Abstract
Background
Lupus anticoagulants (LA) are one laboratory criterion for classification of antiphospholipid syndrome, with presence of vascular thrombosis and/or pregnancy/fetal morbidity being clinical criteria. The presence of LA is detected (or excluded) by laboratory testing, with the activated partial thromboplastin time and dilute Russell's viper venom time the most commonly used tests. Given the association of thrombosis with LA, it is no surprise that anticoagulants are used to treat or manage such patients.
Objectives
To review and discuss interferences from anticoagulants on LA testing, and strategies to mitigate these.
Methods
This narrative review assessed interference from commonly used anticoagulants, focusing on LA testing while on direct oral anticoagulants (DOACs), including use of DOAC neutralizers.
Results
The classical anticoagulants comprise vitamin K antagonists such as warfarin, and heparins, predominantly unfractionated heparin and low molecular weight heparin (LMWH). DOACs have emerged with favorable efficacy and safety. These comprise two classes: direct anti‐thrombin (anti‐IIa; dabigatran) or direct anti‐Xa (rivaroxaban, apixaban, edoxaban) agents. All anticoagulants affect clotting assays, although there are differences in effects according to anticoagulant and assay. Nevertheless, because of such interferences, anticoagulants can lead to false‐negative or false‐positive LA findings. Several strategies can mitigate such interferences, including avoidance of testing while patients are on such anticoagulants, temporarily switching to an anticoagulant (i.e., LMWH) with less assay interference, testing for LA at nadir levels of anticoagulants, and/or use of anticoagulant neutralizers.
Conclusion
Whilst the best approach is to avoid LA testing on patients taking anticoagulants; if unavoidable, testing may be facilitated by various mitigating strategies.
Keywords: apixaban, clinical laboratory techniques, dabigatran, direct oral anticoagulants, DOACs, lupus anticoagulant, rivaroxaban
Essentials.
Testing for lupus anticoagulants (LA) is common.
As LA is associated with thrombosis, many tested patients are on anticoagulant therapy.
Anticoagulant therapy interferes with LA assays and may yield false positive and negative results.
Strategies to deal with anticoagulant interferences in LA testing are discussed.
1. INTRODUCTION
Lupus anticoagulants (LA) represent one of the laboratory criteria for patient classification as “definite” antiphospholipid (antibody) syndrome (APS), 1 with presence of antibodies against cardiolipin (aCL) or beta‐2‐glycoprotein I (aB2GPI) representing alternate (or additional) laboratory criteria. Clinical criteria for APS comprise vascular thrombosis and/or pregnancy/fetal morbidity. 1 In turn, LA, aCL, and aB2GPI represent autoantibodies directed against phospholipids, generally in complex with a cofactor, which may be B2GPI or prothrombin. The term “lupus anticoagulant” is actually a (double) misnomer because these antibodies are associated with thrombosis, and the “anticoagulant” effect is solely expressed in in vitro assays, generally observed as a prolongation of clotting times; second, LA are not a significant feature of most cases of lupus, and the association with lupus evolved from initial case descriptions. 2 , 3 , 4 The presence of LA is detected (or excluded) by laboratory testing. 5 , 6 Although expanded on later in this review, the most common tests used for assessing LA are the activated partial thromboplastin time (aPTT) and the dilute Russell's viper venom time (dRVVT). However, there may be a number of other tests that are used in addition or in place of these common tests. 7 For example, the silica clotting time (SCT) represents a form of LA‐sensitive aPTT that may be used in place of a standard LA aPTT. 8 In addition, assays such as Taipan snake venom time and Textarin time are insensitive to some of the anticoagulants that compromise dRVVT and aPTT, and assays such as dilute prothrombin time (dPT) can detect LA unreactive in dRVVT and aPTT. 7
2. A SHORT OVERVIEW OF ANTICOAGULANTS AND THEIR EFFECT ON LA AND OTHER COAGULATION TESTING
Anticoagulants represent a class of drugs that are predominantly used for treatment and/or prevention of thrombosis. 9 , 10 Accordingly, it should come as no surprise that they may be used to treat or prevent thrombosis in patients with symptomatic APS, or otherwise clinically symptomatic and found to be positive for LA. The classical anticoagulants comprise the vitamin K antagonists (VKAs) such as warfarin, and the heparins, predominantly unfractionated heparin (UFH) and low molecular weight heparin (LMWH). Because UFH and LMWH represent parenteral agents that need to be administered by injection (either intravenously [UFH] or subcutaneously [LMWH and sometimes UFH]), VKAs (administered orally) have for long represented the anticoagulant of choice for extended or long‐term treatment. More recently, a separate class of anticoagulants, namely the direct oral anticoagulants (DOACs), have been developed that have favorable efficacy and safety compared with the classical anticoagulant agents. These comprise two separate classes, being direct anti‐thrombin (anti‐IIa; dabigatran) or direct anti‐Xa (e.g., rivaroxaban, apixaban, edoxaban) agents. 9 , 10 , 11 , 12 Although DOACs are not the preferred anticoagulant for thrombotic APS, especially for patients with a high‐risk APL profile (i.e., so‐called triple positive), it is not uncommon to perform testing for LA as part of thrombophilia screens in patients treated with DOACs, or indeed other anticoagulants.
Of relevance to this review, it should be recognized that all anticoagulants, both classical and DOACs, can have affect clot‐based assays, inclusive of aPTT, dRVVT, and SCT; this is summarized in Table 1. The anticoagulants may have some differential effects on these tests, and indeed also on other common coagulation tests such as prothrombin time (PT) and thrombin time (TT) (Table 1). Such differential effects should be noted by clinicians requesting LA testing and laboratories performing such tests. Thus, anticoagulants can adversely impact tests used for LA detection/exclusion, and thus lead to potential false‐positive and false‐negative LA findings. This may then affect future choice of anticoagulant and duration of treatment, with risk of adverse outcome if based on an incorrect premise. Alternatively, the observed test patterns in patients where the anticoagulant may not be known may be useful to help identify the anticoagulant in use. For example, only the heparins and anti‐Xa agents (including the DOACs apixaban, rivaroxaban, edoxaban) will yield activity in anti‐Xa assays, whereas only anti‐IIa agents (such as dabigatran and UFH) will affect the TT. But moreover, no two anticoagulants have exactly the same profile regarding effects on coagulation assays (Table 1).
TABLE 1.
Effect of anticoagulants on tests used to investigate lupus anticoagulant (LA) as well as other routine coagulation tests
| Anticoagulant | aPTT | SCT | dRVVT | PT | TT | Anti‐Xa assay |
|---|---|---|---|---|---|---|
| Unfractionated heparin | ↑–↑↑↑ (concentration dependent; most reagents do not contain neutralizer) |
↔ (up to ~1 U/ml if contains heparin neutralizer) ↑ (if exceeds or no neutralizer) |
↔ (up to ~1 U/ml if contains heparin neutralizer) ↑ (if exceeds neutralizer) |
↔ (up to ~1 U/ml if contains heparin neutralizer) ↑ (if exceeds neutralizer) |
↑↑↑ | ↑–↑↑↑ (concentration dependent) |
| LMWH | ↑ |
↔ (if contains heparin neutralizer) ↑ (if no neutralizer) |
↔ (if contains heparin neutralizer) | ↔ (if contains heparin neutralizer) | ↑ | ↑–↑↑↑ (concentration dependent) |
| VKAs | ↑ | ↑ | ↑↑ | ↑↑↑ | ↔ | ↔ |
| Dabigatran | ↑↑ | ↑ | ↑↑ | ↑ | ↑↑↑ | ↔ |
| Rivaroxaban | ↑ | ↑↑ | ↑↑↑ | ↑↑ | ↔ | ↑↑↑ |
| Apixaban | ↔–↑ (assay dependent) | ↑ | ↑ (but LA ratio may fall because effect greater on confirmed reagents) | ↔–↑ (assay dependent) | ↔ | ↑↑↑ |
| Edoxaban | ↔–↑ (assay dependent) | ↑ | ↑ | ↔–↑ (assay dependent) | ↔ | ↑↑↑ |
↑, prolongs (the more ↑, the greater the prolongation); ↔, no effect.
Abbreviations: aPTT, activated partial thromboplastin time; dRVVT, dilute Russell's viper venom time; LMWH, low molecular weight heparin; PT, prothrombin time; SCT, Silica clotting time; TT, thrombin time; VKA, vitamin K agonist.
In regard to LA assays, such interferences can lead to both false‐positive and false‐negative LA results. 5 In recognition of such assay interference, manufacturers have produced reagents for dRVVT (and some aPTT and SCT reagents) that are resistant to heparin within their therapeutic level (generally up to around 1 U/ml heparin) by using heparin “neutralizers” (e.g., heparinase, protamine, polybrene). However, most aPTT reagents do not contain such neutralizers, and indeed, many aPTT reagents purposely exclude such additives because they may alternately be used as surrogate markers of UFH level for patients treated therapeutically. 13 , 14 No test reagent manufacturer currently includes any neutralizers to VKAs or to DOACs in any commercial assay. However, some DOAC neutralizers exist, and this is expanded on later.
3. LUPUS ANTICOAGULANTS GUIDELINES
There are now a plethora of recent guidelines advising on laboratory test procedures to aid in the detection (or exclusion) of LA. 5 , 6 , 15 , 16 , 17 The most widely used guidelines have been developed by the LA Scientific Standardisation Committee (SSC) of the International Society on Thrombosis and Haemostasis (ISTH), of which the latest were published in 2020. 5 , 17 Perhaps less well‐known is that these guidelines build on previous iterations, 18 , 19 , 20 in particular each previous version in sequence. Additional recent guidelines on LA testing are available from the Clinical and Laboratory Standards Institute (CLSI 6 ) and the British Committee for Standards in Haematology 15 , 16 . There are both similarities and differences in the recommended testing approaches between the guidelines. 21 , 22 , 23 Of some relevance to the current review, at the time of the earlier 2009 ISTH guidelines, 18 VKAs and heparin represented the predominant available anticoagulants, with the modern DOACs only emerging in the early 2010s. 9 , 10 , 24 , 25 Thus, the earlier 2009 and 2012 LA guidelines only provided guidance on LA testing in the absence or presence of VKAs and heparin 15 , 18 (Table 2; Table S1). Although the 2014 CLSI guidelines did to some extent cover testing in the presence of DOACs, 6 the main recommendation was to avoid such testing on patients, given the known test interferences, and the difficulty in discriminating true LA from false (“DOAC‐induced”) LA. Of additional relevance, an update to the British Society for Haematology guidelines published in 2020 do provide some guidance on LA testing in the presence of DOACs 16 (Table 2; Table S1). Also, the recent 2020 ISTH guidelines 5 , 17 do cover testing in anticoagulated patients, with the latter 17 providing the most guidance on LA testing in the presence of DOACs (Table 2; Table S1).
TABLE 2.
Recommendations and comments from guidelines on investigation of lupus anticoagulant (LA) while on anticoagulant therapy
| Guideline | Unfractionated heparin | LMWH | VKAs | DOACs |
|---|---|---|---|---|
| ISTH 2009 18 | Some commercial dRVVT and aPTT reagents contain neutralizers able to quench heparin up to 0.8 U/ml. LA screening not possible if heparin level exceeds reagent neutralization capacity | Screening for LA in patients treated with LMWH is possible. However, the effect on LA assays may vary depending on the ratio between FXa to FIIa activity of each LMWH preparation | Interpretation of results on patients on VKAs is difficult because of prolonged basal clotting times. Laboratory procedures should be performed 1–2 wk after treatment discontinuation or when INR <1.5. Bridging VKA discontinuation with LMWH is recommended, with the last dose of LMWH administered more than 12 h before blood is drawn for LA testing. Alternatively, for INRs between 1.5 and <3.0, a 1:1 dilution of patient plasma and PNP can be considered. However, result interpretation may be difficult, and the LA titer will be diluted twofold | The effect of direct thrombin or FXa inhibitors on LA assays is unknown |
| BCSH 2012 15 |
LA tests should not be performed in patients receiving therapeutic doses of UFH because of potential erroneous results. Low‐dose subcutaneous UFH and LMWH have less effect on the dRVVT and most commercial reagents contain heparin neutralizers sufficient to cover prophylactic doses. If positive results are obtained from aCL or aB2GPI assays, these are sufficient for the diagnosis of APS |
LA testing is not recommended in patients receiving VKA. Brief discontinuation of VKA therapy for diagnostic purposes is not a high‐risk strategy in most instances. Performing testing on equal volume mixtures of patient and normal plasma may be informative. Because of the dilution effect, negative testing in mixing studies does not exclude the presence of a LA. Alternate assays to dRVVT can be considered. If positive results are obtained from aCL or aB2GPI assays, these are sufficient for the diagnosis of APS | Not mentioned | |
| CLSI 2014 6 | If possible, samples from patients treated with UFH should not be screened with the aPTT or SCT unless treated with a heparin neutralizer. Most commercially available dRVVT screening reagents contain a heparin neutralizer that permits testing in the presence of UFH. However, samples containing high UFH levels may give incorrect results | LMWHs, depending upon their composition may prolong the aPTT and therefore results should be interpreted with caution. However, in certain patient populations that are at high risk for APS and treated with LMWH, there is no alternative but to test in the presence of the drug | If possible, VKA samples should not be screened with the aPTT because correct interpretation of test results is difficult. Most patients on VKAs also have prolonged SCT and dRVVT complicating interpretation | DTIs and factor FXa inhibitors (e.g., rivaroxaban) give prolonged dRVVT results that show only partial correction in a screening mixing test |
| ISTH 2020 5 | Whenever possible, blood for LA detection should be collected in patients not receiving any anticoagulant treatment | |||
| Heparins interfere with LA clotting assays; however, although UFH and enoxaparin affect the dRVVT at supra‐therapeutic anti‐Xa levels, they may not lead to false‐positive LA in a three‐step procedure. Some reagents contain heparin neutralizers, but it is important to verify the levels of heparins that are quenched in these reagents. Samples should be taken, when feasible, at least 12 h after the last dose of LMWH was administered and as near as possible to the next dose | Taipan/Ecarin tests are less affected by VKAs. Recommendations for their general use awaits the provision of independent evidence. Dilution of patient plasma into PNP is not a reliable solution in patients on VKA (false‐negative or false‐positive LA results may occur) | Taipan/Ecarin tests are less affected by anti‐FXa DOACs. Recommendations for their general use awaits the provision of independent evidence. If feasible to briefly interrupt DOAC anticoagulation, LA testing can be performed after checking the level of DOAC. DOAC adsorption may be considered in DOAC treated patients | ||
| ISTH 2020 17 | Some brands of LMWH, depending on their anti‐FXa/FIIa ratio, may result in sizeable prolongation of clotting tests and may affect LA detection. UFH clearly affects LA assays, especially aPTT‐based tests, with false‐positive screening and mixing results. However, at low anti‐FXa UFH activity levels, application of the three‐step procedure does not produce false‐positive LA | Although dilution of the test plasma into PNP is widely used, it is not robust enough to help making diagnosis of LA and both false‐negative or false‐positive results may occur |
In patients on DOACs, on a pragmatic empirical basis, LA testing may be undertaken at least 48 h after the last dose, and longer in patients with renal impairment, although DOAC levels should also be checked. DOAC neutralizers can be considered |
|
| BCSH 2020 16 | Not mentioned | Although LMWH have little effect on LA tests, this may be dependent on LMWH type and reagent. Therefore, possible interferences should be considered even if using reagents with heparin neutralizers. Samples should be taken just before the next dose of LMWH to minimize effects | Not mentioned | aPTT or dRVVT based tests should not be used to detect LA on samples from patients taking DOACs when there is a detectable drug level. There is insufficient evidence to recommend alternative tests for detection of LA in the presence of DOACs. Some studies have suggested absorption methods to remove DOACs are effective, but these methods require further validation |
Text includes modifications to promote clarity and brevity. The authors apologize if this causes any misinterpretation of the original guidance. Additional descriptive text is available in Table S1.
Abbreviations: aB2GPI, anti‐beta 2 glycoprotein I antibodies; aCL, anticardiolipin antibodies; APS, antiphospholipid (antibody) syndrome; aPTT, activated partial thromboplastin time; BCSH, British Committee for Standards in Haematology; CLSI, Clinical and Laboratory Standards Institute; DOAC, direct oral anticoagulant; dRVVT, dilute Russell's viper venom time; DTIs, direct thrombin inhibitor; F, factor; INR, International Normalized Ratio; ISTH, International Society on Thrombosis and Haemostasis; LA, lupus anticoagulant; LMWH, low molecular weight heparin; PNP, pooled normal plasma; PT, prothrombin time; TT, thrombin time; UFH, unfractionated heparin; VKAs, vitamin K antagonists.
4. ANTICOAGULANT NEUTRALIZERS
All LA guidelines recognize the use of heparin neutralizers (e.g., heparinase, protamine sulphate, polybrene) in use in LA reagents, predominantly dRVVT reagents, 5 , 6 , 15 , 16 , 17 able to quench therapeutic levels of heparin (up to ~1 U/ml), and thus enable some LA testing without heparin influence on clotting tests in most clinical situations. However, the guidelines correctly caveat that should heparin levels exceed the reagent's neutralizing ability, some residual effects may be observed, potentially leading to false‐positive LA findings. Such heparin neutralizers are not present in most aPTT reagents because, in general, most aPTT reagents are used to assist in the monitoring of heparin therapy, 13 , 14 and thereby are made purposely sensitive to heparin. As an alternative to a heparin neutralizer in the aPTT reagent, at least one manufacturer has produced a CaCl2 reagent with added heparin neutralizers (http://haematex.com/hrrs.html#title_bar). This then permits use of standard aPTT reagents (without added neutralizers) for both heparin monitoring (use of standard CaCl2) and for LA investigation (CaCl2 with heparin neutralizer), assuming that such aPTT reagents are otherwise suitable for said purposes.
There is no means to specifically “neutralize” the effect of VKAs because such anticoagulants act in vivo to alter the vitamin K–dependent coagulation factors (II, VII, IX, X) and thereby reduce their activity, thus ultimately affecting all clotting assays in which such factors are represented. In vivo, patient overexposure to VKAs, thus yielding very high International Normalized Ratio (INR) values can be mitigated by use of vitamin K and/or factor replacement therapy. However, there is no specific agent available to alter a VKA effect in vitro, although, to some extent, neutralization of VKA effects can be achieved by performing tests as mixtures with normal plasma. This acts to correct the loss of factor II, VII, IX, and X “deficiency” caused by VKA use, and thus provides a means of assessing LA somewhat free of the VKA effect. Indeed, this was a recommended strategy in the 2009 ISTH LA guidelines 18 (Table 2; Table S1). However, this strategy has lost favor in the revised 2020 ISTH LA guidelines 5 , 17 (Table 2; Table S1) because some experts believe this may lead to false‐negative or false‐positive LA findings. Nevertheless, it may remain the only option available for laboratories faced with assessing LA in a VKA‐treated patient.
Given experience with heparin neutralizers in dRVVT assays, to negate the effect of therapeutic heparin and permit more accurate detection/exclusion of LA, it should therefore come as no surprise that manufacturers have now produced “DOAC neutralizers” for similar in vitro application. However, as stated earlier, such neutralizers have not yet been included in any LA assay by manufacturers of aPTT, dRVVT, or SCT, but rather represent a separate laboratory step before LA testing by such assays. There are four main commercial products available. The first reported 26 is called DOAC‐Stop and was produced locally in Australia by Thomas Exner at his research and manufacturing facility of Haematex in Sydney. As a historical link to LA guidelines, readers may be interested to know that Exner was lead author of the 1991 ISTH LA guidelines, 21 as well as authoring dozens of other papers on LA. The product and its use have now been reported in several studies 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 (Table 3; Table S2). The product represents a form of activated charcoal, and one pellet of the commercial product can remove a therapeutic level of all the DOACs (dabigatran, rivaroxaban, and apixaban being those most well studied) from 1 ml of citrate anticoagulated plasma. In brief, after adding one pellet to 1 ml of plasma and mixing, the treated plasma is then centrifuged to pellet out the black charcoal (complexed to the DOAC), and laboratory testing then progressed on the “DOAC‐free” supernatant plasma. Another activated charcoal‐based product called DOAC‐Remove has subsequently been produced by another commercial manufacturer (5‐Diagnostics, Switzerland), and seems to have similar features to DOAC‐Stop 38 , 39 , 44 , 45 , 46 , 47 , 48 , 49 , 50 (Table 3; Table S2). A third product is available from a third manufacturer (Stago Diagnostics) and is called DOAC‐filter 51 , 52 (Table 3; Table S2). A different type of filter has recently been released for sale by 5‐Diagnostics, called DP‐filter; studies on the device appear to only have been published in abstract form. 53 , 54 An additional publication discusses the use of another activated charcoal product 55 in this setting, but is not clearly reflective of the use of any of the four commercial products mentioned previously. A series of reviews on this topic have also been published. 56 , 57 , 58 , 59 , 60
TABLE 3.
Summary data from select studies reporting on DOAC neutralization studies
| Study | Summary of findings | Comments/author conclusions |
|---|---|---|
| DOAC‐Stop | ||
| Exner et al 2018 26 | DOAC‐Stop tested on normal and a range of abnormal plasmas using aPTT, dRVVT, PT/INR, including LA samples. DOAC‐Stop found to remove dabigatran, apixaban, rivaroxaban and edoxaban with minimal effect on any of the (mainly clotting) tests | Original description of DOAC‐Stop, and indeed any DOAC‐neutralization for LA testing (and other coagulation assays) |
| Jacquemin et al 2018 27 | Assessed DOAC‐Stop compared with idarucizumab, a humanized antibody fragment that binds dabigatran and acts as an in vivo antidote. DOAC‐Stop as effective as idarucizumab to neutralize dabigatran in a variety of assays and did not interfere with detection of LA | Idarucizumab would represent a very expensive way to neutralize dabigatran for laboratory tests |
| Kopatz et al 2018 28 | Normal pooled plasma spiked with apixaban, dabigatran, edoxaban, or rivaroxaban assessed for thrombin generation in the presence and absence of DOAC‐Stop. DOAC‐Stop effectively removed DOACs, but leaving the DOAC‐Stop‐treated plasma slightly more procoagulant | Although not related to LA, “a minor DOAC‐independent increase in thrombin generation response in the DOAC‐Stop‐treated sample should be taken into account” in relation to other potential hemostasis test results |
| Exner et al 2019 29 | This study aimed to investigate the specificity of an DOAC‐Stop on a range of other anticoagulants using the aPTT. In addition to extracting DOACs, DOAC‐Stop also bound argatroban and lepirudin, but had no effect on heparin, enoxaparin or danaparoid. Among other aPTT‐inhibiting agents, DOAC‐Stop also extracted protamine, aprotinin, and polymyxin | Important follow‐up study, showing additional potential utility for DOAC‐Stop, as well as potential confounders |
| Platton and Hunt 30 | Investigated DOAC‐Stop effects on a range of hemostasis assays on plasmas collected from patients on rivaroxaban or apixaban and enabled more accurate interpretation of coagulation assays (PT, aPTT, DOAC‐specific anti‐Xa assay, factor VIII, and dRVVT) before and after sample treatment | DOAC‐Stop significantly removed the effects of rivaroxaban and apixaban and reduced the number of false‐positive LA interpretations with rivaroxaban. There was no effect on results from patients not anticoagulated. Complete reversal of the anti‐Xa effect did not occur in every sample |
| Ząbczyk et al 31 | Assessed the impact of DOAC‐Stop, reversing in vitro effects of DOACs, on LA testing in 75 anticoagulated VTE patients (50 on rivaroxaban, 20 on dabigatran, and 5 on apixaban) | Authors concluded that DOAC‐Stop did not adversely influence LA testing in APS patients, and effectively reduced plasma DOAC concentrations leading to appropriate dRVVT results in up to 97% of VTE patients |
| Favaloro et al 2019 33 | Assessed cross‐laboratory (n = 82) testing of four samples to investigate whether rivaroxaban‐induced interference in LA testing could be neutralized: (A) A pool of normal plasma (LA‐negative control); (B) sample A spiked with rivaroxaban (200 ng/ml) to create rivaroxaban‐induced interference (LA “false”‐positive sample); (C) sample B subsequently treated with a commercial DOAC‐neutralizer (DOAC‐Stop); (D) sample B treated with andexanet alfa (200 μg/ml) | DOAC‐Stop was able to neutralize the false LA activity induced by rivaroxaban. In contrast, although andexanet alfa negated the rivaroxaban‐prolonged LA ratio, it did not fully correct clot times, leaving some residual LA interference, and requiring additional testing to investigate prolonged clotting times |
| Favresse et al 2018 34 | Investigated the effect of DOAC‐Stop on thrombophilia assays (antithrombin, protein S, protein C, LA, APCR) using 135 DOAC‐treated patients (38 apixaban, 40 dabigatran, 15 edoxaban, and 42 rivaroxaban) and 20 control patients. DOAC‐Stop treatment was mostly effective to overcome the effect of DOACs on aPTT‐LA and dRVVT tests. False‐positive results (up to 75%) from DOACs observed with LA tests fell to zero after DOAC‐Stop treatment, regardless of the DOAC considered | Authors concluded that DOAC‐Stop appeared to be an effective and simple way to overcome the interference of DOAC on coagulation tests and should facilitate the interpretation of thrombophilia screening tests in patients taking DOACs |
| Slavik et al 2019 35 | Evaluated the effectiveness of DOAC‐Stop using 60 (20 apixaban, 20 dabigatran, and 20 rivaroxaban) patients treated with DOACs and using high‐performance liquid chromatography‐coupled tandem mass spectrometry. DOAC‐Stop eliminated dabigatran from 99.5%, rivaroxaban from 97.9%, and apixaban from 97.1% of samples | Authors concluded that residual DOAC amounts did not exceed 2.7 ng/ml for dabigatran, 10.9 ng/ml for rivaroxaban, or 13.03 ng/ml for apixaban, “which are safe values that do not affect either screening or special coagulation tests” |
| De Kesel and Devreese 2020 38 | Assessed the ability of DOAC‐Stop to overcome DOAC interference in LA assays in a representative patient cohort (DOAC, n = 43; VKA, n = 2; heparins, n = 21; no anticoagulants, n = 63). Also, apixaban (30–933 ng/ml), edoxaban (31–1060 ng/ml), rivaroxaban (35–1020 ng/ml), and dabigatran (20–360 ng/ml) were spiked to normal plasma | Authors concluded that DOAC‐Stop limits DOAC interference in LA assays, but that DOAC measurements should be performed in treated samples because incomplete removal may occur. Applying DOAC‐Stop to VKA‐ or heparin‐containing, or non‐anticoagulated samples may lead to erroneous LA results. Therefore, DOAC‐Stop should only be used in plasma from DOAC‐treated patients |
| Monteyne et al 2020 39 | Comparative study of DOAC‐Stop and DOAC‐Remove on a range of assays, including the aPTT, in the absence of DOACs | “aPTT results should be interpreted carefully after treatment with DOAC Stop/Remove as there is a risk for falsely prolonged clotting times” |
| Riva et al 2021 40 | Assessed the effect of DOAC‐Stop on a range of assays (including aPTT and dRVVT) using plasma spiked with various DOACs or parenteral agents | False‐positive LA results obtained with rivaroxaban were normalized with DOAC‐Stop. No effect was observed on the indirect factor Xa inhibitors |
| Baker et al 2021 41 | Authors aimed to evaluate DOAC‐Stop for the removal of DOAC interference in LA testing in 73 samples from patients on DOAC therapy, along with samples from 40 LA positive and negative control patients not on therapy, using aPTT, SCT, and dRVVT. DOAC‐Stop markedly reduced DOAC interference from test samples but had no effect on LA testing in the absence of DOAC therapy, permitting the identification of all LA positive and negative controls | Authors concluded that DOAC‐Stop removed false positives and false negatives resulting from DOAC interference and allowed the identification of patients meeting criteria for the diagnosis of APS by LA testing, as well as the detection of patients on rivaroxaban who are triple positive for APS |
| Úlehlová et al 2021 42 | 31 patient samples spiked with dabigatran, rivaroxaban, or apixaban using concentrations that influenced LA screening tests and thus mask the presence of LA. DOAC levels before and after DOAC‐Stop were determined by functional assays and LC‐MS analysis. The results of LA‐positive samples show significant differences between functional tests and the LC‐MS method both before and after DOAC binding | The presence of LA affects the determination of DOAC by functional tests, and in such cases, it is necessary to use LC‐MS to determine DOAC values accurately. Thus, in patients treated with DOAC who develop LA of medium and higher titers, the authors do not recommend checking DOAC levels with functional tests |
| DOAC‐Remove | ||
| Cox‐Morton et al 2019 44 | DOAC‐Remove did not interfere with coagulation testing in normal plasma or in patients on DOAC with a known LA in 1566 routine patient samples tested. DOAC‐Remove prevented 5% of patients having a false LA detected. DOAC did not significantly affect the LA aPTT ratio, protein S antigen, or protein C activity | Authors concluded DOAC‐Remove reversed DOAC effects on hemostasis assays and aids diagnostic accuracy |
| Jourdi et al 2019 46 | Authors evaluated DOAC‐Remove in dRVVT testing in patient samples: 49 apixaban, 48 rivaroxaban, 24 dabigatran, and 30 none. DOAC‐Remove did not affect dRVVT results in non‐DOAC patients, whereas it resulted in DOAC concentrations <20 ng/ml in 82%, 98%, and 100% of apixaban, rivaroxaban, and dabigatran samples, respectively. DOAC‐Remove corrected DOAC interference with dRVVT assays in 76%, 85%, and 95% of the patients, respectively | Authors recommend the use of DOAC‐Remove for every rivaroxaban sample, whereas it might only be used in positive apixaban and dabigatran samples. A residual DOAC interference cannot be ruled out in case of persisting dRVVT positive results after treatment with DOAC‐Remove |
| Favre et al 2021 48 | 61 referred patients on anticoagulant treatment receiving either DOACs (n = 47: n = 27 rivaroxaban, n = 18 apixaban, n = 2 dabigatran), unfractionated heparin (UFH; n = 7) or LMWH (n = 7); plus 9 patients without anticoagulant treatment | No significant differences between PT, aPTT, fibrinogen, aPTT‐LA, dRVVT, protein C, or protein S before and after the addition of DOAC‐Remove for patients not taking DOACs. Treatment caused aPTT‐LA and dRVVT screen tests falsely positive to became negative |
| Skaugen et al 2021 49 | Study aimed to establish performance characteristics of DOAC‐Remove for neutralization of the effects of rivaroxaban and apixaban in LA testing using samples spiked with rivaroxaban or apixaban and testing by dRVVT, aPTT, and dPT. DOAC‐Remove neutralized rivaroxaban and apixaban concentrations as high as 415 and 333 ng/ml, respectively | Authors concluded that DOAC‐Remove has acceptable performance characteristics for neutralizing effects of rivaroxaban and apixaban for LA testing in the dRVVT and aPTT methods but not in the dPT method |
| Al‐Qawzai et al 2021 50 | 20 samples each from: a control group of non‐anticoagulated patients negative for LA; patients receiving direct factor‐Xa inhibitors (rivaroxaban, apixaban, edoxaban); patients receiving LMWH, dabigatran or argatroban; and patients on warfarin with INR ≥1.5. Testing for PT, aPTT, and TT performed with and without DOAC‐Remove | DOAC‐Remove normalized DOAC and argatroban containing samples |
| DOAC‐Filter | ||
| Farkh et al 2021 51 | Authors evaluated DOAC Filter in 38 rivaroxaban, 41 apixaban, and 68 none patient samples. LA testing was performed using dRVVT and SCT | Authors concluded that DOAC Filter was an easy‐to‐use device allowing FXa inhibitor removal, and thus limiting their interference with LA testing in treated patients |
| Sevenet et al 2020 52 | Study aimed to confirm that DOAC Filter efficiently removes DOACs and to ascertain that coagulation assays are not impacted by filtration. Normal pool plasma (NPP) spiked with DOACs up to 300 ng/ml, with dabigatran etexilate (n = 27), rivaroxaban (n = 35), apixaban (n = 33), and edoxaban (n = 27) or 120 ng/ml for betrixaban (n = 4), and 18 plasma samples from DOAC‐treated patients | Authors conclude that DOAC Filter efficiently removes DOACs from plasma and achieves concentrations below DOAC‐specific assays LoD, except in the case of one apixaban sample. The integrity of plasma is respected, and the cartridge seems not to affect LA diagnosis (NB: Study was from the manufacturer of DOAC Filter) |
| “Activated charcoal” (AC) | ||
| Frans et al 2019 55 | Study evaluated whether AC can be used to resolve DOAC interference on hemostasis tests (anti‐FXa, DTI, PT, aPTT, SCT, dRVVT) using samples from patients receiving DOACs (n = 29), LMWH (n = 10), and VKA (n = 10) | Authors concluded that AC selectively removes DOAC interference on PT, aPTT, and LA assays |
Text includes modifications to promote clarity and brevity. The authors apologize if this causes any misinterpretation of the original material. Additional descriptive text is available in Table S2. See original references reporting data on DOAC neutralization for extended information. Also refer to LA guidelines, 5 , 16 , 18 noting the potential utility of these agents, as well as important caveats (Table 2 and Table S1).
Abbreviations: APCR, activated protein C resistance; aPTT, activated partial thromboplastin time; DOACs, direct oral anticoagulants; dPT, dilute prothrombin time; DTIs, direct thrombin inhibitors; dRVVT, dilute Russell's viper venom time; DTT, diluted thrombin time; INR, international normalized ratio; LA, lupus anticoagulant; LoD, limit of detection; LM‐MS, liquid chromatography coupled with mass spectrometry; LMWH, low molecular weight heparin; PT, prothrombin time; SCT, silica clotting time; TT, thrombin time; UFH, unfractionated heparin; VKAs, vitamin K antagonists; VTE, venous thromboembolism.
Some of the concerns raised with the use of such products include a fear that they may not remove all the DOAC present (especially if super‐therapeutic levels are present), similar to the situation with heparin “exceeding” the capability of heparin neutralizers. This may thus give a false sense of assurance regarding LA testing, and still potentially lead to false‐positive or false‐negative results. A second concern is that these products may have an unknown effect on other components of the test systems, which may in itself adversely affect test results and conclusions around LA presence or absence. For example, historical experience with use of filters in LA testing to help filter out platelets ahead of plasma freezing showed that although such filters successfully removed platelets, some also removed large plasma proteins such as von Willebrand factor, and accordingly also factor VIII. 61 In theory, loss of FVIII could lead to effects on aPTT‐based tests for LA. However, the major concerns related to potential for false diagnosis of von Willebrand disease or hemophilia, should such additional tests be performed on the filtered plasma, for example if LA testing was just one test of a panel performed for investigation of a raised aPTT. Such findings led to withdrawal of recommendations to use filtration devices to remove platelets before sample freezing, and instead to initiate a process of double centrifugation. 18 It is not known if use of the DOAC filter products or other neutralizers will lead to similar or other unwanted effects. Thus, the general recommendation on their use (expanded on later) is to only use such agents in test samples from patients known to be on a DOAC, and to perform specific DOAC testing before and after the use of such agents (to verify DOAC levels before, and absence of DOAC after, use). Of course, all this adds to the complexity, cost, and time taken to investigate patients on DOAC therapy. Use of these products also leads to loss of plasma sample volume, which is often already in short supply given the sample requirements for full investigation of APS or associated conditions. This may be compounded if additional tests are used to help identify the anticoagulant in question. Thus, the best strategy remains to avoid testing LA on patients under DOAC therapy, or if unavoidable, to undertake such testing at trough levels (i.e., collect blood sample just prior to next dose of DOAC), and then potentially use a DOAC neutralizer. However, even these strategies do not guarantee a successful outcome. Additional unknowns include a lack of information about repeat use of neutralizers in case a single use has not removed all the DOAC and whether a mixed approach of DOAC neutralizer/filter provides additional value.
5. GUIDELINE COMMENTARY AND RECOMMENDATIONS ON LA TESTING IN THE PRESENCE OF ANTICOAGULANTS AND ON THE USE OR NOT OF DOAC‐NEUTRALIZERS
As noted previously, given emergence of DOACs in the early 2010s, 9 , 10 only the most recent published guidelines from ISTH, 5 , 17 British Society for Haematology, 16 and CLSI 6 provide recommendations on LA testing in the presence DOACs (Table 2; Table S1). Some of these later guidelines may also provide guidance on LA testing in the presence of classical anticoagulants (VKA, heparins), but greater acknowledgment of these classical anticoagulants is the purview of the earlier guidelines. 15 , 18 Importantly, three of the latest guidelines (ISTH, 5 , 17 BCS 16 ) comment on the use of DOAC‐removal agents. These recommendations are summarized in Table 2, alongside various comments made within the guidelines to help caveat some of the recommendations (Table S1).
6. AUTHORS' PERSONAL VIEWPOINTS ON LA TESTING IN THE PRESENCE OF ANTICOAGULANTS AND ON THE USE OR NOT OF DOAC‐NEUTRALIZERS
One of the authors (E.J.F.) participated in the development of both the CLSI 6 and one of the recent ISTH 5 guidelines, and through his activities in the ISTH also had input into the final version of the 2009 ISTH guidelines. 18 Such guidelines are both evidence‐based (where evidence exists) and eminence‐based (where evidence base is weak or does not exist). Thus, there is a smattering of expert opinion in all LA guidelines. 62 Moreover, the guidelines tend to be consensus‐based (i.e., essentially requiring “support” of the participants), and here, sometimes a majority view may arise that is not reflective of all‐inclusive agreement. 62 All authors have personal experiences and biases; for example, for us having experience around use of DOAC‐Stop 33 , 36 , 37 , 56 but not the other DOAC‐neutralizers/filters. Also, because we run a laboratory that is required to provide a broad diagnostic service, there may be pressure exerted on us by colleagues and other requesting clinicians to perform tests while patients may be on anticoagulant therapy, despite our personal protestations and misgivings. Thus, although we would agree with the guidelines that it is best practice to perform LA testing when patients are not on anticoagulant therapy, this may not always be possible. Examples of reasons where LA testing on anticoagulants may be unavoidable include:
Patient with appropriate clinical condition(s) (e.g., thrombosis, pregnancy morbidity) has tested positive for LA and was subsequently placed on anticoagulant therapy; as per all current guidelines, this initial positive test requires confirmation after 12 weeks, at which time ongoing anticoagulation is likely warranted in most patients.
Patient is on UFH or LMWH therapy and there is consideration for long‐term anticoagulation therapy: is a DOAC suitable or VKA indicated? For example, DOACs are not suitable if a patient is found to be triple positive (positive for aCL, aB2GPI, and LA).
Patient is on a DOAC and found positive for aCL/aB2GPI: should he or sher transition to VKA? (yes, if LA positive).
Patient is on a VKA, but possibility of transitioning to a DOAC has arisen. Is a DOAC acceptable for this patient?
In such situations, our recommended approach would entail a different approach based on the anticoagulant in question, as summarized here:
If possible, do not test patients while they are on anticoagulant therapy (i.e., perform testing when patients are not on anticoagulation therapy, at least 48 h after ceasing DOACs, 1 week after ceasing VKA 17 ).
If required to test while on anticoagulant therapy, find out which anticoagulant the patient is on. If the patient is unconscious/unavailable or the clinician is unavailable/does not know, perform routine coagulation assays and/or specific anticoagulant assays to determine anticoagulant (assess test patterns) if sample volume permits.
If required to test while on anticoagulant therapy, test at nadir levels (i.e., test sample taken before next dose of LMWH or DOAC). However, be aware that even at trough DOAC levels, a lack of DOAC effect on LA testing cannot be guaranteed, even if a DOAC neutralizer is used.
If clinically feasible, consider transitioning patient from VKA or DOAC to LMWH therapy and test LA while on LMWH therapy. However, this needs to be assessed on a case‐by‐case basis and may not be a clinically safe strategy for some patients. In addition, complete suspension of anticoagulant therapy to assess LA is not recommended and may lead to catastrophic consequences.
If a patient is on LMWH therapy, then LA testing is possible; however, be aware that LMWH may have an effect on aPTT and SCT tests. Interpret results accordingly and caveat with suitable comment.
If a patient is on UFH and needs to be assessed for LA, be aware that the UFH will affect the aPTT assay, and may affect the dRVVT if UFH exceeds the heparin‐neutralizing ability. Consider transitioning to LMWH or else using a heparin‐resistant aPTT (or CaCl2). Interpret results accordingly and caveat with suitable comment.
If the patient on a VKA, and if transitioning him or her to LMWH therapy is not feasible, consider performance of LA as mixing study. Note, however, that although this was identified as a recommendation in the 2009 ISTH guidelines, it is not a recommendation in the 2020 ISTH guidelines because of the potential to miss “weak” LA. Interpret results accordingly and caveat with suitable comment.
If the patient is on a DOAC, and transitioning to LMWH not feasible, test at nadir levels (i.e., on sample collected just before next dose), and use a DOAC neutralizer. Interpret results accordingly and caveat with suitable comment. Testing the DOAC level before and after the neutralizer will provide some evidence of DOAC‐free LA testing.
As a brief overview, based on our experience, we would also proffer the following. As already noted, different DOACs have variable effects on aPTT, dRVVT, and other routine assays such as PT and TT (Table 1). Particularly, although all DOACs can prolong the aPTT, the extent of prolongation is both DOAC and reagent dependent (Figure 1). Dabigatran affects the aPTT more than rivaroxaban, and apixaban affects the aPTT the least of the three. In terms of reagent dependence and LA testing, the effect can perhaps be highlighted using a common reagent pair used for such testing, Siemens FSL (LA sensitive) and FS (LA insensitive). FS tends to be more affected than FSL with all the DOACs (Figure 1); however, their relative sensitivity compared with other aPTT reagents differs according to the DOAC. The three DOACs also differ in regard to dRVVT sensitivity (Figure 2). Here, rivaroxaban affects dRVVT more than dabigatran, with apixaban showing least affect. However, the effects on screen and confirm reagent testing also differ, such that rivaroxaban, and to a lesser extent dabigatran, affects the screen more than the confirm, thus potentially yielding an abnormal LA ratio (or a false LA result; Figure 2). In contrast, apixaban affects the confirm more than the screen, thus potentially yielding a reduced LA ratio at “within therapy” levels, that in a patient with a weak LA can lead to a false‐negative result. That apixaban can lead to a false‐negative LA finding has also been inferred from studies using ex vivo samples 38 , 63 ; however, such false‐negative phenomena are harder to prove than false positives because they are reliant on finding studies using rare potentially weak LA patients on apixaban therapy. Additional local information, looking at comparative assay ratios for PT, aPTT, and dRVVT (Figure 3), provides additional context. In general, the dRVVT is affected more by the DOACs than the aPTT or PT, but there is variability in extent and relative prolongations among the assays.
FIGURE 1.
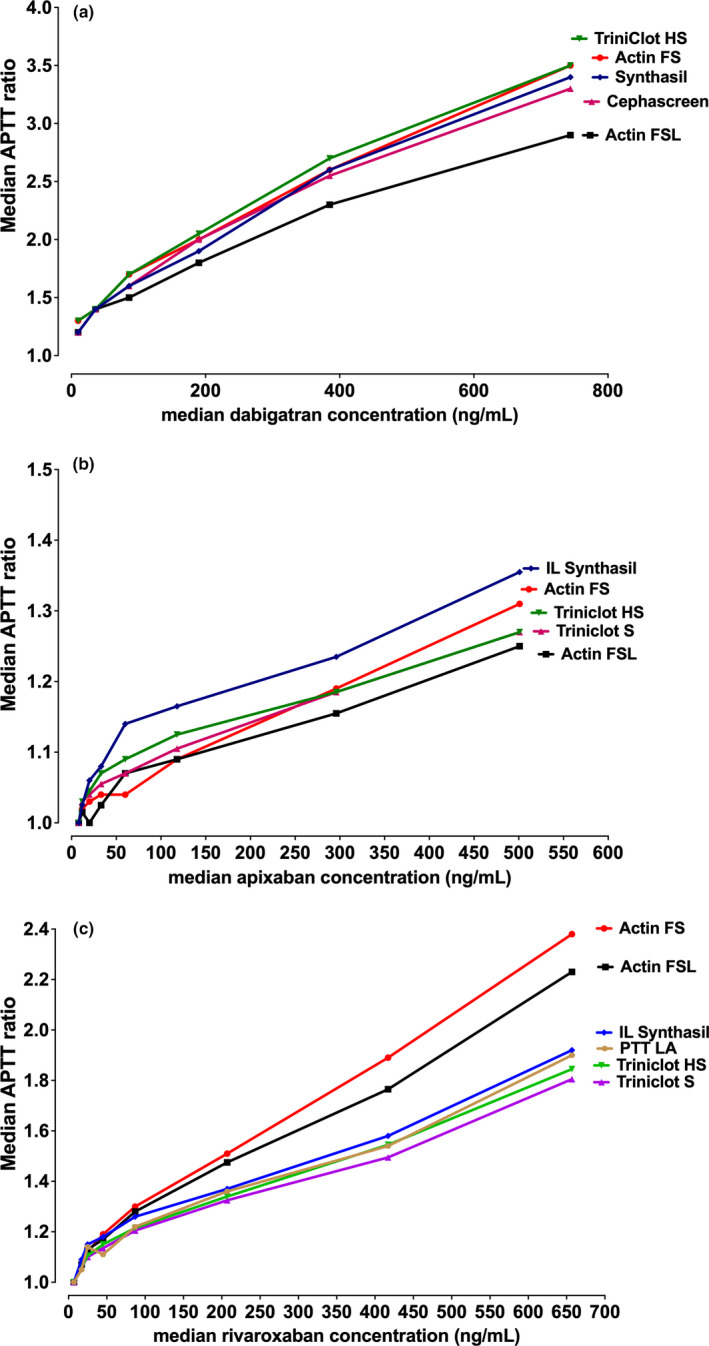
Summarizing the effect of direct oral anticoagulants (DOACs) on the activated partial thromboplastin time (aPTT). An original figure highlighting historical data in which the lead author performed in collaboration with the Royal College of Pathologists of Australasia Quality Assurance Program (RCPQAP) 64 , 65 and showing differential effects on various commercial aPTT reagents according to type of DOAC. The aPTT data are shown as APTT ratios
FIGURE 2.
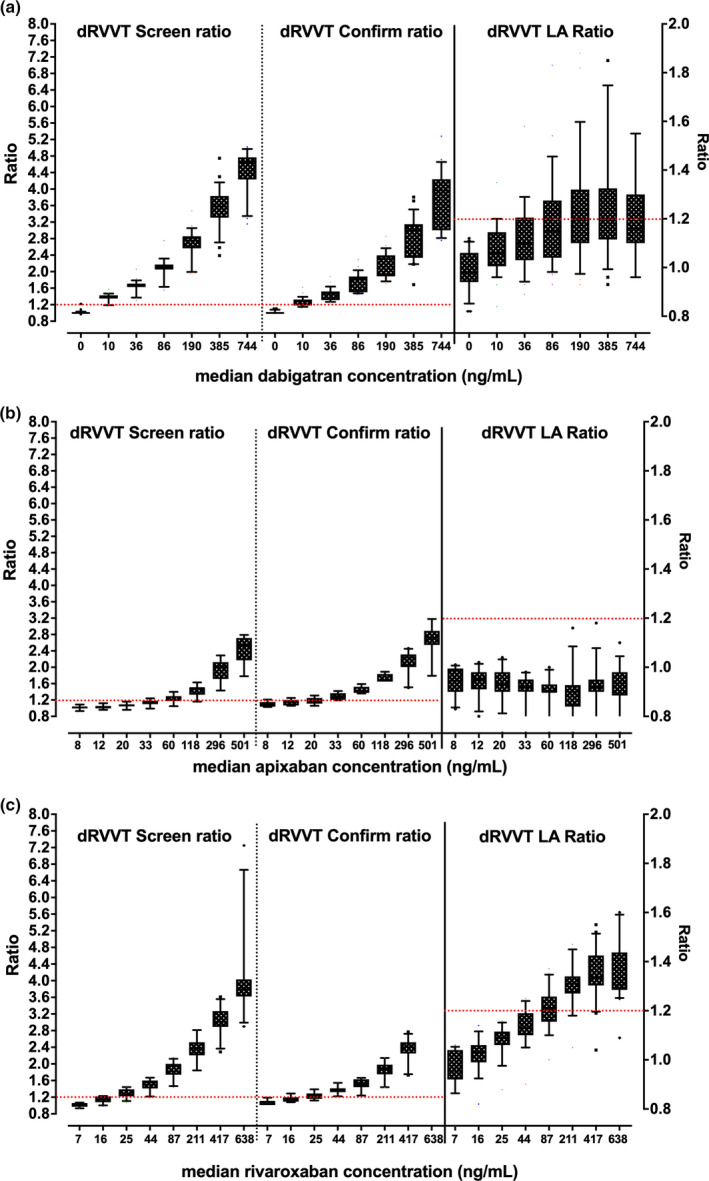
Summarizing the effect of direct oral anticoagulants (DOACs) on the dilute Russell's viper venom time (dRVVT). An original figure highlighting historical data in which the lead author performed in collaboration with the Royal College of Pathologists of Australasia Quality Assurance Program (RCPQAP) 64 , 65 and showing differential effects according to type of DOAC. Data shown as dRVVT screen and confirm ratios (left y‐axis) and arising dRVVT screen/confirm ratio (right y‐axis), using box and whiskers showing 10th–90th percentiles
FIGURE 3.
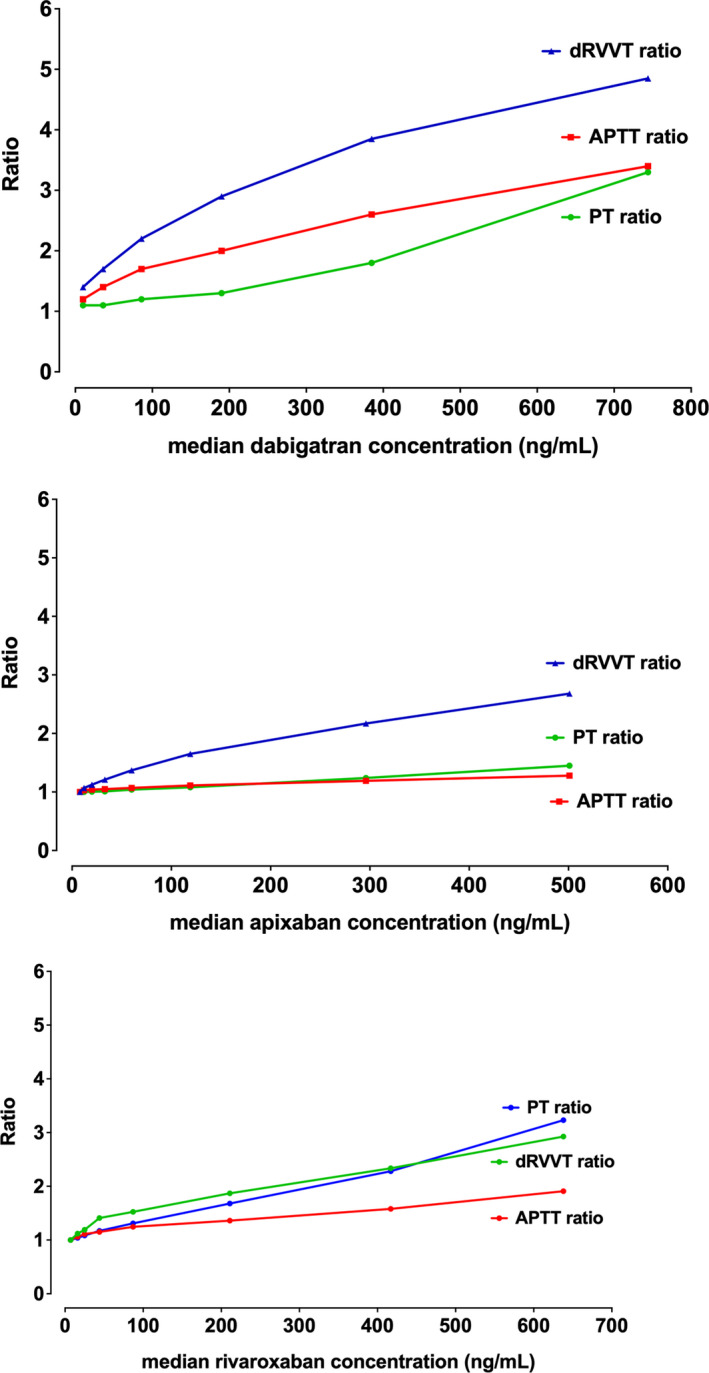
Summarizing the effect of direct oral anticoagulants (DOACs) on the various coagulation assays. An original figure highlighting historical data in which the lead author performed in collaboration with the Royal College of Pathologists of Australasia Quality Assurance Program (RCPQAP) 64 , 65 and showing differential effects on the three assays according to type of DOAC. Data shown as comparative activated partial thromboplastin time (aPTT), dilute Russell's viper venom time (dRVVT), and prothrombin time (PT) ratios. In general, a ratio above 1.2 can be considered as “abnormal”
In regard to DOAC neutralizers, our experience with DOAC‐Stop has shown several noteworthy findings related to LA testing, 33 as was also highlighted within the ISTH SSC guidance on LA detection in anticoagulated patients. 17 First, when rivaroxaban was added to pooled normal plasma, this (as expected) caused clotting time prolongation for most LA tests performed by participants of an external quality assessment program and generated falsely elevated dRVVT screen/confirm ratio results that mimicked the presence of LA. Second, when the rivaroxaban plasma sample was treated with DOAC‐Stop, results showed correction of the prolongation of the clotting time and the screen/confirm ratio for most LA tests. Notably, all study participants correctly identified the rivaroxaban plasma treated with DOAC‐Stop as LA‐negative. Third, andexanet‐alfa, an in vivo antidote for rivaroxaban, when added to the rivaroxaban plasma in vitro was able to correct the prolonged clotting time induced by rivaroxaban. It also corrected the screen/confirm ratio, but to such an extent (i.e., overcorrection) that such reduction in LA ratio could potentially lead to a false‐negative LA in those patients with weak positive LA while on rivaroxaban, should andexanet‐alfa be used as an in vitro DOAC neutralizer. Thus, in summary, andexanet‐alfa is not recommended as an in vitro DOAC neutralizer ahead of LA testing. The effect of in vivo use of andexanet‐alfa on LA test patterns from treated patients is to our knowledge unknown.
7. CONCLUSION
The investigation of LA represents a common activity for hemostasis laboratories. The presence of LA is detected (or excluded) by laboratory testing, with the aPTT and the dRVVT being most commonly used. Anticoagulants are commonly used to treat or manage thrombosis, which may include many patients being investigated for LA. All anticoagulants will affect the assays used to investigate LA, but to variable extent. Ideally, investigation of LA will occur at a time when patients are not on an anticoagulant. However, should this be unavoidable, there are several strategies available to mitigate anticoagulant interferences, including the use of various anticoagulant neutralizers. As an alternative to LA testing while on anticoagulant therapy, some authors instead advocate for performance of anti–phosphatidyl‐serine/prothrombin (aPS/PT) antibodies, which can be used as a surrogate test for LA and is not affected by anticoagulants. 66 , 67 , 68 The premise for such use is that most patients with APS and triple positivity are also positive in aPS/PT (tetra‐positive aPL), 67 and that aPS/PT more than aβ2GPI is responsible for LA activity in these patients. 68 Figure 4 provides an algorithm that summarises the sentiments expressed in this review, representing a potential approach to the investigation of LA when a patient is on anticoagulant therapy.
FIGURE 4.
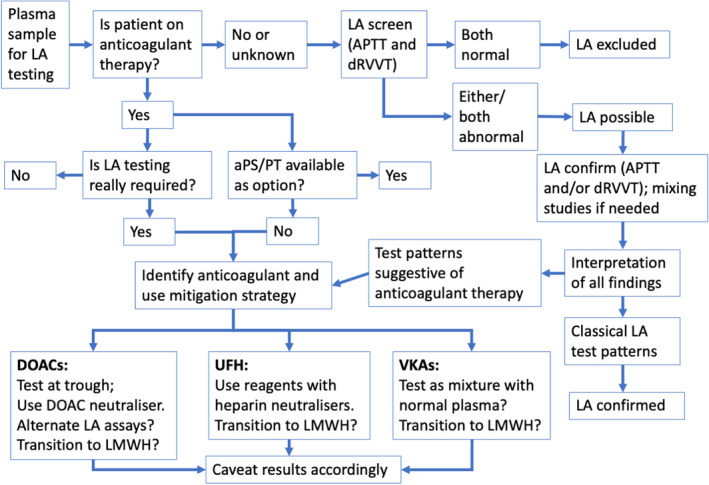
One potential algorithm to support the identification/exclusion of lupus anticoagulants from patients on anticoagulant therapy and applying some of the recommendations from the current review. The algorithm is based on the authors' personal preferences, but also considers options used by other workers in the field
RELATIONSHIP DISCLOSURE
The authors have no competing interests.
AUTHOR CONTRIBUTIONS
Emmanuel J. Favaloro wrote the original draft of this manuscript. Both authors contributed content, helped revise the manuscript, and approved its submission. The views expressed herein are those of the authors and are not necessarily those of NSW Health Pathology or the Royal College of Pathologists of Australasia Quality Assurance Program.
Supporting information
Table S1‐S2
ACKNOWLEDGMENTS
The authors thank various current and past employees of NSW Health Pathology and the Royal College of Pathologists of Australasia Quality Assurance Program for past contributions permitting reuse of some data for educational purposes in the current manuscript. There was no specific funding for this work. NSW Health Pathology, as the employer of the authors, is acknowledged for in‐kind support that permitted some allocation of time for its completion.
Favaloro EJ, Pasalic L. Lupus anticoagulant testing during anticoagulation, including direct oral anticoagulants. Res Pract Thromb Haemost. 2022;6:e12676. doi: 10.1002/rth2.12676
Handling Editor: Pantep Angchaisuksiri
DATA AVAILABILITY STATEMENT
Please contact the lead author.
REFERENCES
- 1. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295‐306. doi: 10.1111/j.1538-7836.2006.01753.x [DOI] [PubMed] [Google Scholar]
- 2. Conley CL. Disorders of the blood in disseminated lupus erythematosus. Am J Med. 1952;13(1):1‐2. [DOI] [PubMed] [Google Scholar]
- 3. Pengo V, Bison E, Denas G, Jose SP, Bracco A, Banzato A. The paradox of the lupus anticoagulant: history and perspectives. Semin Thromb Hemost. 2014;40(8):860‐865. doi: 10.1055/s-0034-1395158 [DOI] [PubMed] [Google Scholar]
- 4. Thachil J. Doctor, Do I have lupus? Time to reconsider the lupus anticoagulant terminology. Semin Thromb Hemost. 2021;47(7):892‐894. doi: 10.1055/s-0041-1728787 [DOI] [PubMed] [Google Scholar]
- 5. Devreese KMJ, de Groot PG, de Laat B, et al. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis: update of the guidelines for lupus anticoagulant detection and interpretation. J Thromb Haemost. 2020;18(11):2828‐2839. doi: 10.1111/jth.15047 [DOI] [PubMed] [Google Scholar]
- 6. Clinical and Laboratory Standards Institute (CLSI) . Laboratory testing for the lupus anticoagulant; approved guideline. CLSI document H60‐A. Wayne, PA: CLSI, 2014.
- 7. Moore GW. Alternative assays to dRVVT and aPTT for lupus anticoagulant detection. Am J Hematol. 2020;95(8):992‐998. doi: 10.1002/ajh.25836 [DOI] [PubMed] [Google Scholar]
- 8. Tripodi A, Chantarangkul V. Lupus anticoagulant testing: activated partial thromboplastin time (APTT) and silica clotting time (SCT). Methods Mol Biol. 2017;1646:177‐183. doi: 10.1007/978-1-4939-7196-1_15 [DOI] [PubMed] [Google Scholar]
- 9. Favaloro EJ, Pasalic L, Lippi G. Replacing warfarin therapy with the newer direct oral anticoagulants, or simply a growth in anticoagulation therapy? Implications for pathology testing. Pathology. 2017;49(6):639‐643. doi: 10.1016/j.pathol.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 10. Lippi G, Gosselin R, Favaloro EJ. Current and emerging direct oral anticoagulants: state‐of‐the‐art. Semin Thromb Hemost. 2019;45(5):490‐501. doi: 10.1055/s-0039-1692703 [DOI] [PubMed] [Google Scholar]
- 11. Gosselin RC, Adcock DM, Bates SM, et al. International Council for Standardization in Haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2018;118(3):437‐450. doi: 10.1055/s-0038-1627480 [DOI] [PubMed] [Google Scholar]
- 12. Douxfils J, Adcock D, Bates SM, et al. 2021 update of the International Council for Standardization in Haematology recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2021;121(8):1008‐1020. doi: 10.1055/a-1450-8178 [DOI] [PubMed] [Google Scholar]
- 13. Baluwala I, Favaloro EJ, Pasalic L. Therapeutic monitoring of unfractionated heparin ‐ trials and tribulations. Expert Rev Hematol. 2017;10(7):595‐605. doi: 10.1080/17474086.2017.1345306 [DOI] [PubMed] [Google Scholar]
- 14. Favaloro EJ, Kershaw G, Mohammed S, Lippi G. How to optimize Activated Partial Thromboplastin Time (APTT) testing: solutions to establishing and verifying normal reference intervals and assessing APTT reagents for sensitivity to heparin, lupus anticoagulant, and clotting factors. Semin Thromb Hemost. 2019;45(1):22‐35. doi: 10.1055/s-0038-1677018 [DOI] [PubMed] [Google Scholar]
- 15. Keeling D, Mackie I, Moore GW, Greer IA, Greaves M, British Committee for Standards in Haematology . Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157(1):47‐58. doi: 10.1111/j.1365-2141.2012.09037.x [DOI] [PubMed] [Google Scholar]
- 16. Arachchillage DRJ, Gomez K, Alikhan R, et al. Addendum to British Society for Haematology Guidelines on Investigation and Management of Antiphospholipid syndrome, 2012 (Br. J. Haematol. 2012; 157: 47‐58): use of direct acting oral anticoagulants. Br J Haematol. 2020;189(2):212‐215. doi: 10.1111/bjh.16308 [DOI] [PubMed] [Google Scholar]
- 17. Tripodi A, Cohen H, Devreese KMJ. Lupus anticoagulant detection in anticoagulated patients. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020;18(7):1569‐1575. doi: 10.1111/jth.14846 [DOI] [PubMed] [Google Scholar]
- 18. Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2009;7(10):1737‐1740. doi: 10.1111/j.1538-7836.2009.03555.x [DOI] [PubMed] [Google Scholar]
- 19. Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for the diagnosis of lupus anticoagulants: an update. On behalf of the Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the ISTH. Thromb Haemost. 1995;74:1185‐1190. [PubMed] [Google Scholar]
- 20. Exner T, Triplett DA, Taberner D, Machin SJ. Guidelines for testing and revised criteria for lupus anticoagulants. SSC Subcommittee for the Standardization of Lupus Anticoagulants. Thromb Haemost. 1991;65(3):320‐322. [PubMed] [Google Scholar]
- 21. Favaloro EJ, Wong RCW. Antiphospholipid antibody testing for the antiphospholipid syndrome: a synopsis of challenges and recent guidelines. Pathology. 2014;46(6):481‐495. doi: 10.1097/PAT.0000000000000142 [DOI] [PubMed] [Google Scholar]
- 22. Moore GW. Commonalities and contrasts in recent guidelines for lupus anticoagulant detection. Int J Lab Hematol. 2014;36(3):364‐373. doi: 10.1111/ijlh.12227 [DOI] [PubMed] [Google Scholar]
- 23. Moore GW. Recent guidelines and recommendations for laboratory detection of lupus anticoagulants. Semin Thromb Hemost. 2014;40(2):163‐171. doi: 10.1055/s-0033-1364185 [DOI] [PubMed] [Google Scholar]
- 24. Favaloro EJ. Anticoagulant therapy: present and future. Semin Thromb Hemost. 2015;41(2):109‐112. doi: 10.1055/s-0035-1546828 [DOI] [PubMed] [Google Scholar]
- 25. Favaloro EJ, Pasalic L, Lippi G. Oral anticoagulation therapy: an update on usage, costs and associated risks. Pathology. 2020;52(6):736‐741. doi: 10.1016/j.pathol.2020.05.006 [DOI] [PubMed] [Google Scholar]
- 26. Exner T, Michalopoulos N, Pearce J, Xavier R, Ahuja M. Simple method for removing DOACs from plasma samples. Thromb Res. 2018;163:117‐122. doi: 10.1016/j.thromres.2018.01.047 [DOI] [PubMed] [Google Scholar]
- 27. Jacquemin M, Toelen J, Feyen L, et al. The adsorption of dabigatran is as efficient as addition of idarucizumab to neutralize the drug in routine coagulation assays. Int J Lab Hematol. 2018;40(4):442‐447. doi: 10.1111/ijlh.12807 [DOI] [PubMed] [Google Scholar]
- 28. Kopatz WF, Brinkman HJM, Meijers JCM. Use of DOAC Stop for elimination of anticoagulants in the thrombin generation assay. Thromb Res. 2018;170:97‐101. doi: 10.1016/j.thromres.2018.08.014 [DOI] [PubMed] [Google Scholar]
- 29. Exner T, Ahuja M, Ellwood L. Effect of an activated charcoal product (DOAC Stop™) intended for extracting DOACs on various other APTT‐prolonging anticoagulants. Clin Chem Lab Med. 2019;57(5):690‐696. [DOI] [PubMed] [Google Scholar]
- 30. Platton S, Hunt C. Influence of DOAC Stop on coagulation assays in samples from patients on rivaroxaban or apixaban. Int J Lab Hematol. 2019;41(2):227‐233. doi: 10.1111/ijlh.12950 [DOI] [PubMed] [Google Scholar]
- 31. Ząbczyk M, Kopytek M, Natorska J, Undas A. The effect of DOAC‐Stop on lupus anticoagulant testing in plasma samples of venous thromboembolism patients receiving direct oral anticoagulants. Clin Chem Lab Med. 2019;57(9):1374‐1381. [DOI] [PubMed] [Google Scholar]
- 32. Exner T, Favresse J, Lessire S, Douxfils J, Mullier F. Clotting test results correlate better with DOAC concentrations when expressed as a "Correction Ratio"; results before/after extraction with the DOAC Stop reagent. Thromb Res. 2019;179:69‐72. [DOI] [PubMed] [Google Scholar]
- 33. Favaloro EJ, Gilmore G, Arunachalam S, Mohammed S, Baker R. Neutralising rivaroxaban induced interference in laboratory testing for lupus anticoagulant (LA): a comparative study using DOAC Stop and andexanet alfa. Thromb Res. 2019;180:10‐19. doi: 10.1016/j.thromres.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 34. Favresse J, Lardinois B, Sabor L, et al. Evaluation of the DOAC‐Stop® procedure to overcome the effect of DOACs on several thrombophilia screening tests. TH Open. 2018;2(2):e202‐e209. doi: 10.1055/s-0038-1657785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Slavik L, Jacova J, Friedecky D, et al. Evaluation of the DOAC‐stop procedure by LC‐MS/MS assays for determining the residual activity of dabigatran, rivaroxaban, and apixaban. Clin Appl Thromb Hemost. 2019;25:107602961987255. doi: 10.1177/1076029619872556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Favaloro EJ, Gilmore G, Bonar R, et al. Reducing the effect of DOAC interference in laboratory testing for factor VIII and factor IX: a comparative study using DOAC Stop and andexanet alfa to neutralize rivaroxaban effects. Haemophilia. 2020;26(2):354‐362. doi: 10.1111/hae.13930 [DOI] [PubMed] [Google Scholar]
- 37. Favaloro EJ, Gilmore G, Bonar R, et al. Laboratory testing for activated protein C resistance: rivaroxaban induced interference and a comparative evaluation of andexanet alfa and DOAC Stop to neutralise interference. Clin Chem Lab Med. 2020;58(8):1322‐1331. doi: 10.1515/cclm-2019-1160 [DOI] [PubMed] [Google Scholar]
- 38. De Kesel PM, Devreese KMJ. Direct oral anticoagulant adsorption: impact on lupus anticoagulant testing‐review of the literature and evaluation on spiked and patient samples. J Thromb Haemost. 2020;18(8):2003‐2017. doi: 10.1111/jth.14894 [DOI] [PubMed] [Google Scholar]
- 39. Monteyne T, De Kesel P, Devreese KMJ. Interference of DOAC stop and DOAC remove in the thrombin generation assay and coagulation assays. Thromb Res. 2020;192:96‐99. doi: 10.1016/j.thromres.2020.04.044 [DOI] [PubMed] [Google Scholar]
- 40. Riva N, Vella K, Hickey K, et al. The effect of DOAC‐Stop on several oral and parenteral anticoagulants. Int J Lab Hematol. 2021;43(4):O171‐O175. doi: 10.1111/ijlh.13487 [DOI] [PubMed] [Google Scholar]
- 41. Baker SA, Jin J, Pfaffroth C, Vu T, Zehnder JL. DOAC‐Stop in lupus anticoagulant testing: direct oral anticoagulant interference removed in most samples. Res Pract Thromb Haemost. 2021;5(2):314‐325. doi: 10.1002/rth2.12472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Úlehlová J, Piskláková B, Ivanovová E, et al. Evaluation of the determination of dabigatran, rivaroxaban, and apixaban in lupus anticoagulant‐positive patients. Diagnostics (Basel). 2021;11(11):2027. doi: 10.3390/diagnostics11112027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dirienzo L, Vitulli A, Mancazzo F, et al. Differential effect of direct oral anticoagulants on thrombin generation and fibrinolysis in patients with atrial fibrillation and venous thromboembolism. Blood Transfus. 2021. doi: 10.2450/2021.0153-21. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cox‐Morton S, MacDonald S, Thomas W. A diagnostic solution for haemostasis laboratories for patients taking direct oral anticoagulants using DOAC‐Remove. Br J Haematol. 2019;187(3):377‐385. doi: 10.1111/bjh.16091 [DOI] [PubMed] [Google Scholar]
- 45. Kopytek M, Ząbczyk M, Malinowski KP, Undas A, Natorska J. DOAC‐Remove abolishes the effect of direct oral anticoagulants on activated protein C resistance testing in real‐life venous thromboembolism patients. Clin Chem Lab Med. 2020;58(3):430‐437. doi: 10.1515/cclm-2019-0650 [DOI] [PubMed] [Google Scholar]
- 46. Jourdi G, Delrue M, Stepanian A, et al. Potential usefulness of activated charcoal (DOAC remove®) for dRVVT testing in patients receiving Direct Oral AntiCoagulants. Thromb Res. 2019;184:86‐91. doi: 10.1016/j.thromres.2019.11.001 [DOI] [PubMed] [Google Scholar]
- 47. Jilma‐Stohlawetz P, Lysy K, Sunder‐Plassmann R, et al. Limitations of a calibrated, quantitative APC‐R assay under routine conditions. Int J Lab Hematol. 2021;43(2):318‐323. doi: 10.1111/ijlh.13378 [DOI] [PubMed] [Google Scholar]
- 48. Favre R, Zia‐Chahabi S, Talb Y, de Gunzburg N, Flaujac C. Direct oral anticoagulant neutralization by activated charcoal DOAC‐remove for thrombophilia screening. Blood Coagul Fibrinolysis. 2021;32(5):356‐358. doi: 10.1097/MBC.0000000000001040 [DOI] [PubMed] [Google Scholar]
- 49. Skaugen JM, Sayre C, Hassett AC, et al. Performance characteristics of DOAC‐remove for neutralization of the effects of apixaban and rivaroxaban in lupus anticoagulant assays. Am J Clin Pathol. 2021:aqab149. doi: 10.1093/ajcp/aqab149. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 50. Al‐Qawzai Z, Dale C, Dave M, Yartey N, Platton S. Effect of DOAC‐Remove on coagulation screening assays in samples from patients receiving oral or parenteral anticoagulation. Int J Lab Hematol. 2021. doi: 10.1111/ijlh.13743. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 51. Farkh C, Ellouze S, Gounelle L, et al. A diagnostic solution for lupus anticoagulant testing in patients taking direct oral FXa inhibitors using DOAC filter. Front Med (Lausanne). 2021;31(8):683357. doi: 10.3389/fmed.2021.683357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sevenet PO, Cucini V, Hervé T, et al. Evaluation of DOAC Filter, a new device to remove direct oral anticoagulants from plasma samples. Int J Lab Hematol. 2020;42(5):636‐642. doi: 10.1111/ijlh.13267 [DOI] [PubMed] [Google Scholar]
- 53. Bouvy C, Evrard J, Siriez R, et al. P220: Removal of DOACs from plasma: performance comparison and pre‐analytical considerations of three different devices. ECTH 2018 Abstract Book 2018.
- 54. Gheldof D, Delvigne AS, Bouvy C, Douxfils J, Dogné JM. A rapid, practical and ergonomic device to prepare plasma sample free of platelets and direct oral anticoagulant for routine hemostasis tests. PB0141. Res Pract Thromb Haemost. 2019;1(3):139‐140. [Google Scholar]
- 55. Frans G, Meeus P, Bailleul E. Resolving DOAC interference on aPTT, PT, and lupus anticoagulant testing by the use of activated carbon. J Thromb Haemost. 2019;17(8):1354‐1362. doi: 10.1111/jth.14488 [DOI] [PubMed] [Google Scholar]
- 56. Exner T, Rigano J, Favaloro EJ. The effect of DOACs on laboratory tests and their removal by activated carbon to limit interference in functional assays. Int J Lab Hematol. 2020;42(suppl 1):41‐48. doi: 10.1111/ijlh.13196 [DOI] [PubMed] [Google Scholar]
- 57. Darlow J, Mould H. Thrombophilia testing in the era of direct oral anticoagulants. Clin Med (Lond). 2021;21(5):e487‐e491. doi: 10.7861/clinmed.2020-1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Siriez R, Dogné JM, Gosselin R, Laloy J, Mullier F, Douxfils J. Comprehensive review of the impact of direct oral anticoagulants on thrombophilia diagnostic tests: practical recommendations for the laboratory. Int J Lab Hematol. 2021;43(1):7‐20. doi: 10.1111/ijlh.13342 [DOI] [PubMed] [Google Scholar]
- 59. Moser KA, Smock KJ. Direct oral anticoagulant (DOAC) interference in hemostasis assays. Hematology. 2021;2021(1):129‐133. doi: 10.1182/hematology.2021000241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gosselin RC. Review of coagulation preanalytical variables with update on the effect of direct oral anticoagulants. Int J Lab Hematol. 2021;43(suppl 1):109‐116. doi: 10.1111/ijlh.13585 [DOI] [PubMed] [Google Scholar]
- 61. Favaloro EJ, Mohammed A, Coombs R, Mehrabani PA. Filtered plasma as a potential cause of clinical misdiagnosis: inappropriate testing in a haematology laboratory. Br J Biomed Sci. 1995;52:243‐248. [PubMed] [Google Scholar]
- 62. Wong RC, Adelstein S, Gillis D, Favaloro EJ. Development of consensus guidelines for anticardiolipin and lupus anticoagulant testing. Semin Thromb Hemost. 2005;31(1):39‐48. doi: 10.1055/s-2005-863804 [DOI] [PubMed] [Google Scholar]
- 63. Favaloro EJ, Mohammed S, Curnow J, Pasalic L. Laboratory testing for lupus anticoagulant (LA) in patients taking direct oral anticoagulants (DOACs): potential for false positives and false negatives. Pathology. 2019;51(3):292‐300. doi: 10.1016/j.pathol.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 64. Bonar R, Favaloro EJ, Mohammed S, Pasalic L, Sioufi J, Marsden K. The effect of dabigatran on haemostasis tests: a comprehensive assessment using in‐vitro and ex‐vivo samples. Pathology. 2015;47(4):355‐364. doi: 10.1097/PAT.0000000000000252 [DOI] [PubMed] [Google Scholar]
- 65. Bonar R, Favaloro EJ, Mohammed S, et al. The effect of the direct factor Xa inhibitors apixaban and rivaroxaban on haemostasis tests: a comprehensive assessment using in vitro and ex vivo samples. Pathology. 2016;48(1):60‐71. doi: 10.1016/j.pathol.2015.11.025 [DOI] [PubMed] [Google Scholar]
- 66. Pengo V. Additional laboratory tests to improve on the diagnosis of antiphospholipid syndrome: response from Pengo. J Thromb Haemost. 2020;18(11):3118‐3119. doi: 10.1111/jth.15026 [DOI] [PubMed] [Google Scholar]
- 67. Pengo V, Del Ross T, Ruffatti A, et al. Lupus anticoagulant identifies two distinct groups of patients with different antibody patterns. Thromb Res. 2018;172:172‐178. doi: 10.1016/j.thromres.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 68. Cattini MG, Bison E, Pontara E, Cheng C, Denas G, Pengo V. Tetra positive thrombotic antiphospholipid syndrome: major contribution of anti phosphatidyl‐serine/prothrombin antibodies to Lupus anticoagulant activity. J Thromb Haemost. 2020;18(5):1124. doi: 10.1111/jth.14765 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2
Data Availability Statement
Please contact the lead author.


