Abstract
Bacteroides thetaiotaomicron is a human gut bacterium that holds promise for delivery of therapies in the gut microbiome1. Therapeutic bacteria would benefit from the ability to turn on different programs of gene expression in response to conditions inside and outside of the gut; however, the availability of regulatory parts and methods to combine them have been limited in B. thetaiotaomicron2-5. We report implementation of Cello circuit design automation software6 for this species. First, we characterize a set of genome-integrated NOT/NOR gates based on sgRNAs (CRISPR/Cas9) to inform a Bt user constraint file (UCF) for Cello. Then, logic circuits are designed to integrate sensors that respond to bile-acid and anhydrotetracycline (aTc), including one created to distinguish between environments associated with bioproduction, the human gut, and after release. This circuit was found to be stable under laboratory conditions for at least 12 days and function in bacteria associated with a primary colonic epithelial monolayer in an in vitro human gut model system.
Engineered bacteria can function as “smart” therapeutics to deliver a drug or vaccine, to detect or prevent infections, and to respond to physiological changes7. Although it is possible to express a therapeutic payload continuously, it would be useful if bacteria could be engineered to respond to environmental signals, for example to trigger drug release in a particular microenvironment, maintain a treatment at specific levels, or to switch a therapy on or off in the body8-11. Bacteroides are Gram-negative obligate anaerobes that comprise a third of the bacteria found in fecal material12-19. They are attractive candidate vectors for therapies due to their stable colonization of the mammalian colon and modulation of the host immune system. They have been studied as treatments of colitis and autism and strains have been engineered to improve engraftment and bio-containment20-22. Tools for genetic manipulation have been reported2-4,23, but predictively combining these parts to create more complex functions, including genetic circuits, remains a challenge.
Bacteria residing in the body can be engineered to respond to in situ conditions, small molecules present in food or water, and distinguish disease states, including inflammation, fever, intestinal bleeding, tumors, and pathogens2,3,20,24-34. Small molecule sensors have been developed for Bacteroides species to induce expression when colonized in a host2,3,20. As an endogenous signal, bile acids offer a means to identify regions within the gut. Gradients of bile acid variants are formed as they are released into the duodenum and are chemically modified by bacteria as they transit the length of the intestines35-40. The human large intestine has ~500 mM bile acids, a third of which is deoxycholic acid (DCA)41. Changes in bile acid composition have been correlated with the onset of colon/liver cancer, genetic disorders, obesity, diabetes, and inflammation36,42-44. Some pathogenic bacteria use TetR homologues to respond to bile acids45-47. Based on a bacterial regulator, a synthetic bile acid sensor has been constructed in mammalian cells48.
Sensors alone can only produce a simple response. Genetic circuits can implement more complex signal processing, for example, logic to identify an environment, periodic drug delivery, diagnostic memory, or communicating to control toxin release4,26,32,49,50. Cello (www.cellocad.org) was developed to automate circuit design and make it easier to incorporate synthetic regulation into genetic engineering projects6. The inputs to Cello are the sensors, the desired circuit function (in Verilog), and a “User Constraint File” (UCF) that specifies organism, genetic location, gate technology and mapping constraints51. The first UCF (Eco1C1G1T1) is specific to E. coli NEB10β, for circuits on a p15a plasmid, and contains 12 repressor-based NOR/NOT gates. Cello uses the response function of the gates (how the output promoter changes as a function of the input promoter) to connect them to create the specified circuit. Connecting gates to each other and to sensors requires that their inputs/outputs share a common signal carrier, defined to be the RNA polymerase (RNAP) flux and measured in relative promoter units (RPUs) as a ratio to a reference promoter52. Designed circuits function as specified in E. coli; however, it is not possible to simply use Eco1C1G1T1 for other bacterial species.
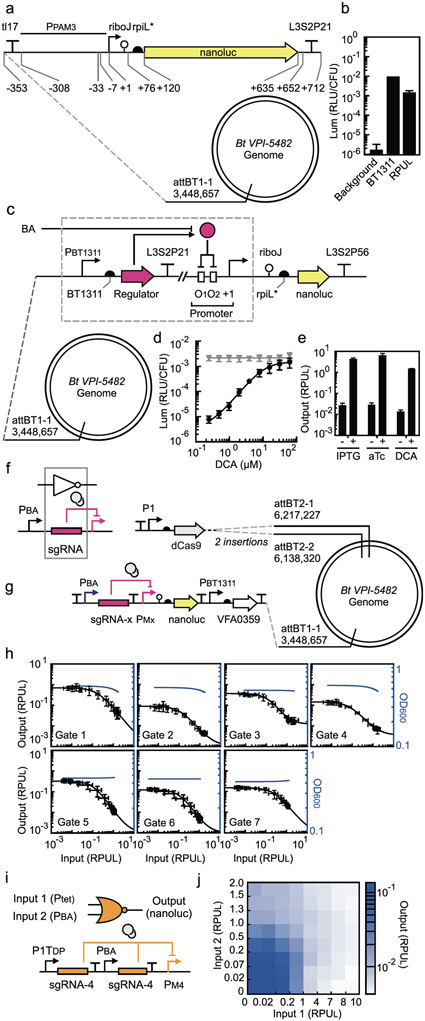
The first step in implementing circuit design automation for a new host is to define a reference promoter to use when characterizing sensor and gate promoters in RPUs. E. coli promoters do not work well in Bacteroides, so we selected a different constitutive promoter for this purpose (PPAM3) and a reference strain was constructed (B. thetaiotaomicron strain MT768) (Figure 1a and Supplementary Figure S1)2. As green fluorescent protein (GFP) requires oxygen for chromophore maturation and is only visible in Bacteroides when highly expressed4 we opted to use luciferase (nanoluc) as our reporter gene53. Luminescence from PPAM3 is measured and normalized by cell density (Online Methods). The resulting value is defined as RPUL = 1 and used to normalize the activity of other promoters (Figure 1b).
Figure 1: Sensor, NOT and NOR gate characterization in B. thetaiotaomicron.
(a) Schematic of the promoter standard (PPAM3) used to measure RPUL (B. thetaiotaomicron MT768). (b) The luminescence activity of the strong promoter PBT1311 (sourced from ref28) and the background luminescence from B. thetaiotaomicron (strain MT767) is compared to the RPUL standard (Online Methods). (c) The design of the bile acid sensors and its location in the genome (B. thetaiotaomicron MT329) is shown. The “regulator” gene represents the bile acid binding proteins tested and the corresponding operator is placed in two locations in the promoter. (d) The response of the bile acid sensor to DCA is shown (Online Methods). The grey line is the response of the parent promoter PcfxA. (e) The responses of the sensors to no inducer and the presence of inducer (500 μM IPTG, 100 ng/μL aTc, and 62.5 μM DCA) are shown (B. thetaiotaomicron MT770, MT771, and MT772) (Online Methods). These values are used as the inputs to Cello for circuit design. For (a), (b), and (c), the sequences of genetic parts are provided in Supplementary Table S4. The data represent the average of three replicates collected on different days and error bars correspond to the standard deviation between these measurements. (f) The inverter function is performed by dCas9 (grey circles) directed to a promoter by a sgRNA. The output promoter of the bile acid sensor (PBA) is used to characterize gate function. (g) The characterization system for measuring the response functions of NOT gates is shown. dCas9 is constitutively expressed from the P1 promoter. The bile acid sensor is used to turn on the NOT gate (PBA). (Note that the strains also contain the aTc- and IPTG- sensors that are removed here for clarity, see Supplementary Figure S13). Each NOT gate (x from 1 to 7) required the construction of a different strain for characterization: B. thetaiotaomicron MT773-MT775, MT782, MT804-MT806. The sequences of the NOT gates, including all component parts, are provided in Supplementary Table S5. (h) The response function for each NOT gate is shown (Online Methods). The input to the gate is the bile acid sensor induced by DCA (left to right): 0, 0.4, 0.6, 1.0, 1.7, 2.9, 4.9, 8.1, 13.5, 22.5, 37.5, and 62.5 μM. The input (x-axis) is converted to RPUL by separately measuring the activity of PBA for these concentrations (Supplementary Figure S14). The black lines are fits to the Hill function (parameters in Table 1). The blue lines show the impact of gate induction on cell growth (a regression curve is shown, fit to the OD600 data in Supplementary Figure S15). The data represent the average of three replicates collected on different days, and error bars correspond to the standard deviation between these measurements. (i) The “split” design of the gate is shown, where each input promoter drives a separate copy of the same gRNA. (j) The luminescence of the NOR gate is shown as a function of the activity of the two input promoters. The bile acid sensor was converted from μM DCA to RPUL as described in part (h) (bottom to top, 0, 0.9, 1.9, 3.9, 7.8, 15.6, 31.25, 62.5 μM DCA). The second input was induced by aTc and converted to RPUL similarly (left to right, 0, 0.4, 1.0, 2.6, 6.4, 16, 40, 100 ng/μL of aTc) (Supplementary Figure S19). The data represent the average of three replicates collected on different days.
Next, we constructed a bile acid sensor. First, we compiled a list of known and putative repressors that respond to bile acids, including CmeR (C. jejuni) and BreR (Vibrio cholera) and six putative regulators (VFA0359, BCAS0007, Bamb6005, VFA0088, Smlt2112, and Xcc0233)45,47,48,54,55. A sensor was constructed based on each repressor by placing the gene under the control of a constitutive promoter (Figure 1c). An output promoter was designed by placing the operator sequence between the −33 and −7 region of a constitutive promoter (PcfxA). The sensors were integrated into attBT1-1. Cells were grown in the presence and absence of 62.5 μM DCA for six hours in TYG media under anaerobic conditions and then the promoter activity was measured (Supplementary Figure S2 and Online Methods). The sensor based on VFA0359 produced a superior response, with a low basal activity, 440-fold dynamic range, and half-maximum at 8 μM DCA (Figure 1d and Supplementary Figure S3). This sensor was evaluated for its response to other bile acids and it was found DCA > LCA > CDCA > CA (Supplementary Figure S4 and Supplementary Table 1). No response for conjugated bile acids (TCA and GCA) was observed (not shown).
We tested the VFA0359 bile acid sensor for crosstalk with the aTc- and IPTG- sensors previously developed for B. thetaiotaomicron2,3. Each sensor was divided such that the repressors and the output promoter/reporter were integrated into different locations in the B. thetaiotaomicron genome. All three repressors were transcribed from the PBT1311 constitutive promoter and the cassette was inserted into attBT-1. The output promoters (PBA, PLacO23, and P1TDP) drive nanoluc expression and are integrated at one (DCA) or two (IPTG and aTc) sites in the genome. The sensors were evaluated for their response to DCA, IPTG, and aTc and no crosstalk was observed (Figure 1e, Supplementary Figure S5-S7). There is also no crosstalk between repressors and promoters (Supplementary Figure S5-S7).
Genetic circuits can be constructed by combining gates that perform simpler logic operations. We chose to base the gate technology on the 2-input NOR function, which has two input promoters and one output promoter. Previously, we used protein repressors to implement the inversion function56, but we found these do not produce sufficient dynamic range when moved to the B. thetaiotaomicron genome (not shown). As an alternative, we implement this function using CRISPRi by having a small guide RNA (sgRNA) direct deactivated Cas9 (dCas9) to repress a target promoter (Figure 1f). This approach has been used previously by ourselves and others to build genetic logic in E. coli and S. cerevisiae2,57-60. While toxicity has been observed when dCas9 is expressed in E. coli58, no growth impact is observed in B. thetaiotaomicron (Supplementary Figure S8).
Each gate is based on a different sgRNA. The sgRNAs were designed by randomizing the DNA recognition sequence and computationally scanning the genome to ensure its absence (Online Methods). Repressible promoters were designed by placing the corresponding target sequence in different positions in PBF2884 and screening for the highest repression, which occurs just upstream of the −7 position (PMsite3) (Supplementary Figure S9). When 62.5 μM DCA is added to induce sgRNA transcription, this leads to a 20-fold reduction in luminescence (Online Methods). Additional sgRNAs were designed to create an orthogonal set of seven gates (Supplementary Figure S10 and S11) (Online Methods). While a much larger library is possible, we found in E. coli that additional sgRNAs above 7 cannot be used in a single circuit without significantly drawing down the dCas9 resource and reducing the gate response to <10-fold58,61.
Gates require strong terminators to avoid read-through to other gates or chromosomal genes. In addition, each gate should use a different terminator to avoid repeated DNA sequences that might cause recombination while constructing or carrying the circuit. Terminators have not been well-characterized in Bacteroides, so we chose several strong terminators from an E. coli library62 and designed a system to quantify their strength in B. thetaiotaomicron (Online Methods and Supplementary Figure S12).
NOT gates were constructed based on the sgRNAs, promoters, and terminators. The expression of dCas9 is controlled by the constitutive P1 promoter (Figure 1g and Supplementary Figure S13). The response function of each gate was measured by increasing the concentration of DCA and measuring the response of the gate output promoter in RPUL (Figure 1h and Supplementary Figures S14, S15) (Online Methods). However, in order to predict how to connect gates, the input also has to be reported in RPUL, not DCA concentration. To correct the response functions, a separate strain (B. thetaiotaomicron strain MT772) was constructed to measure the activity of the PBA promoter as a function of DCA and these data are used to correct the x-axes of the response functions (Supplementary Figure S14). The corrected response functions were fit to a mathematical form, from which the parameters extracted (Table 1).
Table 1:
Response function parameters for the NOT gates
| Parametersa | ||||
|---|---|---|---|---|
| Gates | y max | y min | K | n |
| 1 | 0.70 | 0.001 | 0.19 | 1.3 |
| 2 | 0.09 | 0.001 | 0.11 | 1.3 |
| 3 | 0.36 | 0.011 | 0.13 | 1.5 |
| 4 | 0.15 | 0.003 | 0.08 | 1.2 |
| 5 | 0.33 | 0.001 | 0.11 | 1.5 |
| 6 | 0.12 | 0.001 | 0.11 | 1.5 |
| 7 | 0.15 | 0.001 | 0.27 | 1.3 |
The mathematical form of the response function is y = ymin + (ymax-ymin)Kn/(xn+Kn), where x is the input and y is the output (in RPU).
The NOT gates can be converted to NOR gates by adding a second input promoter. To avoid the roadblocking that can occur with tandem promoters23, we opted to construct NOR gates by creating a duplicate copy of the sgRNA (and its associated terminator) and placing each under the control of a different input promoter (Figure 1i and Supplementary Figure S16, S17). NOR gates are characterized by a 2-dimensional response function (Figure 1j), but the design automation algorithm is simplified if it can be based on their 1-dimensional NOT response functions (Figure 1h) and using the sum of the input promoter activities. Experiments were designed to test whether this is sufficiently accurate and, indeed, the NOT gate response functions can be used (Supplementary Figure S16).
The DNA sequence, parameterization of the response function, and growth impact (OD600) of each gate were combined to build a UCF for B. thetaiotaomicron (Bth1C1G1T1, Supplementary File 1). The specified organism is B. thetaiotaomicron VPI-5482 with a cassette in attBT2-1 and attBT2-2 for the constitutive expression of dCas9. EUGENE rules are included in the UCF to specify that the gates appear in the forward direction upstream of the output (Supplementary Figure S18). A final OR gate is implemented by splitting the last promoter to drive two copies of the output gene (e.g., nanoluc). The UCF specifies that the circuit be inserted into attBT1-1.
Cello was used to design logic circuits that can integrate the bile acid and aTc sensors. The logic operation can be specified in Verilog, either as a desired truth table or a specific wiring diagram. Once run, algorithms optimize the assignment of sgRNAs to each gate and then assemble the sequences to form the linear DNA encoding the circuit. Predictions are made for the expression of the output gene in response to all combinations of inputs (the presence and absence of aTc and DCA). Because the response functions are based on luciferase measurements, they represent cell population averages.
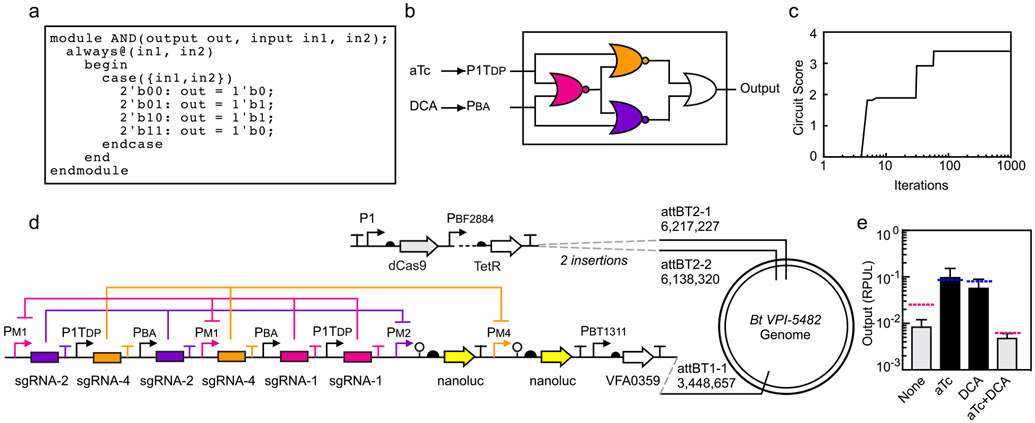
First, we designed a circuit that implements XOR logic: the output is on when either, but not both, inputs are on. Based on the truth table, written in Verilog (Figure 2a), a logic minimization algorithm identifies the optimal wiring diagram (Figure 2b). Then, the response functions of the sgRNA gates are used to calculate the optimal sgRNA for each position of the wiring diagram. This search is performed using a simulated annealing algorithm that seeks to maximize the difference between the highest output that should be off and the lowest output that should be on (Circuit Score) (Figure 2c). Then, the EUGENE rules are used to convert the assignment to a linear DNA sequence (Figure 2d). The predicted responses are shown as the colored dashed lines in Figure 2e. Note that these predictions were made prior to the construction of the circuit. The B. thetaiotaomicron strain containing the circuit was constructed exactly as specified. The response to the different combinations of inducers was measured and found to match closely to the predicted values (Figure 2e).
Figure 2: Automated design of an XOR circuit.
(a) The XOR gate is specified using the Verilog description language. (b) Cello identifies the wiring diagram of NOR/OR gates that produces the XOR function. The two sensors are defined (aTc and DCA) as well as their OFF/ON states in RPUL (Figure 1e). Each gate is then assigned a sgRNA (colors). (c) The simulated annealing run for gate assignment is shown. Circuit score is defined by the lowest output state that should be on divided by the highest output state that should be off. Different sgRNAs are permuted between gates until it converges on the solution shown in part b. (d) The genome organization of the circuit, as specified by Cello. Note that the IPTG sensor is also present at the attBT2-1/-2 sites (dots, see Supplementary Figure S20). (e) The colored lines show the predicted response to different combinations of inputs (the presence and absence of 100 ng/μL aTc and 62.5 μM DCA). The lines are colored blue for the on states and red for the off states. The predictions are made by Cello by mathematically combining the gate response functions. The experimental measurements are shown as grey and black bars for the off and on states, respectively (Online Methods). The data represent the average of three replicates collected on different days and error bars correspond to the standard deviation between these measurements.
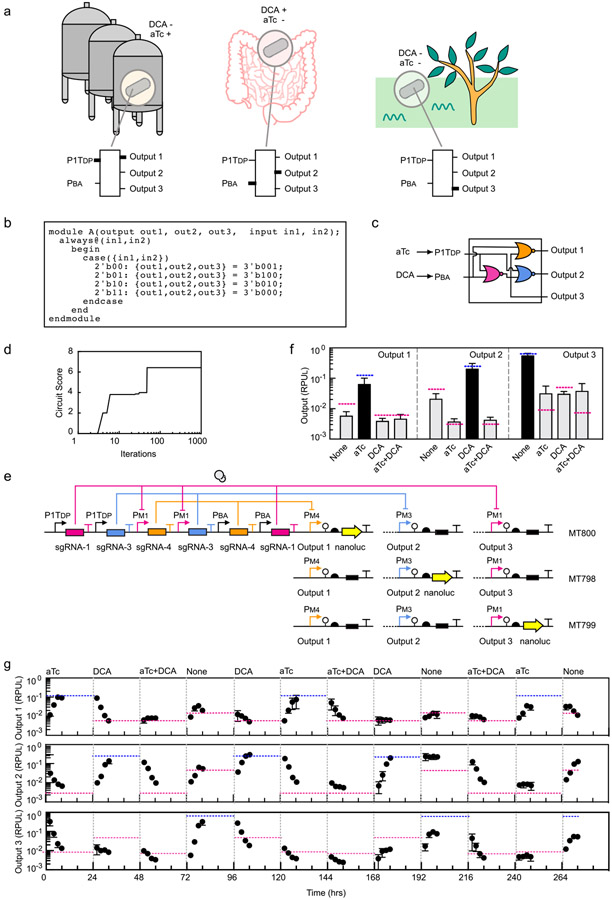
Therapeutic bacteria would benefit from the ability to turn on different programs of gene expression at different stages. This can be achieved by multi-input multi-output logic that integrate a set of sensors to identify the environment. As a proof-of-principle, we envisioned a circuit designed to respond to signals corresponding to the conditions of a fermenter (inducer, but no bile acid), the gut environment (bile acid, but no inducer), and when expelled into the environment post-treatment (no inducer and no bile acid) (Figure 3a). Each of these is assigned a different output promoter that could then be used to control desired genes in each of these conditions. The desired logic was written in Verilog (Figure 3b) and Cello designed the corresponding circuit and DNA sequence (Figure 3c and 3d). To measure the outputs using the same reporter, three strains were constructed connecting each output promoter to nanoluc. Note that all three strains have the same circuit and all three output promoters, but null DNA sequences replace nanoluc for those not being measured (Figure 3e). The output of the circuit closely matches the predictions across all 12 states representing the 4 combinations of inputs and 3 outputs (Figure 3f).
Figure 3: A circuit designed to integrate two sensors and control three programs of gene expression.
(a) The hypothetical inspiration for the circuit design is illustrated (see main text). An output promoter for each combination of inputs allows different genes to be expressed under these conditions. The thick lines in the circuit indicate when the input promoters (aTc, P1TDP; DCA, PBA) or outputs are on. (b) The Verilog specification of the truth table corresponding to the desired logic is shown. (c) Logic minimization algorithms identified the circuit diagram to produce the desired truth table using NOR gates. (d) The simulated annealing run in Cello assigned sgRNAs to each gate. The best set found are shown in part c. (e) The circuit was constructed as specified and inserted into the genome at the same position as shown in Figure 2d. The same sensors and dCas9 cassette are inserted into the attBT2-1/-2 sites (Supplementary Figure S20). Three strains were constructed to report on the three output promoter activities (Output 1, Bt MT800; Output 2, Bt MT798; Output 3, Bt MT799). The black box indicates that the sequence was replaced with a null ATGTAA for promoters that are not being measured. (f) Outputs of the 2-input 3-output circuits are shown. The predicted values for the three outputs are shown as blue (high) or red (low) lines. The inputs are the presence or absence of 100 ng/μL aTc and 62.5 μM DCA. The bars show the experimentally measured outputs expected to be low (grey) or high (black). The data represent the average of three replicates collected on different days and error bars correspond to the standard deviation between these measurements. (g) A timecourse is shown where the circuit is switched between states over more than two weeks (Online Methods). Each of the outputs was measured using the three reporter strains. The grey lines indicate when the transitions between inducer combinations occurred and the concentrations used are the same as above. The blue and red dashed lines are the steady-state values predicted by Cello for each combination of inputs.
To assess the long-term genetic stability of our circuits, strains were grown for 12 days and switched between states in a random progression in an anaerobic chamber. Cells were not carefully maintained in exponential phase during this growth experiment, with periods of being left in the anaerobic chamber for extended times. This was done to stress the cells and to passage them through different growth conditions and nutrient levels. The circuits performed as predicted during this period, with no signs of breakage (Figure 3g). The characteristic times for the circuit to go from off→on and on→off state are ~6 hours.
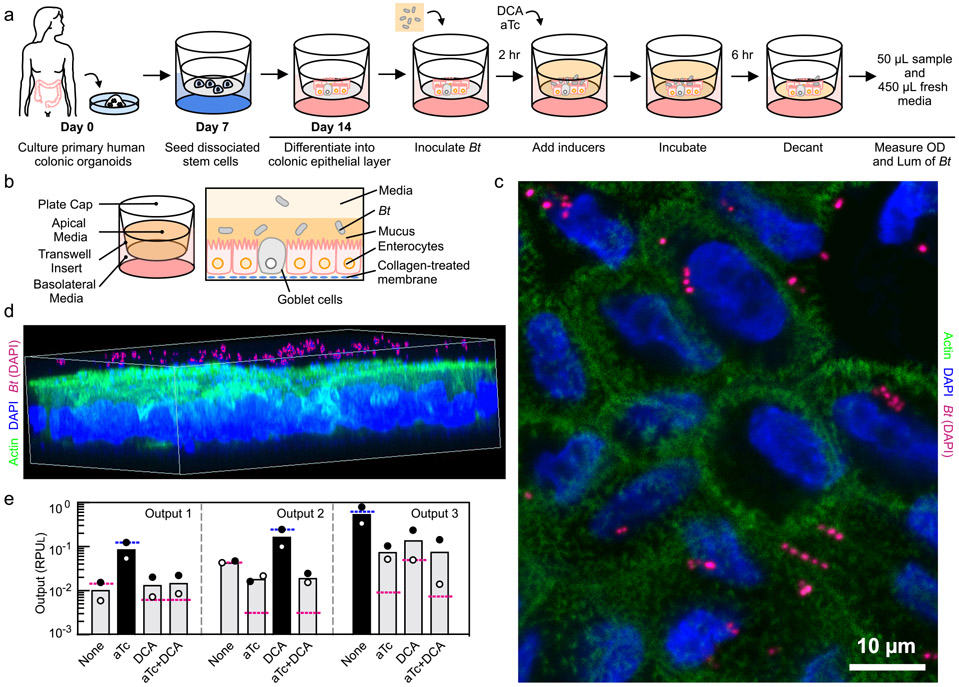
A human gastrointestinal model63 was used to test circuit function in the context of B. thetaiotaomicron cells adhered to a human epithelial monolayer. The monolayer covering the intestinal surface is faithfully recapitulated using primary colonic stem cell organoids that differentiate into absorptive, goblet, and enteroendocrine cells (Figure 4a) (Online Methods)64. Unlike models derived from cancer cell lines, the mucosal layer is secreted from goblet cells and more accurately reflects the coating of the human epithelium to which B. thetaiotaomicron naturally adheres (Figure 4b)65. Upon adhering, B. thetaiotaomicron undergoes global changes in its transcriptome, seeks alternative carbon sources, and slows in growth65. Molecules secreted by cells in the epithelial layer, such as antimicrobial peptides, also impact the B. thetaiotaomicron transcriptome66. Using the gastrointestinal model allows us to prototype the performance of circuits in a realistic model of this cellular and environmental context.
Figure 4: Coculture of B. thetaiotaomicron with colonic epithelial monolayer.
(a) Schematic description of the steps to perform the B. thetaiotaomicron (MT798, MT799 and MT800; see Table S2) and colonic epithelia coculture experiment (Online Methods). (b) An illustration of the human colonic epithelia and B. thetaiotaomicron co-culture system. (c) Top view of the monolayer stained with DAPI (blue) and anti-Actin (green). B. thetaiotaomicron was false colored from blue to pink to highlight their location (see Supplementary Figure S21 for original image). (d) 3D rendering of the monolayer stained with DAPI and anti-Actin (See Supplementary Figure S22 for original image). (e) Outputs of the 2-input 3-ouput circuit harbored by B. thetaiotaomicron co-cultured with human colonic epithelia are shown. The predicted values for the three outputs are shown as blue (high) or red (low) lines. Inputs are the presence or absence of 100 ng/μL aTc and 62.5 μM DCA. The bars show the experimentally measured outputs expected to be low (grey) or high (black). The data represent the average of two replicates collected on different days and white/black circles correspond to each measurement.
Circuit performance was evaluated using the gastrointestinal model for the 2-input 3-output logic circuit. The epithelial monolayer is lined on a transwell insert with growth-supporting media for mammalian cells on the basolateral side (Figure 4a). Intrinsic to the evaluation of a circuit is the need to test the response to many combinations of inducers. To this end, the transwell system is ideal because the same epithelial monolayer can be established in a 12-well plate and the circuit response can be tested under identical conditions. Stem cells are seeded on a collagen-coated membrane of a transwell and differentiated for approximately one week (Figure 4a and 4b). Separately, an overnight culture of B. thetaiotaomicron is resuspended in fresh pre-reduced YCFA media, of which 500 μL of this is introduced into the apical side of the monolayer and cultured for two hours (Online Methods). DAPI and immunostaining of actin filaments indicate B. thetaiotaomicron cells are localized to the apical side (Figure 4c,d and Supplementary Figure S21, S22). The monolayer was verified to be intact as shown by the brightfield images with no observable holes and transepithelial electrical resistance (TEER) values in the ‘tight’ GI epithelia range (Supplementary Figure S23)67.
After the bacteria adhere, the genetic circuit is induced by adding fresh media containing the appropriate combinations of DCA and aTc. The cells are cultured with the inducers for 6 hours and the non-adhered cells are removed by pipetting. B. thetaiotaomicron cells located at the bottom of the apical chamber closely associated with the monolayer were harvested by removing the top 450 μL of the culture and mixing the remaining 50 μL with 450 μL of fresh media. Their OD and luminescence were measured in the same manner as those samples in TYG culture. As seen in Figure 4e, the output of the circuit closely matched that of the in vitro results (Figure 3f) across all 12 states representing the 4 combinations of inputs and 3 outputs (Figure 4e).
We have created a flexible platform to design genetic circuits for B. thetaiotaomicron, and proven its utility by designing a circuit that integrates sensors that responds to a signal of gastrointestinal health and a small molecule pharmaceutical. While the UCF only has 7 gates, these could be used to build ~1012 circuits and could be connected to other, user-specified sensors. The circuit output is a reporter gene, but this could be replaced with a gene encoding a therapeutic protein, or gene clusters that build a chemically-complex antibiotic or immunomodulator. To date, genetic circuit design automation has only been enabled for a laboratory strain of a model organism (E. coli) and a plasmid-based system6. Here, we demonstrate that extending circuit design automation to a new species for which there are relatively few genetic tools does not require extensive host characterization. Several circuits were constructed using Cello and, while they were designed to function under specific culture conditions, they were found to function correctly when prototyped under realistic conditions that represent the complexity and uniqueness of the human gut. Our human gastrointestinal model was able to rapidly evaluate the multiple states of the circuit, but this came at the cost of only being able to study the circuit response for eight hours, after which bacterial overgrowth compromised the monolayer. More detailed microphysiological systems could be used to evaluate circuits for up to weeks68,69. Further, the complexity of the epithelial layer could be increased to study specific phenomena, such as the response of bacteria residing in crypts, the role of peristalsis and shear stress, molecular transport into the bloodstream, and interactions with the gut-brain axis. The ability to design circuits to specification and then rapidly prototype them under realistic environment will aid the development of living, responsive human therapeutics.
Online Methods
Strains, Plasmids, and Media.
Bacteroides thetaiotaomicron VPI-5482 wildtype (ATCC 29148) strain was used. The backbone vectors, pNBU1-erm and pNBU2-tetQ, were provided by Timothy Lu (MIT) and Michael Fischbach (Stanford), respectively. All B. thetaiotaomicron strains were cultured in Tryptone-Yeast Extract Glucose (TYG) broth or Brain Heart Infusion (BHI; BD BBL BHI agar #211065) agar supplemented with 10% Horse Blood (Hemostat DHB500). 1L of TYG broth contains 10 g of Tryptone Peptone (BD Bacto #211705), 5 g Yeast Extract (BD Difco #210929), 2 g of Glucose (Fisher #50-99-7), 100 mL of 1 M KPO4 [pH 7.2; 1 L of the 1 M Dibasic (VWR #0705-500G) in H2O and ~430mL of the 1M Monobasic (Sigma 795488-500G) in H2O were mixed to achieve pH 7.2], 40 mL of TYG Salt Solution [0.5 g MgSO47H2O (USB Corp. #18651), 10 g NaHCO3 (Fisher Scientific S233), 2 g of NaCl (Amresco X190) dissolved in 1 L of H2O], 1 mL of 0.8% CaCl2 (Sigma #C1016-500G) in H2O , 1 mL of 0.4 mg/mL of FeSO4 (Amresco #0387-500G) in H2O, 4 mL of 0.25mg/mL of Resazurin (Sigma #R7017-1G) in H2O, 1 mL of histidine-hematin solution [1.2 μg/ml hematin 12mg of hematin (Sigma #H3281-1G) was dissolved in 10mL of 0.2 M histidine (Sigma #H7875-25G) in H2O adjusted to pH 8 and filter sterilized], 0.5 g of L-cysteine (Sigma #30089-25G), and 1 mL of 1 mg/mL of Menadione (Sigma #M5625-25G) in 100% EtOH. L-cysteine was resuspended in water (1ml per 25mg of L-cysteine) and filter sterilized (0.22uM filter Pall PN4192) immediately prior to inoculation. Sterile histidine hematin solution, menadione, and L-cysteine were added to the autoclaved media immediately prior to inoculation. Media were pre-reduced (left overnight inside the anaerobic chamber) before inoculation unless otherwise noted. Antibiotics were added as appropriate: erythromycin (25 μg/mL; Acros Organics #114-07-8), tetracycline (2 μg/mL; Goldbio #64-75-5) and gentamicin (200 μg/mL; Goldbio #1405-41-0). Antibiotics for B. thetaiotaomicron were only used for post-conjugation selection, and not during growth. The following inducer concentrations were used as appropriate unless otherwise specified: 500 μM IPTG (Goldbio #367-93-1), 100 ng/ml aTc (Acros Organics #13803-65-1), and 62.5 μM DCA (Sigma #D2510-10G). Other bile acids used were CA (Sigma #C9282-25G), CDCA (Sigma #C8261-1G), LCA (Sigma #L6250-10G), TCA (Sigma #T4009-1G) and GCA (Sigma #G7132-1G). Escherichia coli S17-1 ƛ pir (from Timothy Lu) or the EC100D pir (VWR #75927-934) was used to propagate NBU1- and NBU2-based constructs, and were grown at 37 °C, shaken aerobically in LB (BD Difco #244620) broth or LB agar (BD Bacto #214010) supplemented with 100 μg/mL carbenicillin (Goldbio #4800-94-6) for post-transformation selection and growth.
Conjugation into B. thetaiotaomicron and genome engineering.
Constructs based on the pNBU1 and pNBU2 plasmids (Supplementary Figure S24) were integrated into the Bacteroides genome by conjugation using E. coli S17-1 ƛ pir. pNBU1 is based on a Bacteroides mobilizable transposon NBU1, containing intN1 that mediates site specific recombination of the attN1 site of pNBU1 and the attBT1-1 site in the B. thetaiotaomicron genome (located at the 3’ end of tRNA-Leu gene). pNBU1 contains a mutated attN1 where the −2 site has been changed from a G to a C, which has been shown to increase integration frequency. An overnight culture of E. coli S17-1 ƛ pir and B. thetaiotaomicron was mixed at a 1:1 ratio in TYG and spot plated on BHI + 10% horse blood agar plates. The matings were incubated aerobically at 37°C. After >16 hours, the cells were scraped and serially streaked onto BHI blood agar plates supplemented with gentamicin and erythromycin for pNBU1 or gentamicin and tetracycline for pNBU2 and incubated anaerobically at 37 °C. Resultant colonies that appeared after 24 - 48 hours were verified for site specific integration by performing PCR using genome and vector specific primers. When both pNBU2 and pNBU1 plasmids were to be integrated into B. thetaiotaomicron, pNBU2 was conjugated first followed by pNBU1. All strains constructed for this work are listed in Supplementary Table S2, and their maps illustrated in Supplementary Figure S24.
Anaerobic growth.
All B. thetaiotaomicron strains were grown statically in the Forced Air Incubator (Coy Laboratory Model 2000 #8543025) at 37°C inside the anaerobic chamber (Coy Laboratory; Vinyl Anaerobic Chamber Type A Glove Box #7000000). O2 in the anaerobic chamber was constantly removed by the Palladium Catalyst (Coy Laboratory #6501050), which was renewed weekly by incubating in the 90°C oven for overnight or longer. Nitrogen (Airgas #NIUHP300) was used to purge air inside the airlock and to maintain a positive pressure in the anaerobic chamber. The O2 and the H2 level in the anaerobic chamber was monitored using the CAM-12 Anaerobic Monitor (Coy Laboratory #6250000). The O2 inside the chamber was maintained at 0 ppm, unless the inner door of the airlock was opened to move items in and out of the chamber, in which case low levels of O2 was introduced into the chamber but decreased rapidly. H2 was maintained above 1.5% inside the chamber for proper functioning of the palladium catalyst by adding 5% H2, 20% CO2, N2 gas mix (Airgas Custom Mix, #X03NI75C2O05502). When moving items in and out of the chamber, the default P1 program (2 cycles of vacuum and purge gas (N2), followed by 1 cycle of vacuum and gas mix) was used.
Luciferase Assay and RPUL.
Overnight B. thetaiotaomicron culture in TYGs were diluted 1:100 in fresh TYG and aliquots (180 μL) of B. thetaiotaomicron cultures were taken when the OD600 was between 0.4 to 0.7 (approximately 6 hours). From this culture, 15 μL was mixed with 15 μL of nanoluc reaction buffer (Promega Nano-Glo Luciferase System N1120; with substrate added in 1:50 ratio). The OD600 and luminescence were measured using Synergy H1 Hybrid Reader (BioTek #8041000). For luminescence reads, 1 s at a gain setting of 100 was used. Both measurements were read on a 96-well flat bottom microtiter plate (Nunc #165305). The Relative Luminescence Unit (RLU/CFU) was calculated by dividing the raw luminescence value by CFU, which was calculated from OD600 using a standard curve generated from plotting OD600 versus CFU. To convert luciferase activity to RPUL, an RPUL standard strain (B. thetaiotaomicron strain MT768) was defined (Figure 1a, 1b). Luminescence (RLU/CFU) was converted into RPUL by dividing the luminescence (RLU/CFU) by the luminescence (RLU/CFU) of the RPUL strain. See Life Sciences Reporting Summary.
Sensor characterization.
DNA encoding CmeR, BreR and the BreR homologs were codon optimized for B. thetaiotaomicron VPI-5482 using Jcat and synthesized as gBlocks (IDT). Cultures of B. thetaiotaomicron containing the sensors were serially streaked onto BHI blood agar plates. A single colony was picked after 24 hours and inoculated into 2 mL of TYG (not prereduced) in 14 mL Falcon tubes (Fisher #14-959-11B) and incubated in the anaerobic chamber at 37°C statically. Overnight cultures were diluted 1:100 the next day and grown for 6 hr in 800 μL of pre-reduced TYG with or without appropriate inducers in 96 deep well plates statically (USA Scientific 1896-2000). Aliquots (180 μL) were taken after 6 hours and luciferase and OD600 were measured (described above). Sensor crosstalk experiments were performed in the same manner. Fold-induction for chemical crosstalk was calculated by dividing the luminescence (RLU/CFU) of the induced culture by that of the uninduced culture. When used as a figure axis label, the term ‘Repression (−repressor/+repressor)’ refers to the division of the normalized luminescence (RLU/CFU) of a strain lacking a repressor (−repressor) by that measured from a strain containing a repressor (+repressor).
Terminator characterization in B. thetaiotaomicron.
The luminescence (RLU/CFU) is measured for a strain containing an expression cassette and a strain with a terminator in between the promoter and open reading frame (Supplementary Figure S12). Glycerol stocks of B. thetaiotaomicron containing the terminators were serially streaked onto BHI blood agar plates. A single colony was picked after 24 hours and inoculated into 2 mL of TYG (not prereduced) in 14 mL Falcon tubes (Fisher #14-959-11B) and incubated in the anaerobic chamber statically. Overnight cultures were diluted 1:100 the next day and grown for 6 hr in 800 μL of pre-reduced TYG with or without appropriate inducers in 96 deep well plates statically (USA Scientific 1896-2000). Aliquots (180 μL) were taken after 6 hours and luciferase and OD600 were measured (described above).
sgRNA design.
A 20bp random DNA sequence including the TANNTTTG was generated using the Random DNA Sequence Generator (http://www.faculty.ucr.edu/~mmaduro/random.htm; GC content probability parameter of 0.5) and they were scanned against the Bacteroides thetaiotaomicron VPI-5482 genome via BLASTn to look for off-target sites in the genome that could potentially be affected. No 20 nucleotide sequence had homology to a PAM adjacent locus within the genome.
Orthogonality of sgRNAs.
All possible combinations of the 7 sgRNA and 7 sgRNA-operator promoters were constructed and conjugated into B. thetaiotaomicron strain MT724, resulting in a total of 49 strains (B. thetaiotaomicron MT809-MT857). Glycerol stocks of B. thetaiotaomicron containing the sgRNA and sgRNA-operator promoter pairs were serially streaked onto BHI blood agar plates. A single colony was picked after 24 hours and inoculated into 2 mL of TYG (not prereduced) in 14 mL Falcon tubes (Fisher #14-959-11B) and incubated in the anaerobic chamber statically. Overnight cultures were diluted 1:100 the next day and grown for 6 hr in 800 μL of pre-reduced TYG with or without appropriate inducers in 96 deep well plates statically (USA Scientific 1896-2000). Aliquots (180 μL) were taken after 6 hours and luciferase and OD600 were measured (described above). Fold repression was calculated by dividing the luminescence of the uninduced by the luminescence of the induced B. thetaiotaomicron culture.
Gate characterization.
The gate measurement plasmids, the RPUL standard and the bile acid sensor (to convert DCA concentrations to input promoter activity) were all conjugated into B. thetaiotaomicron strain MT724. Glycerol stocks of B. thetaiotaomicron containing the gates, the RPUL standard, and the bile acid sensor were serially streaked onto BHI blood agar plates. A single colony was picked after 24 hours and inoculated into 2 mL of TYG (not prereduced) in 14 mL Falcon tubes (Fisher #14-959-11B) and incubated in the anaerobic chamber statically. Overnight cultures were diluted 1:100 the next day and grown for 6 hr in 800 μL of pre-reduced TYG in 96 deep well plates statically (USA Scientific 1896-2000), under a series of DCA concentrations (62.5, 37.5, 22.5, 13.5, 8.1, 4.9, 2.9, 1.7, 1.0, 0.6, 0.4 and 0 μM). Aliquots (180 μL) were taken after 6 hours and luciferase and OD600 were measured. The activity of the input promoter against the output promoter was plotted in RPUL and Hill Function was fit to the data using Prism’s Nonlinear Regression tool and the resulting fit parameters are presented in Table 1. Input thresholds IL (input low) and IH (input high), which determines the connectivity of two NOT gates, was initially calculated as the 0.5x the maximum and 2x the minimum output, respectively, as described by Nielsen and coworkers6. However, for Bth1C1G1T1 UCF, the values were changed to have an IL of 0.001 and IH of 0.2 to increase the number of circuit design solutions that could be found.
Circuit designs using Cello.
The Cello software and code are available open source (https://github.com/CIDARLAB/cello). Cello was run locally using the Cello API, and the growth score cutoff was set to 0.75. A UCF file (Bth1C1G1T1.UCF.json) was used (Supplementary File 1). Eugene rules are applied to map gates to linear DNA sequences. In order to assign output gate to the 3’ position, rules are included in Eugene such that all the logic gates are assigned before the output gate (written in Eugene as “gate_M1 BEFORE gate_nanoluc”). The order of logic gates is random, which is the default option by Eugene. Orientation of each transcription unit is set to be in the same direction and all forward (written in Eugene as “ALL_FORWARD”). 0.03 RPUL for no aTc, 6.4 RPUL for 100 ng/ml aTc, 0.01 RPUL for no DCA and 1.5RPUL for 62.5μM DCA was used.
Characterizing Circuit Behavior in a Timecourse.
Single colonies of B. thetaiotaomicron strains harboring RPUL and the 2-input 3-output circuit (B. thetaiotaomicron MT798, MT799, and MT800 corresponding to reporting output 2, 3, and 1, respectively) were picked and inoculated into TYG (not reduced) without inducers overnight. The next day, 1 mL of the culture was spun down inside the anaerobic chamber (VWR Galaxy minicentrifuge) and the pellets were washed in 1 mL of pre-reduced TYG with aTc twice, and diluted 1:50 into 800 μL of TYG with the same media. OD600 and luminescence measurements were taken as described every two hours for 8 hours immediately after inoculating in a new input state. After 8 hours, the culture was kept in the anaerobic chamber at 37°C overnight. The next day, the culture was spun down anaerobically, and again washed twice but with pre-reduced TYG with inducers corresponding to the next input state (DCA only), and diluted 1:50 in TYG with the same media. Again, OD600 and luminescence measurements were taken every 2 hours for 8 hours immediately after the input state transition. This process was repeated for all possible permutations.
Medium for culturing primary colonic epithelial monolayer.
Primary colonic epithelial monolayer was maintained by a base medium (DMEM/F12 (Gibco, 12634-010 500 mL) with 5 mL Glutamax -I (Gibco, 35050-061), 5 mL HEPES (Gibco, 15630-080), and 5 mL Pen-Strep (Gibco, 15140-148), an organoid growth medium (Wnt, R-spondin, Noggin (WRN) conditioned medium provided by Boston Children’s Hospital (David Breault), base medium, and 1X B-27 Supplement 50X (Gibco, 17504-001), 1X N-2 Supplement 100X (Gibco, 17502-001), 10 mM nicotinamide (Sigma-Aldrich, N0636), 500 μM N-acetyl cysteine (Sigma-Aldrich, A9165), 10 μM Y-27632 dihydrochloride (Biogems, 1293823), 10 μM SB202190 (Biogems, 1523072; Tocris, 1264), 500 nM A 83-01 (Biogems, 9094360), 50 ng/mL murine EGF (PeproTech, AF-315-09), human [Leu15]-Gastrin I (Sigma-Aldrich, G9145), and 5 nM prostaglandin E2 (Biogems, 3632464)), a seeding medium (Seeding medium composition: 65% WRN conditioned medium, 32% base medium, 1X B-27 Supplement 50X, 1X N-2 Supplement 100X, 500 μM N-acetyl cysteine, 10 μM SB202190, 2.5 μM thiazovivin, 500 nM A 83-01, 50 ng/mL murine EGF, human [Leu15]-Gastrin I, 5 nM prostaglandin E2) and a differentiation medium (Differentiation medium composition: 20% R-spondin 1 conditioned medium, 80% base medium (antibiotic free), 1X B-27 Supplement 50X, 1X N-2 Supplement 100X, 500 μM N-acetyl cysteine, 500 nM A 83-01, 100 ng/mL human noggin (PeproTech, 120-10C), 50 ng/mL murine EGF, and human [Leu15]-Gastrin I.
Gut epithelial monolayer
The colon monolayer model was derived from primary human colonic stem cell organoids. The cell organoid establishment and maintenance were performed according to previously described methods by the Yilmaz lab at the Koch Institute/MIT. Normal appearing region of the endoscopic tissue biopsies was collected from the rectosigmoid colon of a de-identified individual (30 yr Diverticulitis patient) at the Massachusetts General Hospital, upon the donor’s informed consent. Methods were carried out in accordance to the Institutional Review Board of Boston Children’s Hospital (protocol number IRB-P00000529) and the Koch Institute Institutional Review Board Committee as well as the Massachusetts Institute of Technology Committee on the Use of Humans as Experimental Subjects. Cell organoids were maintained in Matrigel droplets and passaged every seven days at a ratio of 1:3. A media change was performed at day 4 after passaging. To prepare the monolayer, organoids were collected into a 15-mL conical tube at day 7. Organoids were pelleted by centrifugation (1000 g × 5min, 4°C) and then media removal via aspiration. After that, the Cell Recovery Solution (Corning, 354253; 1mL per 100 μL Matrigel) was added to disrupt the organoid pellet, and then the organoid suspension was incubated on ice for 45-60 min. The organoid suspension was then pelleted and resuspended with 1 mL pre-warmed PBS−/− (Gibco, 10010-023) containing 2.5mg/mL Trypsin (Sigma, T4549) and 0.45 mM EDTA (Ambion, AM9260G). The resuspended organoids were placed in a 37 °C water bath for 5 min and then manually dissociated into single cells using a 1000-μL pipette with a bent tip. Cells were counted with TrypanBlue and automated cell counter (Invitrogen). Cells were diluted to a density of 600,000 cells per mL in seeding media and 500 μL was added into the apical side of each 12-well coated transwell (surface area: 1.12 cm2) and 1.5 mL cell-free seeding medium was added to the basolateral side. Prior to seeding, transwells were coated with rat tail collagen I (Gibco, A10483-01, 50 μg/mL in PBS) for 1-2 hours in the incubator, then was washed with PBS immediately before adding the cells.
Coculture of primary colonic epithelial monolayer and B. thetaiotaomicron.
Differentiated monolayers were used for the coculture with B. thetaiotaomicron. At day 3 after seeding, the monolayers were differentiated by exchanging antibiotic-free base medium on the apical side and differentiation medium on the basolateral side. The differentiated monolayers (4-5 days after cell differentiation) were used for epithelia-B. thetaiotaomicron coculture. B. thetaiotaomicron cells were grown as described above except TYG medium or BHI agar plates were replaced by yeast casitone fatty acids (YCFA) medium (AS-680, Anaerobe Systems) or YCFA agar plates (AS-675, Anaerobe Systems). TYG media was not used because preliminary experiments suggested TYG media alone compromises the monolayer. B. thetaiotaomicron cells were resuspended in 500 μL of YCFA containing no inducers; and then added into the apical side of the monolayer. Prior to adding B. thetaiotaomicron cells, the apical media was aspirated. The monolayers were then immediately transferred into the incubator (5% CO2, 37°C). After incubating for 2 hours, inducers were added to the media and incubated for another 6 hours. After 6 hours, 450 μL of the top apical media was removed, and 50 μL of the media containing the cells settling on the surface of the epithelial cells were diluted and mixed with 450 μL of fresh YCFA. OD600 and luminescence were measured as described above. After harvesting B. thetaiotaomicron, transepithelial electrical resistance (TEER) was measured by adding 500 μl PBS and placing the transwell into the EndOhm chamber connected to the EVOM2 Epithelial Voltohmmeter. See Life Sciences Reporting Summary.
Immunofluorescent staining and imaging
The monolayer was fixed with 4% formaldehyde for 10 min. To block the non-specific binding, the fixed cells were then cultured with BlockAid™ blocking solution (Thermo Fisher B10710) for 1 h with gentle shaking (50 rpm). Afterwards, cells were stained with conjugated phalloidin (ab176753) and 4′,6-diamidino-2-phenylindole (DAPI) diluted in blockade solution and incubated at 4 °C overnight with aluminum foil cover. Cells were washed twice the next day with PBS, 5 min each. Then the membrane with monolayer was cut out from the transwell insert, mounted on a glass and then covered with thin circular glass slip after adding a drop of antifade solution. Imaging was performed in Zeiss LSM 880 confocal microscope. The 3D image was rendered using the software Zen 2.3 SP1 FP3, version 14.0.20.201 (Zeiss). Brightfield images were taken with EVOS cell imaging system. For Figure 4c and 4d, the bacteria in the image were specifically false colored from the DAPI blue color to pink using GIMP-2.10 to highlight the bacteria relative to the mammalian cells. Brightness and contrast were also increased.
Supplementary Material
Acknowledgements
We thank M. Mimee (MIT) and T. Lu (MIT) for providing us the NBU1 based Bacteroides shuttle vector pNBU1-Erm. We thank M. Fischbach (Stanford) for providing us the NBU2 based Bacteroides shuttle vector pNBU2-tetQ. Primary human colonic epithelial cells were obtained by generous contributions from the laboratory of O. Yilmaz (MIT). We thank K. Schneider and C. Wright for technical assistance. This work was supported by the National Institute of Health P50 grant (P50-GM098792), Office of Naval Research Multidisciplinary University Research Initiatives Program (N00014-13-1-0074), Defense Agency Research Projects Agency Synergistic Discovery and Design (SD2; FA8750-17-C-0229), National Science Foundation Semiconductor Synthetic Biology for Information Processing and Storage Technologies (SemiSynBio; CCF-1807575) program and NIH R01EB021908.
Footnotes
Competing interests
C.A.V. and M.T. have filed a provisional patent based on this work.
Code Availability
The Cello software and codes are available open source (https://github.com/CIDARLAB/cello).
Data Availability
Genetic parts and the UCF file Bth1C1G1T1 are available as Supplementary Information. The DNA sequences for the following plasmids are deposited into Genbank: pMT405 (MN991273); pMT406 (MN991274); pMT423 (MN991275); pMT444 (MN991276); pMT445 (MN991277); pMT447 (MN991278); pMT448 (MN991279); pMT449 (MN991280); pMT450 (MN991281); pMT451 (MN991282); pMT455 (MN991283); pMT462 (MN991284); pMT468 (MN991285); pMT469 (MN991286); pMT470 (MN991287); pMT492 (MN991288); pMT493 (MN991289); pMT494 (MN991290).
References
- 1.Claesen J & Fischbach MA Synthetic Microbes As Drug Delivery Systems. ACS Synth. Biol 4, 358–364, doi: 10.1021/sb500258b (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mimee M, Tucker A,C, Voigt C,A & Lu T,K Programming a Human Commensal Bacterium, Bacteroides thetaiotaomicron, to Sense and Respond to Stimuli in the Murine Gut Microbiota. Cell Systems 1, 62–71, doi: 10.1016/j.cels.2015.06.001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim B, Zimmermann M, Barry NA & Goodman AL Engineered Regulatory Systems Modulate Gene Expression of Human Commensals in the Gut. Cell 169, 547–558.e515, doi: 10.1016/j.cell.2017.03.045 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitaker WR, Shepherd ES & Sonnenburg JL Tunable Expression Tools Enable Single-Cell Strain Distinction in the Gut Microbiome. Cell 169, 538–546.e512, doi: 10.1016/j.cell.2017.03.041 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruder WC, Lu T & Collins JJ Synthetic Biology Moving into the Clinic. Science 333, 1248–1252, doi: 10.1126/science.1206843 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Nielsen AA et al. Genetic circuit design automation. Science 352, aac7341, doi: 10.1126/science.aac7341 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Ozdemir T, Fedorec AJH, Danino T & Barnes CP Synthetic Biology and Engineered Live Biotherapeutics: Toward Increasing System Complexity. Cell Systems 7, 5–16, doi: 10.1016/j.cels.2018.06.008 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Lee JW, Chan CTY, Slomovic S & Collins JJ Next-generation biocontainment systems for engineered organisms. Nat Chem Biol 14, 530–537, doi: 10.1038/s41589-018-0056-x (2018). [DOI] [PubMed] [Google Scholar]
- 9.Piraner DI, Abedi MH, Moser BA, Lee-Gosselin A & Shapiro MG Tunable thermal bioswitches for in vivo control of microbial therapeutics. Nat Chem Biol 13, 75–80, doi: 10.1038/nchembio.2233 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Isabella VM et al. Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nature Biotechnology 36, 857–864, doi: 10.1038/nbt.4222 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Riglar DT & Silver PA Engineering bacteria for diagnostic and therapeutic applications. Nature Reviews Microbiology 16, 214–225, doi: 10.1038/nrmicro.2017.172 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Wexler AG & Goodman AL An insider's perspective: Bacteroides as a window into the microbiome. Nature Microbiology 2, 17026, doi: 10.1038/nmicrobiol.2017.26 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwalm ND & Groisman EA Navigating the Gut Buffet: Control of Polysaccharide Utilization in Bacteroides spp. Trends in Microbiology 25, 1005–1015, doi: 10.1016/j.tim.2017.06.009 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Faith JJ et al. The Long-Term Stability of the Human Gut Microbiota. Science 341, 1237439, doi: 10.1126/science.1237439 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donaldson GP, Lee SM & Mazmanian SK Gut biogeography of the bacterial microbiota. Nature Reviews Microbiology 14, 20–32, doi: 10.1038/nrmicro3552 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson A et al. A Gut Commensal-Produced Metabolite Mediates Colonization Resistance to Salmonella Infection. Cell Host & Microbe 24, 296–307.e297, doi: 10.1016/j.chom.2018.07.002 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell Alistair B. et al. A Type VI Secretion-Related Pathway in Bacteroidetes Mediates Interbacterial Antagonism. Cell Host & Microbe 16, 227–236, doi: 10.1016/j.chom.2014.07.007 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramakrishna C et al. Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis. Nat Commun 10, doi: 10.1038/s41467-019-09884-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wegorzewska MM et al. Diet modulates colonic T cell responses by regulating the expression of a Bacteroides thetaiotaomicron antigen. Science Immunology 4, eaau9079, doi: 10.1126/sciimmunol.aau9079 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamady ZZR et al. Xylan-regulated delivery of human keratinocyte growth factor-2 to the inflamed colon by the human anaerobic commensal bacterium Bacteroides ovatus. Gut 59, 461–469, doi: 10.1136/gut.2008.176131 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Round JL & Mazmanian SK Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. PNAS 107, 12204–12209, doi: 10.1073/pnas.0909122107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shepherd ES, DeLoache WC, Pruss KM, Whitaker WR & Sonnenburg JL An exclusive metabolic niche enables strain engraftment in the gut microbiota. Nature 557, 434–438, doi: 10.1038/s41586-018-0092-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi Lei S. et al. Repurposing CRISPR as an RNA-Guided Platform for Sequence-Specific Control of Gene Expression. Cell 152, 1173–1183, doi: 10.1016/j.cell.2013.02.022 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daeffler KNM et al. Engineering bacterial thiosulfate and tetrathionate sensors for detecting gut inflammation. Molecular Systems Biology 13, 923, doi: 10.15252/msb.20167416 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riglar DT et al. Engineered bacteria can function in the mammalian gut long-term as live diagnostics of inflammation. Nature Biotechnology 35, 653–658, doi: 10.1038/nbt.3879 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Archer EJ, Robinson AB & Süel GM Engineered E. coli That Detect and Respond to Gut Inflammation through Nitric Oxide Sensing. ACS Synth. Biol 1, 451–457, doi: 10.1021/sb3000595 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Hwang IY et al. Engineered probiotic Escherichia coli can eliminate and prevent Pseudomonas aeruginosa gut infection in animal models. Nat Commun 8, 1–11, doi: 10.1038/ncomms15028 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y et al. Robust bioengineered 3D functional human intestinal epithelium. Scientific Reports 5, 1–11, doi: 10.1038/srep13708 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leschner S et al. Identification of tumor-specific Salmonella Typhimurium promoters and their regulatory logic. Nucleic Acids Res 40, 2984–2994, doi: 10.1093/nar/gkr1041 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozdemir T Design and Construction of Therapeutic Bacterial Sensors in Escherichia coli Nissle 1917. 173. [Google Scholar]
- 31.Kotula JW et al. Programmable bacteria detect and record an environmental signal in the mammalian gut. PNAS 111, 4838–4843, doi: 10.1073/pnas.1321321111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S et al. Quorum Sensing Can Be Repurposed To Promote Information Transfer between Bacteria in the Mammalian Gut. ACS Synth. Biol 7, 2270–2281, doi: 10.1021/acssynbio.8b00271 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Chen JX et al. Development of Aspirin-Inducible Biosensors in Escherichia coli and SimCells. Appl Environ Microbiol 85, doi: 10.1128/AEM.02959-18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mimee M, Citorik RJ & Lu TK Microbiome therapeutics - Advances and challenges. Adv Drug Deliv Rev 105, 44–54, doi: 10.1016/j.addr.2016.04.032 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao L et al. A selective gut bacterial bile salt hydrolase alters host metabolism. eLife 7, doi: 10.7554/eLife.37182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridlon JM, Kang DJ, Hylemon PB & Bajaj JS Bile Acids and the Gut Microbiome. Current opinion in gastroenterology 30, 332–338, doi: 10.1097/MOG.0000000000000057 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridlon Jason M., S. C. H. S. B. D.-J. K., Phillip & Hylemon B Consequences of bile salt biotransformations by intestinal bacteria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Degirolamo C, Modica S, Palasciano G & Moschetta A Bile acids and colon cancer: Solving the puzzle with nuclear receptors. Trends in Molecular Medicine 17, 564–572, doi: 10.1016/j.molmed.2011.05.010 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Kawamata Y et al. A G Protein-coupled Receptor Responsive to Bile Acids. J. Biol. Chem 278, 9435–9440, doi: 10.1074/jbc.M209706200 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Hofmann AF & Hagey LR Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell. Mol. Life Sci 65, 2461–2483, doi: 10.1007/s00018-008-7568-6 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamilton JP et al. Human cecal bile acids: concentration and spectrum. American Journal of Physiology - Gastrointestinal and Liver Physiology 293, G256–G263, doi: 10.1152/ajpgi.00027.2007 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Li T & Chiang JYL Bile acids as metabolic regulators. Curr Opin Gastroenterol 31, 159–165, doi: 10.1097/MOG.0000000000000156 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ung KA, Gillberg R, Kilander A & Abrahamsson H Role of bile acids and bile acid binding agents in patients with collagenous colitis. Gut 46, 170–175 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernstein H, Bernstein C, Payne CM, Dvorakova K & Garewal H Bile acids as carcinogens in human gastrointestinal cancers. Mutat. Res 589, 47–65, doi: 10.1016/j.mrrev.2004.08.001 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Cerda-Maira FA, Ringelberg CS & Taylor RK The Bile Response Repressor BreR Regulates Expression of the Vibrio cholerae breAB Efflux System Operon. J. Bacteriol 190, 7441–7452, doi: 10.1128/JB.00584-08 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gunn JS Mechanisms of bacterial resistance and response to bile. Microbes and Infection 2, 907–913, doi: 10.1016/S1286-4579(00)00392-0 (2000). [DOI] [PubMed] [Google Scholar]
- 47.Lin J et al. Bile Salts Modulate Expression of the CmeABC Multidrug Efflux Pump in Campylobacter jejuni. J Bacteriol 187, 7417–7424, doi: 10.1128/JB.187.21.7417-7424.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rössger K, Charpin-El-Hamri G & Fussenegger M Bile acid-controlled transgene expression in mammalian cells and mice. Metabolic Engineering 21, 81–90, doi: 10.1016/j.ymben.2013.11.003 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Din MO et al. Synchronized cycles of bacterial lysis for in vivo delivery. Nature 536, 81–85, doi: 10.1038/nature18930 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riglar DT et al. Bacterial variability in the mammalian gut captured by a single-cell synthetic oscillator. Nat Commun 10, 1–12, doi: 10.1038/s41467-019-12638-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oberortner E, Bhatia S, Lindgren E & Densmore D A Rule-Based Design Specification Language for Synthetic Biology. J. Emerg. Technol. Comput. Syst 11, 25:21–25:19, doi: 10.1145/2641571 (2014). [DOI] [Google Scholar]
- 52.Kelly JR et al. Measuring the activity of BioBrick promoters using an in vivo reference standard. Journal of Biological Engineering 3, 4, doi: 10.1186/1754-1611-3-4 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hall MP et al. Engineered Luciferase Reporter from a Deep Sea Shrimp Utilizing a Novel Imidazopyrazinone Substrate. ACS Chemical Biology 7, 1848–1857, doi: 10.1021/cb3002478 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuthbertson L, Ahn Sang K. Nodwell Justin R. Deglycosylation as a Mechanism of Inducible Antibiotic Resistance Revealed Using a Global Relational Tree for One-Component Regulators. Chemistry & Biology 20, 232–240, doi: 10.1016/j.chembiol.2012.11.011 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Cerda-Maira FA, Kovacikova G, Jude BA, Skorupski K & Taylor RK Characterization of BreR Interaction with the Bile Response Promoters breAB and breR in Vibrio cholerae. J Bacteriol 195, 307–317, doi: 10.1128/JB.02008-12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stanton BC et al. Genomic mining of prokaryotic repressors for orthogonal logic gates. Nat Chem Biol 10, 99–105, doi: 10.1038/nchembio.1411 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nielsen AAK & Voigt CA Multi-input CRISPR/Cas genetic circuits that interface host regulatory networks. Molecular Systems Biology 10, n/a-n/a, doi: 10.15252/msb.20145735 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang S & Voigt CA Engineered dCas9 with reduced toxicity in bacteria: implications for genetic circuit design. Nucl. Acids Res 46, 11115–11125, doi: 10.1093/nar/gky884 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gander MW, Vrana JD, Voje WE, Carothers JM & Klavins E Digital logic circuits in yeast with CRISPR-dCas9 NOR gates. Nat Commun 8, 15459, doi: 10.1038/ncomms15459 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Y et al. Synthesizing AND gate genetic circuits based on CRISPR-Cas9 for identification of bladder cancer cells. Nat Commun 5, 5393, doi: 10.1038/ncomms6393 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Chen P-Y, Qian Y & Del Vecchio D in 2018 IEEE Conference on Decision and Control (CDC). 4333–4338. [Google Scholar]
- 62.Chen Y-J et al. Characterization of 582 natural and synthetic terminators and quantification of their design constraints. Nat Meth 10, 659–664, doi: 10.1038/nmeth.2515 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Bein A et al. Microfluidic Organ-on-a-Chip Models of Human Intestine. Cellular and Molecular Gastroenterology and Hepatology 5, 659–668, doi: 10.1016/j.jcmgh.2017.12.010 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kozuka K et al. Development and Characterization of a Human and Mouse Intestinal Epithelial Cell Monolayer Platform. Stem Cell Reports 9, 1976–1990, doi: 10.1016/j.stemcr.2017.10.013 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li H et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat Commun 6, 1–13, doi: 10.1038/ncomms9292 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fofanova TY et al. A novel human enteroid-anaerobe co-culture system to study microbial-host interaction under physiological hypoxia. bioRxiv, 555755, doi: 10.1101/555755 (2019). [DOI] [Google Scholar]
- 67.Srinivasan B et al. TEER measurement techniques for in vitro barrier model systems. J Lab Autom 20, 107–126, doi: 10.1177/2211068214561025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shah P et al. A microfluidics-based in vitro model of the gastrointestinal human–microbe interface. Nat Commun 7, 11535, doi: 10.1038/ncomms11535 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jalili-Firoozinezhad S et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat Biomed Eng 3, 520–531, doi: 10.1038/s41551-019-0397-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genetic parts and the UCF file Bth1C1G1T1 are available as Supplementary Information. The DNA sequences for the following plasmids are deposited into Genbank: pMT405 (MN991273); pMT406 (MN991274); pMT423 (MN991275); pMT444 (MN991276); pMT445 (MN991277); pMT447 (MN991278); pMT448 (MN991279); pMT449 (MN991280); pMT450 (MN991281); pMT451 (MN991282); pMT455 (MN991283); pMT462 (MN991284); pMT468 (MN991285); pMT469 (MN991286); pMT470 (MN991287); pMT492 (MN991288); pMT493 (MN991289); pMT494 (MN991290).