Abstract
Anti-RNA Polymerase III antibodies are the most frequent anti-nuclear antibodies in systemic sclerosis, after anti-centromere and anti-Topoisomerase I. Considering their specificity for systemic sclerosis, they have been included in 2013 American College of Rheumatology/European League Against Rheumatism classification criteria for systemic sclerosis. They were first identified in 1993 using an immunoprecipitation method; the subsequent diffusion of commercial assays, based on the enzyme-linked immunosorbent assay or multiplex line immunoblot techniques, has allowed an increasing number of systemic sclerosis patients to be tested for this autoantibody; nevertheless, the diffusion of this test in systemic sclerosis patients is probably still sub-optimal. Anti-RNA Polymerase III antibodies have been associated with important clinical manifestations: rapid and diffuse cutaneous involvement, joint contractures, scleroderma renal crisis, gastric antral vascular ectasia and malignancies synchronous to systemic sclerosis onset. Moreover, other possible clinical associations, including pulmonary hypertension, still need confirmation. Since the correct approach for screening for anti- RNA Polymerase III antibodies in patients with suspected or definite systemic sclerosis is still debated, possible strategies are proposed here. Moreover, issues that are still controversial are discussed, including the interpretation of multiple simultaneous positivity for anti-RNA Polymerase III antibodies and other autoantibodies in line immunoassay, and the possible relevance of anti-RNA Polymerase III antibodies titre.
Keywords: Autoantibodies, enzyme-linked immunosorbent assay, immunoblotting, immunoprecipitation, RNA polymerase III, scleroderma, systemic
Introduction
The presence of circulating antinuclear autoantibodies (ANA) is one of the distinctive features of systemic sclerosis (SSc). Since their first identification by Kuwana et al. 1 in 1993, antibodies reactive with RNA polymerase III (anti-RNAP3) were demonstrated to be a highly specific biomarker to SSc with relevant clinical associations, in particular with diffuse cutaneous involvement (dcSSc) and scleroderma renal crisis (SRC).2–5
These findings were afterwards confirmed by other independent studies, but for several years, the use of anti-RNAP3 in clinical practice was greatly limited by the cumbersome procedures required by the immunoprecipitation (IP) assay used in the first investigations. The establishment of sensitive and specific immunoassay systems was made possible by the identification of the major antigenic site universally recognized by anti-RNAP3-positive SSc sera.6,7 This evolution led to the development of commercial kits using recombinant antigenic fragments, which are now available as in vitro diagnostics in many countries.4,7–9 This allowed the identification of previously unnoticed clinical features in with anti-RNAP3 positive patients, including gastric antral vascular ectasia (GAVE), and close temporal relationship between SSc onset and malignancies. 5
Nevertheless, there are some clues that screening for anti-RNAP3 is not always applied in SSc patients. For example, in a recent analysis of the European Scleroderma Trials and Research (EUSTAR) registry, the largest multicentre cohort of patients with SSc, performed in March 2014, data on anti-RNAP3 were available only in 4986 of 11,399 (43.7%) patients fulfilling either the 1980 American College of Rheumatology (ACR) or the 2013 ACR/European League Against Rheumatism (EULAR) classification criteria for SSc, even if information on anti-RNAP3 could be recorded in the registry since December 2008. 10
In this review, recent data supporting the need for anti-RNAP3 screening 11 in patients with suspected or definite SSc are discussed, and possible algorithms for screening and future directions for research are proposed.
Sensitivity of anti-RNAP3 for SSc
A recent meta-analysis of 30 studies, assessing the prevalence of anti-RNAP3 in SSc patients worldwide and the potential factors of variability, 12 confirmed that anti-RNAP3 are one of the most frequent antinuclear antibodies in SSc, after anticentromere (ACA) and anti-topoisomerase I (anti-Topo I). The overall pooled prevalence was 11% (95% confidence interval (CI), 8–14), with a high degree of heterogeneity, part of which was explained by geography influence. In fact, geographical areas of low prevalence of anti-RNAP3 among SSc patients (3%–10%) were identified: these included Southern and Central Europe (12 studies from France, Italy, Belgium, Switzerland and Germany) and Asia (5 studies from Japan, South Korea and Singapore; a small study from China reported a slightly higher prevalence). Conversely, geographical areas at high prevalence (15%–22%) included Northern Europe (4 studies from the United Kingdom, Sweden and Denmark), North America (6 studies from the United States and Canada) and Australia. Only few and discordant data were available from Central and Southern America, and no data at all from Africa. Only few studies included data about ethnicity; however, some American studies reported a lower frequency of anti-RNAP3 in African than in Caucasian patients,13,14 suggesting that ethnicity may at least partially explain the variability of anti-RNAP3 prevalence. 12
Specificity of anti-RNAP3 for SSc
The high specificity of diagnostic tests for anti-RNAP3 for SSc suggested by the first investigators using the IP assays was then confirmed by several studies applying the newly developed immunoassays.4,8,9,15,16 Interestingly, during the development of the 2013 ACR/EULAR classification criteria for SSc, anti-RNAP3 were positive in 27/268 (10%) SSc patients in the criteria validation cohort, as compared with 0/137 in patients with an SSc-like disorder.17,18
Do we need to identify anti-RNAP3 in patients with suspected SSc?
On the basis of a broad consensus among experts, anti-RNAP3 were included together with ACA and anti-Topo I among SSc-related autoantibodies as a specific item in the 2013 ACR-EULAR SSc classification criteria for their specificity and sensitivity.
Some doubts have been raised on the opportunity to routinely test anti-RNAP3 in the context of the suspect of systemic autoimmune diseases. 19 On the other hand, ACR and EULAR experts strongly support the notion that anti-RNAP3 can be helpful in the diagnosis and classification of SSc when a specific clinical suspect is present. 18 An example of clinical setting inducing the suspect of SSc may be represented by patients with VEDOSS red flags (Raynaud’s phenomenon and puffy fingers, or ANA positivity), 20 but other scenarios are also possible.
Do we need to identify anti-RNAP3 in definite SSc patients?
The utility of testing for anti-RNAP3 antibodies in patients with SSc derives from the peculiar clinical correlations of these autoantibodies. As we will see, all these associations were confirmed by independent studies conducted in different countries.
Association with rapidly progressive skin involvement and joint contractures
The association of anti-RNAP3 with the dcSSc subset is well known.1–5 For example, in the EUSTAR registry 58% anti-RNAP3+ SSc patients had dcSSc compared with 28% anti-RNAP3-. 10 The skin involvement among anti-RNAP3+ SSc patients is characterized by rapid progression. In fact, the time interval between the onset of Raynaud’s phenomenon and the first symptom other than Raynaud’s phenomenon (or the SSc diagnosis), as well the one between the first SSc-related symptoms and the peak of modified Rodnan skin score (mRSS), is shorter in anti-RNAP3-positive SSc patients than in patients with anti-Topo I or ACA.10,21,22 These observations were recently confirmed by a multicentre prospective observational study of 326 patients with early dcSSc in which mRSS was assessed every 3 months for 12–24 months. 23 Moreover, all patients with anti-RNAP3+ developing a total mRSS > 20 points did so within 3 years after onset of Raynaud’s phenomenon, 22 and 90% of them reached their peak of mRSS within the first 2 years of the disease. 10 These data suggest that, after the first few years of the disease, skin involvement does not further progress in anti-RNAP3-positive patients. Indeed, in many patients, skin thickness may regress during the follow-up, even without treatment. 24
Finally, the frequency of joint contractures was significantly higher in anti-RNAP3+ patients than in anti-RNAP3- in the EUSTAR registry (46% vs 30%, respectively). 10
Association with SRC
Patients with anti-RNAP3 positivity have the highest risk for developing SRC compared to SSc patients with other autoantibodies:1–5 some recent data from the EUSTAR registry, 10 and large cohorts from the United States and Japan,25,26 indicate that around 50% of patients with SRC have anti-RNP3 antibodies. Lower incidence of SRC in Japan, France or Italy, when compared with the United Kingdom and North America, may reflect the lower prevalence of anti-RNAP3 antibody within these SSc populations.5,12
Conversely, in these recent studies, the prevalence of SRC among anti-RNAP3+ SSc patients during the disease course ranges from 12% to 24%.10,26 It is noteworthy that the time from SSc onset to SRC is shorter in patients with anti-RNAP3 antibodies than in those with anti-Topo I+.25,27
Association with GAVE
As previously mentioned, new clinical associations with anti-RNAP3 have emerged thanks to the larger availability of diagnostic tests. The association with GAVE (or ‘watermelon stomach’) was first suggested by a small single-centre study, 28 and subsequently confirmed by data from an EUSTAR case-control study (49 SSc with GAVE vs 93 SSc without GAVE, with an odds ratio (OR) of 4.6 for anti-RNAP3+ patients), 29 and independently, by a multicentre Australian study. 30 In another EUSTAR case-control study, in which 158 anti-RNAP3+ were compared with 199 anti-RNAP3-patients matched for sex, age, disease subset and duration, GAVE was identified (in most cases because it was clinically relevant for anaemia or evident bleeding) in 8% of anti-RNAP3+ versus 1% anti-RNAP3 patients. 10 Similar to what has been reported for rapid progression of cutaneous involvement and SRC, GAVE was more frequently observed during the first phases of diseases.28,29
Association with cancer synchronous to SSc onset
The association of anti-RNAP3 antibodies with cancer diagnosed in close temporal relationship to SSc onset was the most relevant information provided by studies on anti-RNAP3 in the last decade. First identified by Shah et al. 31 in a single-centre American study, in which anti-RNAP3 were evaluated both by IP and enzyme-linked immunosorbent assay (ELISA), this observation was afterwards confirmed in several single-centre cohorts coming from different geographical areas (Italy, the United Kingdom, the United States, Australia and Japan).32–36
Moreover, the association with synchronous malignancies was confirmed in a multicentre case-control study from the EUSTAR registry: anti-RNAP3+ SSc patients had an OR of 7.38 of having a diagnosis of cancer within an interval between 6 months before and 12 months after SSc onset, as compared with matched anti-RNAP3-negative patients. 10 The frequency of cancer diagnosis within 2 years before or after SSc onset in anti-RNAP3+ patients was 9%, and the number of anti-RNAP 3+ patients needed to screen to find 1 synchronous cancer at SSc diagnosis was 17. 10 Considering that the majority of cancers in these patients were diagnosed within a short interval between and after SSc onset, no available data so far suggest that the risk of cancer is extended beyond this time interval.10,34
This EUSTAR study provided additional information on the characteristics of cancers in these patients: breast cancers were largely the most prevalent type in females, in accordance to what observed in a previous British study; 34 malignancies other than breast cancer were instead much more frequent in males than females. Finally, the study evaluated whether anti-RNAP3+ patients with synchronous cancer have different characteristics as compared to anti-RNAP3+ without synchronous cancer demonstrating that patients with older age or dcSSc were particularly at risk for cancer. 10
It is beyond the scope of this article to review the mechanistic link between cancer and the development of SSc. 37 However, we cannot fail to mention that the close temporal relationship between cancer and SSc among anti-RNAP3+ patients led to the hypothesis that SSc could represent a para-neoplastic disorder in this patients’ subset.38,39 This hypothesis was supported by the presence of genetic abnormalities (missense mutations or loss of heterozygosity) in the RPC155/POLR3A gene in the cancer tissues from anti-RNAP3+ SSc patients, but not in cancers from other SSc patients. Moreover, the presence of mutation-specific T-cell and B-cell immune responses cross-reacting with both mutated and wild-type RNAP3 protein was also demonstrated. 40
Notably, in a study of 50 women with breast cancer without rheumatic diseases, none had positivity for anti-RNAP3, confirming the specificity of these autoantibodies for SSc and suggesting that it may be a cancer biomarker only in patients with this disease. 41
Clinical associations of anti-RNAP3: some open issues (interstitial lung disease and pulmonary hypertension)
Most cohort studies described no association between anti-RNAP3 and interstitial lung disease (ILD) or pulmonary hypertension (PH). Accordingly, in a recent prospective observational study from Norway, 49% of 33 anti-RNAP3+ SSc patients showed no evidence of ILD development during a 8-year mean follow-up. 42 However, 18% of these anti-RNAP3+ patients developed extensive ILD at High Resolution Computed Tomography (HRCT), indicating that the issue cannot be neglected in all cases. Indeed, previous studies reported that the prevalence of ILD among anti-RNAP3+ SSc patients was higher than in ACA+ patients.32,43 Unlike the other clinical manifestations associated with anti-RNAP3, in the Norwegian cohort, extensive ILD tended to develop later on in the disease course than in patients with anti-Topo I. Although different mechanisms for fibrosis may be hypothesized, this latter observation might have been influenced by the significant shorter disease duration at the baseline for anti-RNAP3+ patients as compared with anti-Topo I+. 44
Finally, anti-RNAP3 are generally not associated with PH, but in the large single-centre cohort from the Royal Free Hospital in London, these autoantibodies, even if not associated with the development of PH in unadjusted analysis, were shown to be an independent predictor of PH, when correcting for other variables. 45 It should be noted that the risk was calculated including together the development of catheterization-proven pulmonary arterial hypertension, either associated with ILD or not. 45 This very important observation should be verified in other, possibly multicentre, cohorts.
How to identify anti-RNAP3 in SSc patients?
Anti-RNAP3 were first identified using an IP assay,1,2 which is still considered the gold standard. However, although good for research proposals, this method is time-consuming and unsuitable for screening and other routine applications. The availability of commercial immunoassay kits based on the ELISA or multiplex line immunoassay (LIA) methods contributed to the diffusion of this test for the diagnosis and subsetting of SSc patients. Both methods were proven to be sensitive and specific, practical and rapid.8,9,15,16,30 Each method has some pros and cons, and their use depends on local consuetude and facilities availability. Briefly, ELISA is cheaper (reagents-only real-life cost in our Hospital: 5 Euro/test) and semiquantitative, whereas LIA is more expensive (reagents-only real-life cost in our Hospital: 40 Euro/test), and only poorly semiquantitative, but it allows the simultaneous evaluation of multiple autoantibodies other than anti-RNAP3.
The most relevant information for the purpose of this review is that the clinical associations of anti-RNAP3 (diffuse subset, renal crisis, joint contractures, synchronous cancer) in different studies were confirmed whatever the method used (IP, ELISA or LIA).
Nevertheless, there are still some open issues, which will be discussed below.
How to interpret multiple positive LIA results?
A simultaneous positivity for multiple autoantibodies represent a frequent issue, when analysing SSc sera by LIA,15,16,30 challenging the common opinion that autoantibodies in SSc are mutually exclusive. In a large cross-sectional study, evaluating 505 Australian SSc patients with a commercial LIA, 16% of them were found to be anti-RNAP3+. Among them, 66% showed multiple positivity, but this was always explained by non SSc-specific autoantibodies (most frequently anti-Ro52/TRIM, observed in 30% of the cases); none of these patients had instead simultaneous positivity for ACA or anti-Topo I. 30 Moreover, in the same study, classical anti-RNAP3 clinical associations were confirmed also in patients with multiple positivity, 30 suggesting that the clinicians should be guided by the presence of anti-RNAP3 in these patients, irrespectively from the presence of other autoantibodies.
On the other hand, no definitive data is available in the very rare cases in which anti-RNAP3 are associated with the other major SSc-specific Abs (anti-Topo I, ACA). However, in another recent cross-sectional study, 76 SSc patients positive for either ACA or anti-Topo I were tested for the presence of anti-RNAP3, which were found positive only in one ACA+ serum (1.3%), confirming that is a very rare scenario. 46 Notably, the clinical features of this patient were closer to the one described in patients with anti-RNAP3 (dcSSc, joint contractures and a concomitant cancer).
Is anti-RNAP3 antibody titre important?
There are some clues that clinical correlations with anti-RNAP3 are stronger in patients with high antibody titre: for example, SRC is better correlated with ELISA higher titres 26 and mRSS is higher in patients with high anti-RNAP3 as measured by ELISA,4,26,47 or with stronger LIA reactivity. 30 The association with GAVE is also more evident in these cases. 30
Moreover, small series or case reports described a reduction or negativization of anti-RNAP3 titre correlated with clinical improvement, either spontaneously during the disease course 47 or after immunosuppressive therapy, particularly with rituximab.48–50
It should be considered that higher titres of anti-RNAP3 are more frequent in the earlier phases of disease,30,47 in which the associated clinical manifestations usually appear. Indeed, there is currently no evidence for the utility to check anti-RNAP3 titre during follow-up.
Conclusion
Why is it important to screen for anti-RNAP3? (which are the clinical implications?)
The data from the literature presented here demonstrated that anti-RNAP3 not only are frequent SSc-specific autoantibodies but also are associated with a peculiar clinical phenotype which may be severe, particularly in the earlier phases of the disease.
This information may be important for patients’ management, indicating the need of a careful follow-up during the first phases of the disease leading to early consideration for aggressive disease-modifying therapies. 5 Moreover, these patients may need early physical and occupational therapy to prevent or reduce joint contractures. Anti-RNAP3+ patients have high risk for SRC and therefore should be educated to recognize and refer symptoms of SRC and to regular monitor blood pressure at home. Close follow-up of renal function and complete blood count (that could also detect anaemia associated with GAVE) is mandatory.
Finally, since these patients have a significantly increased risk of cancer within a short interval of SSc onset, they may benefit from cancer screening at the time of diagnosis. 5 The optimal approach for this screening may be guided by the recommendations proposed by 82 EUSTAR experts using a Delphi exercise. 10 The tests required should include screening for breast cancer in all female patients, and non-invasive tests guided by clinical suspicion for other malignancies for all patients, with particular attention to patients with older age and dcSSc.
How to screen SSc patients for anti-RNAP3?
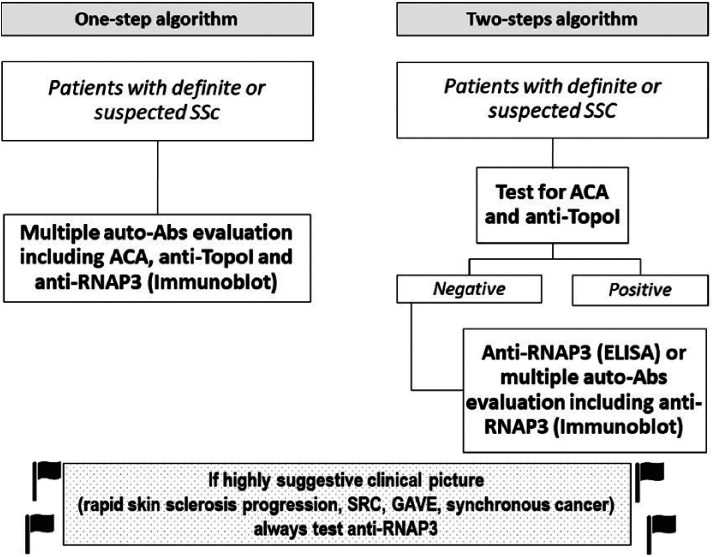
Since clinical association for anti-RNAP3 are generally independent from the laboratory methods of detection and they are relatively not expensive, the strategy for screening of anti-RNAP3 may depend on local factors such as availability of the diagnostic tools. At least two possible algorithms may be considered (Figure 1):
Figure 1.
Proposed algorithms for anti-RNAP3 screening in patients with definite or supected SSc.
A one-step algorithm, in which patients with definite, or strongly suspected, SSc are evaluated by a multiple autoantibody assay (e.g. LIA) that includes anti-RNAP3 as well as ACA and anti-Topo I.
A two-step algorithm in which patients are first checked for ACA and anti-Topo I, and then, if negative for these more frequent SSc-specific autoantibodies, evaluated for anti-RNAP3 by a specific ELISA, or for multiple less frequent autoantibodies, including anti-RNAP3, by a LIA.
The latter strategy takes into consideration the rare coexistence of anti-RNAP3 with the other two major SSc-specific autoantibodies.
However, we would strongly recommend that when SSc patients present with a clinical picture highly suggestive for the presence of anti-RNAP3 (e.g. rapid skin sclerosis progression, SRC, GAVE, synchronous cancer), they should be always tested for anti-RNAP3.
Final remarks and future directions
Screening for anti-RNAP3 is useful for patients with suspected or confirmed SSc, being important for management of patients. Different laboratory methods seem to have the same reliability as far as clinical correlations, and they are relatively not expensive (it should be noted that so far there is no indication to repeat the test in the follow-up).
Large prospective studies are needed to establish other possible rare clinical correlations, the optimal screening for cancer, the characteristics of cancer patients, the possible effect of removal of cancer on SSc or the possible specific effect of different therapies in this subset of SSc. Regarding laboratory issues, future researches are needed to clarify the possible value of monitoring anti-RNAP3 titre and the clinical significance of the very rare cases of simultaneous positivity with ACA+ and anti-TopoI+.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- 1. Kuwana M, Kaburaki J, Mimori T, et al. Autoantibody reactive with three classes of RNA polymerases in sera from patients with systemic sclerosis. J Clin Invest 1993; 91: 1399–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Okano Y, Steen VD, Medsger TA. Autoantibody reactive with RNA polymerase III in systemic sclerosis. Ann Intern Med 1993; 119: 1005–1013. [DOI] [PubMed] [Google Scholar]
- 3. Bunn CC, Denton CP, Shi-Wen X, et al. Anti-RNA polymerases and other autoantibody specificities in systemic sclerosis. Br J Rheumatol 1998; 37: 15–20. [DOI] [PubMed] [Google Scholar]
- 4. Kuwana M, Okano Y, Pandey JP, et al. Enzyme-linked immunosorbent assay for detection of anti-RNA polymerase III antibody: analytical accuracy and clinical associations in systemic sclerosis. Arthritis Rheum 2005; 52: 2425–2432. [DOI] [PubMed] [Google Scholar]
- 5. Kuwana M. A to-do list at diagnosis of systemic sclerosis with positive anti-RNA polymerase III antibodies. J Rheumatol 2017; 44: 550–552. [DOI] [PubMed] [Google Scholar]
- 6. Kuwana M, Okano Y, Kaburaki J, et al. Autoantibodies to RNA polymerases recognize multiple subunits and demonstrate cross-reactivity with RNA polymerase complexes. Arthritis Rheum 1999; 42: 275–284. [DOI] [PubMed] [Google Scholar]
- 7. Kuwana M, Kimura K, Kawakami Y. Identification of an immunodominant epitope on RNA polymerase III recognized by systemic sclerosis sera: application to enzyme-linked immunosorbent assay. Arthritis Rheum 2002; 46: 2742–2747. [DOI] [PubMed] [Google Scholar]
- 8. Parker JC, Burlingame RW, Webb TT, et al. Anti-RNA polymerase III antibodies in patients with systemic sclerosis detected by indirect immunofluorescence and ELISA. Rheumatology 2008; 47: 976–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Satoh T, Ishikawa O, Ihn H, et al. Clinical usefulness of anti-RNA polymerase III antibody measurement by enzyme-linked immunosorbent assay. Rheumatology 2009; 48: 1570–1574. [DOI] [PubMed] [Google Scholar]
- 10. Lazzaroni MG, Cavazzana I, Colombo E, et al. Malignancies in patients with anti-RNA polymerase III antibodies and systemic sclerosis: analysis of the EUSTAR cohort and possible recommendations for screening. J Rheumatol 2017; 44: 639–647. [DOI] [PubMed] [Google Scholar]
- 11. Wilson JMG, Jungner G. Principles and practice of screening for disease. Geneva: WHO, 1968. [Google Scholar]
- 12. Sobanski V, Dauchet L, Lefèvre G, et al. Prevalence of anti-RNA polymerase III antibodies in systemic sclerosis: new data from a French cohort and a systematic review and meta-analysis. Arthritis Rheumatol 2014; 66: 407–417. [DOI] [PubMed] [Google Scholar]
- 13. Steen V, Domsic RT, Lucas M, et al. A clinical and serologic comparison of African American and Caucasian patients with systemic sclerosis. Arthritis Rheum 2012; 64: 2986–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gelber AC, Manno RL, Shah AA, et al. Race and association with disease manifestations and mortality in scleroderma: a 20-year experience at the Johns Hopkins Scleroderma Center and review of the literature. Medicine 2013; 92: 191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maes L, Blockmans D, Verschueren P, et al. Anti-PM/Scl-100 and anti-RNA-polymerase III antibodies in scleroderma. Clin Chim Acta 2010; 411: 965–971. [DOI] [PubMed] [Google Scholar]
- 16. Villalta D, Imbastaro T, Di Giovanni S, et al. Diagnostic accuracy and predictive value of extended autoantibody profile in systemic sclerosis. Autoimmun Rev 2013; 12: 14–20. [DOI] [PubMed] [Google Scholar]
- 17. van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann Rheum Dis 2013; 172: 1747–1755. [DOI] [PubMed] [Google Scholar]
- 18. Fransen J, Pope J, Baron M, et al. Response to: ‘are autoantibodies to RNA-polymerase III to be incorporated in routine diagnostic laboratory algorithms for systemic autoimmune rheumatic diseases?’ by Jan Damoiseaux. Ann Rheum Dis 2014; 73: e30. [DOI] [PubMed] [Google Scholar]
- 19. Damoiseaux J. Are autoantibodies to RNA-polymerase III to be incorporated in routine diagnostic laboratory algorithms for systemic autoimmune rheumatic diseases? Ann Rheum Dis 2014; 73: e29. [DOI] [PubMed] [Google Scholar]
- 20. Avouac J, Fransen J, Walker UA, et al. Preliminary criteria for the very early diagnosis of systemic sclerosis: results of a Delphi consensus study from EULAR scleroderma trials and research group. Ann Rheum Dis 2011; 70(3): 476–481. [DOI] [PubMed] [Google Scholar]
- 21. Cavazzana I, Ceribelli A, Airò P, et al. Anti-RNA polymerase III antibodies. A marker of systemic sclerosis with rapid onset and skin thickening progression. Autoimmun Rev 2009; 8: 580–584. [DOI] [PubMed] [Google Scholar]
- 22. Wirz EG, Jaeger VK, Allanore Y, et al. Incidence and predictors of cutaneous manifestations during the early course of systemic sclerosis: a 10-year longitudinal study from the EUSTAR database. Ann Rheum Dis 2016; 75: 1285–1292. [DOI] [PubMed] [Google Scholar]
- 23. Herrick AL, Peytrignet S, Lunt M, et al. Patterns and predictors of skin score change in early diffuse systemic sclerosis from the European Scleroderma Observational Study. Ann Rheum Dis 2018; 77: 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Domsic RT, Medsger TA., Jr. Autoantibodies and their role in scleroderma clinical care. Curr Treatm Opt Rheumatol 2016; 2(3): 239–251. [Google Scholar]
- 25. Nguyen B, Assassi S, Arnett FC, et al. Association of RNA polymerase III antibodies with scleroderma renal crisis. J Rheumatol 2010; 37(5): 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamaguchi Y, Kodera M, Matsushita T, et al. Clinical and immunologic predictors of scleroderma renal crisis in Japanese systemic sclerosis patients with anti-RNA polymerase III autoantibodies. Arthritis Rheumatol 2015; 67: 1045–1052. [DOI] [PubMed] [Google Scholar]
- 27. Codullo V, Cavazzana I, Bonino C, et al. Serologic profile and mortality rates of scleroderma renal crisis in Italy. J Rheumatol 2009; 36: 1464–1469. [DOI] [PubMed] [Google Scholar]
- 28. Ceribelli A, Cavazzana I, Airò P, et al. Anti-RNA polymerase III antibodies as a risk marker for early gastric antral vascular ectasia (GAVE) in systemic sclerosis. J Rheumatol 2010; 37: 1544. [DOI] [PubMed] [Google Scholar]
- 29. Ghrénassia E, Avouac J, Khanna D, et al. Prevalence, correlates and outcomes of gastric antral vascular ectasia in systemic sclerosis: a EUSTAR case-control study. J Rheumatol 2014; 41: 99–105. [DOI] [PubMed] [Google Scholar]
- 30. Patterson KA, Roberts-Thomson PJ, Lester S, et al. Interpretation of an extended autoantibody profile in a well characterized Australian systemic sclerosis (scleroderma) cohort using principal components analysis. Arthritis Rheumatol 2015; 67: 3234–3244. [DOI] [PubMed] [Google Scholar]
- 31. Shah AA, Rosen A, Hummers L, et al. Close temporal relationship between onset of cancer and scleroderma in patients with RNA polymerase I/III antibodies. Arthritis Rheum 2010; 62: 2787–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Airò P, Ceribelli A, Cavazzana I, et al. Malignancies in Italian patients with systemic sclerosis positive for anti-RNA polymerase III antibodies. J Rheumatol 2011; 38: 1329–1334. [DOI] [PubMed] [Google Scholar]
- 33. Nikpour M, Hissaria P, Byron J, et al. Prevalence, correlates and clinical usefulness of antibodies to RNA polymerase III in systemic sclerosis: a cross-sectional analysis of data from an Australian cohort. Arthritis Res Ther 2011; 13: R211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moinzadeh P, Fonseca C, Hellmich M, et al. Association of anti-RNA polymerase III autoantibodies and cancer in scleroderma. Arthritis Res Ther 2014; 16: R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saigusa R, Asano Y, Nakamura K, et al. Association of anti-RNA polymerase III antibody and malignancy in Japanese patients with systemic sclerosis. J Dermatol 2015; 42: 524–527. [DOI] [PubMed] [Google Scholar]
- 36. Shah AA, Hummers LK, Casciola-Rosen L, et al. Examination of autoantibody status and clinical features associated with cancer risk and cancer-associated scleroderma. Arthritis Rheumatol 2015; 67: 1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shah AA, Casciola-Rosen L. Mechanistic and clinical insights at the scleroderma-cancer interface. J Scleroderma Relat Disord 2017; 2: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shah AA, Rosen A. Cancer and systemic sclerosis: novel insights into pathogenesis and clinical implications. Curr Opin Rheumatol 2011; 23(6): 530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shah AA, Casciola-Rosen L, Rosen A. Cancer-induced autoimmunity in the rheumatic diseases. Arthritis Rheumatol 2015; 67: 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Joseph CG, Darrah E, Shah AA, et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science 2014; 343: 152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shah AA, Rosen A, Hummers LK, et al. Evaluation of cancer-associated myositis and scleroderma autoantibodies in breast cancer patients without rheumatic disease. Clin Exp Rheumatol 2017; 35(Suppl. 106): S71–S74. [PMC free article] [PubMed] [Google Scholar]
- 42. Hoffmann-Vold AM, Midtvedt O, Tennoe AH, et al. Cardiopulmonary disease development in anti-RNA polymerase III positive systemic sclerosis; comparative analyses from an unselected, prospective patient cohort. J Rheumatol 2017; 44: 459–465. [DOI] [PubMed] [Google Scholar]
- 43. Motegi S, Toki S, Yamada K, et al. Demographic and clinical features of systemic sclerosis patients with anti-RNA polymerase III antibodies. J Dermatol 2015; 42: 189–192. [DOI] [PubMed] [Google Scholar]
- 44. Nihtyanova SI, Denton CP. Scleroderma lung involvement, autoantibodies, and outcome prediction: the confounding effect of time. J Rheumatol 2017; 44: 404–406. [DOI] [PubMed] [Google Scholar]
- 45. Nihtyanova SI, Schreiber BE, Ong VH, et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol 2014; 66: 1625–1635. [DOI] [PubMed] [Google Scholar]
- 46. Benyamine A, Bertin D, Heim X, et al. Sh I antibodies? Eur J Intern Med 2017; 44: e42–e44. [DOI] [PubMed] [Google Scholar]
- 47. Nihtyanova SI, Parker JC, Carol M, et al. A longitudinal study of anti-RNA polymerase III antibody levels in systemic sclerosis. Rheumatology 2009; 48: 1218–1221. [DOI] [PubMed] [Google Scholar]
- 48. Lafyatis R, Kissin E, York M, et al. B cell depletion with rituximab in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum 2009; 60(2): 578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bonroy C, Smith V, Deschepper E, et al. Specific antinuclear antibody level changes after B cell depletion therapy. J Rheumatol 2016; 3(1): 247–249. [DOI] [PubMed] [Google Scholar]
- 50. Dall’Ara F, Lazzaroni MG, Antonioli CM, et al. Systemic sclerosis with anti-RNA polymerase III positivity following silicone breast implant rupture: possible role of B-cell depletion and implant removal in the treatment. Rheumatol Int 2017; 37(5): 847–851. [DOI] [PubMed] [Google Scholar]