ABSTRACT.
The authors recently reported that long-lasting insecticidal nets (LLINs) distributed in Papua New Guinea (PNG) between 2013 and 2019, exhibited severely diminished efficacy to knock down and kill susceptible Anopheles mosquitoes. This coincided with a rise in malaria observed in PNG since 2015. Here, the authors show that LLIN bioefficacy is increased by heating LLINs prior to WHO cone bioassays. Unused LLINs with low bioefficacy, delivered to PNG in 2019, were heated to 120°C for 5 minutes. Cone bioassays were performed before and at 1 hour, 7 days, and 30 days after heating. This led to a significant increase in 24-hour mortality from 17% to 61% and 60-minute knock down from 31% to 72%. The effect was sustained over 30 days. Bioassays are crucial in quality assurance of LLIN products. Our findings indicate that bioefficacy of LLINs can be increased by heating. This may have implications for quality assurance procedures used to assess LLINs.
Accounting for more than 50% of the annual global malaria control budget, long-lasting insecticidal nets (LLINs) are estimated to have prevented 1.5 billion malaria infections and saved 6.8 million lives between 2000 and 2015.1,2 Currently, more than 200 million LLINs are distributed to recipient countries per year. Nevertheless, malaria rates are on the rise again in many countries and global estimates of morbidity and mortality have stagnated after decades of continuous decline.2
The number of LLIN products available to donors has increased considerably over the last 10 years.3 Normally, new candidate LLIN products undergo WHO-supervised assessment in a prequalification (PQ) process. The PQ process involves extensive validation of candidate product bio- efficacy, and chemical and physical properties under laboratory and operational conditions.3,4 The most widely used and robust tests to measure LLIN bioefficacy, in the PQ process and in postmarketing surveillance, are standardized WHO cone bioassays, in which pyrethroid-susceptible mosquitoes are exposed to LLIN samples.4 Adequate bioefficacy is indicated if, after 3 minutes of exposure to a LLIN that has been washed 20 times, 95% of mosquitoes are knocked down within 60 minutes, or 80% of mosquitoes are found dead within 24 hours.4 Once products have been PQ-listed, they are eligible for donor-funded procurement, which sustains the vast majority of the LLIN market.3 Postmarketing, LLIN quality assurance monitoring includes predelivery inspections testing total chemical active ingredient (AI) content, wash retention of the AI, and physical strength of LLINs.3
Despite this quality assurance framework, we have recently shown that PermaNet 2.0® (Vestergaard, Lausanne, Switzerland), LLINs with severely diminished bioefficacy, were distributed in Papua New Guinea (PNG) between 2013 and 2019, unnoticed by regulatory authorities.5 Intriguingly, despite predelivery inspection reports indicating adequate total AI content, the bioefficacy of these LLINs was low and highly variable in standardized WHO cone bioassays, with an average 24-hour mortality of just 39.4% for new nets that had never been used or washed.5 A study from Nicaragua, published in early 2021, showed similar deficiencies for the same LLIN product, with strikingly low mortality rates for pyrethroid-susceptible mosquitoes (< 20%) just 6 months after distribution.6 These findings stand in stark contrast to the bioefficacy of PermaNet 2.0® manufactured prior to 2013, which was consistently 100%, even after extended periods of usage.7
Recently, Yang et al. showed that technical-grade deltamethrin, which is the insecticidal AI in 9 out of 22 currently prequalified LLIN products including PermaNet 2.0®, can unfold significantly increased insecticidal potency after short heating for 5 minutes at 120°C, which is slightly above the melting point of deltamethrin (98°C).8 The authors attributed this effect to a change in deltamethrin structure from crystalline to amorphous.
Inspired by these novel findings and the notion of “heat regeneration,” sometimes mentioned in documentation associated with LLIN bioefficacy testing, especially after washing,9 we performed similar experiments with new and unwashed PermaNet 2.0® LLINs delivered to PNG in 2019. The LLINs used in this study were the same that exhibited very low bioefficacy in WHO cone bioassays upon arrival in PNG.5 The purpose of these experiments was to assess whether heating these LLINs in a similar way as described by Yang et al. for the technical-grade AI would increase bioefficacy, as measured in WHO standard cone bioassays.8 Being able to increase bioefficacy of LLIN products using such a simple method may hold opportunities of how to improve the efficacy of these important vector control commodities.8 However, the ability to manipulate the outcomes of WHO cone bioassays may also compromise standardized quality assurance processes.
World Health Organization cone bioassays were conducted in strict adherence to WHO guidelines.4 Briefly, pyrethroid susceptible Anopheles farauti colony mosquitoes were exposed to LLIN material for 3 minutes in WHO standard cones (5 mosquitoes per cone, 20 mosquitoes per LLIN section). A total of N = 5 sections (25 × 25 cm) per LLIN were tested (four sides and roof), that is, a total of N = 100 mosquitoes per net. Overall, N = 12 PermaNet 2.0® LLINs manufactured in 2019 originating from various batches were tested, resulting in a total of N = 60 tested sections (12 nets × 5 sections per net).
LLIN sections were heated for 5 minutes in a laboratory oven set to 120°C. Sections were allowed to cool for 1 hour before conducting WHO standard cone bioassays.
Outcomes of WHO cone bioassays (24-hour mortality and 60-minute knock down) were determined by calculating the proportions of exposed mosquitoes either dead (after 24 hours) or knocked down (after 60 minutes). Results were averaged for the N = 5 sections per net. To determine whether there were statistically significant differences before and after heating, Wilcoxon’s matched pairs tests (preceded by a Friedman test), were conducted between baseline and the results obtained on days 0, 7, and 30 after heating.
We observed a substantial, permanent, and statistically significant increase in both 24-hour mortality and 60-minute knock down after LLINs were heated for 5 minutes at 120°C. Specifically, while nets prior to heating exhibited a median of 17% (11–84%) 24-hour mortality and 31% (13–75%) 60-minute knock down, this increased to a median of 61% (34–98%) 24-hour mortality and 72% (59–100%) 60-minute knock down (Figure 1), respectively, directly after heating, that is, a 2- to 3-fold increase. The effect was sustained for 30 days, after which samples exhibited a median of 78% (30–96%) 24-hour mortality and 73% (41–96%) 60-minute knock down (Figure 1).
Figure 1.
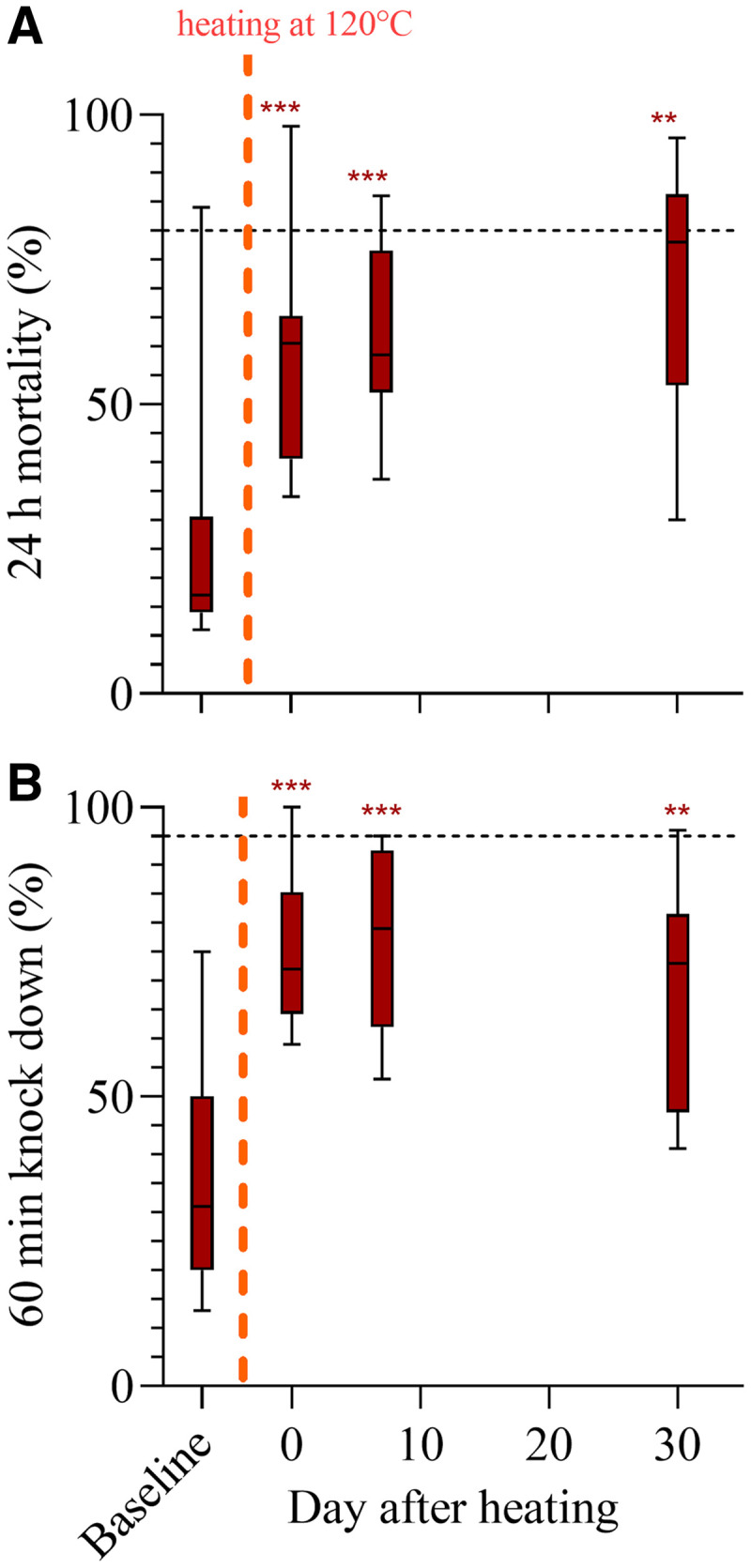
Effect of heating to 120°C on bioefficacy of PermaNet 2.0® (manufactured in 2019) as observed in WHO standard cone bioassays. Panel A shows 24-hour mortality before and after heating for 5 minutes. Panel B shows 60-minute knock down before and after heating for 5 minutes. Significance is indicated by ** ≤ 0.01 and *** ≤ 0.001 using Wilcoxon matched pairs tests (following Friedman tests) comparing post-heating results back to baseline. Standard box-and-whisker plots are presented for the N = 12 long-lasting insecticidal nets (LLINs), showing the median and ranges. The dashed black lines indicate WHO-ratified performance thresholds for LLINs < 3 years in use or washed ≤ 20 times. This figure appears in color at www.ajtmh.org.
Previous studies have shown that bioassay conditions, including ambient temperature, humidity, and the angle of the bioassay cones, can influence mortality and knock down.10 This study shows that the bioefficacy of LLINs, as measured using standardized WHO cone bioassays, can be substantially altered by heating the net material prior to conducting the assays. This observation has important implications on several levels. First, it is possible that an optimal treatment regimen exists (i.e., time and temperature of heating) that would allow for a complete rescue of bioefficacy in products that are currently observed to exhibit diminished performance. Further research is needed to identify these optimal conditions for various LLIN products. However, it is unknown in how far the heating process affects other important parameters such as wash resistance and physical strength of LLIN products. Thus, further studies would be required to determine whether it is advisable to apply this heating procedure to LLINs that are currently observed to exhibit diminished bioefficacy, or to used nets to potentially regenerate some of their insecticidal potency.
Second, our observations imply that bioefficacy can be manipulated by applying simple procedures. Bioefficacy testing is essential in LLIN quality assurance, especially as we have shown that total AI content may not always be correlated with the ability of a product to kill or knock down mosquitoes.5 PQ procedures and postmarket monitoring regulations for LLINs should incorporate this knowledge, ideally by implementing well-validated and standardized methods to measure the surface bioavailable proportion of AI at multiple stages of the product evaluation process, including in recipient countries.11
It is currently unclear what is causing the increased mortality and knock down in the heat-treated LLINs. One hypothesis is that deltamethrin is converted into a more potent form by heating, as recently shown by Yang et al.8 Thus, it could be that the surface bioavailable AI on the LLINs in this study was converted into the more potent form through the application of the heating process. Future studies using electron microscopy-based techniques may be able to elucidate this possibility further.
Alternatively, it is possible that heating facilitates the diffusion of AI to the LLIN surface, thereby increasing surface bioavailability. This has been observed for other LLIN products subsequent to washing, albeit using different temperature and time regimens (e.g., 60°C for 4 hours).9,12 The practical relevance of heat regeneration of LLINs is unclear, as it appears unlikely that end users in resource-limited settings would widely apply the required procedures subsequent to washing.
In conclusion, here we present thought-provoking evidence that heating LLINs coated with deltamethrin can substantially and permanently increase bioefficacy observed with WHO cone bioassays. Our observations are indicative and limited to one brand of LLINs. Results should not be assumed to apply to other brands without further testing. Future studies should include a range of products with different AIs and net materials.
ACKNOWLEDGMENTS
The authors would like to thank the staff of the PNG National Malaria Program, Rotarians Against Malaria PNG, and the PNG Institute of Medical Research for their support.
REFERENCES
- 1. World Health Organization , 2017. Ten Years in Public Health, 2007–2017: Report by Dr. Margaret Chan, Director-General, World Health Organization. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2. World Health Organization , 2021. World Malaria Report 2020. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 3.Karl S Freeman T Katusele M Moore SJ , 2021. Quality control of long-lasting insecticidal nets: are we neglecting it? Trends Parasitol (formal acceptance date March 5th 2021). [DOI] [PubMed]
- 4. World Health Organization , 2013. Guidelines for Laboratory and Field Testing of Long-Lasting Insecticidal Nets. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 5.Vinit R Timinao L Bubun N Katusele M Robinson LJ Kaman P Sakur M Makita L Reimer L Schofield L , 2020. Decreased bioefficacy of long-lasting insecticidal nets and the resurgence of malaria in Papua New Guinea. Nat Commun 11: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villalta EL Soto Bravo AM Vizcaino L Dzuris N Delgado M Green M Smith SC Lenhart A Macedo de Oliveira A , 2021. Evaluation of the durability and use of long‐lasting insecticidal nets in Nicaragua. Malar J 20: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katusele M Gideon G Thomsen EK Siba PM Hetzel MW Reimer LJ , 2014. Long-lasting insecticidal nets remain efficacious after five years of use in Papua New Guinea. P N G Med J 57: 86–93. [PubMed] [Google Scholar]
- 8.Yang J Erriah B Hu CT Reiter E Zhu X Lopez-Mejias V Carmona-Sepulveda IP Ward MD Kahr B , 2020. A deltamethrin crystal polymorph for more effective malaria control. Proc Natl Acad Sci USA 117: 26633–26638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gimnig JE Lindblade KA Mount DL Atieli FK Crawford S Wolkon A Hawley WA Dotson EM , 2005. Laboratory wash resistance of long-lasting insecticidal nets. Trop Med Int Health 10: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 10.Owusu HF Müller P , 2016. How important is the angle of tilt in the WHO cone bioassay? Malar J 15: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green MD Atieli F Akogbeto M , 2009. Rapid colorimetric field test to determine levels of deltamethrin on PermaNet surfaces: association with mosquito bioactivity. Trop Med Int Health 14: 381–388. [DOI] [PubMed] [Google Scholar]
- 12.Jaramillo GI Robledo PC Mina NJ Munoz JA Ocampo CB , 2011. Comparison of the efficacy of long-lasting insecticidal nets PermaNet(R) 2.0 and Olyset(R) against Anopheles albimanus under laboratory conditions. Mem Inst Oswaldo Cruz 106: 606–612. [DOI] [PubMed] [Google Scholar]


