Abstract
Background
Gynaecological cancers account for 15% of newly diagnosed cancer cases in women worldwide. In recent years, increasing evidence demonstrates that traditional approaches in perioperative care practice may be unnecessary or even harmful. The enhanced recovery after surgery (ERAS) programme has therefore been gradually introduced to replace traditional approaches in perioperative care. There is an emerging body of evidence outside of gynaecological cancer which has identified that perioperative ERAS programmes decrease length of postoperative hospital stay and reduce medical expenditure without increasing complication rates, mortality, and readmission rates. However, evidence‐based decisions on perioperative care practice for major surgery in gynaecological cancer are limited. This is an updated version of the original Cochrane Review published in Issue 3, 2015.
Objectives
To evaluate the beneficial and harmful effects of perioperative enhanced recovery after surgery (ERAS) programmes in gynaecological cancer care on length of postoperative hospital stay, postoperative complications, mortality, readmission, bowel functions, quality of life, participant satisfaction, and economic outcomes.
Search methods
We searched the following electronic databases for the literature published from inception until October 2020: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, PubMed, AMED (Allied and Complementary Medicine), CINAHL (Cumulative Index to Nursing and Allied Health Literature), Scopus, and four Chinese databases including the China Biomedical Literature Database (CBM), WanFang Data, China National Knowledge Infrastructure (CNKI), and Weipu Database. We also searched four trial registration platforms and grey literature databases for ongoing and unpublished trials, and handsearched the reference lists of included trials and accessible reviews for relevant references.
Selection criteria
We included randomised controlled trials (RCTs) that compared ERAS programmes for perioperative care in women with gynaecological cancer to traditional care strategies.
Data collection and analysis
Two review authors independently screened studies for inclusion, extracted the data and assessed methodological quality for each included study using the Cochrane risk of bias tool 2 (RoB 2) for RCTs. Using Review Manager 5.4, we pooled the data and calculated the measures of treatment effect with the mean difference (MD), standardised mean difference (SMD), and risk ratio (RR) with a 95% confidence interval (CI) to reflect the summary estimates and uncertainty.
Main results
We included seven RCTs with 747 participants. All studies compared ERAS programmes with traditional care strategies for women with gynaecological cancer. We had substantial concerns regarding the methodological quality of the included studies since the included RCTs had moderate to high risk of bias in domains including randomisation process, deviations from intended interventions, and measurement of outcomes.
ERAS programmes may reduce length of postoperative hospital stay (MD ‐1.71 days, 95% CI ‐2.59 to ‐0.84; I2 = 86%; 6 studies, 638 participants; low‐certainty evidence). ERAS programmes may result in no difference in overall complication rates (RR 0.71, 95% CI 0.48 to 1.05; I2 = 42%; 5 studies, 537 participants; low‐certainty evidence). The certainty of evidence was very low regarding the effect of ERAS programmes on all‐cause mortality within 30 days of discharge (RR 0.98, 95% CI 0.14 to 6.68; 1 study, 99 participants). ERAS programmes may reduce readmission rates within 30 days of operation (RR 0.45, 95% CI 0.22 to 0.90; I2 = 0%; 3 studies, 385 participants; low‐certainty evidence). ERAS programmes may reduce the time to first flatus (MD ‐0.82 days, 95% CI ‐1.00 to ‐0.63; I2 = 35%; 4 studies, 432 participants; low‐certainty evidence) and the time to first defaecation (MD ‐0.96 days, 95% CI ‐1.47 to ‐0.44; I2 = 0%; 2 studies, 228 participants; low‐certainty evidence). The studies did not report the effects of ERAS programmes on quality of life. The evidence on the effects of ERAS programmes on participant satisfaction was very uncertain due to the limited number of studies. The adoption of ERAS strategies may not increase medical expenditure, though the evidence was of very low certainty (SMD ‐0.22, 95% CI ‐0.68 to 0.25; I2 = 54%; 2 studies, 167 participants).
Authors' conclusions
Low‐certainty evidence suggests that ERAS programmes may shorten length of postoperative hospital stay, reduce readmissions, and facilitate postoperative bowel function recovery without compromising participant safety. Further well‐conducted studies are required in order to validate the certainty of these findings.
Keywords: Female, Humans, Length of Stay, Neoplasms, Perioperative Care, Postoperative Complications, Postoperative Complications/epidemiology, Postoperative Complications/prevention & control, Quality of Life
Plain language summary
Perioperative enhanced recovery after surgery programmes for women with gynaecological cancers
Background Gynaecological cancers lead to a significant amount of morbidity and mortality. Surgery, either by laparoscopic (key‐hole surgery) or open surgical techniques, is one of the most important approaches in the treatment of gynaecological cancer. Well‐planned perioperative care (care at or around the time of surgery) is vital for recovery following surgery.
In recent years, researchers and doctors have suggested that many aspects of traditional perioperative care practice may be unnecessary or even harmful. For example, the use of oral laxative and enema could result in preoperative abnormalities in the levels of sodium, potassium or calcium, along with dehydration. The enhanced recovery after surgery (ERAS) programme aims to reduce surgical stress and avoid harmful aspects of traditional perioperative care. It has been introduced gradually to various surgical fields, particularly bowel surgery. ERAS programmes may help recovery after surgery, shorten time in hospital, and save hospital costs without putting the person at greater risk. However, less is known about the effects of ERAS programmes in women with gynaecological cancer. This review aims to evaluate the benefits and harms of perioperative ERAS programmes in gynaecological cancer care.
Study characteristics
We searched both Chinese and English databases (up to October 2020) and found seven trials of 747 women with gynaecological cancer, including cervical cancer, uterine cancer, ovarian cancer, and endometrial cancer. Five studies only recruited women with suspected or confirmed gynaecological cancer and two studies also included a small group of women with a benign or borderline tumour. Three studies recruited women who underwent laparotomy (where a surgeon makes one large incision in the abdomen) and two studies included those who underwent laparoscopic surgery (a minimally invasive procedure that requires only small incisions). Two of the studies used both types of surgery. Women then received either perioperative ERAS programmes or traditional care.
Key results
ERAS programmes may reduce time in hospital after the operation and readmission rates within 30 days of surgery. ERAS programmes may speed up recovery of bowel functions following surgery, measured by time to when the woman first breaks wind or opens her bowels. There may be no increase in complications within 30 days of operation using ERAS programmes. Due to limited evidence, we are very uncertain about the effects of ERAS programmes on death from any cause within 30 days of operation, or on how satisfied women were with their care. We did not find any evidence about their quality of life. ERAS might not increase hospital costs, but the evidence was very uncertain.
Conclusions
ERAS programmes may shorten time in hospital after the operation, reduce postoperative readmission rates, and facilitate bowel function recovery without compromising the safety of women with gynaecological cancer, although we have limited confidence in the findings due to the quality of the studies. Future well‐conducted studies may increase the certainty of these findings.
Summary of findings
Summary of findings 1. Enhanced recovery after surgery programme compared to traditional care for women with gynaecological cancers.
| Enhanced recovery after surgery programme compared to traditional care for women with gynaecological cancers | ||||||
| Patient or population: women with gynaecological cancers Setting: hospital‐based gynaecological cancer perioperative care Intervention: enhanced recovery after surgery programme Comparison: traditional care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | What this means | |
| Risk with traditional care | Risk with enhanced recovery after surgery programme | |||||
| Length of postoperative hospital stay | The mean length of postoperative hospital stay was 7.87 days | MD ‐1.71 days (‐2.59 to ‐0.84) | ‐ | 638 (6 RCTs) | ⊕⊕⊝⊝ low a,b | Enhanced recovery after surgery programmes might result in a reduction in length of postoperative hospital stay. |
| Overall postoperative complications (within 30 days of operation) | Study population | RR 0.71 (0.48 to 1.05) | 537 (5 RCTs) | ⊕⊕⊝⊝ low c,d | Enhanced recovery after surgery programmes might result in no difference in overall complications within 30 days of operation. | |
| 333 per 1000 | 237 per 1000 (160 to 350) | |||||
| Mortality (within 30 days of discharge) | Study population | RR 0.98 (0.14 to 6.68) | 99 (1 RCT) | ⊕⊝⊝⊝ very lowd,e | The evidence was very uncertain about the effect of the enhanced recovery after surgery programme on all‐cause mortality within 30 days of discharge . | |
| 41 per 1000 | 40 per 1000 (6 to 273) | |||||
| Readmission (within 30 days of operation) | Study population | RR 0.45 (0.22 to 0.90) | 385 (3 RCTs) | ⊕⊕⊝⊝ low b,d | Enhanced recovery after surgery programmes might reduce readmission within 30 days of operation. | |
| 126 per 1000 | 57 per 1000 (28 to 114) | |||||
| Time to first flatus (bowel function) | The mean time to first flatus (bowel function) was 1.80 days | MD ‐0.82 days (‐1.00 to ‐0.63) | ‐ | 432 (4 RCTs) | ⊕⊕⊝⊝ low d,f | Enhanced recovery after surgery programmes might result in reduction in time to first flatus. |
| Time to first defaecation (bowel function) | The mean time to first defaecation (bowel function) was 3.97 days | MD ‐0.96 days (‐1.47 to ‐0.44) | ‐ | 228 (2 RCTs) | ⊕⊕⊝⊝ low d,g | Enhanced recovery after surgery programmes might reduce time to first defaecation. |
| Participant satisfaction | ‐ | SMD 0.92 (0.06 to 1.78) | ‐ | 228 (2 RCTs) | ⊕⊝⊝⊝ very low a,b,c,d | The evidence was very uncertain about the effect of enhanced recovery after surgery programmes on participant satisfaction. |
| Economic outcomes | ‐ | SMD ‐0.22 (‐0.68 to 0.25) | ‐ | 167 (2 RCTs) | ⊕⊝⊝⊝ very low b,c,d | The evidence was very uncertain about the effect of enhanced recovery after surgery programmes on hospital costs. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aInconsistency downgraded one level because the proportion of the variability in effect estimates that is due to true heterogeneity is considerable. bRisk of bias downgraded one level because of suspected deviations from intended interventions or high risk of confounding bias and information bias. cImprecision downgraded one level due to inclusion of appreciable benefit and no effect. dPublication bias downgraded because data were reported by a limited number of studies. eImprecision downgraded two levels as CI of pooled estimate includes substantial benefit, substantial harm and no effect. fInconsistency downgraded one level as one study that could not be included in the pooled analysis reported that no significant difference was found regarding the distribution of the time to first flatus between groups. gIndirectness downgraded one level due to one of the two RCTs including participants with both benign and malignant tumours. Pooled results changed when excluding this study.
Background
This review is an update of a previous Cochrane Review (Lu 2015).
Description of the condition
Gynaecological cancers consist of cancers of the cervix uteri, corpus uteri, ovary, vulva, and vagina, and the five‐year gynaecological cancer prevalence rate is > 7.7% worldwide (WHO 2021). There were over 1.39 million new cases of gynaecological cancers in 2020, accounting for 15.2% of all new cancer cases in women. About 15% of women with cancer died of gynaecological cancers in 2020, of which ovarian cancer was found to be associated with a worse prognosis compared to other gynaecological cancers (Ledford 2019; WHO 2021). Cervical cancer is the most prevalent type of gynaecological cancer, making up 6.5% of new cases every year. It has become the fourth most common cancer in women, following breast, colorectal, and lung cancer (Arbyn 2020; WHO 2021). The age‐standardised incidence and mortality rates of cervical cancer are 13.3 and 7.3 per 100,000 persons worldwide, respectively (Arbyn 2020; WHO 2021).
Surgery, either by laparoscopic or open surgical techniques, is one of the most important approaches in the treatment of gynaecological cancers (Zalewski 2018). Other treatments include chemotherapy and radiotherapy, along with other multimodal therapies. Perioperative care, which is influenced by specific care strategies, impacts recovery following surgery. Traditional perioperative care may consist of preoperative overnight fasting and mechanical bowel preparation, intravenous fluids prior to surgery or anaesthesia, receiving pelvic drains during operation, receiving nasogastric tubes, and beginning a graduated oral intake until bowel sounds return after surgery. However, more evidence‐based results have shown that many of these traditional perioperative approaches are unnecessary or may even be harmful.
For example, with people who underwent colorectal surgery, the use of mechanical bowel preparation was not associated with a lower risk of mortality, wound infection, anastomotic leak, or reoperation as compared to those who had no mechanical bowel preparation (Cao 2012; Dahabreh 2015). Moreover, the use of mechanical bowel preparation may lead to adverse events due to preoperative dehydration and electrolyte imbalance, so impede the recovery process (Arnold 2015). Preoperative overnight fasting may also be problematic because it may cause postoperative complications such as insulin resistance (ASAC 2017). A Cochrane Review assessing the effects of preoperative carbohydrate treatment has shown that the treatment was associated with less postoperative insulin resistance, faster bowel function recovery, and shorter length of hospital stay, without compromising safety in adult participants who underwent abdominal, orthopaedic, and cardiac surgeries (Smith 2014).
With increasing concerns regarding postoperative recovery and comfort, and the development of enhanced recovery strategies, it is imperative to re‐examine the necessity and appropriateness of traditional approaches and to establish if current practice is supported by clinical evidence in the perioperative care of women with gynaecological cancers.
Description of the intervention
Enhanced Recovery After Surgery (ERAS), also known as Enhanced Recovery Programme (ERP), or fast track (FT) surgery, was first incorporated in colorectal surgeries in the 1990s (Kehlet 2008). ERAS refers to a multidisciplinary, multimodal approach to minimise the physiologic deviations induced by surgery, and to avoid traditional aspects of perioperative care that have documented harm (Kehlet 1997; Ljungqvist 2017). The implementation of ERAS usually requires co‐operation and co‐ordination among surgeons, anaesthetists, an ERAS co‐ordinator (often a nurse or a physician assistant), and staff from units that care for people undergoing surgery, based on the consensus on their ERAS protocols (Ljungqvist 2017).
With continued development in the past two decades, ERAS has been widely accepted and adopted in colorectal, gastric, lung, liver, pancreatic, urological, head and neck surgeries (Li S 2017; Li Z 2017; Rouxel 2019; Spanjersberg 2011; Watson 2020). Specialists also developed ERAS guidelines for different types of diseases or surgeries by synthesising direct evidence in their areas, indirect evidence related to their areas, and expert opinions (Ljungqvist 2017; Melloul 2020). The components of different ERAS programmes vary in different settings but usually address preoperative patient assessment and counselling, avoidance of premedication, fasting and bowel preparation, appropriate use of drains and catheters, hypothermia prevention, multimodal analgesia, postoperative early oral intake, and early mobilisation (Nicholson 2014).
Recently, the ERAS® Society published updated ERAS guidelines on gynaecological oncology care and stated the latest consensus recommendations for ERAS practice (Nelson 2019). They listed a total of 21 domains, including preadmission information, education, counselling, preoperative optimisation, prehabilitation, preoperative bowel preparation, urinary drainage management, and early mobilisation. However, a recent international study showed that although 80% of the participants agreed that ERAS pathways could improve outcomes for people undergoing surgery, only 37% of them reported that ERAS was implemented at their institutions, with a range from 10% in participants from Africa to 38% in participants from Europe (Bhandoria 2020). It is obvious that, in many instances, high quality data were unavailable, and the recommendation was formed based on evidence from other surgical disciplines where abdominal surgeries are routinely performed (Nelson 2019). Furthermore, best ERAS evidence‐based practices for people with different characteristics (e.g. different types of gynaecological cancers) or undergoing different kinds of surgeries (e.g. laparotomy and laparoscopic surgery) are still unclear.
How the intervention might work
A growing body of evidence outside of gynaecological cancers, especially in colorectal cancers, has indicated that ERAS facilitates recovery by minimising physiologic stress after surgery and decreasing length of hospital stay and costs without increasing adverse outcomes. Shorter time to first flatus and defaecation, and earlier acceptance of a solid diet were observed in people with colorectal cancer from ERAS groups when compared with those in traditional care groups in previous randomised controlled trials (Ni 2019; Spanjersberg 2011). People receiving ERAS programmes also performed better on postoperative early mobilisation (Liu 2020; Spanjersberg 2011).
People who underwent colorectal cancer surgeries and received an ERAS intervention usually had shorter postoperative hospital stays as compared to people who received traditional care (Li J 2019; Ljungqvist 2017; Ni 2019; Spanjersberg 2011). Due to the reduction in length of hospital stay and postoperative complications, people receiving ERAS programmes had lower hospitalisation costs compared with those receiving traditional care (Baimas‐George 2020; Li J 2019). Additionally, ERAS implementation is not associated with increased postoperative complication rates and may even be associated with decreased complication rates and severity as compliance with ERAS programmes increases (Li J 2019; Ljungqvist 2017; Ni 2019; Pisarska 2016; Spanjersberg 2011). As such, ERAS implementation is not related to higher readmission rates or mortality (Baimas‐George 2020; Lohsiriwat 2019; Rouxel 2019). From the perspectives of people undergoing different kinds of surgeries, ERAS protocols did not negatively impact quality of life (Leon Arellano 2020; Wu 2019). Participant satisfaction with ERAS programmes was also generally high across different studies (Debono 2019; Liu 2020).
Though the effects of ERAS implementation on long‐term outcomes are unclear at present due to a lack of evidence, a recent study reported that ERAS programmes may facilitate long‐term survival of people with colorectal cancer, as the implementation of ERAS was associated with receiving on‐time adjuvant chemotherapy after surgery (Hassinger 2019).
Why it is important to do this review
Implementation of perioperative ERAS programmes in people with colorectal, gastric, lung, liver, and pancreatic cancer has been examined and supported by several systematic reviews and trials (Li S 2017; Li Z 2017; Rouxel 2019; Spanjersberg 2011; Watson 2020). The main components of ERAS practice for women with gynaecological cancer are mainly extended from other diseases such as colorectal oncology (Nelson 2019), and evidence‐based decisions on major surgery for gynaecological cancers remain limited. Women with gynaecological cancers may have to deal with various challenges, including concerns about physical well‐being (e.g. menopausal symptoms, bowel and urinary complications, lymphoedema, and pelvic pain) and cancer‐related psychosocial issues, such as perceptions of having an altered body image, compared with people from other cancer groups (Ringwald 2017; Sekse 2019). Therefore, we deem it important to assess the benefits and harms of ERAS programmes for women with gynaecological cancer, and to develop tailored pathways for managing the care of women with gynaecological cancer.
Objectives
To evaluate the beneficial and harmful effects of perioperative enhanced recovery after surgery (ERAS) programmes in gynaecological cancer care on length of postoperative hospital stay, postoperative complications, mortality, readmission, bowel functions, quality of life, participant satisfaction, and economic outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and cluster‐RCTs that assessed the effects of ERAS programmes for women with gynaecological cancer. Although there has been a wealth of evidence from non‐RCTs (e.g. before‐after design trials) on the topic, we only focused on RCTs because randomisation prevents systematic differences (confounders) between baseline characteristics of participants in different intervention groups, and claims about cause and effect based on the findings can be made with greater confidence than those from non‐RCTs.
Types of participants
We included women aged ≥18 years old with indications for non‐emergency surgical treatment of gynaecological cancers. Gynaecological cancers consist of cervical, uterine, ovarian, fallopian, vulval, vaginal, and endometrial cancers. Studies including both laparoscopic and open surgical techniques were eligible for inclusion.
Some studies include all women with gynaecological tumours, regardless of whether the tumour is benign or malignant. To avoid the loss of data if such studies were excluded, we also included studies in which the majority (>50%) of the women were diagnosed with gynaecological cancers and the others were diagnosed with benign gynaecological tumours. This meant that we would include some women with benign tumours who did not meet the eligibility criteria, so we used sensitivity analysis to assess the impact of this decision on the review's findings (see Sensitivity analysis).
Types of interventions
We compared any ERAS programmes with traditional recovery strategies in perioperative care for women with gynaecological cancer. The ERAS® Society published the latest consensus reviews of ERAS practice for gynaecological oncology in their updated guidelines (Nelson 2019). A total of 21 domains with recommendations were listed, including 17 domains related to ERAS pre‐, intra‐, and postoperative practices and four related to ERAS management.
Perioperative ERAS practices consist of preadmission information, education, and counselling; preoperative optimisation; prehabilitation; preoperative bowel preparation; preoperative fasting and carbohydrate treatment; pre‐anaesthetic medication; venous thromboembolism prophylaxis; surgical site infection reduction bundles (including antimicrobial prophylaxis, skin preparation, prevention of hypothermia, avoidance of drains/tubes, and control of perioperative hyperglycaemia); standard anaesthetic protocol; nausea and vomiting prophylaxis; minimally invasive surgery; perioperative fluid management/goal‐directed fluid therapy; perioperative nutrition; prevention of postoperative ileus; opioid sparing multimodal postoperative analgesia; urinary drainage management; and early mobilisation. The other four domains related to ERAS management consist of patient‐reported outcomes, including functional recovery, role of ERAS in pelvic exenteration and hyperthermic intraperitoneal chemotherapy, discharge pathways, and ERAS audit and reporting. We defined an eligible ERAS strategy as consisting of at least four domains related to ERAS practice (Chambers 2014). We considered traditional care strategies that did not adopt an ERAS protocol as comparators. Traditional care strategies may consist of preoperative overnight fasting, mechanical and antibiotic bowel preparation, intravenous fluids prior to surgery or anaesthesia, receiving pelvic drains during operation, receiving nasogastric tubes, and beginning a graduated oral intake until bowel sounds return after surgery. In this review, eligible comparators were traditional care strategies which did not consist of any ERAS components, which included some ERAS components without a standardised ERAS protocol, or which were implemented before the introduction of an ERAS protocol.
Types of outcome measures
Primary outcomes
Length of postoperative hospital stay (i.e. the number of days from the day of surgery to discharge).
Postoperative complication rate within 30 days of operation, for example: acute confusion, nausea and vomiting, postoperative fever, secondary haemorrhage, atelectasis (i.e. the lack of gas exchange within alveoli owing to blood consolidation), pneumonia, wound infection, wound or anastomosis dehiscence (i.e. breakdown of the stitches), embolism and deep vein thrombosis, urinary retention, urinary tract infection, bowel obstruction owing to fibrinous adhesions, paralytic ileus, incisional hernia, persistent fistula (i.e. an abnormal connection or passageway between two organs or vessels that normally do not connect).
Early and late postoperative mortality from all causes (early mortality is defined as death within 30 days of operation; late mortality is defined as death within two to three months of operation).
Secondary outcomes
Readmission rate within 30 days of operation.
Bowel functions, including time to first flatus and time to first defaecation.
Quality of life, measured by a validated scale such as Medical Outcomes Study 36‐item Short Form Health Survey (SF‐36) and World Health Organization Quality of Life‐100 (WHOQOL‐100).
Participant satisfaction with hospital care.
Economic outcomes, including cost of care, direct, or indirect related costs.
Search methods for identification of studies
Electronic searches
We searched 11 electronic databases.
This search was run for the original review in 2010 and subsequent searches were run in May 2012, November 2014, and October 2020. The Cochrane Information Specialist helped to define the search strategies for this update (Appendix 1, Appendix 2, and Appendix 3) and searched the following electronic databases:
The Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 10);
MEDLINE via Ovid (1946 to October week 2, 2020);
Embase via Ovid (1980 to 2020, week 42).
We also searched other English databases including PubMed, AMED (Allied and Complementary Medicine), CINAHL (Cumulative Index to Nursing and Allied Health Literature), and Scopus in October 2020, and adapted the search strategies accordingly. Additionally, we searched four Chinese databases including the China Biomedical Literature Database (CBM), WanFang Data, China National Knowledge Infrastructure (CNKI), and Weipu Database. The search strategy for Chinese databases is shown in Appendix 4. There were no language, date, or publication status limitations for literature inclusion.
Searching other resources
Unpublished and Grey literature
We searched the following registries for ongoing trials: clinicaltrials.gov, www.who.int/clinical-trials-registry-platform/the-ictrp-search-portal, www.isrctn.com, and www.cancer.gov/about-cancer/treatment/clinical-trials/search. We contacted the main investigators of relevant trials for further information about the ongoing studies.
We also searched the following grey literature databases for relevant sources such as reports, dissertations, and conference abstracts: www.greynet.org/opensiglerepository.html, www.wolterskluwer.com/en/solutions/ovid/99, and www.ntis.gov.
Handsearching
We handsearched the reference lists of all relevant trials obtained by the searches to identify any further trials.
Data collection and analysis
Selection of studies
We downloaded all records retrieved from the electronic databases and imported them into Covidence, a Cochrane‐recommended tool to support authors in the stages of screening and data collection. After removing duplicates, two review authors (XL and JZ) screened the titles and abstracts independently. They excluded any studies that clearly did not meet the inclusion criteria and obtained copies of the full text of potentially relevant references. Two review authors (XL and JZ) then independently examined the full texts to assess their eligibility, resolving any disagreements by discussion between themselves. If consensus could not be reached, we included a third reviewer (JPC) to make recommendations for potential eligibility. The review authors documented reasons for exclusion. We drew a PRISMA flow diagram to display the selection process (Figure 1).
1.
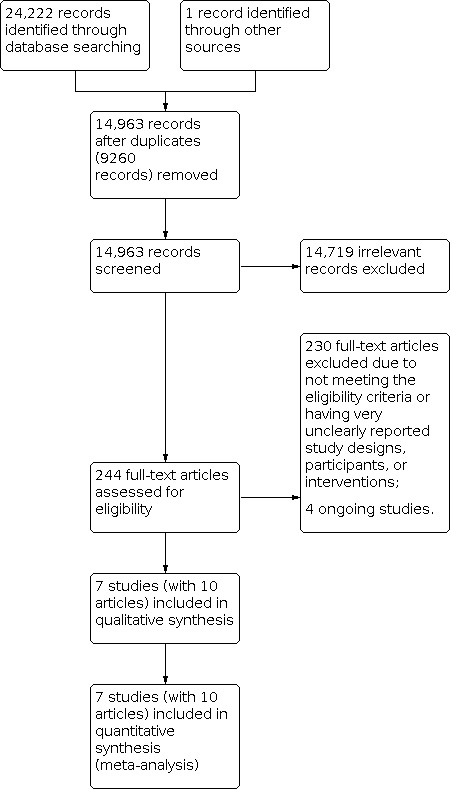
Study flow diagram
Data extraction and management
We developed a data extraction list according to the Cochrane Handbook for Systematic Reviews of Interventions (Li T 2019), and embedded it in Covidence. Two review authors (XL and JZ) independently extracted the following data from the included studies.
Study information: source of funding; duration, timing and location of the trial; author's contact details; study registration information; and potential conflicts of interest.
Methods: study design; details of randomisation and blinding; and methods used to address missing data.
Participants: inclusion and exclusion criteria; group differences in participants' demographic characteristics (e.g. age, socioeconomic status) and the prognostic factors for surgical recovery (e.g. smoking, comorbidities, American Society of Anaesthesiology (ASA) classification, and obesity) at baseline; type of gynaecological cancer; and type of surgical procedure.
Intervention and control: components of ERAS programmes according to the guidelines from the ERAS® Society (Nelson 2019); perioperative care in the control group; compliance with the interventions; and other identical perioperative co‐interventions in both groups.
Outcomes: prespecified primary and secondary outcomes, including their definitions, measurement tools and timing of outcome measurements; and the raw data of the outcomes.
We compared the extracted data and resolved any differences by discussion, or by including a third review author (SHL) if necessary. We attempted to contact authors to obtain missing or unclear information when necessary.
For dichotomous outcomes, we extracted the number of participants experiencing the event and the totals in each group of the trials. For continuous outcomes, we extracted the means with standard deviations (SDs). For some outcomes reported as medians with ranges or interquartile ranges (IQRs), we transformed the medians to means with SDs by applying the formulae provided by Luo 2018 and Wan 2014. To facilitate comparisons between trials, we converted variables that could have been reported in different metrics to a common metric. For example, we converted hours to days.
Assessment of risk of bias in included studies
Two review authors (XL and JZ) independently assessed the risk of bias of the included RCTs for all primary and secondary outcomes prespecified in the section Types of outcome measures. They resolved any disagreements by discussion between themselves or by involvement of a third review author (SHL). They assessed the risk of bias using an Excel tool developed from version two of the Cochrane risk of bias tool for randomised trials (RoB 2) and the criteria specified in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
This included assessment of the following domains. The first domain was assessed at the study level, and the other four domains were assessed at the outcome level.
Bias arising from the randomisation process. This domain addresses issues about the allocation sequence, allocation concealment, and baseline differences between intervention and control groups that may suggest a problem with the randomisation process.
Bias due to deviations from intended interventions. In this review, the effect of interest is that of assignment to intervention. Therefore, we addressed the issues of whether the participants and the intervention providers delivering the interventions were aware of participants' assigned intervention during the trial, and whether there were deviations that did not reflect usual practice and affected the outcome.
Bias due to missing outcome data. This domain addresses whether the data for outcomes are available for all, or nearly all, participants randomised and, if applicable, the proportions of or the reasons for missingness in the outcomes.
Bias in measurement of the outcome. This domain addresses the appropriateness of measurement tools, measurement differences across groups, and the blinding of outcome assessors.
Bias in selection of the reported result. This domain addresses whether the analyses are in accordance with the protocols and whether there is selective reporting of a particular outcome or analysis.
There is a series of 'signalling questions' with five response options for each domain: 'yes', 'probably yes', 'probably no', 'no', and 'no information'. Two review authors answered the signalling questions according to the instructions in the Cochrane Handbook (Higgins 2019), and the guideline for the RoB 2 tool (riskofbias.info/). We based our judgements on the risk of bias for each individual domain and the overall risk of bias for a result on algorithms that map responses to the signalling questions or the risk of bias judgements within domains to an overall judgement for a domain or a result. Possible judgments were: 'low risk of bias', 'some concerns', or 'high risk of bias'. We also recorded the supporting information for each judgement made. Responses to each signalling question within each domain across different outcomes are stored as supplemental files and deposited in an online repository.
Results are summarised in the risk of bias section of this review (located after the Characteristics of included studies) and in traffic lights on the forest plots. We interpreted the results of meta‐analyses in light of the findings with respect to risk of bias for the included studies.
Measures of treatment effect
For continuous outcomes (i.e. length of postoperative hospital stay, time to first flatus and time to first defaecation indicating bowel function, participant satisfaction, and cost) we analysed data reported using the same measures with the mean difference (MD), and used the standardised mean difference (SMD) when measures were different. For dichotomous outcomes (i.e. complications, death, and readmission), we used the risk ratio (RR) to present results. We did not identify ordinal outcome data or time‐to‐event outcome data in the included studies. We used a 95% confidence interval (CI) for all measures of treatment effect to reflect the uncertainty of the summary estimates.
Unit of analysis issues
The unit of analysis was individuals. We did not identify any cluster‐RCTs in this systematic review.
Dealing with missing data
Missing outcome data from individual participants
We attempted to extract data from intention‐to‐treat analyses or for the outcomes among participants who were assessed at end point. We analysed only the available data and addressed the risk of bias due to incomplete outcome data in the risk of bias tool (RoB 2).
Missing summary data
Summary data for an outcome in a form that can be included in a meta‐analysis, for example, the SDs for continuous outcomes, may be missing. We obtained the missing data from other statistics, including standard errors, CIs, statistics, and P values. We also imputed the means and SDs from the medians with ranges and IQRs (see Data extraction and management). We performed sensitivity analyses to assess the results by removing studies with imputed means and SDs.
Missing study‐level characteristics for subgroup analyses
We conducted subgroup analyses by grouping studies with different characteristics. If necessary information was not available for some studies, we contacted the investigators to request missing data whenever possible and addressed the potential impact on the findings of the review in the discussion section.
Assessment of heterogeneity
We assessed heterogeneity between studies by visual inspection of forest plots and a formal statistical test of the significance of the heterogeneity. We employed I2statistic methods. We assumed the level of heterogeneity in the outcomes was substantial when the estimate of I2 reached 50%, and considerable when I2 reached 75% (Higgins 2019). If there was evidence of substantial heterogeneity, we investigated the possible reasons for this by subgroup analyses (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
We searched the protocols of every included study report to detect selective non‐reporting biases. If we could not find the protocols, we compared outcomes stated in the method section with the outcomes reported in the result section of the studies. When there were 10 or more studies included in a meta‐analysis, we planned to use funnel plots to assess the possibility that the results from studies with small‐sized or insignificant effects were missing from the meta‐analysis (Page 2019).
Data synthesis
If sufficient (usually two or more) clinically similar studies were available, we pooled their results in meta‐analyses. We considered that the intervention effects across studies had minimal similarity, so we used random‐effects models with inverse variance weighting for all meta‐analyses. For studies that could not be included in the meta‐analysis due to heterogeneity, we described the results narratively.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses if possible, grouping the trials by:
different types of surgical procedures (i.e. laparotomy versus laparoscopic surgery);
different types of gynaecological cancers;
different ERAS programmes with different ERAS components.
We considered factors such as age, stages of cancer, length of follow‐up and adjusted/unadjusted analysis in the interpretation of any heterogeneity.
Sensitivity analysis
We identified some studies that included a proportion of participants who did not meet the eligibility criteria (diagnosed as benign tumour). Thus, we conducted sensitivity analyses by removing studies with mixed participants to test whether the pooled results were influenced. To deal with missing summary data such as means with SDs, we imputed the data and used them in meta‐analyses. We performed sensitivity analyses to assess the results by removing studies with imputed data (see Dealing with missing data). We also carried out sensitivity analyses by excluding studies with high risk of bias.
Summary of findings and assessment of the certainty of the evidence
Two review authors (XL and JZ) independently assessed the certainty of the evidence with the GRADE profiler Guideline Development Tool (GRADEpro GDT). They resolved any disagreements by discussion or by involvement of a third review author (JPC). We categorised the certainty of the evidence as high, moderate, low, or very low, according to the assessment of its risk of bias, inconsistency, imprecision, publication bias, and magnitude of the effect. We included the following outcomes in the main summary of findings table: length of postoperative hospital stay, postoperative complications within 30 days of operation, mortality within 30 days of discharge, readmission within 30 days of operation, time to first flatus, time to first defaecation, participant satisfaction, and economic outcomes (Table 1). We have presented the evidence for the other outcomes related to postoperative complications (such as nausea and vomiting) in the additional Table 2. We exported the summary of findings tables from GRADEpro GDT.
1. GRADE assessment results on evidence about postoperative complications within 30 days of operation.
| Enhanced recovery after surgery programme compared to traditional care for women with gynaecological cancers | ||||||
|
Patient or population: women with
gynaecological cancers Setting: hospital‐based gynaecological cancer perioperative care Intervention: enhanced recovery after surgery programme Comparison: traditional care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | What this means | |
| Risk with traditional care | Risk with enhanced recovery after surgery programme | |||||
| Postoperative acute confusion | 20 per 1000 | 20 per 1000 (1 to 311) | RR 0.98 (0.06 to 15.23) | 99 (1 RCT) | ⊕⊝⊝⊝ very low a,b | The evidence is very uncertain about the effect of the ERAS programme on postoperative acute confusion. |
| Postoperative nausea and vomiting | 257 per 1000 | 134 per 1000 (85 to 216) | RR 0.52 (0.33 to 0.84) | 642 (6 RCTs) | ⊕⊕⊕⊝ moderate c | ERAS programmes likely reduced postoperative nausea and vomiting. |
| Postoperative fever | 167 per 1000 | 133 per 1000 (40 to 448) | RR 0.80 (0.24 to 2.69) | 60 (1 RCT) | ⊕⊝⊝⊝ very low a,b,d | The evidence is very uncertain about the effect of the ERAS programme on postoperative fever. |
| Postoperative secondary haemorrhage | 13 per 1000 | 12 per 1000 (2 to 80) | RR 0.94 (0.14 to 6.29) | 309 (3 RCTs) | ⊕⊝⊝⊝ very low a,b | The evidence is very uncertain about the effect of ERAS programmes on postoperative secondary haemorrhage. |
| Postoperative pneumonia | 29 per 1000 | 12 per 1000 (2 to 79) | RR 0.41 (0.06 to 2.75) | 217 (2 RCTs) | ⊕⊝⊝⊝ very low a,b | The evidence is very uncertain about the effect of ERAS programmes on postoperative pneumonia. |
| Postoperative wound infection | 70 per 1000 | 9 per 1000 (1 to 161) | RR 0.13 (0.01 to 2.29) | 107 (1 RCT) | ⊕⊝⊝⊝ very low a,b | The evidence is very uncertain about the effect of the ERAS programme on postoperative wound infection. |
| Postoperative anastomosis dehiscence | 20 per 1000 | 40 per 1000 (4 to 427) | RR 1.96 (0.18 to 20.92) | 99 (1 RCT) | ⊕⊝⊝⊝ very low a,b | The evidence is very uncertain about the effect of the ERAS programme on postoperative anastomosis dehiscence. |
| Postoperative embolism and deep vein thrombosis | 28 per 1000 | 4 per 1000 (0 to 75) | RR 0.14 (0.01 to 2.64) | 206 (2 RCTs) | ⊕⊝⊝⊝ very low a,b | The evidence is very uncertain about the effect of ERAS programmes on postoperative embolism and deep vein thrombosis. |
| Postoperative ileus | 97 per 1000 | 55 per 1000 (29 to 104) | RR 0.57 (0.30 to 1.07) | 492 (4 RCTs) | ⊕⊕⊝⊝ low b,e | ERAS programmes result in no difference in postoperative ileus. |
| Postoperative colorectal anastomotic fistula | 20 per 1000 | 40 per 1000 (4 to 427) | RR 1.96 (0.18 to 20.92) | 99 (1 RCT) | ⊕⊝⊝⊝ very low a,b | The evidence is very uncertain about the effect of the ERAS programme on postoperative colorectal anastomotic fistula. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aImprecision downgraded two levels due to very small sample size or low incidence of events and inclusion of appreciable benefit and harm in confidence intervals. bPublication bias downgraded because data were reported by limited number of studies. cInconsistency downgraded because the pooled results changed substantially when excluding a small study with positive results (Ho 2020). dRisk of bias downgraded one level because of suspected deviations from intended interventions. eImprecision downgraded one level as confidence interval of pooled estimate includes appreciable benefit and no effect
Results
Description of studies
Studies that we included and excluded from this review, and ongoing studies, are described in tables (see Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies).
Results of the search
We initially obtained a total of 24,223 records from electronic searching and other resources. After removal of duplicates, two review authors screened 14,963 records independently. They sought a total of 244 full texts for detailed assessment after title and abstract screening. We excluded 230 full‐text articles and identified seven eligible studies (with 10 references) (Dickson 2017; Ferrari 2020; Ho 2020; Sánchez‐Iglesias 2020; Shi 2020; Zhang 2016; Zhou 2020), and four ongoing RCTs (ChiCTR1900025117; NCT02864277; NCT03640299; NCT04063072). See Figure 1 for the process of record screening and study selection.
Included studies
The review included seven studies with 747 participants. Details of the included studies are shown in the Characteristics of included studies table.
Three studies were conducted in China (Shi 2020; Zhang 2016; Zhou 2020), one in the USA (Dickson 2017), one in Spain (Sánchez‐Iglesias 2020), one in Italy (Ferrari 2020), and one in Malaysia (Ho 2020). The settings of the studies included the departments of gynaecologic oncology or the departments of obstetrics and gynaecology at university hospitals or medical centres (Dickson 2017; Ferrari 2020; Zhou 2020), comprehensive cancer centres (Ho 2020; Shi 2020), a general hospital (Zhang 2016), and a referral centre for gynaecologic oncology (Sánchez‐Iglesias 2020). All studies were conducted in a single centre. The studies took place between 2012 and 2019.
1. Design
We included seven RCTs (Dickson 2017; Ferrari 2020; Ho 2020; Sánchez‐Iglesias 2020; Shi 2020; Zhang 2016; Zhou 2020). All the RCTs were prospective parallel studies.
2. Participants
2.1 Age
All studies reported the mean ages of women, which were between 48.4 to 57.3 years old.
2.2 Diagnosis
Five studies only recruited participants with suspected or confirmed gynaecological cancer (Ho 2020; Sánchez‐Iglesias 2020; Shi 2020; Zhang 2016; Zhou 2020). The other two studies recruited participants with benign, borderline, or malignant gynaecologic tumours, of which the proportions of participants with malignant tumours were 51.1% (Ferrari 2020), and 64.1% (Dickson 2017). Of the five studies including participants with cancer, two studies only included participants with one type of cancer: ovarian cancer (Sánchez‐Iglesias 2020); and cervical cancer (Zhang 2016). Five studies consisted of participants with different types of cancer, including cervical cancer, uterine cancer, ovarian cancer, endometrial cancer, and carcinosarcoma (Dickson 2017; Ferrari 2020; Ho 2020; Shi 2020; Zhou 2020). Some researchers recruited specific groups of participants based on the stage of cancer (e.g. according to the International Federation of Gynecology and Obstetrics (FIGO) stages of gynaecological cancer); for example, Zhang 2016 only recruited participants with stage I‐II cancer; and Sánchez‐Iglesias 2020 recruited participants with advanced stages of cancer (FIGO stages IIb‐IVa, and relapses).
2.3 Type of surgery
Three studies only recruited participants undergoing laparotomy (Dickson 2017; Ho 2020; Sánchez‐Iglesias 2020). Two studies only recruited participants undergoing laparoscopic surgeries (Zhang 2016; Zhou 2020). Two studies included participants undergoing both laparotomies and laparoscopic surgeries in both the intervention and control groups (Ferrari 2020; Shi 2020).
2.4 Exclusion criteria
Reported exclusion criteria included participants with severe complications or dysfunctions (Ferrari 2020; Ho 2020; Sánchez‐Iglesias 2020; Shi 2020; Zhang 2016; Zhou 2020), undergoing emergency/unplanned surgeries (Dickson 2017; Ho 2020; Sánchez‐Iglesias 2020), with poor physical condition that put them at high risk from undergoing anaesthesia and surgery (i.e. American Society of Anesthesiologists level ≥ 4) (Dickson 2017; Ferrari 2020; Sánchez‐Iglesias 2020; Zhou 2020), receiving an anti‐tumour treatment such as radiotherapy or chemotherapy before operation (Zhang 2016; Zhou 2020), and undergoing surgery for vulval cancer (due to short hospital stay, usually < 1 day; Dickson 2017). For other details, see the Characteristics of included studies table.
3. Interventions
According to the 17 domains specified in the updated ERAS® Society guidelines that relate to ERAS pre‐, intra‐, and postoperative practices for gynaecological oncology (Nelson 2019), we depicted the exact ERAS components adopted in each of the included studies (Figure 2). ERAS components varied among included studies and the number of domains adopted ranged between 8 and 15 (median = 12). The most widely adopted domains (reported in all seven studies) were: preadmission information, education and counselling, preoperative fasting and carbohydrate treatment, early feeding after operation (i.e. perioperative nutrition domain), and early mobilisation after operation. Another five domains were adopted in six of the seven studies: limited preoperative mechanical bowel preparation, prevention of hypothermia, nausea and vomiting prophylaxis, perioperative fluid management/goal‐directed fluid treatment and opioid sparing postoperative analgesics. Only one study reported the intervention on prehabilitation in its ERAS pathway (Zhou 2020).
2.

ERAS domains adopted in the included studies (intervention group)
Four studies reported the level of compliance with ERAS components (Dickson 2017; Ferrari 2020; Ho 2020; Sánchez‐Iglesias 2020). Overall, postoperative compliance rates were generally high (usually ≥70%). Components with relatively lower compliance rates (< 70%) included avoidance of peritoneal drainage (61.4%) and energy intake on postoperative day 0 (65.1%), which were reported by Ferrari 2020.
As described in the included studies, three studies applied traditional care strategies (e.g. preoperative overnight fasting and mechanical bowel preparation) with no ERAS components (Dickson 2017; Sánchez‐Iglesias 2020; Zhang 2016). Four studies applied traditional care strategies with several ERAS components (in addition to minimally invasive surgery) but without an ERAS protocol (Ferrari 2020; Ho 2020; Shi 2020; Zhou 2020). The review authors addressed sufficient discrimination between the intervention and control groups in these four studies through displaying compliance to the intervention components or by adding an explanation for the situation.
4. Outcomes
The studies reported data for length of postoperative hospital stay, postoperative complications within 30 days of operation, mortality from all causes within 30 days of discharge (rather than our planned outcome of within 30 days of operation), readmission within 30 days of operation, bowel functions (including time to first flatus and time to first defaecation), participant satisfaction, and economic costs. No study reported the outcome that described five types of postoperative complications (i.e. urinary retention, urinary tract infection, incisional hernia, atelectasis, and wound dehiscence), and none reported quality of life.
Excluded studies
There were some studies that did not meet all the criteria, but which a reader might plausibly expect to see among the included studies; we selected and listed 28 excluded studies and detailed the primary reason for their exclusion in the Characteristics of excluded studies table. Two were excluded because less than 50% of the participants were diagnosed with gynaecological cancer (Miller 2015; Wijk 2014). Four studies examined only a single component of ERAS (Belavy 2013; Feng 2008; Gerardi 2008; Janda 2014), while two studies compared participant outcomes between the first year and the second year after the implementation of ERAS programmes (Mukhopadhyay 2015; Pather 2011). The remaining 20 studies were non‐RCTs.
Risk of bias in included studies
The risk of bias assessments for each outcome, including all domain judgements and support for judgement, are presented in the risk of bias section of this review (located after the Characteristics of included studies) and in traffic lights on the forest plots. To assess detailed responses to each signalling question within each domain across different outcomes, please use the following link (doi.org/10.7910/DVN/NRKF38).
Length of postoperative hospital stay
This outcome had a high overall risk of bias. Other than Ferrari 2020, all the studies reporting this outcome had a high risk of bias due to bias in measurement of outcomes (Ho 2020; Sánchez‐Iglesias 2020; Shi 2020; Zhang 2016; Zhou 2020 ), or deviations from intended interventions (Zhou 2020). We assessed the outcomes reported in Ferrari 2020, as well as Zhang 2016 and Zhou 2020, as having 'some concerns' about the randomisation process because they only reported the generation of allocation sequence and did not provide further details on allocation concealment.
Postoperative complications within 30 days of operation
Risk of bias of outcomes related to postoperative complications across all studies was similar and predominately assessed as having 'some concerns'. The only exception was postoperative fever (only reported in Zhou 2020), which we judged to be at high risk of bias due to deviations from intended interventions.
Early and late mortality from all causes within 30 days of operation
Only Sánchez‐Iglesias 2020 reported early mortality from all causes within 30 days of discharge. We assessed this outcome as having 'some concerns' due to deviations from intended interventions.
Readmission rate within 30 days of operation
This outcome had a high overall risk of bias due to bias in measurement of outcomes (Ho 2020; Sánchez‐Iglesias 2020). We also assessed this outcome as having 'some concerns' due to randomisation process (Ferrari 2020), and deviations from intended interventions (Sánchez‐Iglesias 2020).
Bowel function recovery
We assessed the time to first flatus and time to first defaecation, which are observer‐reported outcomes not involving judgement, as having 'some concerns' regarding randomisation process (Ferrari 2020; Zhang 2016; Zhou 2020), and reporting bias (Zhang 2016; Zhou 2020), and having 'some concerns' (Zhang 2016) or a high risk of bias (Zhou 2020) due to deviations from intended interventions.
Participant satisfaction with hospital care
We assessed the outcome as having a high overall risk of bias due to deviations from intended interventions (Zhou 2020), and having 'some concerns' on the randomisation process, measurement, and reporting bias.
Economic outcomes
We assessed the outcome as having a high overall risk of bias due to deviations from intended interventions and measurement of the outcomes (Shi 2020; Zhou 2020), and having 'some concerns' about the randomisation process and reporting bias (Zhou 2020).
Effects of interventions
See: Table 1
1. Length of postoperative hospital stay
All studies included data on length of postoperative hospital stay, abstracted from medical records and reported in different forms. Four studies reported the means with SDs (Ho 2020; Shi 2020; Zhang 2016; Zhou 2020), one reported the median with IQR (Ferrari 2020), one reported the median with range (Sánchez‐Iglesias 2020), and one reported the median with a 95% confidence interval (Dickson 2017).
In each of the included studies, women in the ERAS and control groups had identical discharge criteria. The criteria judged by the gynaecologists included: tolerance to solid foods without discomfort such as nausea and vomiting (in all seven studies), absence of complications such as fever and haemorrhage (in all seven studies), adequate control of pain (in six studies), ability to mobilise independently (in five studies), passage of stool or flatus (in four studies), and participants’ agreement to be discharged (in three studies).
ERAS programmes could result in a reduction in the length of postoperative hospital stay, with an MD of ‐1.71 days (95% CI ‐2.59 to ‐0.84; I2 = 86%; 6 studies, 638 participants; low‐certainty evidence; Analysis 1.1). However, considerable heterogeneity was detected. We conducted meta‐analyses because the directions of the effect were consistent in the majority of included studies. It was shown that ERAS programmes reduced length of postoperative hospital stay in women undergoing laparotomy (MD ‐1.92 days, 95% CI ‐2.99 to ‐0.85; I2 = 92%; 3 studies, 312 participants) and laparoscopic surgeries (MD ‐1.81, 95% CI ‐3.34 to ‐0.29; I2 = 82%; 3 studies, 219 participants), without significant subgroup differences detected (P = 0.91, I2= 0%; Analysis 1.2). The results of sensitivity analyses indicated that the pooled result was not substantially affected by the inclusion of a study with both benign and malignant oncology patients (Analysis 1.3), or introducing transformed mean values (Analysis 1.4). We did not pool the data Dickson 2017 because the median with 95% CI reported could not be transformed to mean with SD. Dickson 2017 found that there was no difference between the ERAS and traditional care groups in length of postoperative hospital stay in people undergoing laparotomy (median = 3.0 in both groups; P = 0.36).
1.1. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 1: Outcome 1: length of postoperative hospital stay
1.2. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 2: Outcome 1: length of postoperative hospital stay—subgroup analysis—surgery type
1.3. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 3: Outcome 1: length of postoperative hospital stay—sensitivity analysis 1 (excluding studies on participants with both benign and malignant tumours)
1.4. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 4: Outcome 1: length of postoperative hospital stay—sensitivity analysis 2 (excluding studies introducing transformed outcome value)
2. Postoperative complications
All seven studies reported postoperative complications within 30 days of operation. We did not find any studies that reported data on postoperative urinary retention, urinary tract infection, incisional hernia, atelectasis, or wound dehiscence, which were prespecified in the protocol.
2.1 Overall complications
Five studies reported an overall complication rate during primary stay and after surgery up to 30 days (Dickson 2017; Ferrari 2020; Sánchez‐Iglesias 2020; Shi 2020; Zhou 2020). We found that there might be no difference in overall complication rates with ERAS programmes within 30 days of operation (RR 0.71, 95% CI 0.48 to 1.05; I2 = 42%; 5 studies, 537 participants; low‐certainty evidence; Analysis 1.5) in both types of surgery (Analysis 1.6). The results of sensitivity analyses showed that the pooled result was consistent after excluding studies with participants with both benign and malignant tumours (Analysis 1.7).
1.5. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 5: Outcome 2.1: overall postoperative complications
1.6. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 6: Outcome 2.1: overall postoperative complications—subgroup analysis—surgery type
1.7. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 7: Outcome 2.1: overall postoperative complications—sensitivity analysis (excluding studies on participants with both benign and malignant tumours)
2.2 Acute confusion
One RCT reported the occurrence of acute confusion (i.e. delirium; Sánchez‐Iglesias 2020) and no significant difference was reported between groups (RR 0.98, 95% CI 0.06 to 15.23; 99 participants; very low‐certainty evidence; Analysis 1.8).
1.8. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 8: Outcome 2.2: postoperative complication—acute confusion
2.3 Nausea and vomiting
Six studies reported postoperative nausea and vomiting (emesis) (Dickson 2017; Ferrari 2020; Ho 2020; Shi 2020; Zhang 2016; Zhou 2020). ERAS programmes likely reduced the occurrence of postoperative nausea and vomiting (RR 0.52, 95% CI 0.33 to 0.84; I2 = 42%; 6 studies, 642 participants; moderate‐certainty evidence; Analysis 1.9). However, the subgroup analysis found ERAS programmes did not reduce the occurrence of postoperative nausea and vomiting in participants who underwent laparotomy (RR 0.54, 95% CI 0.19 to 1.51; I2 = 84%; 2 studies, 221 participants) or laparoscopic surgeries (RR 0.53, 95% CI 0.22 to 1.25; I2 = 0%; 2 studies, 146 participants), without significant subgroup differences detected (P = 0.97, I2= 0%; Analysis 1.10). The results of sensitivity analyses indicated that the pooled results were not affected by the inclusion of studies with participants with both benign and malignant tumours (Analysis 1.11).
1.9. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 9: Outcome 2.3: postoperative complication—nausea and vomiting
1.10. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 10: Outcome 2.3: postoperative complication—nausea and vomiting—subgroup analysis—surgery type
1.11. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 11: Outcome 2.3: postoperative complication—nausea and vomiting—sensitivity analysis (excluding studies on participants with both benign and malignant tumours)
2.4 Fever
Only one RCT (Zhou 2020) reported the effects of ERAS programmes on postoperative fever (RR 0.80, 95% CI 0.24 to 2.69; 60 participants; very low‐certainty evidence; Analysis 1.12). The evidence was very uncertain due to serious risk of bias, imprecision and publication bias.
1.12. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 12: Outcome 2.4: postoperative complication—fever
2.5 Secondary haemorrhage
Three studies reported the complication of postoperative secondary haemorrhage (Dickson 2017; Sánchez‐Iglesias 2020; Shi 2020). Pooled results indicated that the evidence on the effect of ERAS programmes on postoperative secondary haemorrhage was very uncertain, due to imprecision and publication bias (RR 0.94, 95% CI 0.14 to 6.29; I2 = 0%; 3 studies, 309 participants; very low‐certainty evidence; Analysis 1.13).
1.13. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 13: Outcome 2.5: postoperative complication—secondary haemorrhage
2.6 Pneumonia
Two studies reported the complication of postoperative pneumonia (Ho 2020; Sánchez‐Iglesias 2020). Pooled results indicated that the evidence on the effect of ERAS programmes on postoperative pneumonia was very uncertain, due to imprecision and publication bias (RR 0.41, 95% CI 0.06 to 2.75; I2 = 0%; 2 studies, 217 participants; very low‐certainty evidence; Analysis 1.14).
1.14. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 14: Outcome 2.6: postoperative complication—pneumonia
2.7 Wound infection
Only Shi 2020 reported the outcome of wound infection. The evidence on the effect of ERAS programmes on the occurrence rate of wound infection was very uncertain (RR 0.13, 95% CI 0.01 to 2.29; 1 study, 107 participants; very low‐certainty evidence; Analysis 1.15).
1.15. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 15: Outcome 2.7: postoperative complication—wound infection
2.8 Anastomosis dehiscence
Only Sánchez‐Iglesias 2020 reported the outcome of anastomosis dehiscence. The evidence on the effect of ERAS programmes on the occurrence of anastomosis dehiscence was very uncertain (RR 1.96, 95% CI 0.18 to 20.92; 1 study, 99 participants; very low‐certainty evidence; Analysis 1.16).
1.16. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 16: Outcome 2.8: postoperative complication—anastomosis dehiscence
2.9 Embolism and deep vein thrombosis
Two studies reported data on embolism and deep vein thrombosis (Sánchez‐Iglesias 2020; Shi 2020). The evidence on the effect of ERAS programmes on the occurrence rate of embolism and deep vein thrombosis was very uncertain (RR 0.14, 95% CI 0.01 to 2.64; 2 studies, 206 participants; very low‐certainty evidence; Analysis 1.17).
1.17. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 17: Outcome 2.9: postoperative complication—embolism and deep vein thrombosis
2.10 Ileus
Four studies reported data on postoperative ileus (Ferrari 2020; Ho 2020; Sánchez‐Iglesias 2020; Shi 2020). ERAS programmes may have no effect on the incidence of postoperative ileus (RR 0.57, 95% CI 0.30 to 1.07; I2 = 0%; 4 studies, 492 participants; low‐certainty evidence; Analysis 1.18). The pooled result was similar when excluding the study that included both participants with benign and malignant tumours from the sensitivity analysis (RR 0.65, 95% CI 0.34 to 1.26; I2 = 0%; 3 studies, 324 participants = 324; Analysis 1.19).
1.18. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 18: Outcome 2.10: postoperative complication—ileus
1.19. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 19: Outcome 2.10: postoperative complication—ileus—sensitivity analysis (excluding studies on participants with both benign and malignant tumours)
2.11 Colorectal anastomotic fistula
Only Sánchez‐Iglesias 2020 reported data on postoperative colorectal anastomotic fistula. The evidence was very uncertain about the effect of ERAS programmes on the rate of postoperative colorectal anastomotic fistula (RR 1.96, 95% CI 0.18 to 20.92; 1 study, 99 participants; very low‐certainty evidence; Analysis 1.20).
1.20. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 20: Outcome 2.11: postoperative complication—colorectal anastomotic fistula
3. Mortality
Only Sánchez‐Iglesias 2020 reported early mortality from all causes. The study reported this outcome as mortality within 30 days of discharge, rather than our planned outcome of within 30 days of operation. The evidence was very uncertain about the effect of ERAS programmes on all‐cause mortality within 30 days of discharge (RR 0.98, 95% CI 0.14 to 6.68; 1 study, 99 participants; very low‐certainty evidence; Analysis 1.21). There were no data on late mortality, which was defined as death within two to three months in all causes.
1.21. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 21: Outcome 3: mortality
4. Readmission
Three studies reported readmission rates within 30 days of operation (Ferrari 2020; Ho 2020; Sánchez‐Iglesias 2020). ERAS programmes may reduce the risk of readmission within 30 days of operation (RR 0.45, 95% CI 0.22 to 0.90; I2 = 0%; 3 studies, 385 participants; low‐certainty evidence; Analysis 1.22). The pooled result was similar when excluding the study whose participants had both benign and malignant tumours (RR 0.35, 95% CI 0.15 to 0.80; I2 = 0%; 2 studies, 217 participants; Analysis 1.23).
1.22. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 22: Outcome 4: readmission
1.23. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 23: Outcome 4: readmission—sensitivity analysis (excluding studies on participants with both benign and malignant tumours)
5. Bowel function
Five studies reported the outcomes related to bowel function, including time to first flatus and time to first defaecation.
5.1 Time to first flatus
Three studies reported the time to first flatus in means with SDs (Ho 2020; Zhang 2016; Zhou 2020), one reported only the mean (Ferrari 2020), and one reported the number of participants who had their first flatus on postoperative day one, day two, day three, and day four or beyond (Dickson 2017). ERAS programmes may result in a reduction in the time to first flatus (MD ‐0.82 days, 95% CI ‐1.00 to ‐0.63; I2 = 35%; 4 studies, 432 participants; low‐certainty evidence; Analysis 1.24). However, Dickson 2017, whose data could not be pooled with the other studies, reported no significant difference in the distributions of the time to first flatus. Subgroup analyses showed that ERAS programmes reduced the time to first flatus in participants who underwent laparotomy surgery (MD ‐1.03 days, 95% CI ‐1.30 to ‐0.76; 1 study, 118 participants) and laparoscopic surgery (MD ‐0.74 days, 95% CI ‐0.87 to ‐0.61; I2 = 0%; 2 studies, 146 participants), with significant subgroup differences detected (P = 0.06, I2= 71.7%; Analysis 1.25). The pooled results were substantially similar when excluding the study with both benign and malignant oncology participants (Analysis 1.26).
1.24. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 24: Outcome 5.1: bowel function—time to first flatus
1.25. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 25: Outcome 5.1: bowel function—time to first flatus—subgroup analysis—surgery type
1.26. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 26: Outcome 5.1: bowel function—time to first flatus—sensitivity analysis (excluding studies on participants with both benign and malignant tumours)
5.2 Time to first defaecation
As for the time to first defaecation, Zhou 2020 reported the mean with a SD and Ferrari 2020 reported the mean without a SD. ERAS programmes may reduce the time to first defaecation (MD ‐0.96 days, 95% CI ‐1.47 to ‐0.44; I2 = 0%; 2 studies, 228 participants; low‐certainty evidence; Analysis 1.27).
1.27. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 27: Outcome 5.2: bowel function—time to first defaecation
6. Participant satisfaction
Different measurement tools were used by Ferrari 2020 and Zhou 2020 to survey participant satisfaction with hospital care. Ferrari 2020 investigated participant satisfaction 24 hours after surgery and at discharge using Quality of Recovery‐15 (QoR‐15), which has 15 items with acceptable reliability and validity (Stark 2013). Zhou 2020 adopted a self‐developed 10‐item questionnaire surveying participant satisfaction with nursing service, competencies, health education, and ward management. The total scores ranged from 20 to 100. The Cronbach's alpha of the scale was 0.855 in Zhou 2020. Further information about the questionnaire was not reported in the literature.
The evidence was very uncertain about the effect of ERAS programmes on participant satisfaction due to serious risk of bias, imprecision, inconsistency and publication bias (SMD 0.92, 95% CI 0.06 to 1.78; I2 = 86%; 2 studies, 228 participants; very low‐certainty evidence; Analysis 1.28).
1.28. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 28: Outcome 6: participant satisfaction
7. Economic outcomes
Three studies reported economic outcomes (Sánchez‐Iglesias 2020; Shi 2020; Zhou 2020). Shi 2020 and Zhou 2020 measured the total hospital expenditures but did not provide detailed information on the constitution of the expenditures. The evidence was very uncertain about the effect of ERAS programmes on economic costs (SMD ‐0.22, 95% CI ‐0.68 to 0.25; I2 = 54%; 2 studies, 167 participants; very low‐certainty evidence; Analysis 1.29). However, one study that was not included in the pooled analysis due to lack of usable data regarding actual cost saving indicated that the ERAS protocol resulted in an annual cost reduction of 13,330 euros for 100 patients in the best scenario (Sánchez‐Iglesias 2020).
1.29. Analysis.

Comparison 1: ERAS programmes versus traditional care, Outcome 29: Outcome 7: economic outcomes
Discussion
Summary of main results
In this review, we searched for RCTs comparing ERAS programmes with traditional care strategies for women with gynaecological cancer after surgery. We included seven RCTs with 747 women. Primary outcomes reported in trials included length of postoperative hospital stay, postoperative complications, and mortality within 30 days of discharge. Secondary outcomes included readmission within 30 days of operation, return of bowel function postsurgery, participant satisfaction and cost.
Primary outcomes
ERAS programmes may result in a reduction in the length of postoperative hospital stay (MD ‐1.71 days, 95% CI ‐2.59 to ‐0.84; I2 = 86%; 6 studies, 638 participants; low‐certainty evidence).
ERAS programmes may result in no difference in overall postoperative complication rates (RR 0.71, 95% CI 0.48 to 1.05; I2 = 42%; 5 studies, 537 participants; low‐certainty evidence). As for the other postoperative complications, ERAS programmes likely reduced the occurrence of postoperative nausea and vomiting (RR 0.52, 95% CI 0.33 to 0.84; I2 = 42%; 6 studies, 642 participants; moderate‐certainty evidence); the evidence was very uncertain for other complications such as acute confusion, fever, second haemorrhage, pneumonia, wound infection, anastomosis dehiscence, embolism and deep vein thrombosis, and colorectal anastomotic fistula due to serious risk of bias, imprecision, and publication bias. ERAS programmes have no effect on the incidence of postoperative ileus (RR 0.57, 95% CI 0.30 to 1.07; I2 = 0%; 4 studies, 492 participants; low‐certainty evidence). The evidence was very uncertain about the effect of ERAS programmes on all‐cause mortality within 30 days of discharge.
Secondary outcomes
ERAS programmes may reduce readmission rates within 30 days of operation (RR 0.45, 95% CI 0.22 to 0.90; I2 = 0%; 3 studies, 385 participants; low‐certainty evidence).
ERAS programmes may facilitate recovery of postoperative bowel functions, as indicated by the reduction of time to first flatus (MD ‐0.82 days, 95% CI ‐1.00 to ‐0.63; I2 = 35%; 4 studies, 432 participants; low‐certainty evidence) and time to first defaecation (MD ‐0.96 days, 95% CI ‐1.47 to ‐0.44; I2 = 0%; 2 studies, 228 participants; low‐certainty evidence).
The evidence for the effects of ERAS programmes on participant satisfaction was very uncertain due to the limited number of studies found. Adoption of ERAS strategies might not increase medical expenditure. However, the evidence was of very low certainty (SMD ‐0.22, 95% CI ‐0.68 to 0.25; I2 = 54%; 2 studies, 167 participants).
Intervention compliance is important for multi‐component interventions such as ERAS. The overall compliance rates (reported in four out of seven studies) were generally high (usually ≥ 70%) with the exception of some components, such as early feeding and avoidance of peritoneal drainage. However, bias might exist since the low compliance rates of the interventions might not be reported by other researchers. Domains adopted in the majority of ERAS programmes included preadmission information, education and counselling, preoperative fasting and carbohydrate treatment, early feeding after operation (i.e. perioperative nutrition domain), early mobilisation after operation, limited preoperative mechanical bowel preparation, prevention of hypothermia, nausea and vomiting prophylaxis, perioperative fluid management/goal‐directed fluid treatment, and opioid sparing postoperative analgesia.
Overall completeness and applicability of evidence
The overall completeness and applicability of the evidence available for the effects of ERAS programmes for women with gynaecological cancer is currently inadequate. Caution is needed when interpreting the evidence.
We included seven RCTs and found four ongoing RCTs. Evidence was insufficient for determining the effects of ERAS programmes in women undergoing different types of surgeries or with different types of gynaecological cancers. Outcomes, especially postoperative complications, were only reported by a limited number of studies with very low incidence of events. Data on participant satisfaction and economic outcomes were sparse, and there were no data on quality of life.
Moreover, the domains included in different ERAS protocols varied across trials despite the adoption of common domains by researchers. We were unable to assess the effects of a single intervention domain or compare the effects of different combinations of intervention domains due to the limited number of trials. However, we deem it important to assess the ERAS components adopted because, as a multidisciplinary programme, ERAS is a considerable investment dependent on workforce and material resources needed to embed every domain of ERAS into clinical practice.
Quality of the evidence
We used the GRADE assessment tool to assess the certainty of evidence for each outcome, and downgraded due to risk of bias, inconsistency, imprecision, and publication bias.
Only four of the seven RCTs reported allocation concealment. The other three studies reported the generation of allocation sequence but did not provide further details on allocation concealment. With the exception of one study, which excluded the investigators from the clinical management of participants (Ferrari 2020), none of the other studies blinded the participants, intervention providers, or outcome assessors. Though it might be hard to adopt the blinding method in such trials due to the nature of the intervention, this may cause serious risk of performance bias and information bias, especially on some outcomes that reflected the judgement or the decision of outcome assessors. We could not find the prespecified study protocol of one RCT in which reporting biases might have occurred.
For length of postoperative hospital stay, time to first flatus, and participant satisfaction, we downgraded for inconsistency because the proportion of the variability in effect estimates due to true heterogeneity was considerable. Although we included two studies that recruited participants with benign, borderline, and malignant gynaecologic tumours, we did not downgrade the certainty of evidence in most cases due to indirectness because the results of sensitivity analyses showed that the overall results of the outcomes were not affected by the inclusion of participants with mixed types of cancer. We only downgraded the evidence on the time to first defaecation due to indirectness because the result changed when excluding one of the two included RCTs that enrolled participants with both benign and malignant tumours. For postoperative complications, mortality, participant satisfaction, and cost, we downgraded for imprecision because wide confidence intervals included appreciable benefit to no effect or harm, or the studies had very small sample sizes or low incidence of events. For postoperative complications, mortality, readmission, time to first flatus, time to first defaecation, participant satisfaction, and cost, we downgraded for publication bias indicated by the limited number of studies.
Potential biases in the review process
To prevent bias in the review process, the search was guided by the Cochrane Gynaecological Cancer Review Group. We also conducted a comprehensive search of the literature in other databases, which were not accessible to our Information Specialist. No restrictions such as language, date, or publication status were applied to the search. Two review authors independently undertook study screening and selection, data extraction, risk of bias assessment, and evidence certainty assessment in adherence with the Cochrane Handbook. The two independent review authors resolved any disagreements by discussion and included a third review author when consensus could not be reached.
However, it is possible that there is publication bias because we did not identify any studies in languages other than English and Chinese.
Agreements and disagreements with other studies or reviews
We found six systematic reviews related to our topic (De Groot 2016; Kalogera 2019; Lindemann 2017; Nelson 2014; Shakir 2017; Song 2020). Four reviews included women undergoing gynaecologic surgery for benign and malignant diseases, where the studies in women with gynaecological cancers constituted only a small proportion (De Groot 2016; Kalogera 2019; Nelson 2014; Song 2020). The other two reviews focused on women with ovarian cancer (Lindemann 2017; Shakir 2017). However, in Lindemann 2017, only 34% of the study participants had ovarian, fallopian tube, or peritoneal cancer. The Shakir 2017 only identified three non‐RCTs, which was far from adequate to draw a conclusion. Four reviews focused on open abdominal gynaecological surgeries, and the others used either minimally invasive surgery only (Kalogera 2019), or both laparotomy and laparoscopy surgery (Song 2020).
All reviews compared the effect of ERAS programmes to traditional care on outcomes such as overall length of postoperative hospital stay, postoperative complications, and readmission rates. De Groot 2016 and Song 2020 restricted inclusion to only studies investigating pathways that contained at least four ERAS components, which was similar to our review. Lindemann 2017 defined an ERAS programme as the inclusion of more than one component in a clinical pathway; the number of ERAS components identified in the included studies ranged from 3 to 14.
The studies included in these reviews were almost all non‐RCTs, while only a limited number of RCTs were found in the studies by Lindemann 2017 and Song 2020. However, those RCTs either addressed a single ERAS intervention rather than an ERAS protocol (Lindemann 2017), or ERAS interventions conducted in people with benign gynaecological diseases (Song 2020).
The findings of our review were in line with the aforementioned reviews: ERAS programmes may result in a reduction in the length of postoperative hospital stay (De Groot 2016; Kalogera 2019; Lindemann 2017; Nelson 2014; Shakir 2017; Song 2020), and shorten the time needed for postoperative bowel function recovery (Song 2020). However, ERAS programmes may have no apparent effect on postoperative complications and mortality rates (De Groot 2016; Kalogera 2019; Nelson 2014; Song 2020). Preadmission information, education and counselling, early feeding after operation, and early mobilisation after operation have been identified as the most commonly adopted ERAS components across studies included in both our review and the previous reviews. However, heterogeneity in the ERAS programmes and the paucity of high‐quality evidence limit clinical decision‐making on whether and how to adopt ERAS programmes for women with gynaecological cancers. Our review adds to the current evidence by using a systematic literature search to identify RCTs on ERAS programmes relevant to gynaecological cancer patients in order to provide up‐to‐date evidence needed to make timely healthcare decisions.
Authors' conclusions
Implications for practice.
The implementation of an enhanced recovery after surgery (ERAS) programme in perioperative care for women with gynaecological cancer is likely to be safe according to the findings of this updated review. ERAS may be effective in shortening length of postoperative hospital stay, accelerating the recovery of postoperative bowel functions, and reducing readmissions without increasing complications, death or expenses. However, these findings are based on evidence of moderate to very low certainty.
It is important to determine the components of an ERAS programme. Since the studies included in this review adopted various ERAS components in their programmes, healthcare institutions and providers should consider the adoption of one specific component carefully on the basis of more evidence and organisational context. It might be effective to include the following components that were widely implemented in the trials of this review: preadmission information, education and counselling, preoperative fasting and carbohydrate treatment, early feeding after operation, early mobilisation after operation, limited preoperative mechanical bowel preparation, prevention of hypothermia, nausea and vomiting prophylaxis, perioperative fluid management/goal‐directed fluid treatment, and opioid sparing postoperative analgesia. Organisational context should be taken into consideration when planning an ERAS programme, because the implementation of ERAS requires co‐ordination and co‐operation by a multidisciplinary team of gynaecologists, oncologists, anaesthesiologists, endocrinologists, dieticians, and nurses spanning the whole perioperative period.
The findings of this review, as well as a recent international survey, showed that some recommendations from ERAS guidelines, such as early feeding and avoidance of peritoneal drainage, had relatively low adherence (Bhandoria 2020). Effective measures such as personnel education, audit, and feedback taken to encourage compliance or adherence to ERAS components are also recommended. Previous studies suggested that improving nursing staff compliance with ERAS guidelines through effective measures such as education sessions could be a potential means to improve postoperative outcomes (Ardò 2018; Wickenbergh 2020).
Implications for research.
There were insufficient high quality studies in this area to generate informative evidence on the implementation of ERAS programmes. Further well‐designed and well‐conducted RCTs are expected. A prespecified intervention protocol is highly recommended.
More attention should be paid to outcome measurements, with particular emphasis on participant‐reported outcomes. In ERAS programmes, it is crucial for people undergoing surgery to receive information about why certain actions should be carried out, so as to encourage active participation in their own care (Aasa 2013). However, we found that participant‐reported outcomes such as satisfaction, quality of life, symptoms, and functional recovery outcomes in early, intermediate, and late postoperative phases are still lacking. Specifically, we did not find any long‐term outcomes that were measured beyond 30 days after operation, either reported by participants or healthcare providers. Furthermore, more data on cost‐effectiveness outcomes are needed to assess the economic impact of ERAS programme implementation. In an oncological setting, data on time to adjuvant treatment following surgery would also be useful, since reduction in delays to further treatment is likely to have a beneficial effect on patient outcomes. Effects on overall survival would also be interesting, although it is unlikely that large enough studies would be performed to reach adequate power.
What's new
| Date | Event | Description |
|---|---|---|
| 18 November 2020 | New citation required and conclusions have changed | Seven randomised controlled trials included. |
History
Protocol first published: Issue 1, 2010 Review first published: Issue 6, 2010
| Date | Event | Description |
|---|---|---|
| 9 April 2021 | New search has been performed | New author team. |
| 19 March 2015 | New search has been performed | Searched update November 2014. Text updated accordingly. |
| 19 March 2015 | New citation required but conclusions have not changed | No new studies identified for inclusion. |
| 5 November 2012 | New citation required but conclusions have not changed | No new studies identified. |
| 1 May 2012 | New search has been performed | A new search was run in May 2012. |
Risk of bias
Risk of bias for analysis 1.1 Outcome 1: length of postoperative hospital stay.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Ferrari 2020 | Some concerns | Quote: "We randomly assigned patients to undergo standard
perioperative care (SPC) or ERAS protocol." "There were
no baseline differences between the 2 study groups." The authors just mentioned randomisation without providing detailed information about the randomisation process and allocation concealment method. |
Low risk of bias | Quote: "A randomised trial on ERAS is difficult to
perform because running SPC and ERAS protocol
simultaneously carries the risk of mixing the elements.
Hence, to avoid any unintentional biases, the
investigators were excluded from the clinical management
of the patients. Furthermore, we split the surgical,
anaesthesiological, and nurse teams involved in the
study, without the possibility of changes in the team's
composition, and we enrolled the patients minimising the
overlapping of the hospitalisation." The intervention providers took measures to avoid deviations. In addition, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. |
Low risk of bias | Quote: "We excluded from analysis 2 patients in the SPC
group and 6 patients in the ERAS protocol group either
because they withdrew the consent before receiving the
allocated treatment or because we planned the surgery
for the wrong week." Data were available for nearly all participants randomised and results were not likely biased by missing data. |
Low risk of bias | Quote: "The investigators were excluded from the clinical management of the patients." | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT03347409). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Lack of information on randomisation. |
| Ho 2020 | Low risk of bias | Quote: "Consenting subjects were randomised into two
groups before baseline assessment: intervention group
(CHO‐P) and control group (CO). Randomization was done
by a computer‐generated number randomisation which was
prepared by an independent statistician. The allocation
of randomised numbers was concealed in sealed envelopes
by the study coordinator. The envelope was only opened
after consent and before the baseline assessment."
"There was no statistically significant difference among
any baseline variables between groups." The allocation sequence was random and concealed until participants were enrolled and assigned to interventions. The baseline differences between intervention and control groups did not suggest a problem with the randomisation process. |
Low risk of bias | Quote: "All randomised RCT subjects were included in
analysis on an intention‐to‐treat(ITT) basis. " The interventions for the two groups were very clear. It is likely that no deviations from the intended intervention arose because of the experimental context. An appropriate analysis was used to estimate the effect of assignment to intervention. |
Low risk of bias | The data were available for all participants randomised. | High risk of bias | The length of stay reflected the decisions made by the intervention provider, where recording of the decisions does not involve any judgement, but where the decision itself can be influenced by knowledge of intervention received. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT03667755). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | High risk of bias | Blinding of outcome assessors. |
| Sánchez‐Iglesias 2020 | Low risk of bias | Quote: "Simple randomisation was carried out (by a
hospital staff member blinded to the study) using
computer generated random allocation for the ERAS or CM
group and concealed in a numbered, opaque envelope.
Participants were then randomised (i.e. given an
envelope) to either the ERAS or the CM arm during the
preoperative visit." "Both groups were similar with
respect to demographic data, including age, body mass
index and CC index, as well as for surgery indication,
histological type and FIGO stage." The allocation sequence was random and concealed until participants were enrolled and assigned to interventions. The baseline differences between intervention and control groups did not suggest a problem with the randomisation process. |
Some concerns | The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. However, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. | Low risk of bias | Quote: "Eleven patients did not receive laparotomic
cytoreductive surgery and were excluded from the study
after randomisation: one experienced an episode of
Takotsubo cardiomyopathy (before laparoscopy), four had
unresectable tumours and six had initial stages of the
disease and underwent minimally incisive surgery." Data were reasonably complete. Eleven patients were excluded after randomisation because they were identified as ineligible. |
High risk of bias | The length of stay reflected the decisions made by the intervention provider, where recording of the decisions does not involve any judgement, but where the decision itself can be influenced by knowledge of intervention received. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT02172638). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | High risk of bias | Blinding of participants, providers, and outcome assessors. |
| Shi 2020 | Low risk of bias | Quote: "Computer‐generated random numbers and list of any factors for stratification. To reduce predictability of a random sequence, details of any planned restriction should be provided in a separate document that is unavailable to those who enroll participants or assign interventions." | Some concerns | Quote: "Data collectors are not involved in the clinical
management of patients to ensure statistical validity
and reliability." "The surgical technique is
standardised for the treatment team, and patients'
families are not blinded to the study." "Operating
surgeons, attending physicians, nursing staff, and
patients and families cannot be blinded in this study,
as the procedures differ between groups; however,
outcome assessors will not be blinded." "All surgeries
are performed by the same team of surgeons, and patients
are treated and nursed by the same treatment team during
the pre‐operative period." "In the full analysis set,
patients will be analysed as randomised according to the
intentionto‐treat principle. The intention‐to‐treat
principle implies that the analysis includes all
randomised patients. The per protocol analysis set will
include all patients without major protocol deviation.
Deviations from the protocol will be assessed as major
or minor, and patients with major deviations from the
protocol will be excluded from the per protocol
analysis." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. |
Low risk of bias | Quote: "In the full analysis set, patients will be
analysed as randomised according to the
intention‐to‐treat principle." Data were reasonably complete. |
High risk of bias | The length of stay reflected the decisions made by the intervention provider, where recording of the decisions does not involve any judgement, but where the decision itself can be influenced by knowledge of intervention received. | Low risk of bias | Protocol registered in clinicaltrial.gov: NCT02687412; A protocol published in 2016: Cui L, Shi Y, Zhang GN (2016). Fast‐track surgery after gynaecological oncological surgery: study protocol for a prospective randomised controlled trial. Trials, 17:597. | High risk of bias | Blinding of participants, providers, and outcome assessors. |
| Zhang 2016 | Some concerns | The allocation sequence was randomised using a random number table. However, no detailed information about allocation sequence concealing was reported. | Some concerns | The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. | Low risk of bias | Data were reasonably complete. | High risk of bias | The length of stay reflected the decisions made by the intervention provider, where recording of the decisions does not involve any judgement, but where the decision itself can be influenced by knowledge of intervention received. | Some concerns | No pre‐registered protocol or a pre‐specified statistical analysis plan was found. However, the outcome measurements and analyses were clearly defined. There is no indication of selection of the reported analysis from among multiple analyses or selection of the cohort or subgroups for analysis and reporting on the basis of the results. | High risk of bias | Lack of information on randomisation, blinding of participants, providers, and outcome assessors, and no pre‐registered protocol or statistical plan. |
| Zhou 2020 | Some concerns | The allocation sequence was randomised using a random number table. However, no detailed information about allocation sequence concealment was reported. | High risk of bias | Quote: "Advertisements were put out all over the
department, which may cause deviations from the intended
intervention." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. Participants from different groups lived in the same department. Due to the advertisements posted all over the department, there is evidence, or strong reason to believe, that the trial context led to failure in implementing the protocol interventions in the control group and instead led to the implementation of interventions not allowed by the protocol. |
Low risk of bias | Data were reasonably complete. | High risk of bias | The length of stay reflected the decisions made by the intervention providers, where recording of the decisions does not involve any judgement, but where the decision itself can be influenced by knowledge of intervention received. | Some concerns | No pre‐registered protocol or statistical analysis plan was found. However, the outcome measurements and analyses were clearly defined. There is no indication of selection of the reported analysis from among multiple analyses or selection of the cohort or subgroups for analysis and reporting on the basis of the results. | High risk of bias | Failure in implementing the protocol interventions due to trial context, lack of information on randomisation, blinding of participants, providers, and outcome assessors, and no pre‐registered protocol or statistical plan. |
Risk of bias for analysis 1.5 Outcome 2.1: overall postoperative complications.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Dickson 2017 | Some concerns | Quote: "Patients were randomised (1:1) at the time of
preoperative evaluation to receive either enhanced
recovery after surgery or usual care using blocked
randomisation with varying block sizes and sequentially
numbered sealed opaque envelopes to mitigate selection
bias." The randomisation process was clearly described and conducted. However, significant difference on the presence of "other malignancy" was found. |
Some concerns | Quote: "All patients were counselled about the potential
risks and benefits of the enhanced recovery after
surgery protocol before being offered enrolment." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. |
Low risk of bias | Data were reasonably complete. Nine patients were excluded after randomisation because they were confirmed as ineligible. | Some concerns | The outcomes were according to the records of intervention providers, who were not blinded and might involve some judgement on clinical definitions. | Low risk of bias | The data were analysed in accordance with a prespecified analysis plan (clinicaltrial.gov: NCT01705288). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Baseline imbalances and blinding of participants, providers, and outcome assessors. |
| Ferrari 2020 | Some concerns | Quote: "We randomly assigned patients to undergo standard
perioperative care (SPC) or ERAS protocol." "There were
no baseline differences between the 2 study groups." The authors just mentioned randomisation without providing detailed information about the randomisation process and allocation concealment method. |
Low risk of bias | Quote: "A randomised trial on ERAS is difficult to
perform because running SPC and ERAS protocol
simultaneously carries the risk of mixing the elements.
Hence, to avoid any unintentional biases, the
investigators were excluded from the clinical management
of the patients. Furthermore, we split the surgical,
anaesthesiological, and nurse teams involved in the
study, without the possibility of changes in the team's
composition, and we enrolled the patients minimising the
overlapping of the hospitalisation." The intervention providers took measures to avoid deviations. In addition, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. |
Low risk of bias | Quote: "We excluded from analysis 2 patients in the SPC
group and 6 patients in the ERAS protocol group either
because they withdrew the consent before receiving the
allocated treatment or because we planned the surgery
for the wrong week." Data were available for nearly all participants randomised and results were not likely biased by missing data. |
Low risk of bias | Quote: "The investigators were excluded from the clinical management of the patients." | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT03347409). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Lack of information on randomisation. |
| Sánchez‐Iglesias 2020 | Low risk of bias | Quote: "Simple randomisation was carried out (by a
hospital staff member blinded to the study) using
computer generated random allocation for the ERAS or CM
group and concealed in a numbered, opaque envelope.
Participants were then randomised (i.e. given an
envelope) to either the ERAS or the CM arm during the
preoperative visit." "Both groups were similar with
respect to demographic data, including age, body mass
index and CC index, as well as for surgery indication,
histological type and FIGO stage." The allocation sequence was random and concealed until participants were enrolled and assigned to interventions. The baseline differences between intervention and control groups did not suggest a problem with the randomisation process. |
Some concerns | The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. However, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. | Low risk of bias | Quote: "Eleven patients did not receive laparotomic
cytoreductive surgery and were excluded from the study
after randomisation: one experienced an episode of
Takotsubo cardiomyopathy (before laparoscopy), four had
unresectable tumours and six had initial stages of the
disease and underwent minimally incisive surgery." Data were reasonably complete. Eleven patients were excluded after randomisation because they were identified as ineligible. |
Some concerns | The outcomes were according to the records of intervention providers who were not blinded and might involve some judgement on clinical definitions. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT02172638). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | The participants, providers, and outcome assessors were not blinded to the intervention. |
| Shi 2020 | Low risk of bias | Quote: "Computer‐generated random numbers and list of any factors for stratification. To reduce predictability of a random sequence, details of any planned restriction should be provided in a separate document that is unavailable to those who enroll participants or assign interventions." | Some concerns | Quote: "Data collectors are not involved in the clinical
management of patients to ensure statistical validity
and reliability." "The surgical technique is
standardised for the treatment team, and patients'
families are not blinded to the study." "Operating
surgeons, attending physicians, nursing staff, and
patients and families cannot be blinded in this study,
as the procedures differ between groups; however,
outcome assessors will not be blinded." "All surgeries
are performed by the same team of surgeons, and patients
are treated and nursed by the same treatment team during
the pre‐operative period." "In the full analysis set,
patients will be analysed as randomised according to the
intention‐to‐treat principle. The intention‐to‐treat
principle implies that the analysis includes all
randomised patients. The per protocol analysis set will
include all patients without major protocol deviation.
Deviations from the protocol will be assessed as major
or minor, and patients with major deviations from the
protocol will be excluded from the per protocol
analysis." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. |
Low risk of bias | Quote: "In the full analysis set, patients will be
analysed as randomised according to the
intention‐to‐treat principle." Data were reasonably complete. |
Some concerns | The outcomes were according to the records of intervention providers, who were not blinded and might involve some judgement on clinical definitions. | Low risk of bias | Protocol registered in clinicaltrial.gov: NCT02687412; A protocol published in 2016: Cui L, Shi Y, Zhang GN (2016). Fast‐track surgery after gynaecological oncological surgery: study protocol for a prospective randomised controlled trial. Trials, 17:597. | Some concerns | The participants, providers, and outcome assessors were not blinded to the intervention. |
| Zhou 2020 | Some concerns | The allocation sequence was randomised using a random number table. However, no detailed information about allocation sequence concealment was reported. | High risk of bias | Quote: "Advertisements were put out all over the
department, which may cause deviations from the intended
intervention." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. Participants from different groups lived in the same department. Due to the advertisements posted all over the department, there is evidence, or strong reason to believe, that the trial context led to failure in implementing the protocol interventions in the control group and instead led to the implementation of interventions not allowed by the protocol. |
Low risk of bias | Data were reasonably complete. | Some concerns | The outcomes were monitored by the surgical gynaecological team, who were not blinded and might involve some judgement on clinical definitions. | Some concerns | No pre‐registered protocol or statistical analysis plan was found. However, the outcome measurements and analyses were clearly defined. There is no indication of selection of the reported analysis from among multiple analyses or selection of the cohort or subgroups for analysis and reporting on the basis of the results. | High risk of bias | Failure in implementing the protocol interventions due to trial context, lack of information on randomisation, blinding of participants, providers, and outcome assessors, and no pre‐registered protocol or statistical plan. |
Risk of bias for analysis 1.8 Outcome 2.2: postoperative complication—acute confusion.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Sánchez‐Iglesias 2020 | Low risk of bias | Quote: "Simple randomisation was carried out (by a
hospital staff member blinded to the study) using
computer generated random allocation for the ERAS or CM
group and concealed in a numbered, opaque envelope.
Participants were then randomised (i.e. given an
envelope) to either the ERAS or the CM arm during the
preoperative visit." "Both groups were similar with
respect to demographic data, including age, body mass
index and CC index, as well as for surgery indication,
histological type and FIGO stage." The allocation sequence was random and concealed until participants were enrolled and assigned to interventions. The baseline differences between intervention and control groups did not suggest a problem with the randomisation process. |
Some concerns | The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. However, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. | Low risk of bias | Quote: "Eleven patients did not receive laparotomic
cytoreductive surgery and were excluded from the study
after randomisation: one experienced an episode of
Takotsubo cardiomyopathy (before laparoscopy), four had
unresectable tumours and six had initial stages of the
disease and underwent minimally incisive surgery." Data were reasonably complete. Eleven patients were excluded after randomisation because they were identified as ineligible. |
Some concerns | The outcomes were according to the records of intervention providers who were not blinded and might involve some judgement on clinical definitions. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT02172638). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Blinding of participants, providers, and outcome assessors. |
Risk of bias for analysis 1.9 Outcome 2.3: postoperative complication—nausea and vomiting.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Dickson 2017 | Some concerns | Quote: "Patients were randomised (1:1) at the time of
preoperative evaluation to receive either enhanced
recovery after surgery or usual care using blocked
randomisation with varying block sizes and sequentially
numbered sealed opaque envelopes to mitigate selection
bias." The randomisation process was clearly described and conducted. However, significant difference on the presence of "other malignancy" was found. |
Some concerns | Quote: "All patients were counselled about the potential
risks and benefits of the enhanced recovery after
surgery protocol before being offered enrolment." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. |
Low risk of bias | Data were reasonably complete. Nine patients were excluded after randomisation because they were confirmed as ineligible. | Some concerns | The reports on nausea came directly from participants about how they feel. This is potentially influenced by knowledge of intervention received. | Low risk of bias | The data were analysed in accordance with a prespecified analysis plan (clinicaltrial.gov: NCT01705288). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Baseline imbalances and blinding of participants, providers, and outcome assessors. |
| Ferrari 2020 | Some concerns | Quote: "We randomly assigned patients to undergo standard
perioperative care (SPC) or ERAS protocol." "There were
no baseline differences between the 2 study groups." The authors just mentioned randomisation without providing detailed information about the randomisation process and allocation concealment method. |
Low risk of bias | Quote: "A randomised trial on ERAS is difficult to
perform because running SPC and ERAS protocol
simultaneously carries the risk of mixing the elements.
Hence, to avoid any unintentional biases, the
investigators were excluded from the clinical management
of the patients. Furthermore, we split the surgical,
anaesthesiological, and nurse teams involved in the
study, without the possibility of changes in the team's
composition, and we enrolled the patients minimising the
overlapping of the hospitalisation." The intervention providers took measures to avoid deviations. In addition, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. |
Low risk of bias | Quote: "We excluded from analysis 2 patients in the SPC
group and 6 patients in the ERAS protocol group either
because they withdrew the consent before receiving the
allocated treatment or because we planned the surgery
for the wrong week." Data were available for nearly all participants randomised and results were not likely biased by missing data. |
Some concerns | The reports on nausea came directly from participants about how they feel. This is potentially influenced by knowledge of intervention received. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT03347409). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Lack of information on randomisation, blinding of outcome assessors. |
| Ho 2020 | Low risk of bias | Quote: "Consenting subjects were randomised into two
groups before baseline assessment: intervention group
(CHO‐P) and control group (CO). Randomization was done
by a computer‐generated number randomisation which was
prepared by an independent statistician. The allocation
of randomised numbers was concealed in sealed envelopes
by the study coordinator. The envelope was only opened
after consent and before the baseline assessment."
"There was no statistically significant difference among
any baseline variables between groups." The allocation sequence was random and concealed until participants were enrolled and assigned to interventions. The baseline differences between intervention and control groups did not suggest a problem with the randomisation process. |
Low risk of bias | Quote: "All randomised RCT subjects were included in
analysis on an intention‐to‐treat(ITT) basis. " The interventions for the two groups were very clear. It is likely that no deviations from the intended intervention arose because of the experimental context. An appropriate analysis was used to estimate the effect of assignment to intervention. |
Low risk of bias | The data were available for all participants randomised. | Some concerns | The outcomes were monitored by the surgical gynaecological team who were not blinded and might involve some judgement on clinical definitions. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT03667755). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Blinding of outcome assessors. |
| Shi 2020 | Low risk of bias | Quote: "Computer‐generated random numbers and list of any factors for stratification. To reduce predictability of a random sequence, details of any planned restriction should be provided in a separate document that is unavailable to those who enroll participants or assign interventions." | Some concerns | Quote: "Data collectors are not involved in the clinical
management of patients to ensure statistical validity
and reliability." "The surgical technique is
standardised for the treatment team, and patients'
families are not blinded to the study." "Operating
surgeons, attending physicians, nursing staff, and
patients and families cannot be blinded in this study,
as the procedures differ between groups; however,
outcome assessors will not be blinded." "All surgeries
are performed by the same team of surgeons, and patients
are treated and nursed by the same treatment team during
the pre‐operative period." "In the full analysis set,
patients will be analysed as randomised according to the
intention‐to‐treat principle. The intention‐to‐treat
principle implies that the analysis includes all
randomised patients. The per protocol analysis set will
include all patients without major protocol deviation.
Deviations from the protocol will be assessed as major
or minor, and patients with major deviations from the
protocol will be excluded from the per protocol
analysis." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. |
Low risk of bias | In the full analysis set, patients will be analysed as
randomised according to the intention‐to‐treat
principle." Data were reasonably complete. |
Some concerns | The reports on nausea came directly from participants about how they feel. This is potentially influenced by knowledge of intervention received. | Low risk of bias | Protocol registered in clinicaltrial.gov: NCT02687412; A protocol published in 2016: Cui L, Shi Y, Zhang GN (2016). Fast‐track surgery after gynaecological oncological surgery: study protocol for a prospective randomised controlled trial. Trials, 17:597. | Some concerns | Blinding of participants, providers, and outcome assessors. |
| Zhang 2016 | Some concerns | The allocation sequence was randomised using a random number table. However, no detailed information about allocation sequence concealing was reported. | Some concerns | The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. | Low risk of bias | Data were reasonably complete. | Some concerns | The reports on nausea came directly from participants about how they feel. This is potentially influenced by knowledge of intervention received. | Some concerns | No pre‐registered protocol or a pre‐specified statistical analysis plan was found. However, the outcome measurements and analyses were clearly defined. There is no indication of selection of the reported analysis from among multiple analyses or selection of the cohort or subgroups for analysis and reporting on the basis of the results. | Some concerns | Lack of information on randomisation, blinding of participants, providers, and outcome assessors, and no pre‐registered protocol for statistical plan. |
| Zhou 2020 | Some concerns | The allocation sequence was randomised using a random number table. However, no detailed information about allocation sequence concealment was reported. | High risk of bias | Quote: "Advertisements were put out all over the
department, which may cause deviations from the intended
intervention." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. Participants from different groups lived in the same department. Due to the advertisements posted all over the department, there is evidence, or strong reason to believe, that the trial context led to failure in implementing the protocol interventions in the control group and instead led to the implementation of interventions not allowed by the protocol. |
Low risk of bias | Data were reasonably complete. | Some concerns | The reports on nausea were coming directly from participants about how they feel. This is potentially influenced by knowledge of intervention received. | Some concerns | No pre‐registered protocol or statistical analysis plan was found. However, the outcome measurements and analyses were clearly defined. There is no indication of selection of the reported analysis from among multiple analyses or selection of the cohort or subgroups for analysis and reporting on the basis of the results. | High risk of bias | Lack of information on randomisation, blinding of participants, providers, and outcome assessors, failure in implementing the protocol interventions due to trial context, and no pre‐registered protocol or statistical plan. |
Risk of bias for analysis 1.12 Outcome 2.4: postoperative complication—fever.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Zhou 2020 | Some concerns | The allocation sequence was randomised using a random number table. However, no detailed information about allocation sequence concealment was reported. | High risk of bias | Quote: "Advertisements were put out all over the
department, which may cause deviations from the intended
intervention." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. Participants from different groups lived in the same department. Due to the advertisements posted all over the department, there is evidence, or strong reason to believe, that the trial context led to failure in implementing the protocol interventions in the control group and instead led to the implementation of interventions not allowed by the protocol. |
Low risk of bias | Data were reasonably complete. | Some concerns | The outcomes were monitored by the surgical gynaecological team, who were not blinded and might involve some judgement on clinical definitions. | Some concerns | No pre‐registered protocol or statistical analysis plan was found. However, the outcome measurements and analyses were clearly defined. There is no indication of selection of the reported analysis from among multiple analyses or selection of the cohort or subgroups for analysis and reporting on the basis of the results. | High risk of bias | Lack of information on randomisation, blinding of participants, providers, and outcome assessors, failure in implementing the protocol interventions due to trial context, and no pre‐registered protocol or statistical plan. |
Risk of bias for analysis 1.13 Outcome 2.5: postoperative complication—secondary haemorrhage.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Dickson 2017 | Some concerns | Quote: "Patients were randomised (1:1) at the time of
preoperative evaluation to receive either enhanced
recovery after surgery or usual care using blocked
randomisation with varying block sizes and sequentially
numbered sealed opaque envelopes to mitigate selection
bias." The randomisation process was clearly described and conducted. However, significant difference on the presence of "other malignancy" was found. |
Some concerns | Quote: "All patients were counselled about the potential
risks and benefits of the enhanced recovery after
surgery protocol before being offered enrolment." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. |
Low risk of bias | Data were reasonably complete. Nine patients were excluded after randomisation because they were confirmed as ineligible. | Some concerns | The outcomes were according to the records of intervention providers, who were not blinded and might involve some judgement on clinical definitions. | Low risk of bias | The data were analysed in accordance with a prespecified analysis plan (clinicaltrial.gov: NCT01705288). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Baseline imbalances and blinding of participants, providers, and outcome assessors. |
| Sánchez‐Iglesias 2020 | Low risk of bias | Quote: "Simple randomisation was carried out (by a
hospital staff member blinded to the study) using
computer generated random allocation for the ERAS or CM
group and concealed in a numbered, opaque envelope.
Participants were then randomised (i.e. given an
envelope) to either the ERAS or the CM arm during the
preoperative visit." "Both groups were similar with
respect to demographic data, including age, body mass
index and CC index, as well as for surgery indication,
histological type and FIGO stage." The allocation sequence was random and concealed until participants were enrolled and assigned to interventions. The baseline differences between intervention and control groups did not suggest a problem with the randomisation process. |
Some concerns | The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. However, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. | Low risk of bias | Data were reasonably complete. Eleven patients were excluded after randomisation because they were identified as ineligible. | Some concerns | The outcomes were according to the records of intervention providers who were not blinded and might involve some judgement on clinical definitions. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT02172638). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Blinding of participants, providers, and outcome assessors. |
| Shi 2020 | Low risk of bias | Quote: "Computer‐generated random numbers and list of any factors for stratification. To reduce predictability of a random sequence, details of any planned restriction should be provided in a separate document that is unavailable to those who enroll participants or assign interventions." | Some concerns | Quote: "Data collectors are not involved in the clinical
management of patients to ensure statistical validity
and reliability." "The surgical technique is
standardised for the treatment team, and patients'
families are not blinded to the study." "Operating
surgeons, attending physicians, nursing staff, and
patients and families cannot be blinded in this study,
as the procedures differ between groups; however,
outcome assessors will not be blinded." "All surgeries
are performed by the same team of surgeons, and patients
are treated and nursed by the same treatment team during
the pre‐operative period." "In the full analysis set,
patients will be analysed as randomised according to the
intention‐to‐treat principle. The intention‐to‐treat
principle implies that the analysis includes all
randomised patients. The per protocol analysis set will
include all patients without major protocol deviation.
Deviations from the protocol will be assessed as major
or minor, and patients with major deviations from the
protocol will be excluded from the per protocol
analysis." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. |
Low risk of bias | Data were reasonably complete. | Some concerns | The outcomes were according to the records of intervention providers, who were not blinded and might involve some judgement on clinical definitions. | Low risk of bias | Protocol registered in clinicaltrial.gov: NCT02687412; A protocol published in 2016: Cui L, Shi Y, Zhang GN (2016). Fast‐track surgery after gynaecological oncological surgery: study protocol for a prospective randomised controlled trial. Trials, 17:597. | Some concerns | Blinding of participants, providers, and outcome assessors. |
Risk of bias for analysis 1.14 Outcome 2.6: postoperative complication—pneumonia.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Ho 2020 | Low risk of bias | Quote: "Consenting subjects were randomised into two
groups before baseline assessment: intervention group
(CHO‐P) and control group (CO). Randomization was done
by a computer‐generated number randomisation which was
prepared by an independent statistician. The allocation
of randomised numbers was concealed in sealed envelopes
by the study coordinator. The envelope was only opened
after consent and before the baseline assessment."
"There was no statistically significant difference among
any baseline variables between groups." The allocation sequence was random and concealed until participants were enrolled and assigned to interventions. The baseline differences between intervention and control groups did not suggest a problem with the randomisation process. |
Low risk of bias | Quote: "All randomised RCT subjects were included in
analysis on an intention‐to‐treat(ITT) basis. " The interventions for the two groups were very clear. It is likely that no deviations from the intended intervention arose because of the experimental context. An appropriate analysis was used to estimate the effect of assignment to intervention. |
Low risk of bias | The data were available for all participants randomised. | Some concerns | The outcomes were monitored by the surgical gynaecological team who were not blinded and might involve some judgement on clinical definitions. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT03667755). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Blinding of outcome assessors. |
| Sánchez‐Iglesias 2020 | Low risk of bias | Quote: "Simple randomisation was carried out (by a
hospital staff member blinded to the study) using
computer generated random allocation for the ERAS or CM
group and concealed in a numbered, opaque envelope.
Participants were then randomised (i.e. given an
envelope) to either the ERAS or the CM arm during the
preoperative visit." "Both groups were similar with
respect to demographic data, including age, body mass
index and CC index, as well as for surgery indication,
histological type and FIGO stage." The allocation sequence was random and concealed until participants were enrolled and assigned to interventions. The baseline differences between intervention and control groups did not suggest a problem with the randomisation process. |
Some concerns | The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. However, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. | Low risk of bias | Data were reasonably complete. Eleven patients were excluded after randomisation because they were identified as ineligible. | Some concerns | The outcomes were according to the records of intervention providers who were not blinded and might involve some judgement on clinical definitions. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT02172638). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Blinding of participants, providers, and outcome assessors. |
Risk of bias for analysis 1.15 Outcome 2.7: postoperative complication—wound infection.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Shi 2020 | Low risk of bias | Quote: "Computer‐generated random numbers and list of any factors for stratification. To reduce predictability of a random sequence, details of any planned restriction should be provided in a separate document that is unavailable to those who enroll participants or assign interventions." | Some concerns | Quote: "Data collectors are not involved in the clinical
management of patients to ensure statistical validity
and reliability." "The surgical technique is
standardised for the treatment team, and patients'
families are not blinded to the study." "Operating
surgeons, attending physicians, nursing staff, and
patients and families cannot be blinded in this study,
as the procedures differ between groups; however,
outcome assessors will not be blinded." "All surgeries
are performed by the same team of surgeons, and patients
are treated and nursed by the same treatment team during
the pre‐operative period." "In the full analysis set,
patients will be analysed as randomised according to the
intention‐to‐treat principle. The intention‐to‐treat
principle implies that the analysis includes all
randomised patients. The per protocol analysis set will
include all patients without major protocol deviation.
Deviations from the protocol will be assessed as major
or minor, and patients with major deviations from the
protocol will be excluded from the per protocol
analysis." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. |
Low risk of bias | Data were reasonably complete. | Some concerns | The outcomes were according to the records of intervention providers, who were not blinded and might involve some judgement on clinical definitions. | Low risk of bias | Protocol registered in clinicaltrial.gov: NCT02687412; A protocol published in 2016: Cui L, Shi Y, Zhang GN (2016). Fast‐track surgery after gynaecological oncological surgery: study protocol for a prospective randomised controlled trial. Trials, 17:597. | Some concerns | Blinding of participants, providers, and outcome assessors. |
Risk of bias for analysis 1.16 Outcome 2.8: postoperative complication—anastomosis dehiscence.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Sánchez‐Iglesias 2020 | Low risk of bias | Quote: "Simple randomisation was carried out (by a
hospital staff member blinded to the study) using
computer generated random allocation for the ERAS or CM
group and concealed in a numbered, opaque envelope.
Participants were then randomised (i.e. given an
envelope) to either the ERAS or the CM arm during the
preoperative visit." "Both groups were similar with
respect to demographic data, including age, body mass
index and CC index, as well as for surgery indication,
histological type and FIGO stage." The allocation sequence was random and concealed until participants were enrolled and assigned to interventions. The baseline differences between intervention and control groups did not suggest a problem with the randomisation process. |
Some concerns | The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. However, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. | Low risk of bias | Data were reasonably complete. Eleven patients were excluded after randomisation because they were identified as ineligible. | Some concerns | The outcomes were according to the records of intervention providers who were not blinded and might involve some judgement on clinical definitions. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT02172638). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Blinding of participants, providers, and outcome assessors. |
Risk of bias for analysis 1.17 Outcome 2.9: postoperative complication—embolism and deep vein thrombosis.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Sánchez‐Iglesias 2020 | Low risk of bias | Quote: "Simple randomisation was carried out (by a
hospital staff member blinded to the study) using
computer generated random allocation for the ERAS or CM
group and concealed in a numbered, opaque envelope.
Participants were then randomised (i.e. given an
envelope) to either the ERAS or the CM arm during the
preoperative visit." "Both groups were similar with
respect to demographic data, including age, body mass
index and CC index, as well as for surgery indication,
histological type and FIGO stage." The allocation sequence was random and concealed until participants were enrolled and assigned to interventions. The baseline differences between intervention and control groups did not suggest a problem with the randomisation process. |
Some concerns | The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. However, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. | Low risk of bias | Data were reasonably complete. Eleven patients were excluded after randomisation because they were identified as ineligible. | Some concerns | The outcomes were according to the records of intervention providers who were not blinded and might involve some judgement on clinical definitions. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT02172638). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Blinding of participants, providers, and outcome assessors. |
| Shi 2020 | Low risk of bias | Quote: "Computer‐generated random numbers and list of any factors for stratification. To reduce predictability of a random sequence, details of any planned restriction should be provided in a separate document that is unavailable to those who enroll participants or assign interventions." | Some concerns | Quote: "Data collectors are not involved in the clinical
management of patients to ensure statistical validity
and reliability." "The surgical technique is
standardised for the treatment team, and patients'
families are not blinded to the study." "Operating
surgeons, attending physicians, nursing staff, and
patients and families cannot be blinded in this study,
as the procedures differ between groups; however,
outcome assessors will not be blinded." "All surgeries
are performed by the same team of surgeons, and patients
are treated and nursed by the same treatment team during
the pre‐operative period." "In the full analysis set,
patients will be analysed as randomised according to the
intention‐to‐treat principle. The intention‐to‐treat
principle implies that the analysis includes all
randomised patients. The per protocol analysis set will
include all patients without major protocol deviation.
Deviations from the protocol will be assessed as major
or minor, and patients with major deviations from the
protocol will be excluded from the per protocol
analysis." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. |
Low risk of bias | Data were reasonably complete. | Some concerns | The outcomes were according to the records of intervention providers, who were not blinded and might involve some judgement on clinical definitions. | Low risk of bias | Protocol registered in clinicaltrial.gov: NCT02687412; A protocol published in 2016: Cui L, Shi Y, Zhang GN (2016). Fast‐track surgery after gynaecological oncological surgery: study protocol for a prospective randomised controlled trial. Trials, 17:597. | Some concerns | Blinding of participants, providers, and outcome assessors. |
Risk of bias for analysis 1.18 Outcome 2.10: postoperative complication—ileus.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Ferrari 2020 | Some concerns | Quote: "We randomly assigned patients to undergo standard
perioperative care (SPC) or ERAS protocol." "There were
no baseline differences between the 2 study groups." The authors just mentioned randomisation without providing detailed information about the randomisation process and allocation concealment method. |
Low risk of bias | Quote: "A randomised trial on ERAS is difficult to
perform because running SPC and ERAS protocol
simultaneously carries the risk of mixing the elements.
Hence, to avoid any unintentional biases, the
investigators were excluded from the clinical management
of the patients. Furthermore, we split the surgical,
anaesthesiological, and nurse teams involved in the
study, without the possibility of changes in the team's
composition, and we enrolled the patients minimising the
overlapping of the hospitalisation." The intervention providers took measures to avoid deviations. In addition, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. |
Low risk of bias | Quote: "We excluded from analysis 2 patients in the SPC
group and 6 patients in the ERAS protocol group either
because they withdrew the consent before receiving the
allocated treatment or because we planned the surgery
for the wrong week." Data were available for nearly all participants randomised and results were not likely biased by missing data. |
Low risk of bias | Quote: "The investigators were excluded from the clinical management of the patients." | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT03347409). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Lack of information on randomisation. |
| Ho 2020 | Low risk of bias | Quote: "Consenting subjects were randomised into two
groups before baseline assessment: intervention group
(CHO‐P) and control group (CO). Randomization was done
by a computer‐generated number randomisation which was
prepared by an independent statistician. The allocation
of randomised numbers was concealed in sealed envelopes
by the study coordinator. The envelope was only opened
after consent and before the baseline assessment."
"There was no statistically significant difference among
any baseline variables between groups." The allocation sequence was random and concealed until participants were enrolled and assigned to interventions. The baseline differences between intervention and control groups did not suggest a problem with the randomisation process. |
Low risk of bias | Quote: "All randomised RCT subjects were included in
analysis on an intention‐to‐treat (ITT) basis. " The interventions for the two groups were very clear. It is likely that no deviations from the intended intervention arose because of the experimental context. An appropriate analysis was used to estimate the effect of assignment to intervention. |
Low risk of bias | The data were available for all participants randomised. | Some concerns | The outcomes were monitored by the surgical gynaecological team who were not blinded and might involve some judgement on clinical definitions. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT03667755). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Blinding of outcome assessors. |
| Sánchez‐Iglesias 2020 | Low risk of bias | Quote: "Simple randomisation was carried out (by a
hospital staff member blinded to the study) using
computer generated random allocation for the ERAS or CM
group and concealed in a numbered, opaque envelope.
Participants were then randomised (i.e. given an
envelope) to either the ERAS or the CM arm during the
preoperative visit." "Both groups were similar with
respect to demographic data, including age, body mass
index and CC index, as well as for surgery indication,
histological type and FIGO stage." The allocation sequence was random and concealed until participants were enrolled and assigned to interventions. The baseline differences between intervention and control groups did not suggest a problem with the randomisation process. |
Some concerns | The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. However, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. | Low risk of bias | Data were reasonably complete. Eleven patients were excluded after randomisation because they were identified as ineligible. | Some concerns | The outcomes were according to the records of intervention providers who were not blinded and might involve some judgement on clinical definitions. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT02172638). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Blinding of participants, providers, and outcome assessors. |
| Shi 2020 | Low risk of bias | Quote: "Computer‐generated random numbers and list of any factors for stratification. To reduce predictability of a random sequence, details of any planned restriction should be provided in a separate document that is unavailable to those who enroll participants or assign interventions." | Some concerns | Quote: "Data collectors are not involved in the clinical
management of patients to ensure statistical validity
and reliability." "The surgical technique is
standardised for the treatment team, and patients'
families are not blinded to the study." "Operating
surgeons, attending physicians, nursing staff, and
patients and families cannot be blinded in this study,
as the procedures differ between groups; however,
outcome assessors will not be blinded." "All surgeries
are performed by the same team of surgeons, and patients
are treated and nursed by the same treatment team during
the pre‐operative period." "In the full analysis set,
patients will be analysed as randomised according to the
intention‐to‐treat principle. The intention‐to‐treat
principle implies that the analysis includes all
randomised patients. The per protocol analysis set will
include all patients without major protocol deviation.
Deviations from the protocol will be assessed as major
or minor, and patients with major deviations from the
protocol will be excluded from the per protocol
analysis." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. |
Low risk of bias | Data were reasonably complete. | Some concerns | The outcomes were according to the records of intervention providers, who were not blinded and might involve some judgement on clinical definitions. | Low risk of bias | Protocol registered in clinicaltrial.gov: NCT02687412; A protocol published in 2016: Cui L, Shi Y, Zhang GN (2016). Fast‐track surgery after gynaecological oncological surgery: study protocol for a prospective randomised controlled trial. Trials, 17:597. | Some concerns | Blinding of participants, providers, and outcome assessors. |
Risk of bias for analysis 1.20 Outcome 2.11: postoperative complication—colorectal anastomotic fistula.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Sánchez‐Iglesias 2020 | Low risk of bias | Quote: "Simple randomisation was carried out (by a
hospital staff member blinded to the study) using
computer generated random allocation for the ERAS or CM
group and concealed in a numbered, opaque envelope.
Participants were then randomised (i.e. given an
envelope) to either the ERAS or the CM arm during the
preoperative visit." "Both groups were similar with
respect to demographic data, including age, body mass
index and CC index, as well as for surgery indication,
histological type and FIGO stage." The allocation sequence was random and concealed until participants were enrolled and assigned to interventions. The baseline differences between intervention and control groups did not suggest a problem with the randomisation process. |
Some concerns | The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. However, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. | Low risk of bias | Data were reasonably complete. Eleven patients were excluded after randomisation because they were identified as ineligible. | Some concerns | The outcomes were according to the records of intervention providers who were not blinded and might involve some judgement on clinical definitions. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT02172638). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Blinding of participants, providers, and outcome assessors. |
Risk of bias for analysis 1.21 Outcome 3: mortality.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Sánchez‐Iglesias 2020 | Low risk of bias | Quote: "Simple randomisation was carried out (by a
hospital staff member blinded to the study) using
computer generated random allocation for the ERAS or CM
group and concealed in a numbered, opaque envelope.
Participants were then randomised (i.e. given an
envelope) to either the ERAS or the CM arm during the
preoperative visit." "Both groups were similar with
respect to demographic data, including age, body mass
index and CC index, as well as for surgery indication,
histological type and FIGO stage." The allocation sequence was random and concealed until participants were enrolled and assigned to interventions. The baseline differences between intervention and control groups did not suggest a problem with the randomisation process. |
Some concerns | The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. However, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. | Low risk of bias | Data were reasonably complete. Eleven patients were excluded after randomisation because they were identified as ineligible. | Low risk of bias | The survival outcomes were obtained directly from patients and do not involve any judgement. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT02172638). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Blinding of participants and providers. |
Risk of bias for analysis 1.22 Outcome 4: readmission.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Ferrari 2020 | Some concerns | Quote: "We randomly assigned patients to undergo standard
perioperative care (SPC) or ERAS protocol." "There were
no baseline differences between the 2 study groups." The authors just mentioned randomisation without providing detailed information about the randomisation process and allocation concealment method. |
Low risk of bias | Quote: "A randomised trial on ERAS is difficult to
perform because running SPC and ERAS protocol
simultaneously carries the risk of mixing the elements.
Hence, to avoid any unintentional biases, the
investigators were excluded from the clinical management
of the patients. Furthermore, we split the surgical,
anaesthesiological, and nurse teams involved in the
study, without the possibility of changes in the team's
composition, and we enrolled the patients minimising the
overlapping of the hospitalisation." The intervention providers took measures to avoid deviations. In addition, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. |
Low risk of bias | Data were available for nearly all participants randomised and results were not likely biased by missing data. | Low risk of bias | Quote: "The investigators were excluded from the clinical management of the patients." | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT03347409). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Lack of information on randomisation. |
| Ho 2020 | Low risk of bias | Quote: "Consenting subjects were randomised into two
groups before baseline assessment: intervention group
(CHO‐P) and control group (CO). Randomization was done
by a computer‐generated number randomisation which was
prepared by an independent statistician. The allocation
of randomised numbers was concealed in sealed envelopes
by the study coordinator. The envelope was only opened
after consent and before the baseline assessment."
"There was no statistically significant difference among
any baseline variables between groups." The allocation sequence was random and concealed until participants were enrolled and assigned to interventions. The baseline differences between intervention and control groups did not suggest a problem with the randomisation process. |
Low risk of bias | Quote: "All randomised RCT subjects were included in
analysis on an intention‐to‐treat(ITT) basis. " The interventions for the two groups were very clear. It is likely that no deviations from the intended intervention arose because of the experimental context. An appropriate analysis was used to estimate the effect of assignment to intervention. |
Low risk of bias | The data were available for all participants randomised. | High risk of bias | The readmission reflected the decisions made by the intervention provider, where recording of the decisions does not involve any judgement, but where the decision itself can be influenced by knowledge of intervention received. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT03667755). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | High risk of bias | Blinding of outcome assessors. |
| Sánchez‐Iglesias 2020 | Low risk of bias | Quote: "Simple randomisation was carried out (by a
hospital staff member blinded to the study) using
computer generated random allocation for the ERAS or CM
group and concealed in a numbered, opaque envelope.
Participants were then randomised (i.e. given an
envelope) to either the ERAS or the CM arm during the
preoperative visit." "Both groups were similar with
respect to demographic data, including age, body mass
index and CC index, as well as for surgery indication,
histological type and FIGO stage." The allocation sequence was random and concealed until participants were enrolled and assigned to interventions. The baseline differences between intervention and control groups did not suggest a problem with the randomisation process. |
Some concerns | The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. However, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. | Low risk of bias | Data were reasonably complete. Eleven patients were excluded after randomisation because they were identified as ineligible. | High risk of bias | The readmission reflected the decisions made by the intervention provider, where recording of the decisions does not involve any judgement, but where the decision itself can be influenced by knowledge of intervention received. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT02172638). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | High risk of bias | Blinding of participants, providers, and outcome assessors. |
Risk of bias for analysis 1.24 Outcome 5.1: bowel function—time to first flatus.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Ho 2020 | Low risk of bias | Quote: "Consenting subjects were randomised into two
groups before baseline assessment: intervention group
(CHO‐P) and control group (CO). Randomization was done
by a computer‐generated number randomisation which was
prepared by an independent statistician. The allocation
of randomised numbers was concealed in sealed envelopes
by the study coordinator. The envelope was only opened
after consent and before the baseline assessment."
"There was no statistically significant difference among
any baseline variables between groups." The allocation sequence was random and concealed until participants were enrolled and assigned to interventions. The baseline differences between intervention and control groups did not suggest a problem with the randomisation process. |
Low risk of bias | Quote: "All randomised RCT subjects were included in
analysis on an intention‐to‐treat(ITT) basis. " The interventions for the two groups were very clear. It is likely that no deviations from the intended intervention arose because of the experimental context. An appropriate analysis was used to estimate the effect of assignment to intervention. |
Low risk of bias | The data were available for all participants randomised. | Low risk of bias | The outcomes on bowel function were obtained from patients directly and do not involve any judgement. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT03667755). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Low risk of bias | Low risk of bias in all domains. |
| Ferrari 2020 | Some concerns | Quote: "We randomly assigned patients to undergo standard
perioperative care (SPC) or ERAS protocol." "There were
no baseline differences between the 2 study groups." The authors just mentioned randomisation without providing detailed information about the randomisation process and allocation concealment method. |
Low risk of bias | Quote: "A randomised trial on ERAS is difficult to
perform because running SPC and ERAS protocol
simultaneously carries the risk of mixing the elements.
Hence, to avoid any unintentional biases, the
investigators were excluded from the clinical management
of the patients. Furthermore, we split the surgical,
anaesthesiological, and nurse teams involved in the
study, without the possibility of changes in the team's
composition, and we enrolled the patients minimising the
overlapping of the hospitalisation." The intervention providers took measures to avoid deviations. In addition, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. |
Low risk of bias | Quote: "We excluded from analysis 2 patients in the SPC
group and 6 patients in the ERAS protocol group either
because they withdrew the consent before receiving the
allocated treatment or because we planned the surgery
for the wrong week." Data were available for nearly all participants randomised and results were not likely biased by missing data. |
Low risk of bias | The outcomes on bowel function were obtained from patients directly and do not involve any judgement. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT03347409). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Lack of information on randomisation. |
| Zhang 2016 | Some concerns | The allocation sequence was randomised using a random number table. However, no detailed information about allocation sequence concealing was reported. | Some concerns | The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. | Low risk of bias | Data were reasonably complete. | Low risk of bias | The outcomes on bowel function were obtained from patients directly and do not involve any judgement. | Some concerns | No pre‐registered protocol or a pre‐specified statistical analysis plan was found. However, the outcome measurements and analyses were clearly defined. There is no indication of selection of the reported analysis from among multiple analyses or selection of the cohort or subgroups for analysis and reporting on the basis of the results. | Some concerns | Lack of information on randomisation, blinding of participants, providers, and outcome assessors, and no pre‐registered protocol for statistical plan. |
| Zhou 2020 | Some concerns | The allocation sequence was randomised using a random number table. However, no detailed information about allocation sequence concealment was reported. | High risk of bias | Quote: "Advertisements were put out all over the
department, which may cause deviations from the intended
intervention." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. Participants from different groups lived in the same department. Due to the advertisements posted all over the department, there is evidence, or strong reason to believe, that the trial context led to failure in implementing the protocol interventions in the control group and instead led to the implementation of interventions not allowed by the protocol. |
Low risk of bias | Data were reasonably complete. | Low risk of bias | The outcomes on bowel function were obtained from patients directly and do not involve any judgement. | Some concerns | No pre‐registered protocol or statistical analysis plan was found. However, the outcome measurements and analyses were clearly defined. There is no indication of selection of the reported analysis from among multiple analyses or selection of the cohort or subgroups for analysis and reporting on the basis of the results. | High risk of bias | Lack of information on randomisation, blinding of participants and providers, failure in implementing the protocol interventions due to trial context, and no pre‐registered protocol or statistical plan. |
Risk of bias for analysis 1.27 Outcome 5.2: bowel function—time to first defaecation.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Ferrari 2020 | Some concerns | Quote: "We randomly assigned patients to undergo standard
perioperative care (SPC) or ERAS protocol." "There were
no baseline differences between the 2 study groups." The authors just mentioned randomisation without providing detailed information about the randomisation process and allocation concealment method. |
Low risk of bias | Quote: "A randomised trial on ERAS is difficult to
perform because running SPC and ERAS protocol
simultaneously carries the risk of mixing the elements.
Hence, to avoid any unintentional biases, the
investigators were excluded from the clinical management
of the patients. Furthermore, we split the surgical,
anaesthesiological, and nurse teams involved in the
study, without the possibility of changes in the team's
composition, and we enrolled the patients minimising the
overlapping of the hospitalisation." The intervention providers took measures to avoid deviations. In addition, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. |
Low risk of bias | Quote: "We excluded from analysis 2 patients in the SPC
group and 6 patients in the ERAS protocol group either
because they withdrew the consent before receiving the
allocated treatment or because we planned the surgery
for the wrong week." Data were available for nearly all participants randomised and results were not likely biased by missing data. |
Low risk of bias | The outcomes on bowel function were obtained from patients directly and do not involve any judgement. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT03347409). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Lack of information on randomisation. |
| Zhou 2020 | Some concerns | The allocation sequence was randomised using a random number table. However, no detailed information about allocation sequence concealment was reported. | High risk of bias | Quote: "Advertisements were put out all over the
department, which may cause deviations from the intended
intervention." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. Participants from different groups lived in the same department. Due to the advertisements posted all over the department, there is evidence, or strong reason to believe, that the trial context led to failure in implementing the protocol interventions in the control group and instead led to the implementation of interventions not allowed by the protocol. |
Low risk of bias | Data were reasonably complete. | Low risk of bias | The outcomes on bowel function were obtained from patients directly and do not involve any judgement. | Some concerns | No pre‐registered protocol or statistical analysis plan was found. However, the outcome measurements and analyses were clearly defined. There is no indication of selection of the reported analysis from among multiple analyses or selection of the cohort or subgroups for analysis and reporting on the basis of the results. | High risk of bias | Lack of information on randomisation, blinding of participants and providers, failure in implementing the protocol interventions due to trial context, and no pre‐registered protocol or statistical plan. |
Risk of bias for analysis 1.28 Outcome 6: participant satisfaction.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Ferrari 2020 | Some concerns | Quote: "We randomly assigned patients to undergo standard
perioperative care (SPC) or ERAS protocol." "There were
no baseline differences between the 2 study groups." The authors just mentioned randomisation without providing detailed information about the randomisation process and allocation concealment method. |
Low risk of bias | Quote: "A randomised trial on ERAS is difficult to
perform because running SPC and ERAS protocol
simultaneously carries the risk of mixing the elements.
Hence, to avoid any unintentional biases, the
investigators were excluded from the clinical management
of the patients. Furthermore, we split the surgical,
anaesthesiological, and nurse teams involved in the
study, without the possibility of changes in the team's
composition, and we enrolled the patients minimising the
overlapping of the hospitalisation." The intervention providers took measures to avoid deviations. In addition, an appropriate analysis (intention‐to‐treat analysis) was used to estimate the effect of assignment to intervention. |
Low risk of bias | Quote: "We excluded from analysis 2 patients in the SPC
group and 6 patients in the ERAS protocol group either
because they withdrew the consent before receiving the
allocated treatment or because we planned the surgery
for the wrong week." Data were available for nearly all participants randomised and results were not likely biased by missing data. |
Some concerns | The reports on satisfaction came directly from participants about how they feel. This is potentially influenced by knowledge of intervention received. | Low risk of bias | The data were analysed in accordance with a pre‐specified analysis plan (clinicaltrial.gov: NCT03347409). The results were unlikely to have been selected from multiple eligible outcome measurements or multiple eligible analyses of the data. | Some concerns | Lack of information on randomisation and blinding of outcome assessors. |
| Zhou 2020 | Some concerns | The allocation sequence was randomised using a random number table. However, no detailed information about allocation sequence concealment was reported. | High risk of bias | Quote: "Advertisements were put out all over the
department, which may cause deviations from the intended
intervention." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. Participants from different groups lived in the same department. Due to the advertisements posted all over the department, there is evidence, or strong reason to believe, that the trial context led to failure in implementing the protocol interventions in the control group and instead led to the implementation of interventions not allowed by the protocol. |
Low risk of bias | Data were reasonably complete. | Some concerns | The reports on satisfaction were coming directly from participants about how they feel. This is potentially influenced by knowledge of intervention received. | Some concerns | No pre‐registered protocol or statistical analysis plan was found. However, the outcome measurements and analyses were clearly defined. There is no indication of selection of the reported analysis from among multiple analyses or selection of the cohort or subgroups for analysis and reporting on the basis of the results. | High risk of bias | Lack of information on randomisation, blinding of participants, providers, and outcome assessors, failure in implementing the protocol interventions due to trial context, and no pre‐registered protocol or statistical plan. |
Risk of bias for analysis 1.29 Outcome 7: economic outcomes.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Shi 2020 | Low risk of bias | Quote: "Computer‐generated random numbers and list of any factors for stratification. To reduce predictability of a random sequence, details of any planned restriction should be provided in a separate document that is unavailable to those who enroll participants or assign interventions." | Some concerns | Quote: "Data collectors are not involved in the clinical
management of patients to ensure statistical validity
and reliability." "The surgical technique is
standardised for the treatment team, and patients'
families are not blinded to the study." "Operating
surgeons, attending physicians, nursing staff, and
patients and families cannot be blinded in this study,
as the procedures differ between groups; however,
outcome assessors will not be blinded." "All surgeries
are performed by the same team of surgeons, and patients
are treated and nursed by the same treatment team during
the pre‐operative period." "In the full analysis set,
patients will be analysed as randomised according to the
intention‐to‐treat principle. The intention‐to‐treat
principle implies that the analysis includes all
randomised patients. The per protocol analysis set will
include all patients without major protocol deviation.
Deviations from the protocol will be assessed as major
or minor, and patients with major deviations from the
protocol will be excluded from the per protocol
analysis." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. |
Low risk of bias | Quote: "In the full analysis set, patients will be
analysed as randomised according to the
intention‐to‐treat principle." Data were reasonably complete. |
High risk of bias | The outcome reflected the decisions made by the intervention provider, where recording of the decisions does not involve any judgement, but where the decision itself can be influenced by knowledge of intervention received. | Low risk of bias | Protocol registered in clinicaltrial.gov: NCT02687412; A protocol published in 2016: Cui L, Shi Y, Zhang GN (2016). Fast‐track surgery after gynaecological oncological surgery: study protocol for a prospective randomised controlled trial. Trials, 17:597. | High risk of bias | Blinding of participants, providers, and outcome assessors. |
| Zhou 2020 | Some concerns | The allocation sequence was randomised using a random number table. However, no detailed information about allocation sequence concealment was reported. | High risk of bias | Quote: "Advertisements were put out all over the
department, which may cause deviations from the intended
intervention." The participants were aware of their assigned intervention during the trial and the carers and people delivering the interventions were aware of participants' assigned intervention during the trial. Participants from different groups lived in the same department. Due to the advertisements posted all over the department, there is evidence, or strong reason to believe, that the trial context led to failure in implementing the protocol interventions in the control group and instead led to the implementation of interventions not allowed by the protocol. |
Low risk of bias | Data were reasonably complete. | High risk of bias | The outcome reflected the decisions made by the intervention providers, where recording of the decisions does not involve any judgement, but where the decision itself can be influenced by knowledge of intervention received. | Some concerns | No pre‐registered protocol or statistical analysis plan was found. However, the outcome measurements and analyses were clearly defined. There is no indication of selection of the reported analysis from among multiple analyses or selection of the cohort or subgroups for analysis and reporting on the basis of the results. | High risk of bias | Lack of information on randomisation, blinding of participants, providers, and outcome assessors, failure in implementing the protocol interventions due to trial context, and no pre‐registered protocol or statistical plan. |
Acknowledgements
We would like to thank Jo Morrison for clinical and editorial advice; Jo Platt for designing the search strategy; and Gail Quinn, Clare Jess and Tracey Harrison for their contributions to the editorial process.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
We wish to acknowledge the authors of the original version of this review; Lu D, Wang X, and Shi G.
The authors and Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Team, are grateful to the following peer reviewers for their time and comments: Sue Golby, Andrew Nordin, Nithya Ratnavelu.
Special thanks to Ms. Vivien Daum, Dietitian of University of Pécs, Clinical Centre, Hungary, who helped to translate one study from German (Eberhart 2008).
Appendices
Appendix 1. CENTRAL search strategy
CENTRAL
#1 MeSH descriptor Uterine Neoplasms explode all trees #2 MeSH descriptor Ovarian Neoplasms explode all trees #3 MeSH descriptor Fallopian Tube Neoplasms explode all trees #4 MeSH descriptor Vaginal Neoplasms explode all trees #5 MeSH descriptor Vulvar Neoplasms explode all trees #6 (endometr* or uter* or cervi* or ovar* or vagin* or fallopian* or vulva* or gynae* or gyne*) near/5 (cancer* or neoplas* or carcinom* or malignan* or tumour* or tumour*) #7 (#1 OR #2 OR #3 OR #4 OR #5 OR #6) #8 Any MeSH descriptor with qualifier: SU #9 MeSH descriptor Gynecologic Surgical Procedures explode all trees #10 MeSH descriptor Laparoscopy explode all trees #11 surg* or operat* or laparoscop* or hysterectomy or ovariectomy or salpingostomy #12 (#8 OR #9 OR #10 OR #11) #13 (#7 AND #12) #14 MeSH descriptor Perioperative Care explode all trees #15 MeSH descriptor Preoperative Care explode all trees #16 MeSH descriptor Convalescence explode all trees #17 MeSH descriptor Length of Stay explode all trees #18 ERAS #19 fast track #20 (enhanced or early) and (rehabilitat* or recover* or convalesc*) #21 (#14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20) #22 (#13 AND #21)
Appendix 2. MEDLINE search strategy
MEDLINE Ovid
1 exp Uterine Neoplasms/ 2 exp Ovarian Neoplasms/ 3 exp Fallopian Tube Neoplasms/ 4 exp Vaginal Neoplasms/ 5 exp Vulvar Neoplasms/ 6 ((endometr* or uter* or cervi* or ovar* or vagin* or fallopian* or vulva* or gynae* or gyne*) adj5 (cancer* or neoplas* or carcinom* or malignan* or tumour* or tumour*)).mp. 7 1 or 2 or 3 or 4 or 5 or 6 8 surgery.fs. 9 exp Gynecologic Surgical Procedures/ 10 exp Laparoscopy/ 11 (surg* or operat* or laparoscop* or hysterectomy or ovariectomy or salpingostomy).mp. 12 8 or 9 or 10 or 11 13 7 and 12 14 exp Perioperative Care/ 15 exp Preoperative Care/ 16 exp Convalescence/ 17 exp "Length of Stay"/ 18 ERAS.mp. 19 fast track.mp. 20 ((enhanced or early) and (rehabilitat* or recover* or convalesc*)).mp. 21 14 or 15 or 16 or 17 or 18 or 19 or 20 22 13 and 21 23 randomised controlled trial.pt. 24 controlled clinical trial.pt. 25 randomized.ab. 26 placebo.ab. 27 drug therapy.fs. 28 randomly.ab. 29 trial.ab. 30 groups.ab. 31 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 32 (animals not (humans and animals)).sh. 33 31 not 32 34 22 and 33
Key: mp = title, original title, abstract, name of substance word, subject heading word, fs = floating subheading, ab = abstract, sh = medical subject heading
Appendix 3. EMBASE search strategy
EMBASE Ovid
1 exp uterus cancer/ 2 exp ovary tumour/ 3 exp uterine tube tumour/ 4 exp vagina tumour/ 5 exp vulva tumour/ 6 ((endometr* or uter* or cervi* or ovar* or vagin* or fallopian* or vulva* or gynae* or gyne*) adj5 (cancer* or neoplas* or carcinom* or malignan* or tumour* or tumour*)).mp. 7 or/1‐6 8 su.fs. 9 exp gynaecologic surgery/ 10 exp laparoscopy/ 11 (surg* or operat* or laparoscop* or hysterectomy or ovariectomy or salpingostomy).mp. 12 or/8‐11 13 7 and 12 14 exp perioperative period/ 15 exp preoperative care/ 16 exp peroperative care/ 17 exp postoperative care/ 18 exp "length of stay"/ 19 ERAS.mp. 20 fast track.mp. 21 ((enhance or early) and (rehabilitat* or recover* or convalesc*)).mp. 22 exp convalescence/ 23 or/14‐22 24 13 and 23 25 exp controlled clinical trial/ 26 randomized.ab. 27 placebo.ab. 28 dt.fs. 29 randomly.ab. 30 trial.ab. 31 groups.ab. 32 or/25‐31 33 24 and 32
Key: mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name, ab = abstract, fs = floating subheading
Appendix 4. CBM search strategy
#1 "快速康复"[常用字段:智能] OR "加速康复"[常用字段:智能] OR "ERAS"[常用字段:智能] #2 "妇科"[常用字段:智能] OR "外阴"[常用字段:智能] OR "子宫颈"[常用字段:智能] OR "子宫"[常用字段:智能] OR "输卵管"[常用字段:智能] OR "子宫内膜"[常用字段:智能] OR "快速康复"[常用字段:智能] #3 "肿瘤"[不加权:扩展] #4 #1 AND #2 AND #3
Data and analyses
Comparison 1. ERAS programmes versus traditional care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Outcome 1: length of postoperative hospital stay | 6 | 638 | Mean Difference (IV, Random, 95% CI) | ‐1.71 [‐2.59, ‐0.84] |
| 1.2 Outcome 1: length of postoperative hospital stay—subgroup analysis—surgery type | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 Laparotomy surgery | 3 | 312 | Mean Difference (IV, Random, 95% CI) | ‐1.92 [‐2.99, ‐0.85] |
| 1.2.2 Laparoscopic surgery | 3 | 219 | Mean Difference (IV, Random, 95% CI) | ‐1.81 [‐3.34, ‐0.29] |
| 1.3 Outcome 1: length of postoperative hospital stay—sensitivity analysis 1 (excluding studies on participants with both benign and malignant tumours) | 5 | 470 | Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐2.44, ‐0.60] |
| 1.4 Outcome 1: length of postoperative hospital stay—sensitivity analysis 2 (excluding studies introducing transformed outcome value) | 4 | 371 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐2.58, ‐0.02] |
| 1.5 Outcome 2.1: overall postoperative complications | 5 | 537 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.48, 1.05] |
| 1.6 Outcome 2.1: overall postoperative complications—subgroup analysis—surgery type | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.6.1 Laparotomy surgery | 2 | 202 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.54, 1.79] |
| 1.6.2 Laparoscopic surgery | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.34, 1.55] |
| 1.7 Outcome 2.1: overall postoperative complications—sensitivity analysis (excluding studies on participants with both benign and malignant tumours) | 3 | 266 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.42, 1.13] |
| 1.8 Outcome 2.2: postoperative complication—acute confusion | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.9 Outcome 2.3: postoperative complication—nausea and vomiting | 6 | 642 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.33, 0.84] |
| 1.10 Outcome 2.3: postoperative complication—nausea and vomiting—subgroup analysis—surgery type | 4 | 367 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.28, 0.96] |
| 1.10.1 Laparotomy surgery | 2 | 221 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.19, 1.51] |
| 1.10.2 Laparoscopic surgery | 2 | 146 | Risk Ratio (IV, Random, 95% CI) | 0.53 [0.22, 1.25] |
| 1.11 Outcome 2.3: postoperative complication—nausea and vomiting—sensitivity analysis (excluding studies on participants with both benign and malignant tumours) | 4 | 371 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.24, 0.54] |
| 1.12 Outcome 2.4: postoperative complication—fever | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.13 Outcome 2.5: postoperative complication—secondary haemorrhage | 3 | 309 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.14, 6.29] |
| 1.14 Outcome 2.6: postoperative complication—pneumonia | 2 | 217 | Risk Ratio (IV, Random, 95% CI) | 0.41 [0.06, 2.75] |
| 1.15 Outcome 2.7: postoperative complication—wound infection | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.16 Outcome 2.8: postoperative complication—anastomosis dehiscence | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.17 Outcome 2.9: postoperative complication—embolism and deep vein thrombosis | 2 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.18 Outcome 2.10: postoperative complication—ileus | 4 | 492 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.30, 1.07] |
| 1.19 Outcome 2.10: postoperative complication—ileus—sensitivity analysis (excluding studies on participants with both benign and malignant tumours) | 3 | 324 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.34, 1.26] |
| 1.20 Outcome 2.11: postoperative complication—colorectal anastomotic fistula | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.21 Outcome 3: mortality | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.22 Outcome 4: readmission | 3 | 385 | Risk Ratio (IV, Random, 95% CI) | 0.45 [0.22, 0.90] |
| 1.23 Outcome 4: readmission—sensitivity analysis (excluding studies on participants with both benign and malignant tumours) | 2 | 217 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.15, 0.80] |
| 1.24 Outcome 5.1: bowel function—time to first flatus | 4 | 432 | Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐1.00, ‐0.63] |
| 1.25 Outcome 5.1: bowel function—time to first flatus—subgroup analysis—surgery type | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.25.1 Laparotomy surgery | 1 | 118 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.30, ‐0.76] |
| 1.25.2 Laparoscopic surgery | 2 | 146 | Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐0.87, ‐0.61] |
| 1.26 Outcome 5.1: bowel function—time to first flatus—sensitivity analysis (excluding studies on participants with both benign and malignant tumours) | 3 | 264 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.03, ‐0.58] |
| 1.27 Outcome 5.2: bowel function—time to first defaecation | 2 | 228 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.47, ‐0.44] |
| 1.28 Outcome 6: participant satisfaction | 2 | 228 | Std. Mean Difference (IV, Random, 95% CI) | 0.92 [0.06, 1.78] |
| 1.29 Outcome 7: economic outcomes | 2 | 167 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.68, 0.25] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dickson 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | A total of 103 participants (intervention: 51,
control: 52; cancer: 64.1%) with a planned
laparotomy on the gynaecologic oncology service,
irrespective of their presumed diagnosis, were
considered eligible Exclusion criteria include < 19 years old; pregnant; undergoing a procedure other than laparotomy; scheduled to be discharged on the same day of surgery; chronic narcotic pain medication user; ASA score ≥ 3; any condition that would exclude women from undergoing regional anaesthesia Mean age (years): 56 in the control group and 55.4 in the ERAS group Diagnosis: 34% benign, 1.9% borderline, and 64.1% cancer Surgery type: laparotomy |
|
| Interventions | Intervention: a standardised enhanced recovery after
surgery protocol Control: current surgical practices. Any or all ERAS tenets are permitted for use. |
|
| Outcomes | Length of postoperative hospital stay (median with
95% CI) Overall postoperative complication rate within 30 days of operation, nausea and vomiting and secondary haemorrhage Bowel function: time to first flatus |
|
| Notes | ||
Ferrari 2020.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | A total of 174 participants were randomised to the
ERAS group (n = 87) and standard perioperative care
group (n = 87) with 57.1% of participants diagnosed
with cancer. Women were eligible for enrolment if their age was ≥ 18 years and ≤ 75 years, and they were a candidate for major gynaecologic surgery for benign disease (including leiomyomas, endometriosis, and functional pathology of the uterus) or malignant disease (including endometrial cancer or advanced‐stage ovarian cancer) that considers at least total hysterectomy with either an endoscopic or an open approach. Exclusion criteria: people with ASA risk ≥ 4 or with planned discharge from the intensive care unit, Karnofsky performance status < 70, contraindication to locoregional anaesthesia (i.e. coagulative disorders), organ failure or severe dysfunction, history of previous or current alcohol or drug abuse, a comorbidity‐polypharmacy score ≥ 22, and presence of a psychiatric condition or language barrier. People affected by cervical cancer, given the early findings of the Laparoscopic Approach to Cervical Cancer trial, were excluded to avoid future unbalanced accrual regarding surgical access. Mean age (years): 55.7 in all the participants; 54.9 in the control group and 56.5 in the ERAS group Diagnosis: 42.9% benign, 28.6% endometrial cancer, and 28.6% ovarian cancer Surgery type: both laparotomy (56.6%) and laparoscopic (43.4%) surgery Surgical procedure: bilateral pelvic lymph nodes dissection (30.4%); paraaortic lymph nodes dissection (7.7%); omentectomy (28.6%); bowel resection (7.8%); diaphragm resection (2.4%) |
|
| Interventions | Intervention: an enhanced recovery after surgery
protocol Control: standardised perioperative care without ERAS practices such as avoidance of bowel preparation |
|
| Outcomes | Length of postoperative hospital stay Overall postoperative complication rate within 30 days of operation, nausea and vomiting, ileus Bowel functions: time to first flatus, time to first defaecation Readmission rate within 30 days of operation Participant satisfaction |
|
| Notes | ||
Ho 2020.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | A total of 118 Malaysians aged 18 or above
(intervention: 62, control: 56) who were diagnosed
with gynaecologic cancer stage 1 to stage 4 and were
candidates for elective operation treatments were
included.
Exclusion Criteria: not a candidate
for elective operation treatments; aged < 18
years old; diagnosed with diabetes mellitus/chronic
kidney disease/heart disease/chronic liver disease;
allergic to milk/soy/whey protein; participating in
another intervention study Mean age (years): 51.2 in the control group and 49.5 in the ERAS group Diagnosis: 42.4% ovarian cancer, 33.9% endometrial cancer, 17.8% cervical cancer, and 5.9% uterine cancer Surgery type: laparotomy Surgery procedure: 65.3% total abdominal hysterectomy bilateral salpingo‐oophorectomy, 20.3% salpingo‐oophorectomy, 9.3% radical hysterectomy, 5.1% debulking tumour. |
|
| Interventions | Intervention group: fast‐track recovery surgery with
whey protein infused carbohydrate loading and early
oral feeding. Participants will be carbohydrate
loaded with a whey protein infused carbohydrate
drink and started on early oral feeding
postoperation. Control group: traditional care such as fasting at least 12 hours preoperatively, allowing clear fluid once bowel sound presented and following a transition diet postoperatively. |
|
| Outcomes | Length of postoperative hospital stay Postoperative complications within 30 days of operation: nausea and vomiting, ileus and pneumonia Bowel function: time to first flatus Readmission within 30 days of operation |
|
| Notes | ||
Sánchez‐Iglesias 2020.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | A total of 99 participants (intervention: 50,
control: 49) who are 18 years or above, with
clinical or radiological suspicion of advanced
disease or relapses, diagnosed with advanced ovarian
cancer (FIGO Stages III‐IV, and relapses) tributary
to laparotomy surgical management and with informed
consent. Exclusion criteria: physical status classification of ASA >III; presence of active ischemics heart disease; advanced cirrhosis (Child‐Pugh class B or C); severe psychiatric condition (patient not capable of giving her informed consent properly, not capable or not willing to attend follow‐up visits); urgent surgery due to acute complications Mean age (years): 57.33 in total; 57.24 in the control group and 57.8 in the ERAS group Diagnosis: ovarian cancer Surgery type: laparotomy Surgery procedure: rectosigmoid resection (42%), small bowel resection (8%); splenectomy (9%); and hepatectomy (5%) |
|
| Interventions | Intervention: the ERAS protocol was based on the
evidence available at the time at the start of the
study in 2014, as well as on the guidelines
published by the ERAS® Society, and adapted for
gynaecologic oncology patients. Control: participants in the control group received up‐to‐date traditional management, including anterograde mechanical bowel preparation (Fosfosoda), mechanical bowel preparation (retrograde), anxiolytic premedication, a strict diet with no solids or liquids starting from the night before surgery, intraoperative anaesthetic management as per the standard protocol for major oncological surgery, prophylactic nasogastric intubation until the beginning of peristalsis, and postoperative analgesia using an epidural technique or intravenous analgesia with major opioids. |
|
| Outcomes | Length of postoperative hospital stay Overall postoperative complication rate within 30 days of operation, acute confusion, secondary haemorrhage, pneumonia, anastomosis dehiscence, DVT/VTE, ileus, colorectal anastomotic fistula All‐cause mortality within 30 days of discharge Readmission Economic outcomes |
|
| Notes | ||
Shi 2020.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | A total of 107 participants (intervention: 50,
control: 57) with cervical, endometrial, or ovarian
cancers scheduled for gynaecological oncology
surgery (including radical hysterectomy and
lymphadenectomy, hysterectomy and lymphadenectomy,
and cytoreductive procedures for both open and
laparoscopic surgery) were included. Exclusion criteria: people ≥ 71 years, with a documented infection at the time of surgery, ileus at the time of surgery, hypo‐coagulability, psychological disorders, alcohol dependence, or drug abuse history, primary nephrotic or hepatic disease, severe hypertension defined as systolic blood pressure ≥ 160 mmHg and diastolic blood pressure > 90 mmHg Mean age (years): 55.25 in the control group and 48.62 in the ERAS group Diagnosis: 52.3% cervical cancer, 21.5% endometrial cancer, and 26.2% ovarian cancer Surgery type: 81.3% laparotomy and 18.7% laparoscopic surgery Surgery procedure: no information |
|
| Interventions | Intervention: ERAS. Control: traditional management without ERAS components. |
|
| Outcomes | Length of postoperative hospital stay Overall postoperative complication rate within 30 days of operation, nausea and vomiting, embolism and deep vein thrombosis, ileus, wound infection, secondary haemorrhage Economic outcomes |
|
| Notes | ||
Zhang 2016.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | A total of 86 participants (intervention: 43,
control: 43) with cervical cancer (Stage IA2‐II)
undergoing laparoscopic extensive hysterectomy and
pelvic lymphadenectomy. Exclusion criteria: preoperative neoadjuvant chemotherapy; abnormal liver and kidney function, hypoproteinaemia; a history of autoimmune diseases, nephritis, hypertension, diabetes, and cardiovascular and cerebrovascular diseases; severe organ dysfunction; switching to laparotomy; and other conditions that may affect the person's immune function and stress response. Mean age (years): 48.36 in the control group and 49.23 in the ERAS group. Diagnosis: cervical cancer. Surgery type: laparoscopic surgery. Surgery procedure: extensive hysterectomy and pelvic lymphadenectomy. |
|
| Interventions | Intervention: an ERAS protocol Control: traditional care without ERAS components |
|
| Outcomes | Length of postoperative hospital stay Postoperative complication within 30 days of operation: nausea and vomiting Bowel function: time to first flatus |
|
| Notes | ||
Zhou 2020.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | A total of 60 participants (intervention: 30,
control: 30) aged 18 to 70 with gynaecological
malignant tumours (i.e. cervical cancer, ovarian
cancer, endometrial cancer, fallopian tube cancer,
uterine sarcoma, choriocarcinoma; ASA stages I‐III)
who underwent minimally invasive surgery.
Participants should be willing to attend long‐term
follow‐up visits. Exclusion criteria: people who had contraindications to surgery; had a history of other cancers, radiation therapy, or chemotherapy; had severe complications including cardiovascular diseases, diabetes, and liver/kidney dysfunctions; and had a history of mental disorders Mean age (years): 50.03 in the control group and 50.43 in the ERAS group Diagnosis: 10% endometrial cancer, 76.7% cervical cancer, 5% ovarian cancer, 1.7% sarcoma of uterus, and 6.6% borderline ovarian tumours Surgery type: laparoscopic surgery Surgery procedure: no information |
|
| Interventions | Intervention: an enhanced recovery after surgery
protocol Control: traditional perioperative care without ERAS practices such as avoidance of bowel preparation |
|
| Outcomes | Length of postoperative hospital stay Overall postoperative complication rate within 30 days of operation,fever, nausea and vomiting Bowel functions: time to first flatus and defaecation Participant satisfaction Economic outcomes |
|
| Notes | ||
ASA: American Society of Anesthesiologists ; CI: confidence interval; DVT: deep vein thrombosis; ERAS: enhanced recovery after surgery; FIGO: International Federation of Gynecology and Obstetrics; VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agarwal 2019 | Non‐RCT |
| Belavy 2013 | Only examined epidural analgesia. |
| Bergstrom 2018 | Non‐RCT |
| Bernard 2021 | Non‐RCT |
| Bisch 2018 | Non‐RCT |
| Boitano 2018 | Non‐RCT |
| Chapman 2016 | Non‐RCT |
| deGroot 2014 | Non‐RCT |
| Eberhart 2008 | Non‐RCT |
| Feng 2008 | Only investigated early enteral feeding. |
| Gerardi 2008 | Mainly investigated early feeding. |
| Janda 2014 | Only investigated early oral intake. |
| Kalogera 2013 | Non‐RCT |
| Kay 2020 | Non‐RCT |
| Lambaudie 2017 | Non‐RCT |
| Marx 2006 | Non‐RCT |
| Mendivil 2018 | Non‐RCT |
| Meyer 2018 | Non‐RCT |
| Miller 2015 | The study only included participants with non‐malignant lesions. |
| Mukhopadhyay 2015 | Study compared outcomes between the first year and the second year after the implementation of enhanced recovery programme. |
| Myriokefalitaki 2016 | Non‐RCT |
| Pather 2011 | The study compared the perioperative outcomes between people undergoing total laparoscopic hysterectomy and people undergoing fast‐track open hysterectomy. |
| Renaud 2019 | Non‐RCT |
| Sanad 2019 | Non‐RCT |
| Schwartz 2019 | Non‐RCT |
| Shen 2019 | Non‐RCT |
| Wijk 2014 | Most of the participants were not diagnosed with cancer. Only 28% of the participants from the pre‐ERAS group and 38% of the participants from ERAS group were people with cancer. |
| Zhang 2019 | Non‐RCT |
ERAS: enhanced recovery after surgery; RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1900025117.
| Study name | Enhanced recovery after laparoscopic surgery (ERAS) in gynaecologic oncology: a prospective, randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: aged 18 to 75 years; undergoing a
selective laparoscopic surgery for malignant
gynaecological conditions; American Society of
Anesthesiologists (ASA) classification I‐II; have
signed the informed consent. Exclusion criteria: preoperative ileus; with surgery contraindications including acute infection, severe cardiovascular, coagulation abnormalities and psychological disorders; having NSAIDs contraindications including peptic ulcer, history of NSAIDs allergy and aspirin asthma; benign gynaecological diseases. |
| Interventions | ERAS perioperative intervention vs traditional perioperative intervention |
| Outcomes |
|
| Starting date | August 2019 |
| Contact information | China, Peking Department of Obstetrics & Gynecology, Peking Union Medical College Hospital Shuaifuyuan Wangfujing Street, Dongcheng District, Beijing, China Study leader:Lingya Pan Tel: +86 13910217876 Email: panly@pumch.cn Applicant:Wei Wang Tel: +86 13511073307 Email: vivianwang_pumch@sina.com |
| Notes | Estimated study completion date: March 2021 |
NCT02864277.
| Study name | Enhanced recovery after surgery (ERAS) program versus conventional perioperative strategies in patients undergoing gynecologic oncologic surgery: a randomized controlled trial |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: women between the ages of 18 and 70 years; fluent in English language; known or suspected diagnosis of gynaecologic malignancy (including ovarian, endometrial, and cervical cancers); scheduled for elective laparotomy; medically eligible for major surgical procedure. Exclusion criteria: unable to mobilise independently preoperatively; adults unable to consent (such as the cognitively impaired); minors < 18 years of age; pregnant women; prisoners; people having emergency surgery. |
| Interventions | ERAS (no detailed information) vs traditional strategy. |
| Outcomes | 1. Total length of hospital stay (days) (up to 10
days) 2. Achievement of postoperative milestones (30 days) 3. Surgical complications (30 days) 4. Readmission rates (2 weeks and 30 days) 5. Time to adjuvant treatment (60 days) 6. 30‐day mortality rates (30 days) 7. Participant‐reported satisfaction (14 days) 8. Progression‐free survival (2 years) 9. Preoperative vs postoperative day # 1 C‐Reactive Protein (CRP) (24 hours post surgery) 10. Preoperative vs postoperative day # 1 Interleukin‐6 (IL‐6) levels (24 hours post surgery) |
| Starting date | August 2016 |
| Contact information | United States, Illinois
Prentice Women's
Hospital, Chicago, Illinois, United States,
60611
Contact: Shireen Ahmad, M.D. Tel:
312‐472‐3585 Email:
sah704@northwestern.edu
Contact: Robert
McCarthy, PharmD Tel: 312‐695‐4976 Email: r‐mccarthy@northwestern.edu |
| Notes | Other Study ID Numbers: STU00203177; estimated study completion date: December 2021 |
NCT03640299.
| Study name | The safety and efficacy of enhanced recovery after surgery on clinical and immune outcomes for gynecological oncology |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: newly pathological diagnosed and suspected gynaecologic tumours, including cervical cancer, ovarian cancer, endometrial cancer, fallopian tube cancer, uterine carcinosarcoma, and choriocarcinoma; ages range from 18 to 70; body mass index of between 18 and 35; American Society of Anesthesiologists (ASA) grading of I to III; no history of abdominal surgery and severe organ dysfunction such as heart and lung. Exclusion criteria: unwillingness to participate; inability to give written informed consent; absolute contraindication for surgery; history of other malignancies, radiotherapy and chemotherapy; uncontrollable cardiovascular and cerebrovascular diseases, diabetes, and liver and kidney dysfunction. |
| Interventions | ERAS vs no intervention: traditional treatment procedure |
| Outcomes | 1. Total length of hospital stay (days) (up to 20 days) 2. Operation Time (hour) (day of surgery) 3. Intraoperative blood loss (mL) (day of surgery) 4. Intraoperative fluid transfusion units (mL) (day of surgery) 5. Intraoperative urinary volume (mL) (day of surgery) 6. Intraoperative blood transfusion (mL) (day of surgery) 7. Visual Analog Scale (VAS) for pain (up to 5 days after surgery) 8. Postoperative nausea and vomiting status (up to 5 days after surgery) 9. Time of off‐bed activity per day (1 week) 10. Distance of off‐bed activity per day (1 week) 11. First exhaust defaecation time (up to 3 days after surgery) 12. Albumin (before the operation; on postoperative days 1, 3 and 5) 13. Prealbumin (before the operation; on postoperative days 1, 3 and 5) 14. WBC (before the operation; on postoperative days 1, 3 and 5) 15. Neutrophil count (NEUT) (before the operation; on postoperative days 1, 3 and 5) 16. C‐reactive protein (CRP) (before the operation; on postoperative days 1, 3 and 5) 17. CD4+ (before the operation; on postoperative days 1, 3 and 5) 18. CD8+ (before the operation; on postoperative days 1, 3 and 5) 19. CD4+ / CD8+ (before the operation; on postoperative days 1, 3 and 5) 20. Hospitalisation expense (up to 1 month after surgery) 21. Anxiety indicators (before the operation; on postoperative day 5) 32. Sleep quality indicators (before the operation; on postoperative day 5) 23. Postoperative complications (up to 1 month after surgery) |
| Starting date | August 2018 |
| Contact information | Shanghai First Maternity and Infant
Hospital
Shanghai, Shanghai, China,
201204
Contact: Xiaoqing Guo, Doctor Tel:
+86‐181117203488 Email: Xiaoqing_Guo@tongji.edu.cn Contact: Na Liu, Doctor Tel: +87‐15601745699 Email: Na_Liu@tongji.edu.cn |
| Notes | Estimated study completion date: 31 December 2021 |
NCT04063072.
| Study name | ERAS (Enhanced Recovery After Surgery) protocol implementation in Piedmont region for hysterectomy of benign or malignant tumors of the uterus. A stepped‐wedge cluster randomized clinical trial |
| Methods | Cross‐over, cluster‐randomised clinical trial |
| Participants | Inclusion criteria: all the hospital wards within the Piemonte region performing hysterectomy; all the participants receiving an elective hysterectomy for benign or malignant tumours of the uterus. Exclusion criteria: hospital wards performing less than 20 expected cases per year; emergency hysterectomy; hysterectomy for pelvic floor disorders; high severity cases not allowing ERAS protocol implementation (i.e. American Society of Anesthesiologists score: ASA V). |
| Interventions | ERAS vs no intervention with usual care |
| Outcomes | 1. Length of stay (12 days after admission)
2.
Recovery after surgery (24 hours after surgery) 3. Complications (30 days after discharge) 4. Transfer to intensive care unit (30 days after surgery) 5. Emergency visits after discharge (30 days after discharge) 6. Hospital admissions after discharge (30 days after discharge) 7. Reintervention (30 days after surgery) 8. Participant satisfaction (15 days after discharge) 9. Healthcare costs (30 days after discharge) |
| Starting date | September 2019 |
| Contact information | Italy
Ospedale Regina Montis
Regalis
Mondovì, Italy Contact: Andrea Puppo, MD Tel: 0039 0174677 ext 470 Email: andrea.puppo@aslcn1.it Contact: Marco Camanni, MD Tel: 0039 011 7095 ext 2531 Email: marco.camanni@aslcittaditorino.it |
| Notes | Estimated study completion date: 31 January 2021 |
ASA: American Society of Anesthesiologists; ERAS: enhanced recovery after surgery; NSAIDS: non‐steroidal anti‐inflammatory drugs;
Differences between protocol and review
We modified the inclusion criteria based on the protocol developed by the previous author team before updating this review.
Type of participants
In the protocol, it was stated that the participants should be diagnosed with gynaecological cancer. However, we found some studies that only partially overlapped with our target population. These studies included participants diagnosed with gynaecologic benign tumour and cancer simultaneously, and did not report separate data from the eligible section of the population. In this review, to avoid the loss of data if these studies were excluded, we applied a less stringent rule and decided to include studies in which the majority (usually >50%) of the participants were diagnosed with gynaecological cancer. Concerning the applicability of including participants who do not meet the eligibility criteria, we conducted sensitivity analyses to test whether the pooled results were influenced by removing studies with mixed participants from the analyses.
Types of comparators
In the protocol, it was stated that we would include as comparators traditional care strategies that do not adhere to the ideas or components of ERAS. In the review, we decided to extend the criteria to reflect the circumstances of clinical practice, by which physicians/surgeons may adopt several ERAS components at their discretion in traditional care when a standardised ERAS programme is not actively introduced to their institutions.
Risk of bias assessment tool
In the protocol, the risk of bias in included studies was planned to be assessed by the first version of the RoB tool (Higgins 2011). Since the previous systematic review did not include any studies and new tools have become available since it was published, we applied the RoB 2 tool when assessing RCTs. The newly‐developed tool reflects current understanding of how the causes of bias influence study results, and the most appropriate ways that risk can be assessed (Higgins 2019).
Contributions of authors
Janita Pak Chun Chau: developed the search strategy; selected which studies to include; carried out the analysis; interpreted the analysis; drafted the final review.
Xu Liu: developed and ran the search strategy; obtained copies of studies; selected which studies to include; extracted data from studies; assessed methodological quality for each included study; entered data into RevMan; carried out the analysis; interpreted the analysis; drafted the final review.
Suzanne Hoi Shan Lo: extracted data from studies; assessed methodological quality for each included study; interpreted the analysis; drafted the final review.
Wai Tong Chien: interpreted the analysis; drafted the final review.
Sze Ki Hui: interpreted the analysis; drafted the final review.
Kai Chow Choi: interpreted the analysis; drafted the final review.
Jie Zhao: selected which studies to include; extracted data from studies; assessed methodological quality for each included study.
Sources of support
Internal sources
No sources of support provided
External sources
-
NIHR, UK
This project was supported by the National Institute for Health Research (NIHR), via Cochrane infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Group.
Declarations of interest
Janita Pak Chun Chau: none known Xu Liu: none known Suzanne Hoi Shan Lo: none known Wai Tong Chien: none known Sze Ki Hui: none known Kai Chow Choi: none known Jie Zhao: none known
Edited (conclusions changed)
References
References to studies included in this review
Dickson 2017 {published data only}
- Dickson EL, Stockwell E, Geller MA, Vogel RI, Mullany SA, Ghebre R, et al.Enhanced recovery program and length of stay after laparotomy on a gynecologic oncology service: a randomized controlled trial. Obstetrics and Gynecology 2017;129:355-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrari 2020 {published data only}
- Ferrari F, Forte S, Sbalzer N, Zizioli V, Mauri M, Maggi C, et al.Validation of an enhanced recovery after surgery protocol in gynecologic surgery: an Italian randomized study. American Journal of Obstetrics & Gynecology 2020;223:e1-14. [DOI: 10.1016/j.ajog.2020.07.003] [DOI] [PubMed] [Google Scholar]
Ho 2020 {published data only}
- Ho CY, Ibrahim Z, Zaid ZA, Daud ZA, Yusop NB, Omar J, et al.Impact of enhanced recovery after surgery with preoperative whey protein-infused carbohydrate loading and postoperative early oral feeding among surgical gynecologic cancer patients: an open-labelled randomized controlled trial. Nutrients 2020;12:264. [DOI: 10.3390/nu12010264] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CY, Ibrahim Z, Zaid ZA, Daud ZA, Yusop NB.Fast-track-recovery surgery with a whey protein-infused carbohydrate-loading drink pre-operatively and early oral feeding postoperatively among surgical gynaecological cancer patients: Study protocol of an open labelled, randomised controlled trial. Trials 2020;21:533. [DOI: 10.1186/s13063-020-04462-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sánchez‐Iglesias 2020 {published data only}
- Sánchez-Iglesias JL, Carbonell-Socias M, Pérez-Benavente MA, Clua SM, Manrique-Muñoz S, Gorriz MG, et al.PROFAST: a randomised trial implementing enhanced recovery after surgery for high complexity advanced ovarian cancer surgery. European Journal of Cancer 2020;136:149-158. [DOI: 10.1016/j.ejca.2020.06.011] [DOI] [PubMed] [Google Scholar]
Shi 2020 {published and unpublished data}
- Cui L, Shi Y, Zhang GN.Fast-track surgery after gynaecological oncological surgery: study protocol for a prospective randomised controlled trial. Trials 2016;17:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Cui L, Zhang G, Shi Y, Wang D, Yang Z.Fast-track surgery after gynaecological oncological surgery: a prospective randomized trial. Research Square 2020 June 1 [preprint version 1]. [DOI: 10.21203/rs.3.rs-22182/v1] [DOI]
Zhang 2016 {published data only}
- Zhang SM, Wang YK, Chen L.Effect of fast track surgery on immune function after laparoscopic radical hysterectomy in patients with cervical cancer. Chinese Journal of Practical Gynecology and Obstetrics 2015;31(8):754-8. [Google Scholar]
- Zhang SM.The effect of enhanced recovery after surgery on patients undergoing laparoscopic radical resection of cervical cancer [Doctoral dissertation]. China, Qingdao: Qingdao University, 2016. [cdmd.cnki.com.cn/Article/CDMD-11065-1016772898.htm] [Google Scholar]
Zhou 2020 {published data only}
- Zhou Y, Liu Y, Jiang H, Zhou Y, Zhou S.Application of enhanced recovery after surgery nursing in patients with minimally invasive surgery of gynecological malignant tumors. Chinese Journal of Modern Nursing 2020;26(12):1582-6. [DOI: 10.3760/cma.j.cn115682-20190716-02531] [DOI] [Google Scholar]
References to studies excluded from this review
Agarwal 2019 {published data only}
- Agarwal R, Rajanbabu A, Nitu PV, Goel G, Madhusudanan L, Unnikrishnan UG.A prospective study evaluating the impact of implementing the ERAS protocol on patients undergoing surgery for advanced ovarian cancer. International Journal of Gynecological Cancer 2019;29:605-12. [DOI] [PubMed] [Google Scholar]
Belavy 2013 {published data only}
- Belavy D, Janda M, Baker J, Obermair A.Epidural analgesia is associated with an increased incidence of postoperative complications in patients requiring an abdominal hysterectomy for early stage endometrial cancer. Gynecologic Oncology 2013;131(2):423-9. [DOI] [PubMed] [Google Scholar]
Bergstrom 2018 {published data only}
- Bergstrom JE, Scott ME, Alimi Y, Yen T-T, Hobson D, Machado KK, et al.Narcotics reduction, quality and safety in gynecologic oncology surgery in the first year of enhanced recovery after surgery protocol implementation. Gynecologic Oncology 2018;149:554-9. [DOI] [PubMed] [Google Scholar]
Bernard 2021 {published data only}
- Bernard L, McGinnis JM, Su J, Alyafi M, Palmer D, Potts L, et al.Thirty-day outcomes after gynecologic oncology surgery: a single-center experience of enhanced recovery after surgery pathways. Acta Obstetricia et Gynecologica Scandinavica 2021;100(2):353-61. [DOI: 10.1111/aogs.14009] [DOI] [PubMed] [Google Scholar]
Bisch 2018 {published data only}
- Bisch SP, Wells T, Gramlich L, Faris P, Wang X, Tran DT, et al.Enhanced Recovery After Surgery (ERAS) in gynecologic oncology: system-wide implementation and audit leads to improved value and patient outcomes. Gynecologic Oncology 2018;151:117-23. [DOI] [PubMed] [Google Scholar]
Boitano 2018 {published data only}
- Boitano TK, Smith HJ, Rushton T, Johnston MC, Lawson P, Leath CA, et al.Impact of enhanced recovery after surgery (ERAS) protocol on gastrointestinal function in gynecologic oncology patients undergoing laparotomy. Gynecologic Oncology 2018;151:282-6. [DOI] [PubMed] [Google Scholar]
- Gentry ZL, Boitano TK, Smith HJ, Eads DK, Russell JF, Straughn JM Jr.The financial impact of an enhanced recovery after surgery (ERAS)protocol in an academic gynecologic oncology practice. Gynecologic Oncology 2020;156:284-7. [DOI: 10.1016/j.ygyno.2019.11.017] [DOI] [PubMed] [Google Scholar]
Chapman 2016 {published data only}
- Chapman JS, Roddy E, Ueda S, Brooks R, Chen LL, Chen LM.Enhanced recovery pathways for improving outcomes after minimally invasive gynecologic oncology surgery. Obstetrics and Gynecology 2016;128:138-44. [DOI] [PubMed] [Google Scholar]
deGroot 2014 {published data only}
- Groot JJ, Es LE, Maessen JM, Dejong CH, Kruitwagen RF, Slangen BF.Diffusion of enhanced recovery principles in gynecologic oncology surgery: is active implementation still necessary? Gynecologic Oncology 2014;134:570-5. [DOI] [PubMed] [Google Scholar]
Eberhart 2008 {published data only}
- Eberhart LH, Koch T, Ploger B, Wagner U, Wulf H, Zwiorek L, et al.Enhanced recovery after major gynaecological surgery for ovarian cancer - an objective and patient-based assessment of a traditional versus a multimodal "fast track" rehabilitation programme. Anasthesiologie und Intensivmedizin 2008;49(4):180-94. [Google Scholar]
Feng 2008 {published data only}
- Feng S, Chen L, Wang G, Chen A, Qiu Y.Early oral intake after intra-abdominal gynecological oncology surgery. Cancer Nursing 2008;31(3):209-13. [DOI] [PubMed] [Google Scholar]
Gerardi 2008 {published data only}
- Gerardi MA, Santillan A, Meisner B, Zahurak ML, Diaz Montes TP, Giuntoli RL 2nd, et al.A clinical pathway for patients undergoing primary cytoreductive surgery with rectosigmoid colectomy for advanced ovarian and primary peritoneal cancers. Gynecologic Oncology 2008;108(2):282-6. [DOI] [PubMed] [Google Scholar]
Janda 2014 {published data only}
- Janda M, Graves N, Bauer J, Baker J, Obermair A.Quality of life and nutritional status after early enteral feeding versus standard care after surgery for advanced epithelial ovarian cancer. International Journal of Gynecological Cancer 2014;24(9):45. [Google Scholar]
Kalogera 2013 {published data only}
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, Trabuco E, Lovely JK, Dhanorker S, et al.Enhanced recovery in gynecologic surgery. Obstetrics and Gynecology 2013;122:319-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kay 2020 {published data only}
- Kay AH, Venn M, Urban R, Gray HJ, Goff B.Postoperative narcotic use in patients with ovarian cancer on an enhanced recovery after surgery (ERAS) pathway. Gynecologic Oncology 2020;156:624-8. [DOI: 10.1016/j.ygyno.2019.12.018] [DOI] [PubMed] [Google Scholar]
Lambaudie 2017 {published data only}
- Lambaudie E, Nonneville A, Brun C, Laplane C, N'Guyen Duong L, Boher J-M, et al.Enhanced recovery after surgery program in Gynaecologic Oncological surgery in a minimally invasive techniques expert center. BMC surgery 2017;17:136-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marx 2006 {published data only}
- Marx CI, Rasmussen T, Jakobsen DH, Ottosen C, Lundvall L, Ottesen BS, et al.Accelerated course after operation for ovarian cancer. Ugeskrift for Laeger 2006;168:1533-6. [PubMed] [Google Scholar]
- Marx CI, Rasmussen T, Jakobsen DH, Ottosen C, Lundvall L, Ottesen BS, et al.The effect of accelerated rehabilitation on recovery after surgery for ovarian malignancy. Acta Obstetricia et Gynecologica 2006;85:488-92. [DOI] [PubMed] [Google Scholar]
Mendivil 2018 {published data only}
- Mendivil AA, Busch JR, Richards DC, Vittori H, Goldstein BH.The impact of an enhanced recovery after surgery program on patients treated for gynecologic cancer in the community hospital setting. International Journal of Gynecological Cancer 2018;28:581-5. [DOI] [PubMed] [Google Scholar]
Meyer 2018 {published data only}
- Harrison RF, Li Y, Guzman A, Pitcher B, Rodriguez-Restrepo A, Cain KE, et al.Impact of implementation of an enhanced recovery program in gynecologic surgery on healthcare costs. American Journal of Obstetrics and Gynecology 2020;222:e1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer LA, Lasala J, Iniesta MD, Nick AM, Munsell MF, Shi Q, et al.Effect of an enhanced recovery after surgery program on opioid use and patient-reported outcomes. Obstetrics and Gynecology 2018;132:281-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2015 {published data only}
- Miller EC, McIsaac DI, Chaput A, Antrobus J, Shenassa H, Lui A.Increased postoperative day one discharges after implementation of a hysterectomy enhanced recovery pathway: a retrospective cohort study. Journal Canadien d'Anesthesie 2015;62(5):451-60. [DOI] [PubMed] [Google Scholar]
Mukhopadhyay 2015 {published data only}
- Mukhopadhyay D, Khalil Razvi.Enhanced recovery programme in gynaecology: outcomes of a hysterectomy care pathway. BMJ Quality Improvement Reports 2015;4(1):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Myriokefalitaki 2016 {published data only}
- Myriokefalitaki E, Smith M, Ahmed AS.Implementation of enhanced recovery after surgery (ERAS) in gynaecological oncology. Archives of Gynecology and Obstetrics 2016;294:137-43. [DOI] [PubMed] [Google Scholar]
Pather 2011 {published data only}
- Pather S, Loadsman JA, Mansfield C, Rao A, Arora V, Philp S, et al.Perioperative outcomes after total laparoscopic hysterectomy compared with fast-track open hysterectomy - a retrospective case-control study. Australian and New Zealand Journal of Obstetrics and Gynaecology 2011;51(5):393-6. [DOI] [PubMed] [Google Scholar]
Renaud 2019 {published data only}
- Renaud MC, Belanger L, Lachapelle P, Gregoire J, Sebastianelli A, Plante M.Effectiveness of an enhanced recovery after surgery program in gynaecology oncologic surgery: a single-centre prospective cohort study. Journal of Obstetrics and Gynaecology Canada 2019;41:436-42. [DOI] [PubMed] [Google Scholar]
Sanad 2019 {published data only}
- Sanad AS, El-Gindi E, El-Khateeb RR, Abdl-Ghany AM, Abdelrazik A, Mousa A, et al.Implementation of enhanced recovery after surgery for endometrial carcinoma: a non-randomized controlled trial. Indian Journal of Public Health Research and Development 2019;10:1979-84. [Google Scholar]
Schwartz 2019 {published data only}
- Schwartz AR, Lim S, Broadwater G, Cobb L, Valea F, Marosky Thacker J, et al.Reduction in opioid use and postoperative pain scores after elective laparotomy with implementation of enhanced recovery after surgery protocol on a gynecologic oncology service. International Journal of Gynecological Cancer 2019;29:935-43. [DOI] [PubMed] [Google Scholar]
Shen 2019 {published data only}
- Shen X.The application of enhanced recovery after surgery in laparoscopic radical hysterectomy of cervical cancer [Masters thesis]. China, Chongqing: Chongqing Medical University, 2019. [cdmd.cnki.com.cn/Article/CDMD-10631-1019660791.htm] [Google Scholar]
Wijk 2014 {published data only}
- Wijk L, Franzen K, Ljungqvist O, Nilsson K.Implementing a structured Enhanced Recovery after Surgery (ERAS) protocol reduces length of stay after abdominal hysterectomy. Acta Obstetricia et Gynecologica Scandinavica 2014;93(8):749-56. [DOI] [PubMed] [Google Scholar]
Zhang 2019 {published data only}
- Zhang CY, Ouyang L.The effect of the enhanced recovery after surgery in the radical operation of cervical cancer. Chinese Journal of Minimally Invasive Surgery 2019;19:145-8, 177. [Google Scholar]
References to ongoing studies
ChiCTR1900025117 {published data only}
- ChiCTR1900025117.Enhanced recovery after laparoscopic surgery (ERAS) in gynecologic oncology: a prospective, randomized controlled trial. www.chictr.org.cn/com/25/showprojen.aspx?proj=42068 (first received 12 August 2019).
NCT02864277 {published data only}
- NCT02864277.Enhanced recovery after surgery gynecology oncology (ERAS) [Enhanced recovery after surgery (ERAS) program versus conventional perioperative strategies in patients undergoing gynecologic oncologic surgery: A randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT02864277 (first received 12 August 2016).
NCT03640299 {published data only}
- NCT03640299.The safety and efficacy of enhanced recovery after surgery on clinical and immune outcomes for gynecological oncology. clinicaltrials.gov/show/NCT03640299 (first received 21 August 2018). [Google Scholar]
NCT04063072 {published data only}
- NCT04063072.ERAS (Enhanced Recovery After Surgery) protocol omplementation in Piedmont region for hysterectomy. (ERAS-Gyneco) [ERAS (Enhanced Recovery After Surgery) protocol implementation in Piedmont region for hysterectomy of benign or malignant tumors of the uterus: A stepped-wedge cluster randomized clinical trial]. clinicaltrials.gov/show/NCT04063072 (first received 21 August 2019). [Google Scholar]
Additional references
Aasa 2013
- Aasa A, Hovbäck M, Berterö CM.The importance of preoperative information for patient participation in colorectal surgery care. Journal of Clinical Nursing 2013;22:1604-12. [DOI: 10.1111/jocn.12110] [DOI] [PubMed] [Google Scholar]
Arbyn 2020
- Arbyn M, Weiderpass E, Bruni L, Sanjosé S, Saraiya M, Ferlay J, et al.Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Global Health 2020;8(2):e191-e203. [DOI: 10.1016/S2214-109X(19)30482-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ardò 2018
- Ardò NP, Loizzi D, Panariti S, Piccinin I, Sollitto F.Enhanced recovery pathways in thoracic surgery from Italian VATS group: nursing care program. Journal of Thoracic Disease 2018;10:S529-34. [DOI: 10.21037/jtd.2017.12.85] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arnold 2015
- Arnold A, Aitchison LP, Abbott J.Preoperative mechanical bowel preparation for abdominal, laparoscopic, and vaginal surgery: a systematic review. Journal of Minimally Invasive Gynecology 2015;22:737-52. [DOI] [PubMed] [Google Scholar]
ASAC 2017
- American Society of Anesthesiologists Committee.Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists task force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology 2017;126(3):376-93. [DOI] [PubMed] [Google Scholar]
Baimas‐George 2020
- Baimas-George M, Cochran A, Tezber K, Kirks RC, Addor V, Baker E, et al.A 2-year experience with enhanced recovery after surgery: evaluation of compliance and outcomes in pancreatic surgery. Journal of Nursing Care Quality 2021;36(2):E24-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bhandoria 2020
- Bhandoria GP, Bhandarkar P, Ahuja V, Maheshwari A, Sekhon RK, Gultekin M, et al.Enhanced Recovery After Surgery (ERAS) in gynecologic oncology: an international survey of peri-operative practice. International Journal of Gynecological Cancer 2020;30:1471-8. [DOI: 10. 1136/ ijgc-2020- 001683] [DOI] [PubMed] [Google Scholar]
Cao 2012
- Cao F, Li J, Li F.Mechanical bowel preparation for elective colorectal surgery: updated systematic review and meta-analysis. International Journal of Colorectal Disease 2012;27(6):803-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chambers 2014
- Chambers D, Paton F, Wilson P, Eastwood A, Craig D, Fox D, et al.An overview and methodological assessment of systematic reviews and meta-analyses of enhanced recovery programmes in colorectal surgery. BMJ open 2014;4(5):e005014. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence.Version accessed 27 October 2020. Melbourne, Australia: Veritas Health Innovation. Available at www.covidence.org.
Dahabreh 2015
- Dahabreh IJ, Steele DW, Shah N, Trikalinos TA.Oral mechanical bowel preparation for colorectal surgery: systematic review and meta-analysis. Diseases of the Colon and Rectum 2015;58(7):698-707. [PMID: ] [DOI] [PubMed] [Google Scholar]
Debono 2019
- Debono B, Corniola MV, Pietton R, Sabatier P, Hamel O, Tessitore E.Benefits of Enhanced Recovery After Surgery for fusion in degenerative spine surgery: impact on outcome, length of stay, and patient satisfaction. Neurosurgical Focus 2019;46(4):E6. [PMID: ] [DOI] [PubMed] [Google Scholar]
De Groot 2016
- De Groot JJ, Ament SM, Maessen JM, Dejong CH, Kleijnen JM, Slangen BF.Enhanced recovery pathways in abdominal gynecologic surgery: a systematic review and meta-analysis. Acta Obstetricia et Gynecologica Scandinavica 2016;95:382-95. [DOI: 10.1111/aogs.12831] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT.Version accessed 6 August 2016. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Hassinger 2019
- Hassinger TE, Mehaffey JH, Martin AN, Bauer-Nilsen K, Turrentine FE, Thiele RH, et al.Implementation of an enhanced recovery protocol is associated with on-time initiation of adjuvant chemotherapy in colorectal cancer. Diseases of the Colon and Rectum 2019;62(11):1305-15. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al.The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA.Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:205-228. [Google Scholar]
Kalogera 2019
- Kalogera E, Glaser GE, Kumar A, Dowdy SC, Langstraat CL.Enhanced recovery after minimally invasive gynecologic procedures with bowel surgery: a systematic review. Journal of Minimally Invasive Gynecology 2019;26:288-98. [DOI: 10.1016/j.jmig.2018.10.016] [DOI] [PubMed] [Google Scholar]
Kehlet 1997
- Kehlet H.Multimodal approach to control postoperative pathophysiology and rehabilitation. British Journal of Anaesthesia 1997;78(5):606-17. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kehlet 2008
- Kehlet H.Fast-track colorectal surgery. Lancet 2008;371:791-3. [DOI: 10.1016/S0140-6736(08)60357-8] [DOI] [PubMed] [Google Scholar]
Ledford 2019
- Ledford LR, Lockwood S.Scope and epidemiology of gynecologic cancers: an overview. Seminars in Oncology Nursing 2019;35(2):147-50. [DOI: 10.1016/j.soncn.2019.03.002] [DOI] [PubMed] [Google Scholar]
Leon Arellano 2020
- Leon Arellano M, Tejedor P, Guadalajara H, Ortega M, Garcia Olmo D, Pastor C.Evolution of perioperative quality of life in patients under enhanced recovery after surgery care in colorectal cancer. Revista espanola de enfermedades digestivas: organo oficial de la Sociedad Espanola de Patologia Digestiva 2020;112(2):127-32. [PMID: ] [DOI] [PubMed] [Google Scholar]
Li J 2019
- Li J, Kong XX, Zhou JJ, Song YM, Huang XF, Li GH, et al.Fast-track multidisciplinary treatment versus conventional treatment for colorectal cancer: a multicenter, open-label randomized controlled study. BMC cancer 2019;19(1):988. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindemann 2017
- Lindemann K, Kok PS, Stockler M, Jaaback K, Brand A.Enhanced recovery after surgery for advanced ovarian cancer: a systematic review of interventions trialed. International Journal of Gynecological Cancer 2017;27:1274-82. [DOI: 10.1097/IGC.0000000000000981] [DOI] [PubMed] [Google Scholar]
Li S 2017
- Li S, Zhou K, Che G, Yang M, Su J, Shen C, et al.Enhanced recovery programs in lung cancer surgery: systematic review and meta-analysis of randomized controlled trials. Cancer Management and Research 2017;9:657-70. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li T 2019
- Li T, Higgins JP, Deeks JJ, editor(s).Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:109-42. [Google Scholar]
Liu 2020
- Liu B, Liu S, Wang Y, Zhao L, Zheng T, Chen L, et al.Enhanced recovery after intraspinal tumor surgery: a single-institutional randomized controlled study. World Neurosurgery 2020;136:e542-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Li Z 2017
- Li Z, Wang Q, Li B, Bai B, Zhao Q.Influence of enhanced recovery after surgery programs on laparoscopy-assisted gastrectomy for gastric cancer: a systematic review and meta-analysis of randomized control trials. World Journal of Surgical Oncology 2017;15(1):207. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ljungqvist 2017
- Ljungqvist O, Scott M, Fearon KC.Enhanced recovery after surgery: a review. JAMA Surgery 2017;152(3):292-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lohsiriwat 2019
- Lohsiriwat V, Jitmungngan R.Enhanced recovery after surgery in emergency colorectal surgery: review of literature and current practices. World Journal of Gastrointestinal Surgery 2019;11(2):41-52. [PMID: ] [DOI] [PMC free article] [PubMed]
Luo 2018
- Luo D, Wan X, Liu J, Tong T.Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Statistical Methods in Medical Research 2018;27:1785-1805. [DOI] [PubMed] [Google Scholar]
Melloul 2020
- Melloul E, Lassen K, Roulin D, Grass F, Perinel J, Adham M, et al.Guidelines for perioperative care for pancreatoduodenectomy: enhanced recovery after surgery (ERAS) recommendations 2019. World Journal of Surgery 2020;44(7):2056-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nelson 2014
- Nelson G, Kalogera E, Dowdy SC.Enhanced recovery pathways in gynecologic oncology. Gynecologic Oncology 2014;135:586-94. [DOI: 10.1016/j.ygyno.2014.10.006] [DOI] [PubMed] [Google Scholar]
Nelson 2019
- Nelson G, Bakkum-Gamez J, Kalogera E, Glaser G, Altman A, Meyer LA, et al.Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations - 2019 update. International Journal of Gynecological cancer 2019;29(4):651-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ni 2019
- Ni X, Jia D, Chen Y, Wang L, Suo J.Is the enhanced recovery after surgery (ERAS) program effective and safe in laparoscopic colorectal cancer surgery? A meta-analysis of randomized controlled trials. Journal of Gastrointestinal Surgery 2019;23(7):1502-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nicholson 2014
- Nicholson A, Lowe MC, Parker J, Lewis SR, Alderson P, Smith AF.Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. British Journal of Surgery 2014;101(3):172-88. [PMID: ] [DOI] [PubMed] [Google Scholar]
Page 2019
- Page MJ, Higgins JP, Sterne JA.Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook.
Pisarska 2016
- Pisarska M, Pedziwiatr M, Malczak P, Major P, Ochenduszko S, Zub-Pokrowiecka A, et al.Do we really need the full compliance with ERAS protocol in laparoscopic colorectal surgery? A prospective cohort study. International Journal of Surgery 2016;36(Pt A):377-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ringwald 2017
- Ringwald J, Marwedel L, Junne F, Ziser K, Schaffeler N, Gerstner L, et al.Demands and needs for psycho-oncological eHealth interventions in women with cancer: cross-sectional study. JMIR Cancer 2017;3(2):e19. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rouxel 2019
- Rouxel P, Beloeil H.Enhanced recovery after hepatectomy: a systematic review. Anaesthesia, Critical Care & Pain Medicine 2019;38(1):29-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sekse 2019
- Sekse RJ, Dunberger G, Olesen ML, Osterbye M, Seibaek L.Lived experiences and quality of life after gynaecological cancer - an integrative review. Journal of Clinical Nursing 2019;28(9-10):1393-421. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shakir 2017
- Shakir B.A systematic review on Enhanced Recovery After Surgery (ERAS) pathways designed for women undergoing elective open abdominal surgery due to advanced ovarian cancer [Masters thesis]. Lithuanian University of Health Sciences, 2017. [hdl.handle.net/20.500.12512/104524] [Google Scholar]
Smith 2014
- Smith MD, McCall J, Plank L, Herbison GP, Soop M, Nygren J.Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database of Systematic Reviews 2014, Issue 8. Art. No: CD009161. [DOI: 10.1002/14651858.CD009161.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2020
- Song Z, Wang Y, Zhang D, Zhou Y.Enhanced Recovery After Surgery (ERAS) in gynaecology: a meta-analysis.. Authorea Preprints 23 August 2020. [DOI: 10.22541/au.159818470.01003468] [DOI] [Google Scholar]
Spanjersberg 2011
- Spanjersberg WR, Reurings J, Keus F, Laarhoven CJ.Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database of Systematic Reviews 2011, Issue 2. Art. No: CD007635. [DOI: 10.1002/14651858.CD007635.pub2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stark 2013
- Stark PA, Myles PS, Burke JA.Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology 2013;118:1332-40. [DOI: 10.1097/ALN.0b013e318289b84b] [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T.Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 2020
- Watson LJ, Ewers C.Enhanced recovery after head and neck cancer surgery: a review of current literature. Current Opinion in Otolaryngology & Head and Neck Surgery 2020;28(3):161-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
WHO 2021
- World Health Organization International Agency for Research on Cancer.Globocan 2020 Population fact sheets (World) [ ]. gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf 2021 .
Wickenbergh 2020
- Wickenbergh E, Nilsson L, Bladh M, Kjølhede P, Wodlin NB.Agreements on perceived use of principles for Enhanced Recovery AfterSurgery between patients and nursing staff in a gynecological ward. European Journal of Obstetrics & Gynecology and Reproductive Biology 2020;250:216-23. [DOI: 10.1016/j.ejogrb.2020.04.014] [DOI] [PubMed] [Google Scholar]
Wu 2019
- Wu X, Kong W, Zhu Q, Wang W, Xu H, Zhou S, et al.Improved perioperative quality of life in endoscopic sinus surgery by application of enhanced recovery after surgery. Therapeutics and Clinical Risk Management 2019;15:683-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zalewski 2018
References to other published versions of this review
Lu 2015
- Lu D, Wang X, Shi G.Perioperative enhanced recovery programmes for gynaecological cancer patients. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD008239. [DOI: 10.1002/14651858.CD008239.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


