Abstract
Children were initially considered unsusceptible to severe COVID-19. Our knowledge after two years has changed dramatically, but there are still many unknowns. Here, we report the current knowledge about why children generally experience a milder COVID-19 course and highlight research questions about pediatric infection that require answers.
Children were initially considered unsusceptible to severe COVID-19. Our knowledge after two years has changed dramatically, but there are still many unknowns. Here, we report the current knowledge about why children generally experience a milder COVID-19 course and highlight research questions about pediatric infection that require answers.
Main text
The first cluster of severe pneumonia caused by a new betacoronavirus, the 2019 novel coronavirus (2019-nCoV), was reported in Wuhan (Hubei province, China) in November 2019. From the start of the COVID-19 pandemic, few papers described characteristics of pediatric infection. Currently, children of all ages appear to be susceptible to COVID-19, and no significant gender difference has been reported. Early data from China suggested that only 1%–2% of COVID-19 patients were children. We now know that SARS-CoV-2 infection in children has been largely underestimated as a result of limited testing practices. In addition, there are currently sporadic data on specific pediatric risk groups, such as those living with HIV, those with other immunosuppressive conditions (including cancer patients), or children with chronic heart or lung diseases (such as cystic fibrosis). The transmissibility of SARS-CoV-2 from children (whether symptomatic or asymptomatic) to other children and adults, including members of their own household, is still unclear and requires further investigation.1 Data collected during public health emergencies are inevitably subject to reporting biases, variations in case definitions, and variable population coverage. Therefore, one must be cautious when generalizing widely from early studies; several important knowledge gaps remain about COVID-19 in children. One of the most remarkable features of SARS-CoV-2 infection is the wide range of outcomes that follow virus exposure, ranging from asymptomatic infection to mild, severe, and fatal COVID-19.
Distinct features of pediatric SARS-CoV-2 infection
Since the COVID-19 pandemic’s very beginning, the pediatric population seemed to suffer fewer symptoms than adults. Many reports showed how children infected by SARS-CoV-2 usually developed a milder disease than adults. Understanding the reasons for this milder disease expression is important to identify new potential assets and approaches for the development of new therapies to reduce the spread of the virus and disease morbidity across all populations. However, as the pandemic expanded, it became clear that some children (including those without underlying health problems) did, indeed, develop severe disease that required intensive care. There are many theories to explain the differences between adults and children, and many of them remain merely speculative because of the relatively recent history of the disease and the lack of robust experimental data.
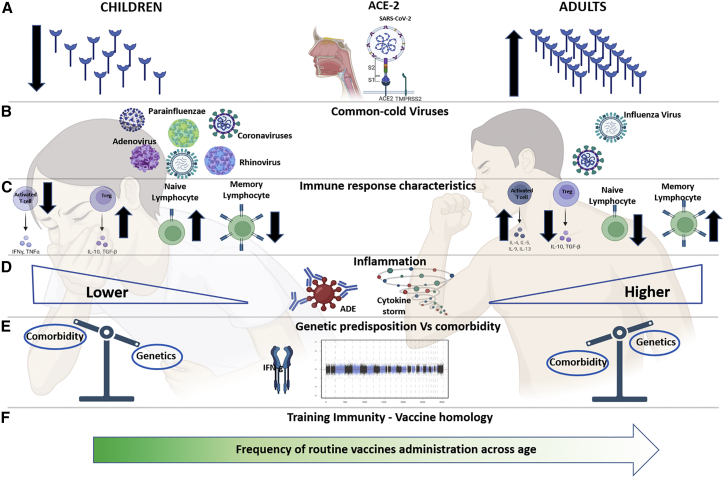
Reduced COVID-19 severity in children may be due to a lower rate of comorbidities compared to the elderly. However, another important hypothesis focuses on the mechanism of SARS-CoV-2 cell entry through the angiotensin-converting enzyme (ACE) 2 receptor. Indeed, it has been speculated that the higher frequency of such receptors in the airways of adults compared to children could represent a possible explanation for COVID-19’s milder course during childhood (Figure 1A). In addition, it has been shown how, apart from age, the ACE2-angiotensin system is affected by many other factors, including sex, smoking, diet, vitamin D, body-mass index, drug use, and comorbidities including diabetes mellitus, chronic obstructive pulmonary disease, hypertension, and IFN-γ levels.2,3 However, a direct role of different ACE2 distribution has not been confirmed, since most literature on the topic is contradictory, and recent works show comparable levels of viral loads regardless of the level of ACE2 expression in infected children.4
Figure 1.
Main theories behind differences between children and adults in SARS-CoV-2 infection
(A) Different expression of ACE-2 between children and adults.
(B) Prior immunity to other common cold viruses including other strains of coronaviruses in children compared to adults.
(C and D) Main differences in terms of immune and inflammatory response in infancy and adulthood.
(E) The different burden of genetic predisposition and comorbidities in children and adults.
(F) Frequency of vaccine administration decreases with age.
ACE-2, angiotensin-converting enzyme 2; ADE, antibody-mediated enhancement. Created with BioRender.com.
Second, an additional possible explanation for the milder clinical course in children might rely on their prior immunity to other common cold viruses, including other strains of coronaviruses (e.g., HCoV-NL63 and HCoV-OC43), which could confer a cross-protective immunity through cross-reactive antibodies presenting neutralizing activity for multiple strains of coronaviruses (Figure 1B). In line with this hypothesis, a recent report also showed a higher cross-reactive, spike-specific immune response to SARS-CoV-2 infection, in terms of neutralizing both Ab- and Ag-specific T cell immune response in children.5 Childhood immune responses are particularly important in an individual’s life, as they form the initial memory B cell pool that shapes future responses. In comparison to adults, children have higher frequencies in their blood of “convergent” B cell clones defined by B cell receptors (BCRs) with high sequence similarity for pathogens they have encountered.6 In line with this, it has been recently demonstrated that children that develop a more severe phenotype also lack antibodies to these common seasonal coronaviruses. Moreover, protection against common human coronaviruses decreases with age, which is in line with the increased susceptibility found in the elderly.
Third, antibodies against SARS-CoV-2 are crucial, when they present neutralizing activity, to control the disease. Conversely, aspecific humoral responses can be detrimental in cases where they induce antibody-mediated enhancement (ADE) inflammation—a mechanism that deserves further attention in the pathogenesis of COVID-19 (Figure 1D). Pre-existing abs against different strains of HCoVs can partially affect neutralizing activity against SARS-CoV-2 and act as a trigger for ADE.7 This could explain both the worse disease course observed in adults and the development of rare hyper-inflammatory conditions in children, such as multisystem inflammatory syndrome in children (MIS-C).
Fourth, it has recently been shown that a relevant proportion of people with a more severe clinical course following SARS-CoV-2 infection show autoantibodies against type I IFN and/or rare genetic variants involved in IFN immunity, suggesting the need for a genome-wide association analysis of host factors determining more severe COVID-19 phenotypes also found in children (Figure 1E).
Fifth, it is well known that lymphocyte memory compartments change dramatically with age—there is a higher percentage of naive cells during the first years of life and an expanded memory compartment with age. This distribution could differentially challenge the immune system upon novel antigenic stimulation in childhood or adulthood. Furthermore, children’s immune responses are skewed toward a Th2 response with a lower amount of pro-inflammatory cytokines compared to the Th1 response more typical of the adult population and associated with an increased disease severity. In line with this hypothesis, a better regulatory activity associated with lower immune activation status has been reported in asymptomatic COVID children (Figure 1C).8
Finally, a fascinating, although unconfirmed, hypothesis is one that confers a protective role to vaccinations routinely performed during infancy. This theory, referred to as “training immunity,” is based on the homology sequence similarity of 30 amino acid residues between the SARS-CoV-2 spike glycoprotein, the fusion (F1) glycoprotein of measles virus, and the envelop (E1) glycoprotein of the Rubella virus induced by the vaccine (Figure 1F).
Multisystem inflammatory syndrome in children
A number of recent studies have clarified that the COVID-19 clinical burden in children is mostly due to over-inflammation and immune cells dysregulation.8,9 It must be noted that an apparently similar hyperinflammation status that characterizes severely ill COVID-19 adults could also be present in children. Critically ill children present with a pro-inflammatory syndrome characterized by a phenotypic picture resembling Kawasaki disease (KD) or toxic shock syndrome possibly related to COVID-19. This new condition related to SARS-CoV-2 infection was initially defined as Kawasaki-like disease. An increasing number of reports found that KD and COVID-19-related pro-inflammatory states share some characteristics and likely a common genetic background but still appear to be clinically different. On May 14, 2020, the Centers for Disease Control and Prevention (CDC) issued a national health advisory to report on cases meeting the criteria for MIS-C. The hyper-inflammation status in MIS-C is also empirically sustained by the efficacy of immunomodulatory therapies such as intravenous immunoglobulin, systemic corticosteroids, and IL-1 receptor antagonists, the latter mainly used as second-line therapy in refractory cases. Considering the severity of this pro-inflammatory condition, we must gain more insight into the pathogenesis of SARS-CoV-2 infection and MIS-C to establish proper treatment strategies and repurpose available drugs targeting signaling and metabolism of immune cells. Few studies have investigated through next generation sequencing how monogenic susceptibility to inflammation may characterize previously healthy children who develop MIS-C after COVID-19. An IFN-γ-skewed response was reported in MIS-C compared to mild COVID-19 patients;10 however, a definitive mechanistic insight related to the host-response and underlying the onset of MIS-C has not been found yet.
SARS-CoV-2 infection in kids: The heart of the problem
Cardiac involvement, well known to be one of the most worrying consequences of MIS-C, was also reported between May and June 2021 in Israeli troops and adolescents who were administered a 2nd dose of mRNA COVID-19 vaccination. Indeed, since then there have been increasing reports of myocarditis and myopericarditis as rare complications of COVID-19 mRNA vaccinations, especially in young-adult and adolescent males.11 The CDC Advisory Committee on Immunization Practices identified a likely association between the two COVID-19 mRNA vaccines from Pfizer-BioNTech and Moderna and cases of myocarditis and pericarditis accounting for 1,226 reports of probable myocarditis/pericarditis cases after >300 million COVID-19 mRNA vaccine doses administered through June 11, 2021. Importantly, most of these cases were reported to be self-limited, without any long-term cardiac sequelae. The severity of the cardiac involvement with MIS-C, both in terms of intensive care need and long-term sequalae, is far worse and not even comparable to cardiac involvement found in children after vaccination. However, the pathogenesis of this condition is still unclear. Overall, it will be crucial to understand if children developing myocarditis after vaccination are those susceptible to MIS-C. Importantly, it has been recently shown how mRNA vaccine against SARS-CoV-2 was able to significantly reduce the incidence of MIS-C.12 In terms of immunogenicity, a higher dose (100 μg) of mRNA vaccine was shown to induce higher CD4 T cell immunity compared to a lower dose (25 μg). There are few hypotheses on how differences in quantity of mRNA delivered through the vaccination could impact safety and possibly heart disease. Among the mRNA vaccinations, the main difference in formulations available for adults stands in the quantity of mRNA delivered with Pfizer-BioNTech (30 μg) versus Moderna (100 μg). Such observations were extremely useful in the context of an immunization schedule designed for children. Indeed, the pediatric population, overlooked for an excessively long period in the SARS-CoV-2 vaccination program, was screened for safety and efficacy in a clinical trial (NCT04816643),13 and both CDC and EMA recently approved the use of Pfizer-BioNTech vaccine in children between 5–11 years old at a lower dosage of mRNA (10 μg). The low-dose efficacy and safety with this population raises the question of whether a personalized and reduced dosage approach should be considered in patients such as teenagers and young adults who experienced cardiac side effects after mRNA vaccines or those patients with a high risk of autoimmunity. Another open question tightly linked to this point regards the booster immunization of patients previously affected by MIS-C. So far, there has been no structured investigation, both in terms of safety and efficacy, on the immunization of these patients. A tailored intervention with adapted timing and dosage may be considered for this particular group (Box 1).
Box 1. Hot research topic in pediatric COVID-19.
Also, the immunization of children younger than 5 years old is crucial: direct benefits of preventing SARS-CoV-2 infection in young children include protection against severe disease, hospitalizations, and severe or long-term complications, such as MIS-C. Indirect benefits include the likelihood of reduced transmission in the home and in school settings, including transmission affecting vulnerable persons. Without effective COVID-19 vaccines for this age group, children could potentially become ongoing reservoirs of infection and sources of newly emerging variants.14 In line with this, the Omicron variant (B.1.1.529), which prevailed among other variants beginning in late 2021, showed a robust spread and an increased virulence among children. It is still unknown whether novel variants may differentially impact pediatric hospitalization, providing further urgency to the need for effective immunization strategies in this population. Indeed, COVID-19 vaccine trials for infants and children older than 6 months are still ongoing. Additional experimental efforts are needed to effectively target younger kids. An extremely well-designed and solid body of evidence with a special regard to the safety profile of vaccine intervention in children is needed. Such data will be crucial to counteract vaccination hesitancy among parents or guardians. Indeed, the proposal to start from lower dosage of mRNA vaccinations would help avoid side effects in children that would dangerously fuel families’ distrust in vaccine intervention. In the meantime, global knowledge of COVID-19 epidemiology, clinical characteristics, and management has continued to evolve since the onset of the pandemic.
Long-term consequences of SARS-CoV-2 infection in children
After the first wave of the pandemic, residual effects of COVID-19 were described in adults: fatigue, dyspnea, chest pain, cognitive disturbances, arthralgia, and decline in quality of life occurring weeks after the acute infection as a result of cellular damage, robust innate immune response with inflammatory cytokine production, and a pro-coagulant state. Therefore, the term “post-acute COVID-19 syndrome” was identified and included persistence of symptoms or development of sequelae beyond 3 or 4 weeks from the onset of acute COVID-19.15 A post-acute outpatient service established in Italy reported persistence of symptoms in 87.4% of 143 adult patients discharged from hospital who recovered from acute COVID-19 at a mean follow-up of 60 days from the onset of the first symptom. Fatigue (53.1%), dyspnea (43.4%), joint pain (27.3%), and chest pain (21.7%) were the most commonly reported symptoms, with 55% of patients continuing to experience 3 or more symptoms. A decline in quality of life, as measured by the EuroQol visual analog scale, was noted in 44.1% of patients in this study. Long-term sequelae of COVID-19 in children have been less well described than in adults. However, such a condition has been characterized as well by symptoms of fatigue, headache, dyspnea, cough, anosmia, etc. In the past couple of months, there have been several publications on this syndrome, but little is known about its prevalence and its associated risk factors, specifically in children and adolescents. This condition was incompletely understood initially, and therefore it did not immediately receive an official name. Nevertheless, patient organizations started complex discussions and movements on social media with various kinds of evidence and advocacy to demonstrate a longer, more complex course of illness and eventually coined the term “long COVID,” which was later also recognized by the World Health Organization. Patient advocacy played a critical role in the naming of long COVID. To date, few data on post-acute COVID-19 children have emerged. A study conducted in Australia on post-acute COVID-19 outcomes in children with mild and asymptomatic COVID-19 reported that the most common post-acute COVID-19 symptoms were mild post-viral cough (6 [4%] of 151 children), fatigue (3 [2%] children), or both post-viral cough and fatigue (1 [1%] child). These findings suggest a difference compared with adults, with whom multisystemic complications have a higher prevalence and severity. An Italian study on post-acute syndrome in children recruited 129 children reporting the following most frequently reported symptoms at 60 days post-acute infection: insomnia (18.6%), respiratory symptoms (including pain and chest tightness) (14.7%), nasal congestion (12.4%), fatigue (10.8%), muscle (10.1%) and joint pain (6.9%), and concentration difficulties (10.1%). These findings are in line with the pattern of symptoms reported in a Swedish cohort. Emerging clinical observations and preliminary research indicate that COVID-19 can also have a long-term impact on mental health in a range of domains, but well-conducted and methodologically robust studies are still lacking. Data collected during the SARS-CoV-2 pandemic infection in the adult population seem to confirm the association between the viral infection and psychiatric disorders, particularly delirium, depression, anxiety, and insomnia. Coronaviruses could induce psychopathological sequelae through direct viral infection of the central nervous system (CNS) or indirectly via an immune response. Moreover, fear of illness, uncertainty of the future, stigma, traumatic memories of severe illness, and social isolation experienced by patients during the COVID-19 pandemic are significant psychological stressors that may interact in defining psychopathological outcome. In the pediatric population, a few studies investigated the psychological and behavioral impact of lockdown and quarantine measures for the COVID-19 pandemic and demonstrated that closing schools, limiting social interactions, imposing travel restrictions, halting sports activities, and transitioning all to online classes have engendered emotional distress, depression, irritability, sleep disturbance, inattention, fear, and anxiety. More recently, preliminary findings from a UK “CLoCk study” suggest that up to one out of seven (14%) children and young people who caught SARS-CoV-2 may have symptoms linked to the virus fifteen weeks later (https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/children-young-people-with-long-covid-clock-study-covid-19-uph/). Overall, these data support the need to better understand the impact of SARS-CoV-2 infection on mental health. Ongoing follow-up of pediatric patients with COVID-19, including assessment of lung and heart function and mental health outcomes, is needed to comprehensively describe long-term outcomes in this population.
Conclusions
Specific treatment for COVID-19 will be a critical tool in the fight against SARS-CoV-2 for the benefit of the patient and, potentially, to reduce viral shedding and onward transmission within the community. Children of all ages are at risk for SARS-CoV-2 infection and some of them for severe disease manifestations. The administration of COVID-19 vaccines could confer both direct and indirect benefits even to younger children (<12 years) and interrupt community transmission. In light of the positive safety and immunogenicity results of recent pediatric mRNA COVID-19 vaccine clinical trials, we highlight the need for initial pediatric clinical trials on novel anti-SARS-CoV-2 treatment strategies to begin in parallel with ongoing adult phase-3 clinical trials. The support of parents aided by comprehensive information and recommendations from their children’s pediatricians will be integral for the success of any COVID-19 vaccine or treatment that is ultimately licensed for children.
Acknowledgments
This study was funded by Fondo 5x1000 2021/2022 to D.A., by Fondo 5x1000 2020/2021 to N.C., and by the project Telescope to P.P.
Author contributions
Conceptualization, D.A., N.C., and P.P.; writing – original draft, D.A., N.C., and P.P.; supervision, P.P.; funding acquisition, D.A., N.C., and P.P.
Declaration of interests
The authors declare no competing interests.
References
- 1.Cotugno N., Ruggiero A., Bonfante F., Petrara M.R., Zicari S., Pascucci G.R., Zangari P., De Ioris M.A., Santilli V., Manno E.C., et al. CACTUS Study Team Virological and immunological features of SARS-CoV-2-infected children who develop neutralizing antibodies. Cell Rep. 2021;34:108852. doi: 10.1016/j.celrep.2021.108852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunyavanich S., Do A., Vicencio A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA. 2020;323:2427–2429. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., et al. HCA Lung Biological Network. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020;181:1016–1035.e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yonker L.M., Neilan A.M., Bartsch Y., Patel A.B., Regan J., Arya P., Gootkind E., Park G., Hardcastle M., St John A., et al. Pediatric Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Clinical Presentation, Infectivity, and Immune Responses. J. Pediatr. 2020;227:45–52.e5. doi: 10.1016/j.jpeds.2020.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowell A.C., Butler M.S., Jinks E., Tut G., Lancaster T., Sylla P., Begum J., Bruton R., Pearce H., Verma K., et al. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nat. Immunol. 2022;23:40–49. doi: 10.1038/s41590-021-01089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang F., Nielsen S.C.A., Hoh R.A., Röltgen K., Wirz O.F., Haraguchi E., Jean G.H., Lee J.-H., Pham T.D., Jackson K.J.L., et al. Shared B cell memory to coronaviruses and other pathogens varies in human age groups and tissues. Science. 2021;372:738–741. doi: 10.1126/science.abf6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan Y., Shang J., Sun S., Tai W., Chen J., Geng Q., He L., Chen Y., Wu J., Shi Z., et al. Molecular Mechanism for Antibody-Dependent Enhancement of Coronavirus Entry. J. Virol. 2020;94:e02015–e02019. doi: 10.1128/JVI.02015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrara M.R., Bonfante F., Costenaro P., Cantarutti A., Carmona F., Ruffoni E., Di Chiara C., Zanchetta M., Barzon L., Donà D., et al. Asymptomatic and Mild SARS-CoV-2 Infections Elicit Lower Immune Activation and Higher Specific Neutralizing Antibodies in Children Than in Adults. Front. Immunol. 2021;12:741796. doi: 10.3389/fimmu.2021.741796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotugno N., Ruggiero A., Pascucci G.R., Bonfante F., Petrara M.R., Pighi C., Cifaldi L., Zangari P., Bernardi S., Cursi L., et al. CACTUS Study Team Virological and immunological features of SARS-COV-2 infected children with distinct symptomatology. Pediatr. Allergy Immunol. 2021;32:1833–1842. doi: 10.1111/pai.13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esteve-Sole A., Anton J., Pino-Ramirez R.M., Sanchez-Manubens J., Fumadó V., Fortuny C., Rios-Barnes M., Sanchez-de-Toledo J., Girona-Alarcón M., Mosquera J.M., et al. Similarities and differences between the immunopathogenesis of COVID-19-related pediatric multisystem inflammatory syndrome and Kawasaki disease. J. Clin. Invest. 2021;131:144554. doi: 10.1172/JCI144554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oster M.E., Shay D.K., Su J.R., Gee J., Creech C.B., Broder K.R., Edwards K., Soslow J.H., Dendy J.M., Schlaudecker E., et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA. 2022;327:331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy M., Recher M., Hubert H., Javouhey E., Fléchelles O., Leteurtre S., Angoulvant F. Multisystem Inflammatory Syndrome in Children by COVID-19 Vaccination Status of Adolescents in France. JAMA. 2022;327:281–283. doi: 10.1001/jama.2021.23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter E.B., Talaat K.R., Sabharwal C., Gurtman A., Lockhart S., Paulsen G.C., Barnett E.D., Muñoz F.M., Maldonado Y., Pahud B.A., et al. Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2021:35–46. doi: 10.1056/NEJMoa2116298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman E. How the unvaccinated threaten the vaccinated for COVID-19: A Darwinian perspective. Proc. Natl. Acad. Sci. U S A. 2021;118 doi: 10.1073/pnas.2114279118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]