringle M, Naharro G and Rubio P, 2011. Trends towards lower antimicrobial susceptibility and characterization of acquired resistance among clinical isolates of Brachyspira hyodysenteriae in Spain. Antimicrobial Agents and Chemotherapy, 55, 3330–3337. 10.1128/AAC.01749-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen S, Willems H, Herbst W, Rohde J and Reiner G, 2014. Mutations in the 50S ribosomal subunit of Brachyspira hyodysenteriae associated with altered minimum inhibitory concentrations of pleuromutilins. Veterinary Microbiology, 172, 223–229. 10.1016/j.vetmic.2014.04.021 [DOI] [PubMed] [Google Scholar]
- Høgh P and Knox B, 1976. Distribution of Treponema hyodysenteriae in Danish pigs as determined by indirect immunofluorescent technique. Proceedings of the International Pig Veterinary Society Congress. Ames, Iowa, USA. [Google Scholar]
- Jacobson M, Fellström C, Lindberg R, Wallgren P and Jensen‐Waern M, 2004. Experimental swine dysentery: comparison between infection models. Journal of Medical Microbiology, 53(Pt. 4), 273–280. 10.1099/jmm.0.05323-0 [DOI] [PubMed] [Google Scholar]
- Jacobson M, Gerth Löfstedt M, Holmgren N, Lundeheim N and Fellström C, 2005. The prevalences of Brachyspira spp. and Lawsonia intracellularis in Swedish piglet producing herds and wild boar population. Journal of Veterinary Medicine. B, Infectious Diseases and Veterinary. Public Health, 52, 386–391. 10.1111/j.1439-0450.2005.00865.x [DOI] [PubMed] [Google Scholar]
- Jansson DS, Bröjer C, Gavier‐Widén D, Gunnarsson A and Fellström C, 2001. Brachyspira spp. (Serpulina spp.) in birds: a review and results from a study of Swedish game birds. Animal Health Research Reviews, 2, 93–100. [PubMed] [Google Scholar]
- Jansson DS, Johansson KE, Olofsson T, Råsbäck T, Vågsholm I, Pettersson B, Gunnarsson A and Fellström C, 2004. Brachyspira hyodysenteriae and other strongly beta‐haemolytic and indole‐positive spirochaetes isolated from mallards (Anas platyrhynchos). Journal of Medical Microbiology, 53(Pt 4), 293–300. 10.1099/jmm.0.05488-0 [DOI] [PubMed] [Google Scholar]
- Jansson DS, Persson M, Zimmerman U and Johansson K‐E, 2011. Phenotypic and genetic diversity among intestinal spirochaetes (genus Brachyspira) in free‐living wild mallards (Anas platyrhynchos) sampled in southern Sweden. Systematic and Applied Microbiology, 34, 566–575. 10.1016/j.syapm.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Jansson DS, Råsbäck T, Fellström C and Feinstein R, 2009. Experimental challenge of mallards (Anas platyrhynchos) with Brachyspira hyodysenteriae and "Brachyspira suanatina" isolated from pigs and mallards. Journal of Comparative Pathology, 141, 211–222. 10.1016/j.jcpa.2009.03.007 [DOI] [PubMed] [Google Scholar]
- Jensen J, Krogh PH and Sverdrup LE, 2003. Effects of the antibacterial agents tiamulin, olanquindox and metronidazole and the anthelmintic ivermectin on the soil invertebrate species Folsomia fimetaria (Collembola) and Enchytraeus crypticus (Enchytraeidae). Chemosphere, 50, 437–443. 10.1016/s0045-6535(02)00336-3 [DOI] [PubMed] [Google Scholar]
- Joens LA, 1980. Experimental transmission of Treponema hyodysenteriae from mice to pigs. American Journal of Veterinary Research, 41, 1225–1226. [PubMed] [Google Scholar]
- Joerling J, Barth SA, Schlez K, Willems H, Herbst W and Ewers C, 2018. Phylogenetic diversity, antimicrobial susceptibility and virulence gene profiles of Brachyspira hyodysenteriae isolates from pigs in Germany. PLoS One, 13, e0190928. 10.1371/journal.pone.0190928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston WT, Dewey CE, Friendship RM, Smart N, McEwen BJ, Stalker M and de Lange CF, 2001. An investigation of the etiology of a mild diarrhea observed in a group of grower/finisher pigs. The Canadian Veterinary Journal, 42, 33–37. 10.4141/cjas62-005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson M, Fellström C, Heldtander MU, Johansson KE and Franklin A, 1999. Genetic basis of macrolide and lincosamide resistance in Brachyspira (Serpulina) hyodysenteriae . FEMS Microbiology Letters, 172, 255–260. 10.1111/j.1574-6968.1999.tb13476.x [DOI] [PubMed] [Google Scholar]
- Kinyon JM, Harris DL and Glock RD, 1977. Enteropathogenicity of various isolates of Treponema hyodysenteriae . Infection and Immunity, 15, 638–646. 10.1128/iai.15.2.638-646.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinyon JM, Harris DL and Glock RD, 1980. Isolation of Treponema hyodysenteriae from Experimentally Infected Pigs at Various Intervals Post‐Inoculation. Proceedings of the 6th International Pig Veterinary Society CongressCopenhagen, Denmark. 232 pp. [Google Scholar]
- Leser TD, Lindecrona RH, Jensen TK, Jensen BB and Møller K, 2000. Changes in bacterial community structure in the colon of pigs fed different experimental diets and after infection with Brachyspira hyodysenteriae . Applied Environmental Microbiology, 66, 3290–3296. 10.1128/AEM.66.8.3290-3296.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahu M, Pasmans F, Vranckx K, De Pauw N, Vande Maele L, Vyt P, Vandersmissen T, Martel A, Haesebrouck F and Boyen F, 2017. Presence and mechanisms of acquired antimicrobial resistance in Belgian Brachyspira hyodysenteriae isolates belonging to different clonal complexes. Veterinary Microbiology, 207, 125–132. 10.1016/j.vetmic.2017.05.022 [DOI] [PubMed] [Google Scholar]
- McFadzean H, Schock A, Stubberfield E, Card RM, Thomson J, Rohde J, Murray L, Velo‐Rego E, Ainsworth H, Barlow AM and Welchman D, 2021. Retrospective analysis of necrotizing typhlitis cases associated with Brachyspira spp. British Rheas. Avian Pathology, 50, 246–256. 10.1080/03079457.2021.1907305 [DOI] [PubMed] [Google Scholar]
- McKean J, Burrough ER and Schwartz KJ, 2013. Bloody scours (Swine Dysentery): A Costly Re‐emerging Disease That Is Preventable. Available online: https://porkgateway.org/resource/bloody‐scours‐swine‐dysentery‐a‐costly‐re‐emerging‐disease‐that‐ispreventable/ [Google Scholar]
- Mhoma JRL, Hampson DJ and Robertson ID< 1992. A serological survey to determine the prevalence of infection with Treponema hyodysenteriae in Western Australia. Australian Veterinary Journal, 69, 81–84. [DOI] [PubMed] [Google Scholar]
- Møller K, Jensen TK, Jorsal SE, Leser TD and Carstensen B, 1998. Detection of Lawsonia intracellularis, Serpulina hyodysenteriae, weakly beta‐haemolytic intestinal spirochaetes, Salmonella enterica, and haemolytic Escherichia coli from swine herds with and without diarrhoea among growing pigs. Veterinary Microbiology, 62, 59–72. 10.1016/s0378-1135(98)00199-0 [DOI] [PubMed] [Google Scholar]
- Moreng NT, Quarles CL, Fagerberg DJ and Moeller DJ, 1980. Pathogenesis and lesions of swine dysentery induced by artificial methods in early weaned pigs. Veterinary Medicine, Small Animal Clinician: VM, SAC, 75, 1841–1844. [PubMed] [Google Scholar]
- Neirynck W, Boyen F, Chantziaras I, Vandersmissen T, Vyt P, Haesebrouck F, Dewulf J and Maes D, 2020. Implementation and evaluation of different eradication strategies for Brachyspira hyodysenteriae . Porcine Health Management, 6, 27. 10.1186/s40813-020-00162-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris SJ, 2019. Hiding in plain sight: colonic spirochetosis in humans. Journal of Bacteriology, 201, e00465–e519. 10.1128/JB.00465-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LD, 1974. Clinical and pathological observations on the experimental passage of swine dysentery. Canadian Journal of Comparative Medicine, 38, 7–13. [PMC free article] [PubMed] [Google Scholar]
- Olson LD, 1995. Survival of Serpulina hyodysenteriae in an effluent lagoon. Journal of the American Veterinary Medical Association, 207, 1470–1472. [PubMed] [Google Scholar]
- Ózsvári L, 2017. Economic significance of swine dysentery control. Magyar Állatorvosok Lapja, 139, 271–275. [Google Scholar]
- Patterson AH, Rubin JE, Fernando C, Costa MO, Harding JCS and Hill JE, 2013. Fecal shedding of Brachyspira spp. on a farrow‐to‐finish swine farm with a clinical history of “Brachyspira hampsonii”–associated colitis. BMC Veterinary Research, 9, 137. 10.1186/1746-6148-9-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson HE, Toribio J‐ALML, Lapidge SJ and Hernández‐Jover M, 2016. Evaluating the risk of pathogen transmission from wild animals to domestic pigs in Australia. Preventive Veterinary Medicine, 123, 39–51. 10.1016/j.prevetmed.2015.11.017 [DOI] [PubMed] [Google Scholar]
- Phillips ND, La T, Adams PJ, Harland BI, Fenwick SG and Hampson DJ, 2009. Detection of Brachyspira hyodysenteriae, Lawsonia intracellularis and Brachyspira pilosicoli in feral pigs. Veterinary Microbiology, 134, 294–299. 10.1016/j.vetmic.2008.08.006 [DOI] [PubMed] [Google Scholar]
- Prášek J, Šperling D, Lobová D, Smola J and Čížek A, 2014. Antibiotic susceptibility of Brachyspira hyodysenteriae isolates from Czech swine farms: a 10‐year follow‐up study. Acta Veterinaria Brno, 83, 3–7. 10.2754/avb201483010003 [DOI] [Google Scholar]
- Pringle M, Fellström C and Johansson K‐E, 2007. Decreased susceptibility to doxycycline associated with a 16S rRNA gene mutation in Brachyspira hyodysenteriae . Veterinary Microbiology, 123, 245–248. 10.1016/j.vetmic.2007.02.019 [DOI] [PubMed] [Google Scholar]
- Pringle M, Landén A, Unnerstad HE, Molander B and Bengtsson B, 2012. Antimicrobial susceptibility of porcine Brachyspira hyodysenteriae and Brachyspira pilosicoli isolated in Sweden between 1990 and 2010. Acta Veterinaria Scandinavica, 54, 54. 10.1186/1751-0147-54-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde J, Kessler M, Baums CG and Amtsberg G, 2004. Comparison of methods for antimicrobial susceptibility testing and MIC values for pleuromutilin drugs for Brachyspira hyodysenteriae isolated in Germany. Veterinary Microbiology, 102, 25–32. 10.1016/j.vetmic.2004.03.023 [DOI] [PubMed] [Google Scholar]
- Rugna G, Bonilauri P, Carra E, Bergamini F, Luppi A, Gherpelli Y, Magistrali CF, Nigrelli A, Alborali GL, Martelli P, La T, Hampson DJ and Merialdi G, 2015. Sequence types and pleuromutilin susceptibility of Brachyspira hyodysenteriae isolates from Italian pigs with swine dysentery: 2003–2012. Veterinary Journal, 203, 115–119. 10.1016/j.tvjl.2014.10.033 [DOI] [PubMed] [Google Scholar]
- Sagartz JE, Swayne DE, Eaton KA, Hayes JR, Amass KD, Wack R and Kramer L, 1992. Necrotizing typhlocolitis associated with a spirochete in rheas (Rhea americana). Avian Diseases, 36, 282–289. [PubMed] [Google Scholar]
- Savage DC, 1980. Colonization by and survival of pathogenic bacteria on intestinal mucosal surfaces. In: Bitton B and Marshall KC (eds.). Adsorption of Microorganisms to Surfaces, Wiley, New York, New York, USA. pp. 175–206. [Google Scholar]
- Sjölund M, Zoric M and Wallgren P, 2014. Financial impact of disease on pig production. Part III. Gastrointestinal disorders. Poster presented at the 6th European Symposium of Porcine Health Management. Sorrento, Italy. [Google Scholar]
- Songer JG, Glock RD, Schwartz KJ and Harris DL, 1978b. Isolation of Treponema hyodysenteriae from sources other than swine. Journal of the American Veterinary Medical Association, 172, 464–466. [PubMed] [Google Scholar]
- Songer JG and Harris DL, 1978a. Transmission of swine dysentery by carrier pigs. American Journal of Veterinary Research, 39, 913–916. [PubMed] [Google Scholar]
- Stege H, Jensen TK, Møller K, Baekbo P and Jorsal SE, 2000. Prevalence of intestinal pathogens in Danish finishing pig herds. Preventive Veterinary Medicine, 46, 279–292. 10.1016/s0167-5877(00)00148-3 [DOI] [PubMed] [Google Scholar]
- Suh DK and Song JC, 2005. Simultaneous detection of Lawsonia intracellularis, Brachyspira hyodysenteriae and Salmonella spp. in swine intestinal specimens by multiplex polymerase chain reaction. Journal of Veterinary Science, 6, 231–237. [PubMed] [Google Scholar]
- SVARM‐Swedres , 2019. Consumption of antibiotics and occurrence of antibiotic resistance in Sweden. National surveillance report on AMR data in Sweden in 2018. Available online: https://www.folkhalsomyndigheten.se/contentassets/fb80663bc7c94d678be785e3360917d1/swedres‐svarm‐2019.pdf [Google Scholar]
- Trott DJ, Atyeo RF, Lee JI, Swayne DA, Stoutenburg JW and Hampson DJ, 1996. Genetic relatedness amongst intestinal spirochaetes isolated from rats and birds. Letters in Applied Microbiology, 23, 431–436. 10.1111/j.1472-765x.1996.tb01352.x [DOI] [PubMed] [Google Scholar]
- Trott DJ and Hampson DJ, 1998. Evaluation of day‐old specific pathogen‐free chicks as an experimental model for pathogenicity testing of intestinal spirochaete species. Journal of Comparative Pathology, 118, 365–381. 10.1016/s0021-9975(07)80012-0 [DOI] [PubMed] [Google Scholar]
- Walczak K, Friendship R, Brockoff E, Greer A and Poljak Z, 2017. Treatment rates for injectable tiamulin and lincomycin as an estimate of morbidity in a swine herd with endemic swine dysentery. The Canadian Veterinary Journal, 58, 472–481. [PMC free article] [PubMed] [Google Scholar]
- Wilberts BL, Arruda PH, Warneke HL, Erlandson KR, Hammer JM and Burrough ER, 2014. Cessation of clinical disease and spirochete shedding after tiamulin treatment in pigs experimentally infected with "Brachyspira hampsonii". Research in Veterinary Science, 97, 341–347. 10.1016/j.rvsc.2014.08.004 [DOI] [PubMed] [Google Scholar]
- Wilberts BL, Warneke HL, Bower LP, Kinyon JM and Burrough ER, 2015. Comparison of culture, polymerase chain reaction, and fluorescent in situ hybridization for detection of Brachyspira hyodysenteriae and "Brachyspira hampsonii" in pig feces. Journal of Veterinary Diagnostic Investigation, 27, 41–46. 10.1177/1040638714563064 [DOI] [PubMed] [Google Scholar]
- Windsor RS and Simmons JR, 1981. Investigation into the spread of swine dysentery in 25 herds of East Anglia and assessment of its economic significance in five herds. The Veterinary Record, 109, 482–484. 10.1136/vr.109.22.482 [DOI] [PubMed] [Google Scholar]
- Zeeh F, Klausmann S, Masserey Y, Nathues H, Perreten V and Rohde J, 2018. Isolation of Brachyspira hyodysenteriae from a crow (Corvus corone) in close proximity to commercial pigs. Veterinary Journal, 236, 111–112. 10.1016/j.tvjl.2018.05.002 [DOI] [PubMed] [Google Scholar]
- Zmudzki J, Szczotka A, Nowak A, Strzelecka H, Grzesiak A and Pejsak Z, 2012. Antimicrobial susceptibility of Brachyspira hyodysenteriae isolated from 21 Polish farms. Polish Journal of Veterinary Sciences, 15, 259–265. 10.2478/v10181-011-0143-3 [DOI] [PubMed] [Google Scholar]


