Abstract
Systemic sclerosis is the main systemic fibrotic disease with unknown etiology characterized by peripheral microvascular injury, activation of immune system, and wide-spread progressive fibrosis. Microparticles can be derived from any cell type during normal cellular differentiation, senescence, and apoptosis, and also upon cellular activation. Carrying along a broad range of surface cytoplasmic and nuclear molecules of originating cells, microparticles are closely implicated in inflammation, thrombosis, angiogenesis, and immunopathogenesis. Recently, microparticles have been proposed as biomarkers of endothelial injury, which is the primary event in the genesis of tissue fibrosis. Microparticles may have a role in fostering endothelial to mesenchymal transition, thus giving a significant contribution to the development of myofibroblasts, the most important final effectors responsible for tissue fibrosis and fibroproliferative vasculopathy. Thanks to potent profibrotic mediators, such as transforming growth factor beta, platelet-derived growth factor, high mobility group box 1 protein, nicotinamide adenine dinucleotide phosphate oxidase 4, and antifibrotic agents, such as matrix metalloproteinases, microparticles may play an opposite role in fibrosis.
Keywords: Systemic sclerosis, microparticles, biomarkers, endothelial damage, fibrosis
Introduction
Fibrotic diseases are one of the leading causes of mortality due to the scarce therapeutic options available today.1,2 Despite numerous recent advances in the understanding of various complex mechanisms responsible for the pathogenesis and progression of fibrotic diseases, this topic still remains elusive. 3 The pathogenetic pathways leading to fibrosis are multiple, but a classic model of fibrotic diseases is represented by systemic sclerosis (SSc). This disease is characterized by a complex pathophysiology 4 which is not only represented by an immune dysfunction but also by the peculiar involvement of the microvascular system. 5 The sufferance of the endothelial cells (ECs) covering the vessel wall is a pivotal event in the disease pathogenesis also for the endothelial transition into myofibrobalsts (EndoMT). 6 Therefore, the endothelium injury may be considered as a primary event which leads to tissue fibrosis. For this reason, the markers of endothelial injury have been studied at large, but none of them has been identified as useful and reliable marker of the endothelial sufferance so far.
Microparticles (MPs) can regulate vascular thrombosis, angiogenesis, vascular reactivity, and inflammation. 7 Epigenetic studies contributed to better understanding of MPs and its potential role in fibrosis.8–10 It is well known that MPs have been also proposed as markers of endothelial involvement, and their role in the genesis and maintenance of fibrosis is recently hypothesized.11,12
The aim of this review is to assess the importance of MPs in the pathogenetic cascade involved in tissue fibrosis.
MPs: state of the art
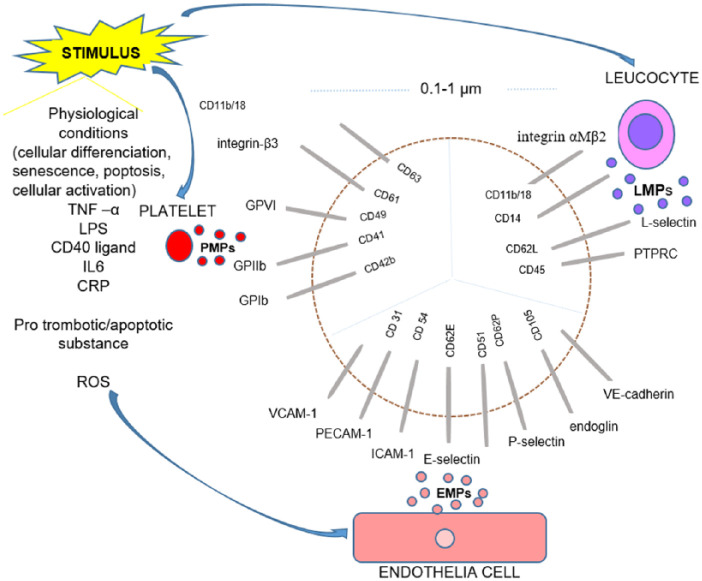
In the literature, MPs, also called microvesicles and ectosomes, are heterogeneous population of membrane-coated vesicles generated from the cells via outward blebbing of the plasma membrane under both physiological and pathological conditions.13–15 Although MPs may be distinguished from other extracellular vesicles (such as exomes and apoptotic bodies) according to the mechanism of formation and their content, the most commonly used differentiating parameter is size. MPs are typically defined as 0.1–1 µm in diameter, while exomes are smaller (approximately 40–100 nm). Apoptotic bodies are much larger compared to both exomes and MPs, with size of 1–5 µm. 14
MPs are generated and released during different biological processes, including not only normal cellular differentiation, senescence, or apoptosis but also upon cellular activation following stimulation with proinflammatory cytokines (i.e. tumor necrosis factor α (TNF-α), lipopolysaccharides (LPS), soluble CD40 ligand, interleukin-6 (IL-6), IL-1α, and C-reactive protein (CRP)), prothrombotic (i.e. thrombin, collagen, proteinase-activated receptor agonists, and plasminogen activator inhibitor-1ß (PAI-1)), or proapoptotic substances and exposure to high shear stress (Figure 1).13,16–19
Figure 1.
Schematic presentation of MPs surface main components.
EMPs: endothelial cells–derived microparticles; PMPs: platelet-derived microparticles; LMPs: leukocyte-derived microparticles; GPVI: glycoprotein VI; GPIIb: glycoprotein IIb; GPIbα: glycoprotein Ibα; VCAM-1: vascular cell adhesion molecule-1; PECAM-1: platelet endothelial cell adhesion molecule-1; ICAM-1: intercellular cell adhesion molecule-1; E-selectin: endothelial selectin; CD51: vitronectin receptor; P-selectin: platelet selectin; L selectin: leukocyte selectin.
These small particles (0.1 and 1 µm in diameter) may be distinguished from other groups of cell-derived vesicles because the MPs membrane and its proteins originate from their parental cells, reflecting both the type and state of their cellular origin.13,14 Thus, MPs express a broad range of surface cytoplasmic and nuclear molecules that are incorporated into membrane-bound structures including bioactive lipids, integrins, cytokines, and enzymes.13,14,16,20 MPs contain DNA, RNA, including micro RNA (miRNA), histones, and damage-associated molecular patterns (DAMPs).21,22 Once MPs are released into circulation, they bind and fuse with their target cells through receptor/ligand interaction and act as biological vectors. 13
MPs can deliver miRNA into recipient cells, where the exogenous miRNA may regulate target gene expression and modulate the function of recipient cells. Furthermore, immune complexes with autoantigens presented by MPs may induce immune response; in addition, the particles themselves may coordinate functions of different cells via both autocrine and paracrine ways. Thus, MPs are found as central mediators of a communication network for the local and systemic intercellular exchange of biological information and cell–cell interaction.17,23 Some components of MPs are selectively enriched compared to their cell of the origin, and even more, the composition and the function of MPs not only depend on the cellular origin but also on the inducing triggers and the microenvironment of the parental cell.23,24
Although circulating MPs can be derived from virtually any cell type, including immune cells, they are most commonly originated from the vasculature and circulating blood cells: endothelial cells (endothelial cells–derived microparticles (EMPs)), platelets (platelet-derived microparticles (PMPs)), leukocytes (leukocyte-derived micropaticles (LMPs)), and vascular smooth muscle cells. 13 The components on the MPs surface are the most notable, since they allow detection by flow cytometry (FC). Thus, EMPs may display platelet endothelial cell adhesion molecule-1 (PECAM-1; CD31), vascular endothelial cadherin (VE-cadherin; CD144), vitronectin receptor (CD51), ICAM-1 (CD54), vascular cell adhesion molecule-1 (VCAM-1; CD106), endothelial selectin (E-selectin; CD62E), platelet selectin (P-selectin; CD62P), or endoglin (CD105). 25 PMPs can exhibit glycoprotein ibα polypeptide (GPIbα; also known as CD42b), glycoprotein IIb (GPIIb; CD41), glycoprotein VI (GPVI; CD49), integrin-β3 (CD61), and the lysosomal markers such as CD68 or CD63, while LMPs may express protein tyrosine phosphatase receptor type C (PTPRC; CD45), CD14, L-selectine (CD62L), and integrin αMβ2 (CD11b/18)25–27.
The expression of different antigens on the MPs surface depends on the state of the origin cell. Thus, the activated ECs increase expression of inducible antigens on EMPs (e.g. CD62E, CD106, and CD54), while ECs undergoing apoptosis enhance expression of constitutive antigens (e.g. CD31 and CD105) and increase binding of annexinV.13,28
Presence of different components/antigens suggests a wide range of MPs activities, mainly in hemostasis, inflammation, vascular reactivity, and angiogenesis.4,9,29–33
Increased number of EMPs and PMPs are found in patients with antiphospholipid syndrome, suggesting a role of MPs in thrombotic events and pregnancy complications. 7 PMPs could be considered as a biomarker for the risk of thrombosis or miscarriage in individuals with antiphospholipid antibodies. 34 Enhanced TF expression on both EMPs and LMPs has been shown in systemic lupus erythematosus (SLE) patients, indicating an active role of TF-positive MPs in thrombosis. 35
Furthermore, EMPs vesiculation correlate with IL-6 release, showing that close relationship between endothelial vesiculation and classical inflammatory pathway exist and that MPs are implicated in inflammation. 9
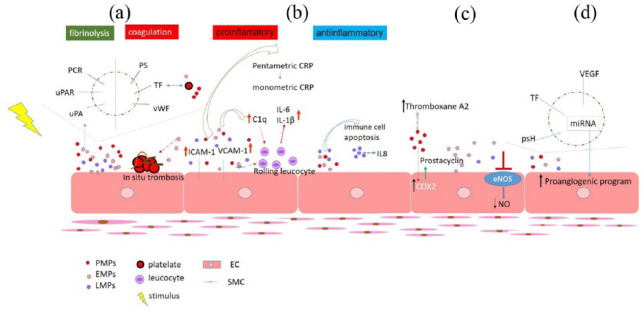
Generally, MPs can affect many different processes of the vasculature. PMPs may induce the expression of cyclooxygenase 2, leading to the release of the vasodilatative mediator prostacyclin. On the contrary, PMPs may contain the arachidonic acid metabolite thromboxane A2, which increases vascular contraction. Furthermore, EMPs have an endothelial-dependent vasodilatation effect influencing directly vascular tone (Figure 2).36,37
Figure 2.
Schematic presentation of possible microparticles activity: (a) Hemostatic properties of MPs. Platelet aggregation and spreading of procoagulant potential; (b) Induction and amplification of inflammation. Immune cell apoptosis and production of anti-inflammatory mediators; (c) Vascular reactivity and endothelial dysfunction; and (d) Angiogenesis.
MPs: microparticles; PS: phosphatidylserine; TF: tissue factor; vWF: large von Willebrand factor; PCR: protein C receptor; uPAR: urokinase-type plasminogen activator receptor; ICAM-1: intercellular adhesion molecule 1; VCAM-1: vascular cell adhesion molecule-1; C1q: complement component 1q; COX 2: cyclooxygenase 2; NO: nitric oxide; eNOS: endothelial nitric oxide synthase; psH: protein sonic Hedgehog; PMPs: platelet-derived MPs; EMPs: endothelial cells–derived MPs; LMPs: leukocyte-derived MPs: EC: endothelial cell; SMC: smooth muscle cell.
MPs may promote endothelial dysfunction by impairing the endothelial nitric oxide (NO) pathway and inducing proinflammatory response (Figure 2). 38 . EMPs are considered as a new useful and reliable marker of endothelial dysfunction. Furthermore, it has been shown that PMPs can stimulate angiogenesis and revascularization in ischemic heart disease. 10
Despite increasing scientific and clinical interest, methodology for MPs assessment is still an area of great debate, which is impeded by technological issues.39,40 Nevertheless, many different methods of MPs detection in biological samples have been described in the literature so far.40,41 No standardized protocols are available for the isolation, detection, and characterization of MPs.
All preanalytical steps, from blood sampling to sample freezing, should be considered as a source of variation in MPs analysis. For instance, isolation of MPs from blood is affected by the following: venepuncture and the diameter of the needle; time between blood collection and first centrifugation, which should be within 30 min to 1 h from sampling, no more than 2 h; type of anticoagulant used; and freezing and storage of the samples (storage no more than 1 year after freezing until analysis at a temperature below 80°C), thawing, centrifugation, and washing procedures.40,42–44 After blood collection, platelets need to be removed from the plasma in order to avoid cellular activation, leading to involuntary production of MPs. Centrifugation protocols for preparing platelet-free plasma and isolated MPs have major influences on MP analysis. The common centrifugation parameters used for MPs isolation vary between 1500g and 10,000g for 5–20 min in the first centrifugation step intended to remove cells and large particles (including platelets), followed by 13,000g–100,000g for 30–60 min to exclude residual platelets obtaining MPs pellet. These differences in centrifugation speed and time greatly affect the final MP counts. It has been shown that initial low-speed centrifugation between 1200g and 2000g for 15–20 min could effectively remove erythrocytes, platelets, and large membranous fragments, whereas speeds >2000g lead to a substantial loss of MPs.40,45 Sometimes, in order to remove residual platelets, filtration with 0.8-µm porous membranes is used; 46 however, filtration may activate platelets and induce MPs fragmentation, leading to serious loss of MPs. 45
Isolation of MPs in SSc studies have included either one step (vary from 1500g to 2000g, 10–15 min) 47–49 or two step (first: 200g–1800g, 10 min and second: 800g–20,000g, 6–10 min) centrifugation.50–53 After centrifugation and freeze-thaw steps had been done, one more centrifugation with two steps (1500g for 5 min and 100,000g for 20 min) was performed in one study. 49
Arising concern is that isolation could result in getting MPs contaminated with exosomes; apoptotic blebs; protein aggregates, including immune complexes; the presence of lipoprotein particles; and small platelets within the size range of MPs.46,54,55 In spite of these limitations, the combination of differential centrifugation and FC has proven to be invaluable for the detection of MPs.49–51,53,56–59
Different optical and non-optical detection methods have been utilized for the assessment/quantification of MPs, including immunoassays, FC, electron microscopy, atomic force microscopy, and dynamic light scattering.40,41
FC analysis of blood MPs appears to be the most favored analytical method of identification, quantification, and size assessment of the microvesicles.40,60 MPs are typically detected in terms of size by FC based on the intensity of light scattering and exposure of phosphatidylserine (PS), an “eat me signal” for the immune system, identified by staining with annexin V and further characterized with fluorescent-labeled antibodies against specific surface antigens.13,39–41,60,61 One of the biggest FC limitation is MPs size detection, regarding the fact that MPs are too small and heterogeneous in size to be detected and clusters of small MPs might be counted as one event.39,40 Furthermore, immune complexes can overlap with size of MPs appearing as MPs by FC. 46 Quantification of MPs could be done with detection of PS-rich surface; annexin V (AnxV) binding is often used as identifier.39,40,46,60–62 The mechanisms of generating AnxV non-binding MPs (AnxV-MPs) are not fully known, but because PS exposure is a typical feature of apoptosis, it may be hypothesized that AnxV– MPs are generated by cellular activation. However, a significant proportion of the MPs are annexin V negative, suggesting either heterogeneity in the mechanism of production or the presence of PS at concentrations below the limits of detection. As an alternative, lactadherin could be used with higher affinity for PS and the potential advantage of non-calcium dependent binding to PS compared to AnxV. BODIPY maleimide, calcein AM, and SYTO 13 are other alternative probes that could detect all circulating MPs also.13,39,63,64 Identification the MPs origin with fluorescent-labeled antibodies also has limitation in that antibodies are dissimilar according to specificity and sensitivity for specific antigens. 39 Finally, the standardization of both preanalytic and analytic methods still remains a significant challenge. 40
The link between inflammation, coagulation, and fibrosis is well documented. Even low-grade persistent inflammation is enough to promote fibrosis. Alike, plasma coagulation cascade proteases are also involved in fibrosis via induction of profibrotic molecules. 65 However, our knowledge of MPs in the development of fibrosis is unclear.
Fibrosis: state of the art
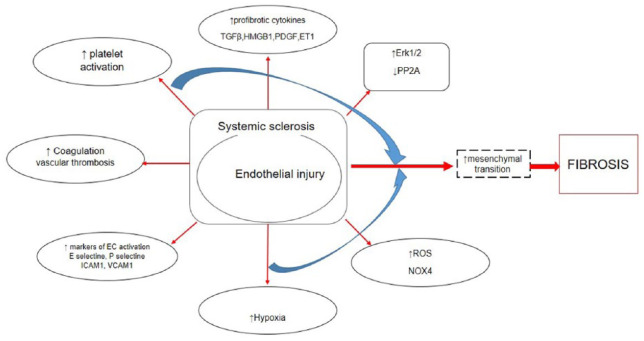
Fibrosis is characterized by excessive deposition of extracellular matrix (ECM) proteins, mainly collagen and fibronectin, in response to injury and is quite important in wound healing. However, when fibrotic process is chronically active, it could lead to permanent tissue remodeling and significant organ impairment. 5 It has been already known that fibrosis is a feature of different connective tissue diseases (CTDs) and is the hallmark of the main systemic fibrotic disease—SSc. SSc is a complex, multifaceted CTD of unknown etiology, characterized by a peripheral microvascular injury spreading into progressive fibrosis of the skin and multiple internal organs. 5 We have already pointed out that endothelial injury is a key pathological event in SSc, contributing to enhanced leukocyte, platelet activation, and coagulation pathways; production of proinflammatory and profibrotic cytokines; generation of reactive oxygen species (ROS); defective angiogenesis; and vasculogenesis, resulting in development of severe vasculopathy and further fibrosis (Figure 3).5,66,67
Figure 3.
Illustration of the link between endothelial injury and fibrosis.
SSc-associated interstitial lung disease (ILD) and pulmonary arterial hypertension (PAH) are the leading causes of impaired quality of life and mortality in SSc.1–3,68 It has been reported that endothelium and pericyte activation, telocytes loss, aberrant immune responses, endoplasmic reticulum stress, and chronic tissue injury are involved in the initiation of fibrosis in SSc.69–71 Although the pathogenesis of SSc fibrosis is still elusive, strong evidence suggests that myofibroblasts (MFs) are the main final effectors responsible for tissue fibrosis and fibroproliferative vasculopathy (Figure 3). 72 These cells contribute to the progressive increase in tissue stiffness, further enhancing the profibrotic process. 73
Extensive investigations have revealed that MFs originate from multiple cellular sources, including resident tissue fibroblasts, bone marrow–derived circulating fibroblast precursors (also known as fibrocytes), and epithelial cells via a phenotypic transition into mesenchymal cells (EMT), by which epithelial cells modify adhesive properties and polarity and acquire ECM-producing MFs features. Other cell types such as pericytes, adipocytes, pleural mesothelial cells, or macrophages are also potential sources of MFs.72,74 More recent studies have shown that another source of activated MFs are ECs that have acquire a mesenchymal phenotype through a endothelial to mesenchymal transition (EndoMT) procces. Today, we know that EndoMT plays an important role during several pathological conditions, including cardiac, pulmonary, and renal fibrosis; carcinoma-associated interstitial fibrosis; idiopathic portal hypertension; intestinal fibrosis; and diabetic nephropathy.72,74–77 Moreover, EndoMT may play a role in the development of tissue fibrosis and fibroproliferative vasculopathy in SSc. 72 One more evidence which supports this hypothesis is that EndoMT may occur in SSc dermal endothelium, contributing the development of dermal fibrosis. 6 During EndoMT, ECs become detached from endothelial layer; change their morphologic characteristics; lose their specific EC markers such as CD31/PECAM-1, large von Willebrand factor (vWF), occluding, and VE-cadherin; and initiate the expression of mesenchymal/myofibroblast phenotype characterized by the expression of alpha smooth muscle actin (α-SMA), vimentin, S100A4/fibroblast-specific protein-1, and type I collagen (CI). It has been shown that EndoMT may support loss of microvascular EC and thus contribute to capillary rarefaction, leading to chronic tissue ischemia and amplifying further fibrotic process.72,78,79
Multiple pathways are implicated in fibrotic process. The potent profibrotic transforming growth factor beta (TGF-β) has been highlighted as a key player in fibrosis as well as in EndoMT and EMT, confirmed both in vitro and in vivo studies.78,80,81 TGF-β-regulated genes are expressed in the skin and the lung of patients with SSc, and the extent of the cytokine expression correlates with the disease activity. 82 Thrombospondin-1 (TSP-1) is important in controlling TGF-β activation in fibrotic diseases. 83 Smad-dependent and Smad-independent pathways and numerous transcriptional regulators such as Snail, Snail2 (or Slug), Twist, and some members of Zeb family of proteins are implicated in EndoMT.84,85 According to Smad-independent pathway, extracellular signal-regulated protein kinase (Erk) 1/2 has been suggested to have an important role in fibrosis by regulating MF transdifferentiation, cell proliferation, and survival, as well as matrix synthesis. Erk1/2 pathway is induced by TGF-β in dermal fibroblasts and ECs. 86 The protein phosphatase 2A (PP2A) dephosphorylates and blocks activation of ERK1/2. 87 The PP2A mRNA and protein expression are significantly reduced in SSc fibroblasts and correlate with an increase in ERK1/2 phosphorylation and collagen expression. 88 Furthermore, aberrant activation of Notch, Sonic Hedgehog, and Wnt/13-catenin pathways may lead to various pathological consequences, including the development of fibrotic diseases via, in part, EndoMT (Figure 3).89–91
EndoMT may be mediated by other potential factors including platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), insulin-derived growth factor, connective tissue growth factor (CTGF), and endotelin-1 (ET-1). 92 It has been proven that ET-1 is capable of generating EndoMT either by itself or in combination with TGF-β, and despite this, the synergistic interaction with TGF-β is still widely supported. CTGF is a common target for both ET-1 and TGF-β. In different cells type, CTGF regulates cell proliferation, apoptosis, migration, mesencymal cell activation, and ECM accumulation. 93
Hypoxia represents a potent stimulus for the generation of various growth factors and ROS, which influence the fate of ECs, promoting mesenchimal transition and fibrosis. 94 Skin hypoxia has been documented in SSc patients. 95 Oxidative stress is implicated in various features of idiopathic pulmonary fibrosis (IPF), liver fibrosis, and SSc, mediated by ROS. Mainly, ROS production derives from the activation of the nicotinamide adenine dinucleotide phosphate oxidase (NOX) family. NOX4 has a central role in the initiation and maintenance of fibrosis. Increased expression of NOX4 transcripts and also level of NOX4 in affected SSc skin have been demonstrated. 96 Moreover, TGF-β1-mediated expression of NOX4 is closely related to myofibroblastic differentiation; on the contrary, MF differentiation is dependent on the generation of ROS by NOX4, suggesting that ROS and TGF-β1 are essential for manifestation of the MF phenotype (Figure 3).97,98. Activation of HIF-1 signaling in renal epithelial cells may promote fibrogenesis by increasing expression of ECM modifying and by facilitating EMT.99,100 Furthermore, activation of HIF-1α in dermal fibroblasts of SSc may upregulate CTGF expression, contributing to the progression of skin fibrosis. 101
Several studies have shown a significant deregulation of miRNAs involved in angiogenesis, vascular repair, and endothelial homeostasis. 102 In SSc, several miRNAs are associated with kTGF-β and CI expression. Thus, microRNA-29a and miRNA-196a can supress CI gene expression, but they are downregulated in SSc, suggesting that their low-level expression may promote upregulation of CI by TGF-β in SSc fibrogenesis. Moreover, levels of miRNA-196 inversly correlate with prevalence of pitting scars and modified Rodnan skin thickness score (mRSS) score.103,104 The downregulation of miRNA let7a leads to the exessive CI expression and, recently, has been shown that treatment with let7a improves the skin fibrosis in SSc. 105 Several studies have proven that using strategy with combination of miRNAs can transdifferentiate fibroblasts into cardiomyocytes or neuronal tissue. However, the rate of reprogramming fibroblasts is low and insufficient to translate into a clinical setting.106,107 Recently, it has been demonstrated that MPs derived from endothelial progenitor cells could protect the kidney from ischemic acute injury by miRNA-dependent reprogramming of hypoxic resident renal cells to a regenerative program. 108
Circulating blood cells can mediate various features in the fibrotic diseases, through pleiotropic functions. Platelets are critical players in SSc pathogenesis. They represent source of different profibrotic signals such as VEGF, TGF-β, PDGF, and serotonin. 109 Platelets also contain high mobility group box 1 (HMGB1) protein (Figure 3). This protein plays multiple roles in the pathogenesis of inflammatory and autoimmune diseases and mediates processes that range from inflammation to repair. It targets various immunologically relevant systems, including p53, nuclear factor (NF)-κB, the glucocorticoid receptor, and the receptor for advanced glycation end products (RAGE). The signaling of HMGB1 or with its receptors plays a crucial role in mediating fibrotic diseases in liver, renal, lung, and myocardial.11,109 Further studies have revealed that serum HMGB1 level in SSc is higher compared with healthy controls and control mice, while SSc patients with elevated HMGB1 level have more frequent involvement of several organs and immunological abnormalities than those with normal level. 110 The bioactive HMGB1 can stimulate neutrophils to generate ROS via P-selectin, thus contributing to increased vessel inflammation in SSc and oxidative stress development. The oxidation of HMGB1 further amplified its ability to activate neutrophils. It has been demonstrated that HMGB1+ MPs purified from SSc patients activate in vitro healthy neutrophils maintaining of sterile inflammation in SSc patients. 111 Moreover, the activation of HMGB1 is associated with the loss of telocytes.70,71 Finally, HMGB1 may contribute to EMT and EndoMT in various fibrotic diseases (Figure 3). 11
Data from diverse fibrosis models indicate that matrix metalloproteinases (MMP) may modulate a range of biological processes, especially those related to immunity and tissue repair and/or remodeling, having both inhibitory and stimulatory roles in fibrosis. 112 Since MMP3, MMP2, and MMP9 might stimulate EMT, MMP9 can activate TGF-β, contributing to enhance the pool of active TGF-β. MMP7-deficient mouses are protected from bleomicyn-induced lung fibrosis, suggesting profibrotic role of this MMPs. Even though, in lung fibrosis model, at the beginning MMP7s facilitate neutrophil influx and activation, leading to epithelial damage and an enhanced fibrotic environment, later epithelial-derived MMP-7s promote resolution by attracting an influx of immunosuppressive leukocytes reflecting also antifibrotic role. In a model of liver fibrosis, MMP-1 and MMP-13 lead to resolution of fibrosis.112,113 In a SSc patient, MMP-7 serum level is higher than those in control group, and patients with lung fibrosis have higher levels than those without. Interestingly, fibroblasts in early stage of SSc exhibit higher levels of MMP-1, MMP-3, and TIMP-1, unlike the gene expression of MMP-1, MMP-2, and MMP-3, which is decreased in fibroblast from SSc patients with mild stage of disease. MMP-9 concentration positively correlates with the mRSS, and one of the sources of MMP-9 is dermal fibroblast.114,115
Is there a link between MPs and fibrosis?
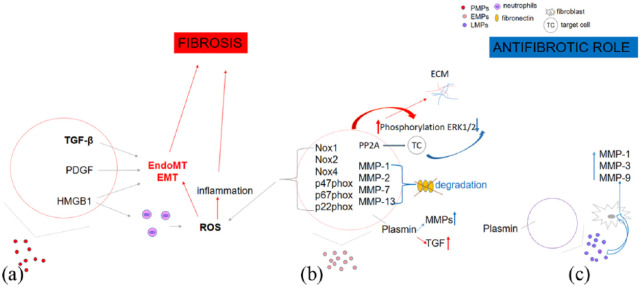
A few important findings suggest direct implication of MPs in fibrosis and possibly a role in EMT and EndoMT. PMPs secretome contains a range of vasoactive mediators favoring vasoconstriction (e.g. thromboxane), growth factors (TGF-β and PDGF), and HMGB1 protein that may contribute to fibrosis.9,11 Very new precious data have shown that PMPs from SSc patients interact with neutrophils in vitro and in mice, promoting neutrophil autophagy and leading to generation of neutrophil extracellular traps (NETs). 116 Taken together, all of these data may support the role of PMPs in EndoMT due to fact that NETosis itself is contributing to EndoMT. 117 Furthermore, EMPs may contain Nox1, Nox2, Nox4, p47phox, p67phox, and p22phox and have the ability to produce ROS through Nox-dependent processes. Moreover, EMPs can increase phosphorylation of ERK1/2 and Src via ROS-independent way, contributing to increased ECM accumulation. A recent proteomic analysis found that PP2A, which is present in human EMPs, may be transferred to target cells, giving insight to decreased ERK1/2 phosphorylation, but this has not been shown experimentally yet. 8 In contrast, MPs from LPS-treated THP-1 monocytes cell may induce phosphorylation of ERK1/2, activation of the nuclear factor-B pathway, and expression of cell adhesion molecules ICAM-1, VCAM-1, and E-selectin, promoting proinflamatory and profibrotic role (Figure 4). 118
Figure 4.
Illustration of the link between microparticles and fibrosis: (a) Platelet-derived microparticles (PMPs) secretome may contribute to fibrosis via EndoMT, EMT procces, and ROS-mediated pathway; (b) Fibrotic and antifibrotic role of endothelial cells–derived microparticles (EMPs); and (c) Profibrotic and antifibrotic properties of leukocyte-derived microparticles (LMPs).
ECM: extracellular matrix; EMT: epithelial to mesenchymal transition; EndoMT: endothelial to mesenchymal transition; ERK1/2: extracellular signal-regulated protein kinase 1/2; HMGB1: High mobility group box 1; NOX: NADP(H) oxidase; p47phox, p67phox, p22phox: NADP(H) oxidase subunits; PP2A: protein phosphatase 2A; ROS: reactive oxygen species.
Podocyte-derived MPs might increase p38 and Smad3 phosphorylation and expression of the ECM proteins in proximal tubule epithelial cells, suggesting their role in EMT and tubular fibrosis. 119 EMT could be fostered by EMPs coming from activated ECs via increasing the expression of HIF-α/VEGF-A in a COX-2/EP2 receptor dependent manner. 120
As MPs contain proteolotic enzymes, it is possible that MPs may contribute to alteration of the ECM and cleavage of signaling molecules. For example, MPs derived from microvascular ECs contain MMP-1, MMP-2, MMP-7, and MMP-13 and may degrade fibronectin in vitro. 121 Furthermore, EMPs have ability to bind and activate both endogenous and exogenous proMMP-2, leading to vascular matrix remodeling. 122 Very new data have proven that LMPs derived from T cells and monocytes potently induce the synthesis of MMP-1, MMP-3, MMP-9, and MMP-13 in fibroblasts in a time-dependent manner. On the contrary, no contraregulatory induction of the expression of tissue inhibitors of MMPs was observed. 14 In SSc, the inverse correlation between the mRSS and values of total EMPs and PMPs has been demonstrated (Figure 4).49,50
EMPs and LMPs may generate on the surface plasmin, which has both profibrotic and antifibrotic properties by activating TGF beta on one hand and on the other hepatocyte growth factors and MMPs (Figure 4). 123
MMPs and plasmin influence migration capacity of cells which could be implicated in fibrosis. MPs promote fibroblast activation and migration. ROS, namely H2O2, may increase the generation of procoagulant MPs, TF-bearing MPs, by alveolar epithelial cells that could activate local synthesis of factor Xa leading to PAR-1-mediated activation of fibroblasts and a profibrotic response. 124 Furthermore, EMPs isolated from idiopathic pulmonary patients might induce migration capacity of the lung fibroblast, increasing formation of F-actin fibers, by their fibrinolytic activity contributing to fibrogenesis. 125 EMPs are elevated among IPF patients with severe reduced diffusing capacity of the lung for carbon monoxide (DLCO), while TF-bearing MPs negatively correlate with both forced vital capacity (FVC) and DLCO, 125 suggesting their implication in lung fibrosis disease. Evidence that MPs are involved in pathogenesis of lung fibrosis also comes from the finding that LMPs might lead to extensive entry of neutrophils into airways and aggregation at the epithelial surface of the respiratory tract in cystic fibrosis patients. 126 Regarding the well-known profibrotic effect of IL17,127,128 PMPs could be considered as antifibrotic mediators through their ability to prevent the differentiation of Tregs into IL-17 and IFN-γ—producing cells in P-selectin-dependent manner. 129 Moreover, PMPs may deliver miRNA let7a to the ECs and reduce production of TSP-1, possibly influencing TGF-β pathway. 130
The role of MPs in SSc
Recently, a few research groups reported plasma levels of MPs and their clinical association in SSc patients giving divergent results.47–53,59,111,116,131 EMPs, PMPs, and LMPs are predominantly investigated, and labeling antigens for detection-specific MPs population has been dissimilar across studies. Furthermore, some studies have shown that patients with SSc have increased concentration of MPs compared to healthy controls,48–52,111,116,131 while others have demonstrated opposite results (Table 1).47,59
Table 1.
Labeling of MPs and differences across studies.
| Labeling | SSc vs HC | lSSc vs HC | dSSc vs HC | lSSc vs dSSc | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| tMP | ↑** | ↑* | ↑* | Guiducci et al. 49 | ||||||
| ↓** | ns | ns | Iversen et al. 59 | |||||||
| AnxV− MPs | Total Total fraction |
↓ns ↑* |
Iversen et al. 59 | |||||||
| CD62E+ | ↑** | ↑* | ↑* | ↑ns | Michalska-Jakubus et al. 50 | |||||
| AnxV+ MPs | Total | ↓* | Iversen et al. 59 | |||||||
| CD62E+ | ↑** | ↑* | ↑* | ↑ns | Michalska-Jakubus et al. 50 | |||||
| CD31+/CD42b– | ↓* | Jung et al. 47 | ||||||||
| CD31+/CD42b– | ↑ns | McCarthy et al. 51 | ||||||||
| CD31+/CD42b+ or CD31–/CD42b+ | ↑** | McCarthy et al. 51 | ||||||||
| EMPs | CD144+ | ↑** | ↑* | ↑** | ↑ns | Guiducci et al. 49 | ||||
| CD146+ a CD146+ |
↓*
↑ns |
↑ns |
↑ns |
Iversen et al. 59 | ||||||
| CD31+/CD42b– | ↑** | ↑* | ↑* | ↑ns | Michalska-Jakubus et al. 50 | |||||
| CD51+ | ↑* | ↑* | ↑* | |||||||
| PMPs | CD42+ | ↑** | ↑* | ↑* | ↑ns | Guiducci et al. 49 | ||||
|
b
CD42a+ CD42a+ |
↑ns ↓* |
↑** |
↑ns | ↑ns | Iversen et al. 59 Nomura et al. 52 | |||||
| CD61+HMGB1+ | ↑** | 111,116,131 | ||||||||
| LMPs |
c
CD14+ d CD3+ |
↑**
↑* |
↑*
↑* |
↑*
↑* |
↑ns ↑ns |
Guiducci et al. 49 | ||||
| CD45+ e CD45+ |
↓*
↑ns |
↑ns | ↑ns | Iversen et al. 59 | ||||||
HC: healthy controls; tMP: total number of MPs; AnxV: annexin V; MP: microparticles; EMPs: endothelial cells–derived microparticles; PMPs: platelet-derived microparticles; LMPs: leukocyte-derived micropaticles; SSc: systemic sclerosis; lSSc: limited systemic sclerosis; dSSc: diffuse systemic sclerosis.
Fraction of AnxV−CD146+.
Fraction of AnxV−CD42a+.
Monocytes.
T cells.
Fraction of AnxV−CD45+.
p < .05; **p < .001; ns p ⩾ .05.
Even though some of them have investigated same population of MPs, heterogeneity of the studies with respect to eligibility criteria, study population, methods, and choice of outcome statistics make the comparison difficult.
In spite of the study differences, the association of MPs with hemostasis disturbance, microangiopathy, disease activity, inflammation, and organ involvement in SSc might be speculated.
Hemostasis
As a common feature, particles expose PS, as a consequence of membrane flipping during apoptosis, which can bind and activate different coagulation factors promoting conversion of prothrombin into thrombin. Furthermore, MPs may express tissue factor (TF) and vWF multimers, which may initiate the extrinsic coagulation pathway and promote platelet aggregation.29,30,132 EMPs from activated ECs may trigger TF-dependent thrombin formation in vitro and thrombus formation in vivo. 133 In addition, TF can be transferred between MPs and different cell types, spreading procoagulant potential. 134
Recent studies have demonstrated that EMPs expose endothelial protein C receptor, urokinase-type plasminogen activator, and its receptor, suggesting that these particles also have anticoagulant properties (Figure 2). 12
Concomitant to the changes in the SSc endothelial lining, platelets undergo activation. Enhanced activation of platelets, increased tendency to aggregation, and activation of coagulative cascade have long been observed in SSc patients.109,135,136 Different stimulus may influence the emergence of procoagulant MPs. An oxidative stimulus, namely H2O2, increases the production of procoagulant MPs by alveolar epithelial cells in culture. Increased number of TF-bearing MPs has been found in SSc interstitial lung disease. 124
Vascular health and microangiopathy
Few lines of evidence support the hypothesis that certain MPs subpopulation can induce angiogenesis and vascular remodeling. It has been postulated that expressing the VEGF, TF, and the protein sonic Hedgehog can define MPs as proangiogenic structures. Since MPs contain miRNA, they are able to activate a proangiogenic program in ECs. 4 Incubation of human microvascular endothelial cell line (HMEC-1) with THP-1 MPs leads to transfer of miRNA 150 from MP to recipient EC promoting angiogenesis, leading finally to the developed capillary-like structures out of existing blood vessels. 31 EMPs may transfer miRNA to ECs promoting angiogenesis. 32 In addition, activated subtype of EMPs positively correlate with number of ramified capillaries, indicating their role in angiogenesis and vessel regeneration. 33 In contrast, high levels of EMPs isolated from human umbilical vein ECs may reduce angiogenesis, while low concentration of EMPs stimulate formation of capillary-like structure (Figure 2). 9
Recently, it has been shown within SSc patients that the active nailfold videocapillaroscopy (NVC) pattern is associated with higher concentration of activated E-selectin-positive EMPs (CD62+AnxV–) compared to early microvascular involvement. This subpopulation of EMPs is also increased in patients with specific microvascular alterations: pericapillary edema and giant capillaries or frequent microhemorrhages, confirming that endothelial activation is enhanced in the active phase of SSc-related microangiopathy and also suggesting that their increased concentration might be a sensitive marker for early EC dysfunction. Furthermore, activated EMPs may reflect early step of angiogenesis since they positively correlate with number of ramified capillaries. Total number of EMPs is associated with the overall number of microvessels reflecting the severity of avascularizations. The confirmation proof of this is that the total number of EMPs is decreased in late NVC compared to early pattern and inversely correlates with number of ramified loops in SSc patients. 50 Apoptotic MPs phenotype (AnxV+ MP) has shown positive association with the avascular and microvasculopathy scores objected by NVC in the autoimmune disease patients with Raynaud’s phenomenon, reflecting the existence of critical tissue hypoxia. 48 Furthermore, it has been shown that both higher annexin-positive EMPs and PMPs levels are associated with better digital perfusion assessed using laser speckle contrast imaging (LSCI) in patients with primary RP and SSc, reflecting vascular perfusion across diseases. 51
It is well known that calcinosis and digital ulcers are associated with the late NVC pattern.137,138 Regarding this, we could expect that in patients with this features of disease, total number of EMPs is decreased or apoptotic phenotype increased. Indeed, the significantly decreased numbers of both total MPs shading from various cells and PMPs have been demonstrated in patients with present cutaneus ulcers. 49 Furthermore, total EMPs levels also tended to be lower in SSc patients with active digital ulcers, 50 and higher levels of apoptotic EMPs (CD31+/CD42b–AnxV+) are associated with a history of digital ulceration/pitting. 51 In contrast, patients with calcinosis have increased level of activated (CD146+AnxV–) EMPs subpopulation. 53
Inflammation and disease activity
MPs can activate complement cascade (C1q), enhance leukocyte rolling, and stimulate the release of broad proinflammatory mediators (e.g. IL-6 and IL-1 β; Figure 2). IL-6 has been implicated in the pathogenesis of SSc via stimulation of fibroblasts to produce excess collagen and glycosaminoglycan, but to the best of our knowledge, no study so far has tested the role of MPs bearing IL6 in SSc. 139 Interaction between EMPs and naïve EC triggers proinflammatory response by upregulation of ICAM-1, messenger RNA (mRNA) expression, and solubile ICAM-1 shedding from target cells. Furthermore, EMPs which are triggered by transforming growth factor-alpha increase the release of solubile ICAM-1 secretion from ECs, enhancing the endothelial response to inflammation. This paracrine effect of MPs could not be observed using EMPs from unstimulated ECs, suggesting that these MPs may be both a consequence and a cause of the inflammatory response. 9
In inflammation state, MPs have ability to transfer chemokine receptors and arachidonic acid between cells, leading to induction of adhesion molecules such as intracellular adhesion molecule-1 (ICAM-1) and VCAM-1. Adhesion molecules on EMPs can mediate adhesion of monocytes to ECs in vitro, leading to the maintenance of inflammation (Figure 2). Several studies have demonstrated increased sVCAM-1, sICAM1, sE-selectin, and sP-selectin levels in serum of SSc patients compared with the healthy controls,66,140,141 raising the question: do MPs have together with adhesion molecules role in SSc pathogenesis. It has been found that sE- and sP-selectins 142 strongly correlate with either fraction (F) of AnxV-negative EMPs or PMPs in SSc with difference regarding to subtypes. In patients with diffuse systemic sclerosis (dSSc), association between F EMPs and both sP and sE selectins was observed, while in limited systemic sclerosis (lSSc), the only association was between PMPs and sP. 59
MPs are capable of converting pentametric CRP into proinflamatory monometric CRP. Furthermore, MPs containing CRP monomers can bind to the surface of ECs and generate proinflamatory signals in vitro. 143 In early inflammation, MPs may also induce immune cell apoptosis and the production of anti-inflammatory mediators such as IL8 predominately from LMPs (Figure 2). 144 CRP is one of the revised European Scleroderma Trials and Research group (EUSTAR) index component. 145 The highest number of total MPs has been found in a SSc patient with elevated CRP and an increased disease activity score of 3.5. 49 In spite of no study showing significant correlation between MPs and EScSG disease activity index score49,50 so far, some data suggest that this association might exist. Thus, C3 complement, one of the EScSG component and possible marker of vascular injury, 146 inversely correlates with values of activated EMPs (CD62+AnxV–). 50
Recently, strong association has been demonstrated between EMPs (CD62+) levels and perivascular soft tissue inflammation, visualized by fluorescence optical imaging (FOI) in SSc patients. 47 An enhancement of fluorescence optical contrast media has been observed in vivo in the inflammatory tissue as visualized by FOI with an excellent correlation to histopathology. 147
Organ involvement
The levels of EMPs, MPs, and PMPs total number inversely correlate with the severity of skin involvement assessed by mRSS,49,50 the best validated outcome measure for skin fibrosis in SSc. 146 Lower levels of PMPs and total MPs have been reported in patients with mRSS ⩾10, 49 indicating that numbers of MPs could be associated with milder dermal fibrosis in SSc.
Recently, it has been shown that worse lung function measured by DLCO and FVC correlates with higher levels of both AnxV non-binding EMPs and LMPs, and these findings have been dissimilar in patients with limited and diffuse disease. In dSSc, increased concentration of both AnxV– EMPs and LMPs is related to a reduction of FVC, whereas in lSSc, the same MPs are associated with a reduction of DLCO. Furthermore, increased AnxV– EMPs have been found in patients with x-ray-confirmed lung fibrosis compared to cases without (frequency of ILD was higher in lSSc group). 53 The significantly increased concentration of both PMPs and monocytes-derived microparticles (mMPs) has been found in SSc patients with interstitial pneumonia (IP). PMPs-enhanced rsCD40L may stimulate the activation of monocytes and promote the production of mMPs from THP-1 sugessting the role of MPs in pathophysiology of progressive SSc with IP. 52 Furthermore, oxidized extracellular HMGB1, soluble or associated to PMPs, may amplify activation of neutrophils. Activated leukocytes and membrane HMGB1 are elevated in SSc patients with PAH or with diffuse subtype of disease. 111 PAH is associated with a specific pattern of platelets activation and higher fraction of HMGB1+ PMPs. 131
Conclusion
Although the knowledge about the role of MPs in fibrosis has recently advanced considerably, this research area still presents a great number of challenges. There is now accumulating evidence of the multiple faces of MPs as conveyors of cell information with major role in inflammation, thrombosis, and angiogenesis. MPs are undoubtedly implicated in immunopathogenesis. At present, the attention may be focused for the first time on the fact that MPs may have different behaviors. In fact, they can be antifibrotic and profibrotic as well. These particles may contribute to EndoMT and EMT via different mediators such as TGF-β, PDGF, and HMGB1 protein. Furthermore, they may directly produce ROS through Nox-dependent processes, leading to the development and maintenance of oxidative stress, which is an important trigger of fibrosis. MPs are implicated in microangiopathy and clinical features of SSc. On the contrary, MPs contain proteolotic enzymes, such as MMPs involved into ECM degradation. Moreover, these particles may induce the synthesis in fibroblast of some MMPs, thus enhancing the ECM degradation process. Expressing VEGF, TF, and the protein sonic Hedgehog, MPs may promote new vessel formation. The proven antifibrotic activity of MPs might serve as new therapeutic targets, opening new research avenues. More studies are warranted to provide novel insights into the world of MPs, to disclose their real potential as factors with a regenerative as well as an antifibrotic role.
Acknowledgments
The authors state that the contribution is original and is not submitted elsewhere.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jelena Čolić  https://orcid.org/0000-0002-8418-3056
https://orcid.org/0000-0002-8418-3056
References
- 1. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 2012; 18(7): 1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Friedman SL, Sheppard D, Duffield JS, et al. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med 2013; 5(167): 167sr1. [DOI] [PubMed] [Google Scholar]
- 3. Wei J, Bhattacharyya S, Tourtellotte WG, et al. Fibrosis in systemic sclerosis: emerging concepts and implications for targeted therapy. Autoimmun Rev 2011; 10(5): 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Varga J, Trojanowska M, Kuwana M. Pathogenesis of systemic sclerosis: recent insights of molecular and cellular mechanisms and therapeutic opportunities. J Scleroderma Relat Disord 2017; 2(3): 137–152. [Google Scholar]
- 5. Matucci-Cerinic M, Kahaleh B, Wigley FM. Review: evidence that systemic sclerosis is a vascular disease: vascular origins of scleroderma. Arthritis Rheum 2013; 65(8): 1953–1962. [DOI] [PubMed] [Google Scholar]
- 6. Manetti M, Romano E, Rosa I, et al. Endothelial-to-mesenchymal transition contributes to endothelial dysfunction and dermal fibrosis in systemic sclerosis. Ann Rheum Dis 2017; 76(5): 924–934. [DOI] [PubMed] [Google Scholar]
- 7. Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol 2010; 6(1): 21–29. [DOI] [PubMed] [Google Scholar]
- 8. Burger D, Turner M, Munkonda MN, et al. Endothelial microparticle-derived reactive oxygen species: role in endothelial signaling and vascular function. Oxid Med Cell Longev 2016; 2016: 5047954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCarthy EM, Wilkinson FL, Parker B, et al. Endothelial microparticles: pathogenic or passive players in endothelial dysfunction in autoimmune rheumatic diseases? Vascul Pharmacol 2016; 86: 71–76. [DOI] [PubMed] [Google Scholar]
- 10. Li J, Zhang Y, Liu Y, et al. Microvesicle-mediated transfer of MicroRNA-150 from monocytes to endothelial cells promotes angiogenesis. J Biol Chem 2013; 288(32): 23586–23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol 2011; 31(1): 27–33. [DOI] [PubMed] [Google Scholar]
- 12. Li L-C, Gao J, Li J. Emerging role of HMGB1 in fibrotic diseases. J Cell Mol Med 2014; 18(12): 2331–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roos MA, Gennero L, Denysenko T, et al. Microparticles in physiological and in pathological conditions. Cell Biochem Funct 2010; 28(7): 539–548. [DOI] [PubMed] [Google Scholar]
- 14. Burger D, Schock S, Thompson CS, et al. Microparticles: biomarkers and beyond. Clin Sci 2013; 124(7): 423–441. [DOI] [PubMed] [Google Scholar]
- 15. Nusbaum P, Laine C, Seveau S, et al. Early membrane events in polymorphonuclear cell (PMN) apoptosis: membrane blebbing and vesicle release, CD43 and CD16 down-regulation and phosphatidylserine externalization. Biochem Soc Trans 2004; 32(Pt. 3): 477–479. [DOI] [PubMed] [Google Scholar]
- 16. Morel O, Morel N, Freyssinet J-M, et al. Platelet microparticles and vascular cells interactions: a checkpoint between the haemostatic and thrombotic responses. Platelets 2008; 19(1): 9–23. [DOI] [PubMed] [Google Scholar]
- 17. Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res 2010; 107(9): 1047–1057. [DOI] [PubMed] [Google Scholar]
- 18. Terrisse AD, Puech N, Allart S, et al. Internalization of microparticles by endothelial cells promotes platelet/endothelial cell interaction under flow: internalization of microparticles by endothelial cells. J Thromb Haemost 2010; 8(12): 2810–2819. [DOI] [PubMed] [Google Scholar]
- 19. Takano K, Asazuma N, Satoh K, et al. Collagen-induced generation of platelet-derived microparticles in whole blood is dependent on ADP released from red blood cells and calcium ions. Platelets 2004; 15(4): 223–229. [DOI] [PubMed] [Google Scholar]
- 20. Biro E, Akkerman JWN, Hoek FJ, et al. The phospholipid composition and cholesterol content of platelet-derived microparticles: a comparison with platelet membrane fractions. J Thromb Haemost 2005; 3(12): 2754–2763. [DOI] [PubMed] [Google Scholar]
- 21. Hreggvidsdottir HS, Ostberg T, Wahamaa H, et al. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol 2009; 86(3): 655–662. [DOI] [PubMed] [Google Scholar]
- 22. Pisetsky D. Cell death in the pathogenesis of immune-mediated diseases: the role of HMGB1 and DAMP-PAMP complexes. Swiss Med Wkly, http://doi.emh.ch/smw.2011.13256 [DOI] [PMC free article] [PubMed]
- 23. Wang Y, Chen L, Liu M. Microvesicles and diabetic complications—novel mediators, potential biomarkers and therapeutic targets. Acta Pharmacol Sin 2014; 35(4): 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sims PJ, Faioni EM, Wiedmer T, et al. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem 1988; 263(34): 18205–18212. [PubMed] [Google Scholar]
- 25. AbidHussein MN, Meesters EW, Osmanovic N, et al. Antigenic characterization of endothelial cell-derived microparticles and their detection ex vivo. J Thromb Haemost 2003; 1(11): 2434–2443. [DOI] [PubMed] [Google Scholar]
- 26. Horstman LL, Jy W, Jimenez JJ, et al. New horizons in the analysis of circulating cell-derived microparticles. Keio J Med 2004; 53(4): 210–230. [DOI] [PubMed] [Google Scholar]
- 27. Pluskota E, Woody NM, Szpak D, et al. Expression, activation, and function of integrin M 2 (Mac-1) on neutrophil-derived microparticles. Blood 2008; 112(6): 2327–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jimenez JJ, Jy W, Mauro LM, et al. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb Res 2003; 109(4): 175–180. [DOI] [PubMed] [Google Scholar]
- 29. Burnier L, Fontana P, Kwak BR, et al. Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost 2009; 101(3): 439–451. [PubMed] [Google Scholar]
- 30. Morel O, Toti F, Hugel B, et al. Procoagulant microparticles: disrupting the vascular homeostasis equation. Arterioscler Thromb Vasc Biol 2006; 26(12): 2594–2604. [DOI] [PubMed] [Google Scholar]
- 31. Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 2007; 110(7): 2440–2448. [DOI] [PubMed] [Google Scholar]
- 32. Murtha LA, Schuliga MJ, Mabotuwana NS, et al. The processes and mechanisms of cardiac and pulmonary fibrosis. Front Physiol, http://journal.frontiersin.org/article/10.3389/fphys.2017.00777/full [DOI] [PMC free article] [PubMed]
- 33. Brill A, Dashevsky O, Rivo J, et al. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res 2005; 67(1): 30–38. [DOI] [PubMed] [Google Scholar]
- 34. Jy W, Tiede M, Bidot CJ, et al. Platelet activation rather than endothelial injury identifies risk of thrombosis in subjects positive for antiphospholipid antibodies. Thromb Res 2007; 121(3): 319–325. [DOI] [PubMed] [Google Scholar]
- 35. Mobarrez F, Vikerfors A, Gustafsson JT, et al. Microparticles in the blood of patients with systemic lupus erythematosus (SLE): phenotypic characterization and clinical associations. Sci Rep, http://www.nature.com/articles/srep36025 [DOI] [PMC free article] [PubMed]
- 36. Amabile N. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol 2005; 16(11): 3381–3388. [DOI] [PubMed] [Google Scholar]
- 37. Pfister SL. Role of platelet microparticles in the production of thromboxane by rabbit pulmonary artery. Hypertension 2004; 43(2): 428–433. [DOI] [PubMed] [Google Scholar]
- 38. VanWijk MJ, VanBavel E, Sturk A, et al. Microparticles in cardiovascular diseases. Cardiovasc Res 2003; 59(2): 277–287. [DOI] [PubMed] [Google Scholar]
- 39. Lacroix R, Robert S, Poncelet P, et al. Overcoming limitations of microparticle measurement by flow cytometry. Semin Thromb Hemost 2010; 36(8): 807–818. [DOI] [PubMed] [Google Scholar]
- 40. Gradziuk M, Radziwon P. Methods for detection of microparticles derived from blood and endothelial cells. Acta Haematol Pol 2017; 48(4): 316–329. [Google Scholar]
- 41. Van Der Pol E, Hoekstra AG, Sturk A, et al. Optical and non-optical methods for detection and characterization of microparticles and exosomes: detection and characterization of microparticles and exosomes. J Thromb Haemost 2010; 8(12): 2596–2607. [DOI] [PubMed] [Google Scholar]
- 42. Yuana Y, Bertina R, Osanto S. Pre-analytical and analytical issues in the analysis of blood microparticles. Thromb Haemost 2011; 105(3): 396–408. [DOI] [PubMed] [Google Scholar]
- 43. Jayachandran M, Miller VM, Heit JA, et al. Methodology for isolation, identification and characterization of microvesicles in peripheral blood. J Immunol Methods 2012; 375(1–2): 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Burger D, Oleynik P. Isolation and characterization of circulating microparticles by flow cytometry. In:Touyz RM, Schiffrin EL. (eds) Hypertension. New York: Springer, 2017, pp. 271–81. [DOI] [PubMed] [Google Scholar]
- 45. Dinkla S, Brock R, Joosten I, et al. Gateway to understanding microparticles: standardized isolation and identification of plasma membrane-derived vesicles. Nanomedicine 2013; 8(10): 1657–1668. [DOI] [PubMed] [Google Scholar]
- 46. Gyorgy B, Modos K, Pallinger E, et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood 2011; 117(4): e39–e48. [DOI] [PubMed] [Google Scholar]
- 47. Jung C, Drummer K, Oelzner P, et al. The association between endothelial microparticles and inflammation in patients with systemic sclerosis and Raynaud’s phenomenon as detected by functional imaging. Clin Hemorheol Microcirc 2016; 61(3): 549–557. [DOI] [PubMed] [Google Scholar]
- 48. Wu Hua CC, Chen JY, Chang YW, et al. The role of endothelial microparticles in autoimmune disease patients with Raynaud’s phenomenon. J Microbiol Immunol Infect 2017; 50(6): 857–862. [DOI] [PubMed] [Google Scholar]
- 49. Guiducci S, Distler JHW, Jungel A, et al. The relationship between plasma microparticles and disease manifestations in patients with systemic sclerosis. Arthritis Rheum 2008; 58(9): 2845–2853. [DOI] [PubMed] [Google Scholar]
- 50. Michalska-Jakubus M, Kowal-Bielecka O, Smith V, et al. Plasma endothelial microparticles reflect the extent of capillaroscopic alterations and correlate with the severity of skin involvement in systemic sclerosis. Microvasc Res 2017; 110: 24–31. [DOI] [PubMed] [Google Scholar]
- 51. McCarthy EM, Moreno-Martinez D, Wilkinson FL, et al. Microparticle subpopulations are potential markers of disease progression and vascular dysfunction across a spectrum of connective tissue disease. BBA Clin 2017; 7: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nomura S, Inami N, Ozaki Y, et al. Significance of microparticles in progressive systemic sclerosis with interstitial pneumonia. Platelets 2008; 19(3): 192–198. [DOI] [PubMed] [Google Scholar]
- 53. Iversen LV, Ullman S, Østergaard O, et al. Cross-sectional study of soluble selectins, fractions of circulating microparticles and their relationship to lung and skin involvement in systemic sclerosis. BMC Musculoskelet Disord 2015, http://bmcmusculoskeletdisord.biomedcentral.com/articles/10.1186/s12891-015-0653-8 [DOI] [PMC free article] [PubMed]
- 54. Gyorgy B, Szabo TG, Pasztoi M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 2011; 68(16): 2667–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Araldi E, Kramer-Albers EM, Hoen EN, et al. International society for extracellular vesicles: first annual meeting, April 17–21, 2012: ISEV-2012. J Extracell Vesicles 2012; 1: 26082071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hind E, Heugh S, Ansa-Addo EA, et al. Red cell PMVs, plasma membrane-derived vesicles calling out for standards. Biochem Biophys Res Commun 2010; 399(4): 465–469. [DOI] [PubMed] [Google Scholar]
- 57. Aatonen M, Gronholm M, Siljander PR. Platelet-derived microvesicles: multitalented participants in intercellular communication. Semin Thromb Hemost 2012; 38(1): 102–113. [DOI] [PubMed] [Google Scholar]
- 58. Rank A, Nieuwland R, Delker R, et al. Surveillance of megakaryocytic function by measurement of CD61-exposing microparticles in allogeneic hematopoietic stem cell recipients: microparticles in hematopoietic transplantation. Clin Transplant 2011; 25(3): E233–E242. [DOI] [PubMed] [Google Scholar]
- 59. Iversen L, Ostergaard O, Ullman S, et al. Circulating microparticles and plasma levels of soluble E- and P-selectins in patients with systemic sclerosis. Scand J Rheumatol 2013; 42(6): 473–482. [DOI] [PubMed] [Google Scholar]
- 60. Jimenez JJ, Jy W, Horstman LL, et al. Measuring circulating cell-derived microparticles. J Thromb Haemost 2004; 2(10): 1850–1851. [DOI] [PubMed] [Google Scholar]
- 61. Shet AS. Characterizing blood microparticles: technical aspects and challenges. Vasc Health Risk Manag 2008; 4(4): 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pisetsky DS, Ullal AJ, Gauley J, et al. Microparticles as mediators and biomarkers of rheumatic disease. Rheumatology 2012; 51(10): 1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Distler JHW, Huber LC, Gay S, et al. Microparticles as mediators of cellular cross-talk in inflammatory disease. Autoimmunity 2006; 39(8): 683–690. [DOI] [PubMed] [Google Scholar]
- 64. Dasgupta SK, Guchhait P, Thiagarajan P. Lactadherin binding and phosphatidylserine expression on cell surface-comparison with annexin A5. Transl Res 2006; 148(1): 19–25. [DOI] [PubMed] [Google Scholar]
- 65. Mutsaers SE, Bishop JE, McGrouther G, et al. Mechanisms of tissue repair: from wound healing to fibrosis. Int J Biochem Cell Biol 1997; 29(1): 5–17. [DOI] [PubMed] [Google Scholar]
- 66. Kuryliszyn-Moskal A, Klimiuk PA, Sierakowski S. Soluble adhesion molecules (sVCAM-1, sE-selectin), vascular endothelial growth factor (VEGF) and endothelin-1 in patients with systemic sclerosis: relationship to organ systemic involvement. Clin Rheumatol 2005; 24(2): 111–116. [DOI] [PubMed] [Google Scholar]
- 67. Abraham D, Distler O. How does endothelial cell injury start? The role of endothelin in systemic sclerosis. Arthritis Res Ther 2007; 9(Suppl. 2): S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Allanore Y, Simms R, Distler O, et al. Systemic sclerosis. Nat Rev Dis Primer 2015; 1: 15002. [DOI] [PubMed] [Google Scholar]
- 69. Ho YY, Lagares D, Tager AM, et al. Fibrosis—a lethal component of systemic sclerosis. Nat Rev Rheumatol 2014; 10(7): 390–402. [DOI] [PubMed] [Google Scholar]
- 70. Manetti M, Rosa I, Messerini L, et al. A loss of telocytes accompanies fibrosis of multiple organs in systemic sclerosis. J Cell Mol Med 2014; 18(2): 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Manetti M, Guiducci S, Ruffo M, et al. Evidence for progressive reduction and loss of telocytes in the dermal cellular network of systemic sclerosis. J Cell Mol Med 2013; 17(4): 482–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jimenez SA, Piera-Velazquez S. Endothelial to mesenchymal transition (EndoMT) in the pathogenesis of systemic sclerosis-associated pulmonary fibrosis and pulmonary arterial hypertension. Matrix Biol 2016; 51: 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hinz B. Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep 2009; 11(2): 120–126. [DOI] [PubMed] [Google Scholar]
- 74. Manetti M, Guiducci S, Matucci-Cerinic M. The origin of the myofibroblast in fibroproliferative vasculopathy: does the endothelial cell steer the pathophysiology of systemic sclerosis. Arthritis Rheum 2011; 63(8): 2164–2167. [DOI] [PubMed] [Google Scholar]
- 75. Good RB, Gilbane AJ, Trinder SL, et al. Endothelial to mesenchymal transition contributes to endothelial dysfunction in pulmonary arterial hypertension. Am J Pathol 2015; 185(7): 1850–1858. [DOI] [PubMed] [Google Scholar]
- 76. Arciniegas E, Frid MG, Douglas IS, et al. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2007; 293(1): L1–L8. [DOI] [PubMed] [Google Scholar]
- 77. Kizu A, Medici D, Kalluri R. Endothelial–mesenchymal transition as a novel mechanism for generating myofibroblasts during diabetic nephropathy. Am J Pathol 2009; 175(4): 1371–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nicolosi PA, Tombetti E, Maugeri N, et al. Vascular remodelling and mesenchymal transition in systemic sclerosis. Stem Cells Int 2016; 2016: 4636859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Piera-Velazquez S, Mendoza F, Jimenez S. Endothelial to mesenchymal transition (EndoMT) in the pathogenesis of human fibrotic diseases. J Clin Med 2016; 5(4): 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li Z, Jimenez SA. Protein kinase Cδ and c-Abl kinase are required for transforming growth factor β induction of endothelial-mesenchymal transition in vitro. Arthritis Rheum 2011; 63(8): 2473–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cooley BC, Nevado J, Mellad J, et al. TGF-signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci Transl Med 2014; 6(227): 227ra34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lafyatis R. Transforming growth factor β—at the centre of systemic sclerosis. Nat Rev Rheumatol 2014; 10(12): 706–719. [DOI] [PubMed] [Google Scholar]
- 83. Murphy-Ullrich JE, Suto MJ. Thrombospondin-1 regulation of latent TGF-β activation: a therapeutic target for fibrotic disease. Matrix Biol 2018; 68–69: 28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Medici D, Potenta S, Kalluri R. Transforming growth factor-β2 promotes Snail-mediated endothelial–mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling. Biochem J 2011; 437(3): 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Piera-Velazquez S, Jimenez SA. Molecular mechanisms of endothelial to mesenchymal cell transition (EndoMT) in experimentally induced fibrotic diseases. Fibrogenesis Tissue Repair 2012; 5(Suppl. 1): S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nakerakanti S. The role of TGF-β receptors in Fibrosis. Open Rheumatol J 2012; 6(1): 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu Q, Hofmann PA. Protein phosphatase 2A-mediated cross-talk between p38 MAPK and ERK in apoptosis of cardiac myocytes. Am J Physiol Heart Circ Physiol 2004; 286(6): H2204–H2212. [DOI] [PubMed] [Google Scholar]
- 88. Samuel GH, Bujor AM, Nakerakanti SS, et al. Autocrine transforming growth factor β signaling regulates extracellular signal-regulated kinase 1/2 phosphorylation via modulation of protein phosphatase 2A expression in scleroderma fibroblasts. Fibrogen Tiss Rep 2010; 3(1): 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Horn A, Palumbo K, Cordazzo C, et al. Hedgehog signaling controls fibroblast activation and tissue fibrosis in systemic sclerosis. Arthritis Rheum 2012; 64(8): 2724–2733. [DOI] [PubMed] [Google Scholar]
- 90. Louvi A, Artavanis-Tsakonas S. Notch and disease: a growing field. Semin Cell Dev Biol 2012; 23(4): 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bergmann C, Distler JHW. Canonical Wnt signaling in systemic sclerosis. Lab Invest 2016; 96(2): 151–155. [DOI] [PubMed] [Google Scholar]
- 92. Pattanaik D, Brown M, Postlethwaite BC, et al. Pathogenesis of systemic sclerosis. Front Immunol 2015; 6: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cipriani P, Di Benedetto P, Ruscitti P, et al. The endothelial-mesenchymal transition in systemic sclerosis is induced by endothelin-1 and transforming growth factor- and may be blocked by macitentan, a dual endothelin-1 receptor antagonist. J Rheumatol 2015; 42(10): 1808–1816. [DOI] [PubMed] [Google Scholar]
- 94. Xu X, Tan X, Tampe B, et al. Snail is a direct target of hypoxia-inducible factor 1α (HIF1α) in hypoxia-induced endothelial to mesenchymal transition of human coronary endothelial cells. J Biol Chem 2015; 3290(27): 16653–16664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Beyer C, Schett G, Gay S, et al. Hypoxia in the pathogenesis of systemic sclerosis. Arthritis Res Ther 2009; 11(2): 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Piera-Velazquez S, Makul A, Jiménez SA. Increased expression of NAPDH oxidase 4 in systemic sclerosis dermal fibroblasts: regulation by transforming growth factor β: increased nox-4 in systemic sclerosis fibroblasts. Arthritis Rheumatol 2015; 67(10): 2749–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cucoranu I. NAD(P)H oxidase 4 mediates transforming growth factor- 1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 2005; 97(9): 900–907. [DOI] [PubMed] [Google Scholar]
- 98. Hecker L, Vittal R, Jones T, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 2009; 15(9): 1077–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Higgins DF, Kimura K, Bernhardt WM, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest, http://content.the-jci.org/articles/view/30487 [DOI] [PMC free article] [PubMed]
- 100. Xiong A, Liu Y. Targeting hypoxia inducible factors-1α as a novel therapy in fibrosis. Front Pharmacol 2017, http://journal.frontiersin.org/article/10.3389/fphar.2017.00326/full [DOI] [PMC free article] [PubMed]
- 101. Hong K-H, Yoo S-A, Kang S-S, et al. Hypoxia induces expression of connective tissue growth factor in scleroderma skin fibroblasts. Clin Exp Immunol 2006; 146(2): 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zampetaki A, Kiechl S, Drozdov I, et al. Plasma MicroRNA profiling reveals loss of endothelial MiR-126 and Other MicroRNAs in type 2 diabetes. Circ Res 2010; 107(6): 810–817. [DOI] [PubMed] [Google Scholar]
- 103. Maurer B, Stanczyk J, Jungel A, et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum 2010; 62(6): 1733–1743. [DOI] [PubMed] [Google Scholar]
- 104. Honda N, Jinnin M, Kajihara I, et al. TGF-mediated downregulation of MicroRNA-196a Contributes to the constitutive upregulated type I collagen expression in scleroderma dermal fibroblasts. J Immunol 2012; 188(7): 3323–3331. [DOI] [PubMed] [Google Scholar]
- 105. Makino K, Jinnin M, Hirano A, et al. The downregulation of microRNA let-7a contributes to the excessive expression of type I collagen in systemic and localized scleroderma. J Immunol 2013; 190(8): 3905–3915. [DOI] [PubMed] [Google Scholar]
- 106. Jayawardena TM, Egemnazarov B, Finch EA, et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res 2012; 110(11): 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Xue Y, Ouyang K, Huang J, et al. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated MicroRNA circuits. Cell 2013; 152(1–2): 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cantaluppi V, Gatti S, Medica D, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia–reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int 2012; 82(4): 412–427. [DOI] [PubMed] [Google Scholar]
- 109. Ntelis K, Solomou EE, Sakkas L, et al. The role of platelets in autoimmunity, vasculopathy, and fibrosis: implications for systemic sclerosis. Semin Arthritis Rheum 2017; 47(3): 409–417. [DOI] [PubMed] [Google Scholar]
- 110. Yoshizaki A, Komura K, Iwata Y, et al. Clinical significance of serum HMGB-1 and sRAGE levels in systemic sclerosis: association with disease severity. J Clin Immunol 2009; 29(2): 180–189. [DOI] [PubMed] [Google Scholar]
- 111. Maugeri N, Rovere-Querini P, Baldini M, et al. Oxidative stress elicits platelet/leukocyte inflammatory interactions via HMGB1: a candidate for microvessel injury in sytemic sclerosis. Antioxid Redox Sig 2014; 20(7): 1060–1074. [DOI] [PubMed] [Google Scholar]
- 112. Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech 2014; 7(2): 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Pardo A, Selman M. Role of matrix metaloproteases in idiopathic pulmonary fibrosis. Fibrogen Tiss Rep 2012; 5(Suppl. 1): S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kuroda K, Shinkai H. Gene expression of types I and III collagen, decorin, matrix metalloproteinases and tissue inhibitors of metalloproteinases in skin fibroblasts from patients with systemic sclerosis. Arch Dermatol Res 1997; 289(10): 567–572. [DOI] [PubMed] [Google Scholar]
- 115. Moinzadeh P, Krieg T, Hellmich M, et al. Elevated MMP-7 levels in patients with systemic sclerosis: correlation with pulmonary involvement: letter to the editor. Exp Dermatol 2011; 20(9): 770–773. [DOI] [PubMed] [Google Scholar]
- 116. Maugeri N, Capobianco A, Rovere-Querini P, et al. Platelet microparticles sustain autophagy-associated activation of neutrophils in systemic sclerosis. Sci Transl Med 2018; 10: eaao3089. [DOI] [PubMed] [Google Scholar]
- 117. Pieterse E, Rother N, Garsen M, et al. Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arterioscler Thromb Vasc Biol 2017; 37(7): 1371–1379. [DOI] [PubMed] [Google Scholar]
- 118. Wang J-G, Williams JC, Davis BK, et al. Monocytic microparticles activate endothelial cells in an IL-1 -dependent manner. Blood 2011; 118(8): 2366–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Munkonda MN, Akbari S, Landry C, et al. Podocyte-derived microparticles promote proximal tubule fibrotic signaling via p38 MAPK and CD36. J Extracell Vesicles 2018; 7(1): 1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Fernandez-Martinez AB, Torija AV, Carracedo J, et al. Microparticles released by vascular endothelial cells increase hypoxia inducible factor expression in human proximal tubular HK-2 cells. Int J Biochem Cell Biol 2014; 53: 334–342. [DOI] [PubMed] [Google Scholar]
- 121. Distler JHW, Jungel A, Huber LC, et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc Natl Acad Sci U S A 2005; 102(8): 2892–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lozito TP, Tuan RS. Endothelial cell microparticles act as centers of matrix metalloproteinsase-2 (MMP-2) activation and vascular matrix remodeling. J Cell Physiol 2012; 227(2): 534–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lacroix R, Plawinski L, Robert S, et al. Leukocyte- and endothelial-derived microparticles: a circulating source for fibrinolysis. Haematologica 2012; 97(12): 1864–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Novelli F, Neri T, Tavanti L, et al. Procoagulant, tissue factor-bearing microparticles in bronchoalveolar lavage of interstitial lung disease patients: an observational study. PLoS ONE 2014; 9(4): e95013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Bacha NC, Blandinieres A, Rossi E, et al. Endothelial microparticles are associated to pathogenesis of idiopathic pulmonary fibrosis. Stem Cell Rev 2018; 14(2): 223–235. [DOI] [PubMed] [Google Scholar]
- 126. Asef A, Mortaz E, Jamaati H, et al. Immunologic role of extracellular vesicles and exosomes in the pathogenesis of cystic fibrosis. Tanaffos 2018; 17(2): 66–72. [PMC free article] [PubMed] [Google Scholar]
- 127. Bălănescu P, Lădaru A, Bălănescu E, et al. IL-17, IL-6 and IFN-γ in systemic sclerosis patients. Rom J Intern Med 2015; 53(1): 46–51. [DOI] [PubMed] [Google Scholar]
- 128. Sziksz E, Pap D, Lippai R, et al. Fibrosis related inflammatory mediators: role of the IL-10 cytokine family. Mediators Inflamm 2015; 2015: 764641–764615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Dinkla S, vanCranenbroek B, vanderHeijden WA, et al. Platelet microparticles inhibit IL-17 production by regulatory T cells through P-selectin. Blood 2016; 127(16): 1976–1986. [DOI] [PubMed] [Google Scholar]
- 130. Anene C, Graham AM, Boyne J, et al. Platelet microparticle delivered microRNA-Let-7a promotes the angiogenic switch. Biochim Biophys Acta Mol Basis Dis 2018; 1864(8): 2633–2643. [DOI] [PubMed] [Google Scholar]
- 131. Maugeri N, Franchini S, Campana L, et al. Circulating platelets as a source of the damage-associated molecular pattern HMGB1 in patients with systemic sclerosis. Autoimmunity 2012; 45(8): 584–587. [DOI] [PubMed] [Google Scholar]
- 132. Jy W, Jimenez JJ, Mauro LM, et al. Endothelial microparticles induce formation of platelet aggregates via a von Willebrand factor/ristocetin dependent pathway, rendering them resistant to dissociation: EMP-platelet interaction. J Thromb Haemost 2005; 3(6): 1301–1308. [DOI] [PubMed] [Google Scholar]
- 133. Abid Hussein MN, Böing AN, Biró E, et al. Phospholipid composition of in vitro endothelial microparticles and their in vivo thrombogenic properties. Thromb Res 2008; 121(6): 865–871. [DOI] [PubMed] [Google Scholar]
- 134. Freyssinet J-M. Cellular microparticles: what are they bad or good for? J Thromb Haemost 2003; 1(7): 1655–1662. [DOI] [PubMed] [Google Scholar]
- 135. Postlethwaite AE, Chiang TM. Platelet contributions to the pathogenesis of systemic sclerosis. Curr Opin Rheumatol 2007; 19(6): 574–579. [DOI] [PubMed] [Google Scholar]
- 136. Ntelis K, Bogdanos D, Dimitroulas T, et al. Platelets in systemic sclerosis: the missing link connecting vasculopathy, autoimmunity, and fibrosis? Curr Rheumatol Rep 2019; 21(5), http://link.springer.com/10.1007/s11926-019-0815-z [DOI] [PubMed] [Google Scholar]
- 137. Avouac J, Vallucci M, Smith V, et al. Correlations between angiogenic factors and capillaroscopic patterns in systemic sclerosis. Arthritis Res Ther 2013; 15(2): R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Morardet L, Avouac J, Sammour M, et al. Late nailfold videocapillaroscopy pattern associated with hand calcinosis and acro-osteolysis in systemic sclerosis: NVC patterns with hand calcinosis and acro-osteolysis in SSc. Arthritis Care Res 2016; 68(3): 366–373. [DOI] [PubMed] [Google Scholar]
- 139. Yousif M, Habib R, Esaely H, et al. Interleukin-6 in systemic sclerosis and potential correlation with pulmonary involvement. Egypt J Chest Dis Tuberc 2015; 64(1): 237–241. [Google Scholar]
- 140. Thakkar V, Patterson KA, Stevens W, et al. Increased serum levels of adhesion molecules ICAM-1 and VCAM-1 in systemic sclerosis are not specific for pulmonary manifestations. Clin Rheumatol 2018; 37(6): 1563–1571. [DOI] [PubMed] [Google Scholar]
- 141. Cossu M, Andracco R, Santaniello A, et al. Serum levels of vascular dysfunction markers reflect disease severity and stage in systemic sclerosis patients. Rheumatology 2016; 55(6): 1112–1116. [DOI] [PubMed] [Google Scholar]
- 142. Mostmans Y, Cutolo M, Giddelo C, et al. The role of endothelial cells in the vasculopathy of systemic sclerosis: a systematic review. Autoimmun Rev 2017; 16(8): 774–786. [DOI] [PubMed] [Google Scholar]
- 143. Habersberger J, Strang F, Scheichl A, et al. Circulating microparticles generate and transport monomeric C-reactive protein in patients with myocardial infarction. Cardiovasc Res 2012; 96(1): 64–72. [DOI] [PubMed] [Google Scholar]
- 144. Gasser O. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 2004; 104(8): 2543–2548. [DOI] [PubMed] [Google Scholar]
- 145. Valentini G, Iudici M, Walker UA, et al. The European scleroderma trials and research group (EUSTAR) task force for the development of revised activity criteria for systemic sclerosis: derivation and validation of a preliminarily revised EUSTAR activity index. Ann Rheum Dis 2017; 76(1): 270–276. [DOI] [PubMed] [Google Scholar]
- 146. Valentini G. European multicentre study to define disease activity criteria for systemic sclerosis. II. Identification of disease activity variables and development of preliminary activity indexes. Ann Rheum Dis 2001; 60(6): 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hansch A, Frey O, Sauner D, et al. In vivo imaging of experimental arthritis with near-infrared fluorescence: in vivo imaging of AIA with NIRF. Arthritis Rheum 2004; 50(3): 961–967. [DOI] [PubMed] [Google Scholar]