Abstract
Mesenchymal stromal cells, commonly referred to as MSCs, have emerged as a promising cell-based therapy for a range of autoimmune diseases thanks to several therapeutic advantages. Key among these are: 1) the ability to modulate innate and adaptive immune responses and to promote tissue regeneration, 2) the ease of their isolation from readily accessible tissues and expansion at scale in culture, 3) their low immunogenicity enabling use as an allogeneic “off-the-shelf” product, and 4) MSC therapy’s safety and feasibility in humans, as demonstrated in more than one thousand clinical trials. Evidence from preclinical studies and early clinical trials indicate the therapeutic potential of MSCs and their derivatives for efficacy in ocular autoimmune diseases such as autoimmune uveoretinitis and Sjögren’s syndromerelated dry eye disease. In this review, we provide an overview of the current understanding of the therapeutic mechanisms of MSCs, and summarize the results from preclinical and clinical studies that have used MSCs or their derivatives for the treatment of ocular autoimmune diseases. We also discuss the challenges to the successful clinical application of MSC therapy, and suggest strategies for overcoming them.
Keywords: dry eye disease, mesenchymal stem cell, mesenchymal stromal cell, ocular autoimmune disease, Sjögren’s syndrome, uveitis, uveoretinitis
1. Introduction
Mesenchymal stromal cells, commonly abbreviated as MSCs, are a heterogeneous population of the spindle-shaped, plastic-adherent cells isolated from various connective tissues including bone marrow (BM), adipose tissue (AT) and umbilical cord (UC). MSCs are further defined by their expression of several mesodermal markers, their lack of hematopoietic or endothelial markers, their capability to differentiate into the three mesodermal lineages (osteoblasts, chondrocytes and adipocytes) under in vitro conditions (Viswanathan et al., 2019). MSCs can also differentiate into other types of cells and be readily expanded in vitro. Therefore, MSCs were initially of interest as easily accessible autologous stem cells to replace damaged tissue-resident cells in diseases such as osteogenesis imperfecta (Horwitz et al., 1999) and cartilage defect (Noel et al., 2002). However, the prominent feature of MSCs that has prompted a large number of preclinical and clinical studies is their hitherto unexpected inhibition of T lymphocyte proliferation both in vitro (Di Nicola et al., 2002) and in vivo (Bartholomew et al., 2002). Subsequent to these observations, the immunosuppressive functions of MSCs were supported by seminal clinical trials in which BM-derived MSCs were shown to treat acute steroid-resistant graft-versushost disease (GVHD) in patients (Le Blanc et al., 2004; Le Blanc et al., 2008).
The indication that MSCs suppress the immune response opened up the possibility of using them for the treatment of autoimmune diseases driven primarily by Th1 and Th17 cells. This possibility was first tested in mice with experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (MS). The results demonstrated that intravenous (IV) administration of human or syngeneic mouse MSCs significantly ameliorated the clinical course of EAE, reduced demyelination and leukocyte infiltration, induced T cell anergy, and stimulated oligodenrogenesis (Zappia et al., 2005; Zhang et al., 2005). The efficacy of MSCs in modulating immune response and enhancing tissue regeneration has since been examined in numerous preclinical models and clinical trials for a broad range of autoimmune diseases, such as inflammatory bowel disease, MS, type 1 diabetes, systemic lupus erythematosus, rheumatoid arthritis and systemic sclerosis. Currently, autoimmune diseases comprise one of the most frequent indications among 1199 MSC clinical trials registered internationally (https://celltrials.org/public-cells-data/msc-trials-20112018/65). At the time of the writing of this review, approximately 150 MSC-based clinical trials targeting autoimmune diseases have been registered in the official database of the US National Institutes of Health (NIH) (http://clinicaltrials.gov/); most of these trials are phase I/II, less than 5% being phase III trials.
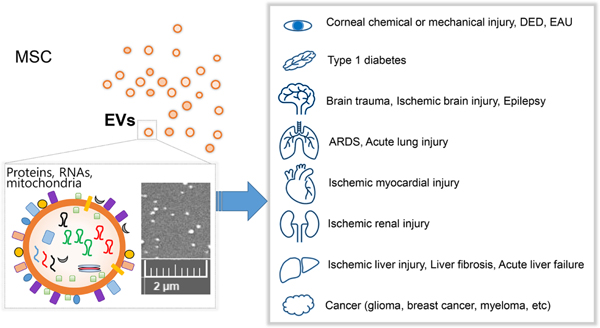
With regard to ocular autoimmune diseases, multiple groups have explored the therapeutic potential and mechanism of action of MSCs mainly in animal models of experimental autoimmune uveoretinitis (EAU) and Sjögren’s syndrome (SS)-related dry eye disease (DED). Evidence from preclinical studies have suggested that exogenous administration of MSCs attenuates the development and progression of autoimmune inflammation in the eye through repression of Th1 and Th17 cells and induction of immunoregulatory cells, leading to protection of ocular structure and function. Clinical trials with allogeneic MSCs or their extracellular vesicles (EVs) are underway for the treatment of DED in patients with chronic GVHD (NCT04213248) and SS (NCT03878628, NCT04615455). As the mainstay of treatment for ocular autoimmune inflammation is still limited to the use of corticosteroids and cytotoxic immunosuppressive drugs, novel MSC-based approaches aimed at both restoring immune homeostasis and fostering tissue regeneration would hold promise for the development of effective cell-derived therapeutics for ocular autoimmune diseases.
In this review, first, we present an overview of the therapeutic mechanisms of MSCs. Second, we review the results from preclinical and clinical studies with MSCs and their derivatives for the treatment of ocular autoimmune diseases. Third, we describe the risks and challenges that may impede successful clinical translation of MSCs. Finally, we discuss strategies for overcoming these hurdles and optimizing MSC-based therapies for clinical application.
2. Paradigm evolution in the therapeutic mechanism of MSCs
MSCs, given their potential to differentiate into other cell types in vitro, were originally expected to promote tissue repair by directly replacing damaged cells. Engraftment and differentiation of MSCs were indeed observed under certain conditions after their local administration in large numbers; one example is their injection into the knees of rabbits with surgically induced cartilage defects (Koga et al., 2008). However, most studies have demonstrated no evidence of robust engraftment and/or differentiation of MSCs in vivo after systemic administration. Moreover, administration of conditioned media derived from MSC cultures has been shown to reproduce many of the therapeutic effects of MSCs (Jabbehdari et al., 2020; Oh et al., 2008; Oh et al., 2014b; Rabiee et al., 2020), and furthermore, proteomic analysis has revealed that MSC secretome contains a wide array of trophic factors that are vital to tissue regeneration and immune regulation (for a review, see Lee et al., 2011). Based on these observations, the paradigm has evolved from the original presumption, that MSCs repair tissues through their transdifferentiation, to the current paradigm that MSCs exert their therapeutic effects through paracrine factors that they produce upon cross-talk with the microenvironment (Prockop and Oh, 2012a). Over the past decade, research thus has shifted towards identifying the therapeutic factors of MSCs and harnessing their paracrine activity to stimulate tissue repair and modulate excessive immune response.
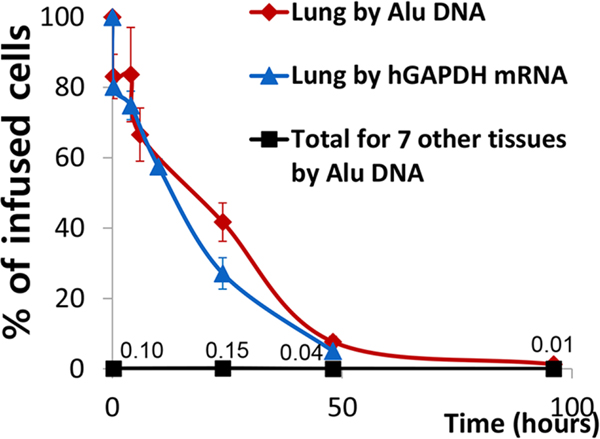
Our group was one of the first to shape the current paradigm. We performed IV infusion of human BM-derived MSCs into immunodeficient NOD/scid mice after induction of myocardial infarction (MI), and observed that MSCs decreased inflammatory response and improved cardiac function despite transient and small engraftment of MSCs in the damaged heart (Lee et al., 2009a). To understand the kinetics of MSC survival, we quantitated the number of MSCs in the major organs of mice with time after IV injection by using a real-time PCR assay for Alu sequence, a human genetic marker that is sensitive enough to detect hundreds of human cells in mouse tissues (Figure 1). The results showed that up to 85% of human MSCs were trapped in the lung within a few minutes post-infusion. Meanwhile, less than 1% of the infused MSCs briefly appeared in the heart after 24 to 48 hours, and a mere 0.01% of MSCs were found after 96 hours in peripheral organs including the heart and spleen. Similar observations were reported with syngeneic mouse MSCs that had been infused intraperitoneally or intravenously into immunocompetent mice (Gao et al., 2001; Leibacher and Henschler, 2016).
Figure 1.
Kinetics of live MSCs in the lung and 7 other tissues after IV infusion of 2 × 106 human MSCs into mice.
The kinetics and redistribution of human BM-derived MSCs after IV administration into mice were evaluated by a real-time PCR assay for human Alu sequence and GAPDH. About 85% of the human BM-derived MSCs were initially trapped in the lung within a few minutes after IV infusion and disappeared with a half-life of 24 hours. The cells did not appear in any significant numbers in other tissues, 0.04% of the infused MSCs having been recovered after 48 hours and 0.01% after 96 hours.
Reprinted with permission from Cell Stem Cell. Lee et al., 2009. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5, 54–63. Copyright © 2009 Elsevier Inc.
To investigate how MSCs trapped in the lung could act at distance to rescue the heart, we analyzed the transcriptome of the infused MSCs by microarrays and compared it with that of control MSCs before infusion (Lee et al., 2009a). Amongst the upregulated genes in MSCs after IV administration, TNFα-stimulated gene/protein-6 (TSG-6) was of particular interest, given its status as a multifunctional protein with anti-inflammatory and tissue-protective properties (Day and Milner, 2019). Remarkably, transient transduction of MSCs with TSG-6 siRNA abrogated the therapeutic benefits of MSCs in mice with MI, and infusion of recombinant human TSG-6 replicated the effects of MSCs. In a further mechanistic study, we found that TSG-6 secreted by MSCs inhibited pro-inflammatory activation of resident macrophages through downregulation of Toll-like receptor (TLR)-2/NF-κB signaling, thereby reducing the production of chemokines and recruitment of neutrophils (Choi et al., 2011). Consistent with these results, the role of TSG-6 in mediating the therapeutic action of MSCs has been confirmed by subsequent studies utilizing various animal models of human diseases of the pancreas (He et al., 2016; Kota et al., 2013; Li et al., 2018), intestines (An et al., 2020; Song et al., 2017; Song et al., 2018b; Yang et al., 2019), skin (Liu et al., 2016; Qi et al., 2014), kidney (Chen et al., 2019), lung (Chaubey et al., 2018; Wang et al., 2018), liver (Wan et al., 2020), brain (Jha et al., 2019; Liu et al., 2019; Roura et al., 2020) and eye (Ko et al., 2016; Lee et al., 2014; Oh et al., 2012b; Roddy et al., 2011; Song et al., 2018a; Yun et al., 2017). Moreover, an elegant study by Romano et al. demonstrated that MSCs derived from BM of TSG-6−/− mice displayed severe perturbation in the expression of transcription factors, cytokines and genes related to key biological pathways (Romano et al., 2019). TSG-6−/− MSCs differed from wild-type MSCs in terms of morphology, reduced-size EVs, decreased cell-proliferative rate, loss of differentiation potential, and increased pro-inflammatory activity. Therefore, the results collectively indicate that TSG-6 does not only mediate the therapeutic effects of MSCs but also plays a crucial role in the maintenance of their biological properties.
Several factors, in addition to TSG-6, that have been reported to derive from activated MSCs also have been implicated in MSC-mediated immunomodulation (for reviews, see Lee et al., 2011; Prockop and Oh, 2012b; Spees et al., 2016). Among them, indoleamine-2,3-dioxygenase (IDO), an enzyme that catabolizes l-tryptophan and blocks T cell proliferation (Lee et al., 2002), is one of the most widely investigated. It was shown that both human and murine MSCs upregulated the expression of functional IDO in response to the pro-inflammatory cytokine IFN-γ, and that IDO activity was required for MSCs to suppress T cell proliferation and induce monocyte polarization into immunosuppressive macrophages (English et al., 2007; Francois et al., 2012; Meisel et al., 2004). Programmed death-ligand 1 (PD-L1) and Fas ligand (FasL), the molecules regulating T cell activation and apoptosis, also have been shown to be secreted by human and mouse MSCs and mediate the effects of MSCs on the suppression of T cell proliferation, induction of T cell apoptosis and expansion of regulatory T (Treg) cells (Akiyama et al., 2012; Davies et al., 2017; Gu et al., 2013). Moreover, TGF-β, hepatocyte growth factor (HGF), prostaglandin E2 (PGE2) and nitric oxide (NO) have been implicated as MSC-derived suppressors of T cell response (Aggarwal and Pittenger, 2005; Di Nicola et al., 2002; Ren et al., 2008; Sato et al., 2007). Of note, NO has been shown to mediate only the function of murine MSCs, whereas IDO mediates the immunosuppressive action of human or monkey MSCs (Ren et al., 2009).
Although many studies have identified different factors as effectors of the immunomodulatory effects of MSCs, it is a common finding that MSCs are activated to secrete therapeutic factors in response to inflammatory stimuli in the injury microenvironment. For instance, the expression of IDO, PD-L1, FasL and TSG-6 in cells is transcriptionally induced by activation of NF-κB and interferon regulatory factors (Antonangeli et al., 2020; Gowrishankar et al., 2015; Hsu et al., 1999; Robinson et al., 2005), and these transcription factors are activated by inflammatory cytokines and TLRs. Therefore, it can be postulated that MSCs in an inflammatory microenvironment release various therapeutic factors, and that the intricate interactions among these factors as well as the actions of individual factors contribute in concert to the beneficial effects of MSCs. In support for this notion, a recent study by Wang et al. discovered a direct link between IDO and TSG-6 in MSCs, and demonstrated that kynurenic acid, an IDO metabolite, controlled the anti-inflammatory effects of human UC-derived MSCs through binding to the TSG-6 promoter and upregulation of TSG-6 expression (Wang et al., 2018).
3. MSCs for the treatment of autoimmune DED
3.1. Lessons from sterile corneal injury models
3.1.1. Anti-inflammatory effects of MSCs
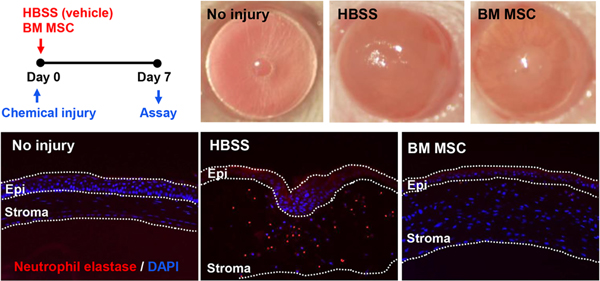
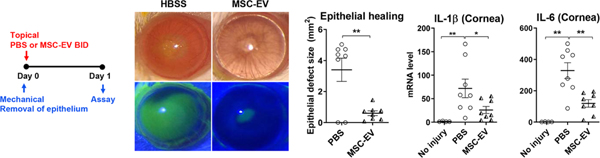
We have long observed that exogenous application of MSCs or MSC-derived factors promotes corneal epithelial regeneration and preserves corneal transparency after sterile injury to the cornea. To examine the effect of MSCs on the cornea, we employed a rodent model of corneal chemical injury whereby the corneal and limbal epithelia were mechanically removed after 30 sec exposure to ethanol. As in sterile injuries to other organs (Shen et al., 2013), serial waves of neutrophil infiltration into the cornea ensue after chemical injury, and cellular triggers of this sterile neutrophilic inflammation involve cornea-resident macrophages (Oh et al., 2012a). In both rat and mouse models, corneal inflammation was suppressed and corneal epithelial healing was facilitated by intraperitoneal (IP) or IV injection of human BM MSCs (Roddy et al., 2011), by IV injection of human induced pluripotent stem cell (iPSC)-derived MSCs (Yun et al., 2017), and by topical application of allogeneic rat MSCs (Oh et al., 2008) (Figure 2). Moreover, some of the beneficial effects on the cornea were reproduced by topical application of MSC-conditioned medium (Oh et al., 2008), thus supporting the role for MSC-secreted paracrine factors. Consistent with our observation in MI mice (Lee et al., 2009a), knockdown of TSG-6 largely abrogated the effects of human BM or iPSC-derived MSCs on the cornea following chemical injury (Roddy et al., 2011; Yun et al., 2017), whereas overexpression of TSG-6 in human MSCs augmented them (Lee et al., 2014). Also, in the same model, the effects of MSCs were essentially replicated by IV infusion (Roddy et al., 2011), topical instillation (Roddy et al., 2011) and intracameral injection of recombinant human TSG-6 (Oh et al., 2010).
Figure 2.
Anti-inflammatory effects of MSCs on the cornea.
Human BM-derived MSCs or vehicle (Hank’s balanced salt solution, HBSS) were administered into mice via tail vein injection immediately after sterile injury to the cornea. Seven days later, the cornea was evaluated clinically for opacity and histologically for neutrophil infiltration. MSCs significantly reduced the development of corneal opacity and neutrophil infiltration.
Reprinted with permission from Cytotherapy. Yun et al., 2017. Comparison of the anti-inflammatory effects of induced pluripotent stem cell-derived and bone marrow-derived mesenchymal stromal cells in a murine model of corneal injury. Cytotherapy 19, 28–35. Copyright © 2017 Elsevier Inc.
3.1.2. Anti-apoptotic and pro-proliferative effects of MSCs
Furthermore, we found that the therapeutic effects of MSCs are attributable not only to the anti-inflammatory action on the cornea but also to the anti-apoptotic and pro-proliferative effects on corneal epithelial progenitor cells. By using an in vitro culture system of corneal epithelial cells and an ex vivo tissue culture system of the whole cornea, we demonstrated that the conditioned medium derived from human BM MSCs, but not from human dermal fibroblasts, stimulated the proliferation of human corneal epithelial progenitor cells in culture following ethanol injury, while inhibiting apoptosis (Oh et al., 2014b). In addition, the MSC-conditioned medium accelerated the wound healing of the corneal epithelium in tissue cultures of rabbit corneas after ethanol and mechanical injuries to the epithelium. The protective effect of an MSC-conditioned medium on the corneal epithelium was partly accounted for by reference to stanniocalcin-1 (STC-1), an anti-apoptotic protein secreted by MSCs (Block et al., 2009). In separate studies, we additionally found that STC-1 was effective in protecting photoreceptors and retinal ganglion cells against oxidative damage and apoptotic death in rat models of inherited retinal degeneration (Roddy et al., 2012) and optic nerve transection (Kim et al., 2013).
3.1.3. Anti-angiogenic effects of MSCs
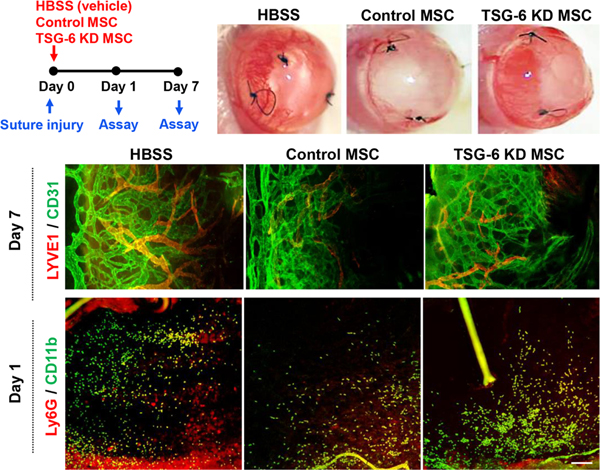
One of the concerns regarding the use of MSCs in corneal disease is that they produce pro-angiogenic factors that might cause in-growth of new blood vessels in the cornea, thereby threatening corneal transparency. In fact, we observed in vitro that MSCs secreted an increased level of vascular endothelial growth factors after coculturing with ethanol-injured corneal epithelial cells (Oh et al., 2009). However, we also found in vivo that systemic infusion or topical application of MSCs rather inhibited corneal angiogenesis in animal models of suture-induced corneal neovascularization (Song et al., 2018a) and corneal chemical injury (Oh et al., 2008). The anti-angiogenic effect of MSCs on the cornea observed in vivo was largely due to the suppression of pro-angiogenic monocytes/macrophages by MSCs in a TSG-6dependent manner (Figure 3) (Song et al., 2018a).
Figure 3.
Anti-angiogenic effects of MSCs on the cornea through TSG-6-mediated suppression of inflammation.
Corneal neovascularization was induced in mice by suture application, and human BM-derived MSCs transfected with either TSG-6 siRNA (TSG-6 KD MSC) or control scrambled siRNA (control MSC) were intravenously administered (day 0). At day 1, corneal inflammation was evaluated by immunostaining of corneal whole-mounts for CD11b and Ly6G. At day 7, corneal new vessel growth was assessed by clinical observation and immunostaining for CD31 and LYVE-1. Both corneal inflammation and neovascularization were markedly suppressed by control MSCs, but not by TSG-6 KD MSCs or the vehicle (Hank’s balanced salt solution, HBSS).
Reprinted with permission from Molecular Therapy. Song et al., 2018. Mesenchymal Stromal Cells Inhibit Inflammatory Lymphangiogenesis in the Cornea by Suppressing Macrophage in a TSG-6-Dependent Manner. Mol. Ther. 26, 162–172. Copyright © 2017 The American Society of Gene and Cell Therapy.
In agreement with our findings, many other groups have reported the effectiveness of BM-, AT- or corneal stroma-derived MSCs in the promotion of corneal regeneration in a variety of sterile injury models in mice, rats and rabbits (for a review, see Sahu et al., 2019). Hence, the demonstration that MSCs protect corneal epithelial cells both through immune modulation and stimulation of corneal epithelial regeneration makes MSCs and MSC-derived factors promising candidates for a novel therapy of SS-related DED, an autoimmune disease characterized by progressive lymphocyte infiltration into the lacrimal gland (LG) and ocular surface, leading to aqueous deficiency dry eye (ADDE) and ocular surface epithelial defects.
3.2. Effects of MSCs in preclinical models of SS-related DED
Early research on MSCs and SS focused on the effects of MSCs on salivary gland dysfunction and xerostomia. It was reported that IV administration of allogeneic BM MSCs, despite transient or no engraftment, restored salivary secretion and reduced T and B cell infiltration into the salivary gland while increasing FoxP3+ Treg cells in NOD mice, an animal model of secondary SS (Khalili et al., 2012; Xu et al., 2012). Similarly, IV infusion of UC MSCs was effective in treating SS patients, without adverse effects, by increasing the salivary flow rate, ameliorating refractory hemolytic anemia, and alleviating autoimmune hepatitis (Xu et al., 2012). As with the effects on the salivary gland and xerostomia, therapeutic effects of systemic administration of MSCs on the LG and DED have been demonstrated in several models of SS-related DED.
In 13-week-old NOD mice, Aluri et al. performed a single IP injection of mouse BM MSCs (1 × 106 cells derived from C57BL/6 mice), and found a sustained improvement in aqueous tear production over 4 weeks and a decrease in the size of lymphocytic foci in the LG without any change in the number of FoxP3+ Treg cells (Aluri et al., 2017). Abughanam et al. carried out, in 8-week-old NOD mice, IV injections of either mouse BM MSCs (2 × 106 cells derived from C57BL/6 mice) or MSC-extracts (prepared by repetitive freezing-thawing of the same number of MSCs) once weekly for 4 consecutive weeks, and observed that both MSCs and MSC-extracts preserved the lacrimal flow rate and maintained corneal epithelial thickness during 16 weeks of follow-up (Abughanam et al., 2019). The size of lymphocytic foci and the infiltration of B220+ or BAFF+ B cells into the LG were reduced in MSC-treated mice at 16 weeks, while FoxP3+ Treg cells were increased. The expression of a proliferation marker in the LG was upregulated in MSC-treated mice, while an apoptosis marker was downregulated, indicating LG regeneration. Also, the serum levels of anti-SSA/Ro autoantibodies were significantly lowered by MSC treatment. Therefore, both studies in the NOD mouse model demonstrate that systemic administration of MSCs has long-lasting effects on the LG by suppressing inflammation and enhancing regeneration.
The immunomodulatory effects of MSCs have been elucidated in more detail in rabbits where autoimmune dacryoadenitis was induced by IV transfer of activated peripheral blood lymphocytes autoreactive to LG epithelial cells. This model elicits severe lymphocytic infiltration into the LG, leading to chronic DED that persists until 6 months after adoptive transfer of activated lymphocytes and closely mimics human SS. In the first set of experiments, Li et al. intravenously injected allogeneic AT MSCs (1 × 107 cells) into rabbits at days 1, 3, 5, 7 and 9 after adoptive transfer of autoreactive lymphocytes (Li et al., 2016). Since DED develops 3 to 5 days after the transfer, this MSC treatment protocol was designed to evaluate the preventive effects of MSCs on autoimmune DED. IV MSCs markedly reduced immune cell infiltrates and pro-inflammatory cytokine levels in the LG. Specifically, Th1/Th17 cell responses in the LG and the spleen were downregulated by MSCs, while the proportion and function of CD4+FoxP3+ Treg cells were upregulated. As a consequence, the secretory function of the LG was preserved and clinical signs of DED on the ocular surface were alleviated in MSC-treated animals, as evaluated clinically by increases in aqueous tear volume and tear break-up time (TBUT) and by a decrease in fluorescein ocular staining scores. In a second series of experiments, the same group intravenously administered either human UC MSCs or rabbit AT MSCs (1 × 107 cells) into rabbits for 5 consecutive days, starting either at the time of autoreactive lymphocyte transfer (preventive protocol) or at 2 weeks after transfer (therapeutic protocol) (Lu et al., 2020). Human UC MSCs were as effective as allogeneic AT MSCs in preventing the development of autoimmune dacryoadenitis when administered prior to disease manifestation. Moreover, IV MSCs administered after disease onset reduced the severity of dacryoadenitis and DED, as examined clinically and histologically. The number of lymphocytic foci in the conjunctiva and the LG was decreased by MSCs. Likewise, the mRNA levels of pro-inflammatory molecules (TNF-α, IL-1β, IL-6, matrix metalloproteinases-2 and −9) in the LG were downregulated by MSCs, whereas the transcript levels of anti-inflammatory cytokines (IL-10, TGF-β) were upregulated. Another notable finding is that the markers for classically activated M1 macrophages, inducible nitric oxide synthase (iNOS) and monocyte chemoattractant protein-1 (MCP-1), were decreased in the MSC-treated LGs, while the markers for alternatively activated M2 macrophages, arginase 1 (Arg1) and CD206, were increased, reflecting the conversion of proinflammatory M1 macrophages to anti-inflammatory M2 macrophages by MSC treatment. Further mechanistic studies revealed that MSCs activated the P13K/AKT pathway in macrophages to elicit M2 macrophage polarization. Therefore, these data suggest that MSCs, by suppressing excessive immune responses and inducing regulatory immune cells, have both preventive and therapeutic effects on autoimmune dacryoadenitis and DED.
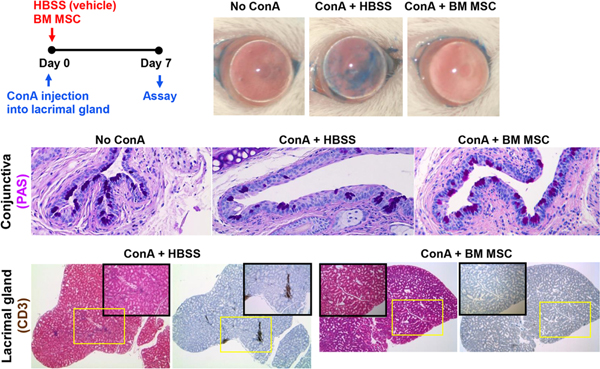
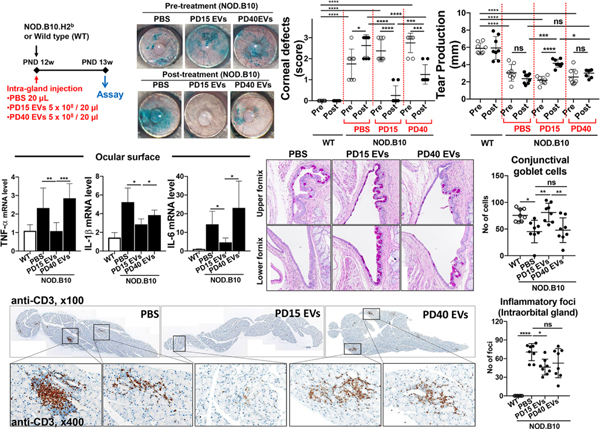
We adopted a more direct approach to investigate the effect of MSCs on LG inflammation (Lee et al., 2015c). We injected concanavalin A (ConA), a T cell mitogen, directly into the intraorbital LG in BALB/c mice, which induced infiltration of Th1 cells into the LG and the upregulation of T cell-derived cytokines (IL-2 and IFN-γ) in the gland and on the ocular surface. This Th1-mediated immune response in the LG subsequently led to a reduction in aqueous tear production and an increase in punctate corneal epithelial erosions, i.e. the generation of LG inflammation-related DED. Immediately after ConA injection, we administered either human BM MSCs, syngeneic mouse BM MSCs or human dermal fibroblasts into the periorbital space around the intraorbital gland (1 × 103 cells or 1 × 105 cells). Both human and mouse MSCs, but not fibroblasts, reduced the number of Th1 cells and the levels of Th1 cytokines in the LG and on the ocular surface without affecting FoxP3+ Treg cells. In addition, tear production and conjunctival goblet cell counts were increased, and corneal epithelial erosions were decreased in MSC-treated mice relative to the vehicle- or fibroblast-treated controls (Figure 4). No significant numbers of MSCs were detected in the intraorbital LG one or 7 days after periorbital injection. Therefore, our data suggest that MSCs, regardless of little engraftment, exert durable effects on the suppression of LG inflammation, thereby alleviating DED.
Figure 4.
Effects of MSCs on LG inflammation-induced DED.
LG inflammation was induced by an injection of ConA, the prototypic T cell mitogen, into the intraorbital gland in mice. Subsequently, syngeneic mouse BM-derived MSCs or vehicle (Hank’s balanced salt solution, HBSS) were administered into the periorbital space. ConA injection induced CD3 T cell infiltration into the LG, severe epithelial defects in the cornea, and a reduction in conjunctival goblet cells. Notably, MSCs suppressed LG inflammation. Consequent to that, MSCs significantly preserved the corneal epithelium and conjunctival goblet cells as assessed by corneal lissamine green dye staining and conjunctival periodic acid–Schiff (PAS) staining, respectively.
Reprinted with permission from Molecular Therapy. Lee et al., 2015. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol. Ther. 23, 139–146. Copyright © 2015 The American Society of Gene and Cell Therapy.
Dietrich et al. further elucidated the effect of exogenous MSCs on LG regeneration in a duct ligation model whereby the secretory duct of the extraorbital LG was ligated in C57BL/6J mice (Dietrich et al., 2019). Although a duct ligation model is not directly related to autoimmune diseases, duct ligation generates severe inflammatory reaction in the LG, leading to profound loss of functional LG tissue and reduction of tear secretion, similarly to the findings observed in autoimmune SS-related DED. In the study by Dietrich et al., duct ligation was released after 3 days, and MSCs, which had been isolated from murine extraorbital LGs, were injected directly into the extraorbital LG (2.5 × 105 cells). MSCs repressed immune response in the LG as demonstrated by decreases in macrophage infiltration and TNF-α expression. Notably, the amount of vital acinar structures in the gland was significantly increased, and the number of apoptotic cells was decreased in MSC-treated mice relative to the vehicle-treated controls, an indication that local injection of LG-derived MSCs promoted LG regeneration and suppressed LG inflammation. Unlike our observation, however, the presence of MSCs was observed in the LG until 21 days after the intragland injection of the cells. This dissimilarity might have arisen from the differences in the tissue source and species origin of MSCs or in the injection route of the cells.
Collectively, the data presented above demonstrate that MSCs attenuate LG inflammation, facilitate LG regeneration, and thereby treat DED.
3.3. Effects of MSCs on spontaneous keratoconjunctivitis sicca in dogs
Therapeutic benefits of MSCs in autoimmune DED have been successfully demonstrated in dogs. Keratoconjunctivitis sicca (KCS) spontaneously develops in up to 35% of dogs in a way similar to autoimmune DED in human SS patients (Barabino and Dana, 2004; Kaswan et al., 1998; Williams, 2008). Canine KCS is characterized by lymphocyte infiltration into the LG, increased apoptosis in lacrimal acinar and conjunctival epithelial cells, multiple autoantibodies against lacrimal antigens, and more severe clinical profiles of DED than those described in human KCS (Barabino and Dana, 2004; Williams, 2008).
The safety and feasibility of transconjunctival injection of MSCs into normal dogs were first demonstrated in a study by Park et al. (Park et al., 2013). The injections of allogeneic AT MSCs into the main LG and the accessory LG of the third eyelid (2 × 106 cells per gland) were repeated 6 times at one-week intervals. During 9 weeks of clinical observation, there were no adverse effects except transient conjunctival congestion and chemosis. No significant histologic lesions were noted on pathologic examination of various systemic organs and ocular tissues when evaluated 9 weeks after MSC injection. In another study by Villatoro et al., the therapeutic effects of allogeneic AT MSCs were investigated in dogs with naturally occurring KCS refractory to conventional treatments with corticosteroids, tacrolimus, cyclosporine and artificial tears (Villatoro et al., 2015). MSCs were injected once into the main LG (5 × 106 cells) and into the third eyelid LG (3 × 106 cells) of KCS dogs. The effects of a single MSC injection were long-lasting: tear secretion persistently improved over the course of 9 months of follow-up, and the DED scores, as assigned by clinical signs such as ocular discharge, hyperemia and corneal changes, showed a sustained decrease without systemic or ocular complications. Bittencourt et al. obtained similar results with a lower number of allogeneic AT MSCs (1 × 106 MSCs) (Bittencourt et al., 2016). A single injection of lower-dose MSCs significantly increased tear production and alleviated clinical signs in KCS dogs, and the beneficial effects were maintained during the 12-month follow-up (Figure 5). There were no adverse effects immediately after injection or over the course of short- and long-term follow-ups. In a recent study by Sgrignoli et al., anti-inflammatory effects of MSCs on the ocular surface were further identified in KCS dogs (Sgrignoli et al., 2019). Allogeneic AT MSCs were topically applied in the conjunctival sac (1 × 106 cells in 50 μL of one drop) every 7 days for one month (in total, 4 applications), and the dogs were followed up for 6 months. Significant improvement was observed in clinical signs as reflected by decreases in conjunctival discharge, corneal opacity, corneal neovascularization and corneal pigmentation. Both tear production and tear quality, as measured by Schirmer test and TBUT, were increased. No clinical signs of rejection or ocular discomfort were detected. Remarkably, the density of neutrophils in the palpebral conjunctiva was reduced compared with their state prior to MSC treatment, and additionally, the expression of pro-inflammatory markers (CD4, IL-1, IL-6, TNF-α) in the palpebral conjunctiva and third eyelid gland was reduced, while the goblet cell density in the conjunctiva was increased. As in previous studies, the effects of MSC treatment for the first one month persisted until the end of the study (i.e. 6 months) in most animals.
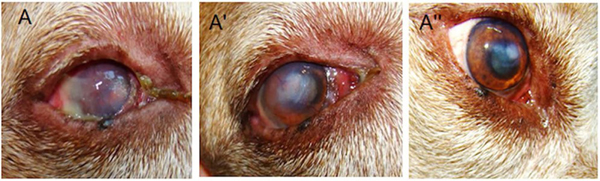
Figure 5.
Effects of MSCs on naturally-occurring canine KCS.
Allogeneic AT-derived MSCs were injected into the dorsal LG and the third eyelid LG in dogs with naturally occurring KCS. Prior to MSC transplantation, there was ocular discharge, conjunctival hyperemia, corneal opacity and vascularization, as well as Schirmer test results of 2 mm/min, all of which are clinical signs of KCS (A). MSCs dramatically reduced clinical signs of KCS both in the short term (28 days after MSC treatment, A’) and in the long term (12 months after MSC treatment, A”).
Reprinted with permission from Cell Medicine. Bittencourt et al., 2016. Allogeneic Mesenchymal Stem Cell Transplantation in Dogs With Keratoconjunctivitis Sicca. Cell Med. 8, 63–77. Copyright © 2016 Cognizant, LLC.
Although the studies noted above followed different therapeutic regimens (treatment frequency, cell dose and injection site), the results all point to the conclusion that the immunomodulatory and therapeutic effects of MSCs are long-lasting.
3.4. Clinical trials with MSCs in DED related to GVHD and SS
The therapeutic efficacy of MSCs in the treatment of DED was first reported from a clinical study involving chronic GVHD patients. After IV transplantation of allogeneic BM MSCs, the symptoms and signs of DED improved in 12 of 22 patients with refractory DED secondary to chronic GVHD, as assessed by dry eye scores based on the NIH consensus criteria, ocular surface disease index scores (OSDI) and Schirmer test (Weng et al., 2012).
Recently, the results of the first clinical trial of MSCs for the treatment of SS-associated DED were published. Møller-Hansen and colleagues performed one transconjunctival injection of allogeneic AT MSCs into the LG (2.2 × 107 cells/mL, the injection volume being 50% of the LG volume corresponding to 0.1 to 0.2 mL) in 7 patients with severe ADDE due to primary or secondary SS, and followed the patients for 16 weeks (Moller-Hansen et al., 2020). The treatment was well-tolerated, and no adverse events were observed. The most notable finding was a rapid, dramatic, persistent improvement in dry eye symptoms as measured by OSDI scores. A significant improvement was also observed in clinical signs of DED: tear osmolarity was decreased, TBUT was lengthened, tear production (Schirmer results) was increased, and corneal staining showed a decreasing trend following MSC treatment. Importantly, these beneficial effects were sustained for up to 16 weeks after a single injection of MSCs. Two patients had donor-specific HLA class II antibodies before treatment, and two developed de novo donor-specific HLA class I antibodies after treatment. However, no patients showed symptoms or signs indicative of immunization. Moreover, the development of donor-specific antibodies had no influence on the therapeutic effects of MSCs, which has also been seen in other MSC studies (Kot et al., 2019). Hence, the results of an intra-gland injection of allogeneic HLA-unmatched MSCs in SS-related DED patients, alongside preclinical data, demonstrate a therapeutic potential for the use of allogeneic MSCs in DED treatment.
4. MSCs for the treatment of autoimmune uveoretinitis
The ability of MSCs to control ocular autoimmune inflammation has also been established by extensive experiments with animal models of EAU, a prototypic T cell-mediated autoimmune disease in the retina that represents autoimmune uveitis in humans (for a review, see Diedrichs-Mohring et al., 2018). EAU can be reproducibly induced in rodents by active immunization with a retinal antigen such as interphotoreceptor retinoid-binding protein (IRBP) or by adoptive transfer of antigen-primed T cells or antigen-pulsed dendritic cells (Agarwal and Caspi, 2004; Agarwal et al., 2012; Caspi et al., 2008; Diedrichs-Mohring et al., 2018). The typical histological appearance of EAU closely resembles that of human uveitis. Moreover, the disease course has been well-characterized in each EAU model (Agarwal and Caspi, 2004; Caspi et al., 2008; Diedrichs-Mohring et al., 2018). Hence, the EAU model is wellsuited and has thus been widely used to study the role of MSCs in the treatment of ocular autoimmune diseases.
4.1. Effects of MSCs on EAU
Several studies have demonstrated that systemic (IP or IV) administration of MSCs produces beneficial effects in various EAU models with different clinical courses (acute or chronic, monophasic or relapsing-remitting).
In 2011, Zhang et al. first reported the therapeutic potential of MSCs in autoimmune uveitis (Zhang et al., 2011). They demonstrated that IV injections of BM-derived MSCs for 3 consecutive days (5 × 106 cells per day) delayed the disease onset and attenuated the severity of EAU as assessed by clinical and histological scores in Lewis rats where EAU had been induced by immunization with IRBP peptide R14. MSC treatment inhibited the proliferation of T cells isolated from EAU rats and reduced the secretion of Th1 and Th17 cytokines while increasing IL-10 production. The beneficial effects were similar between syngeneic (Lewis rat-derived) and allogeneic (Wistar rat-derived) MSCs. Additionally, the optimal time window for MSC treatment was determined. MSCs were effective in preventing and treating EAU when administered before disease onset (simultaneously with immunization), during the early phase of disease manifestation, or at the peak of disease. However, MSC administration during the chronic phase after disease stabilization did not ameliorate EAU, an indication that the immunologic mechanism MSCs affect is not active in the chronic phase of disease.
Meanwhile, the therapeutic and preventive benefits of MSCs also were demonstrated in a recurrent EAU model. As autoimmune uveitis is usually a chronic and recurrent disease, adoptive transfer of T cells specific to IRBP peptides (R14 or R16) has been used to induce spontaneous, relapsing-remitting uveitis in rodents with unpredictable recurrences of intraocular inflammation (Agarwal et al., 2012; Diedrichs-Mohring et al., 2008; Diedrichs-Mohring et al., 2018; Shao et al., 2005). Zhang et al. induced recurrent uveitis in Lewis rats by IV transfer of T cells that had been collected from R16-immunized rats and then incubated with antigen-presenting cells (APCs) with R16 stimulation (Zhang et al., 2014). Allogeneic BM MSCs from Wistar rats were injected for 3 consecutive days (5 × 106 cells per day), starting either at the time of transfer (before disease onset) or at the time of clinical disease manifestation. MSCs, administered in one course before or after disease onset, sufficed to inhibit the progression and relapse incidence of EAU. Moreover, a single course of MSC treatment around the time of disease onset was more effective in controlling inflammation, reducing recurrences, and protecting the retina than was long-term treatment with dexamethasone. In a subsequent study, MSCs were administered after the second attack of EAU in the same model (Zhao et al., 2016). Delayed treatment with MSCs during the recurrent phase significantly reduced the severity of EAU, preserved the retinal structure and photoreceptors, and improved retinal function as assessed by electroretinography (ERG), suggesting that MSCs are also beneficial for advanced cases of recurrent uveitis.
4.2. Therapeutic mechanisms of MSCs
4.2.1. Th1/Th17 inhibition and Treg induction
To understand the mechanism underlying the beneficial effects of MSCs on EAU, Li et al. performed a time-course study and tracked the number of Th17 and Treg cells in the spleen and eye in an IRBP (R16)-induced EAU model in Lewis rats after IV administration of allogeneic MSCs for 3 consecutive days (5 × 106 cells per day) (Li et al., 2013). MSCs, infused either before disease onset or at the disease peak, decreased the number of Th17 cells in both the spleen and the eye at all time-points while increasing the number of CD4+CD25+FoxP3+ Treg cells consistently during the entire disease course. Since Th17 cells cause whereas Treg cells inhibit autoimmune responses (Lee, 2018), these results suggest that MSCs regulate Th17/Treg balance, thereby leading to EAU alleviation.
Another study by Tasso et al. confirmed the effects of MSCs on Treg cell induction and EAU development in a C57BL/6 (H-2b) mouse model characterized by later onset and longer duration of predominantly posterior uveitis as compared with acute panuveitis in a rat model (Tasso et al., 2012). The results were dramatic. A single IP injection of syngeneic BM MSCs (5 × 106 cells) at the time of IRBP immunization prevented the development of EAU almost completely; only 2 of 14 MSC-treated mice developed EAU, whereas 14 of 16 MSC-untreated mice developed the disease. MSC treatment significantly increased the frequency of CD4+CD25+FoxP3+ Treg cells in the spleen of the EAU mice. Treg cells isolated from MSC-treated EAU mice inhibited the proliferation of IRBP-primed T cells from EAU mice, while Treg cells isolated from MSC-untreated mice did not. Moreover, in a transwell coculture experiment, MSCs induced an expansion of antigen-specific, functional Treg cells in a paracrine fashion by secreting TGF-β.
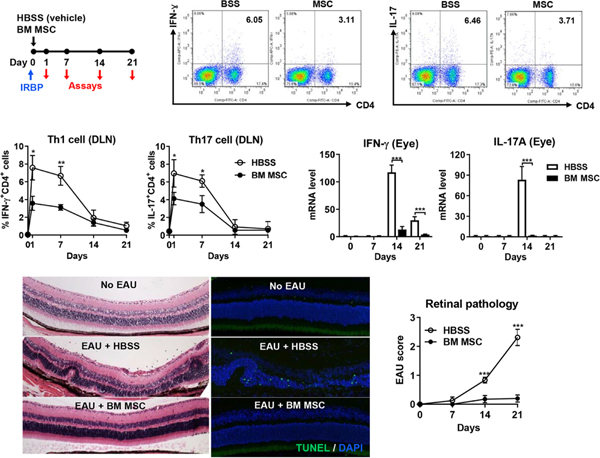
We obtained similar results with human MSCs in the same murine EAU model. A single IP or IV injection of human or syngeneic mouse BM-derived MSCs immediately after IRBP immunization was sufficient to block EAU development in C57BL/6 (H-2b) mice (Figure 6) (Lee et al., 2015a; Oh et al., 2014a). IP and IV administration were equally effective. Human and mouse MSCs achieved similar effects. We injected 1 × 106 cells, with which only 12.5% of immunized mice developed EAU, in contrast to 87.5% of mice that developed EAU without MSC treatment. This efficacy of 1 × 106 MSCs is comparable to that of 5 × 106 MSCs observed in the study by Tasso et al (Tasso et al., 2012). Additionally, we made a serial analysis of cellular, molecular and histologic profiles in EAU mice with or without MSC treatment. The kinetics demonstrated a clear sequence in pathogenic events during EAU progression (Lee et al., 2015a; Oh et al., 2014a): 1) at days 1 and 7, Th1 and Th17 cells were increased in draining lymph nodes (DLNs); 2) at day 14, Th1 and Th17 cells were infiltrated into the retina with as yet little damage to the retinal architecture; 3) at day 21, the retinal structure was destroyed histologically (and in fact, histological changes in the retina have been found to correlate with clinical changes of the fundus in EAU mice (Copland et al., 2008)). Of note, systemic MSC administration reduced the frequencies of Th1 and T17 cells, pathogenic effectors of autoimmune uveitis, in DLNs during the early phase of disease (at days 1 and 7), consequently leading to decreased retinal infiltration of T cells and protection of the retinal structure (Lee et al., 2015a; Oh et al., 2014a). Contrary to previous reports (Li et al., 2013; Tasso et al., 2012; Zhang et al., 2011), MSCs did not increase the number of CD4+CD25+FoxP3+ Treg cells in DLNs, peripheral blood or spleen (Lee et al., 2015a; Oh et al., 2014a).
Figure 6.
Effects of MSCs on EAU.
Human BM-derived MSCs or vehicle (Hank’s balanced salt solution, HBSS) were administered into C57BL/6 (H-2b) mice either intraperitoneally or intravenously immediately after EAU induction via IRBP immunization. Both IFN-γ+CD4+ Th1 cells and IL-17+CD4+ Th17 cells were significantly reduced in DLNs by MSC treatment. Accordingly, the transcript levels of IFN-γ and IL-17A in the eye were decreased by MSCs, and the retinal architecture was preserved with a smaller number of TUNEL+ apoptotic cells, all of which indicate the protection against EAU.
Reprinted with permission from The Journal of Immunology. Lee et al. 2015. Mesenchymal stem/stromal cells protect against autoimmunity via CCL2-dependent recruitment of myeloid-derived suppressor cells. J. Immunol. 194, 3634–3645.
Copyright © 2015 The American Association of Immunologists, Inc. and from Mediators of Inflammation. Oh et al., 2014. Intraperitoneal infusion of mesenchymal stem/stromal cells prevents experimental autoimmune uveitis in mice. Mediators Inflamm. 2014, 624640.
The effects and mechanisms of MSCs have also been tested in an EAU model in B10.RIII (H-2r) mice, a strain that is more conducive to EAU induction and develops more severe disease than the C57BL/6 (H-2b) strain after IRBP immunization (Cortes et al., 2008). Similarly to that seen in C57BL/6 (H-2b) mice (Lee et al., 2015a; Oh et al., 2014a; Tasso et al., 2012), Kimbrel et al. demonstrated that a single IP injection of human embryonic stem cell-derived MSCs (5 × 106 cells) at the time of immunization (day 0) suppressed the development of EAU in B10.RIII (H-2r) mice (Kimbrel et al., 2014). The same group subsequently showed that the secretion of Th17-associated cytokines (IL-17, IL-22, IL-6) was lower in splenocytes collected at the peak of disease (day 14) from MSC-treated EAU mice than in those obtained from MSC-untreated EAU mice (Qin et al., 2018), an indication that the Th17 response in mice at day 14 was repressed by MSCs that had been injected at day 0. However, they did not find any increase in CD4+FoxP3+ Treg cells in the spleen or DLNs in the MSC-treated group, which is consistent with our findings (Lee et al., 2015a; Oh et al., 2014a).
In a study by Chen et al., CD73 on mouse AT MSCs was shown to contribute to T cell inhibition in EAU by cooperating with CD39 and CD73 on activated T cells to produce adenosine, a molecule having T cell-suppressive effects (Chen et al., 2016).
4.2.2. Monocytes/macrophages as intermediary cells for MSC action
From the experiments discussed above, it is apparent that in EAU models, MSCs alleviate disease severity and prevent recurrence by suppressing Th1 and T17 cells, and that the immunosuppressive effects of MSCs are long-lasting. However, several paradoxical observations made with regard to MSCs render explanation of their therapeutic mechanisms difficult.
One such observation is that MSCs had a short metabolic half-life after systemic administration in experimental animals, but had long-term effects on immune responses such as inhibition of Th1/Th17 cells and induction of Treg cells. In a C57BL/6 mouse model of EAU, we found that only 1.8% of MSCs were present in ocular DLNs 24 hours after IV administration of human MSCs (Lee et al., 2015a). Although the numbers of MSCs detected in tissues beyond the lung were small and the engraftment was temporary, MSCs acted at a distance and exerted durable effects on EAU. Another observation is that almost identical results were obtained in EAU models when human (Kimbrel et al., 2014; Lee et al., 2015a; Oh et al., 2014a; Qin et al., 2018), allogeneic (Li et al., 2013; Zhang et al., 2011; Zhang et al., 2014; Zhao et al., 2016) or syngeneic MSCs (Lee et al., 2015a; Tasso et al., 2012; Zhang et al., 2011) were utilized. These findings had originally been interpreted under the assumption that MSCs were immune-privileged, but subsequent investigations supported the notion that exogenously administered MSCs are recognized by the recipient’s immune system and can be rejected under mismatched conditions (Ankrum et al., 2014). To reconcile the observations that MSCs have a short window of therapeutic activity but suppress adaptive immune responses that can take weeks to develop, we have investigated whether the immunosuppressive effects of MSCs might be transmitted by longer-lived innate immune cells that act as intermediaries to control the activation of the adaptive immune system.
Among innate immune cells, monocytes and macrophages are good candidates for mediation of the therapeutic action of MSCs, due specifically to their distinct characteristics as important regulators of immune response. One of these characteristics is their presence in all tissues. Monocytes, arising from myeloid progenitors in BM, comprise 10% of circulating leukocytes in the peripheral blood and promptly migrate from the blood to the tissue of injury where they subsequently differentiate into macrophages. Macrophages reside in all tissues, either as tissueresident cells, which are mostly derived from the yolk sac and take up residence during embryogenesis, or as monocyte-derived cells, which are differentiated from monocytes infiltrating into tissues upon injury (for a review, see Ginhoux and Guilliams, 2016). Another distinct characteristics of monocytes and macrophages is their functional diversity and plasticity. After injury, monocytes and macrophages change their phenotypes and functions with chameleon-like ease and play critical roles in the initiation, maintenance and resolution of inflammation and tissue repair (for reviews, see Locati et al., 2020; Wynn and Vannella, 2016). In recent years, a more active role of monocytes and macrophages in the regulation of the adaptive immune system, in addition to their role as sentinel and effector cells, has emerged as one of the immunoregulatory mechanisms by which an organism induces tolerance and restores homeostasis after injury.
In research over the past decade, we have identified cells from the monocyte/macrophage lineage as intermediaries in the therapeutic action of MSCs.
4.2.2.1. Effects of MSCs on myeloid-derived suppressor cells
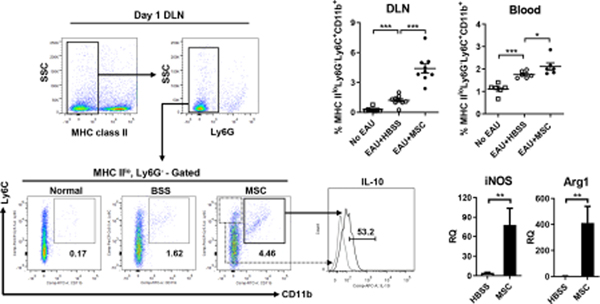
In the first series of experiments (Lee et al., 2015a), we infused human or syngeneic mouse BM-derived MSCs via the tail vein into C57BL/6 mice immediately after IRBP immunization (day 0). By serially monitoring Th1 and Th17 cells, we found an initial expansion of Th1 and Th17 cells in DLNs during days 1 to 7, followed by an increase of Th1 and Th17 cells in the blood at day 7, and by a later infiltration of the cells into the retina, with a peak at day 14. As expected, MSC treatment repressed Th1 and Th17 immune responses in all tissues during the entire disease course, leading to the near-complete protection of the eye against EAU. Next, we explored DLNs at day 1, an initial site of Th1 and Th17 cell activation. About 1.8% of MSCs were detected in DLNs 24 hours after IV administration. Simultaneously, there was a marked increase of IL-10-expressing, monocytic MHC class II−CD11b+Ly6C+Ly6Glo cells in DLNs of MSC-treated EAU mice, along with the upregulation of iNOS and Arg1 (Figure 7). In culture, these CD11b+Ly6C+ cells suppressed the proliferation of CD4+ T cells and differentiation into Th1 and Th17 cells, while inducing CD4+ cell apoptosis. In mice, adoptive transfer of the MSC-induced CD11b+Ly6C+ cells ameliorated EAU, whereas depletion of CD11b+Ly6C+ cells abrogated the effects of MSCs. We defined the MSC-induced CD11b+Ly6C+ cells as myeloid-derived suppressor cells (MDSCs) on the basis of recommendations for MDSC nomenclature: an expression of both CD11b and Gr-1 (Ly6C or Ly6G) markers and the activity to inhibit T cells (Bronte et al., 2016). Additional experiments revealed that the mobilization of MDSCs to DLNs of EAU mice was dependent on MCP-1/CCL2 (chemokine (C-C motif) ligand 2) expressed by MSCs (Lee et al., 2015a). Together, the results suggest that MSCs induce the recruitment of monocytic MDSCs to DLNs, the sites of immune reaction, in a paracrine manner.
Figure 7.
MSCs increased monocytic MDSCs in DLNs and peripheral blood in mice with EAU.
One day after EAU induction and MSC IV administration, the increase of IL-10-expressing MHC IIloLy6G−Ly6ChiCD11b+ cells was noted in DLNs and peripheral blood in mice. Also, there were up to 80- and 400-fold increases in iNOS and Arg1, the molecules known to mediate the immunosuppressive functions of MDSCs, in DLNs of the MSC-treated mice.
Reprinted with permission from The Journal of Immunology. Lee et al. 2015. Mesenchymal stem/stromal cells protect against autoimmunity via CCL2-dependent recruitment of myeloid-derived suppressor cells. J. Immunol. 194, 3634–3645. Copyright © 2015 The American Association of Immunologists, Inc.
The role of MDSCs in autoimmune uveitis has been identified in several studies. We showed that MDSCs were implicated in the resolution of inflammation in EAU mice and in human patients with autoimmune posterior uveitis (Jeong et al., 2018; Kerr et al., 2008). Tu et al. demonstrated that retinal pigment epithelial cells induced the differentiation of MDSCs from BM progenitors in C57BL/6 mice and thus controlled immune reactions in the retina in EAU mice (Tu et al., 2012).
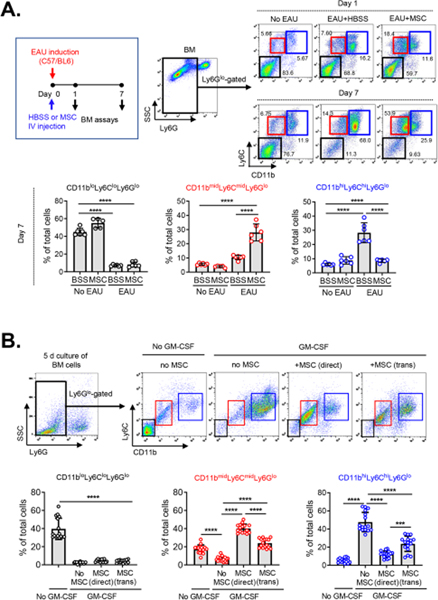
In a subsequent series of experiments (Lee et al., 2020), we explored whether MSCs might affect monocytic MDSC differentiation as well as recruitment. Since BM is the primary site of MDSC expansion, we examined BM cells for CD11b, Ly6C and Ly6G markers in C57BL/6 mice at days 1 and 7 after IRBP immunization and concurrent IV MSC treatment. A critical observation was that a distinct subset of CD11bmidLy6CmidLy6Glo cells was induced in BM by MSC treatment, whereas most of the Ly6Glo cells differentiated into CD11bhiLy6ChiLy6Glo cells in EAU mice without MSC treatment (Figure 8A). However, MSCs did not affect BM cell differentiation in naïve mice without EAU. These findings were recapitulated in vitro. Both direct and transwell cocultures of BM cells with MSCs induced a prominent subset of CD11bmidLy6CmidLy6Glo cells under granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulation, while most BM cells differentiated toward CD11bhiLy6ChiLy6Glo cells under GM-CSF without MSCs (Figure 8B). The MSC-induced CD11bmidLy6CmidLy6Glo cells had a transcriptome profile distinct from CD11bhiLy6ChiLy6Glo cells, as analyzed by bulk RNA sequencing. Surface marker and molecular analysis revealed that the CD11bmidLy6CmidLy6Glo cells differed from CD11bhiLy6ChiLy6Glo cells by low expression of MHC class II and costimulatory molecules (CD40, CD80, CD86) and high production of immunoregulatory molecules (IL-10, Arg1, NO, TGF-β). Functionally, the MSC-induced CD11bmidLy6CmidLy6Glo cells were not responsive to lipopolysaccharides (LPS) in contrast to CD11bhiLy6ChiLy6Glo cells that underwent marked pro-inflammatory activation in response to LPS. Furthermore, the MSC-induced CD11bmidLy6CmidLy6Glo cells inhibited T cell proliferation and activation in vitro, and adoptive transfer of the cells attenuated EAU development in mice. Therefore, CD11bmidLy6CmidLy6Glo cells induced in BM by MSCs can be defined as MDSCs. Further mechanistic studies demonstrated that the differentiation of CD11bmidLy6CmidLy6Glo MDSCs was partly dependent on PGE2 and HGF secretion from MSCs.
Figure 8.
MSCs induced differentiation of monocytic MDSCs in BM.
A. In C57BL/6 mice, human BM MSCs were injected via the tail vein after EAU induction via IRBP immunization. Examination at days 1 and 7 revealed that the MSCs increased a distinct subset of CD11bmidLy6CmidLy6Glo cells in BM while reducing the number of CD11bhiLy6ChiLy6Glo cells.
B. In culture, BM cells were extracted from C57BL/6 mice and cocultured with human BM MSCs in a direct coculture or transwell system under GM-CSF stimulation for 5 days. Both direct and transwell cocultures with MSCs induced a distinct subset of CD11bmidLy6CmidLy6Glo cells, while most of Ly6Glo BM cells differentiated into CD11bhiLy6ChiLy6Glo cells without MSCs.
Reprinted with permission from JCI Insight. Lee et al., 2020. Mesenchymal stromal cells induce distinct myeloid-derived suppressor cells in inflammation. JCI Insight 5, e136059. Copyright © 2020 American Society for Clinical Investigation.
As in the EAU model, MDSCs have also been found to mediate therapeutic effects of MSCs in non-ocular disease models such as allogeneic heart transplantation (Obermajer et al., 2014), bleomycin-induced lung injury (Su et al., 2018), and acute GVHD (Yang et al., 2020).
Altogether, the data indicate that MSCs induce the differentiation and recruitment of MDSCs under inflammatory conditions, and that MDSCs are key intermediaries in mediating the immunosuppressive effects of MSCs. As MDSCs are inherently heterogeneous cell populations, we are currently analyzing MSC-induced MDSCs by means of single-cell RNA sequencing.
4.2.2.2. Modulatory effects of MSCs on macrophages
In another effort to explain how the therapeutic effects of MSCs extend beyond the persistence of the cells, we discovered that intravenously infused MSCs impacted monocytes/macrophages in the lung, which in turn exerted therapeutic activities in the long-term.
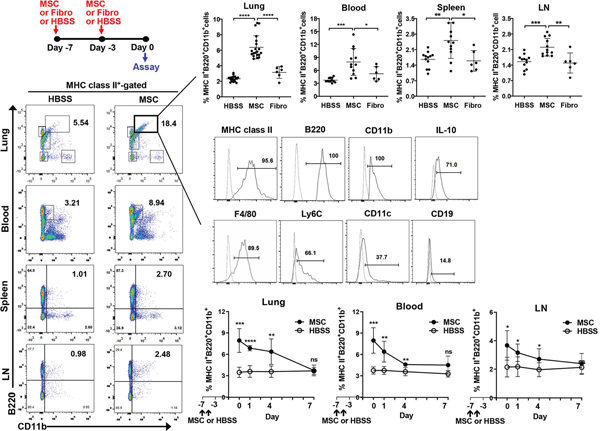
We observed that pretreatment of C57BL/6 mice with IV MSCs 7 and 3 days before IRBP immunization (days −7 and −3) significantly protected them from EAU (Ko et al., 2016). Although there were no MSCs in the lung at the time of IRBP challenge (day 0), the effect of MSC pretreatment on EAU was dramatic: only 2 of 9 MSC-pretreated mice developed EAU (clinical score 1) at day 21, whereas all 9 vehicle-pretreated mice and all 9 fibroblast-pretreated mice developed more severe EAU (clinical score 1 or higher) at day 21. These results indicate the involvement of endogenous cells in mediating MSC effects. To search for a specific cell population affected by MSCs, we analyzed the lung, the site where most MSCs were trapped early after IV infusion (Lee et al., 2009a), at day 0 (the day of EAU induction or 3 days after the second MSC treatment). In the result, MHC class II+B220+CD11b+ cells, which also expressed IL-10, F4/80 and Ly6C, were found to be significantly increased in the MSC-pretreated lung (Figure 9). Time-course analysis showed that a significantly higher number of MHC class II+B220+CD11b+ cells was present in the peripheral blood and ocular DLNs as well as in the lung until day 4, suggesting that the cells persisted in mice until at least 7 days after IV MSC injection (Figure 9). The MSCinduced lung B220+CD11b+ cells were a morphologically homogenous population of monocytes, suppressed CD4+ T cell proliferation, and blocked Th1 and Th17 cell differentiation. Importantly, adoptive transfer of the MSC-induced B220+CD11b+ macrophages prevented EAU development in secondary recipient mice, and deletion of macrophages in the lung negated the effects of MSC pretreatment on EAU. Microarray and qPCR validation further identified TSG-6 as the most upregulated gene in the lung after MSC treatment. The induction of immunosuppressive MHC class II+B220+CD11b+ macrophages in the lung was dependent on TSG-6 expression by MSCs. Therefore, the data indicate that MSCs precondition lung monocytes/macrophages toward immunosuppressive phenotypes, thereby inducing immune tolerance. In a subsequent study, we determined whether the immunosuppressive MHC class II+B220+CD11b+ cells induced in the lung by MSCs originate from circulating Ly6C+ monocytes or lung resident macrophages (Ko et al., 2017). Depletion of circulating CD11b+Ly6C+ monocytes, but not CD11b+Ly6G+ granulocytes, reversed the effects of MSCs on the induction of MHC class II+B220+CD11b+ macrophages in the lung and on EAU prevention. These results suggest that CD11b+Ly6C+ monocytes are precursors to MSC-induced MHC class II+B220+CD11b+ immunosuppressive macrophages and thus are required for MSC-induced immune tolerance in EAU.
Figure 9.
MSCs increased a distinct population of MHC class II+B220+CD11b+ cells in the lung.
At days −7 and −3, human BM MSCs, dermal fibroblasts (Fibro) or vehicle (Hank’s balanced salt solution) were injected intravenously into naive BALB/c mice. The analysis at day 0 demonstrated higher percentages of MHC class II+B220+CD11b+ cells in the lung, peripheral blood, spleen and cervical lymph nodes (LN) of MSC-pretreated mice, compared with HBSS- or Fibro-pretreated mice. The MSC-induced MHC class II+B220+CD11b+ cells expressed high levels of IL-10, F4/80 and Ly6C and a moderate level of CD11c. Additional time-course analysis showed that the percentages of MHC class II+B220+CD11b+ cells in the lung, blood and LN remained elevated until day 4 (i.e., 7 days after MSC injection).
Originally published in Proceedings of the National Academy of Sciences of the United States of America. Ko et al., 2016. Mesenchymal stem/stromal cells precondition lung monocytes/macrophages to produce tolerance against allo- and autoimmunity in the eye. Proc. Natl. Acad. Sci. U. S. A. 113, 158–163.
In line with our findings, several groups found the involvement of lung monocytes/macrophages in the effects of MSC pretreatment in other disease models. Nemeth et al., in a sepsis model induced by cecal ligation and puncture, intravenously infused mouse BM MSCs 24 hours before sepsis induction (Nemeth et al., 2009). Similarly to our observation, most of the MSCs were trapped in the lung immediately after IV infusion, and few were detectable in the lung 24 hours later. Nevertheless, MSC pretreatment significantly improved the survival and organ function in sepsis mice. To identify an endogenous cell population responsible for the therapeutic effects of MSCs, the same group further examined the effects of MSCs in Rag2−/− mice that lack mature T and B cells, in mice depleted of natural killer (NK) cells, and in mice depleted of monocytes/macrophages. MSCs were still effective in reducing mortality in the sepsis mice lacking T, B or NK cells, but were no longer effective in the mice depleted of monocytes/macrophages, an indication that lung monocytes/macrophages mediate the therapeutic action of MSCs. Further mechanistic investigation revealed that MSCs reprogrammed macrophages to produce IL-10 by releasing PGE2, and macrophage-derived IL-10 was key to the therapeutic effects of MSCs on sepsis. A similar observation was made with a corneal allotransplant model (Lohan et al., 2018). Lohan et al. administered rat BM MSCs intravenously into rats twice at 7 and 1 days prior to corneal transplantation, and found that MSC pretreatment significantly prolonged the rejection-free graft survival from 0 to 63.6% in a high risk model of corneal transplantation. Consistent with our findings, higher proportions of CD45+CD11b+B220+ cells were observed in the lung 24 hours after the second MSC injection, and the MSC-primed lung monocytes exhibited an immunosuppressive phenotype that suppressed CD4+ and CD8+ T cell proliferation. Hence, these results endorse the conclusion that monocytes/macrophages are intermediary cells for tolerizing effects of MSCs.
In a recent study, we further identified the mechanism by which monocytes/macrophages modulated by MSCs exert therapeutic effects (Ko et al., 2020). In a coculture experiment, MSCs transferred MSC mitochondria-containing EVs to monocyte-derived macrophages, and consequently macrophages were activated to produce amphiregulin (AREG) in a phagocytosis-dependent manner. Notably, macrophage-derived AREG was essential in order for MSC-primed macrophages to suppress Th1 cells and induce Treg cells, both in vitro and in mice with EAU. Because AREG is a member of the epidermal growth factor family that is essential for corneal epithelial cell proliferation, we also evaluated the effects of MSC-primed macrophages and their AREG in a corneal epithelial injury model. As expected, AREG derived from MSC-primed macrophages effectively inhibited apoptosis and enhanced proliferation of corneal epithelial progenitor cells against hyperosmolar stress in vitro and in mice with corneal chemical injury. Hence, it was concluded that macrophages preconditioned by MSCs control immune responses and preserve tissue-resident stem cells through AREG, leading to the restoration of tissue homeostasis.
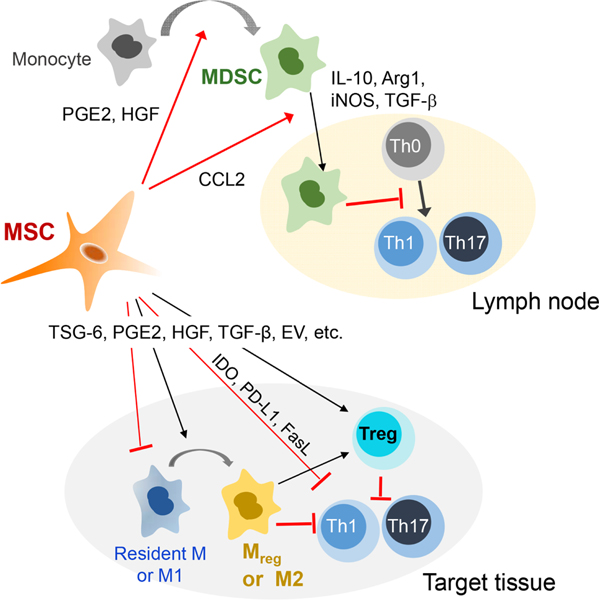
The immunomodulatory mechanisms of MSCs identified thus far are summarized in Figure 10.
Figure 10. Mechanisms of immunomodulation by MSCs.
MSCs exert immunosuppressive effects on autoimmune inflammation through promotion of MDSC expansion and recruitment, induction of macrophage polarization into anti-inflammatory or regulatory phenotypes, inhibition of Th1 and Th17 cells, and upregulation of Treg cells. These immunomodulatory actions of MSCs are mediated in large part by the major immunomodulatory factors that MSCs secrete in response to injury or perturbation of tissue homeostasis.
5. Challenges of MSC therapy
As above-summarized, MSCs have advantages over currently available immunosuppressive agents because a short course of MSC treatment induces long-term therapeutic effects by modulating innate immune cells. Moreover, unlike traditional immunosuppressants, MSCs do not impair the recipients’ antimicrobial immunity, and thus do not increase the risk of infection; they rather enhance host defense against infection and accelerate pathogen clearance (Li et al., 2020; Mei et al., 2010; Mezey and Nemeth, 2015). In addition, MSCs play pro-regenerative and homeostatic roles by promoting tissue-resident stem cells and facilitating tissue repair as well as minimizing collateral tissue damage from excessive inflammation.
There is little controversy that IV administration of MSCs at clinically used doses (1 to 4 × 106 cells/kg) is safe, transient and mild febrile reaction being the only side effect, and is well-tolerated even in patients with obstructive lung allotransplant rejection (Chambers et al., 2017; Galipeau and Sensebe, 2018; Keller et al., 2018). Concerns about spontaneous transformation and genetic instability observed with murine MSCs during culturing have been quelled by the use of human MSCs, because human MSCs cease proliferating after 35–55 population doublings (PD), and translocation is rarely seen at low PD (Prockop and Keating, 2012; Prockop et al., 2017). Since exogenous MSCs disappear before an adaptive immune rejection develops, the immunological incompatibility between donor MSCs and recipient patients is unlikely to impair the therapeutic benefits of MSCs. The possibility and consequence of alloimmunization, however, should be considered under chronic disease conditions where repeated cell injections are necessary. Moreover, although it is commonly known that costimulatory molecules such as CD80, CD86 and CD40 are not expressed or induced in MSCs (Di Nicola et al., 2002; Ryan et al., 2005; Tse et al., 2003), several reports have demonstrated the induction of MHC molecules and costimulatory molecules in MSCs stimulated with inflammatory cytokines, raising a potential role of MSCs as non-professional APCs (Fuentes-Julián et al., 2015; Krampera et al., 2003; Menard et al., 2013; Rafei et al., 2009). It was also reported that MSCs stimulated with a low dose of IFN-γ increased MHC class II expression and failed to suppress T cell proliferation, while stimulation of MSCs with a high dose of IFN-γ yielded the opposite results (Chan et al., 2006). In a similar vein, dexamethasone reduced the inhibitory effect of MSCs on T cell proliferation, and concurrent administration with dexamethasone abrogated the therapeutic effects of MSCs in mice with liver cirrhosis (Chen et al., 2014). These findings might explain the negative outcome of MSC treatment observed in the chronic phases of EAU and EAE with low, persistent inflammatory stimuli (Zappia et al., 2005; Zhang et al., 2011). Hence, different responses of MSCs according to the levels of inflammation should be taken into account when using MSCs in the setting of inflammatory and immune-mediated disorders.
Currently, a major challenge to the clinical use of MSCs is their functional heterogeneity. Indeed, this is the main reason that the majority of MSC clinical trials in autoimmune disease remain in the early stages and only less than 5% are phase III trials.
In vitro-expanded MSCs are intrinsically heterogeneous cell populations, and there are no definitive markers that can be used to purify cells from the mesenchyme to homogeneity. Even single-cell-derived colonies vary in size, differentiation potential, clonogenicity, transcriptomes and proteins (Smith et al., 2004; Ylostalo et al., 2008). Moreover, as MSCs are highly sensitive and responsive to the microenvironment, they dramatically change their molecular characteristics and biological properties during expansion, according to culture conditions. For example, we demonstrated that MSCs, depending on the cell-seeding density, displayed marked changes in cell size, morphology, surface epitopes, differentiation potential, proliferation rate and transcriptome profiles within just a few days (Larson et al., 2008; Lee et al., 2009b). In addition, hypoxia has been reported to elicit different responses in MSC proliferation, differentiation and secretomes (for a review, see Ejtehadifar et al., 2015).
MSCs also exhibit functional heterogeneities according to tissue sources even if the cells are prepared under the same culture condition. In a study by Sakaguchi et al., differentiation potential was compared among MSCs from 5 different tissue sources of healthy human donors (BM, AT, synovium, periosteum and skeletal muscle). BM- and synovium-derived MSCs showed the greatest ability for osteogenesis and chondrogenesis, whereas the frequency of lipid drops was highest in the AT- and synovium- derived MSCs (Sakaguchi et al., 2005). Likewise, numerous studies have demonstrated that MSCs from different tissue sources vary in their immunomodulatory properties as well as differentiation capacities (for a review, see Mattar and Bieback, 2015).
Donor-to-donor heterogeneity is another important issue that impacts the therapeutic efficacy of MSC batches. We demonstrated, in sterile inflammation models such as corneal chemical injury, peritonitis and bleomycin-induced lung injury, that BM MSCs, which had been obtained from different healthy individuals but expanded equally under the same conditions, showed large variations in their anti-inflammatory and therapeutic effects, despite the lack of any differences in cell surface markers commonly used to define MSCs (Lee et al., 2014).
These significant intra-population and donor-to-donor heterogeneities in the biological and functional properties of MSCs, not to mention different manufacturing conditions, can contribute to inconsistent clinical outcomes in human trials. As such, for the success of MSC-based therapies in ocular autoimmune diseases, it is important to standardize the manufacturing protocol so as to reproducibly generate clinically effective and functionally equivalent MSCs. In addition, MSC therapy should be optimized to improve clinical efficacy and minimize variability.
6. Strategies for the optimization of MSC therapy
6.1. MSC preconditioning
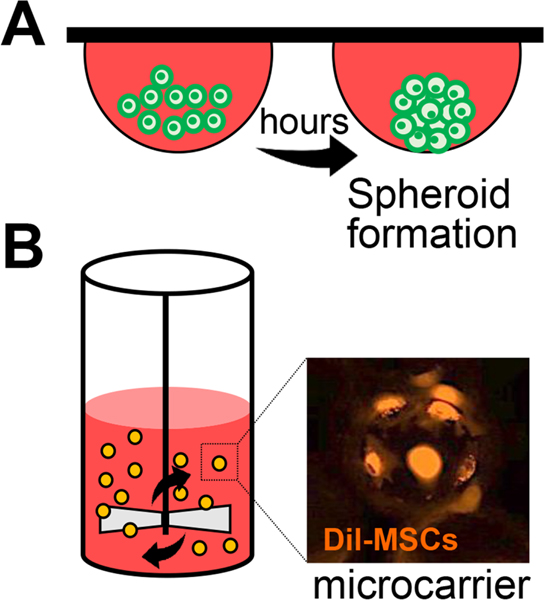
In vitro licensing of MSCs to produce a greater number of therapeutic factors can be used to improve the therapeutic potency of MSCs and to minimize their functional heterogeneity. There are several methods of MSC licensing. One is in vitro stimulation of MSCs with inflammatory cytokines such as IFN-γ or TNF-α. It is well-known that exposure to inflammatory cytokines, IFN-γ in particular, augments the functionality of MSCs by altering their cell morphology (Klinker et al., 2017) or upregulating their expression of immunosuppressive factors including IDO, PGE2, PD-L1 and TSG-6 (Choi et al., 2011; English et al., 2007; Krampera et al., 2013; Polchert et al., 2008). Another method is the genetic manipulation of MSCs to overexpress therapeutic factors using cDNA, CRISPR/Cas9 technology or viruses, but such genetic engineering carries risks of insertional mutations, raising additional safety concerns. Alternatively, MSC expansion under three-dimensional (3D) culture conditions can confer significant therapeutic benefits (Figure 11). Compared with 2D monolayer culture, 3D culture of MSCs, as in a hanging-drop-based scaffold-free system, stirring microcarrier culture system (spin flask or rotating wall vessel), and cell aggregates formed on fabricated membranes, has been found to increase selfrenewal and multipotent differentiation of MSCs, facilitate the engraftment and homing of cells, and boost the production of paracrine factors, leading to the enhanced tissue repair in multiple animal models of human diseases (Bartosh et al., 2010; Goh et al., 2013; Lam et al., 2017; Ylostalo et al., 2012; Zhou et al., 2017). Moreover, the conventional 2D monolayer culture of MSCs complicates the largescale, clinical-grade manufacturing of MSCs because it requires a large amount of media supplemented with human serum or platelet lysate, and thus is cost-intensive. In contrast, a 3D culture system with microcarriers requires a lesser volume of liquid media and is scalable in a closed system. Therefore, the 3D culture system not only improves the therapeutic efficacy of MSCs but also enables their large-scale and cost-effective production for clinical use.
Figure 11.
Three-dimensional (3D) culture methods of MSCs.
A. Hanging drop culture for 3D spheroid formation. MSCs aggregate at the bottom tip of the drop and form a spheroid.
B. Spinner flask bioreactor with microcarriers. MSCs are attached to the surface of the microcarrier and grow as monolayers on the surface of a small sphere. Representative photograph of fluorescence (Dil)-labeled MSCs on microcarriers (Corning) at 4 hours after seeding.
6.2. Biopotency assay
In vitro potency assays to predict clinical efficacy of MSCs and validate the potency of different MSC batches can be used to fend off functional heterogeneities among the cells. Moreover, MSC potency assays as well as identity and purity assays are mandatory for advanced clinical trials and are required to acquire an FDA biologics license application for marketing in the United States (Mendicino et al., 2014).
Thus far, mixed lymphocyte reaction (MLR) assays with peripheral blood mononuclear cells (PBMCs) or T cells have been commonly used to test the immunosuppressive potency of MSCs. In the MLR assay, a large number of MSCs are required to inhibit proliferation of PBMCs or lymphocytes (a few MSC:T-cell or PBMC ratios). In reality, however, most MSCs after systemic administration disappear within no more than a few days, and only a small number of them can encounter lymphocytes. Therefore, it is difficult to assume that the suppressive activity of MSCs in the MLR will reflect their immunosuppressive action in vivo. Indeed, recent studies have found that although late-passage UC-MSCs were more suppressive on PBMC proliferation in the MLR than early-passage UC-MSCs (Zhuang et al., 2015), early-passage MSCs were more effective in prolonging the survival of GVHD patients than were later-passage MSCs (75% vs. 21%) (von Bahr et al., 2012). These results show that the immunosuppressive potency of MSCs as measured with recipient PBMCs in the MLR does not correlate with the actual clinical outcome.
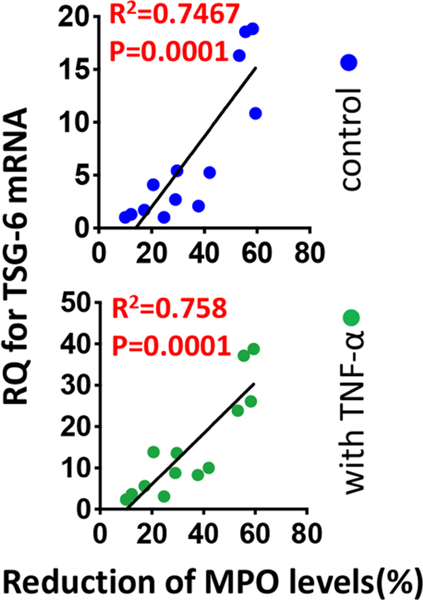
Moreover, the conventional MSC identity assays evaluating clonogenicity, differentiation potential or surface markers did not predict MSCs’ therapeutic potency in our sterile inflammation models (Lee et al., 2014). Instead, we found that the transcript levels of TSG-6 in unstimulated or TNF-α-stimulated MSCs had a positive correlation with the therapeutic potency of MSCs in mice with corneal chemical injury, bleomycin-induced lung injury and peritonitis (Figure 12) (Lee et al., 2014), a clear indication that MSCs’ therapeutic potency is related to their capacity to increase therapeutic factors in response to inflammatory stimuli rather than their proliferation and differentiation capacities.
Figure 12.
TSG-6 as a biomarker predictive of the in vivo anti-inflammatory potency of MSCs.
For identification of a biomarker that predicts the clinical efficacy of MSCs, the level of myeloperoxidase (MPO), a semi-quantitative measure of activated neutrophils, was measured in the cornea in BALB/c mice 3 days after infliction of sterile corneal injury and IV administration of human BM MSCs. Then, the correlation between the efficacy of MSCs in reducing MPO levels in the cornea and the expression by RT-PCR of genes previously linked to the therapeutic benefits of MSCs was evaluated.
Among the analyzed genes, there was a highly-significant correlation between TSG6 transcript levels in MSCs (with and without TNF-α stimulation) and the efficacy of the cells in reducing corneal MPO levels.
Originally published in Proceedings of the National Academy of Sciences of the United States of America. Lee et al., 2014. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc. Natl. Acad. Sci. U. S. A. 111, 16766–16771.
As another potential MSC bioassay, Galleu et al. demonstrated that the ability of recipient-derived cytotoxic cells to induce apoptosis of MSCs in vitro positively predicted clinical response to MSC transfusion in patients with severe steroid-resistant GVHD (Galleu et al., 2017). Also, Klinker et al. identified the morphologic features of MSCs predictive of in vitro immunosuppressive capacity by high-content imaging of MSCs following IFN-γ stimulation (Klinker et al., 2017); however, before determining whether such features can be used as a predictive biomarker of MSC clinical potency, their correlation with MSC therapeutic activity in vivo should be analyzed as well.
For identification of the specific biomarker(s) predictive of MSC potency in suppressing ocular autoimmune inflammation, future research is necessary. Elucidation of the exact mechanisms underlying MSC-mediated immunomodulation in ocular autoimmune diseases would be a key step in successful biopotency assay development.
6.3. Therapy with MSC-derived factors or EVs
It is well-established that MSCs ameliorate disease and maintain homeostasis via secretion of a large panel of bioactive factors and EVs in response to their microenvironment and injury signals. The use of MSC-derived factors as cell-free treatment has several advantages over cell therapy. First, there is no need to transfer cells that may possess mutated or damaged DNA. Second, proteins and EVs are small and readily circulates after IV administration, comparing favorably with MSCs, which are too large to pass through and circulate in lung capillaries. Third, it is easy to control the dose and delivery route for proteins and EVs. Moreover, proteins and EVs can be infused at higher doses and thus delivered to the injured tissue with greater, more efficacious impact. Therefore, efforts are being made to develop MSC-derived factors and EVs as therapeutics for ocular autoimmune diseases.
Among the therapeutic factors secreted by MSCs in an injury microenvironment, we have identified TSG-6 as a potential treatment of SS-related DED. In an NOD.B10.H2b mouse model of primary ocular SS, we found that topical application of recombinant human TSG-6 protein recapitulated to a large extent the therapeutic effects of MSCs on DED (Kim et al., 2016; Lee et al., 2015b). Moreover, TSG-6 dose-dependently promoted the migration of corneal epithelial cells in culture (Lee et al., 2015b). Beneficial therapeutic effects have also been observed with Link_TSG6, which is the recombinant Link module from human TSG-6 protein (Oh, J.Y., Milner, C.M. and Day, A.J. unpublished data). Our data thus indicate that Link_TSG6 has the potential to treat the signs and symptoms of DED.
In recent years, MSC-derived EVs have come into the limelight as a novel alternative to MSC therapy. MSCs secrete EVs containing a large number of RNAs, proteins and mitochondria, which recapitulate a broad range of the therapeutic effects of parent MSCs (Figure 13) (for a review, see Phinney and Pittenger, 2017). In this regard, we demonstrated that MSC-EVs were as effective as their parent MSCs in alleviating immune responses and preventing disease development in animal models of autoimmune diseases (Hai et al., 2018; Shigemoto-Kuroda et al., 2017).
Figure 13.
The potential of MSC-derived EVs as cell-free alternatives to MSCs for the treatment of disease.
MSCs produce EVs which contain a large number of therapeutic molecules including RNAs, proteins and mitochondria. MSC-EVs have been shown to exert therapeutic benefits in a wide range of animal models of human diseases.
In an EAU model, we investigated the effect of human BM MSC-derived EVs isolated by column fractionation on IRBP-induced EAU in C57BL/6 mice (Shigemoto-Kuroda et al., 2017). Either MSC-derived EVs (30 μg containing 15 × 109 EVs per mouse) or MSCs (1 × 106 cells per mouse, the same lot of MSCs from which EVs were produced) were injected once via the tail vein immediately after IRBP immunization (day 0). Examination at day 21 revealed that the MSC-derived EVs suppressed Th1 and Th17 cells and prevented EAU development as effectively as their parent cells did. In culture, MSC-EVs directly inhibited T cell proliferation upon CD3/CD28 stimulation, but did not induce FoxP3+ Treg cells. Also, MSC-EVs downregulated the expression of MHC class II and costimulatory factors (CD40, CD80, CD86) on APCs and hindered APC activation. Similar findings were reported for a Lewis rat model of IRBP (R16)-induced EAU by Bai et al. (Bai et al., 2017). They had administered either human UC MSC-derived EVs (10 to 100 μg) prepared by sequential centrifugation or human dermal fibroblast-derived EVs via periocular injection on a daily basis for 7 consecutive days, starting on the day of disease manifestation (9 days after immunization). MSC-EVs, but not fibroblast-EVs, displayed significant therapeutic effects on the ongoing EAU as examined by clinical observation, retinal histology and ERG.
We also elucidated the effect and mechanism of human BM MSC-derived EVs in an NOD.B10.H2b mouse model exhibiting a phenotype of primary ocular SS (Figure 14) (Kim et al., 2020). In the study, we directly injected 5 × 108 EVs into the intraorbital LG of 12-week-old NOD.B10.H2b mice. MSC-EVs reduced corneal epithelial defects, increased conjunctival goblet cell density, and suppressed the levels of proinflammatory cytokines on the ocular surface. Also, MSC-EVs improved aqueous tear production while reducing the number of CD3+ lymphocytic foci in the LG. Remarkably, a small dose of EVs (5 ×108 EV particles), injected locally into the LG, was sufficient to ameliorate ocular disease, whereas up to 1.5 × 1010 EV particles had been used for systemic IV infusion in EAU mice (Shigemoto-Kuroda et al., 2017). Further mechanistic investigation revealed that EVs derived from early-passage MSCs (15 PD) were more effective than those from late-passage cells (40 PD) in suppressing Th1 and Th17 cytokines in splenocyte cultures and ameliorating DED in NOD.B10.H2b mice (Kim et al., 2020). By comparing molecular profiles between early- and late-passage MSC-EVs, we found that EVs from early-passage MSCs contained higher levels of TGF-β1, pentraxin 3 (PTX3), let-7b and miR-21b. Silencing of these factors in MSCs abrogated the immunosuppressive effects of their EVs in in vitro splenocyte cultures and in NOD.B10.H2b mice, indicating that TGF-β1, PTX3, let-7b and miR-21b are key effectors responsible for EV-mediated immunomodulation. In a separate study, we additionally observed that topical or subconjunctival administration of MSC-EVs had direct effects on the promotion of corneal epithelial healing following epithelial debridement (Figure 15) (Oh, J.Y., unpublished data). Consistent with our results, other groups have demonstrated therapeutic effects of the topical application of human corneal stroma-derived, human placenta-derived, or human AT-derived MSC-EVs in murine models of corneal mechanical wounding (Shojaati et al., 2019), corneal alkali injury (Tao et al., 2019)nd DED (Yu et al., 2020).
Figure 14.
Effects of MSC-derived EVs on SS-related DED.
EVs derived from human BM MSCs were injected locally into the intraorbital LG in NOD.B10.H2b mice exhibiting a phenotype of primary ocular SS. The MSC-EVs were effective in reducing corneal epithelial defects, increasing conjunctival goblet cell density, and suppressing the levels of pro-inflammatory cytokines on the ocular surface. Also, the MSC-EVs improved aqueous tear production and decreased the number of CD3+ lymphocytic foci in the LG. Especially, EVs derived from early-passage MSCs (15 PD) alleviated DED more effectively than did those from late-passage cells (40 PD).
Reprinted with permission from Molecular Therapy. Kim et al., 2020. Comprehensive Molecular Profiles of Functionally Effective MSC-Derived Extracellular Vesicles in Immunomodulation. Mol. Ther. 28, 1628–1644. Copyright © 2020 The American Society of Gene and Cell Therapy.
Figure 15.
Effects of topically applied MSC-EVs on corneal epithelial regeneration.
Topical instillation of EVs derived from human MSCs markedly facilitated corneal epithelial healing in BALB/c mice following mechanical removal of corneal epithelium, as evaluated by fluorescein vital dye staining. The transcript levels of inflammatory cytokines (IL-1β and IL-6) in the cornea also were decreased by the MSC-EVs.
Another advantage of MSC-EVs as a therapeutic is that they can be used as a drug delivery system. Currently, the most popular vehicles of drug delivery are liposomes and polymeric nanoparticles. However, whether liposomes can evade the host immune system with long-circulating stability and without toxicity remains elusive. Polymeric nanoparticles may have better stability than liposomal systems, but their biocompatibility and long-term safety are unresolved issues (for reviews, see Li et al., 2015; Raemdonck et al., 2014). By contrast, EVs have many desirable features of an ideal drug delivery system including biocompatibility, the intrinsic ability to target tissues (Hoshino et al., 2015; Lai et al., 2014; Losche et al., 2004), stability in circulation without losing functional activity, and minimal or no inherent toxicities (Escudier et al., 2005; Maji et al., 2017). Moreover, EVs produced by MSCs have additional benefits for the treatment of disease, specifically by delivering MSCderived therapeutic mRNAs, microRNAs and proteins. Since MSC-EVs express several adhesion molecules (Kim et al., 2020; La Greca et al., 2018), systemically-infused MSC-EVs tend to accumulate in injured tissue (Grange et al., 2014; Otero-Ortega et al., 2018). Furthermore, it is easy to manipulate the contents of MSC-EVs through genetic engineering of MSCs with cDNA, siRNA or virus (Kim et al., 2020; O’Brien et al., 2018), and thus EVs laden with therapeutic factors can be produced from engineered MSCs while avoiding safety concerns related to insertion mutations in genetically engineered cells.
7. Conclusions and future directions
Nearly three decades of research have demonstrated that MSCs have unique properties that can be harnessed for the development of novel therapeutics. In the field of ocular autoimmune disease, preclinical animal data have shown the therapeutic promise of MSCs, and clinical trials are underway to prove the safety and clinical effectiveness of MSCs in patients. The success of clinical trials with MSC-based therapies will eventually depend on a number of critical factors including selection of potent cellular products, establishment of a reproducible manufacturing process, and optimization of treatment protocols tailored to a disease and patient. This process will take much time and rigorous research, as exemplified by the 60-year history that had been required to develop the first successful stem cell therapy: hematopoietic stem cell transplantation. Importantly, since MSCs are a prominent cellular identity that interact with their microenvironment and neighboring cells to prov therapeutic effects, further elucidation of their mechanism of action will equip us with novel translational strategies, and thereby enable us to make better use of MSCs for prevention and treatment of ocular autoimmune diseases.
Highlights.
Mesenchymal stromal cells (MSCs) ameliorate disease by modulating immune response and promoting tissue regeneration.
MSCs exert therapeutic effects on autoimmune uveoretinitis and dry eye disease through Th1 and Th17 inhibition.
MSCs’ long-lasting immunomodulatory effects are partly mediated by monocytes and macrophages.
MSC-derived extracellular vesicles have potential benefits as cell-free therapy for ocular autoimmune diseases.
Acknowledgments
We would like to recognize our postdoctoral advisor, Dr. Darwin J. Prockop, a pioneer in the field of mesenchymal stem/stromal cells. This work would not have been possible without the mentoring we received during our postdoctoral training.
Funding:
This work was supported by the Korea Health Industry Development Institute (HI11C1963, HI12C0191 and HI15C3134 to J.Y.O.), the National Research Foundation of Korea (2014R1A2A1A11050895, 2018R1A2B2004108 and 2021R1A2C3004532 to J.Y.O.), and the National Institutes of Health (1R01EY029350–01A1 and 5R21DE027457–02 to R.H.L.).
Abbreviations
- AT
adipose tissue
- ADDE
aqueous deficiency dry eye
- APC
antigen-presenting cell
- AREG
amphiregulin
- Arg1
arginase 1
- BM
bone marrow
- CCL2
chemokine (C-C motif) ligand 2
- ConA
concanavalin A
- DED
dry eye disease
- DLN
draining lymph node
- EAE
experimental autoimmune encephalomyelitis
- EAU
experimental autoimmune uveoretinitis
- ERG
electroretinography
- EV
extracellular vesicle
- FasL
Fas ligand
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GVHD
graft-versus-host disease
- IDO
indoleamine-2,3-dioxygenase
- iNOS
inducible nitric oxide synthase IP: intraperitoneal
- iPSC
induced pluripotent stem cell
- IRBP
interphotoreceptor retinoid-binding protein
- IV
intravenous
- KCS
keratoconjunctivitis sicca
- LG
lacrimal gland
- LPS
lipopolysaccharides
- MCP-1
monocyte chemoattractant protein-1
- MDSC
myeloid-derived suppressor cell
- MHC
major histocompatibility complex
- MI
myocardial infarction
- MLR
mixed lymphocyte reaction
- MS
multiple sclerosis
- MSC
mesenchymal stromal cell
- NK
natural killer
- NO
nitric oxide
- OSDI
ocular surface disease index
- PBMC
peripheral blood mononuclear cell
- PD
population doublings
- PD-L1
programmed death-ligand 1
- PGE2
prostaglandin E2
- PTX3
pentraxin 3
- SS
Sjögren’s syndrome
- STC-1
stanniocalcin-1
- TBUT
tear break-up time
- TLR
Toll-like receptor
- Treg
regulatory T
- TSG-6
TNFα-stimulated gene/protein-6
- UC
umbilical cord
Footnotes
Declaration of competing interest
J.Y.O. have filed two patent applications, and R.H.L. one patent application, which are in part related to the subject reviewed in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Abughanam G, Elkashty OA, Liu Y, Bakkar MO, Tran SD, 2019. Mesenchymal Stem Cells Extract (MSCsE)-Based Therapy Alleviates Xerostomia and Keratoconjunctivitis Sicca in Sjogren’s Syndrome-Like Disease. Int. J. Mol. Sci. 20, 4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal RK, Caspi RR, 2004. Rodent models of experimental autoimmune uveitis. Methods Mol. Med. 102, 395–419. [DOI] [PubMed] [Google Scholar]
- Agarwal RK, Silver PB, Caspi RR, 2012. Rodent models of experimental autoimmune uveitis. Methods Mol. Biol. 900, 443–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S, Pittenger MF, 2005. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822. [DOI] [PubMed] [Google Scholar]
- Akiyama K., Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S, 2012. Mesenchymal-stem-cell-induced immunoregulation involves FASligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 10, 544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluri HS, Samizadeh M, Edman MC, Hawley DR, Armaos HL, Janga SR, Meng Z, Sendra VG, Hamrah P, Kublin CL, Hamm-Alvarez SF, Zoukhri D, 2017. Delivery of Bone Marrow-Derived Mesenchymal Stem Cells Improves Tear Production in a Mouse Model of Sjogren’s Syndrome. Stem Cells Int. 2017, 3134543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JH, Li Q, Ryu MO, Nam AR, Bhang DH, Jung YC, Song WJ, Youn HY, 2020. TSG-6 in extracellular vesicles from canine mesenchymal stem/stromal is a major factor in relieving DSS-induced colitis. PLoS One 15, e0220756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankrum JA, Ong JF, Karp JM, 2014. Mesenchymal stem cells: immune evasive, not immune privileged. Nat. Biotechnol. 32, 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonangeli F, Natalini A, Garassino MC, Sica A, Santoni A, Di Rosa F, 2020. Regulation of PD-L1 Expression by NF-kappaB in Cancer. Front. Immunol. 11, 584626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Shao H, Wang H, Zhang Z, Su C, Dong L, Yu B, Chen X, Li X, Zhang X, 2017. Effects of Mesenchymal Stem Cell-Derived Exosomes on Experimental Autoimmune Uveitis. Sci. Rep. 7, 4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabino S, Dana MR, 2004. Animal models of dry eye: a critical assessment of opportunities and limitations. Invest. Ophthalmol. Vis. Sci. 45, 1641–1646. [DOI] [PubMed] [Google Scholar]
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R, 2002. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 30, 42–48. [DOI] [PubMed] [Google Scholar]
- Bartosh TJ, Ylostalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H, Prockop DJ, 2010. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. U. S. A. 107, 13724–13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt MK, Barros MA, Martins JF, Vasconcellos JP, Morais BP, Pompeia C, Bittencourt MD, Evangelho KD, Kerkis I, Wenceslau CV, 2016. Allogeneic Mesenchymal Stem Cell Transplantation in Dogs With Keratoconjunctivitis Sicca. Cell Med. 8, 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block GJ, Ohkouchi S, Fung F, Frenkel J, Gregory C, Pochampally R, DiMattia G, Sullivan DE, Prockop DJ, 2009. Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells. 27, 670–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V., Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI, 2016. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 7, 12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi RR, Silver PB, Luger D, Tang J, Cortes LM, Pennesi G, Mattapallil MJ, Chan CC, 2008. Mouse models of experimental autoimmune uveitis. Ophthalmic Res. 40, 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers DC, Enever D, Lawrence S, Sturm MJ, Herrmann R, Yerkovich S, Musk M, Hopkins PM, 2017. Mesenchymal Stromal Cell Therapy for Chronic Lung Allograft Dysfunction: Results of a First-in-Man Study. Stem Cells Transl. Med. 6, 1152–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, Rameshwar P, 2006. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood 107, 48174824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaubey S, Thueson S, Ponnalagu D, Alam MA, Gheorghe CP, Aghai Z, Singh H, Bhandari V, 2018. Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosomeassociated factor TSG-6. Stem Cell Res. Ther. 9, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gan Y, Li W, Su J, Zhang Y, Huang Y, Roberts AI, Han Y, Li J, Wang Y, Shi Y, 2014. The interaction between mesenchymal stem cells and steroids during inflammation. Cell Death Dis. 5, e1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Shao H, Zhi Y, Xiao Q, Su C, Dong L, Liu X, Li X, Zhang X, 2016. CD73 Pathway Contributes to the Immunosuppressive Ability of Mesenchymal Stem Cells in Intraocular Autoimmune Responses. Stem Cells Dev. 25, 337–346. [DOI] [PubMed] [Google Scholar]
- Chen Y, Tang X, Li P, Zhou Y, Xue T, Liu J, Yu C, 2019. Bone Marrow Derived Mesenchymal Stromal Cells Ameliorate Ischemia/Reperfusion Injury-Induced Acute Kidney Injury in Rats via Secreting Tumor Necrosis Factor-Inducible Gene 6 Protein. Biomed. Res. Int. 2019, 9845709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ, 2011. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood 118, 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copland DA, Wertheim MS, Armitage WJ, Nicholson LB, Raveney BJ, Dick AD, 2008. The clinical time-course of experimental autoimmune uveoretinitis using topical endoscopic fundal imaging with histologic and cellular infiltrate correlation. Invest. Ophthalmol. Vis. Sci. 49, 5458–5465. [DOI] [PubMed] [Google Scholar]
- Cortes LM., Mattapallil MJ., Silver PB., Donoso LA., Liou GI., Zhu W., Chan CC., Caspi RR., 2008. Repertoire analysis and new pathogenic epitopes of IRBP in C57BL/6 (H-2b) and B10.RIII (H-2r) mice. Invest. Ophthalmol. Vis. Sci. 49, 19461956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies LC, Heldring N, Kadri N, Le Blanc K, 2017. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells 35, 766–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day AJ, Milner CM, 2019. TSG-6: A multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 78–79, 60–83. [DOI] [PubMed] [Google Scholar]
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM, 2002. Human bone marrow stromal cells suppress Tlymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99, 3838–3843. [DOI] [PubMed] [Google Scholar]
- Diedrichs-Mohring M, Hoffmann C, Wildner G, 2008. Antigen-dependent monophasic or recurrent autoimmune uveitis in rats. Int. Immunol. 20, 365–374. [DOI] [PubMed] [Google Scholar]
- Diedrichs-Mohring M, Kaufmann U, Wildner G, 2018. The immunopathogenesis of chronic and relapsing autoimmune uveitis - Lessons from experimental rat models. Prog. Retin. Eye Res. 65, 107–126. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Ott L, Roth M, Witt J, Geerling G, Mertsch S, Schrader S, 2019. MSC Transplantation Improves Lacrimal Gland Regeneration after Surgically Induced Dry Eye Disease in Mice. Sci. Rep. 9, 18299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejtehadifar M, Shamsasenjan K, Movassaghpour A, Akbarzadehlaleh P, Dehdilani N, Abbasi P, Molaeipour Z, Saleh M, 2015. The Effect of Hypoxia on Mesenchymal Stem Cell Biology. Adv. Pharm. Bull. 5, 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English K, Barry FP, Field-Corbett CP, Mahon BP, 2007. IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol. Lett. 110, 91–100. [DOI] [PubMed] [Google Scholar]
- Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L, 2005. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J. Transl. Med 3, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois M, Romieu-Mourez R, Li M, Galipeau J, 2012. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 20, 187–195. [DOI] [PubMed] [Google Scholar]
- Fuentes-Julián S., Arnalich-Montiel F., Jaumandreu L., Leal M., Casado A., García-Tuñon I., Hernández-Jiménez E., López-Collazo E., De Miguel MP., 2015. Adipose-derived mesenchymal stem cell administration does not improve corneal graft survival outcome. PLoS One 10, e0117945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galipeau J, Sensebe L, 2018. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell. Stem Cell 22, 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS, von Bonin M, Barbieri L, Halai K, Ward S, Weng L, Chakraverty R, Lombardi G, Watt FM, Orchard K, Marks DI, Apperley J, Bornhauser M, Walczak H, Bennett C, Dazzi F, 2017. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 9, eaam7828. [DOI] [PubMed] [Google Scholar]
- Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI, 2001. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 169, 12–20. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Guilliams M, 2016. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity 44, 439–449. [DOI] [PubMed] [Google Scholar]
- Goh TK, Zhang ZY, Chen AK, Reuveny S, Choolani M, Chan JK, Oh SK, 2013. Microcarrier culture for efficient expansion and osteogenic differentiation of human fetal mesenchymal stem cells. Biores Open Access 2, 84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar K, Gunatilake D, Gallagher SJ, Tiffen J, Rizos H, Hersey P, 2015. Inducible but not constitutive expression of PD-L1 in human melanoma cells is dependent on activation of NF-kappaB. PLoS One 10, e0123410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange C, Tapparo M, Bruno S, Chatterjee D, Quesenberry PJ, Tetta C, Camussi G, 2014. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int. J. Mol. Med. 33, 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu YZ, Xue Q, Chen YJ, Yu GH, Qing MD, Shen Y, Wang MY, Shi Q, Zhang XG, 2013. Different roles of PD-L1 and FasL in immunomodulation mediated by human placenta-derived mesenchymal stem cells. Hum. Immunol. 74, 267–276. [DOI] [PubMed] [Google Scholar]
- Hai B, Shigemoto-Kuroda T, Zhao Q, Lee RH, Liu F, 2018. Inhibitory Effects of iPSC-MSCs and Their Extracellular Vesicles on the Onset of Sialadenitis in a Mouse Model of Sjogren’s Syndrome. Stem Cells Int. 2018, 2092315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Hua J, Qian D, Gong J, Lin S, Xu C, Wei G, Meng H, Yang T, Zhou B, Song Z, 2016. Intravenous hMSCs Ameliorate Acute Pancreatitis in Mice via Secretion of Tumor Necrosis Factor-alpha Stimulated Gene/Protein 6. Sci. Rep. 6, 38438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK, 1999. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat. Med. 5, 309–313. [DOI] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K., Minn AJ., Bissell MJ., Garcia BA., Kang Y., Rajasekhar VK., Ghajar CM., Matei I., Peinado H., Bromberg J., Lyden D., 2015. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SC, Gavrilin MA, Lee HH, Wu CC, Han SH, Lai MZ, 1999. NF-kappa B-dependent Fas ligand expression. Eur. J. Immunol. 29, 2948–2956. [DOI] [PubMed] [Google Scholar]
- Jabbehdari S, Yazdanpanah G, Kanu LN, Chen E, Kang K, Anwar KN, Ghassemi M, Hematti P, Rosenblatt MI, Djalilian AR, 2020. Therapeutic Effects of Lyophilized Conditioned-Medium Derived from Corneal Mesenchymal Stromal Cells on Corneal Epithelial Wound Healing. Curr. Eye Res. 45, 1490–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HJ, Lee HJ, Ko JH, Cho BJ, Park SY, Park JW, Choi SR, Heo JW, Yoon SO, Oh JY, 2018. Myeloid-Derived Suppressor Cells Mediate Inflammation Resolution in Humans and Mice with Autoimmune Uveoretinitis. J. Immunol. 200, 1306–1315. [DOI] [PubMed] [Google Scholar]
- Jha KA, Pentecost M, Lenin R, Gentry J, Klaic L, Del Mar N, Reiner A, Yang CH, Pfeffer LM, Sohl N, Gangaraju R, 2019. TSG-6 in conditioned media from adipose mesenchymal stem cells protects against visual deficits in mild traumatic brain injury model through neurovascular modulation. Stem Cell Res. Ther. 10, 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaswan R, Pappas C, Wall K, Hirsh SG, 1998. Survey of canine tear deficiency in veterinary practice. Adv. Exp. Med. Biol. 438, 931–939. [DOI] [PubMed] [Google Scholar]
- Keller CA, Gonwa TA, Hodge DO, Hei DJ, Centanni JM, Zubair AC, 2018. Feasibility, Safety, and Tolerance of Mesenchymal Stem Cell Therapy for Obstructive Chronic Lung Allograft Dysfunction. Stem Cells Transl. Med. 7, 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr EC, Raveney BJ, Copland DA, Dick AD, Nicholson LB, 2008. Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J. Autoimmun. 31, 354–361. [DOI] [PubMed] [Google Scholar]
- Khalili S, Liu Y, Kornete M, Roescher N, Kodama S, Peterson A, Piccirillo CA, Tran SD, 2012. Mesenchymal stromal cells improve salivary function and reduce lymphocytic infiltrates in mice with Sjogren’s-like disease. PLoS One 7, e38615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Lee MJ., Bae EH., Ryu JS., Kaur G., Kim HJ., Kim JY., Barreda H., Jung SY., Choi JM., Shigemoto-Kuroda T., Oh JY., Lee RH., 2020. Comprehensive Molecular Profiles of Functionally Effective MSC-Derived Extracellular Vesicles in Immunomodulation. Mol. Ther. 28, 1628–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Ko JH, Yun JH, Kim JA, Kim TE, Lee HJ, Kim SH, Park KH, Oh JY, 2013. Stanniocalcin-1 protects retinal ganglion cells by inhibiting apoptosis and oxidative damage. PLoS One 8, e63749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Ryu JS, Park SY, Lee HJ, Ko JH, Kim MK, Wee WR, Oh JY, 2016. Comparison of Topical Application of TSG-6, Cyclosporine, and Prednisolone for Treating Dry Eye. Cornea 35, 536–542. [DOI] [PubMed] [Google Scholar]
- Kimbrel EA, Kouris NA, Yavanian GJ, Chu J, Qin Y, Chan A, Singh RP, McCurdy D, Gordon L, Levinson RD, Lanza R, 2014. Mesenchymal stem cell population derived from human pluripotent stem cells displays potent immunomodulatory and therapeutic properties. Stem Cells Dev. 23, 1611–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinker MW, Marklein RA, Lo Surdo JL, Wei CH, Bauer SR, 2017. Morphological features of IFN-gamma-stimulated mesenchymal stromal cells predict overall immunosuppressive capacity. Proc. Natl. Acad. Sci. U. S. A. 114, E2598E2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Lee HJ, Jeong HJ, Kim MK, Wee WR, Yoon SO, Choi H, Prockop DJ, Oh JY, 2016. Mesenchymal stem/stromal cells precondition lung monocytes/macrophages to produce tolerance against allo- and autoimmunity in the eye. Proc. Natl. Acad. Sci. U. S. A. 113, 158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Lee HJ, Jeong HJ, Oh JY, 2017. Ly6C(hi) monocytes are required for mesenchymal stem/stromal cell-induced immune tolerance in mice with experimental autoimmune uveitis. Biochem. Biophys. Res. Commun. 494, 6–12. [DOI] [PubMed] [Google Scholar]
- Ko JH, Kim HJ, Jeong HJ, Lee HJ, Oh JY, 2020. Mesenchymal Stem and Stromal Cells Harness Macrophage-Derived Amphiregulin to Maintain Tissue Homeostasis. Cell Rep. 30, 3806–3820. [DOI] [PubMed] [Google Scholar]
- Koga H, Muneta T, Nagase T, Nimura A, Ju YJ, Mochizuki T, Sekiya I, 2008. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 333, 207–215. [DOI] [PubMed] [Google Scholar]
- Kot M, Baj-Krzyworzeka M, Szatanek R, Musial-Wysocka A, Suda-Szczurek M, Majka M, 2019. The Importance of HLA Assessment in “Off-the-Shelf” Allogeneic Mesenchymal Stem Cells Based-Therapies. Int. J. Mol. Sci. 20, 5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota DJ, Wiggins LL, Yoon N, Lee RH, 2013. TSG-6 produced by hMSCs delays the onset of autoimmune diabetes by suppressing Th1 development and enhancing tolerogenicity. Diabetes 62, 2048–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F, 2003. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101, 3722–3729. [DOI] [PubMed] [Google Scholar]
- Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L, MSC Committee of the International Society for Cellular Therapy (ISCT), 2013. Immunological characterization of multipotent mesenchymal stromal cells--The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 15, 1054–1061. [DOI] [PubMed] [Google Scholar]
- La Greca A., Solari C, Furmento V, Lombardi A, Biani MC, Aban C, Moro L, Garcia M, Guberman AS, Sevlever GE, Miriuka SG, Luzzani C, 2018. Extracellular vesicles from pluripotent stem cell-derived mesenchymal stem cells acquire a stromal modulatory proteomic pattern during differentiation. Exp. Mol. Med. 50, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire CA, Chen JW, Tannous BA, Breakefield XO, 2014. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 8, 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam AT, Li J, Toh JP, Sim EJ, Chen AK, Chan JK, Choolani M, Reuveny S, Birch WR, Oh SK, 2017. Biodegradable poly-epsilon-caprolactone microcarriers for efficient production of human mesenchymal stromal cells and secreted cytokines in batch and fed-batch bioreactors. Cytotherapy 19, 419–432. [DOI] [PubMed] [Google Scholar]
- Larson BL, Ylostalo J, Prockop DJ, 2008. Human multipotent stromal cells undergo sharp transition from division to development in culture. Stem Cells 26, 193–201. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O, 2004. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363, 1439–1441. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O, Developmental Committee of the European Group for Blood and Marrow Transplantation, 2008. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371, 1579–1586. [DOI] [PubMed] [Google Scholar]
- Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL, 2002. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology 107, 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GR, 2018. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 19, 730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Ko JH, Jeong HJ, Ko AY, Kim MK, Wee WR, Yoon SO, Oh JY, 2015a. Mesenchymal stem/stromal cells protect against autoimmunity via CCL2-dependent recruitment of myeloid-derived suppressor cells. J. Immunol. 194, 3634–3645. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Ko JH, Kim HJ, Jeong HJ, Oh JY, 2020. Mesenchymal stromal cells induce distinct myeloid-derived suppressor cells in inflammation. JCI Insight 5, e136059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ., Kim DH., Ryu JS., Ko AY., Ko JH., Kim MK., Wee WR., Khwarg SI., Oh JY., 2015b. Topical TSG-6 Administration Protects the Ocular Surface in Two Mouse Models of Inflammation-Related Dry Eye. Invest. Ophthalmol. Vis. Sci. 56, 5175–5181. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Ko AY, Ko JH, Lee HJ, Kim MK, Wee WR, Khwarg SI, Oh JY, 2015c. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol. Ther. 23, 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, SemprunPrieto L, Delafontaine P, Prockop DJ, 2009a. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5, 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Seo MJ, Pulin AA, Gregory CA, Ylostalo J, Prockop DJ, 2009b. The CD34-like protein PODXL and alpha6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood 113, 816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Oh JY, Choi H, Bazhanov N, 2011. Therapeutic factors secreted by mesenchymal stromal cells and tissue repair. J. Cell. Biochem. 112, 3073–3078. [DOI] [PubMed] [Google Scholar]
- Lee RH, Yu JM, Foskett AM, Peltier G, Reneau JC, Bazhanov N, Oh JY, Prockop DJ, 2014. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc. Natl. Acad. Sci. U. S. A. 111, 16766–16771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibacher J, Henschler R, 2016. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res. Ther. 7, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zhang J, Zu YJ, Nie SF, Cao J, Wang Q, Nie SP, Deng ZY, Xie MY, Wang S, 2015. Biocompatible and biodegradable nanoparticles for enhancement of anti-cancer activities of phytochemicals. Chin. J. Nat. Med. 13, 641652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Yuan L, Ren X, Nian H, Zhang L, Han ZC, Li X, Zhang X, 2013. The effect of mesenchymal stem cells on dynamic changes of T cell subsets in experimental autoimmune uveoretinitis. Clin. Exp. Immunol. 173, 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Song WJ, Ryu MO, Nam A, An JH, Ahn JO, Bhang DH, Jung YC, Youn HY, 2018. TSG-6 secreted by human adipose tissue-derived mesenchymal stem cells ameliorates severe acute pancreatitis via ER stress downregulation in mice. Stem Cell Res. Ther. 9, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Chen W, Huang S, Tang X, Yao G, Sun L, 2020. Mesenchymal Stem Cells Enhance Pulmonary Antimicrobial Immunity and Prevent Following Bacterial Infection. Stem Cells Int. 2020, 3169469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lu X, Sun D, Wang X, Yang L, Zhao S, Nian H, Wei R, 2016. Adipose-Derived Mesenchymal Stem Cells Reduce Lymphocytic Infiltration in a Rabbit Model of Induced Autoimmune Dacryoadenitis. Invest. Ophthalmol. Vis. Sci. 57, 5161–5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Song H., Dua H., Chai J., Yang J., Li X., Yu Y., Zhang X., Hu X., Xiao M., Feng R., Yin H., Hu Q., Yan L., D J., L T., 2016. TSG-6 secreted by human umbilical cord-MSCs attenuates severe burn-induced excessive inflammation via inhibiting activations of P38 and JNK signaling. Sci. Rep. 6, 30121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zeng R, Wang Y, Huang W, Hu B, Zhu G, Zhang R, Li F, Han J, Li Y, 2019. Mesenchymal stem cells enhance microglia M2 polarization and attenuate neuroinflammation through TSG-6. Brain Res. 1724, 146422. [DOI] [PubMed] [Google Scholar]
- Locati M, Curtale G, Mantovani A, 2020. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 15, 123–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohan P, Murphy N, Treacy O, Lynch K, Morcos M, Chen B, Ryan AE, Griffin MD, Ritter T, 2018. Third-Party Allogeneic Mesenchymal Stromal Cells Prevent Rejection in a Pre-sensitized High-Risk Model of Corneal Transplantation. Front. Immunol. 9, 2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losche W, Scholz T, Temmler U, Oberle V, Claus RA, 2004. Platelet-derived microvesicles transfer tissue factor to monocytes but not to neutrophils. Platelets 15, 109–115. [DOI] [PubMed] [Google Scholar]
- Lu X, Li N, Zhao L, Guo D, Yi H, Yang L, Liu X, Sun D, Nian H, Wei R, 2020. Human umbilical cord mesenchymal stem cells alleviate ongoing autoimmune dacryoadenitis in rabbits via polarizing macrophages into an anti-inflammatory phenotype. Exp. Eye Res. 191, 107905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maji S, Yan IK, Parasramka M, Mohankumar S, Matsuda A, Patel T, 2017. In vitro toxicology studies of extracellular vesicles. J. Appl. Toxicol. 37, 310–318. [DOI] [PubMed] [Google Scholar]
- Mattar P, Bieback K, 2015. Comparing the Immunomodulatory Properties of Bone Marrow, Adipose Tissue, and Birth-Associated Tissue Mesenchymal Stromal Cells. Front. Immunol. 6, 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS, Liles WC, Stewart DJ, 2010. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 182, 1047–1057. [DOI] [PubMed] [Google Scholar]
- Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D, 2004. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3dioxygenase-mediated tryptophan degradation. Blood 103, 4619–4621. [DOI] [PubMed] [Google Scholar]
- Menard C, Pacelli L, Bassi G, Dulong J, Bifari F, Bezier I, Zanoncello J, Ricciardi M., Latour M., Bourin P., Schrezenmeier H., Sensebé L., Tarte K., Krampera M., 2013. Clinical-grade mesenchymal stromal cells produced under various good manufacturing practice processes differ in their immunomodulatory properties: standardization of immune quality controls. Stem Cells Dev. 22, 1789–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR, 2014. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell 14, 141–145. [DOI] [PubMed] [Google Scholar]
- Mezey É, Nemeth K, 2015. Mesenchymal stem cells and infectious diseases: Smarter than drugs. Immunol Lett. 168, 208–214. [DOI] [PubMed] [Google Scholar]
- Moller-Hansen M, Larsen AC, Toft PB, Lynggaard CD, Schwartz C, Bruunsgaard H, Haack-Sorensen M, Ekblond A, Kastrup J, Heegaard S, 2020. Safety and feasibility of mesenchymal stem cell therapy in patients with aqueous deficient dry eye disease. Ocul. Surf. 19, 43–52. [DOI] [PubMed] [Google Scholar]
- Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E, 2009. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 15, 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel D, Djouad F, Jorgense C, 2002. Regenerative medicine through mesenchymal stem cells for bone and cartilage repair. Curr. Opin. Investig Drugs 3, 1000–1004. [PubMed] [Google Scholar]
- Obermajer N, Popp FC, Soeder Y, Haarer J, Geissler EK, Schlitt HJ, Dahlke MH, 2014. Conversion of Th17 into IL-17A(neg) regulatory T cells: a novel mechanism in prolonged allograft survival promoted by mesenchymal stem cell-supported minimized immunosuppressive therapy. J. Immunol. 193, 4988–4999. [DOI] [PubMed] [Google Scholar]
- O’Brien KP, Khan S, Gilligan KE, Zafar H, Lalor P, Glynn C, O’Flatharta C, Ingoldsby H, Dockery P, De Bhulbh A, Schweber JR, St John K, Leahy M, Murphy JM, Gallagher WM, O’Brien T, Kerin MJ, Dwyer RM, 2018. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene 37, 2137–2149. [DOI] [PubMed] [Google Scholar]
- Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, Lee JH, 2008. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells 26, 1047–1055. [DOI] [PubMed] [Google Scholar]
- Oh JY, Kim MK, Shin MS, Wee WR, Lee JH, 2009. Cytokine secretion by human mesenchymal stem cells cocultured with damaged corneal epithelial cells. Cytokine 46, 100–103. [DOI] [PubMed] [Google Scholar]
- Oh JY., Roddy GW., Choi H., Lee RH., Ylostalo JH., Rosa RH., Prockop DJ., 2010. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc. Natl. Acad. Sci. U. S. A. 107, 1687516880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JY, Choi H, Lee RH, Roddy GW, Ylostalo JH, Wawrousek E, Prockop DJ, 2012a. Identification of the HSPB4/TLR2/NF-kappaB axis in macrophage as a therapeutic target for sterile inflammation of the cornea. EMBO Mol. Med. 4, 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JY, Lee RH, Yu JM, Ko JH, Lee HJ, Ko AY, Roddy GW, Prockop DJ, 2012b. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol. Ther. 20, 2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JY, Kim TW, Jeong HJ, Lee HJ, Ryu JS, Wee WR, Heo JW, Kim MK, 2014a. Intraperitoneal infusion of mesenchymal stem/stromal cells prevents experimental autoimmune uveitis in mice. Mediators Inflamm. 2014, 624640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JY, Ko JH, Kim MK, Wee WR, 2014b. Effects of mesenchymal stem/stromal cells on cultures of corneal epithelial progenitor cells with ethanol injury. Invest. Ophthalmol. Vis. Sci. 55, 7628–7635. [DOI] [PubMed] [Google Scholar]
- Otero-Ortega L, Gomez de Frutos MC, Laso-Garcia F, Rodriguez-Frutos B, Medina-Gutierrez E, Lopez JA, Vazquez J, Diez-Tejedor E, GutierrezFernandez M, 2018. Exosomes promote restoration after an experimental animal model of intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 38, 767–779. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Park SA, Reilly CM, Wood JA, Chung DJ, Carrade DD, Deremer SL, Seraphin RL, Clark KC, Zwingenberger AL, Borjesson DL, Hayashi K, Russell P, Murphy CJ, 2013. Safety and immunomodulatory effects of allogeneic canine adipose-derived mesenchymal stromal cells transplanted into the region of the lacrimal gland, the gland of the third eyelid and the knee joint. Cytotherapy 15, 1498–1510. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Pittenger MF, 2017. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 35, 851–858. [DOI] [PubMed] [Google Scholar]
- Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B, Bartholomew A, 2008. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur. J. Immunol. 38, 1745–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ, Keating A, 2012. Relearning the lessons of genomic stability of human cells during expansion in culture: implications for clinical research. Stem Cells 30, 1051–1052. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Oh JY, 2012a. Medical therapies with adult stem/progenitor cells (MSCs): a backward journey from dramatic results in vivo to the cellular and molecular explanations. J. Cell. Biochem. 113, 1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ, Oh JY, 2012b. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol. Ther. 20, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ, Oh JY, Lee RH, 2017. Data against a Common Assumption: Xenogeneic Mouse Models Can Be Used to Assay Suppression of Immunity by Human MSCs. Mol. Ther. 25, 1748–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Jiang D, Sindrilaru A, Stegemann A, Schatz S, Treiber N, Rojewski M, Schrezenmeier H., Vander Beken S., Wlaschek M., Bohm M., Seitz A., Scholz N., Durselen L., Brinckmann J., Ignatius A., Scharffetter-Kochanek K., 2014. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J. Invest. Dermatol. 134, 526–537. [DOI] [PubMed] [Google Scholar]
- Qin Y, Chan AM, Chang YL, Matynia A, Kouris NA, Kimbrel EA, Ashki N, Parikh S, Gorin MB, Lanza R, Levinson RD, Gordon LK, 2018. Human Embryonic Stem Cell-Derived Mesenchymal Stromal Cells Decrease the Development of Severe Experimental Autoimmune Uveitis in B10.RIII Mice. Ocul. Immunol. Inflamm. 26, 1228–1236. [DOI] [PubMed] [Google Scholar]
- Rabiee B, Bheri A, Farooq AV, Yazdanpanah G, Jabbehdari S, Ghassemi M, Djalilian AR, 2020. The Effect of Mesenchymal Stem Cell Secretome on Corneal Endothelial Cell Preservation in an Oxidative Injury Model. Cornea 39, 1426–1430. [DOI] [PubMed] [Google Scholar]
- Raemdonck K, Braeckmans K, Demeester J, De Smedt SC, 2014. Merging the best of both worlds: hybrid lipid-enveloped matrix nanocomposites in drug delivery. Chem. Soc. Rev. 43, 444–472. [DOI] [PubMed] [Google Scholar]
- Rafei M, Birman E, Forner K, Galipeau J, 2009. Allogeneic mesenchymal stem cells for treatment of experimental autoimmune encephalomyelitis. Mol Ther. 17, 1799–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y, 2008. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2, 141–150. [DOI] [PubMed] [Google Scholar]
- Ren G, Su J, Zhang L, Zhao X, Ling W, L’huillie A, Zhang J, Lu Y, Roberts AI, Ji W, Zhang H, Rabson AB, Shi Y, 2009. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells 27, 1954–1962. [DOI] [PubMed] [Google Scholar]
- Robinson CM, Hale PT, Carlin JM, 2005. The role of IFN-gamma and TNFalpha-responsive regulatory elements in the synergistic induction of indoleamine dioxygenase. J. Interferon Cytokine Res. 25, 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddy GW., Oh JY, Lee RH, Bartosh TJ, Ylostalo J, Coble K, Rosa RH, Prockop DJ, 2011. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6. Stem Cells 29, 1572–1579. [DOI] [PubMed] [Google Scholar]
- Roddy GW, Rosa RH, Oh JY, Ylostalo JH, Bartosh TJ, Choi H, Lee RH, Yasumura D, Ahern K, Nielsen G, Matthes MT, LaVail MM, Prockop DJ, 2012. Stanniocalcin-1 rescued photoreceptor degeneration in two rat models of inherited retinal degeneration. Mol. Ther. 20, 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano B, Elangovan S, Erreni M, Sala E, Petti L, Kunderfranco P, Massimino L, Restelli S, Sinha S, Lucchetti D, Anselmo A, Colombo FS, Stravalaci M, Arena V, D’Alessio S, Ungaro F, Inforzato A, Izzo AA, Sgambato A, Day AJ, Vetrano S, 2019. TNF-Stimulated Gene-6 Is a Key Regulator in Switching Stemness and Biological Properties of Mesenchymal Stem Cells. Stem Cells 37, 973–987. [DOI] [PubMed] [Google Scholar]
- Roura S, Monguio-Tortajada M, Munizaga-Larroude M, Clos-Sansalvador M, Franquesa M, Rosell A, Borras FE, 2020. Potential of Extracellular VesicleAssociated TSG-6 from Adipose Mesenchymal Stromal Cells in Traumatic Brain Injury. Int. J. Mol. Sci. 21, 6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JM, Barry FP, Murphy JM, Mahon BP, 2005. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A, Foulsham W, Amouzegar A, Mittal SK, Chauhan SK, 2019. The therapeutic application of mesenchymal stem cells at the ocular surface. Ocul. Surf. 17, 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi Y, Sekiya I, Yagishita K, Muneta T, 2005. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 52, 2521–2529. [DOI] [PubMed] [Google Scholar]
- Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K, 2007. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 109, 228–234. [DOI] [PubMed] [Google Scholar]
- Sgrignoli MR, Silva DA, Nascimento FF, Sgrignoli DAM, Nai GA, da Silva MG, de Barros MA, Bittencourt MKW, de Morais BP, Dinallo HR, Foglia BTD, Cabrera WB, Fares EC, Andrade SF, 2019. Reduction in the inflammatory markers CD4, IL-1, IL-6 and TNFalpha in dogs with keratoconjunctivitis sicca treated topically with mesenchymal stem cells. Stem Cell Res. 39, 101525. [DOI] [PubMed] [Google Scholar]
- Shao H, Shi H, Kaplan HJ, Sun D, 2005. Chronic recurrent autoimmune uveitis with progressive photoreceptor damage induced in rats by transfer of IRBP-specific T cells. J. Neuroimmunol. 163, 102–109. [DOI] [PubMed] [Google Scholar]
- Shen H, Kreisel D, Goldstein DR, 2013. Processes of sterile inflammation. J. Immunol. 191, 2857–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto-Kuroda T, Oh JY, Kim DK, Jeong HJ, Park SY, Lee HJ, Park JW., Kim TW., An SY., Prockop DJ., Lee RH., 2017. MSC-derived Extracellular Vesicles Attenuate Immune Responses in Two Autoimmune Murine Models: Type 1 Diabetes and Uveoretinitis. Stem Cell Reports 8, 1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaati G, Khandaker I, Funderburgh ML, Mann MM, Basu R, Stolz DB, Geary ML, Dos Santos A, Deng SX, Funderburgh JL, 2019. Mesenchymal Stem Cells Reduce Corneal Fibrosis and Inflammation via Extracellular VesicleMediated Delivery of miRNA. Stem Cells Transl. Med. 8, 1192–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Pochampally R, Perry A, Hsu SC, Prockop DJ, 2004. Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells 22, 823–831. [DOI] [PubMed] [Google Scholar]
- Song HB, Park SY, Ko JH, Park JW, Yoon CH, Kim DH, Kim JH, Kim MK, Lee RH, Prockop DJ, Oh JY, 2018a. Mesenchymal Stromal Cells Inhibit Inflammatory Lymphangiogenesis in the Cornea by Suppressing Macrophage in a TSG-6-Dependent Manner. Mol. Ther. 26, 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WJ, Li Q, Ryu MO, Ahn JO, Bhang DH, Jung YC, Youn HY, 2017. TSG-6 Secreted by Human Adipose Tissue-derived Mesenchymal Stem Cells Ameliorates DSS-induced colitis by Inducing M2 Macrophage Polarization in Mice. Sci. Rep. 7, 5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WJ, Li Q, Ryu MO, Ahn JO, Bhang DH, Jung YC, Youn HY, 2018b. TSG-6 released from intraperitoneally injected canine adipose tissue-derived mesenchymal stem cells ameliorate inflammatory bowel disease by inducing M2 macrophage switch in mice. Stem Cell Res. Ther. 9, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spees JL, Lee RH, Gregory CA, 2016. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 7, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Yang L, Yin Y, Huang J, Qiao F, Fang Y, Yu L, Wang Y, Zhou K, Wang J, 2018. Bone marrow mesenchymal stem cells tune the differentiation of myeloid-derived suppressor cells in bleomycin-induced lung injury. Stem Cell Res. Ther. 9, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H, Chen X, Cao H, Zheng L, Li Q, Zhang K, Han Z, Han ZC, Guo Z, Li Z, Wang L, 2019. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Corneal Wound Repair. Stem Cells Int. 2019, 5738510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasso R, Ilengo C, Quarto R, Cancedda R, Caspi RR, Pennesi G, 2012. Mesenchymal stem cells induce functionally active T-regulatory lymphocytes in a paracrine fashion and ameliorate experimental autoimmune uveitis. Invest. Ophthalmol. Vis. Sci. 53, 786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC, 2003. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 75, 389–397. [DOI] [PubMed] [Google Scholar]
- Tu Z., Li Y, Smith D, Doller C, Sugita S, Chan CC, Qian S, Fung J, Caspi RR, Lu L, Lin F, 2012. Myeloid suppressor cells induced by retinal pigment epithelial cells inhibit autoreactive T-cell responses that lead to experimental autoimmune uveitis. Invest. Ophthalmol. Vis. Sci. 53, 959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villatoro AJ, Fernandez V, Claros S, Rico-Llanos GA, Becerra J, Andrades JA, 2015. Use of adipose-derived mesenchymal stem cells in keratoconjunctivitis sicca in a canine model. Biomed. Res. Int. 2015, 527926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan S, Shi Y, Galipeau J, Krampera M, Leblanc K, Martin I, Nolta J, Phinney DG, Sensebe L, 2019. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT(R)) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 21, 1019–1024. [DOI] [PubMed] [Google Scholar]
- von Bahr L, Sundberg B, Lonnies L, Sander B, Karbach H, Hagglund H, Ljungman P, Gustafsson B, Karlsson H, Le Blanc K, Ringden O, 2012. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol. Blood Marrow Transplant. 18, 557–564. [DOI] [PubMed] [Google Scholar]
- Wan YM, Li ZQ, Zhou Q, Liu C, Wang MJ, Wu HX, Mu YZ, He YF, Zhang Y, Wu XN, Li YH, Xu ZY, Wu HM, Xu Y, Yang JH, Wang XF, 2020. Mesenchymal stem cells alleviate liver injury induced by chronic-binge ethanol feeding in mice via release of TSG6 and suppression of STAT3 activation. Stem Cell Res. Ther 11, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Cao K, Liu K, Xue Y, Roberts AI, Li F, Han Y, Rabson AB, Wang Y, Shi Y, 2018. Kynurenic acid, an IDO metabolite, controls TSG-6mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 25, 1209–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J, He C, Lai P, Luo C, Guo R, Wu S, Geng S, Xiangpeng A, Liu X, Du X, 2012. Mesenchymal stromal cells treatment attenuates dry eye in patients with chronic graft-versus-host disease. Mol. Ther. 20, 2347–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, 2008. Immunopathogenesis of keratoconjunctivitis sicca in the dog. Vet. Clin. North Am. Small Anim. Pract. 38, 251–268. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Vannella KM, 2016. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 44, 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu O, Ding G, Gao R, Zhang C, Ding Y, Bromberg JS, Chen W, Sun L, Wang S, 2012. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjogren syndrome. Blood 120, 3142–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Feng R, Fu Q, Xu S, Hao X, Qiu Y, Feng T, Zeng Z, Chen M, Zhang S, 2019. Human induced pluripotent stem cell-derived mesenchymal stem cells promote healing via TNF-alpha-stimulated gene-6 in inflammatory bowel disease models. Cell Death Dis. 10, 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Wei Y, Sun R, Lu W, Lv H, Xiao X, Cao Y, Jin X, Zhao M, 2020. Umbilical cord blood-derived mesenchymal stromal cells promote myeloid-derived suppressor cell proliferation by secreting HLA-G to reduce acute graft-versus-host disease after hematopoietic stem cell transplantation. Cytotherapy 22, 718–733. [DOI] [PubMed] [Google Scholar]
- Ylostalo J, Bazhanov N, Prockop DJ, 2008. Reversible commitment to differentiation by human multipotent stromal cells in single-cell-derived colonies. Exp. Hematol. 36, 1390–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylostalo JH, Bartosh TJ, Coble K, Prockop DJ, 2012. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells 30, 2283–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Chen P, Xu J, Liu Y, Li H, Wang L, Di G, 2020. hADSCs derived extracellular vesicles inhibit NLRP3 inflammasome activation and dry eye. Sci. Rep. 10, 14521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun YI, Park SY, Lee HJ, Ko JH, Kim MK, Wee WR, Reger RL, Gregory CA, Choi H, Fulcher SF, Prockop DJ, Oh JY, 2017. Comparison of the anti-inflammatory effects of induced pluripotent stem cell-derived and bone marrow-derived mesenchymal stromal cells in a murine model of corneal injury. Cytotherapy 19, 28–35. [DOI] [PubMed] [Google Scholar]
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A, 2005. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 106, 1755–1761. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB, Mitchell JB, Hammill L, Vanguri P, Chopp M, 2005. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp. Neurol. 195, 16–26. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zheng H, Shao H, Nian H, Zhang Y, Bai L, Su C, Liu X, Dong L, Li X, Zhang X, 2014. Long-term therapeutic effects of mesenchymal stem cells compared to dexamethasone on recurrent experimental autoimmune uveitis of rats. Invest. Ophthalmol. Vis. Sci. 55, 5561–5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ren X, Li G, Jiao C, Zhang L, Zhao S, Wang J, Han ZC, Li X, 2011. Mesenchymal stem cells ameliorate experimental autoimmune uveoretinitis by comprehensive modulation of systemic autoimmunity. Invest. Ophthalmol. Vis. Sci. 52, 3143–3152. [DOI] [PubMed] [Google Scholar]
- Zhao PT., Zhang LJ, Shao H, Bai LL, Yu B, Su C, Dong LJ, Liu X, Li XR, Zhang XM, 2016. Therapeutic effects of mesenchymal stem cells administered at later phase of recurrent experimental autoimmune uveitis. Int. J. Ophthalmol. 9, 1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Chen H, Li H, Wu Y, 2017. 3D culture increases pluripotent gene expression in mesenchymal stem cells through relaxation of cytoskeleton tension. J. Cell. Mol. Med. 21, 1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y, Li D, Fu J, Shi Q, Lu Y, Ju X, 2015. Comparison of biological properties of umbilical cord-derived mesenchymal stem cells from early and late passages: immunomodulatory ability is enhanced in aged cells. Mol. Med. Rep. 11, 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]