Abstract
The interactions of lipopolyamines, a class of structurally unique compounds currently being used as transfection (lipofection) agents, with lipopolysaccharide (LPS) have been characterized. Our studies have demonstrated that 1,3-di-oleoyloxy-2-(6-carboxyspermyl)-propylamide), available commercially as DOSPER, binds to purified LPS with an affinity of about 1/10 that of polymyxin B. This essentially nontoxic compound inhibits, in a dose-dependent manner, LPS-induced activation of the Limulus clotting cascade and the production of tumor necrosis factor alpha (TNF-α) interleukin-6 (IL-6), and nitric oxide from LPS-stimulated J774.A1 cells, a murine macrophage-like cell line. Cytokine inhibition is paralleled by decreased steady-state levels of TNF-α and IL-6 mRNA and inhibits the nuclear translocation of nuclear factor kappa B. These findings suggest that the lipopolyamine compound sequesters LPS, thereby blocking downstream cellular activation events that lead to the production of proinflammatory mediators. Administration of DOSPER to d-galactosamine-sensitized mice challenged either with LPS or with Escherichia coli organisms provided significant protection against lethality both with and without antibiotic chemotherapy. Partial protection is evident in LPS-challenged mice treated with DOSPER as late as 2 to 4 h following the endotoxin challenge. A greater degree of protection is observed in E. coli-challenged animals receiving ceftazidime than in those receiving imipenem, which is probably attributable to the higher levels of LPS released in vivo by the former antibiotic. Potent antiendotoxic activity, low toxicity, and ease of synthesis render the lipopolyamines candidate endotoxin-sequestering agents of potential significant therapeutic value.
It is now well recognized that endotoxins or lipopolysaccharides (LPSs), which are structural components of the outer membranes of gram-negative bacteria (40, 54), play a pivotal role in the sepsis syndrome (44, 55). LPSs, which are released from bacteria either by the body’s natural defense systems or by antibiotics, are recognized by a variety of cell types in the body, important among which is the monocyte/macrophage (26). When these cells sense the presence of LPS, they respond by producing numerous inflammatory mediators, including tumor necrosis factor alpha (TNF-α) (5, 67), interleukin-1 β (IL-1β) (23, 24), and IL-6 (24, 79). Other cells, such as the endothelial cell, produce nitric oxide (38, 76). The production of these mediators, under normal circumstances, is precisely regulated and serves to orchestrate the body’s defense mechanisms. However, the unregulated overproduction of these substances (52) leads to the clinical syndrome termed “septic shock” (1, 7), which is characterized by fever, hypotension, coagulopathy, hemodynamic derangement, tissue hypoperfusion, and multiple-organ failure and which frequently culminates in death (13, 71). It is estimated that more than 300,000 cases of septic shock occur each year in the United States, and at least half of these cases are caused by gram-negative organisms (6). The therapy of septic shock, to date, has primarily been supportive, and specific therapeutic modalities aimed at controlling those pathophysiological mechanisms that lead to the systemic inflammatory response which ultimately manifests in shock are, unfortunately, as yet unavailable (13, 71).
One possible approach to addressing therapeutically the problem of sepsis caused by gram-negative bacteria is to target LPS itself by the use of an agent that would bind to and sequester this potent microbial product, thereby preventing its recognition by the monocyte/macrophage and other effector cells. The strategy of sequestering LPS, historically, has been addressed by the use of either polyclonal or monoclonal antibodies raised against the structurally conserved regions of the molecule (12, 60). However, clinical studies with polyclonal antibodies (2, 82) have been difficult to interpret unequivocally (36). Numerous clinical trials (11, 12, 73, 74, 81) designed to test the therapeutic efficacy of monoclonal antibodies have, to date, failed to establish that the use of such antibodies is of clinical value.
Several LPS-binding proteins of nonimmunologic origin are known to bind to endotoxin and neutralize the effects of endotoxin and are currently being evaluated as candidate therapeutic agents. An endotoxin-binding protein (37, 58) obtained from the horseshoe crab (Limulus polyphemus) and a protein found in neutrophil granules, called bactericidal/permeability-increasing protein (25, 34, 75), are being studied for their potential application in the treatment of septic shock (27, 41). Unfortunately, the production of these proteins for widespread use as therapeutic agents is likely to prove costly and will potentially have a significant effect upon health care costs for the treatment of this disease.
The toxic center of the LPS molecule is a glycolipid moiety called lipid A (54, 78) whose structure is highly conserved and which is therefore very similar among gram-negative bacteria. For these reasons, lipid A presents a logical molecular target for compounds designed to bind to LPS. The anionic and amphiphilic nature of lipid A (56, 78) enables it to bind to numerous substances which are positively charged and which also possess an amphipathic character. We have, over the last several years, characterized the interactions of LPS with a number of classes of cationic amphipathic molecules including proteins (15, 51, 65), peptides (14, 16, 18, 19), pharmaceutical compounds (17, 21), and other synthetic polycationic amphiphiles (20). Adducing the knowledge that we had gained from these studies, we have identified a novel class of cationic amphiphiles, the lipopolyamines, and we report here on the characterization of the endotoxin-binding and -neutralizing properties of some compounds representative of this class of molecules.
MATERIALS AND METHODS
Reagents.
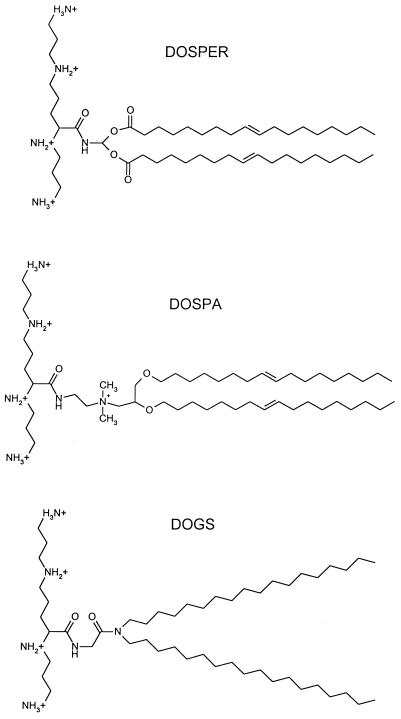
The lipopolyamine compounds DOSPER [1,3-di-oleoyloxy-2-(6-carboxyspermyl)-propylamide] and Transfectam (dioctadecylamidoglycylspermine [DOGS]) were procured from Boehringer Mannheim (Indianapolis, Ind.) and Promega Corporation (Madison, Wis.), respectively. DOSPER was supplied as a clear, aqueous, sterile, buffered suspension bottled under argon at a concentration of 1 mg/ml, and DOGS was supplied as lyophilized material that was reconstituted in ethanol just prior to use. Electrospray mass spectrometry was performed with DOSPER and revealed the presence of both mono- and dioleoyl-substituted compounds. LipofectAMINE, a 3:1 (wt/wt) mixture of the lipopolyamine 2,3-dioleoyloxy-N-[2(sperminecarboxamido)ethyl]-N,N-dimethyl-1-propanaminiumtrifluoroacetate (DOSPA) and the neutral phospholipid dioleoylphosphatidylethanolamine, was purchased from Life Technologies (Gaithersburg, Md.). The chemical structures of these lipopolyamine compounds are shown in Fig. 1. Smooth LPS from Escherichia coli O111:B4, Re LPS, and diphosphoryl lipid A from E. coli K-12 D31m4 were from List Biologicals (Campbell, Calif.). Smooth LPS from Salmonella abortus equi was obtained from Sigma Chemical Co. (St. Louis, Mo.).
FIG. 1.
Chemical structures of the lipopolyamine compounds.
Spectroscopic characterization of the binding of lipopolyamines to LPS and lipid A.
The relative affinities of the interactions of the lipopolyamine compounds with LPS and lipid A were determined by the highly sensitive dansylcadaverine fluorescent probe method (16, 17). Briefly, the binding of the probe to LPS or lipid A results in a blue shift and intensity enhancement in the emission spectrum of dansylcadaverine. Compounds which bind to the lipid A moiety displace the bound probe, resulting in concentration-dependent quenching of fluorescence. The affinities of binding of the compounds were determined from 50% effective doses (ED50) obtained by four-parameter logistic curve fitting of the displacement curves (16, 17) and are expressed relative to that of polymyxin B.
LAL assay.
A quantitative chromogenic version of the Limulus amebocyte lysate (LAL) assay (QCL-1000) from Biowhittaker (Walkersville, Md.) was used. A constant concentration of LPS (4 endotoxin units) was incubated with various doses of the lipopolyamines (or polymyxin B as a control) at 37°C for 10 min in pyrogen-free glass test tubes. A total of 50 μl of this mixture or of the LPS standard was added to equal volumes of the LAL reagent and endotoxin-free water, and the mixture was incubated for a further 10 min at 37°C in a sterile, endotoxin-free, 96-well microtiter plate preequilibrated at 37°C, after which 100 μl of the chromogenic substrate solution was added to each well. The reaction was terminated at 6 min by the addition of 25% acetic acid, and the absorbance at 405 nm was read with a Dynatech MR 5000 plate reader. Free, bioactive LPS in the LPS-lipopolyamine or LPS-polymyxin B mixtures was quantitated from standard curves, which were linear from 0.1 to 2.0 endotoxin units. All samples, standards, and blanks were assayed in quadruplicate.
Cytokine and nitric oxide assays.
The ability of the lipopolyamine compounds to inhibit the LPS-stimulated production of proinflammatory mediators was tested with the murine macrophage-like cell line J774.A1 (American Tissue Type Collection, Washington, D.C.). J774.A1 cells were seeded in a 96-well tissue culture plate at 5 · 105 cells/well. Following overnight culture in RPMI 1640 supplemented with l-glutamine, 10% fetal bovine serum, penicillin, and streptomycin, the cells were stimulated for 8 h with LPS alone (20 ng/ml) or LPS preincubated with graded concentrations of DOSPER, DOGS, or polymyxin B (control). Supernatants were harvested and assayed for TNF-α and IL-6 by specific enzyme-linked immunosorbent assays (Genzyme, Cambridge, Mass.). Nitric oxide was measured as nitrite with the Griess reagent (35).
TNF-α and IL-6 mRNA determination.
J774.A1 cells were plated in 12-well plates at a density of 5 · 106 cells/well. Following overnight culture, the cells were stimulated as indicated above for 2 h. Total RNA was extracted with Trizol (GIBCO BRL, Gaithersburg, Md.) as per the manufacturer’s instructions. Reverse transcription followed by 25 cycles of PCR was carried out with the GeneAmp RNA PCR kit and the GeneAmp 9600 Thermal Controller (Perkin-Elmer, Foster City, Calif.), according to the manufacturer’s instructions. Mouse TNF-α, IL-6, and β-actin primers (Stratagene, La Jolla, Calif.) were used as specified by the vendor. The PCR products were electrophoresed on a 1.6% agarose gel, stained with 0.5 mg of ethidium bromide per ml, and photographed under UV transillumination. Images were analyzed with ITTI 1.31 densitometry software (Interactive Technologies International, St. Petersburg, Fla.).
NFκB nuclear translocation assay.
J774.A1 cells (107) were stimulated with 10 ng of LPS per ml as described above for 45 min, and the nuclear factor kappa B (NFκB) activity in nuclear protein extracts (22) was then determined by electrophoretic mobility shift assays (EMSAs) essentially as published earlier (46, 47). Briefly, the supernatants were aspirated following stimulation and the cells were washed once in ice-cold phosphate-buffered saline (PBS). The cells were then lysed in hypotonic lysis buffer (10 mM HEPES [pH 7.9] containing 10 mM KCl, 1.5 mM MgCl2, and 0.5% Nonidet P-40) for 3 min, and the cellular contents were harvested by scraping with a rubber policeman. The lysis buffer and all other buffers described below contained the following protease inhibitors, which were added after they were freshly prepared: 0.5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 1 μg of pepstatin A per ml, 10 μg each of aprotinin, leupeptin, and type I-S soybean trypsin inhibitor per ml, and 0.1 mM sodium orthovanadate (all from Sigma). All subsequent procedures were carried out at 4°C. The lysate was centrifuged at 3,000 rpm for 5 min, and the pellet was washed twice with lysis buffer. Nuclear proteins were extracted from the pellet with 40 μl of extraction buffer (20 mM HEPES [pH 7.9], 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol) per sample. After vigorous mixing and incubation on ice for 10 min, the suspension was centrifuged at 16,000 × g for 5 min. The supernatant (40 μl) containing the nuclear proteins was diluted with 60 μl of dilution buffer (20 mM HEPES [pH 7.9], 50 mM KCl, 0.2 mM EDTA, 20% glycerol), and the total protein content was determined by the Bradford dye method.
Details of the NFκB-specific oligonucleotide and EMSA have been published earlier (46, 47). The oligonucleotide contains two tandemly arranged NFκB sites (underlined) derived from the human immunodeficiency virus type 1 enhancer region (5′-ATCAGGGACTTTCCGCT-GGGGACTTTCCG-3′) (48, 53), and its complementary strand with a 5′ overhang was obtained from Genosys Inc. The annealed duplex strands were filled in with [γ-32P]dCTP (3,000 Ci/mmol; Du Pont NEN) Klenow polymerase (Life Technologies), and a mixture of dATP, dGTP and dTTP nucleotides and were purified by ethanol precipitation. One microgram of nuclear protein extract was incubated for 30 min at 25°C with 3 μg of poly(dI-dC) · poly(dI-dC) (Pharmacia), bovine serum albumin (3 mg/ml), and ∼4 ng of labeled oligonucleotide (100,000 cpm) in a final volume of 30 μl of EMSA buffer (10 mM Tris-HCl [pH 7.5] containing 200 mM NaCl, 5 mM EDTA, 5 mM β-mercaptoethanol, 20% glycerol, and 0.5% Nonidet P-40). Following the binding reaction, 20 μl of the samples was electrophoresed at 15 V/cm through a 6% nondenaturing polyacrylamide gel that was prepared in 45 mM Tris-borate buffer (pH 8.0) containing 1 mM EDTA. The gels were then dried and processed for autoradiography.
Mouse lethality experiments.
Female, outbred, 9- to 11-week-old CF-1 mice (Charles River, Wilmington, Mass.) weighing between 22 and 28 g were used in the lethality experiments. Upon arrival, the mice were allowed to acclimatize for a week prior to experimentation, were housed at five per cage in a controlled environment at the American Association for Laboratory Animal Care-accredited Kansas University Medical Center Animal Facility, and were fed mouse chow ad libitum. The animals were sensitized to the lethal effects of LPS by d-galactosamine (30–32). d-Galactosamine (800 mg/kg) and LPS (20 ng) were given as a single injection intraperitoneally (i.p.) in freshly prepared PBS. In some experiments, mice received i.p. graded doses of DOSPER diluted in PBS immediately before the d-galactosamine–LPS challenge, while for other mice, a fixed dose of DOSPER (or DOGS) was administered at various times before or after the challenge. Lethality was determined at 24 h.
Other experiments were designed to investigate the possible protective effects of DOSPER on antibiotic-induced in vivo release of LPS from viable gram-negative bacteria (8, 9, 62). In the latter experiments, mice received graded doses of E. coli suspension in PBS; the E. coli suspension had been harvested from Trypticase soy broth at the mid-logarithmic phase and quantitated by turbidimetry at 650 nm (5 · 108 cfu/ml = A650 of 0.65). These mice received, at the same time, by separate i.p. injection, 0.2 ml of either imipenem-cilastin (Merck Inc., Rahway, N.J.) or ceftazidime (Glaxo-Wellcome, Research Triangle Park, N.C.) at concentrations of 2.5 and 5 mg/ml, respectively. DOSPER (40 μg/mouse, 0.2-ml volume) or saline (0.2 ml) was also injected i.p. at the same time. Lethality was determined at 24 h following LPS challenge. The statistical significances of the lethality data were analyzed by the Fisher one-tailed exact probability test.
RESULTS
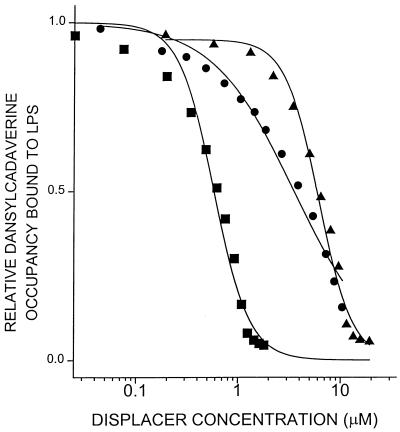
The premise for the evaluation of molecules such as DOSPER and DOGS as LPS-neutralizing agents is that these compounds might possess those physical properties that would enable them to bind to LPS, sequester it, and thereby inhibit the manifestations of endotoxicity. We therefore first characterized the interactions of these compounds with LPS using the dansylcadaverine displacement method (16). Both lipopolyamine compounds displaced the bound fluorescent probe (Fig. 2). The ED50s (displacer concentrations corresponding to 50% probe displacement) for DOSPER and DOGS are 6.1 and 3.5 μM, respectively. Polymyxin B, a peptide antibiotic well recognized for its ability to bind to the lipid A region of LPS (42) and inhibit its toxicity (64), was used as a control substance in these experiments, and its ED50 was determined to be 0.58 μM, consistent with values reported in the literature (16, 57). DOSPER and DOGS also displace dansylcadaverine bound to purified Re LPS and lipid A with comparable affinities (data not shown), verifying that they interact with the lipid A region of LPS. In this initial spectroscopic screening assay, LipofectAMINE also behaved indistinguishably from the other lipopolyamines, with the ED50 of dansylcadaverine displacement being 4.9 μM when corrected for the actual concentration of the active constituent (DOSPA) alone (data not shown). However, this compound was excluded from further characterization because of the presence of substantial amounts of neutral phospholipid which may potentially confound the biological activity of the active constituent.
FIG. 2.
Dansylcadaverine displacement activity. The dansylcadaverine concentration was 15 μM, and the LPS concentration was 20 μM. Experiments were performed in 50 mM Tris-HCl (pH 7.4) with a Hitachi spectrofluorimeter at 25°C. Excitation and emission wavelengths were 340 and 515 nm, respectively. Bandpasses were 5 nm for both monochromators. ●, DOSPER; ▴, DOGS; ■, polymyxin B. Displacement curves were fit by using the ALLFIT four-parameter logistic curve fitting program (45) as described earlier (16, 17).
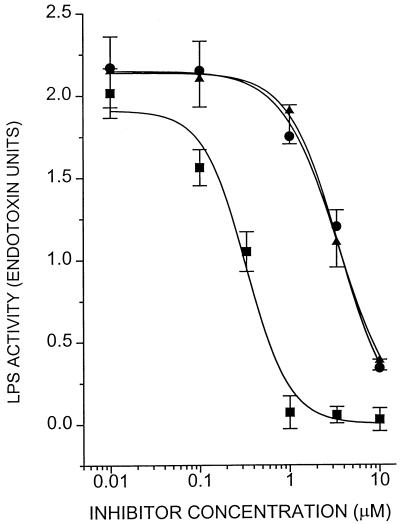
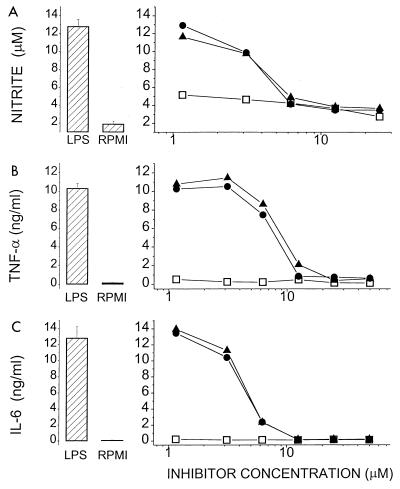
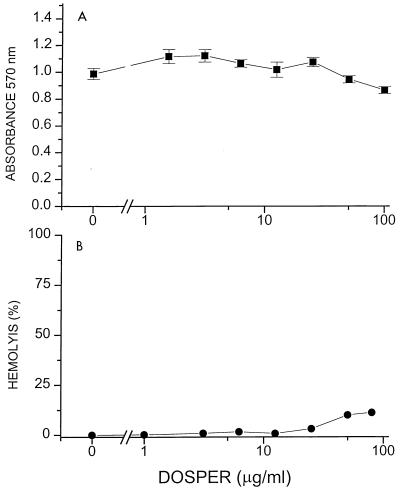
Having established that these molecules bind to LPS, it was necessary to ascertain whether such binding would result in inhibition of the biological activity of the endotoxin molecule. DOSPER and DOGS inhibit LPS-induced activation of LAL in a concentration-dependent manner (Fig. 3); the inhibitory activities of both compounds are indistinguishable, each being about 10-fold lower than that of polymyxin B. The lipopolyamines also inhibit nitrite, TNF-α, and IL-6 production in J774.A1 cells stimulated with LPS (Fig. 4). That the inhibition was not due to toxicity was verified by cell viability studies in which no toxicity was detected for DOSPER even at concentrations of 100 μg/ml (Fig. 5). Furthermore, DOSPER does not display hemolytic activity against human erythrocytes (Fig. 5). This is of particular significance since many cationic amphiphiles are markedly surface active, manifesting as potent membrane-disruptive behavior. We further verified that the inhibitory effect was specific for LPS, since the compounds did not have any effect on the induction of TNF-α, IL-6, or NO in supernatants of J774.A1 cells stimulated with phorbol myristate acetate (data not shown). Because in all of these assays the inhibition profiles of both compounds were very similar, we elected to investigate DOSPER for a more complete profile of its endotoxin-sequestering properties.
FIG. 3.
Inhibition of the chromogenic LAL response to LPS by lipopolyamines. A total of 2.0 endotoxin units of E. coli O111:B4 reference LPS was incubated with increasing concentrations of DOSPER, DOGS, or polymyxin B (control). LPS was quantitated in the samples by extrapolation of standard curves constructed for the reference LPS. Error bars represent standard deviations determined for quadruplicate samples. ●, DOSPER; ▴, DOGS; ■, polymyxin B.
FIG. 4.
Inhibition of nitrite (A), TNF-α (B), and IL-6 (C) secretion in J774.A1 cells stimulated with 20 ng of E. coli O111:B4 LPS per ml in the presence of DOSPER (●), DOGS (▴), or polymyxin B (■). Nitrite was determined by the Griess assay, and the cytokines were quantitated by specific enzyme-linked immunosorbent assays. Datum points are mean values for quadruplicate samples. The bar graphs on the left represent internal controls: RPMI alone (no stimulation) or LPS alone (no inhibitors); error bars correspond to standard deviations for six replicate samples.
FIG. 5.
(A) Cytotoxicity of DOSPER. J774.A1 cells (1.5 · 106/well) were incubated with DOSPER for 6 h. The supernatants were aspirated, 100 μl of RPMI 1640 containing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was added to each well, and the plates were incubated for an additional 2 h. The supernatants were discarded and the cells were solubilized in isopropanol. The absorbance at 570 nm was read in a microplate reader. Datum points represent means ± standard deviations for eight replicate samples. (B) Hemolytic activity of DOSPER. Fresh human saline-washed erythrocytes (type O Rh positive; final hematocrit, 10%) were incubated with DOSPER in saline for 1 h and were then centrifuged. Free hemoglobin in the supernatant was measured at 578 nm and is expressed as a percentage of the total hemoglobin released by hypotonic lysis.
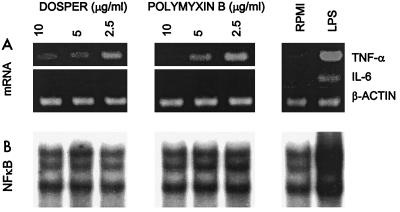
Since an LPS-sequestering agent would be expected to also inhibit early cellular signaling events that precede mediator production in LPS-responsive cells, we examined steady-state TNF-α and IL-6 mRNA levels as well as the nuclear translocation of NFκB, a well-known LPS-activated transcription factor (10, 59, 80). DOSPER completely inhibits IL-6 mRNA expression at all concentrations tested, while its effect on TNF-α mRNA is less pronounced (Fig. 6) but nevertheless significant. This is probably because of the high basal levels of mRNA in J774 cells, as has been observed by us and other investigators. DOSPER also inhibits LPS-induced translocation of cytoplasmic NFκB into the nucleus (Fig. 6). The reduction in band intensity is comparable to that of resting, nonstimulated cells (Fig. 6). The inhibition by DOSPER of NFκB translocation, an early signaling event that precedes cytokine mRNA transcription, substantiates the conclusion that the observed LPS-antagonistic effects of the lipopolyamine compound are, indeed, due to entrapment of free LPS in the form of biologically inactive complexes.
FIG. 6.
(A) Cytokine mRNA (TNF-α or IL-6) in J774.A1 cells stimulated with 20 mg of LPS per ml in the presence or absence of DOSPER or polymyxin B. mRNAs were quantitated by reverse transcription-PCR. (B) NFκB activity in J774.A1 cells. Cells were stimulated for 45 min with 10 ng of LPS per ml in the presence of various amounts of DOSPER or polymyxin B. Resting cells and cells treated with LPS alone (10 ng/ml) without inhibitors served as internal controls (lanes 6 and 7 from the left, respectively). Nuclear protein extracts were subjected to EMSA with a 32P-labeled NFκB oligonucleotide probe.
Having established that DOSPER binds to LPS and inhibits its activity in several in vitro assay systems, it was of importance to examine if the compound would also antagonize LPS effects in vivo, particularly given the susceptibility of DOSPER to hydrolytic cleavage of its acyl chains (see Discussion). We therefore evaluated the activity of the compound in the well-established d-galactosamine-sensitized mouse model of endotoxic shock (30–32, 61–63). The data presented in Table 1 indicate that DOSPER affords protection against LPS-induced lethality. The protection is dose dependent and, of note, is evident even at supralethal LPS doses (e.g., five times the LPS dose inducing 100% lethality). In the experiments described above, DOSPER was administered premixed with LPS so as to facilitate the formation of complexes. In order to determine if DOSPER or DOGS would also bind in vivo to circulating LPS and neutralize its toxicity, additional experiments were performed. In those experiments the lipopolyamine was administered separately at various times with respect to the time of LPS challenge. The results of these experiments are summarized in Table 2. It is clear that DOSPER or DOGS affords nearly complete protection when administered simultaneously with or 15 min before LPS administration (Table 2) but not when the compound is given an hour prior to challenge, probably due to the rapid clearance of the compound. Particularly noteworthy is the fact that partial protection is observed even up to 4 h following LPS challenge, presumably a consequence of the sequestration by DOSPER of circulatory forms of LPS (bound to albumin [15], lipoproteins [28], or other serum proteins [66]).
TABLE 1.
Protective effect of DOSPER in d-galactosamine-sensitized mice challenged with S. abortus equi LPS
| LPS challenge dose (ng/mouse) | No. of dead mice/total no. of mice receiving the following DOSPER dose (μg/mouse):
|
||
|---|---|---|---|
| 0 | 10 | 40 | |
| 0 | 0/5 | 0/5 | |
| 10 | 9/10 | 1/10a | 0/5a |
| 20 | 5/5 | 2/5 | 0/5a |
| 50 | 0/5 | ||
P < 0.005.
TABLE 2.
Effect of time of lipopolyamine administration on LPS-induced lethality in d-galactosamine-sensitized mice
| Groupa | Time of lipopolyamine administrationb | No. of dead mice/total no. of mice tested |
|---|---|---|
| DOSPER | ||
| 1 | Control | 11/12 |
| 2 | −1 h | 10/12 |
| 3 | −15 min | 3/12c |
| 4 | 0 h | 1/12c |
| 5 | +1 h | 7/11 |
| 6 | +2 h | 7/12 |
| 7 | +4 h | 7/12 |
| DOGS | ||
| 1 | Control | 10/10 |
| 2 | −1 h | 6/10 |
| 3 | 0 h | 0/10c |
| 4 | +1 h | 5/10 |
| 5 | +2 h | 9/10 |
| 6 | +4 h | 9/10 |
Each d-galactosamine-sensitized mouse received 20 mg of S. abortus equi LPS.
DOGS or DOSPER dose, 40 μg/mouse. Control animals received 0.2 ml of saline instead of lipopolyamine. 0 h, lipopolyamine was injected into the animals i.p. immediately after LPS and d-galactosamine challenge.
P < 0.005.
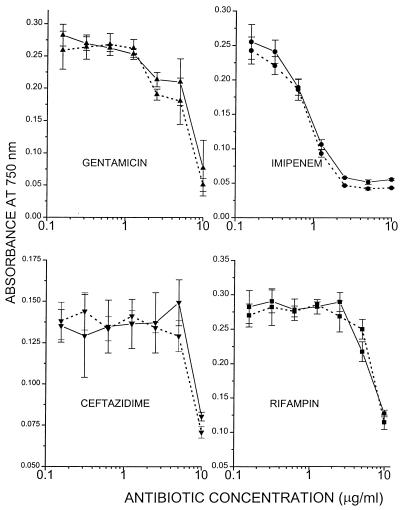
The efficacy of DOSPER was also evaluated in a variant of the d-galactosamine mouse lethality model wherein LPS is released in vivo by the action of antibiotics on gram-negative bacteria (8, 9, 62). Live E. coli O111:B4 organisms were administered along with cell wall-active antibiotics (imipenem or ceftazidime) with or without DOSPER to d-galactosamine-sensitized mice. Imipenem and ceftazidime have different specificities for penicillin-binding proteins on the outer membrane, and therefore, treatment with these drugs results in the release of substantially different amounts of LPS, while they exert very similar microbicidal potencies. Ceftazidime has been shown to cause more LPS release, both in vitro (8) and in vivo (9), than imipenem and therefore provides less protection than imipenem in the d-galactosamine model. We reasoned that if DOSPER were to function as a scavenger of circulating LPS, it would enhance to a greater degree the protection obtained with ceftazidime than that obtained with imipenem. Table 3 summarizes the results obtained in these experiments. As expected, when coadministered with ceftazidime, DOSPER causes a distinct shift to the right in the lethality profiles (combined results obtained with challenge doses of 105 and 106 organisms, 8 of 20 mice for mice receiving ceftazidime plus DOSPER versus 18 of 20 mice for ceftazidime plus vehicle, respectively; P < 0.05), signifying an increase in the 50% lethal dose of more than an order of magnitude of the E. coli challenge dose; this effect is not apparent in the imipenem-treated mice (9 of 19 versus 12 of 20, respectively; P > 0.1; Table 3). Of interest, DOSPER also appears to afford some protection in non-antibiotic-treated mice (4 of 10 mice for DOSPER-treated mice versus 6 of 6 mice when mice were challenged with 104 organisms), which is probably attributable to the sequestration by the compound of LPS released spontaneously by the action of plasma components such as complement (43, 72). DOSPER at concentrations of up to 75 μg/ml has no detectable intrinsic antimicrobial effects on E. coli. Since polycationic molecules have been known to permeabilize the outer membranes of gram-negative bacteria (69, 70), we also evaluated the possible potentiation effects of DOSPER on the microbicidal activities of several classes of antibiotics. DOSPER neither enhances nor inhibits the antimicrobial effect of imipenem, ceftazidime, gentamicin, or rifampin (Fig. 7).
TABLE 3.
Protective effect of DOSPER in d-galactosamine-sensitized mice challenged with E. coli and cell wall-active antibiotics
| E. coli challenge (CFU/mouse) | No. of dead mice/total no. of mice treated with the followinga:
|
|||||
|---|---|---|---|---|---|---|
| Saline | Saline + DOSPER | Imipenem + saline | Imipenem + DOSPER | Ceftazidime + saline | Ceftazidime + DOSPER | |
| 102 | 0/6 | |||||
| 103 | 4/6 | 3/5 | 0/4 | 0/4 | ||
| 104 | 6/6 | 4/10b | 0/10 | 1/10 | ||
| 105 | 9/10 | 6/10 | 2/9 | 8/10 | 2/10b | |
| 106 | 10/10 | 6/10 | 7/10 | 10/10 | 6/10b | |
| 107 | 5/5 | 9/10 | 7/10 | |||
DOSPER (40 μg/mouse) and imipenem (0.5 mg/mouse) or ceftazidime (1.0 mg/mouse) were administered at the same time as the E. coli challenge. Results shown are collated from two separate experiments.
P < 0.005.
FIG. 7.
Effect of DOSPER on antimicrobial activities of antibiotics. A total of 104 CFU of E. coli was cultured in RPMI 1640 (without phenol red or serum) containing graded concentrations of antibiotics with (——) or without (⋯ ⋯) DOSPER (10 μg/ml) in a final volume of 200 μl. Bacterial density was estimated by turbidimetry at 750 nm at 4 h. ●, imipenem; ▴, gentamicin; ■, rifampin; ▾, ceftazidime.
DISCUSSION
In the course of our continuing investigations aimed at elucidating the structural determinants of endotoxin-sequestering agents (14, 16, 17, 19, 21), we had earlier shown that compounds with protonatable positive charges that are so disposed that the distance between them is equivalent to the theoretically determined distance between the two anionic phosphates on the lipid A moiety enable such molecules to bind well to LPS. We had also shown that the presence of additional, appropriately positioned hydrophobic functionalities serve to further enhance binding affinity and stabilize the resultant complexes, presumably due to favorable entropic contributions to the free energy of interaction (17, 21). We had also noted in these early, exploratory studies that the charge distribution profiles in DNA and supramolecular assemblies of lipid A or LPS are similar in certain respects; in both cases the negatively charged phosphate groups occur at regular, periodic intervals of defined distance and geometry (21). Furthermore, several compounds that we observed to bind to LPS with high affinity are known DNA binders. Our interest, therefore, turned to DNA-binding substances, and we found that several of the desirable structural features mentioned above are present in certain members of a novel class of compounds, the lipopolyamines, of which DOSPER and DOGS are representative. The lipopolyamines were originally developed and are currently being used as DNA transfection (lipofection) reagents (3, 4, 29, 33, 39, 77).
The studies described in this paper establish that lipopolyamine compounds such as DOSPER bind to LPS and attenuate its toxic activities. The inhibition of early cellular activation events, namely, NFκB translocation and cytokine mRNA transcription, in conjunction with the inhibition of the more distal response events of cytokine and NO production, strengthen the premise that DOSPER acts via sequestration of LPS. The binding affinity is relatively modest, about an order of magnitude lower than that of polymyxin B. This is to be anticipated since although the intercationic distance of the spermine backbone is optimal for Coulombic interactions with the anionic lipid A phosphate groups (21), the fatty acid substituents impose potentially unfavorable steric properties, as has been observed with spermidinyl derivatives bearing nonterminal hydrophobic substituents (21). Nonetheless, complexes of DOSPER or DOGS and LPS are sufficiently stable in the presence of serum in in vitro conditions and, importantly, also in vivo, so as to significantly inhibit LPS toxicity. Presumably, the acyl substituents of DOSPER serve to stabilize the complex, since other strongly LPS-binding dicationic ligands such as pentamidine (17) (apparent Kd3 0.12 μM), which interact with LPS predominantly via electrostatic interactions (21), completely inhibit the Limulus gelation activity of LPS or lipid A but only weakly block TNF-α, IL-1β, and IL-6 production in cultured human peripheral blood mononuclear cells (18a).
The potential problem of low affinity is offset when the ligand can be used at relatively high concentrations due to mass action effects. In the clinical setting, however, this is not always feasible because of toxicity and the ensuing low therapeutic index. DOSPER is relatively nontoxic. It is devoid of detectable cytotoxic effects on J774.A1 cells up to 100 μg/ml, the maximal concentration tested, and in some mice receiving cumulative doses of 160 μg, no signs of acute toxicity were observed (data not shown). The literature also contains reports that DOSPER has been used in vivo as a transfection agent in pregnant mice at a dose of 400 nmol (0.44 mg/mouse) without any detectable toxicity in the dams or their progeny (68), and U.S. Food and Drug Administration approval has been obtained for the experimental use of at least one lipopolyamine compound as a transfection agent in humans (49, 50). This desirable property of low toxicity appears to be a common attribute of several similar compounds and is likely a consequence both of the susceptibility to hydrolytic cleavage of the ester and amide linkages between the acyl substituents and the spermine backbone and of the inertness of the products of hydrolysis. The lability of the molecule, however, also implies poor pharmacokinetic behavior, and the rapid elimination of the circulatory pool of DOSPER would be consistent with the lack of protection when it is administered 1 h prior to LPS challenge in the d-galactosamine-sensitized mouse model.
It is to be noted that in mice sensitized with d-galactosamine, death typically occurs at between 8 and 12 h (62). Of particular interest, therefore, is the diminished lethality that is apparent when DOSPER is administered even at 4 h following LPS challenge (Table 2). In the in vivo LPS release mouse model, a distinct enhancement of the protective effect of ceftazidime against E. coli challenge is observed (Table 3). When taken together with data previously obtained in this laboratory (8, 9), this observation suggests that the effect is due to the elimination of free LPS released by the action of the antibiotic. The kinetics of LPS release and its subsequent compartmentalization in the peritoneal cavity, portal circulation, and extracellular fluid are as yet poorly understood in this model, and it seems probable that the release and distribution of LPS are relatively slow and sustained processes, unlike when purified LPS is administered as a bolus dose. Given the anticipated short half-life of DOSPER, it is possible that the partial protection observed in this model might be substantially enhanced if the compound is administered as a continual infusion. This remains to be tested.
These data, collectively, present a strong case for the feasibility of sequestering LPS nonimmunologically by using small molecules and emphasizes the potential therapeutic utility of LPS-binding agents of low toxicity such as DOSPER. An understanding of the structural determinants that ascribe endotoxin-binding and -neutralizing activities in potential LPS-binding ligands appears to be amenable to relatively straightforward considerations of the physicochemical aspects of the LPS molecule and of its lipid A moiety. That a lead compound could be identified only on the basis of its structure and then could be evaluated rapidly provides a strong impetus for the systematic and rational development of LPS-neutralizing agents. DOSPER might serve as an excellent starting point, for the simplicity and modularity of its structure lend it to the design of a variety of analogs. A systematic evaluation of such analogs would help incrementally refine the heuristics of designing LPS-sequestering compounds.
ACKNOWLEDGMENTS
This work was supported in part by grants PO1CA54474 from the National Cancer Institute, R37AI23447 from the National Institute of Allergy and Infectious Diseases, and an unrestricted medical grant from Merck & Co., West Point, Pa. S. A. David is a recipient of a Kansas Health Foundation fellowship.
T. Suzuki, Q. Xue, and E. Zuvanich are gratefully acknowledged for their help. We thank Promega Inc. for a generous gift of DOGS.
REFERENCES
- 1.Balk R A, Bone R C. The septic syndrome. Definition and clinical implications. Crit Care Clin. 1989;5:1–8. [PubMed] [Google Scholar]
- 2.Baumgartner J D, Glauser M P, McCutchan J A, Ziegler E J, van Melle G, Klauber M R, Vogt M, Muehlen E, Luethy R, Chiolero R, et al. Prevention of gram-negative shock and death in surgical patients by antibody to endotoxin core glycolipid. Lancet. 1985;ii:59–63. doi: 10.1016/s0140-6736(85)90176-x. [DOI] [PubMed] [Google Scholar]
- 3.Behr J-P. Gene transfer with synthetic cationic amphiphiles: prospects for gene therapy. Bioconjug Chem. 1994;5:382–389. doi: 10.1021/bc00029a002. [DOI] [PubMed] [Google Scholar]
- 4.Behr J-P, Demeneix B, Loeffler J-P, Perez-Mutul J. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc Natl Acad Sci USA. 1989;86:6982–6986. doi: 10.1073/pnas.86.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 6.Bone R C. Gram-negative sepsis: a dilemma of modem medicine. Clin Microbiol Rev. 1993;6:57–68. doi: 10.1128/cmr.6.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bone R C. The sepsis syndrome. Definition and general approach to management. Clin Chest Med. 1996;17:175–181. doi: 10.1016/s0272-5231(05)70307-5. [DOI] [PubMed] [Google Scholar]
- 8.Bucklin S E, Fujihara Y, Leeson M C, Morrison D C. Differential antibiotic-induced release of endotoxin from gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 1994;13(Suppl. 1):43–51. doi: 10.1007/BF02390684. [DOI] [PubMed] [Google Scholar]
- 9.Bucklin S E, Morrison D C. Differences in therapeutic efficacy among cell wall-active antibiotics in a mouse model of gram-negative sepsis. J Infect Dis. 1995;172:1519–1527. doi: 10.1093/infdis/172.6.1519. [DOI] [PubMed] [Google Scholar]
- 10.Cordle S R, Donald R, Read M A, Hawiger J. Lipopolysaccharide induces phosphorylation of MAD3 and activation of c-Rel and related NF-kappaB proteins in human monocytic THP-1 cells. J Biol Chem. 1993;268:11803–11810. [PubMed] [Google Scholar]
- 11.Cross A S. Antiendotoxin antibodies: a dead end. Ann Intern Med. 1994;121:58–60. doi: 10.7326/0003-4819-121-1-199407010-00011. [DOI] [PubMed] [Google Scholar]
- 12.Cross A S, Opal S. Therapeutic intervention in sepsis with antibody to endotoxin: is there a future? J Endotoxin Res. 1994;1:57–69. [Google Scholar]
- 13.Crowley S R. The pathogenesis of septic shock. Heart Lung. 1996;25:124–134. doi: 10.1016/s0147-9563(96)80114-2. [DOI] [PubMed] [Google Scholar]
- 14.David S A, Balaram P, Mathan V I. Interaction of basic amphiphilic polypeptide antimicrobials, gramicidin S, tyrocidin and efrapeptin, with endotoxic lipid A. Med Microbiol Lett. 1993;2:42–47. [Google Scholar]
- 15.David S A, Balaram P, Mathan V I. Characterization of the interaction of lipid A and lipopolysaccharide with human serum albumin: implications for an endotoxin-carrier function for albumin. J Endotoxin Res. 1995;2:99–106. [Google Scholar]
- 16.David S A, Balasubramanian K A, Mathan V I, Balaram P. Analysis of the binding of polymyxin B to endotoxic lipid A and core glycolipid using a fluorescent displacement probe. Biochim Biophys Acta. 1992;1165:147–152. doi: 10.1016/0005-2760(92)90180-4. [DOI] [PubMed] [Google Scholar]
- 17.David S A, Bechtel B, Annaiah C, Mathan V I, Balaram P. Interaction of cationic amphiphilic drugs with lipid A: implications for development of endotoxin antagonists. Biochim Biophys Acta. 1994;1212:167–175. doi: 10.1016/0005-2760(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 18.David S A, Bhattacharjya S, Mathan V I, Balaram P. Elucidation of the conformation of free and LPS-bound polymyxin B nonapeptide in water by 2D-NMR and restrained molecular dynamics methods and molecular modeling of polymyxin-lipid A complex. J Endotoxin Res. 1994;1(Suppl.):A60–A60. [Google Scholar]
- 18a.David, S. A., J. A. Gelfand, and C. A. Dinarello. Unpublished data.
- 19.David S A, Mathan V I, Balaram P. Interaction of melittin with endotoxic lipid A. Biochim Biophys Acta. 1992;1123:269–274. doi: 10.1016/0005-2760(92)90006-h. [DOI] [PubMed] [Google Scholar]
- 20.David S A, Mathan V I, Balaram P. Interactions of linear dicationic molecules with lipid A: structural features that correspond to optimal binding affinity. J Endotoxin Res. 1995;2:325–336. [Google Scholar]
- 21.David S A, Mathan V I, Balaram P. Interactions of linear dicationic molecules with lipid A: structural requisites for optimal binding affinity. J Endotoxin Res. 1995;2:325–336. [Google Scholar]
- 22.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinarello C A. The proinflammatory cytokines interleukin-1 and tumor necrosis factor and treatment of the septic shock syndrome. J Infect Dis. 1991;163:1177–1184. doi: 10.1093/infdis/163.6.1177. [DOI] [PubMed] [Google Scholar]
- 24.Dinarello C A. Cytokines as mediators in the pathogenesis of septic shock. Curr Top Microbiol Immunol. 1996;216:133–165. doi: 10.1007/978-3-642-80186-0_7. [DOI] [PubMed] [Google Scholar]
- 25.Elsbach P, Weiss J. The bactericidal/permeability-increasing protein (BPI), a potent element in host-defense against gram-negative bacteria and lipopolysaccharide. Immunobiology. 1993;187:417–429. doi: 10.1016/S0171-2985(11)80354-2. [DOI] [PubMed] [Google Scholar]
- 26.Evans T J. The role of macrophages in septic shock. Immunobiology. 1996;195:655–659. doi: 10.1016/S0171-2985(96)80029-5. [DOI] [PubMed] [Google Scholar]
- 27.Evans T J, Carpenter A, Moyes D, Martin R, Cohen J. Protective effects of a recombinant amino-terminal fragment of human bactericidal/permeability-increasing protein in an animal model of gram-negative sepsis. J Infect Dis. 1995;171:153–160. doi: 10.1093/infdis/171.1.153. [DOI] [PubMed] [Google Scholar]
- 28.Feingold K R, Funk J L, Moser A H, Shigenaga J K, Rapp J H, Grunfeld C. Role for circulating lipoproteins in protection from endotoxin toxicity. Infect Immun. 1995;63:2041–2046. doi: 10.1128/iai.63.5.2041-2046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Felgner P L, Gadek T R, Holm M, Roman R, Chan H W, Wenz M, Northrop J P, Ringold G M, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galanos C, Freudenberg M A, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;76:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galanos C, Lehmann V, Lüderitz O, Rietschel E T, Westphal O, Brade H, Brade L, Freudenberg M A, Hansen-Hagge T, Lüderitz T, McKenzie G, Schade U, Strittmatter W, Tanamoto K, Zähringer U, Imoto M, Yamamoto M, Shimamoto T, Kusumoto S, Shiba T. Endotoxic properties of chemically synthesized lipid A part structures: comparison of synthetic lipid A precursor and synthetic analogues with biosynthetic lipid A precursor and free lipid A. Eur J Biochem. 1984;140:221–227. doi: 10.1111/j.1432-1033.1984.tb08090.x. [DOI] [PubMed] [Google Scholar]
- 32.Galanos C, Lüderitz O, Rietschel E T, Westphal O, Brade H, Brade L, Freudenberg M A, Schade U F, Imoto M, Yoshimura S, Kusumoto S, Shiba T. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur J Biochem. 1985;148:1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- 33.Gao X, Huang L. Cationic liposome-mediated gene transfer. Gene Therapy. 1995;2:710–722. [PubMed] [Google Scholar]
- 34.Gazzano-Santoro H, Parent J B, Grinna L, Horwitz A, Parsons T, Theofan G, Elsbach P, Weiss J, Conlon P J. High-affinity binding of the bactericidal/permeability-increasing protein and a recombinant amino-terminal fragment to the lipid A region of lipopolysaccharide. Infect Immun. 1992;60:4754–4761. doi: 10.1128/iai.60.11.4754-4761.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green L C, et al. Analysis of nitrate, nitrite and [15-N] nitrate in biological fluids. Anal Biochem. 1982;126:131. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 36.Griesman S E, Johnston C A. Evidence against the hypothesis that antibodies to the inner core of lipopolysaccharides in antisera raised by immunization with enterobacterial deep-rough mutants confer broad-spectrum protection during gram-negative bacterial sepsis. J Endotoxin Res. 1997;4:123–153. [Google Scholar]
- 37.Hoess A, Watson S, Siber G R, Liddington R. Crystal structure of an endotoxin-neutralizing protein from the horseshoe crab, Limulus anti-LPS factor, at 1.5Å resolution. EMBO J. 1993;12:3351–3356. doi: 10.1002/j.1460-2075.1993.tb06008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lefer A M. Endotoxin, cytokines, and nitric oxide in shock. Shock. 1994;1:79–80. doi: 10.1097/00024382-199401000-00014. . (Editorial.) [DOI] [PubMed] [Google Scholar]
- 39.Leventis R, Silvius J R. Interactions of mammalian cells with lipid dispersions containing novel metabolizable cationic amphiphiles. Biochim Biophys Acta. 1990;1023:124–132. doi: 10.1016/0005-2736(90)90017-i. [DOI] [PubMed] [Google Scholar]
- 40.Lüderitz O, Galanos C, Rietschel E T. Endotoxins of gram-negative bacteria. Pharmacol Ther. 1982;15:383–402. doi: 10.1016/0163-7258(81)90051-6. [DOI] [PubMed] [Google Scholar]
- 41.Marra M N, Thornton M B, Snable J L, Wilde C G, Scott R W. Endotoxin-binding and -neutralizing properties of recombinant bactericidal/permeability-increasing protein and monoclonal antibodies HA-1A and E5. Crit Care Med. 1994;22:559–565. doi: 10.1097/00003246-199404000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Morrison D C, Jacobs D M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976;13:813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- 43.Morrison D C, Kline L F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides. J Immunol. 1977;118:362–368. [PubMed] [Google Scholar]
- 44.Morrison D C, Ryan J L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 45.Munson P J, Rodbard D. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 46.Muroi M, Muroi Y, Suzuki T. The binding of immobilized IgG2a to Fc-gamma-2a receptor activates NF-kappa-B via reactive oxygen intermediates and tumor necrosis factor-alpha. J Biol Chem. 1994;269:30561–30568. [PubMed] [Google Scholar]
- 47.Muroi M, Suzuki T. Role of protein kinase A in LPS-induced activation of NF-kappa B proteins of a mouse macrophage-like cell line, J774. Cell Signal. 1993;5:289–298. doi: 10.1016/0898-6568(93)90019-i. [DOI] [PubMed] [Google Scholar]
- 48.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 49.Nabel G J, Chang A, Nabel E G, Plautz G E, Fox B A, Huang L, Shu S. Immunotherapy of malignancy by in vivo gene transfer therapy. Hum Gene Ther. 1992;3:399–410. doi: 10.1089/hum.1992.3.4-399. [DOI] [PubMed] [Google Scholar]
- 50.Nabel G J, Gordon D, Bishop D K, Nickoloff B J, Yang Z Y, Aruga A, Cameron M J, Nabel E G, Chang A E. Immune response in human melanoma after transfer of an allogeneic class I major histocompatibility complex gene with DNA-liposome complexes. Proc Natl Acad Sci USA. 1996;93:15388–15393. doi: 10.1073/pnas.93.26.15388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohno N, Tanida N, Yadomae T. Characterization of complex formation between lipopolysaccharide and lysozyme. Carbohydr Res. 1991;214:115–130. doi: 10.1016/s0008-6215(00)90535-4. [DOI] [PubMed] [Google Scholar]
- 52.Parrillo J E. Pathogenic mechanisms of septic shock. N Engl J Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 53.Pomerantz R J, Feinberg M B, Trono D, Baltimore D. Lipopolysaccharide is a potent monocyte/macrophage-specific stimulator of human immunodeficiency virus type 1 expression. J Exp Med. 1990;172:253–261. doi: 10.1084/jem.172.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raetz C R H. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- 55.Rietschel E T, Kirikae T, Schade F U, Mamat U, Schmidt G, Loppnow H, Ulmer A J, Zähringer U, Seydel U, Di Padova F, Schreier M, Brade H. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 56.Rietschel E T, Kirikae T, Schade U F, Ulmer A J, Holst O, Brade H, Schmidt G, Mamat U, Grimmecke H-D, Kusumoto S, Zähringer U. The chemical structure of bacterial endotoxin in relation to bioactivity. Immunobiology. 1993;187:169–190. doi: 10.1016/S0171-2985(11)80338-4. [DOI] [PubMed] [Google Scholar]
- 57.Schindler M, Osborn M J. Interaction of divalent cations and polymyxin B with lipopolysaccharide. Biochemistry. 1979;18:4425–4430. doi: 10.1021/bi00587a024. [DOI] [PubMed] [Google Scholar]
- 58.Schumann R R, Lamping N, Kirschning C, Knopf H-P, Hoess A, Herrmann F. Lipopolysaccharide binding protein: its role and therapeutical potential in inflammation and sepsis. Biochem Soc Trans. 1994;22:80–82. doi: 10.1042/bst0220080. [DOI] [PubMed] [Google Scholar]
- 59.Shakov A N, Collart M A, Vassalli P, Nedospasov S A, Jongeneel C V. Kappa-B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990;171:35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siegel J P. Antiendotoxin antibodies. Ann Intern Med. 1995;122:315–315. doi: 10.7326/0003-4819-122-4-199502150-00017. [DOI] [PubMed] [Google Scholar]
- 61.Silverstein R, Christoffersen C A, Morrison D C. Modulation of endotoxin lethality in mice by hydrazine sulfate. Infect Immun. 1989;57:2072–2078. doi: 10.1128/iai.57.7.2072-2078.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Silverstein R, Norimatsu M, Morrison D C. Fundamental differences during gram-positive versus gram-negative sepsis become apparent during bacterial challenge of d-galactosamine-treated mice. J Endotoxin Res. 1997;4:173–181. [Google Scholar]
- 63.Silverstein R, Turley B R, Christoffersen C A, Johnson D C, Morrison D C. Hydrazine sulfate protects d-galactosamine-sensitized mice against endotoxin and tumor necrosis factor/cachectin lethality: evidence of a role for the pituitary. J Exp Med. 1991;173:357–365. doi: 10.1084/jem.173.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stokes D C, Shenep J L, Fishman M L, Hidner W K, Bysani G K, Rufus K. Polymyxin B prevents lipopolysaccharide-induced release of tumor necrosis factor-α from alveolar macrophages. J Infect Dis. 1989;160:52–57. doi: 10.1093/infdis/160.1.52. [DOI] [PubMed] [Google Scholar]
- 65.Takada K, Ohno N, Yadomae T. Detoxification of lipopolysaccharide (LPS) by egg white lysozyme. FEMS Immunol Med Microbiol. 1994;9:255–264. doi: 10.1111/j.1574-695X.1994.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 66.Tesh V L, Vukajlovich S W, Morrison D C. Endotoxin interactions with serum proteins: relationship to biological activity. In: Levin J, ten Cate J W, Buller H R, Van Deventer S J H, Strurk A, editors. Bacterial endotoxins: pathophysiological effects, clinical significance, and pharmacological control. New York, N.Y: Alan R. Liss, Inc.; 1988. pp. 47–62. [PubMed] [Google Scholar]
- 67.Tracey K J, Cerami A. Tumor necrosis factor: an updated review of its biology. Crit Care Med. 1993;21:S415–S422. [PubMed] [Google Scholar]
- 68.Tsukamoto M, Ochiya T, Yoshida S, Sugimura T, Terada M. Gene transfer and expression in progeny after intravenous DNA injection into pregnant mice. Nat Genet. 1995;9:243–248. doi: 10.1038/ng0395-243. [DOI] [PubMed] [Google Scholar]
- 69.Vaara M, Vaara T. Polycations as outer membrane disorganizing agents. Antimicrob Agents Chemother. 1983;24:114–122. doi: 10.1128/aac.24.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vaara M, Vaara T. Polycations sensitize enteric bacteria to antibiotics. Antimicrob Agents Chemother. 1983;24:107–113. doi: 10.1128/aac.24.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vincent J L. Definition and pathogenesis of septic shock. Curr Top Microbiol Immunol. 1996;216:1–13. doi: 10.1007/978-3-642-80186-0_1. [DOI] [PubMed] [Google Scholar]
- 72.Vukajlovich S W. Antibody-independent activation of the classical pathway of human serum complement by lipid A is restricted to Re-chemotype lipopolysaccharide and purified lipid A. Infect Immun. 1986;53:480–485. doi: 10.1128/iai.53.3.480-485.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weil M H. Lessons learned from clinical trials on monoclonal antiendotoxin antibody. Arch Intern Med. 1994;154:1183–1184. [PubMed] [Google Scholar]
- 74.Wenzel R P. Anti-endotoxin monoclonal antibodies—a second look. N Engl J Med. 1992;326:1151–1153. doi: 10.1056/NEJM199204233261710. [DOI] [PubMed] [Google Scholar]
- 75.Wilde C G, Seilhamer J J, McGrogan M, Ashton N, Snable J L, Lane J C, Leong S R, Thornton M B, Miller K L, Scott R W, Marra M N. Bactericidal/permeability-increasing protein and lipopolysaccharide (LPS)-binding protein. LPS binding properties and effects on LPS-mediated cell activation. J Biol Chem. 1994;269:17411–17416. [PubMed] [Google Scholar]
- 76.Wright C E, Rees D D, Moncada S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc Res. 1992;26:48–57. doi: 10.1093/cvr/26.1.48. [DOI] [PubMed] [Google Scholar]
- 77.Xu Y, Szoka F C J. Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry. 1996;35:5616–5623. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- 78.Zähringer U, Lindner B, Rietschel E T. Molecular structure of lipid A, the endotoxic center of bacterial lipopolysaccharides. Adv Carbohydr Chem Biochem. 1994;50:211–276. [PubMed] [Google Scholar]
- 79.Zhang Y, Broser M, Rom W. Activation of the interleukin 6 gene by Mycobacterium tuberculosis or lipopolysaccharide is mediated by nuclear factors NF IL 6 and NF-kappa B. Proc Natl Acad Sci USA. 1995;92:3632–3632. doi: 10.1073/pnas.92.8.3632d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng S, Brown M C, Taffet S M. Lipopolysaccharide stimulates both nuclear localization of the nuclear factor kappa-B 50 kDa subunit and loss of the 105 kDa precursor in RAW264 macrophage-like cells. J Biol Chem. 1993;268:17233–17239. [PubMed] [Google Scholar]
- 81.Ziegler E J. Protective antibody to endotoxin core: the emperor’s new clothes? J Infect Dis. 1988;158:286–290. doi: 10.1093/infdis/158.2.286. [DOI] [PubMed] [Google Scholar]
- 82.Ziegler E J, McCutchan J A, Fierer J, Glauser M P, Sadoff J C, Douglas H, Braude A I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982;307:1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]