Abstract
Background
In cardiac transplant recipients, the electrocardiogram (ECG) is a noninvasive measure of early allograft rejection. The ECG can predict an acute cellular rejection, thus shortening the time to recognition of rejection. Earlier diagnosis has the potential to reduce the number and severity of rejection episodes.
Methodology
A systematic literature review was conducted to identify and select the original research reports on using electrocardiography in diagnosing cardiac transplant rejection in accordance with the PRISMA guidelines. Studies included reported sensitivity and specificity of ECG readings in heart transplant recipients during the first post‐transplant year. Data were analyzed with Review manager version 5.4. p‐value was used in testing the significant difference.
Results
After the removal of duplicates, 98 articles were eligible for screening. After the full‐text screening, a total of 17 papers were included in the review based on the above criteria. A meta‐analysis of five studies was done.
Conclusion
In heart transplant recipients, a noninvasive measure of early allograft rejection has the potential to reduce the number and severity of rejection episodes by reducing the time and cost of surveillance of rejection and shortening the time to recognition of rejection.
Keywords: cardio transplant rejection, ECG, heart transplant rejection, rejection diagnosis
Abbreviations
- ECG
Electrocardiography
- EMB
Endomyocardial biopsy
- HT
Heart transplant
- QRS, QTc, ST
ECG waves
- RBBB
Right bundle branch block
1. INTRODUCTION
A well‐established treatment for end‐stage heart failure patients is heart transplantation. The median survival of adult patients who received a heart transplant after the year 2000 is over 12 years 1 —a significant cause of early mortality in acute allograft rejection. The prevalence of allograft rejection has been reported to exceed 13% in the first year following adult heart transplantation. Thus, if the patient survives the first‐year post‐transplant, they are expected to survive at least 15 years. 1 According to the 2011 annual United States data released by the International Society for Heart Lung Transplantation Registry, 26% of heart transplant patients experience at least one rejection episode within the first‐year post‐transplant. The most frequent cause of morbidity and rehospitalization in this patient population remains acute rejection. 2 , 3
The electrocardiogram (ECG) is a simple, cost‐effective, and noninvasive tool used to evaluate the rhythm and electrical activity of the heart. Sensors attached to the skin are used to display the electrical signals generated by your heart on an easy to interpret grid paper. 4 Utilizing ECG readings in heart transplant recipients can predict an acute cellular rejection, thus shortening the time to recognize rejection. A recent study examined serial ECGs in 98 patients within the first‐year post‐heart transplantation. The most common abnormalities were associated with intraventricular conduction delays, with the right bundle branch block (RBBB) being the most prevalent. 5 , 6
In cardiac transplant recipients, a noninvasive measure of early allograft rejection can reduce the number and severity of rejection episodes. ECG can reduce the time to detection and the cost of surveillance of rejection. 6 In this study, we summarize the diagnostic accuracy and criteria of the ECG in the detection of cardiac transplant rejection patients.
2. METHODOLOGY
2.1. Selection of studies
A systematic literature review was conducted to identify all studies about the detection of graft rejection in heart transplant surgeries per the PRISMA guidelines. 7 The online database: Google Scholar, PubMed, and Cochrane were searched from January 1985 to September 2020. Keywords used in the search included (Heart transplant rejection OR Heart transplantation rejection OR Detection of heart transplant rejection OR Cardiac transplant detection OR Cardiac transplantation detection OR Noninvasive ways of detection of cardiac transplant rejection). The screening was completed by Hashim T. Hashim, and Jaffer Sha, with disagreements being resolved by Joseph Varney. There was no restriction on participant's age, gender, or ethnicity, and no restrictions to language written. The references of selected papers were manually checked for additional relating studies. An analysis of the funnel plot was carried out to determine the possibility of bias in the publication in the Review Manager program version 5.4. Inclusion criteria for meta‐analysis were studies that correlated the Endomyocardial biopsy grading to the ECG features.
2.2. Data extraction
Details of the study design, ECG characteristics, endomyocardial biopsy grading, and outcome data, including the QT interval, QTc, QT dispersion, and QTc dispersion, were extracted. The risk of bias was assessed using the Newcastle‐Ottawa Quality Assessment tool.
2.3. Study inclusion
After a comprehensive search of the literature, 170 publications resulted and then became 96 after removal of duplicates. Of these, 51 were eligible for full‐text screening. After the full‐text screening, 18 studies were included in the systematic review and meta‐analysis, as shown in (Figure 1). Six studies were included in the meta‐analysis. The included QT (ms), QTc, QT dispersion, and QTc dispersion outcomes in the meta‐analysis were reported in 2, 3, and 5 studies. The summary of the included studies and risk of bias assessment are shown in Tables 1, 2, 3, respectively.
Figure 1.
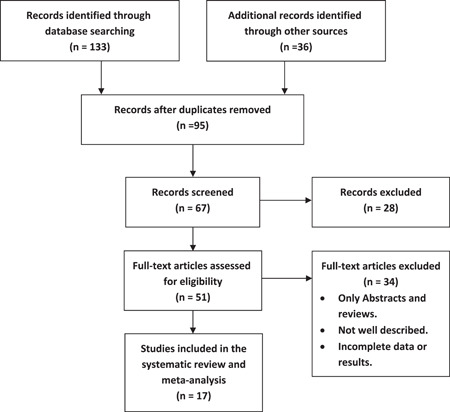
Flow chart of the study selection process
Table 1.
The studies' general information
| The code | The study | References | Year | Country |
|---|---|---|---|---|
| E 1 | Haberl et al. | 8 | 1987 | Germany |
| E 2 | Lacroix et al. | 9 | 1992 | Canada |
| E 3 | Picano Et al. | 10 | 1990 | Italy |
| E 4 | Regueiro‐Abel et al. | 11 | 2002 | Spain |
| E 5 | Kim et al. | 12 | 2019 | South Korea |
| E 6 | Imamura et al. | 13 | 2012 | Japan |
| E 7 | Babuty et al. | 14 | 1994 | France |
| E 8 | Doering et al. | 15 | 2012 | USA |
| E 9 | Grace et al. | 16 | 1991 | UK |
| E 10 | Graceffo et al. | 17 | 1996 | USA |
| E 11 | Grauhan et al. | 18 | 1993 | Germany |
| E 12 | Hicky et al. | 19 | 2018 | USA |
| E 13 | Locke et al. | 20 | 1989 | UK |
| E 14 | Vogt et al. | 21 | 1990 | Germany |
| E 15 | Tenderich et al. | 22 | 2006 | Germany |
| E 16 | Valentino et al. | 23 | 1992 | USA |
| E 17 | Wada et al. | 24 | 1999 | Japan |
| E 18 | Eckart et al. | 25 | 2005 | USA |
Table 2.
The studies' specific data
| Code | Study type | No. of patients | Mean ages | Duration |
|---|---|---|---|---|
| E 1 | Observational | 19 Patients | 40.46 years | 14 days |
| E 2 | Observational | 25 Patients | 44.2 years | 5.2 months |
| E 3 | Observational | 14 Patients | 48.6 years | 24 h |
| E 4 | Observational | 31 Patients | ‐ | 15 months |
| E 5 | Retrospective analysis | 79 Patients | 33.6 years | 5 years |
| E 6 | Case report | 2 Patients | 26 years | I year |
| 48 years | ||||
| E 7 | Review | ‐ | ‐ | ‐ |
| E 8 | Prospective, double‐blind, multi‐center descriptive study | 325 Patients | ‐ | 6 weeks |
| E 9 | Cross‐sectional | 18 Patients | 49 years | 19 days |
| E 10 | Observational | 20 Patients | 47 years | 8 months |
| E 11 | Experimental | 40 Patients | ‐ | 4.5 days |
| E 12 | Observational | 220 Patients | 54 years | 72 h |
| E 13 | Observational | 10 Patients | 38.2 years | ‐ |
| E 14 | Retrospective Study | 13 Patients | 49 years | 3 years |
| E 15 | Observational | 100 Patients | ‐ | 3 months |
| E 16 | Prospective Study | 41 Patients | ‐ | ‐ |
| E 17 | Experimental | ‐ | ‐ | 6 days |
| E 18 | Cohort | 75 | 55.2 years | 7 days |
Table 3.
The ECG's characteristics
| Code | ECG changes | No. of rejection | Outcomes or notes |
|---|---|---|---|
| E 1 | The frequency content of the ST section decreased from 10 to 30 Hz, and the frequency content of the QRS varied from 60 to 150 Hz. | 16 Patients | For the noninvasive diagnosis of acute cardiac rejection after cardiac transplantation, FFT of surface ECGs is encouraging. The mechanism of improvements and the future application of this approach for persistent rejection assessment continue to be assessed. |
| E 2 | In detecting rejection, the root‐mean‐square voltage of the 70‐Hz high pass filtered QRS complex was found to be the most reliable component. | 20 Patients | In the control of heart transplant rejection, the signal‐averaged ECG is useful. Compared with the time‐domain method, frequency domain analysis of the QRS complex would not improve the technique's precision. |
| E 3 | Depression of the ST section in the precordial segments (mostly V3‐V6) and the limb leads. | ‐ | Dipyridamole electrocardiography in the early post‐transplantation era is practical, secure, and affordable, with the potential for noninvasive monitoring of transplantation rejection. |
| E 4 | In the AR group, the QTc dispersion was 40 ± 17 ms. | 31 Patients | Proposals for the use of QTc dispersion for diagnosing AR in HT patients are not confirmed by the findings of this review. |
| E 5 | Longer PR interval RBBB ECG changes. | 3 Patients | Near observation of new RBBB growth at 1‐year post‐HT, associated with a higher rate of new‐onset graft rejection, can be helpful in detecting high‐risk graft rejection patients. |
| E 6 | Disappearance of R waves in I, aVL, and prolongation of wider QRS duration PR intervals and deeper S waves in V5,6 in I, II, aVF deep S wave, poor R progression in all anterior precordial leads, marked PR interval prolongation, and V4‐6 deeper S wave. Also found were ST depression and T wave inversion in I, aVL, and V2‐6. | 2 Patients | While no rejection‐specific ECG changes have been reported so far, the above‐described changes that may represent actual hemodynamic anomalies may be a diagnostic tool for rejection. |
| E 7 | Important changes in the high‐frequency components (between 50 and 110 Hz) of the QRS complex and significant reductions in the low‐frequency components (between 10 and 30 Hz). | ‐ | During acute rejection, improvements in the ECG properties of transplanted hearts were observed, with improvements in intraarticular and auriculoventricuir conduction and decreases in QRS voltage amplitude. These experimental findings should be considered in the development of new methods for detecting cardiac allograft rejection ECGs. |
| E 8 | An expanded QTC interval in recipients of a heart transplant is linked to acute allograft rejection and death. | ‐ | In heart transplant recipients, a noninvasive measure of early allograft rejection has the ability to reduce the number and severity of rejection episodes by reducing the time and cost of monitoring of rejection and shortening the time to identification of rejection. In addition, other ECG parameters important to noninvasive allograft rejection monitoring must be identified to achieve the objectives of the current study and may provide evidence for a randomized controlled trial to assess the feasibility and cost‐effectiveness of this form of noninvasive ECG monitoring as compared with normal EMB surveillance. |
| E 9 | A decrease in the summed QRS voltage in the anterior chest leads and a turn to the right in the QRS frontal vector was also seen in humans and nonspecific repolarization shifts were also seen and drops in the evoked T wave amplitude. | 18 Patients | At the time of transplantation, QT‐driven rate‐responsive units can be implanted with periodic interrogation of these units, theoretically abrogating the need for endomyocardial biopsy. |
| E 10 | Higher frequency QRS voltages. | 20 Patients | The study shows the relative loss of high‐frequency SA‐ECG components in cardiac transplant rejection patients and suggests that SA‐ECG may be useful for noninvasive cardiac transplant rejection assessment. |
| E 11 | ECG voltage amplitude (IMEG) seems to follow a "focal pattern" similar to the histology. | 40 Patients | ‐ |
| E 12 | Important changes in the length of QRS (p < .001), QT (p = .009), QTc (p = .003), and PR (p = .03) cycles, as well as increased odds of development of right bundle block branch (p = .002) and fascicular block (p = .009). | 12 Patients | Electrocardiographic changes following transplant surgery have been linked with mild to serious acute allograft rejection. |
| E 13 | Significant decreases in QRS voltage. | 10 Patients | These results suggest that in the estimation of cardiac rejection, QRS voltage is of highly limited benefit in patients treated with low‐dose triple immunotherapy. |
| E 14 | QRS reduction in the standard ECG. | 13 Patients | A useful screening tool for mild to extreme acute rejection is QRS voltage reduction in a localized region measured by BSPM. |
| E 15 | Prolongation in both QTc time and QTc dispersion of >40 ms. | 100 Patients | ECGs are routinely conducted, QTc time measurements and QTc dispersion can be accurately used to detect acute rejection early after heart transplantation. |
| E 16 | A significantly larger high frequency QRS complex component (50–110 Hz). | 19 Patients | ECG for the diagnosis of acute allograft rejection is a useful noninvasive technique. |
| E 17 | The QRS complex's peak‐to‐peak amplitudes and heart rate are substantially reduced, the power of and the LF is significantly increased. | ‐ | A successful noninvasive marker for early detection of cardiac allograft rejection is heart rate variability study. A responsive means of measuring the effects of immunosuppressive therapy can also be given by this procedure. |
| E 18 | Increased QT dispersion in patients with rejection. | 41 | No statistical significance of QTc−d and severity of rejection. QTc−d should not be considered a sensitive marker for OHT rejection. |
2.4. Analyses
The total number of patients included in the meta‐analysis in the no rejection or mild rejection group is 1733 patients, and the total number of patients in the moderate or severe rejection group is 264 patients.
3. RESULTS
We used random effects due to heterogeneity observed among studies when we used fixed effects. In QT (ms) outcome, the pooled analysis between no or mild rejection and moderate or severe rejection was (MD = 3.80, 95% CI = −18.10 to 25.70, p‐value = .73), we observed heterogeneity that was not solved by random effects, as shown in Figure 1. The pooled analyses between no or mild rejection and moderate or severe rejection in QTc, QT dispersion and QTc dispersions outcomes, were (MD = 18.91, 95% CI = −21.30 to 59.11, p‐value = .36), (MD = −68.54, 95% CI = −195.74 to 58.66, p‐value = .29) and (MD = −41.15, 95% CI = −93.26 to 10.96, p‐value = .12), respectively (Figures S2–S4).
We did subgroup analysis based on the duration of the follow‐up. The two subgroups were from 3 to 6 months and from hospital discharge to 7 days, the heterogeneity was not solved by subgroup analysis and leave one out test in the QTC subgroups and the results (MD = 5.19, 95% CI = −15.55 to 25.92, p‐value = .62) and (MD = 34.67, 95% CI = −30.99 to 100.33, p‐value = .30), respectively (Figure S5).
In the QTc dispersion outcome, the heterogeneity was not solved by subgroup analysis or leave one out test. The results were in the 3–6 months follow‐up subgroup (MD = −68.77, 95% CI = −195.54 to 58.01, p‐value = .29) and in the hospital discharge to 7 days follow‐up subgroup were (MD = 10.00, 95% CI = − 2.17 to 22.17, p‐value = .11) (Figure S6).
No publication bias was observed among included studies, as shown in Figure S7.
Table 1 describes the characteristics data for each study individually and their citations.
Table 2 shows the data of patients and their characteristics, their ages, the type of study, and the duration of the study (the age and the duration are either mean or median).
Table 3 shows the characteristics of the ECG recording and the outcomes of the studies (No. of rejections is the number of patients recognized with ECG).
The rejection was diagnosed with histology findings and biopsies and then compared with the findings of the ECG to give the definitive diagnosis (Figure 2).
Figure 2.
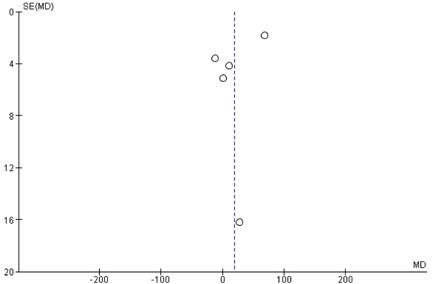
Publication bias
Figure S7 shows the risk of biases and applicability concerns among the studies distributed as high risk, low risk, and unclear risk.
4. DISCUSSION
We found no significant association between heart transplant rejection and QT changes of ECG. The studies included in this review report the rejection of the heart transplant after the surgery with either moderate or severe rejection. The results were assured by the biopsy to compare between the results of the ECG and the histology. A total of 957 patients were identified for heart transplant rejection, with 304 diagnosed by ECG (31.7%). The primary method used for diagnosis was the QRS interval and amplitude (see Table 3). Sensitivity and specificity varied widely between our studies (see Figure S3), potentially showing the user error in ECG placement and reading. To date, the only consistently effective approach available for the diagnosis of cardiac transplant rejection is an endomyocardial biopsy.
The utility of ECG in this population may have various utilities. Preclinical advances in cardiac transplantation have shown that ECG may indicate a beneficial corticosteroid response. 26 After cardiac transplant, the incidence of conduction disorders is well known, and RBBB is the most frequent of these. 27 The occurrence of RBBB within 1 month of cardiac transplant might have different clinical consequences from those with later RBBB incidence. Before the ECG can consistently be used to detect acute allograft rejection, an investigation is still required to assess computerized ECG measurement algorithms.
Given that ECGs are carried out regularly, QTc time and QTc dispersion measurements could be used to accurately detect acute rejection at the early stage after heart transplantation. If further studies confirm our early current findings, it will be possible to implant QT‐driven rate‐sensitive units with periodic interrogation of these units at the time of transplantation. This could nullify the need for endomyocardial biopsy.
5. LIMITATIONS & FUTURE DIRECTION
There is heterogeneity among included studies due to diversity of study designs in the studies included in the meta‐analysis. Few numbers of studies are included in the meta‐analysis due to few data published. Most of the published data are about QT changes with no interest to the other components of the ECG. ECG abnormalities are less sensitive to the mild forms of rejection that occurs with the currently used immunosuppression medications. The use of the SA‐ECG may be combined with the help of the standard ECG to not miss patients with milder rejection who do not have abnormal ECG features. 28 Further studies are needed to determine if the frequency or time domain of the SA‐ECG are better predictors of rejection.
6. CONCLUSION
In heart transplant recipients, the ECG is a noninvasive measure of early allograft rejection. It holds the potential to reduce the number and severity of rejection episodes of rejection. Time‐efficient and low cost make the ECG a good choice for screening, yet specificity and sensitivity varied widely throughout the studies chosen. Reasonings behind this could be as simple as user error. To avoid this shortfall, an algorism for artificial intelligence reading cardiac transplant rejection patients should be created. With the exceedingly high cost of a heart transplant, we feel further investigation is warranted.
We found no significant association between heart transplant rejection and QT changes of ECG. Although some studies reported significant association, other studies did not. There is heterogeneity among studies included in the meta‐analysis, that does not provide conclusive results. More clinical trials are needed to give final conclusion about using ECG as a measure in detecting heart transplant rejections.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
CONSENT FOR PUBLICATION
All the authors provided their consents for publication.
AUTHOR CONTRIBUTIONS
Hashim T. Hashim and Mustafa A. Ramadhan created the idea, wrote the first draft and supervised the work; Joseph Varney, Jaffer Shah, and Shoaib Ahmad did the data collection and studies selection; Karam Ramadan Motawea, Omneya A. Kandil, and Joseph Varney wrote the final draft and did the analysis.
Supporting information
Supplementary information.
Hashim HT, Ramadhan MA, Ahmad S, et al. The role of the electrocardiogram in the recognition of cardiac transplant rejection: A systematic review and meta‐analysis. Clin Cardiol. 2022;45:258‐264. 10.1002/clc.23783
DATA AVAILABILITY STATEMENT
Data can be requested from the corresponding author upon a reasonable request. This manuscript is being submitted on behalf of all authors listed. The authors declare that this manuscript is solely being submitted to your journal and is not currently under review by any other journal.
REFERENCES
- 1. Messer S, Page A, Axell R, et al. Outcome after heart transplantation from donation after circulatory‐determined death donors. J Heart Lung Transplant. 2017;36(12):1311‐1318. [DOI] [PubMed] [Google Scholar]
- 2. Stehlik J, Edwards LB, Kucheryavaya AY, et al. Detection of heart transplant rejection in adults by echocardiographic diastolic indices: a systematic review of the literature. J Am Soc Echocardiogr. 2006;30:1295‐1300. [DOI] [PubMed] [Google Scholar]
- 3. Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI. The registry of the International Society for Heart and Lung Transplantation: Twenty‐eighth Adult Lung and Heart‐Lung Transplant Report—2011. J Heart Lung Transp. 2011;30(10):1104‐1122. 10.1016/j.healun.2011.08.004 [DOI] [PubMed] [Google Scholar]
- 4. Duchateau J, Potse M, Dubois R. Spatially coherent activation maps for electrocardiographic imaging. IEEE Trans Biomed Eng. 2017;64(5):1149‐1156. [DOI] [PubMed] [Google Scholar]
- 5. Duchateau J, Potse M, Dubois R. Electrocardiographic. J Crit Care. 2018;64:145‐150. [Google Scholar]
- 6. Novotný T, et al. Electrocardiographic changes after heart transplantation. Sex and Cardiac Electrophysiology. Academic Press; 2020:231‐236. [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339(1):b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haberl R, Weber M, Reichenspurner H, et al. Frequency analysis of the surface electrocardiogram for recognition of acute rejection after orthotopic cardiac transplantation in man. Circulation. 1987;76:101‐108. [DOI] [PubMed] [Google Scholar]
- 9. Lacroix D, Kacet S, Savard P, et al. Signal‐averaged electrocardiography and detection of heart transplant rejection: comparison of time and frequency domain analyses. J Am Coll Cardiol. 1992;19:553‐558. [DOI] [PubMed] [Google Scholar]
- 10. Picano E, De Pieri G, Salerno JA, et al. Electrocardiographic changes suggestive of myocardial ischemia elicited by dipyridamole infusion in acute rejection early after heart transplantation. Circulation. 1990;81(1):72‐77. 10.1161/01.cir.81.1.72 [DOI] [PubMed] [Google Scholar]
- 11. Tenderich G, Jahanyar J, Zittermann A, et al. Diagnostische Wertigkeit von EKG-Veränderungen bei akuten kardialen Abstoßungsreaktionen nach orthotoper Herztransplantation. Medizinische Klinik. 2006;101(2):99‐106. 10.1007/s00063-006-1014-z [DOI] [PubMed] [Google Scholar]
- 12. Kim JH, Oh J, Kim MJ, et al. Association of newly developed right bundle branch block with graft rejection following heart transplantation. Yonsei Med J. 2019;60:423‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imamura T, Kinugawa K, Shiga T, et al. Reversible abnormality of electrocardiogram as a sign of acute cardiac rejection after orthotropic heart transplantation. J Cardiol Cases. 2012;5:e113‐e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Babuty D, Aupart M, Cosnay P, et al. Electrocardiographic and electrophysiologic properties of cardiac allografts. J Cardiovasc Electrophysiol. 1994;5:1053‐1063. [DOI] [PubMed] [Google Scholar]
- 15. Doering LV, Hickey K, Pickham D, Chen B, Drew BJ. Remote non‐invasive allograft rejection monitoring for heart transplant recipients: study protocol for the novel evaluation with home electrocardiogram and remote transmission (NEW HEART) study. BMC Cardiovasc Disord. 2012;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grace AA, Newell SA, Cary NRB, et al. Diagnosis of early cardiac transplant rejection by fall in evoked T wave amplitude measured using an externalized QT driven rate responsive pacemaker. Pacing Clin Electrophysiol. 1991;14:1024‐1031. [DOI] [PubMed] [Google Scholar]
- 17. Graceffo MA, Robert AO. Cardiac transplant rejection is associated with a decrease in the high‐frequency components of the high‐resolution, signal‐averaged electrocardiogram. Am Heart J. 1996;132(4):820‐826. 10.1016/s0002-8703(96)90317-8 [DOI] [PubMed] [Google Scholar]
- 18. Grauhan O, Warnecke H, Muller J, et al. Intramyocardial electrogram recordings for diagnosis and therapy monitoring of cardiac allograft rejection. Eur J Cardio Thoracic Surg. 1993;7(9):489‐494. 10.1016/1010-7940(93)90279-k [DOI] [PubMed] [Google Scholar]
- 19. Hickey KT, Sciacca RR, Chen B, et al. Electrocardiographic correlates of acute allograft rejection among heart transplant recipients. Am J Crit Care. 2018;27:145‐150. [DOI] [PubMed] [Google Scholar]
- 20. Locke TJ, Karnik R, McGregor CGA, Bexton RS. The value of the electrocardiogram in the diagnosis of acute rejection after orthotopic heart transplantation. Transp Int. 1989;2(3):143‐146. 10.1007/bf02414601 [DOI] [PubMed] [Google Scholar]
- 21. Vogt L, Sigmund M, Kirkpatrick CJ. Non‐invasive detection of acute rejection after cardiac transplantation by means of body surface potential mapping. Proceedings Computers in Cardiology. IEEE; 1990.
- 22. Tenderich G, Jahanyar J, Zittermann A, Schleithoff SS, Wlost S, Körfer R. Diagnostische Wertigkeit von EKG‐Veränderungen bei akuten kardialen Abstoßungsreaktionen nach orthotoper Herztransplantation. Medizinische Klinik. 2006;101:99‐106. [DOI] [PubMed] [Google Scholar]
- 23. Valentino VA, Ventura HO, Abi‐Samra FM, Van Meter CH, Price HL. The signal‐averaged electrocardiogram in cardiac transplantation. A non‐invasive marker of acute allograft rejection. Transplantation. 1992;53(1):124‐127. 10.1097/00007890-199201000-00024 [DOI] [PubMed] [Google Scholar]
- 24. Wada T, Ono K, Hadama T, Uchida Y, Shimada T, Arita M. Detection of acute cardiac rejection by analysis of heart rate variability in heterotopically transplanted rats. J Heart Lung Transpl. 1999;18:499‐509. [DOI] [PubMed] [Google Scholar]
- 25. Eckart RE, Kolasa MW, Khan NA, Kwan MD, Peele ME. Surface electrocardiography and histologic rejection following orthotopic heart transplantation. Ann Noninvas Electrocardiol. 2005;10(1):60‐64. 10.1111/j.1542-474X.2005.00601.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Therrien J, Webb GD, Gatzoulis MA. Reversal of protein losing enteropathy with prednisone in adults with modified Fontan operations: long term palliation or bridge to cardiac transplantation? Heart. 1999;82:241‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tewelde Semhar. Electrocardiographic manifestations of cardiac transplantation. Electrocard Clin Med. 2020;10:377‐381. [Google Scholar]
- 28. Keren A, Gillis AM, Freedman RA, et al. Heart transplant rejection monitored by signal‐averaged electrocardiography in patients receiving cyclosporine. Circulation. 1984;70(3 Pt 2):I124‐I129. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
Data can be requested from the corresponding author upon a reasonable request. This manuscript is being submitted on behalf of all authors listed. The authors declare that this manuscript is solely being submitted to your journal and is not currently under review by any other journal.


