Abstract
Interstitial lung disease is a common manifestation of systemic sclerosis. Systemic sclerosis–associated interstitial lung disease is characterized by progressive pulmonary fibrosis and a reduction in pulmonary function. Effective treatments for systemic sclerosis–associated interstitial lung disease are lacking. In addition to clinical similarities, systemic sclerosis–associated interstitial lung disease shows similarities to idiopathic pulmonary fibrosis in the pathophysiology of the underlying fibrotic processes. Idiopathic pulmonary fibrosis and systemic sclerosis–associated interstitial lung disease culminate in a self-sustaining pathway of pulmonary fibrosis in which fibroblasts are activated, myofibroblasts accumulate, and the excessive extracellular matrix is deposited. Nintedanib is a tyrosine kinase inhibitor that has been approved for the treatment of idiopathic pulmonary fibrosis. In patients with idiopathic pulmonary fibrosis, nintedanib slows disease progression by decreasing the rate of lung function decline. In this review, we summarize the antifibrotic, anti-inflammatory, and attenuated vascular remodeling effects of nintedanib demonstrated in in vitro studies and in animal models of aspects of systemic sclerosis. Nintedanib interferes at multiple critical steps in the pathobiology of systemic sclerosis–associated interstitial lung disease, providing a convincing rationale for its investigation as a potential therapy. Finally, we summarize the design of the randomized placebo-controlled SENSCIS® trial that is evaluating the efficacy and safety of nintedanib in patients with systemic sclerosis–associated interstitial lung disease.
Keywords: Fibroblasts, fibrosis, scleroderma, systemic sclerosis–associated interstitial lung disease, tyrosine kinase inhibitor
Introduction
Systemic sclerosis (SSc) is a rare connective tissue disease associated with high morbidity and mortality.1–3 SSc is characterized by systemic (multi-organ) immunological, vascular, and fibrotic abnormalities, and is highly heterogeneous in its manifestations and clinical course. 2 , 3 Skin thickening, sclerosis, and ulceration, particularly of the fingers, are common and can cause considerable disability, 4 , 5 while the disease can also affect the pulmonary, cardiovascular, esophageal/gastrointestinal, musculoskeletal, and renal systems. 3 Interstitial lung disease (ILD) and pulmonary arterial hypertension are the leading causes of SSc-related death. 6 A meta-analysis of data from 13,529 patients estimated that survival following diagnosis of SSc was 74.9% at 5 years and 62.5% at 10 years. 1
Systemic sclerosis–associated interstitial lung disease (SSc-ILD) is characterized by progressive pulmonary fibrosis, deterioration in lung function, symptoms of cough and dyspnea, and reduced quality of life.7–9 SSc-ILD shows similarities to idiopathic pulmonary fibrosis (IPF), a more common progressive-fibrosing ILD, in its natural history but tends to progress more slowly than IPF and to be associated with a non-specific interstitial pneumonia pattern on high resolution computer tomography (HRCT) rather than usual interstitial pneumonia, as seen in IPF. 10 Acute deteriorations in lung function, while less common than in IPF, may also occur in patients with SSc-ILD. 11 , 12
Current treatment of SSc
There are no approved treatments for SSc, but a multitude of drugs are used to treat certain manifestations of SSc and its related comorbidities. 3 Immunosuppressant therapy is most frequently used, including glucocorticoids, cyclophosphamide (CYC), mycophenolate mofetil (MMF), azathioprine, and methotrexate. 13 , 14 In Scleroderma Lung Study I (SLS I), conducted in 158 patients with SSc-ILD, treatment with CYC for 1 year provided a modest but significant benefit on forced vital capacity (FVC) percent predicted versus placebo, as well as improvements in dyspnea and skin thickening. 15 However, the use of CYC is limited due to its toxicity. In Scleroderma Lung Study II (SLS II), in which 142 patients with SSc-ILD received oral MMF for 2 years or oral CYC for 1 year followed by placebo for 1 year, improvements in FVC percent predicted, dyspnea, and skin thickness were observed at 2 years in both groups, with no significant between-group difference, but with fewer adverse event–related treatment discontinuations in patients receiving MMF versus CYC. 16 The latest treatment guidelines for SSc issued by the European League Against Rheumatism Collaborative Initiative (EULAR) and the EULAR Scleroderma Trials and Research Group (EUSTAR) recommend tailored CYC therapy for SSc-ILD, particularly in the case of progressive disease. 17 These guidelines also recommend that autologous hematopoietic stem cell transplantation (HSCT) should be considered for patients who have rapidly progressive SSc at risk of organ failure, with careful evaluation of the risk–benefit profile for individual patients. HSCT has shown not only considerable benefits in selected patients with SSc but also significant treatment-related mortality. 18 There is a considerable unmet need for new therapies for SSc-ILD with proven efficacy and safety, and several novel compounds, with varying mechanisms of action, are under investigation as potential treatments for this condition. 19
Pathogenesis of SSc and SSc-ILD
The pathogenesis of SSc is extremely complex, but it is believed that the clinical manifestations of SSc result from distinct but highly interdependent processes: (a) vascular damage involving microvascular endothelial cells leading to fibroproliferative vasculopathy and capillary rarefication, perivascular inflammation, and autoimmune activation; (b) abnormalities in the innate and adaptive immune system resulting in autoantibody production, cell-mediated autoimmunity, and the release of profibrotic mediators; and ultimately (c) activation of fibroblasts to myofibroblasts, leading to excessive extracellular matrix (ECM) deposition in skin, blood vessels, and internal organs. 20 , 21
Damage to vascular endothelial cells, which may result from physical trauma, ischemia reperfusion injury, infectious agents, cytotoxic T cells, autoantibodies, or oxidative stress, induces the release of chemokines and growth factors such as endothelins, platelet-derived growth factor, and vascular endothelial growth factor. 22 , 23 These change endothelial permeability and stimulate recruitment and proliferation of leukocytes. These factors and cells would normally control the healing process. However, in patients with SSc, repetitive vascular damage and a failure to resolve the inflammatory response and repair the endothelium evoke adaptive and innate immune mechanisms, including the buildup of macrophages and neutrophils, which recruit lymphocytes to sites of injury. The contribution of endothelial apoptosis to the pathogenesis of SSc is incompletely understood. Apoptosis of endothelial cells might contribute to tissue injury when they are engulfed by macrophages and immature dendritic cells, which then present antigens to T cells, 24 or activate the complement and coagulant pathway, resulting in vasculopathy. 25 , 26 The role of monocytes/macrophages is also debated. Classically activated macrophages of the M1 type and alternatively activated macrophages of the M2 type belong to several subgroups stimulated by mediators like interferon gamma (IFNγ), tumor necrosis factor (TNF), interleukin (IL)-4, IL-6, IL-13, IL-10, and macrophage colony-stimulating factor (M-CSF). M1 and more prominently M2 signatures have been described in the blood, skin, and lung of patients with SSc, but their relevance remains to be defined. 27
Plasma cells secrete specific autoantibodies against host cell antigens and can worsen tissue injury. 28 Chemokines and growth factors originating from the endothelium recruit and activate mesenchymal progenitor cells and resident fibroblasts. Profibrotic factors secreted by activated T cells stimulate the activation of fibroblasts, as well as the synthesis and secretion of ECM. 29 Differentiation of fibroblasts into contractile myofibroblasts expressing alpha smooth muscle actin (αSMA) augments the pathologic processes. Continued production and deposition of ECM components, particularly fibrillar collagen types I and III, within connective tissues causes fibrosis, tissue contraction, and scarring. 30 This inflammation phase involves the activation of T cells and the development of T cells with mainly type 2 T-helper-cell profibrotic cytokine profiles (IL-4, IL-5, and IL-13). Additional T-cell subgroups and B cells, with abnormalities in phenotype and function, are also present. Mesenchymal cell populations, including fibroblasts, pericytes, and circulating progenitor cells, are activated and expanded, further contributing to tissue damage. 31
SSc-ILD shows similarities to IPF in the pathophysiology of the underlying fibrotic cascade. Although the initiating and amplifying events are described to be different, 10 the pathogenesis of both these diseases ends with fibroblast activation, migration, proliferation, and myofibroblast accumulation with excessive ECM deposition, representing a common and self-sustaining final pathway of lung fibrosis. 32 While this suggests that drugs that target fundamental fibrotic pathways in IPF may also be effective in SSc-ILD, the pathological and clinical differences between these diseases mean that robust clinical trials are needed to ascertain the utility of drugs used in IPF in patients with SSc-ILD.
Mechanism of action of nintedanib in SSc and SSc-ILD
Nintedanib is a small-molecule tyrosine kinase inhibitor (TKI), which binds competitively to the adenosine triphosphate (ATP) binding pocket of kinases, blocking their downstream signaling. 33 Nintedanib targets platelet-derived growth factor receptor (PDGFR) α and β, fibroblast growth factor receptor (FGFR) 1–3, and vascular endothelial growth factor receptor (VEGFR) 1–3. 34 , 35 Nintedanib also inhibits Flt-3 (Fms-like tyrosine-protein kinase), Lck (lymphocyte-specific tyrosine-protein kinase), Lyn (tyrosine-protein kinase Lyn), Src (proto-oncogene tyrosine-protein kinase Src) kinases, 34 and the colony-stimulating factor 1 receptor (CSF1R). 36 , 37 Several of these kinase targets, including PDGFR, 38 FGFR, 39 VEGFR, 33 , 40 Src, 41 , 42 and CSF1R 43 are associated with pathogenic processes in lung fibrosis, while the Src-family kinases Lck and Lyn are involved in the activation of T cells and B cells. 44 , 45 Recently, additional potential kinase targets were described for nintedanib, 37 but their relevance for its antifibrotic activity is unknown (Figure 1).
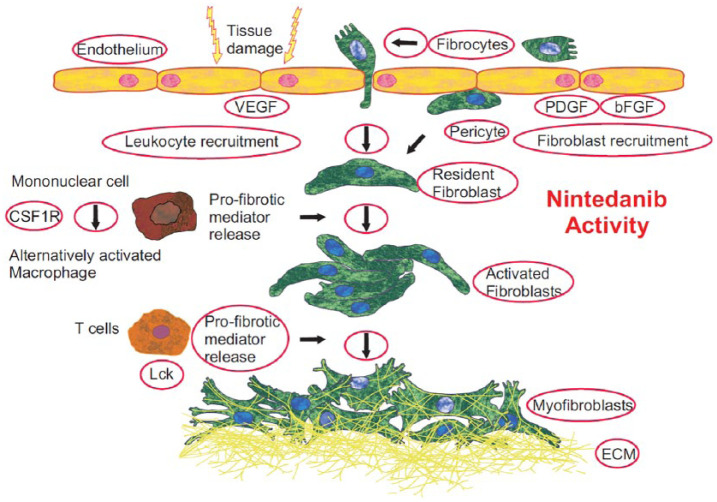
Figure 1.
Effects of nintedanib on pathogenic mechanisms with potential relevance in SSc.
This figure depicts pathogenic mechanisms of SSc that have been shown to be targeted by nintedanib in experiments on human cells or in animal models resembling aspects of SSc and lung fibrosis. Nintedanib potently targets fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor (PDGFR), vascular endothelial growth factor receptor (VEGFR), lymphocyte-specific tyrosine-protein kinase (Lck), and colony-stimulating factor 1 receptor (CSF1R). Nintedanib also exerts vascular effects, that is, inhibits the proliferation of endothelial cells and pericytes. Nintedanib reduces the recruitment of lymphocytes to the lung. Nintedanib inhibits the differentiation and migration of fibrocytes, and the migration, proliferation, and contraction of fibroblasts. By reducing the number of fibroblasts and their transformation to myofibroblasts, the secretion of extracellular matrix (ECM) is reduced. Furthermore, nintedanib blocks the differentiation of alternatively activated macrophages and the release of profibrotic mediators from T cells involved in the initiation of fibrosis.
Nintedanib was originally developed to be a treatment for non-small cell lung cancer by blocking neo-angiogenesis and with it, tumor growth. Preclinical pharmacodynamic exploration revealed that nintedanib attenuates the proliferation of three cell types involved in angiogenesis: endothelial cells, pericytes, and smooth muscle cells. 34 Later, nintedanib was developed as a treatment for IPF, which shows several similarities in pathogenic pathways to cancer. 46 Based on clinical trial data showing that nintedanib reduces the progression of IPF by slowing decline in lung function, 47 , 48 nintedanib has received regulatory approval as a treatment for IPF in many countries worldwide. The antifibrotic effects and safety of nintedanib demonstrated in patients with IPF are regarded as providing proof of concept for its investigation in patients with SSc-ILD.
The mechanism of action of nintedanib in lung fibrosis has been characterized based on in vitro studies and in vivo models. Nintedanib inhibits the proliferation, migration, and contraction of lung fibroblasts from patients with IPF, 33 as well as attenuates the differentiation and migration of profibrotic fibrocytes 49 and the transformation of lung fibroblasts to myofibroblasts. 35 Recently, nintedanib was also shown to restore the elastic modulus of fibrotic matrices to reverse the myofibroblastic phenotype of pericytes. 50 Nintedanib has demonstrated antifibrotic and anti-inflammatory activity in rodent models of pulmonary fibrosis created using various triggers. 33 , 35 , 51 In these models, nintedanib attenuated the accumulation of lymphocytes in bronchoalveolar lavage fluid; reduced levels of IL-1β, the chemokine CXCL1/KC, and the tissue inhibitor of metalloproteinases-1; blocked expression of fibrosis-related marker genes such as transforming growth factor beta 1 (TGFβ1) and procollagen 1; reduced histology scores of inflammation, granuloma formation, and fibrosis in the lungs; reduced lung tissue density and the collagen content of lung tissue; and improved static lung compliance. 33 , 35 , 51 In mice with bleomycin-induced pulmonary fibrosis, nintedanib also attenuated vascular proliferation, resulting in the normalization of the distorted microvascular architecture. 51
Recent in vivo investigations have revealed antifibrotic and anti-inflammatory activities of nintedanib in animal models of aspects of SSc. In bleomycin-induced skin fibrosis, graft versus host disease-induced skin fibrosis, tight skin (fibrillin-1 transgenic), and Fra-2 murine models of SSc, nintedanib reduced myofibroblast accumulation and ECM deposition in skin and lung, attenuated skin and lung fibrosis, and reduced dermal thickening. 52 , 53 In Fra-2+/– transgenic mice, nintedanib attenuated pulmonary vascular remodeling by reducing the number of vascular smooth muscle cells, pulmonary vascular wall thickness, and the number of occluded pulmonary vessels. 53 In addition, nintedanib improved vascular remodeling in the skin of Fra-2+/– mice, with a reduction in the number of endothelial cells undergoing apoptosis and an increase in the number of vessels in the dermis. 53 In in vitro studies, nintedanib has been shown to block the release of profibrotic mediators from human peripheral blood monocytic cells and T cells, 54 and reduce the M2 polarization of human macrophages exposed to macrophage colony-stimulating factor, IL-4, and IL-13. 36 , 53 In experiments in dermal fibroblasts from patients with SSc, nintedanib inhibited fibroblast migration and proliferation; decreased the expression of ECM markers: collagens 1a1 and 1a2, and fibronectin; and reduced transformation of fibroblasts to myofibroblasts as detected by reductions in αSMA and stress fibers. 52 An overview of the preclinical exploration of nintedanib in dermal fibroblasts from patients with SSc and in in vivo models of SSc/SSc-ILD is presented in Tables 1 and 2.
Table 1.
Exploration of nintedanib in dermal fibroblasts from patients with SSc. 52
| Model system/characteristics | Effects of nintedanib |
|---|---|
| TGFβ- and PDGF-induced ECM components and markers of fibroblast to myofibroblast transformation | Collagens 1a1 and 1a2, fibronectin, αSMA, mRNA ↓ Collagen, stress fibers, and TGFβ signaling ↓ |
| TGFβ- and PDGF-induced proliferation and migration of fibroblasts | TGFβ- and PDGF-induced proliferation ↓ TGFβ- and PDGF-induced migration ↓ |
SSc: systemic sclerosis; TGFβ: transforming growth factor beta; PDGF: platelet-derived growth factor; ECM: extracellular matrix; αSMA: alpha smooth muscle actin; ↓: significant reduction (independent of concentration used).
Table 2.
| Model system | Model characteristics | Treatment regimen | Effects of nintedanib |
|---|---|---|---|
| Bleomycin-induced skin fibrosis | Skin damage-induced/inflammation-induced fibrosis | Preventive (weeks 0–3) and therapeutic (weeks 3–6) a |
Skin
Myofibroblast count ↓ Dermal thickness ↓ Hydroxyproline ↓ |
| Graft versus host disease-induced skin fibrosis | Resembles aspects of early inflammatory stage of SSc | Therapeutic (weeks 4–8) a |
Skin
Myofibroblast count ↓ Dermal thickness ↓ Hydroxyproline ↓ |
| Tight skin (fibrillin-1 transgenic) | Resembles aspects of later stage of SSc with less inflammation but early autoantibody production and massive fibrosis | Therapeutic (weeks 5–10) b |
Skin
Myofibroblast count ↓ Hypodermal thickness ↓ Hydroxyproline ↓ |
| Fra-2 (AP-1 family transcription factor +/–) | Resembles aspects of skin and lung fibrosis, including microvascular disease and pulmonary hypertension with typical vascular lesions | Therapeutic (weeks 9–16) b |
Skin
Myofibroblasts count ↓ Dermal thickness ↓ Hydroxyproline ↓ MVEC apoptosis ↓ Capillary loss ↓ M2 macrophages ↓ Lung Myofibroblast count ↓ ECM ↓ Vessel wall thickness ↓ Occluded vessels ↓ VSMC ↓ MVEC apoptosis ↓ Heart Extent of fibrosis ↓ Perivascular inflammation ↓ Endothelial cells apoptosis ↓ |
SSc-ILD: systemic sclerosis–associated interstitial lung disease; MVEC: microvascular endothelial cells; ECM: extracellular matrix; VSMC: vascular smooth muscle cells.
Weeks after induction of pathology.
Weeks after birth.
The comparable effects of nintedanib in animal models of lung fibrosis and SSc suggest that the effective dose may be comparable in patients with IPF and SSc. Nintedanib effectively inhibited fundamental processes of skin fibrosis (i.e. the proliferation and migration of dermal fibroblasts) at concentrations of 100 nM, which are close to the exposure levels achieved in humans (59–74 nmol/L) after steady-state oral dosing of nintedanib 150 mg twice daily in patients with IPF. 55 , 56
Clinical investigation of nintedanib in SSc-ILD
The efficacy and safety of nintedanib in patients with SSc-ILD are being assessed in the SENSCIS® trial (ClinicalTrials.gov: NCT02597933; EudraCT: 2015-000392-28). 57 A total of 580 patients with an age of ⩾18 years with onset of SSc (first non-Raynaud’s symptom), ⩽7 years before screening, ILD (⩾10% fibrosis of the lungs on HRCT), FVC ⩾ 40% predicted, and diffusion capacity of the lung for carbon monoxide 30% to 89% predicted were enrolled. Patients receiving low-dose prednisone and/or stable background therapy with MMF or methotrexate were allowed to participate. While non-clinical data suggest that the potential risk of nintedanib to increase digital ulceration in patients with SSc is low, 53 and studies of patients with IPF who undergo lung transplant following treatment with nintedanib suggest that nintedanib does not have adverse events on wound healing,58–60 given that nintedanib is an inhibitor of the VEGF receptor, patients with >3 fingertip ulcers or a history of severe digital necrosis have been excluded from the trial as a precautionary measure.
Patients in the SENSCIS trial were randomized to receive nintedanib 150 mg twice daily or placebo, stratified by the presence of anti-Scl-70/anti-topoisomerase I antibody, which has been linked with accelerated progression of ILD. 61 The primary endpoint is the annual rate of decline in FVC (mL/year) evaluated over 52 weeks. Key secondary endpoints are absolute changes from baseline in the modified Rodnan skin score (a measure of skin thickening in patients with SSc) and in the St George’s Respiratory Questionnaire total score (a measure of health-related quality of life) at week 52. The SENSCIS trial is due to be completed near the end of 2018.
Conclusion
A high need exists for effective treatments for SSc-ILD. Nintedanib is an approved treatment for IPF, which shows clinical and mechanistic similarities to SSc-ILD. Nintedanib interferes at multiple critical steps in the pathobiology of SSc/SSc-ILD, and has demonstrated anti-inflammatory and antifibrotic activities and attenuated vascular remodeling in several models of SSc/SSc-ILD, providing a strong rationale for its investigation as a treatment for SSc-ILD. The efficacy and safety of nintedanib in patients with SSc-ILD, including patients on certain immunosuppressive regimens, are currently being investigated in the SENSCIS trial.
Acknowledgments
Editorial assistance, supported financially by Boehringer Ingelheim, was provided by Wendy Morris of FleishmanHillard Fishburn, London, UK, during the preparation of this article. The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version.
Footnotes
Declaration of conflicting interests: L.W. and M.G. are employees of Boehringer Ingelheim. J.H.W.D. is a member of a speaker’s bureau and has served on advisory boards for Boehringer Ingelheim; he has also conducted non-clinical studies on behalf of Boehringer Ingelheim and is an investigator for the SENSCIS® trial of nintedanib in patients with systemic sclerosis–associated interstitial lung disease (SSc-ILD). C.P.D. has received consultancy/speakers fees from Actelion, Pfizer, GlaxoSmithKline, Bayer, Sanofi-Aventis, Inventiva, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, and Biogen; received research grant funding from Actelion, Bayer, GlaxoSmithKline, and CSL Behring; and acted as a clinical trial investigator/steering committee member for Bayer, Pfizer, Actelion, Sanofi-Aventis, Inventiva, Boehringer Ingelheim, Roche, and Bristol-Myers Squibb.
Funding: Page processing fees for this manuscript have been paid by Boehringer Ingelheim.
ORCID iD: Lutz Wollin  https://orcid.org/0000-0002-3617-5772
https://orcid.org/0000-0002-3617-5772
References
- 1. Rubio-Rivas M, Royo C, Simeón CP, et al. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin Arthritis Rheum 2014; 44(2): 208–219. [DOI] [PubMed] [Google Scholar]
- 2. Allanore Y, Simms R, Distler O, et al. Systemic sclerosis. Nat Rev Dis Primers 2015; 1: 15002. [DOI] [PubMed] [Google Scholar]
- 3. Denton CP, Khanna D. Systemic sclerosis. Lancet 2017; 390: 1685–1699. [DOI] [PubMed] [Google Scholar]
- 4. Matucci-Cerinic M, Krieg T, Guillevin L, et al. Elucidating the burden of recurrent and chronic digital ulcers in systemic sclerosis: long-term results from the DUO Registry. Ann Rheum Dis 2016; 75(10): 1770–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hudson M, Thombs BD, Steele R, et al. Clinical correlates of quality of life in systemic sclerosis measured with the World Health Organization Disability Assessment Schedule II. Arthritis Rheum 2008; 59(2): 279–284. [DOI] [PubMed] [Google Scholar]
- 6. Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69(10): 1809–1815. [DOI] [PubMed] [Google Scholar]
- 7. Baron M, Sutton E, Hudson M, et al. The relationship of dyspnoea to function and quality of life in systemic sclerosis. Ann Rheum Dis 2008; 67(5): 644–650. [DOI] [PubMed] [Google Scholar]
- 8. Khanna D, Tseng CH, Farmani N, et al. Clinical course of lung physiology in patients with scleroderma and interstitial lung disease: analysis of the Scleroderma Lung Study Placebo Group. Arthritis Rheum 2011; 63(10): 3078–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jaeger VK, Wirz EG, Allanore Y, et al. Incidences and risk factors of organ manifestations in the early course of systemic sclerosis: a longitudinal EUSTAR study. PLoS ONE 2016; 11(10): e0163894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herzog EL, Mathur A, Tager AM, et al. Review: interstitial lung disease associated with systemic sclerosis and idiopathic pulmonary fibrosis: how similar and distinct? Arthritis Rheumatol 2014; 66(8): 1967–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tomiyama F, Watanabe R, Ishii T, et al. High prevalence of acute exacerbation of interstitial lung disease in Japanese patients with systemic sclerosis. Tohoku J Exp Med 2016; 239(4): 297–305. [DOI] [PubMed] [Google Scholar]
- 12. Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med 2016; 194(3): 265–275. [DOI] [PubMed] [Google Scholar]
- 13. Fernandez-Codina A, Walker KM, Pope JE; Scleroderma Algorithm Group. Treatment algorithms for systemic sclerosis according to experts. Arthritis Rheumatol 2018; 70(11): 1820–1828. [DOI] [PubMed] [Google Scholar]
- 14. Adler S, Huscher D, Siegert E, et al. Systemic sclerosis associated interstitial lung disease—individualized immunosuppressive therapy and course of lung function: results of the EUSTAR group. Arthritis Res Ther 2018; 20(1): 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tashkin DP, Elashoff R, Clements PJ, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 2006; 354: 2655–2666. [DOI] [PubMed] [Google Scholar]
- 16. Tashkin DP, Roth MD, Clements PJ, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med 2016; 4(9): 708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kowal-Bielecka O, Fransen J, Avouac J, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis 2017; 76(8): 1327–1339. [DOI] [PubMed] [Google Scholar]
- 18. Eyraud A, Scouppe L, Barnetche T, et al. Efficacy and safety of autologous haematopoietic stem cell transplantation in systemic sclerosis: a systematic review of literature. Br J Dermatol 2018; 178: 650–658. [DOI] [PubMed] [Google Scholar]
- 19. Khanna D, Tashkin DP, Denton CP, et al. Ongoing clinical trials and treatment options for patients with systemic sclerosis-associated interstitial lung disease. Rheumatology. Epub ahead of print 8 June 2018. DOI: 10.1093/rheumatology/key151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Denton CP, Black CM, Abraham DJ. Mechanisms and consequences of fibrosis in systemic sclerosis. Nat Clin Pract Rheumatol 2006; 2(3): 134–144. [DOI] [PubMed] [Google Scholar]
- 21. Pattanaik D, Brown M, Postlethwaite BC, et al. Pathogenesis of systemic sclerosis. Front Immunol 2015; 6: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Distler O, Del Rosso A, Giacomelli R, et al. Angiogenic and angiostatic factors in systemic sclerosis: increased levels of vascular endothelial growth factor are a feature of the earliest disease stages and are associated with the absence of fingertip ulcers. Arthritis Res 2002; 4(6): R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Distler O, Distler JH, Scheid A, et al. Uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis in patients with systemic sclerosis. Circ Res 2004; 95(1): 109–116. [DOI] [PubMed] [Google Scholar]
- 24. Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol 2001; 2(11): 1010–1017. [DOI] [PubMed] [Google Scholar]
- 25. Tsuji S, Kaji K, Nagasawa S. Activation of the alternative pathway of human complement by apoptotic human umbilical vein endothelial cells. J Biochem 1994; 116(4): 794–800. [DOI] [PubMed] [Google Scholar]
- 26. Greeno EW, Bach RR, Moldow CF. Apoptosis is associated with increased cell surface tissue factor procoagulant activity. Lab Invest 1996; 75(2): 281–289. [PubMed] [Google Scholar]
- 27. Stifano G, Christmann RB. Macrophage involvement in systemic sclerosis: do we need more evidence? Curr Rheumatol Rep 2016; 18(1): 2. [DOI] [PubMed] [Google Scholar]
- 28. Sato S, Fujimoto M, Hasegawa M, et al. Altered B lymphocyte function induces systemic autoimmunity in systemic sclerosis. Mol Immunol 2004; 41(12): 1123–1133. [DOI] [PubMed] [Google Scholar]
- 29. O’Reilly S, Hügle T, van Laar JM. T cells in systemic sclerosis: a reappraisal. Rheumatology 2012; 51(9): 1540–1549. [DOI] [PubMed] [Google Scholar]
- 30. Klingberg F, Hinz B, White ES. The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol 2013; 229(2): 298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ebmeier S, Horsley V. Origin of fibrosing cells in systemic sclerosis. Curr Opin Rheumatol 2015; 27(6): 555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bagnato G, Harari S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur Respir Rev 2015; 24(135): 102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wollin L, Wex E, Pautsch A, et al. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J 2015; 45(5): 1434–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res 2008; 68(12): 4774–4782. [DOI] [PubMed] [Google Scholar]
- 35. Wollin L, Maillet I, Quesniaux V, et al. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther 2014; 349(2): 209–220. [DOI] [PubMed] [Google Scholar]
- 36. Tandon K, Herrmann F, Ayaub E, et al. Nintedanib attenuates the polarization of profibrotic macrophages through the inhibition of tyrosine phosphorylation on CSF1 receptor. Am J Respir Crit Care Med 2017; 195: A2397. [Google Scholar]
- 37. Hilberg F, Tontsch-Grunt U, Baum A, et al. Triple angiokinase inhibitor nintedanib directly inhibits tumor cell growth and induces tumor shrinkage via blocking oncogenic receptor tyrosine kinases. J Pharmacol Exp Ther 2018; 364(3): 494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 2004; 15(4): 255–273. [DOI] [PubMed] [Google Scholar]
- 39. Inoue Y, King TE, Jr, Tinkle SS, et al. Human mast cell basic fibroblast growth factor in pulmonary fibrotic disorders. Am J Pathol 1996; 149(6): 2037–2054. [PMC free article] [PubMed] [Google Scholar]
- 40. Barratt SL, Flower VA, Pauling JD, et al. VEGF (vascular endothelial growth factor) and fibrotic lung disease. Int J Mol Sci 2018; 19(5): E1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beyer C, Distler JH. Tyrosine kinase signaling in fibrotic disorders: translation of basic research to human disease. Biochim Biophys Acta 2013; 1832(7): 897–904. [DOI] [PubMed] [Google Scholar]
- 42. Hu M, Che P, Han X, et al. Therapeutic targeting of SRC kinase in myofibroblast differentiation and pulmonary fibrosis. J Pharmacol Exp Ther 2014; 351(1): 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meziani L, Mondini M, Petit B, et al. CSF1R inhibition prevents radiation pulmonary fibrosis by depletion of interstitial macrophages. Eur Respir J 2018; 51(3): 1702120. [DOI] [PubMed] [Google Scholar]
- 44. Straus DB, Weiss A. Genetic evidence for the involvement of the Lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell 1992; 70(4): 585–593. [DOI] [PubMed] [Google Scholar]
- 45. Ochi H, Takeshita H, Suda T, et al. Regulation of B-1 cell activation and its autoantibody production by Lyn kinase-regulated signallings. Immunology 1999; 98(4): 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vancheri C. Idiopathic pulmonary fibrosis and cancer: do they really look similar? BMC Med 2015; 13: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med 2011; 365(12): 1079–1087. [DOI] [PubMed] [Google Scholar]
- 48. Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370(22): 2071–2082. [DOI] [PubMed] [Google Scholar]
- 49. Sato S, Shinohara S, Hayashi S, et al. Anti-fibrotic efficacy of nintedanib in pulmonary fibrosis via the inhibition of fibrocyte activity. Respir Res 2017; 18(1): 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sava P, Ramanathan A, Dobronyi A, et al. Human pericytes adopt myofibroblast properties in the microenvironment of the IPF lung. JCI Insight 2017; 2(24): e96352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ackermann M, Kim YO, Wagner WL, et al. Effects of nintedanib on the microvascular architecture in a lung fibrosis model. Angiogenesis 2017; 20(3): 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huang J, Beyer C, Palumbo-Zerr K, et al. Nintedanib inhibits fibroblast activation and ameliorates fibrosis in preclinical models of systemic sclerosis. Ann Rheum Dis 2016; 75(5): 883–890. [DOI] [PubMed] [Google Scholar]
- 53. Huang J, Maier C, Zhang Y, et al. Nintedanib inhibits macrophage activation and ameliorates vascular and fibrotic manifestations in the Fra2 mouse model of systemic sclerosis. Ann Rheum Dis 2017; 76(11): 1941–1948. [DOI] [PubMed] [Google Scholar]
- 54. Wollin L, Ostermann A, Williams C. Nintedanib inhibits pro-fibrotic mediators with relevance in connective tissue disease-associated interstitial lung diseases. Am J Respir Crit Care Med 2017; 195: A2450. [Google Scholar]
- 55. Mross K, Stefanic M, Gmehling D, et al. Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors. Clin Cancer Res 2010; 16(1): 311–319. [DOI] [PubMed] [Google Scholar]
- 56. Eisen T, Shparyk Y, Macleod N, et al. Effect of small angiokinase inhibitor nintedanib (BIBF 1120) on QT interval in patients with previously untreated, advanced renal cell cancer in an open-label, phase II study. Invest New Drugs 2013; 31(5): 1283–1293. [DOI] [PubMed] [Google Scholar]
- 57. Distler O, Brown KK, Distler JHW, et al. Design of a randomised, placebo-controlled clinical trial of nintedanib in patients with systemic sclerosis-associated interstitial lung disease (SENSCIS™). Clin Exp Rheumatol 2017; 35: 75–81. [PubMed] [Google Scholar]
- 58. Leuschner G, Stocker F, Veit T, et al. Outcome of lung transplantation in idiopathic pulmonary fibrosis with previous anti-fibrotic therapy. J Heart Lung Transplant. Epub ahead of print 5 July 2017. DOI: 10.1016/j.healun.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 59. Lambers C, Boehm PM, Lee S, et al. Effect of antifibrotics on short-term outcome after bilateral lung transplantation. Eur Respir J 2018; 51(6): 1800503. [DOI] [PubMed] [Google Scholar]
- 60. Tuyls S, Verleden SE, Wuyts WA, et al. Determinants of survival in lung transplantation patients with idiopathic pulmonary fibrosis: a retrospective cohort study. Transpl Int 2019; 32(4): 399–409. [DOI] [PubMed] [Google Scholar]
- 61. Assassi S, Sharif R, Lasky RE, et al. Predictors of interstitial lung disease in early systemic sclerosis: a prospective longitudinal study of the GENISOS cohort. Arthritis Res Ther 2010; 12(5): R166. [DOI] [PMC free article] [PubMed] [Google Scholar]