Abstract
Capillaroscopy is a unique method for morphological evaluation of the nailfold capillaries that plays a crucial role for early diagnosis of systemic sclerosis. The first description of the pathological capillaroscopic changes in systemic sclerosis was made by Brown and O’Leary in 1925. Several decades later they have been validated and accepted as a diagnostic criterion in the current 2013 EULAR/ACR classification criteria. This article summarizes the evolving knowledge about the use of nailfold capillaroscopy in systemic sclerosis. Initially, Maricq et al. suggested two major categories of capillaroscopic findings in systemic sclerosis – an ‘active’and ‘slow’ capillaroscopic pattern. Their description and terminology suggested a correlation between capillaroscopic changes and disease activity and progression. In the later classification of Cutolo et al., three phases were defined, i.e. ‘early’, ‘active’ and ‘late’ that reflect the time-related evolution of the capillaroscopic changes suggesting their association with disease duration. Current knowledge about the microvascular changes in systemic sclerosis supports both associations with disease activity and disease duration. The general opinion about the association of capillaroscopic findings with clinical involvement and disease activity in systemic sclerosis is not uniform. This is supposedly because the phase changes of systemic sclerosis–related microangiopathy are almost a universal feature in scleroderma and are not specific for a certain type of an accompaning clinical manifestation. Thus, the speed of progression of microvascular alterations might be a decisive criterion, and in cases of rapid dynamics of capillaroscopic findings, it could be considered as an indicator of disease activity. Interestingly, vascular ‘recovery’ has been observed after treatment with immunosuppressive drugs, haematopoietic stem cell transplantation, and the endothelin receptor antagonist – bosentan. The evolving knowledge about nailfold capillaroscopy in systemic sclerosis will further spread its application from a mainly diagnostic tool to an established, reliable method for evaluation of disease activity, prognosis and therapeutic response.
Keywords: Systemic sclerosis, capillaroscopy, ‘scleroderma’-type capillaroscopic pattern, microangiopathy, diagnostic tool
Introduction
In the nailfold area, capillary loops are oriented parallel to the skin surface that allows visualization of their whole length, while in the other areas they are perpendicular to the skin. Nailfold capillaroscopy is a unique method that differs with noninvasiveness, easy and rapid performance and is the only technique for morphological evaluation of the nailfold capillaries. Its major role in rheumatology is to facilitate the differential diagnosis of Raynaud’s phenomenon. In primary Raynaud’s phenomenon and in systemic sclerosis (SSc) the respective capillaroscopic findings are diagnostic.1–4 The capillaroscopic changes in SSc are specific and can be detected in the vast majority of patients. They include the presence of giant capillaries, haemorrhages, avascular areas, capillary derangement and neoangiogenic capillaries. 5 They were recently validated and accepted as a diagnostic criterion in the current 2013 European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) classification criteria for the disease.3,4 SSc differs with unique pathogenesis that includes microangiopathy, fibrosis of skin and internal organs and autoimmune alterations. The dominant interest in capillaroscopy in SSc is associated with the early appearance of the specific microvascular abnormalities that precede the other pathological events.
Capillaroscopic changes could be also observed in a number of other connective tissue diseases, i.e. in dermato-myositis, mixed connective tissue disease (MCTD), undifferentiated connective tissue disease, systemic lupus erythematosus (SLE), rheumatoid arthritis and overlap syndromes.6,7 Practical issues that are associated with precise differential diagnosis and overlapping capillaroscopic features with SSc are discussed in the current article. Microvascular alterations have been also observed in patients with non-rheumatic diseases such as diabetes mellitus, arterial hypertension and others as well as after prolonged exposure to vibration, chemical agents, ionizing radiation and other occupational factors.6,8,9 However, the capillaroscopic changes in SSc are striking and appear in a definite consequence, and in clinical context, they are accepted as a diagnostic marker of the disease.
Capillaroscopy technique
Videocapillaroscopy is currently the gold standard for morphological assessment of nailfold capillaries. Initially, capillaroscopy has been performed using microscopes (conventional, stereomicroscopes). Other devices that allow bed-side examination such as ophthalmoscope and dermatoscope have been also used,10–12 but provide images with lower quality. Although, generally all the fingers should be evaluated, nailfold capillaries are commonly visualized more clearly at the fourth and fifth fingers due to the greater transparency of the skin of these areas. 10 Adequate patient acclimatization is mandatory for 15–20 min prior the examination at a standard room temperature between 20 and 25°C.7,13 Fingers affected by local recent trauma should be excluded from analysis11,13 as well as those with recent manicure during the previous 4 weeks. The hand should be placed at the heart level, without pressure over the table whose temperature also should be standard to avoid any influence on the circulation. A drop of immersion oil (e.g., cedar oil) should be placed over the nailfold to improve skin transparency. If magnification 200× is used, about a millimetre of the nailfold area is visualized that provides the opportunity for assessment of both capillary distribution and density as well as details in the morphology of single-capillary loops.
The concept for ‘scleroderma’ pattern – its establishment as a ‘unique’ pattern in rheumatology
In 1925, Brown and O’Leary described skin capillaroscopic changes in SSc that included giant capillary loops, elongated capillaries and low capillary number per millimetre. The authors also observed that capillary borders were indistinct with feathery appearance that was associated with decreased skin visibility due to collagen deposition in the skin. 14 In the next years, a number of authors have published their observations on microvascular alterations in scleroderma that included capillary enlargement15–17 and the presence of giant capillary loops, 18 haemorrhages, 16 and reduced capillary number.17,18 Later, Maricq et al. performed extensive research on capillaroscopic changes in SSc. The authors also suggested the term ‘scleroderma’-type capillaroscopic pattern 3 characterized with the presence of giant capillaries, haemorrhages, avascular areas and ramified capillary loops.5,19,20
The ‘scleroderma’-type capillaroscopic pattern is met in the majority of patients with overt SSc (>90% of the cases), which is associated with the high prevalence of Raynaud’s phenomenon in the disease. Maricq et al.19,20 detected ‘scleroderma’ pattern in 82%–95% of SSc patients. In an own study, the specific ‘scleroderma’ pattern of the hands was detected in 97.2% (35/36) of SSc patients.7,21–23
Staging of ‘scleroderma’ pattern
Initially, Maricq et al. 20 suggested the presence of two major categories of capillaroscopic changes in SSc – an ‘active’ and ‘slow’ capillaroscopic pattern. This description has been accompanied by the hypothesis about a correlation between capillaroscopic changes and disease activity and progression. The presence of severe capillary loss with extensive avascular areas and new blood vessel formation was termed ‘active’ type ‘scleroderma’ pattern and was suggested to be associated with disease activity, tendency to disease progression and poor prognosis. High number of giant capillary loops with mild capillary loss was termed ‘slow’-type ‘scleroderma’ pattern and was thought to express lower disease activity 20 (Table 1). Although not establishing it as a distinct pattern subtype, Maricq et al. 24 published a capillary image, in which they mention the existence of ‘early’-type scleroderma pattern in a scleroderma patient with the presence of initial capillary dilations.
Table 1.
| Staging according to Maricq et al. 20 | Staging according to Cutolo et al. 25 |
|---|---|
| ‘Slow’ – high number of giant capillary loops with mild capillary loss.‘Active’ – presence of severe capillary loss with extensive avascular areas and new blood vessel formation. |
‘Early’ – few giant capillaries, few microhaemorrhages, preserved capillary distribution and density. ‘Active’ – the number of megacapillaries and microhaemorrhages is higher; there is moderate loss of capillaries and capillary derangement. ‘Late’ – advanced capillary derangement, extensive avascular areas and neoangiogenic/bushy capillary loops. |
Currently, the classification of ‘scleroderma’-type capillaroscopic changes of Cutolo et al. 25 is widely used and includes three distinct phases, that is, ‘early’, ‘active’ and ‘late’. The ‘early’ phase is characterized with appearance of few giant capillaries and few microhaemorrhages, while capillary distribution and density are preserved. In the ‘active’ phase of ‘scleroderma’-type capillaroscopic changes, the number of megacapillaries and microhaemorrhages is higher, and there is a moderate loss of capillaries and capillary derangement. In the ‘late’ phase, the capillary derangement is advanced, and there are extensive avascular areas as well as neoangiogenic/bushy capillary loops 25 (Table 1). This type of staging as well as the respective terminology mainly reflects the progression of SSc-related microangiopathy during disease evolution and suggests a prominent association with disease duration.
Inhomogeneity of nailfold capillaroscopic changes in SSc
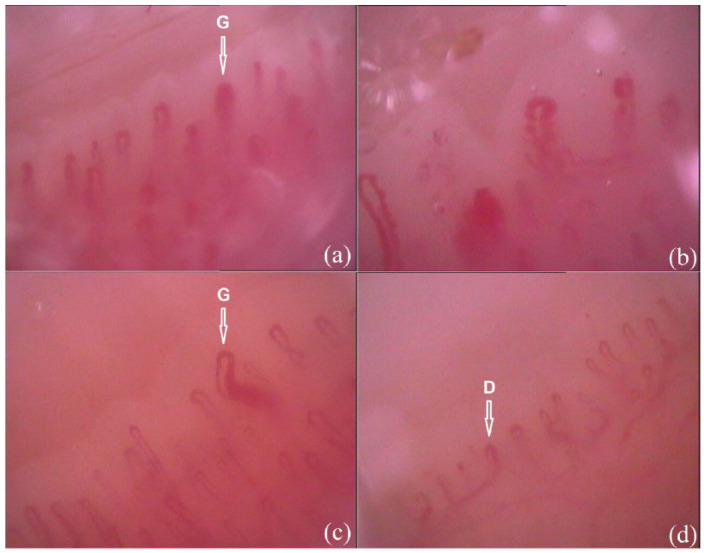
The microangiopathy is a major component of the pathological processes in SSc and affects the vast majority of the patients. Its detection in the nailfold area by means of the nailfold capillaroscopy facilitates early diagnosis of SSc and provides opportunity for monitoring the microvascular changes in the disease course. SSc-related microangiopathy is generalized and could be observed at both fingers and toes as demonstrated in our previous study.7,21 However, the capillaroscopic findings are inhomogeneous.23,24,26 Maricq et al. 24 reported a case of a scleroderma patient, in whom one digit had a normal pattern, while a second digit of the same patient had the characteristic ‘scleroderma’ pattern, and underlined the necessity of examination of all the digits in each patient. Data about the frequency of the phenomenon of inhomogeneity in SSc and about the possible combinations of different microvascular changes at different fingers have not been previously reported. In an own study, ‘scleroderma’-type capillaroscopic changes from different phases as well as combinations between ‘scleroderma’ pattern and normal and/or non-specific findings of different fingers have been observed.23,26 The combinations of ‘scleroderma’-type capillaroscopic changes that were commonly detected were ‘early-active’ and ‘active-late’ stages. 23 This supports the well-known association between the capillaroscopic changes and disease duration in SSc, whose consequence is ‘early’ – first stage, ‘active’ – second stage and ‘late’ – third stage. 25 The phenomenon of inhomogeneity of nailfold capillaroscopic changes in SSc is interesting for clinical practice as it requires detailed examination of the fingers in order to assess the presence of microvascular pathology accurately (Figure 1). Considering the lower capillary visibility of the thumbs, at least fingers from second to fifth (overall eight fingers) bilaterally should be examined. Of note, thumb involvement has been suggested as a clue for secondary nature of Raynaud’s phenomenon. 27 Consequently, especially in cases of clinical involvement of the thumbs, their capillaroscopic findings can also be assessed. In addition, the examination of the toes may also be taken into account and may provide additional diagnostic hints in a proportion of patients.7,21
Figure 1.
Inhomogeneous capillaroscopic findings in 52-year-old woman with SSc with limited cutaneous involvement – CREST syndrome; duration of Raynaud’s phenomenon – 10 years, duration of SSc – 5 years; mild skin involvement with Rodnan skin score – 4 and anti-centromere antibody positive (magnification 200×). (a) ‘Early’-phase ‘scleroderma’-type capillaroscopic pattern of a finger (G-giant capillary loop); (b) ‘active’-phase ‘scleroderma’-type capillaroscopic pattern of a finger; (c) ‘early’-phase ‘scleroderma’-type capillaroscopic pattern of a toe (G-giant capillary loop) and (d) non-specific capillaroscopic findings of a toe with the presence of single dilated loops (D).
Nailfold capillaroscopy and the diagnosis of SSc
The earliest markers of microangiopathy are giant capillaries and microhaemorrhages, which are the keys for early diagnosis of SSc. Observation of a giant capillary loop (with a diameter of either the arterial or the venous limb greater than 50 µm/0.050 mm), even a single one, should be considered as an indicator of initial microangiopathy. 11 Although the target for assessment at the capillaroscopic examination is the distal nailfold capillary row, the detection of a giant capillary loop from proximal rows should be also taken into account. The increase in capillary diameter could be considered as a microvascular response to tissue hypoxia. Of note, increase in diameters of nail fold capillaries has been measured in healthy subjects after acute systemic hypoxia simulated by hypoxicator with an air separation system that delivers lower atmospheric O2 concentration. 28
An interesting question is the ‘evaluable’ capillary diameter. It has been established that only the red blood cell column is visible and could be measured within capillaroscopic images, while the capillary walls can rarely be detected by ordinary light microscopy. Via injection of fluorescein isothiocyanate–human albumin into the brachial artery, the surrounding plasma layer could be measured, which is in direct contact with the capillary wall. Thus, in healthy subjects, it has been found that the ‘true’ capillary diameter of the arterial and venous capillary limb is 4–5 µm wider than the erythrocyte column visualized at conventional nailfold capillaroscopy.29,30 Fluorescein isothiocyanate–human albumin–marked capillary lumen (or true capillary lumen) exceeded the width of the erythrocyte column by 4.2 ± 0.7 µm for the arterial limb (erythrocyte column for the arterial limb 10.8 ± 3.0 µm) and by 4.6 ± 0.8 µm for the venous capillary limb (erythrocyte column for the venous diameter 12.0 ± 2.7 µm). 30 This part of capillary diameters that is ‘invisible’ at the conventional nailfold capillaroscopy has not been measured in patients with SSc. However, the observations in healthy subjects raise the question: ‘if we cannot measure the true capillary diameter, can we really detect the initial appearance of giant capillary loops in time?’ This consideration could potentially change the diagnostic algorithm in cases of early microangiopathy, as borderline values of dilated capillary loops could in fact represent giant capillary loops, specifically if the difference between visible and true capillary diameter is considered. As the true diameter is 4–5 µm wider than those measured at conventional capillaroscopic examination, then an arterial or venous capillary diameter of 45 µm (0.045 mm) may be of diagnostic value as an initial marker of microangiopathy. Thus, capillaries with arterial or venous diameter of 45 µm but below 50 µm could be giant capillary loops according to the value of the true capillary diameter. Association of these dilated capillaries with microhaemorrhages or with the presence of immunological markers should be sought in order to improve the diagnosis of early microangiopathy. In suspicious cases, especially when other pathological findings are absent, a closer follow-up should be recommended.
Of note, it has been observed that capillary dilation is the least sensitive parameter for qualitative analysis. In an own study, comparing the qualitative assessment of two investigators, a statistically significant difference was found for detection of dilated capillaries (p < 0.05), whereas for detection of giant capillaries, avascular areas and haemorrhages, the difference was not statistically significant (p > 0.05). The comparison of the quantitative and qualitative assessments of capillaroscopic images of two investigators demonstrated statistically significant difference between the two methods for the detection of dilated and giant capillaries (p < 0.05) but no significant difference regarding the detection of avascular areas (p > 0.05). These results indicate that quantitative analysis is more precise especially for the detection of capillary dilation. 31 Therefore, especially in borderline cases when the aim is diagnosing of early and very early microangiopathy, the quantitative measurement of capillary diameters should be preferred.
In a recent study of Boulon et al., 32 excellent inter- and intra-observer agreement was also observed between 11 experienced vascular physicians for the diagnosis of ‘scleroderma’ capillaroscopic pattern. The proportion of concordant inter-observer assessments between the 11 observers was 79% (70–87). Seven of the eleven observers rated the same images after 2 weeks and the median proportion of concordant intra-observer evaluations was 97% (89–98). The comparison of each observer with the reference provided a proportion of concordant observations of 79% (70–87). These data illustrate the very good reproducibility of nailfold videocapillaroscopy for the diagnosis of ‘scleroderma’ pattern by experienced vascular physicians, facilitating a reliable diagnosis in SSc patients. 32
Taken together, to enable a very early diagnosis of scleroderma-related microangiopathy, the following considerations need to be emphasized:
In cases of initial capillaroscopic changes, the values of the capillary diameters should be measured quantitatively;
Analysis of capillaroscopic findings of all fingers should be performed, as microvascular capillaroscopic changes are inhomogeneous and even a single giant capillary is of diagnostic value;
Cases with capillary loops with borderline dilation should be extensively analyzed.
Nailfold capillaroscopy and disease duration in SSc
Thе association between capillaroscopic changes and disease duration in SSc is a well-known phenomenon with subsequent appearance of ‘early-’, ‘active-’ and ‘late’-phase ‘scleroderma’-type capillaroscopic pattern. 25 In an own research that included 36 SSc patients (20 with limited, 5 with diffuse cutaneous involvement and 1 patient with overlap), this correlation was confirmed, and in SSc patients with duration of the disease – 4 years or less, classically an ‘early’-phase ‘scleroderma’-type capillaroscopic pattern was common and was found in 50% of the cases (5/10), while a ‘late’ phase was observed in a single patient with diffuse cutaneous involvement (10%). In SSc patients with duration of the disease 5 years or more, ‘early’-phase ‘scleroderma’-type capillaroscopic changes were observed in 26.9% (7/26) of the cases, all of whom were with limited cutaneous involvement. 23 Obviously, disease duration is just one of the numerous factors that affect the dynamics of capillaroscopic changes in SSc (Figures 2 and 3).
Figure 2.
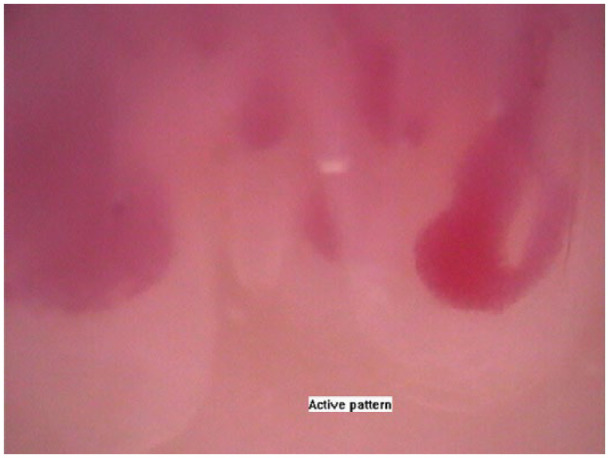
‘Active’-phase ‘scleroderma’-type capillaroscopic pattern in 58-year-old female SSc patient with limited cutaneous involvement; duration of Raynaud’s phenomenon – 20 years, duration of SSc – 20 years; sclerodactyly, mild skin involvement with modified Rodnan skin score – 3, recurrent digital ulcers, telangiectasia and anti-centromere antibody positive; homogeneous capillaroscopic findings – ‘active’-phase ‘scleroderma’-type pattern of all the fingers (magnification 200×).
Figure 3.
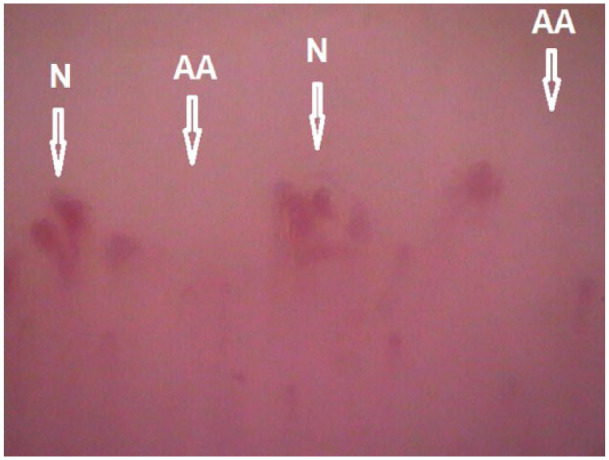
‘Late’-phase ‘scleroderma’-type capillaroscopic pattern in 68-year-old female SSc patient with diffuse cutaneous involvement. The duration of both Raynaud’s phenomenon and SSc is 6 years; modified Rodnan skin score – 46. The patient presents also with pulmonary arterial hypertension at exertion, sicca syndrome and arthritis (N: neoangiogenesis; AA: avascular areas; magnification 200×).
Nailfold capillaroscopy in SSc and its association with clinical picture and immunological profile
The association between disease duration and capillaroscopic changes seems to be a logical consequence of the microvascular evolution. Maricq et al. 20 also suggested the existence of two major subtypes, i.e. ‘slow’ and ‘active’ types, as well as an association between capillaroscopic findings and disease activity, degree of progression and prognosis that is also well reflected by the suggested terminology (‘slow’ type in patients with low disease activity and vice versa). Most of the scientists suggest also a correlation between the type of capillaroscopic changes and extent of cutaneous and visceral involvement in SSc,13,20,33–40 while other authors do not support this idea.41,42
Maricq et al. 24 did not find an association between the degree of capillary changes and the stage of cutaneous disease. The authors observed that in five patients with only modest capillary changes, three had advanced stages of cutaneous involvement, while other three cases with advanced microangiopathy were in the early stages of their cutaneous disease. 24
Cutolo et al. 25 have found that the three phases of ‘scleroderma’-type capillaroscopic changes (‘early’, ‘active’, and ‘late’) could be observed in both disease subtypes with limited and diffuse cutaneous involvement. However, tendency for predominance of the different phases according to the extent of cutaneous involvement has been noted. 13 Capillaroscopic examinations in 241 SSc patients by Cutolo et al. 13 revealed that patients with ‘early’- and ‘active’-phase ‘scleroderma’-type capillaroscopic pattern showed more frequently limited cutaneous form of the disease versus those with ‘late’-phase capillaroscopic changes. Conversely, the cases with ‘late’-phase ‘scleroderma’ pattern were more frequently affected by the diffuse cutaneous form of the disease. 13 In an analysis of a large cohort of SSc patients from the EUSTAR database with available data on subtypes of ‘scleroderma’-type capillaroscopic pattern, 39 a ‘late’ scleroderma pattern was observed more frequently in Scl-70 positive cases with diffuse cutaneous involvement.
Comparing 50 SSc patients with limited and 55 SSc patients with diffuse cutaneous involvement, who had no significant difference in disease duration, Ostojić and Damjanov 38 found that enlarged capillaries without significant capillary loss were more common in patients with limited cutaneous involvement. Very enlarged capillaries with advanced capillary loss were more common in patients with diffuse cutaneous subtype of the disease together with digital ulcers, in the presence of interstitial lung fibrosis, decreased forced vital capacity and diffusing capacity of carbon monoxide (DLCO), oesophageal hypomotility, musculoskeletal impairment and heart and renal involvement. Other features like fingertip osteolysis, telangiectasia and arthritis were observed with equal frequency in the two forms of the disease. 38 Shenavandeh et al. 40 did not find an association between the patterns of capillaroscopy and SSc cutaneous subtypes, but a significant association was evident when the analysis was performed based on the disease duration. The ‘early’-phase ‘scleroderma’ pattern was observed more frequently in patients with early limited cutaneous scleroderma versus the early diffuse cutaneous form, which could be compatible with slower course of the limited cutaneous subtype. Of note, subtle capillaroscopic changes, capillary elongation and capillary tortuosity had an inverse association with some clinical manifestations, and the authors suggested that they might be considered as good prognostic factors. 40
In 91 SSc patients, Bredemeier et al. 35 found an association between higher skin scores, longer disease duration, signs of peripheral ischaemia, esophageal dysfunction, anti-topoisomerase I/anti-Scl-70 antibodies, ground-glass opacities and higher mean avascular scores in nailfold capillaroscopy. The association between ground-glass opacities and higher avascular scores was particularly pronounced in cases with shorter disease duration ⩽5 years. Thus, it has been concluded that the severity of capillaroscopic abnormalities is associated with lung disease activity in SSc, especially in cases with relatively short disease duration. 35 In a study with 103 SSc patients, Caramaschi et al. 37 found a correlation between SSc-related microangiopathy and disease subset, severity of peripheral vascular, skin, heart and lung involvement. ‘Early’ and ‘active’ patterns were more common among patients with limited cutaneous form of SSc, whereas the ‘late’ pattern was more frequent in patients with diffuse cutaneous SSc. 37 Thus, it could be hypothesized that in the diffuse cutaneous form of SSc, accelerated dynamics in microvascular changes could be observed (Figure 3).
Nailfold capillaroscopy and pulmonary arterial hypertension in SSc
An association between capillaroscopic findings in SSc and the pulmonary arterial hypertension (PAH), which is the major cause for increased mortality in scleroderma patients, has been suggested,39,43 but the general expert opinion is not homogeneous.44,45
In 40 SSc patients, a significantly lower capillary density has been found in patients with SSc-related PAH (n = 21) as compared with those without PAH (4.33/mm vs 6.56/mm, p = 0.001), while loop dimensions were equal. Of note, a reduction in capillary density, although to a milder extent, was also noted in patients with idiopathic PAH as compared with healthy controls. A correlation between capillary density and severity of PAH in both SSc-related and idiopathic PAH has been also suggested. 43 In contrast, Greidinger et al., 45 using qualitative assessment of the capillaroscopic changes, found no difference between SSc patients with and without PAH. The authors did not observe “scleroderma-like” capillaroscopic changes in patients with idiopathic PAH. 45
Consequently, the predominant data indicate that peripheral microvascular changes are associated with cutaneous and visceral involvement. However, some studies do not confirm such an association. This is supposedly, because, the phase changes of SSc-related capillaroscopic findings are a universal feature in scleroderma patients and are not specific for a certain type of an accompanying clinical manifestation. However, a dynamic progression of peripheral capillaroscopic findings for short periods of time may indicate disease activity including extensive cutaneous and/or visceral involvement.
Nailfold capillaroscopy and the presence of digital ulcers in SSc
An association between capillaroscopic findings in SSc and the presence of digital ulcers has been suggested. Avascular areas on nailfold capillaroscopy have been proposed as a major risk factor for the development of skin ulcers in SSc in a study that included 130 SSc patients, of whom 110 underwent nailfold capillaroscopic examination. Other factors that were identified to influence development of skin ulcers were diffuse type of cutaneous involvement and systemic inflammation defined by interleukin 6 levels. 46 In an own study, in a group of 36 SSc patients, digital ulcers at the hands were detected in 36% of the cases (13/36). Interestingly, in all of them (13/13), an ‘active’-phase ‘scleroderma’-type capillaroscopic pattern was found at the finger with an active digital ulcer. 22
Prognostic value of nailfold capillaroscopy to predict future clinical outcomes in SSc
In a recent systematic literature review that included 18 studies, Paxton and Pauling 47 evaluated the prognostic value of nailfold capillaroscopic findings in SSc. The authors concluded that most of the studies (89%, 16/18) show positive associations between baseline capillaroscopic changes and clinical outcomes including digital ulcer occurrence/healing, survival, disease progression, calcinosis, skin progression, PAH and/or cardiovascular events. The majority of the evaluated studies were prospective longitudinal studies (13/18, 72%). The duration of the follow-up in the prospective studies varied and was relatively short. 47 Five of the prospective studies (38%) evaluated the association between nailfold capillaroscopy and digital ulcers occurrence and healing within 6 months.47–52 A recent prospective study of Avouac et al. that included 140 SSc patients and a longer period of follow-up (3 years) differs with high quality. 53 It has been suggested that patients with increased number of giant capillaries were at less risk to develop new digital ulcers. Loss of capillaries during the follow-up was found to be an independent marker of overall disease progression, appearance of new digital ulcers, progression of pulmonary vascular involvement, skin fibrosis and worsening of the Medsger severity score. 53
Nailfold capillaroscopy and immunological profile in SSc
The low sensitivity of the old ACR classification criteria (1980) for SSc had stimulated extensive research for improvement in the early diagnosis.54,55 Thus, LeRoy and Medsger 56 proposed the patients with objective signs of Raynaud’s phenomenon and abnormal nailfold capillaroscopic changes or positive SSc-selective autoantibodies (anti-centromere, anti-topoisomerase I/anti-Scl-70, anti-fibrillarin, anti-PM-Scl, anti-fibrillin or anti-RNA polymerase I or III in a titre of 1:100 or higher) to be diagnosed as ‘prescleroderma’ or limited SSc even in the absence of other manifestations of the disease. If Raynaud’s phenomenon is only subjectively reported, both ‘scleroderma’-type nailfold capillary pattern and SSc-selective antibodies should be present. 56 The multi-centre project VEDOSS (Very Early Diagnosis of SSc) has led recently to validation and establishment of a new set of criteria for SSc of EULAR and ACR (2013) with inclusion of both the specific capillaroscopic changes in SSc and SSc-related autoantibodies (anti-centromere, anti-topoisomerase I/anti-Scl-70 and anti-RNA polymerase III).3,4 Thus, the presence of specific ‘scleroderma’-type capillaroscopic changes and SSc-related autoantibodies in patients with Raynaud’s phenomenon are new criteria for diagnosing of SSc.
Less well-known is the question about association of certain autoantibodies with the different phases of the ‘scleroderma’-type capillaroscopic pattern. 13 A significant correlation between the presence of anti-centromere antibodies and the ‘slow’ capillary pattern as defined by Maricq et al. could be shown.20,33 In a study that included 241 SSc patients, Cutolo et al. 13 found anti-centromere antibodies more frequently in the ‘early’ pattern and decreased in the other patterns (‘active’ and ‘late’). Of note, the presence of anti-Scl-70 antibodies was significantly more frequent in patients with both the ‘active’ and ‘late’ phases capillaroscopic pattern versus the ‘early’ phase. Thus, it has been hypothesized that the presence of anti-Scl-70 antibodies might be related to the earlier development of the ‘active’ and ‘late’ phases of ‘scleroderma’-type capillaroscopic pattern. However, no significant correlation was found between the presence of the anti-Scl-70 antibodies and the duration of Raynaud’s phenomenon and SSc. Thus, it has been concluded that although SSc-related autoantibodies (anti-centromere and anti-Scl-70) are not directly linked with the development of a distinct ‘scleroderma’ pattern subtype, they are supposedly associated with the rate of progression of scleroderma microangiopathy. 13
‘Scleroderma’ and ‘scleroderma-like’ pattern
During their extensive research on capillaroscopic findings in connective tissue diseases, Maricq et al.5,19,20 found also components of the ‘scleroderma’ pattern in a group of diseases from the scope of scleroderma-spectrum disorders such as MCTD, undifferentiated connective tissue disease, and dermatomyositis. These changes, commonly termed ‘scleroderma-like’ pattern, based on the analogy with the changes in SSc, in fact establish the ‘scleroderma’ capillaroscopic pattern as a reference pattern in rheumatology.
In MCTD, the ‘scleroderma-like’ capillaroscopic changes have been described in a smaller proportion of patients versus SSc, i.e. in approximately half of the cases.12,19 De Holanda Mafaldo Diogenes et al. 57 have found “scleroderma-like” capillaroscopic changes in 65% -71.5% of cases in a group of 63 MCTD patients at dif-ferent time points during follow-up. Granier et al. 58 observed a similar frequency of “scleroderma-like” pattern in MCTD (63.6% among 22 patients). In MCTD patients, a higher frequency of bushy capillaries was found when compared with both SSc and SLE. 58 Similarly, it has been also suggested that bushy and branching capillaries could be found more frequently in dermatomyositis, although the capillaroscopic changes in dermatomyositis and SSc are usually indistinguishable. 24
In SLE, a ‘scleroderma-like’ capillaroscopic pattern was detected in a lower proportion of patients ranging from 2% to 15%.7,12,19,59–62 An association between the presence of ‘scleroderma-like’ changes and positive anti-U1-RNP antibody was noted that led to the idea that these microvascular changes are a feature of an overlap syndrome with SSc. 61 Of note, in 30 SLE patients aged 14.4 ± 3.3 years, 4 of whom were positive for anti-RNP antibodies, Piotto et al. 63 did not observe any capillaroscopic changes. This suggests the absence of an obligatory association between anti-RNP antibodies and capillaroscopic changes in SLE. In an own study, a ‘scleroderma-like’ pattern was observed in 13% of cases (4/30), all of whom showed symptoms of secondary Raynaud’s phenomenon and high immunological activity without signs of an overlap syndrome. In two of the four patients with a ‘scleroderma’-type pattern, active vasculitis of peripheral vessels was present. Of note, anti-RNP antibodies were positive in a single patient without overlap syndrome and without peripheral vessel vasculitis.7,62 Thus, it could be concluded that ‘scleroderma-like’ changes in SLE patients could be observed without evidence for an overlap with SSc or other connective tissue disease. In our experience “scleroderma-like” pattern could be found in SLE patients who exhibit either active vasculitis of peripheral vessels or only symptoms of secondary Raynaud’s phenomenon. Immunological profile should be assessed in these cases as high immunological activity may accompany these capillaroscopic changes.
It has been suggested that a ‘scleroderma-like’ capillaroscopic pattern could not be found in patients with rheumatoid arthritis.17,59 However, in an own study in 62 rheumatoid arthritis patients with and without Raynaud’s phenomenon, a ‘SSc-like’ pattern was observed in 14.5% of the cases (9/62; 2 males and 7 females). An overlap syndrome (rheumatoid arthritis overlap to SLE) with secondary Raynaud’s phenomenon and secondary vasculitis of peripheral vessels was present in a single case. In the remaining 8 patients, no overlap with other connective tissue disease was detected, but all rheumatoid arthritis patients with ‘SSc-like’ capillaroscopic pattern (9/9) exhibited symptoms of secondary Raynaud’s phenomenon and two of them had vasculitis of peripheral vessels. Based on these observations, it could be concluded that a ‘scleroderma-like’ capillaroscopic pattern could be observed in rheumatoid arthritis patients with secondary Raynaud’s phenomenon with or without vasculitis of peripheral vessels albeit with low frequency, and its presence is not mandatory in the context of overlap syndromes.7,64
The overall conclusion is that ‘scleroderma-like’ capillaroscopic changes may be observed within and beyond the scope of scleroderma-spectrum disorders, and interpretation of the capillaroscopic findings can only be made in a clinical context.
Nailfold capillaroscopy and evaluation of treatment response in SSc
Significant amelioration of capillaroscopic changes in SSc patients in response to treatment has been reported.65–70 A combined therapy with iloprost infusion and oral cyclosporine (2.5 mg/kg/daily) has shown significant positive influence on capillaroscopic changes in SSc after a period of 12 months. 65 Moreover, during a longer 3-year period of follow-up of SSc patients treated with oral cyclosporine (2.5 mg/kg/daily), progressive improvement of capillary lesions was observed in the first 2 years of therapy. 66 Rapid improvement of ‘scleroderma’-type capillaroscopic findings (with capillary haemorrhages, enlarged capillary loops and avascular areas) to almost normal pattern has been reported in a clinical case of SSc after intensive immunosuppression and haematopoietic stem cell transplantation. Mobilization of stem cells was performed using cyclophosphamide (4 g/m2), followed by granulocyte colony-stimulating factor. Conditioning included administration of cyclophosphamide (200 mg/kg) and 7.5 mg/kg rabbit anti-thymocyte globulin followed by stem cell reinfusion. Haematopoietic stem cell transplantation was performed 3 months after mobilization. Five months after the haemato-poietic stem cell transplantation, normalization of capillary number, size and architecture without capillary haemorrhages was observed. 67 Restoration of capillaroscopic pattern from ‘active’ to ‘early ’ phase was also seen in six SSc patients who underwent capillaroscopic examination 3 years after the baseline assessment. Five of them were treated with cyclophosphamide and one patient received methotrexate plus azathioprine. 68 In another study that included 16 patients with severe diffuse cutaneous SSc, the effect of haematopoietic stem cell transplantation and cyclophosphamide treatment on peripheral microangiopathy was assessed. Videocapillaroscopy demonstrated ‘late’-phase ‘scleroderma’-type pattern at baseline, and the follow-up continued 2 years. Six of the patients were treated with haematopoietic stem cell transplantation and 10 with monthly pulse cyclophosphamide (1 g) for 6 months followed by oral cyclophosphamide (50 mg daily) for 6 months. Three months after the haematopoietic stem cell transplantation, the capillaroscopic pattern has changed from ‘late’ into ‘active’ with appearance of frequent giant capillaries (>6/mm), haemorrhages and restoration of avascular areas. The newly appeared ‘active’ pattern was still present also at 6, 12 and 24 months. In patients treated with cyclophosphamide, no modification of the ‘late’-phase capillaroscopic pattern was observed during 24-month follow-up. 69
Bellando-Randone et al. 70 have assessed the effect of bosentan, sildenafil and their combination on capillaroscopic pattern in a retrospective study that included 123 SSc patients. In SSc patients treated with sildenafil, better improvement in hand function and Raynaud’s phenomenon attacks were observed as compared to bosentan, but the capillaroscopic findings worsened in the course of treatment with increase of the percentage of ‘late’ phase ‘scleroderma’ pattern. A significant improvement of capillaroscopic findings was detected in patients treated with bosentan alone or with a combination of bosentan and sildenafil after 3 and 6 months with reduction of the ‘late’- and ‘active’-phase ‘scleroderma’ pattern and an increase of the ‘aspecific’ findings or ‘early’ pattern. In patients, who received a combination therapy with bosentan and sildenafil, the best results were achieved in the earliest phase of SSc, characterized by a lower ACR/EULAR score versus those with more advanced disease. This suggests lower sensitivity of microvascular system to this therapeutic intervention in patients with more advanced clinical involvement. 70 These results can be explained by the crucial role of endothelin-1 in the pathogenesis of scleroderma-related microangiopathy. In SSc patients with ‘late’-phase capillaroscopic changes characterized by capillary loss and advanced fibrosis, Sulli et al. 71 detected higher endothelin-1 plasma levels versus those with ‘early’ phase and controls.
These observations suggest the potential of the capillaroscopic technique to be used for monitoring disease activity and to be applied as a method for investigation of those drugs that influence vascular remodelling and recovery and for evaluation of their long-term therapeutic effect. Currently, an effect on vascular recovery has been reported after treatment with immunosuppressive drugs, haemato-poietic stem cell transplantation and bosentan.
Conclusion
Since the initial description of the “scleroderma” type capillaroscopic pattern, the knowledge about the capillaroscopic abnormalities in SSc is constantly evolving. Currently, the characteristic “scleroderma” type capillaroscopic changes are diagnostic for SSc and it is well known that they go through different phases during disease evolution, but also may have a `reverse’ dynamic after treatment.
Although all three phases of scleroderma-type capillaroscopic pattern, i.e. ‘early, ’ ‘active, ’ and ‘late ’ could be observed in SSc with both limited and diffuse cutaneous involvement, the speed of dynamics of capillaroscopic changes may differ, being higher in rapidly progressive diffuse cutaneous forms of the disease. Of note, the expert opinion about the association between the capillaroscopic findings, clinical involvement and disease activity in SSc is not uniform. This is supposedly because the phase capillaroscopic changes of SSc-related microangiopathy are almost a universal feature in scleroderma patients (>90%) and are not specific for a certain type of an accompanying clinical manifestation. However, the speed of progression of microvascular alterations appears critical, and in cases of a rapid dynamic change of peripheral capillaroscopic findings for short periods of time, this should be considered as an indicator for disease activity including extensive cutaneous and/or visceral involvement as the latter should be evaluated and diagnosed in a clinical context.
In addition, capillaroscopic findings are inhomogeneous and for improvement of the diagnostic potential of nailfold capillaroscopy, detailed assessment of the fingers from second to fifth bilaterally (overall 8 fingers) or all 10 fingers in cases of clinical thumb involvement should be performed. Examination of the toes can also reveal the characteristic capillaroscopic abnormalities and may also be taken into account. Taken together, the evolving knowledge about nailfold capillaroscopy in SSc will further spread its application from a mainly diagnostic tool to an established, reliable and easy-to-perform method for evaluation of disease activity, prognosis and therapeutic response.
Take home messages
Capillaroscopic changes in SSc are diagnostic and are found in the majority of cases with clinically manifest scleroderma (>90%).
Capillaroscopic findings in scleroderma are not homogeneous. Microvascular changes from different phases could be found in different nailfold areas in the same patient as well as combinations between “scleroderma”-type pattern, normal and/or nonspecific findings.
There is an association between the capillaroscopic findings and disease duration in SSc although the time is not the only factor that determines the development of microvascular changes, which suggests the role of disease activity and other factors that may differ interindividually.
SSc-related capillaroscopic findings are almost a universal feature in scleroderma patients and are not specific for a certain type of an accompanying clinical manifestation. Thus, the speed of progression of microvascular alterations should be considered. The rapid dynamic change of peripheral capillaroscopic findings for short periods of time may indicate disease activity including extensive cutaneous and/or visceral involvement, as the latter should be evaluated and diagnosed in a clinical context.
Vascular recovery has been observed after treatment with immunosuppressive drugs, haematopoetic stem cell transplantation and bosentan.
“Scleroderma-like” changes could be observed in other connective tissue diseases with or without clinical sclerodermic features e.g., MCTD, overlap syndromes, undifferentiated connective tissue disease, in a proportion of patients with dermatomyositis. In addition, “scleroderma-like” changes could be also found in SLE and in rheumatoid arthritis, independent of the immunological status of the patients and without any indications for clinical or serological overlap. These conclusions require mandatory interpretation of the capillaroscopic findings in an individual clinical context.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Sevdalina Nikolova Lambova  https://orcid.org/0000-0002-1321-6770
https://orcid.org/0000-0002-1321-6770
References
- 1. LeRoy EC, Medsger TA., Jr. Raynaud’s phenomenon – a proposal for classification. Clin Exp Rheumatol 1992; 10(5): 485–488. [PubMed] [Google Scholar]
- 2. Maverakis E, Patel F, Kronenberg DG, et al. International consensus criteria for the diagnosis of Raynaud’s phenomenon. J Autoimmun 2014; 48-49: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Ann Rheum Dis 2013; 72(11): 1747–1755. [DOI] [PubMed] [Google Scholar]
- 4. van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Arthritis Rheum 2013; 65(11): 2737–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maricq HR, LeRoy EC. Patterns of finger capillary abnormalities in connective tissue disease by ‘wide-field’ microscopy. Arthritis Rheum 1973; 16(5): 619–628. [DOI] [PubMed] [Google Scholar]
- 6. Lambova SN, Muller-Ladner U. Nailfold capillaroscopy within and beyond the scope of connective tissue diseases. Curr Rheumatol Rev 2018; 14(1): 12–21. [DOI] [PubMed] [Google Scholar]
- 7. Lambova SN. The role of capillaroscopy in rheumatology. PhD Dissertation, Justus-Liebig University Giessen, Giessen, 2011. [Google Scholar]
- 8. Chojnowski MM, Felis-Giemza A, Olesińska M. Capillaroscopy – a role in modern rheumatology. Reumatologia 2016; 54(2): 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lambova SN, Müller-Ladner U. The specificity of capillaroscopic pattern in connective autoimmune diseases. A comparison with microvascular changes in diseases of social importance: arterial hypertension and diabetes mellitus. Mod Rheumatol 2009; 19(6): 600–605. [DOI] [PubMed] [Google Scholar]
- 10. Cutolo M, Grassi W, Matucci Cerinic M. Raynaud’s phenomenon and the role of capillaroscopy. Arthritis Rheum 2003; 48(11): 3023–3030. [DOI] [PubMed] [Google Scholar]
- 11. Cutolo M, Pizzorni C, Sulli A. Capillaroscopy. Best Pract Res Clin Rheumatol 2005; 19(3): 437–452. [DOI] [PubMed] [Google Scholar]
- 12. Bergman R, Sharony L, Schapira D, et al. The handheld dermatoscope as a nail-fold capillaroscopic instrument. Arch Dermatol 2003; 139(8): 1027–1030. [DOI] [PubMed] [Google Scholar]
- 13. Cutolo M, Pizzorni C, Tuccio M, et al. Nailfold videocapillaroscopic patterns and serum autoantibodies in systemic sclerosis. Rheumatology 2004; 43(6): 719–726. [DOI] [PubMed] [Google Scholar]
- 14. Brown GE, O’Leary PA. Skin capillaries in scleroderma. Arch Intern Med 1925; 36: 73–88. [Google Scholar]
- 15. Gilje O, O’Leary PA, Baldes EJ. Capillary microscopic examination in skin diseases. AMA Arch Derm Syphilol 1953; 68(2): 136–147. [DOI] [PubMed] [Google Scholar]
- 16. Buchanan IS, Humpston DJ. Nail-fold capillaries in connective-tissue disorders. Lancet 1968; 1(7547): 845–847. [DOI] [PubMed] [Google Scholar]
- 17. Redisch W, Messina EJ, Hughes G, et al. Capillaroscopic observations in rheumatic diseases. Ann Rheum Dis 1970; 29(3): 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rouen LR, Terry EN, Doft BH, et al. Classification and measurement of surface microvessels in man. Microvasc Res 1972; 4(3): 285–292. [DOI] [PubMed] [Google Scholar]
- 19. Maricq HR, LeRoy EC, D’Angelo WA, et al. Diagnostic potential of in vivo capillary microscopy in scleroderma and related disorders. Arthritis Rheum 1980; 23(2): 183–189. [DOI] [PubMed] [Google Scholar]
- 20. Maricq HR, Harper FE, Khan MM, et al. Microvascular abnormalities as possible predictors of disease subsets in Raynaud phenomenon and early connective tissue disease. Clin Exp Rheumatol 1983; 1(3): 195–205. [PubMed] [Google Scholar]
- 21. Lambova SN, Hermann W, Müller-Ladner U. Capillaroscopic pattern at the toes of systemic sclerosis patients: does it ‘tell’ more than those of fingers. J Clin Rheumatol 2011; 17(6): 311–314. [DOI] [PubMed] [Google Scholar]
- 22. Lambova SN, Müller-Ladner U. Capillaroscopic findings in systemic sclerosis – are they associated with disease duration and presence of digital ulcers. Discov Med 2011; 12(66): 413–418. [PubMed] [Google Scholar]
- 23. Lambova SN, Müller-Ladner U. New aspects regarding microvascular abnormalities in systemic sclerosis. Ann Rheum Dis 2012; 71(suppl. 3): 686. [Google Scholar]
- 24. Kenik JG, Maricq HR, Bole GG. Blind evaluation of the diagnostic specificity of nailfold capillary microscopy in the connective tissue diseases. Arthritis Rheum 1981; 24(7): 885–891. [DOI] [PubMed] [Google Scholar]
- 25. Cutolo M, Sulli A, Pizzorni C, et al. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J Rheumatol 2000; 27(1): 155–160. [PubMed] [Google Scholar]
- 26. Lambova SN, Müller-Ladner U. Mosaic capillaroscopic findings in systemic sclerosis. Wien Med Wochenschr 2018; 168(9–10): 248–249. [DOI] [PubMed] [Google Scholar]
- 27. Chikura B, Moore T, Manning J, et al. Thumb involvement in Raynaud’s phenomenon as an indicator of underlying connective tissue disease. J Rheumatol 2010; 37(4): 783–786. [DOI] [PubMed] [Google Scholar]
- 28. Paparde A, Nēringa-Martinsone K, Plakane L, et al. Nail fold capillary diameter changes in acute systemic hypoxia. Microvasc Res 2014; 93: 30–33. [DOI] [PubMed] [Google Scholar]
- 29. Bollinger A, Fagrell B. Clinical capillaroscopy – a guide to its use in clinical research and practice. Toronto, ON, Canada: Hogrefe & Huber Publishers, 1990, pp. 4–5, 44–52. [Google Scholar]
- 30. Mahler F, Nagel G, Saner H, et al. In vivo comparison of the nailfold capillary diameter as determined by using the erythrocyte column and FITC-labelled albumin. Int J Microcirc Clin Exp 1983; 2(2): 147–155. [PubMed] [Google Scholar]
- 31. Lambova SN, Hermann W, Müller-Ladner U. Comparison of qualitative and quantitative analysis of capillaroscopic findings in patients with rheumatic diseases. Rheumatol Int 2012; 32(12): 3729–3735. [DOI] [PubMed] [Google Scholar]
- 32. Boulon C, Blaise S, Lazareth I, et al. Reproducibility of the scleroderma pattern assessed by wide-field capillaroscopy in subjects suffering from Raynaud’s phenomenon. Rheumatology 2017; 56(10): 1780–1783. [DOI] [PubMed] [Google Scholar]
- 33. Chen ZY, Silver RM, Ainsworth SK, et al. Association between fluorescent antinuclear antibodies, capillary patterns, and clinical features in scleroderma spectrum disorders. Am J Med 1984; 77(5): 812–822. [DOI] [PubMed] [Google Scholar]
- 34. Houtman PM, Kallenberg CG, Wouda AA, et al. Decreased nailfold capillary density in Raynaud’s phenomenon: a reflection of immunologically mediated local and systemic vascular disease? Ann Rheum Dis 1985; 44(9): 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bredemeier M, Xavier RM, Capobianco KG, et al. Nailfold capillary microscopy can suggest pulmonary disease activity in systemic sclerosis. J Rheumatol 2004; 31(2): 286–294. [PubMed] [Google Scholar]
- 36. Cutolo M, Sulli A, Secchi ME, et al. Nailfold capillaroscopy is useful for the diagnosis and follow-up of autoimmune rheumatic diseases. A future tool for the analysis of microvascular heart involvement? Rheumatology 2006; 45(suppl 4): iv43–iv46. [DOI] [PubMed] [Google Scholar]
- 37. Caramaschi P, Canestrini S, Martinelli N, et al. Scleroderma patients nailfold videocapillaroscopic patterns are associated with disease subset and disease severity. Rheumatology 2007; 46(10): 1566–1569. [DOI] [PubMed] [Google Scholar]
- 38. Ostojić P, Damjanov N. Different clinical features in patients with limited and diffuse cutaneous systemic sclerosis. Clin Rheumatol 2006; 25(4): 453–457. [DOI] [PubMed] [Google Scholar]
- 39. Ingegnoli F, Ardoino I, Boracchi P, et al. Nailfold capillaroscopy in systemic sclerosis: data from the EULAR scleroderma trials and research (EUSTAR) database. Microvasc Res 2013; 89: 122–128. [DOI] [PubMed] [Google Scholar]
- 40. Shenavandeh S, Haghighi MY, Nazarinia MA. Nailfold digital capillaroscopic findings in patients with diffuse and limited cutaneous systemic sclerosis. Reumatologia 2017; 55(1): 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lefford F, Edwards JC. Nailfold capillary microscopy in connective tissue disease: a quantitative morphological analysis. Ann Rheum Dis 1986; 45(9): 741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lovy M, MacCarter D, Steigerwald JC. Relationship between nailfold capillary abnormalities and organ involvement in systemic sclerosis. Arthritis Rheum 1985; 28(5): 496–501. [DOI] [PubMed] [Google Scholar]
- 43. Hofstee HM, Vonk Noordegraaf A, Voskuyl AE, et al. Nailfold capillary density is associated with the presence and severity of pulmonary arterial hypertension in systemic sclerosis. Ann Rheum Dis 2009; 68(2): 191–195. [DOI] [PubMed] [Google Scholar]
- 44. Sato LT, Kayser C, Andrade LE. Nailfold capillaroscopy abnormalities correlate with cutaneous and visceral involvement in systemic sclerosis patients. Acta Reumatol Port 2009; 34(2A): 219–227. [PubMed] [Google Scholar]
- 45. Greidinger EL, Gaine SP, Wise RA, et al. Primary pulmonary hypertension is not associated with scleroderma-like changes in nailfold capillaries. Chest 2001; 120(3): 796–800. [DOI] [PubMed] [Google Scholar]
- 46. Alivernini S, De Santis M, Tolusso B, et al. Skin ulcers in systemic sclerosis: determinants of presence and predictive factors of healing. J Am Acad Dermatol 2009; 60(3): 426–435. [DOI] [PubMed] [Google Scholar]
- 47. Paxton D, Pauling JD. Does nailfold capillaroscopy help predict future outcomes in systemic sclerosis? A systematic literature review. Semin Arthritis Rheum 2018; 48(3): 482–494. [DOI] [PubMed] [Google Scholar]
- 48. Sebastiani M, Manfredi A, Colaci M, et al. Capillaroscopic skin ulcer risk index: a new prognostic tool for digital skin ulcer development in systemic sclerosis patients. Arthritis Rheum 2009; 61(5): 688–694. [DOI] [PubMed] [Google Scholar]
- 49. Sebastiani M, Manfredi A, Lo Monaco A, et al. Capillaroscopic Skin Ulcers Risk Index (CSURI) calculated with different videocapillaroscopy devices: how its predictive values change. Clin Exp Rheumatol 2013; 31(2 suppl 76): 115–117. [PubMed] [Google Scholar]
- 50. Sebastiani M, Manfredi A, Vukatana G, et al. Predictive role of capillaroscopic skin ulcer risk index in systemic sclerosis: a multicentre validation study. Ann Rheum Dis 2012; 71(1): 67–70. [DOI] [PubMed] [Google Scholar]
- 51. Manfredi A, Sebastiani M, Carraro V, et al. Prediction risk chart for scleroderma digital ulcers: a composite predictive model based on capillaroscopic, demographic and clinico-serological parameters. Clin Hemorheol Microcirc 2015; 59(2): 133–143. [DOI] [PubMed] [Google Scholar]
- 52. Cutolo M, Herrick AL, Distler O, et al. Nailfold videocapillaroscopic features and other clinical risk factors for digital ulcers in systemic sclerosis: a multicenter, prospective cohort study. Arthritis Rheum 2016; 68(10): 2527–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Avouac J, Lepri G, Smith V, et al. Sequential nailfold videocapillaroscopy examinations have responsiveness to detect organ progression in systemic sclerosis. Semin Arthritis Rheum 2017; 47(1): 86–94. [DOI] [PubMed] [Google Scholar]
- 54. Lonzetti LS, Joyal F, Raynauld JP, et al. Updating the American College of Rheumatology preliminary classification criteria for systemic sclerosis: addition of severe nailfold capillaroscopy abnormalities markedly increases the sensitivity for limited scleroderma. Arthritis Rheum 2001; 44(3): 735–736. [DOI] [PubMed] [Google Scholar]
- 55. Hudson M, Taillefer S, Steele R, et al. Improving the sensitivity of the American College of Rheumatology classification criteria for systemic sclerosis. Clin Exp Rheumatol 2007; 25(5): 754–757. [PubMed] [Google Scholar]
- 56. LeRoy EC, Medsger TA., Jr. Criteria for the classification of early systemic sclerosis. J Rheumatol 2001; 28: 1573–1576. [PubMed] [Google Scholar]
- 57. de Holanda Mafaldo Diógenes A, Bonfá E, Fuller R, et al. Capillaroscopy is a dynamic process in mixed connective tissue disease. Lupus 2007; 16(4): 254–258. [DOI] [PubMed] [Google Scholar]
- 58. Granier F, Vayssairat M, Priollet P, et al. Nailfold capillary microscopy in mixed connective tissue disease. Comparison with systemic sclerosis and systemic lupus erythematosus. Arthritis Rheum 1986; 29(2): 189–195. [DOI] [PubMed] [Google Scholar]
- 59. Nagy Z, Czirjak L. Nailfold digital capillaroscopy in 447 patients with connective tissue disease and Raynaud’s disease. J Eur Acad Dermatol Venereol 2004; 18(1): 62–68. [DOI] [PubMed] [Google Scholar]
- 60. Kabasakal Y, Elvins DM, Ring EF, et al. Quantitative nailfold capillaroscopy findings in a population with connective tissue disease and in normal healthy controls. Ann Rheum Dis 1996; 55: 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Furtado RNV, Pucinelli ML, Cristo VV, et al. Scleroderma-like nailfold capillaroscopic abnormalities are associated with anti-U1-RNP antibodies and Raynaud’s phenomenon in SLE patients. Lupus 2002; 11(1): 35–41. [DOI] [PubMed] [Google Scholar]
- 62. Lambova SN, Müller-Ladner U. Capillaroscopic pattern in systemic lupus erythematosus and undifferentiated connective tissue disease: what we still have to learn. Rheumatol Int 2013; 33(3): 689–695. [DOI] [PubMed] [Google Scholar]
- 63. Piotto DG, Len CA, Hilario MO, et al. Nailfold capillaroscopy in children and adolescents with rheumatic disease. Rev Bras Reumatol 2012; 52(5): 722–732. [PubMed] [Google Scholar]
- 64. Lambova SN, Müller-Ladner U. Capillaroscopic pattern in inflammatory arthritis. Microvasc Res 2012; 83(3): 318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Filaci G, Cutolo M, Scudeletti M, et al. Cyclosporin A and iloprost treatment of systemic sclerosis: clinical results and interleukin-6 serum changes after 12 months of therapy. Rheumatology 1999; 38(10): 992–996. [DOI] [PubMed] [Google Scholar]
- 66. Filaci G, Cutolo M, Basso M, et al. Long-term treatment of patients affected by systemic sclerosis with cyclosporin A. Rheumatology 2001; 40(12): 1431–1432. [DOI] [PubMed] [Google Scholar]
- 67. Aschwanden M, Daikeler T, Jaeger KA, et al. Rapid improvement of nailfold capillaroscopy after intense immunosuppression for systemic sclerosis and mixed connective tissue disease. Ann Rheum Dis 2008; 67(7): 1057–1059. [DOI] [PubMed] [Google Scholar]
- 68. Caramaschi P, Volpe A, Pieropan S, et al. Cyclophosphamide treatment improves microvessel damage in systemic sclerosis. Clin Rheumatol 2009; 28(4): 391–395. [DOI] [PubMed] [Google Scholar]
- 69. Miniati I, Guiducci S, Conforti ML, et al. Autologous stem cell transplantation improves microcirculation in systemic sclerosis. Ann Rheum Dis 2009; 68(1): 94–98. [DOI] [PubMed] [Google Scholar]
- 70. Bellando-Randone S, Lepri G, Bruni C, et al. Combination therapy with Bosentan and Sildenafil improves Raynaud’s phenomenon and fosters the recovery of microvascular involvement in systemic sclerosis. Clin Rheumatol 2016; 35(1): 127–132. [DOI] [PubMed] [Google Scholar]
- 71. Sulli A, Soldano S, Pizzorni C, et al. Raynaud’s phenomenon and plasma endothelin: correlations with capillaroscopic patterns in systemic sclerosis. J Rheumatol 2009; 36(6): 1235–1239. [DOI] [PubMed] [Google Scholar]