Abstract
Systemic sclerosis is a connective tissue disorder characterized by microvascular damage and excessive fibrosis of the skin and internal organs. One hallmark of the immunological abnormalities in systemic sclerosis is the presence of anti-nuclear antibodies, which are detected in more than 90% of patients with systemic sclerosis. Anti-centromere antibodies, anti-DNA topoisomerase I antibodies, and anti-RNA polymerase III antibodies are the predominant anti-nuclear antibodies found in systemic sclerosis patients. Other systemic sclerosis–related anti-nuclear antibodies include those targeted against U3 ribonucleoprotein, Th/To, U11/U12 ribonucleoprotein, and eukaryotic initiation factor 2B. Anti-U1 ribonucleoprotein, anti-Ku antibodies, anti-PM–Scl, and anti-RuvBL1/2 antibodies are associated with systemic sclerosis overlap syndrome. Anti-human upstream binding factor, anti-Ro52/TRIM21, anti-B23, and anti-centriole antibodies do not have specificity to systemic sclerosis, but are sometimes detected in sera from patients with systemic sclerosis. Identification of each systemic sclerosis–related antibody is useful to diagnose and predict organ involvement, since the particular type of systemic sclerosis–related antibodies is often predictive of clinical features, severity, and prognosis. The clinical phenotypes are largely influenced by ethnicity. Currently, an immunoprecipitation assay is necessary to detect most systemic sclerosis–related antibodies; therefore, the establishment of an easy, reliable, and simple screening system is warranted.
Keywords: Systemic sclerosis, anti-nuclear antibodies, autoantibodies, clinical features
Introduction
Systemic sclerosis (SSc) is characterized by microvascular damage and excessive fibrosis of the skin and various internal organs. 1 The clinical phenotype of SSc varies among patients. For instance, there are two distinct subtypes of skin involvement, depending upon the extent of the area involved. 2 Limited cutaneous systemic sclerosis (lcSSc) includes those patients in whom skin thickening is restricted to the face and/or distal to the elbow and/or knee, and skin thickening of the upper extremity is limited to the fingers in many cases. 3 In contrast, patients with diffuse-type SSc (diffuse cutaneous systemic sclerosis (dcSSc)) have extensive skin lesions over the elbow and/or knee, and skin lesions on the trunk are often observed. With the exception of pulmonary arterial hypertension (PAH), lcSSc is associated with relatively mild internal organ involvement, whereas dcSSc often leads to more serious complications that include interstitial lung disease (ILD) and scleroderma renal crisis (SRC).
SSc is considered to have an autoimmune etiology due to the following reasons: anti-nuclear antibodies (ANAs) are detected in more than 90% of patients, several potentially pathogenic autoantibodies (autoAbs) that target autoantigens of various components are reported, and there are cases for which immunosuppressive therapies such as cyclophosphamide and autologous stem cell transplantation are effective. ANAs react against a variety of intracellular components. 4 SSc-related ANAs are classified into two subgroups: SSc-specific autoAbs and SSc-associated autoAbs. SSc-specific autoAbs are specifically detected in SSc patients and rarely found in other connective tissue diseases or healthy subjects. This subgroup includes anti-centromere antibodies (ACAs), anti-DNA topoisomerase I (anti-topo I) antibodies (Abs; formerly anti-Scl-70 Abs), anti-RNA polymerase (RNAP) III Abs, anti-U3 ribonucleoprotein (RNP) Abs, anti-Th/To Abs, anti-U11/U12 RNP Abs, anti-eukaryotic initiation factor 2B (eIF2B) Abs, anti-U1 RNP Abs, anti-PM–Scl Abs, anti-Ku Abs, and anti-RuvBL1/2 Abs. Among these autoAbs, anti-U1 RNP Abs, anti-PM–Scl Abs, anti-Ku Abs, and anti-RuvBL1/2 Abs are found in a clinically distinct group of patients with SSc–myositis overlap syndrome. SSc-associated autoAbs are not specific to SSc and occasionally coexist with other SSc-specific and/or other connective tissue disease–related autoAbs. This group includes Abs against human upstream binding factor (hUBF), B23, Ro52/tripartite motif (TRIM) 21, and the centriole proteins. ANAs in patients with SSc exhibit several interesting features. First, the production of ANAs is unique for each patient, and the coexistence of two or more ANAs rarely occurs. 5 Second, once a specific ANA develops, the type and titers of ANA do not change throughout the course of disease. In addition, other SSc-related ANAs do not arise. 6
Both the mechanism by which ANAs are produced and the role of ANAs in the pathogenesis of SSc remain unknown. However, the identification of SSc-specific and SSc-associated autoAbs in each patient is clinically useful to diagnose and evaluate organ involvement, since the particular type of ANA is often indicative of clinical features, severity, and prognosis.
Ethnic differences need to be taken into account when considering the association of ANAs with clinical features, since mounting evidence has revealed that the patient’s genetic background can affect the prevalence and clinical phenotype of SSc. For example, anti-PM–Scl Abs are extremely rare in Japanese SSc patients,7,8 and the association of anti-PM–Scl Abs with SSc is weak in Japanese patients compared to other countries. 9
In this review article, we will review the novel findings of ANAs in patients with SSc, as well as the association of SSc-related ANAs with clinical characteristics.
Detection of antibody
When SSc-related ANAs are suspected, the first step is to confirm the existence of ANA. Indirect immunofluorescence (IIF) staining using HEp-2 cells is recommended for screening for ANA, 10 since the staining pattern and titer help to estimate SSc-related ANA specificities (Figure 1). ACAs and anti-centriole Abs can be identified by IIF, because both Abs exhibit a characteristic staining pattern (Table 1). Anti-U3 RNP Abs, anti-Th/To Abs, anti-PM–Scl Abs, and anti-hUBF Abs produce a nucleolar pattern that is commonly associated with SSc (Table 1). A cytoplasmic pattern is often indicative of myositis-specific autoAbs. While this pattern has been ignored in SSc, it is now necessary to pay attention to this pattern, since anti-eIF2B Abs produce a cytoplasmic staining pattern. 11 Except for ACA and anti-centriole Abs, additional techniques are required to confirm ANA specificities in patients’ sera. Enzyme-linked immunosorbent assay (ELISA) is widely used in routine clinical practice. Although ELISA is excellent in convenience and cost, it is a disadvantage that only limited SSc-related ANAs can be measured and false positives occur frequently. Currently, ELISA systems for ACAs, anti-topo I Abs, anti-RNAP III Abs, and anti-U1 RNP Abs are commercially available. Immunodiffusion assay is another reliable test. Immunoprecipitation (IP) assay is a gold standard for identifying SSc-related ANAs, but only limited facilities adopt this assay due to the complicated procedure. Recently, a line blot assay was used to identify SSc-related ANAs in several studies. Although a line blot assay is easy to apply, physicians should be cautious about interpreting the results, since clinical usefulness of a line blot assay has not been fully established. It is necessary to use validated kits if the results obtained from ELISA and/or a line blot assay are inconsistent with clinical presentation, and re-evaluation using additional detection methods, such as IP assay, is highly recommended. 12
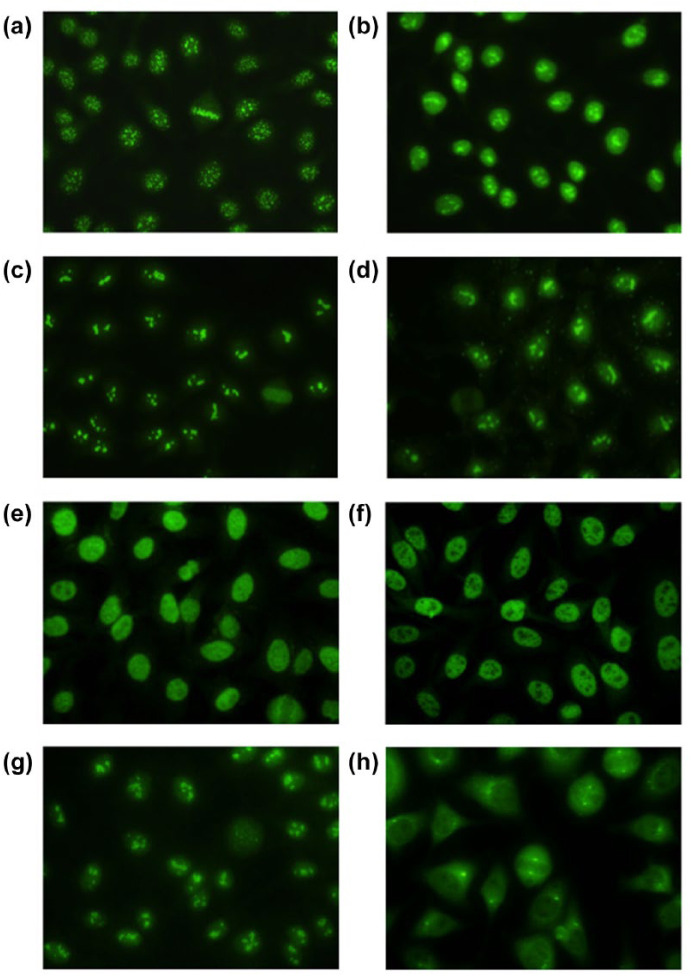
Figure 1.
Indirect immunofluorescence patterns observed on HEp-2 cells stained with (a) anti-centromere antibody (Ab), (b) anti-topoisomerase I Ab, (c) anti-U3 RNP Ab, (d) anti-Th/To Ab, (e) anti-Ku Ab, (f) anti-RuvBL1/2 Ab, (g) anti-hUBF Ab, and (h) anti-centriole Ab sera (original magnification 400×).
Table 1.
Systemic sclerosis–specific autoantibodies.
| Autoantibody | Major autoantigen | IIF |
|---|---|---|
| Anti-centromere | CENP-A, -B, and -C | DC |
| Anti-topoisomerase I | DNA topoisomerase I | Ho + N or H + Sp |
| Anti-RNA polymerase | RNA polymerase I, II, and III | Sp and/or N |
| Anti-U3 RNP | Fibrillarin and other U3 RNP components | N |
| Anti-Th/To | H1/8-2 and Th/7-2 RNA | N |
| Anti-U11/U12 RNP | U11/U12 RNP complex | Sp |
| Anti-eIF2B | Eukaryotic initiation factor 2B | Cyto |
| Anti-U1 RNP | 70 kDa, A and C polypeptides of U1 snRNP | Sp |
| Anti-PM–Scl | PM–Scl-75 and 100 proteins of the human exosome | N |
| Anti-Ku | 80- and 70-kDa DNA binding dimeric protein | Sp |
| Anti-RuvBL1/2 | RuvBL1 and RuvBL2 complex | Sp |
IIF: indirect immunofluorescence staining pattern on HEp-2 cells; snRNP: small nuclear ribonucleoprotein; DC: discrete-speckled; Ho: Homogeneous; N: Nucleolar; Sp: speckled; Cyto: cytoplasmic.
Clinical usefulness of autoAbs in diagnosing early SSc
SSc remains an intractable disease for which no curative therapy has been developed. 13 Once the condition is fully established, SSc is often difficult to treat. Therefore, it is desirable to diagnose and intervene in the SSc patients at an early stage. The Joint Committee of the American College of Rheumatology (ACR) and the European League against Rheumatism (EULAR) developed classification criteria for SSc to overcome the disadvantages of the 1980 ACR preliminary classification criteria.14,15 The ACR/EULAR classification criteria include the presence of ACA, anti-topo I Abs, and anti-RNAP III Abs as one of the items. The presence of SSc-specific autoAbs is a predictive marker in identifying patients in a pre-SSc state who do not yet meet classification criteria for SSc, since SSc-specific autoAbs and nailfold capillary abnormalities are recognized as independent predictors for the future development of SSc in patients with Raynaud’s phenomenon, but without any features of connective tissue diseases. 16 Valentini et al. 17 demonstrated faster progression of SSc in SSc-specific autoAb-positive patients, particularly in those with preclinical internal organ involvement at baseline, compared to SSc-specific autoAb-negative patients.
SSc-specific autoAbs
ACAs
ACAs were first reported by Moroi et al. 18 in 1980. ACAs can be identified by IIF, as they produce a characteristic staining pattern of punctate spots dispersed in the interphase nucleus, localized to the constriction on metaphase chromosomes (Figure 1(a)). This staining pattern is also termed discrete-speckled (DC; Table 1). There are at least six centromeric polypeptides, CENP-A–F, of which CENP-B is the major autoantigen that reacts with virtually all ACA-positive sera19,20 (Table 1). The CENP-B antigen is used in commercially available ELISA systems with adequate sensitivity and specificity.21,22 ACAs are occasionally detected in patients with primary biliary cirrhosis 23 or systemic lupus erythematosus (SLE). 24 ACAs are sometimes found even in healthy individuals without any connective tissue disease–related symptoms. 25 When ACAs are found in patients with Raynaud’s phenomenon without skin thickening, it is predictive of the future development of lcSSc.26,27
The overall frequency of ACAs in SSc patients is 20%–30%. Approximately 30% of Caucasian SSc patients are positive for ACAs, whereas the frequency is lower in African American and Thai patients.28,29
ACAs are associated with limited skin involvement, peripheral vasculopathy, calcinosis, and PAH 30 (Table 2). The frequency and degree of peripheral ischemia vary among different ethnicities. Pitting scars or ulcers occurred in 42%–61% of Caucasian and/or African American patients31–33 and, in contrast, only 11%–17% in Japanese patients.7,8 Digital gangrene is also observed more frequently in Caucasian and/or African American patients. Severe internal organ involvement, such as ILD or SRC, seldom occurs. However, the presence of ACAs can be predictive of the development of PAH at a late stage10,34 and the DETECT algorithm to identify SSc patients at high risk of developing PAH includes ACAs as one of the indices. 35 Generally, ACA-positive SSc patients have a more favorable prognosis than patients with other SSc-related autoAbs, 34 unless ACA-positive patients are suffering from PAH.
Table 2.
Clinical characteristics associated with systemic sclerosis–specific autoantibodies.
| Autoantibody | Subtype | Clinical manifestations |
Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Digital ulcer/gangrene | ILD | PAH | Heart | Renal | Muscle | Joint | |||
| Anti-centromere | lcSSc | 11–61 | 6–20 | 0–19 | 2–16 | 0–1 | 0–3 | 20–60 | Kuwana et al., 7 Steen, 31 and Mitri et al. 32 |
| Anti-topoisomerase I | dcSSc | 41–63 | 67–89 | 0–14 | 9–20 | 4–10 | 6–13 | 36–86 | Kuwana et al., 7 Steen, 31 Satoh et al., 36 and Cavazzana et al. 37 |
| Anti-RNA polymerase | dcSSc | 7–51 | 7–66 | 4–10 | 7–50 | 14–43 | 0–4 | 14–88 | Kuwana et al., 7 Steen, 31 Nikpour et al., 38 and Meyer et al. 39 |
| Anti-U3 RNP | dc=lc | 40–58 | 12–36 | 0–31 | 10–23 | 6–12 | 10–33 | 10–89 | Kuwana et al., 7 Steen, 31 Tormey et al., 40 and Aggarwal et al. 41 |
| Anti-Th/To | lcSSc | 24–29 | 16–48 | 28–32 | 7–21 | 4–5 | 6 | 60 | Kuwana et al., 7 Steen, 31 and Mitri et al. 32 |
| Anti-U11/U12 RNP | dc=lc | NR | 79 | 0 | 24 | 6 | 6 | 79 | Fertig et al. 42 |
| Anti-eIF2B | dcSSc | 50 | 86 | 0 | 67 | NR | 29 | NR | Betteridge et al. 11 |
| Anti-U1 RNP | lcSSc | 30-49 | 22–49 | 14–18 | 3–11 | 0–7 | 20–27 | 52–94 | Kuwana et al. 7 and Steen 31 |
| Anti-PM–Scl | lcSSc | 47 | 17–50 | 3–8 | 6–11 | 4–8 | 35–58 | 58–75 | Steen, 31 D’Aoust et al., 43 and Kaji et al. 44 |
| Anti-Ku | lcSSc | 0–29 | 43–57 | 14–46 | 0–21 | 0–2 | 43–100 | 43 | Kuwana et al., 7 Kaji et al., 44 and Rozman et al. 45 |
| Anti-RuvBL1/2 | dcSSc | 86 | 56 | 12 | 30 | 3 | 57 | NR | Kaji et al. 44 |
ILD: interstitial lung disease; PAH: pulmonary arterial hypertension; RNP: ribonucleoprotein; lcSSc: limited cutaneous systemic sclerosis; dcSSc: diffuse cutaneous systemic sclerosis; NR: not reported.
Values are given as percentages.
Anti-topo I Abs
The autoAbs against a 70- to 100-kDa chromatin-associated protein in patients with SSc were first identified as anti-Scl-70 Abs. 46 This protein was later identified as DNA topoisomerase I 47 (Table 1). The IIF staining pattern of anti-topo I Abs is a combination of a homogeneous with a nucleolar or a homogeneous with a speckled pattern (Figure 1(b)). Anti-topo I Abs were found in about 40% of patients with SSc, although this varies widely among different ethnicities, ranging from 28% to 70%.7,48 Anti-topo I Abs are highly specific to SSc and are rarely found in healthy individuals or in patients with other connective tissue diseases. 48 Coexistence with ACAs occurs in about 0.5% of SSc patients only. 49 Anti-topo I Abs are detected in about 40% of patients with dcSSc, and another study demonstrated that one third of anti-topo I Ab-positive patients had lcSSc. 50
It is well recognized that anti-topo I Abs are strongly associated with a higher risk for severe ILD, resulting in increased mortality (Table 2). 51 ILD occurs in >70% of patients with anti-topo I Abs during the disease course, and about 25% develop severe disease that requires oxygen supplementation. Wells et al. 52 have reported that the percent predicted diffusing capacity of the lungs for carbon monoxide reflected the extent of ILD and, therefore, routine measurement was recommended. SRC, cardiomyopathy, and severe peripheral vasculopathy including digital ulcers/gangrene are also important organ involvement outcomes in patients with anti-topo I Abs.7,31 Anti-topo I Abs are considered to be a marker for poor prognosis, since a uniformly effective therapy for the treatment of ILD has not yet been established.
Serial measurements of antibody titers are not generally considered to be useful for monitoring disease severity in SSc patients. However, several studies have found that anti-topo I Ab levels, as determined by ELISA, were related to disease severity and that conversion to seronegativity resulted in disease remission.53–55
Anti-RNAP III Abs
Major targets of anti-RNAP Abs are RNAP I, II, and III6,56,57 (Table 1). There are four patterns of combination observed for reactivity to anti-RNAP Abs: RNAP I/III, RNAP I/II/III, RNAP III, and RNAP II. 58 Abs to RNAP I and III routinely coexist and this pattern is highly specific for SSc. 58 Anti-RNAP I/III Ab-positive patients sometimes have anti-RNAP II Abs (anti-RNAP I/II/III Abs). Anti-RNAP III Abs alone may be present in only a few cases. Anti-RNAP II Abs alone are detected at a low frequency in SSc patients in combination with anti-topo I Abs, as well as in sera from patients with other connective tissue diseases, including SLE or an overlap syndrome.59,60
The prevalence of anti-RNAP III Abs is influenced by patient ethnicity. In Caucasians in North America and the United Kingdom, 20%–25% of SSc patients are positive for anti-RNAP III Abs, whereas 15.3% (69 out of 451) of Australian Caucasian SSc patients have anti-RNAP III Abs.38,61 On the other hand, only 6%–9% were positive in a French cohort.61,62 An Italian cohort study also reported a low frequency of anti-RNAP III Abs: only 16 of 466 (3%) have anti-RNAP III Abs. 63 In a Japanese population, anti-RNAP III Abs are found in 6%–10%7,8,36 of SSc patients. An ELISA system using recombinant RNAP III as the antigen is currently available and widely used in clinical settings.36,64
Most SSc patients with anti-RNAP III Abs present a diffuse cutaneous form with rapidly progressive skin thickening (Table 2). Nevertheless, many patients experience rapid regression of skin thickening over time, even without treatment. 12 Anti-RNAP III Abs are also strongly associated with SRC independent of ethnicity.31,48,56,65 SRC occurred in 25% of patients with anti-RNAP III Abs in contrast to 12% in other patients. 31 A recent study clarified clinical and immunological predictors of SRC and found that anti-RNAP I/II/III Ab positivity and a higher anti-RNAP III Ab titer, as measured by ELISA, were the independent factors associated with the development of SRC. 65 It has been reported that two anti-RNAP III Ab-positive patients without skin thickness developed SRC. Skin sclerosis was absent at SRC onset, but the two patients eventually developed diffuse and rapidly progressive skin thickening. 66 Conversely, ILD that requires aggressive therapy and severe peripheral ulcers/gangrene rarely occurred in this subgroup. The prognosis of patients with anti-RNAP III Abs was the worst in all SSc patients until angiotensin-converting enzyme (ACE) inhibitors were developed. However, mortality rates in patients with anti-RNAP III Abs have dramatically improved; currently, prognoses for patients with anti-RNAP III Abs are better than those with anti-topo I or anti-U3 RNP Abs. 31 This is due to the fact that patients with anti-RNAP III Abs have a low risk of suffering ILD, and SRC is now more readily treated with ACE inhibitors. 67 Regular, daily monitoring of blood pressure can help with the early diagnosis of SRC, and the prompt introduction of an ACE inhibitor can result in reduced mortality. Another clinical characteristic of patients with anti-RNAP III Abs is gastric antral vascular ectasia (GAVE), also known as watermelon stomach. A case–control study identified anti-RNAP III Abs as a risk factor for GAVE. 68
A recent topic of interest is the association of anti-RNAP III Abs with malignancy. 69 Shah et al. 70 reported a close temporal relationship between the onset of cancer and anti-RNAP I/III positivity in SSc patients. The median duration of SSc at the time of cancer diagnosis is significantly different among SSc-related Ab-based subgroups: –1.2 years for anti-RNAP I/III Abs, +13.4 years for anti-topo I Abs, +11.1 years for ACAs, and +2.3 years for the SSc-specific Ab-negative group. This group also reported that anti-RNAP III positivity and older age at scleroderma onset were significantly associated with a short cancer–scleroderma interval. 71 Joseph et al. 72 reported that novel antigens were encoded by somatically mutated genes in SSc patients with cancer. Genetic alterations of the POLR3A locus that encodes RPC1 were found in anti-RNAP III Ab-positive SSc patients with Abs to RPC1 but not in those without Abs to RPC1, and POLR3A mutations triggered cellular immunity and cross-reactive humoral immune responses in anti-RNAP III Ab-positive patients with cancers.
Anti-U3 RNP Abs
Anti-U3 RNP Abs were first found in sera from SSc patients in 1985. 73 The major autoantigen of anti-U3 RNP Abs is identified as fibrillarin, which is a 34-kDa protein and a component of the nucleolar U3 RNP complex (Table 1). Anti-U3 RNP Abs produce a nucleolar, clumpy IIF staining pattern (Figure 1(c)). 40
The frequency of anti-U3 RNP Abs is around 4%–10% of SSc patients.7,8,31,40,41 Anti-U3 RNP Abs are generally specific to SSc, but have also been described in patients with SLE.10,74 Two thirds of patients with anti-U3 RNP Abs have dcSSc, but one third have the limited cutaneous form. In African American SSc patients, approximately 30% are positive for anti-U3 RNP Abs. 31 Severe internal organ involvement, such as ILD, PAH, cardiomyopathy, and SRC are common in anti-U3 RNP Ab-positive patients, irrespective of dcSSc or lcSSc (Table 2). Anti-U3 RNP Abs were reported to be an independent risk factor for the development of PAH, 75 and PAH is the most common cause of death, leading to an increased mortality in this subgroup. 41 Nishimagi et al. 76 reported that 5 out of 14 patients who experienced severe gastrointestinal tract involvement, including malabsorption syndrome and/or pseudo-obstruction within 2 years of onset of SSc, had anti-U3 RNP Abs. Prognosis in patients with anti-U3 RNP Abs is poor and comparable to that in SSc patients with anti-topo I Abs.
Anti-Th/To Abs (known as anti-7-2RNA Abs)
Okano and Medsger 77 first reported Abs to Th/To (anti-Th/To Abs) in 1990. Anti-Th/To Abs produce a nucleolar, dotty IIF staining pattern (Figure 1(d)). Anti-Th/To autoantigens are RNPs associated with H1/8-2 and Th/7-2 RNAs (Table 1). H1/8-2 is a component of RNase P and TH/7-2 is a component of RNase mitochondrial RNA processing (MRP), and both are RNA processing enzymes78–80 (Table 1). There are at least six subunits consisting of these complexes and a 120-kDa protein contains the major epitope. 81 Anti-Th/To Abs were originally specific for SSc or Raynaud’s disease, but were subsequently detected in patients with localized scleroderma. 82
Anti-Th/To Abs are found in 2%–5% of SSc patients. Anti-Th/To Abs are associated with lcSSc, but their overall prognosis is worse, since anti-Th/To Ab-positive patients have a higher risk for ILD and PAH83,84 (Table 2). Mitri et al. 32 compared the clinical features between ACA and anti-Th/To Abs and found that patients with anti-Th/To Abs were younger and had a shorter disease duration at their first evaluation than those with ACAs. Both subgroups had a higher frequency of PAH (28% of anti-Th/To Abs and 19% of ACAs), but anti-Th/To Ab-positive patients had worse prognoses because anti-Th/To Ab-positive patients were more often suffering from ILD. However, in Japanese SSc patients with anti-Th/To Abs, internal organ involvement is not as severe as in Caucasian patients.7,8
Anti-U11/U12 RNP Abs
Fertig et al. 42 reported 33 patients with anti-U11/U12 RNP Abs who were identified by RNA-IP assay. Anti-U11/U12 RNP Abs produce a speckled nuclear IIF staining (Table 1). Anti-U11/U12 RNP Abs were found in 1%–3% of patients with SSc. The ratio of dcSSc and lcSSc in this cohort was almost 1:1. All 33 patients with anti-U11/U12 RNP Abs had Raynaud’s phenomenon and 82% had gastrointestinal tract involvement. Although none of the 33 patients with this antibody had PAH, nearly 80% of patients with anti-U11/U12 RNP Abs had ILD, which is often severe and rapidly progressive, resulting in an increased mortality (Table 2).
Anti-eIF2B Abs
Anti-eIF2B Abs were recently identified by protein-IP assay. 11 The autoantigen targeted by anti-eIF2B Abs has a molecular weight of 30 kDa. Sera from patients with anti-eIF2B Abs produce a cytoplasmic IIF staining pattern (Table 1). Out of 548 SSc patients, 7 (1.3%) were positive for anti-eIF2B Abs, and six out of seven had dcSSc. ILD was confirmed in six out of seven patients with anti-eIF2B Abs (Table 2). Four out of seven anti-eIF2B Ab-positive patients had overlap features of either myositis or rheumatoid arthritis (RA) (two with myositis and two with RA). SSc specificity and clinical characteristics of anti-eIF2B Abs need to be confirmed in a larger cohort study among different ethnicities.
Anti-U1 RNP Abs
Anti-U1 RNP Abs are directed against the 70-kDa A and C proteins associated with U1 RNA (Table 1). Anti-U1 RNP Abs yield a pure speckled pattern with a high antibody titer. Anti-U1 RNP Abs are the hallmark of mixed connective tissue disease (MCTD), and can occur in SLE in combination with anti-dsDNA Abs or anti-Sm Abs,85,86 but are also detected in patients with SSc comorbidity (range 2%–14%).7,31,48,87 Clinical features of anti-U1 RNP Abs include lcSSc, puffy fingers, Raynaud’s phenomenon, arthritis, and esophageal dysfunction. PAH can occur and cause increased mortality (Table 2). 31 Anti-U1 RNP Abs are generally predictive of a better prognosis, but PAH is the most common cause of death.31,88
Anti-PM–Scl Abs
Anti-PM–Scl Abs were first identified in 1977 in patients with overlap syndrome of polymyositis (PM) and SSc. 89 The PM–Scl antigen consists of 11–16 polypeptides forming antigens 75–100 kDa in size 90 (Table 1). Anti-PM–Scl Abs have a homogeneous nucleolar staining pattern. Anti-PM–Scl Abs are found in 4%–11% of SSc patients overall, but the prevalence of anti-PM–Scl Abs is significantly associated with certain ethnicities, since anti-PM–Scl Abs are strongly associated with HLA DQA1*0501 and HLA DRB1*0301. 91 In fact, anti-PM–Scl Abs are rarely found in non-Caucasian patients. 92 In two Japanese cohort studies, none of the SSc patients were positive for anti-PM–Scl Abs.7,8 Anti-PM–Scl Abs are detected in approximately 25% of SSc–myositis overlap patients, but in only 2% of SSc patients alone.48,83,93 In a Japanese study, nine anti-PM–Scl Ab-positive patients were identified by IP assay, including 4 out of 16 (25%) with undifferentiated connective tissue disease, 3 out of 126 (2.4%) with dermatomyositis, 1 out of 223 (0.4%) with SSc, and 1 out of 88 (1.1%) with Sjögren’s syndrome. 9 Anti-PM–Scl Ab-positive patients often present with subacute myositis, limited cutaneous form SSc, and less serious internal organ involvement 43 (Table 2). Previous reports revealed a good response to low or moderate dosage of corticosteroids, and there is a favorable prognosis in patients with anti-PM–Scl Abs. 94 The prevalence and SSc specificity may be different between Japanese and Caucasian patients. Alternatively, the clinical phenotype of anti-PM–Scl is probably different from “classical” SSc and has the descriptor of sclerodermatomyositis.
Anti-Ku Abs
Mimori et al. 95 initially reported a case of PM/SSc overlap syndrome with anti-Ku Abs in 1994. The Ku autoantigen is now recognized as a heterodimer of 70-kD and 80-kD subunits (Table 1). Anti-Ku Abs present a speckled nuclear staining pattern, but can be distinguished from that produced by anti-U1 RNP Abs, since the nuclei are stained in a reticular pattern that spares the nucleoli (Figure 1(e)). 95 Anti-Ku Abs were originally considered to be specific to SSc, but subsequent studies reported that anti-Ku Abs are also detected in patients with other autoimmune connective tissue diseases, including SLE and overlap syndrome.96,97 Franceschini et al. 97 reported 14 anti-Ku Ab-positive patients; one-half had an overlap syndrome (five with PM/SSc, one with PM/SLE/SSc, and one with PM/SLE). Skin sclerosis is mild in this subgroup, and internal organ involvement is less frequent and mild if present (Table 2). In addition, vascular complications, including digital ulcers or telangiectasia, are not common. 45 Myositis is usually mild with a good response to corticosteroids, leading to a favorable prognosis.
Anti-RuvBL1/2 Abs
Our group reported the clinical characteristics of 37 patients with newly identified anti-RuvBL1/2 Abs in 2014. 44 RuvBL1 and RuvBL2 are highly conserved eukaryotic proteins that form a double hexamer in the nucleoplasm (Table 1). Anti-RuvBL1/2 Abs have a speckled nuclear IIF staining pattern with a high antibody titer (Figure 1(f)). Anti-RuvBL1/2 Abs were detected in 10 out of 588 (1.7%) SSc patients in a Japanese cohort and 27 out of 585 (4.6%) SSc patients in a Pittsburg, PA cohort. Anti-RuvBL1/2 Abs were highly specific to SSc and strongly associated with SSc in overlap with myositis in both the Japanese and Pittsburgh cohorts. The diffuse type is dominant in patients with anti-RuvB1/2 Abs. Compared with other SSc–myositis overlap–related autoAbs (anti-PM–Scl Abs and anti-Ku Abs), anti-RuvBL1/2 Abs were distinctive in terms of its associations with older age at SSc onset, male gender, and a high frequency of the diffuse cutaneous form (Table 2).
SSc-associated autoAbs
Anti-hUBF Abs (formerly anti-NOR90 Abs)
AutoAbs reactive with nucleolus-organizing region (NOR) 90 were first reported in 1987. 98 Later, the autoantigen specificity of this autoAb was identified as hUBF. 99 IIF staining shows that staining is limited to nucleoli and has a coarse speckled pattern (Figure 1(g)). 98 Anti-hUBF Abs are detected not only in SSc, but also in other autoimmune connective tissue diseases such as Raynaud’s disease, Sjögren’s syndrome, RA, and SLE. Anti-hUBF Abs are also found in some malignancies.100–102 Although reported cases with anti-hUBF Abs are limited, previous studies demonstrated that anti-hUBF Abs are probably related to lcSSc, mild organ involvement, and a favorable prognosis. 101 More cases are needed to clarify the clinical characteristics of anti-hUBF Abs.
Anti-Ro52/TRIM21 Abs
Anti-SSA/Ro Abs can occur in SSc patients with concomitant Sjögren’s syndrome. Ro antigens consist of the proteins Ro52 and Ro60.103,104 Ro52 is also termed tripartite motif family of protein 21 (TRIM21). AutoAbs against Ro52/TRIM21 (anti-Ro/TRIM21 Abs) are detected in patients with various connective tissue diseases, especially in SLE and Sjögren’s syndrome. 105 A multicenter cohort study revealed that anti-Ro52/TRIM21 Abs were detected in 20% of the patients and associated with ILD and overlap syndrome. 106 A larger cohort study involving 1574 SSc patients confirmed that 324 (20.6%) patients had anti-Ro52/TRIM21 Abs, an association with ILD and poor survival. 107
Anti-B23 Abs
B23 is one of the most abundant proteins in the nucleolus. It is involved in pre-ribosomal RNA processing and ribosome assembly.108,109 Anti-B23 Abs have a nucleolar IIF pattern. Anti-B23 Abs are detected in <11% of SSc patients and are associated with moderate to severe PAH and anti-U3 RNP Abs. 110
Anti-centriole Abs
Moroi et al. 111 reported two cases of anti-centriole Abs, one with SSc and the other suffering from Raynaud’s phenomenon. Anti-centriole Abs can also be detected by IIF due to a characteristic staining pattern in which each of two dots per cell is located at each side of the visibly grouped chromosomes in mitotic HEp-2 cells (Figure 1(h)). A recent study reported five patients with anti-centriole Abs, 112 all of which were female and had digital ulcers/gangrene. Four of the five (80%) patients had PAH and none of them had active ILD or developed SRC. Anti-centriole Abs may be a marker for a subgroup of severe vasculopathy, such as digital ulcers/gangrene and PAH. More studies are needed to confirm whether anti-centriole Abs are specific to SSc and to report on the clinical characteristics in patients with anti-centriole Abs.
ANA-negative SSc
As described above, ANAs are positive in more than 90% of SSc patients. However, there is a small proportion of SSc patients who are negative for ANAs, and these patients appear to form one unique subgroup. Salazar et al. 113 reported that 208 of 3249 (6.4%) SSc patients were ANA negative in the Scleroderma Family Registry and DNA repository. ANA-negative SSc patients are more likely to be male, less frequently have vasculopathy, such as digital ulcers, telangiectasia, and PAH, and more frequently present with lower gastrointestinal involvement. 113 Meanwhile, the possibility that the ANA-negative patients have other autoAbs that have not been currently identified cannot be ruled out.
Potentially pathogenic autoAbs
Apart from autoAbs that have corresponding antigens in the nucleus or cytoplasm, it has been reported that several autoAbs may have a potentially pathogenic role in tissue fibrosis and vascular damage in SSc.114–116 These autoAbs include autoAbs against the endothelial cell antigens, matrix metalloproteinases (MMPs), and the platelet-derived growth factor receptor (PDGFR).
Anti-endothelial cell Abs
AutoAbs directed to the endothelial cell antigens were initially reported in sera from patients with primary Raynaud’s phenomenon and SSc. 117 Anti-endothelial cell antibodies (AECAs) were detected in 25%–85% of patients with SSc, but are also seen in other connective tissue diseases.117–119 The presence of anti-endothelial cell Abs was associated with severe vascular complications including severe Raynaud’s phenomenon, digital ulcers, and PAH. ILD also frequently occurs in patients with SSc with AECAs. AECA-induced apoptosis of endothelial cells, via activation of the caspase 3 pathway and expression of fibrillin-1, was linked to subsequent autoAb production to fibrillin-1. 120 In another study, purified IgG from AECA-positive SSc and SLE patients with PAH induced significantly higher expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin and production of interleukin (IL)-6, IL-8, and chemokine (C–C motif) ligand 2 (CCL2) on human umbilical vein endothelial cells compared to IgG from AECA-negative patients. 121 Therefore, AECAs may alter the endothelial cell function in SSc to increase adhesion molecules for leukocytes and pro-inflammatory cytokines and chemokines, leading to vascular damage.
Anti-MMP Abs
AutoAbs to MMP-1 122 and MMP-3 123 were reported in the sera of patients with SSc. Anti-MMP-1 and anti-MMP-3 Ab levels were significantly higher in dcSSc than those in lcSSc, and also correlated with fibrosis of the skin, lung, and renal blood vessels. Moreover, IgG anti-MMP-1 and anti-MMP-3 Abs in sera from patients with SSc inhibited MMP collagenase activity. Therefore, anti-MMP-1 and anti-MMP-3 Abs are serological markers that reflect the severity of SSc and contribute to the development of fibrosis by inhibiting collagenase activity and reducing extracellular matrix turnover.
Anti-PDGFR Abs
Baroni et al. 124 reported anti-PDGFR Abs in patients with SSc, but not in healthy controls or patients with SLE, RA, idiopathic pulmonary fibrosis, or primary Raynaud’s phenomenon. These autoAbs may have a pathogenic role, since PDGFR expression is increased by pathologic transforming growth factor (TGF)-β signaling, and binding of PDGFR to anti-PDGFR Abs results in the amplification of the Ras–ERK1/2–ROS cascade, leading to enhanced collagen production.124–126 Further analysis revealed that anti-PDGFRα Abs recognize specific conformational epitopes, leading to a blockade of PDGFRα signaling in patients with SSc, 127 and anti-PDGFR Abs from patients with SSc induced fibrosis in skin-humanized mice. 128 These data provide important information that is critical to elucidating the pathophysiology of SSc. However, neither an agonistic role for anti-PDGFR Abs nor specificity of these autoAbs for SSc was found in other reports.125,129 Therefore, the role of anti-PDGFR Abs in SSc remains to be confirmed.
Conclusion
Since clinical features and prognoses in SSc patients largely vary, it is clinically significant for classifying SSc patients into subgroups based on their autoAb status. Identification of autoAbs in SSc patients is also useful for early diagnosis. Novel findings for each SSc-specific autoAbs are being reported; however, these findings are virtually limited to the SSc-specific autoAbs for which an ELISA procedure has been developed. Most SSc-specific and SSc-associated autoAbs still require IP assays for identification. An easy, reliable, and simple screening system for ANA specificities is needed.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Varga J, Trojanowska M, Kuwana M. Pathogenesis of systemic sclerosis: recent insights of molecular and cellular mechanisms and therapeutic opportunities. J Scleroderma Relat Disord 2017; 2: 137–152. [Google Scholar]
- 2. LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988; 15: 202–205. [PubMed] [Google Scholar]
- 3. Allanore Y. Limited cutaneous systemic sclerosis: the unfairly neglected subset. J Scleroderma Relat Disord 2016; 1: 241–246. [Google Scholar]
- 4. Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol 1989; 44: 93–151. [DOI] [PubMed] [Google Scholar]
- 5. Fanning GC, Welsh KI, Bunn C, et al. HLA associations in three mutually exclusive autoantibody subgroups in UK systemic sclerosis patients. Br J Rheumatol 1998; 37: 201–207. [DOI] [PubMed] [Google Scholar]
- 6. Okano Y. Antinuclear antibody in systemic sclerosis (scleroderma). Rheum Dis Clin North Am 1996; 22: 709–735. [DOI] [PubMed] [Google Scholar]
- 7. Kuwana M, Kaburaki J, Okano Y, et al. Clinical and prognostic associations based on serum antinuclear antibodies in Japanese patients with systemic sclerosis. Arthritis Rheum 1994; 37: 75–83. [DOI] [PubMed] [Google Scholar]
- 8. Hamaguchi Y, Hasegawa M, Fujimoto M, et al. The clinical relevance of serum antinuclear antibodies in Japanese patients with systemic sclerosis. Br J Dermatol 2008; 158: 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muro Y, Hosono Y, Sugiura K, et al. Anti-PM/Scl antibodies are found in Japanese patients with various systemic autoimmune conditions besides myositis and scleroderma. Arthritis Res Ther 2015; 17: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ho KT, Reveille JD. The clinical relevance of autoantibodies in scleroderma. Arthritis Res Ther 2003; 5: 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Betteridge ZE, Woodhead F, Lu H, et al. Brief report: anti-eukaryotic initiation factor 2B autoantibodies are associated with interstitial lung disease in patients with systemic sclerosis. Arthritis Rheum 2016; 68: 2778–2783. [DOI] [PubMed] [Google Scholar]
- 12. Kuwana M. Circulating anti-nuclear antibodies in systemic sclerosis: utility in diagnosis and disease subsetting. J Nippon Med Sch 2017; 84: 56–63. [DOI] [PubMed] [Google Scholar]
- 13. Khanna D, Distler JH, Sandner P, et al. Emerging strategies for treatment of systemic sclerosis. J Scleroderma Relat Disord 2016; 1: 186–193. [Google Scholar]
- 14. Van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann Rheum Dis 2013; 72: 1747–1755. [DOI] [PubMed] [Google Scholar]
- 15. Van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013; 65: 2737–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koenig M, Joyal F, Fritzler MJ, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: a twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum 2008; 58: 3902–3912. [DOI] [PubMed] [Google Scholar]
- 17. Valentini G, Marcoccia A, Cuomo G, et al. Early systemic sclerosis: analysis of the disease course in patients with marker autoantibody and/or capillaroscopic positivity. Arthritis Care Res 2014; 66: 1520–1527. [DOI] [PubMed] [Google Scholar]
- 18. Moroi Y, Peebles C, Fritzler MJ, et al. Autoantibody to centromere (kinetochore) in scleroderma sera. Proc Natl Acad Sci U S A 1980; 77(3): 1627–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Earnshaw WC, Machlin PS, Bordwell BJ, et al. Analysis of anticentromere autoantibodies using cloned autoantigen CENP-B. Proc Natl Acad Sci U S A 1987; 84: 4979–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masumoto H, Masukata H, Muro Y, et al. A human centromere antigen (CENP-B) interacts with a short specific sequence in alphoid DNA, a human centromeric satellite. J Cell Biol 1989; 109: 1963–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rothfield N, Whitaker D, Bordwell B, et al. Detection of anticentromere antibodies using cloned autoantigen CENP-B. Arthritis Rheum 1987; 30: 1416–1419. [DOI] [PubMed] [Google Scholar]
- 22. Tan EM, Smolen JS, McDougal JS, et al. A critical evaluation of enzyme immunoassays for detection of antinuclear autoantibodies of defined specificities. I. Precision, sensitivity, and specificity. Arthritis Rheum 1999; 42: 455–464. [DOI] [PubMed] [Google Scholar]
- 23. Parveen S, Morshed SA, Nishioka M. High prevalence of antibodies to recombinant CENP-B in primary biliary cirrhosis: nuclear immunofluorescence patterns and ELISA reactivities. J Gastroenterol Hepatol 1995; 10: 438–445. [DOI] [PubMed] [Google Scholar]
- 24. Caramaschi P, Biasi D, Manzo T, et al. Anticentromere antibody—clinical associations. A study of 44 patients. Rheumatol Int 1995; 14: 253–255. [DOI] [PubMed] [Google Scholar]
- 25. Gelber AC, Pillemer SR, Baum BJ, et al. Distinct recognition of antibodies to centromere proteins in primary Sjogren’s syndrome compared with limited scleroderma. Ann Rheum Dis 2006; 65(8): 1028–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kallenberg CG, Wouda AA, Hoet MH, et al. Development of connective tissue disease in patients presenting with Raynaud’s phenomenon: a six year follow up with emphasis on the predictive value of antinuclear antibodies as detected by immunoblotting. Ann Rheum Dis 1988; 47: 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takehara K, Soma Y, Igarashi A, et al. Longitudinal study of patients with anticentromere antibody. Dermatologica 1990; 181: 202–206. [DOI] [PubMed] [Google Scholar]
- 28. McNeilage LJ, Youngchaiyud U, Whittingham S. Racial differences in antinuclear antibody patterns and clinical manifestations of scleroderma. Arthritis Rheum 1989; 32: 54–60. [DOI] [PubMed] [Google Scholar]
- 29. Reveille JD, Fischbach M, McNearney T, et al. Systemic sclerosis in 3 US ethnic groups: a comparison of clinical, sociodemographic, serologic, and immunogenetic determinants. Semin Arthritis Rheum 2001; 30: 332–346. [DOI] [PubMed] [Google Scholar]
- 30. Tan EM, Rodnan GP, Garcia I, et al. Diversity of antinuclear antibodies in progressive systemic sclerosis. Anti-centromere antibody and its relationship to CREST syndrome. Arthritis Rheum 1980; 23: 617–625. [DOI] [PubMed] [Google Scholar]
- 31. Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum 2005; 35: 35–42. [DOI] [PubMed] [Google Scholar]
- 32. Mitri GM, Lucas M, Fertig N, et al. A comparison between anti-Th/To- and anticentromere antibody-positive systemic sclerosis patients with limited cutaneous involvement. Arthritis Rheum 2003; 48: 203–209. [DOI] [PubMed] [Google Scholar]
- 33. Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002; 81(2): 139–153. [DOI] [PubMed] [Google Scholar]
- 34. Hesselstrand R, Scheja A, Shen GQ, et al. The association of antinuclear antibodies with organ involvement and survival in systemic sclerosis. Rheumatology (Oxford) 2003; 42(4): 534–540. [DOI] [PubMed] [Google Scholar]
- 35. Coghlan JG, Denton CP, Grunig E, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73: 1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Satoh T, Ishikawa O, Ihn H, et al. Clinical usefulness of anti-RNA polymerase III antibody measurement by enzyme-linked immunosorbent assay. Rheumatology (Oxford) 2009; 48: 1570–1574. [DOI] [PubMed] [Google Scholar]
- 37. Cavazzana I, Ceribelli A, Airo P, et al. Anti-RNA polymerase III antibodies: a marker of systemic sclerosis with rapid onset and skin thickening progression. Autoimmun Rev 2009; 8: 580–584. [DOI] [PubMed] [Google Scholar]
- 38. Nikpour M, Hissaria P, Byron J, et al. Prevalence, correlates and clinical usefulness of antibodies to RNA polymerase III in systemic sclerosis: a cross-sectional analysis of data from an Australian cohort. Arthritis Res Ther 2011; 13: R211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meyer O, De Chaisemartin L, Nicaise-Roland P, et al. Anti-RNA polymerase III antibody prevalence and associated clinical manifestations in a large series of French patients with systemic sclerosis: a cross-sectional study. J Rheumatol 2010; 37: 125–130. [DOI] [PubMed] [Google Scholar]
- 40. Tormey VJ, Bunn CC, Denton CP, et al. Anti-fibrillarin antibodies in systemic sclerosis. Rheumatology (Oxford) 2001; 40: 1157–1162. [DOI] [PubMed] [Google Scholar]
- 41. Aggarwal R, Lucas M, Fertig N, et al. Anti-U3 RNP autoantibodies in systemic sclerosis. Arthritis Rheum 2009; 60(4): 1112–1118. [DOI] [PubMed] [Google Scholar]
- 42. Fertig N, Domsic RT, Rodriguez-Reyna T, et al. Anti-U11/U12 RNP antibodies in systemic sclerosis: a new serologic marker associated with pulmonary fibrosis. Arthritis Rheum 2009; 61: 958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. D’Aoust J, Hudson M, Tatibouet S, et al. Clinical and serologic correlates of anti-PM/Scl antibodies in systemic sclerosis: a multicenter study of 763 patients. Arthritis Rheum 2014; 66: 1608–1615. [DOI] [PubMed] [Google Scholar]
- 44. Kaji K, Fertig N, Medsger TA, et al. Autoantibodies to RuvBL1 and RuvBL2: a novel systemic sclerosis-related antibody associated with diffuse cutaneous and skeletal muscle involvement. Arthritis Care Res 2014; 66: 575–584. [DOI] [PubMed] [Google Scholar]
- 45. Rozman B, Cucnik S, Sodin-Semrl S, et al. Prevalence and clinical associations of anti-Ku antibodies in patients with systemic sclerosis: a European EUSTAR-initiated multi-centre case-control study. Ann Rheum Dis 2008; 67: 1282–1286. [DOI] [PubMed] [Google Scholar]
- 46. Douvas AS, Achten M, Tan EM. Identification of a nuclear protein (Scl-70) as a unique target of human antinuclear antibodies in scleroderma. J Biol Chem 1979; 254: 10514–10522. [PubMed] [Google Scholar]
- 47. Shero JH, Bordwell B, Rothfield NF, et al. High titers of autoantibodies to topoisomerase I (Scl-70) in sera from scleroderma patients. Science 1986; 231: 737–740. [DOI] [PubMed] [Google Scholar]
- 48. Reveille JD, Solomon DH. Evidence-based guidelines for the use of immunologic tests: anticentromere, Scl-70, and nucleolar antibodies. Arthritis Rheum 2003; 49: 399–412. [DOI] [PubMed] [Google Scholar]
- 49. Dick T, Mierau R, Bartz-Bazzanella P, et al. Coexistence of antitopoisomerase I and anticentromere antibodies in patients with systemic sclerosis. Ann Rheum Dis 2002; 61: 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Walker UA, Tyndall A, Czirjak L, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials And Research group database. Ann Rheum Dis 2007; 66: 754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Volkmann ER, Chung A, Tashkin DP. Managing systemic sclerosis-related interstitial lung disease in the modern treatment era. J Scleroderma Relat Disord 2017; 2: 72–83. [Google Scholar]
- 52. Wells AU, Hansell DM, Rubens MB, et al. Fibrosing alveolitis in systemic sclerosis: indices of lung function in relation to extent of disease on computed tomography. Arthritis Rheum 1997; 40: 1229–1236. [DOI] [PubMed] [Google Scholar]
- 53. Henry PA, Atamas SP, Yurovsky VV, et al. Diversity and plasticity of the anti-DNA topoisomerase I autoantibody response in scleroderma. Arthritis Rheum 2000; 43: 2733–2742. [DOI] [PubMed] [Google Scholar]
- 54. Sato S, Hamaguchi Y, Hasegawa M, et al. Clinical significance of anti-topoisomerase I antibody levels determined by ELISA in systemic sclerosis. Rheumatology (Oxford) 2001; 40: 1135–1140. [DOI] [PubMed] [Google Scholar]
- 55. Hamaguchi Y, Fujimoto M, Hasegawa M, et al. Re-emergence of anti-topoisomerase I antibody with exacerbated development of skin sclerosis in a patient with systemic sclerosis. J Am Acad Dermatol 2010; 62: 142–144. [DOI] [PubMed] [Google Scholar]
- 56. Kuwana M, Kaburaki J, Mimori T, et al. Autoantibody reactive with three classes of RNA polymerases in sera from patients with systemic sclerosis. J Clin Invest 1993; 91: 1399–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Okano Y, Steen VD, Medsger TA, et al. Autoantibody reactive with RNA polymerase III in systemic sclerosis. Ann Intern Med 1993; 119: 1005–1013. [DOI] [PubMed] [Google Scholar]
- 58. Kuwana M, Okano Y, Kaburaki J, et al. Autoantibodies to RNA polymerases recognize multiple subunits and demonstrate cross-reactivity with RNA polymerase complexes. Arthritis Rheum 1999; 42: 275–284. [DOI] [PubMed] [Google Scholar]
- 59. Satoh M, Ajmani AK, Ogasawara T, et al. Autoantibodies to RNA polymerase II are common in systemic lupus erythematosus and overlap syndrome. Specific recognition of the phosphorylated (IIO) form by a subset of human sera. J Clin Invest 1994; 94: 1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Harvey GR, Rands AL, McHugh NJ. Anti-RNA polymerase antibodies in systemic sclerosis (SSc): association with anti-topoisomerase I antibodies and identification of autoreactive subunits of RNA polymerase II. Clin Exp Immunol 1996; 105: 468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Meyer OC, Fertig N, Lucas M, et al. Disease subsets, antinuclear antibody profile, and clinical features in 127 French and 247 US adult patients with systemic sclerosis. J Rheumatol 2007; 34: 104–109. [PubMed] [Google Scholar]
- 62. Sobanski V, Dauchet L, Lefevre G, et al. Prevalence of anti-RNA polymerase III antibodies in systemic sclerosis: new data from a French cohort and a systematic review and meta-analysis. Arthritis Rheum 2014; 66: 407–417. [DOI] [PubMed] [Google Scholar]
- 63. Airo P, Ceribelli A, Cavazzana I, et al. Malignancies in Italian patients with systemic sclerosis positive for anti-RNA polymerase III antibodies. J Rheumatol 2011; 38: 1329–1334. [DOI] [PubMed] [Google Scholar]
- 64. Kuwana M, Okano Y, Pandey JP, et al. Enzyme-linked immunosorbent assay for detection of anti-RNA polymerase III antibody: analytical accuracy and clinical associations in systemic sclerosis. Arthritis Rheum 2005; 52: 2425–2432. [DOI] [PubMed] [Google Scholar]
- 65. Hamaguchi Y, Kodera M, Matsushita T, et al. Clinical and immunologic predictors of scleroderma renal crisis in Japanese systemic sclerosis patients with anti-RNA polymerase III autoantibodies. Arthritis Rheum 2015; 67: 1045–1052. [DOI] [PubMed] [Google Scholar]
- 66. Bhavsar SV, Carmona R. Anti-RNA polymerase III antibodies in the diagnosis of scleroderma renal crisis in the absence of skin disease. J Clin Rheumatol 2014; 20: 379–382. [DOI] [PubMed] [Google Scholar]
- 67. Steen VD, Medsger TA., Jr. Long-term outcomes of scleroderma renal crisis. Ann Intern Med 2000; 133: 600–603. [DOI] [PubMed] [Google Scholar]
- 68. Ghrenassia E, Avouac J, Khanna D, et al. Prevalence, correlates and outcomes of gastric antral vascular ectasia in systemic sclerosis: a EUSTAR case-control study. J Rheumatol 2014; 41: 99–105. [DOI] [PubMed] [Google Scholar]
- 69. Shah AA, Casciola-Rosen L. Mechanistic and clinical insights at the scleroderma-cancer interface. J Scleroderma Relat Disord 2017; 2(3): 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shah AA, Rosen A, Hummers L, et al. Close temporal relationship between onset of cancer and scleroderma in patients with RNA polymerase I/III antibodies. Arthritis Rheum 2010; 62: 2787–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shah AA, Hummers LK, Casciola-Rosen L, et al. Examination of autoantibody status and clinical features associated with cancer risk and cancer-associated scleroderma. Arthritis Rheum 2015; 67: 1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Joseph CG, Darrah E, Shah AA, et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science 2014; 343: 152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lischwe MA, Ochs RL, Reddy R, et al. Purification and partial characterization of a nucleolar scleroderma antigen (Mr = 34,000; pI, 8.5) rich in NG, NG-dimethylarginine. J Biol Chem 1985; 260: 14304–14310. [PubMed] [Google Scholar]
- 74. Gunduz OH, Fertig N, Lucas M, et al. Systemic sclerosis with renal crisis and pulmonary hypertension: a report of eleven cases. Arthritis Rheum 2001; 44: 1663–1666. [DOI] [PubMed] [Google Scholar]
- 75. Steen VD, Medsger TA., Jr. Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum 2003; 48: 516–522. [DOI] [PubMed] [Google Scholar]
- 76. Nishimagi E, Tochimoto A, Kawaguchi Y, et al. Characteristics of patients with early systemic sclerosis and severe gastrointestinal tract involvement. J Rheumatol 2007; 34: 2050–2055. [PubMed] [Google Scholar]
- 77. Okano Y, Medsger TA., Jr. Autoantibody to Th ribonucleoprotein (nucleolar 7-2 RNA protein particle) in patients with systemic sclerosis. Arthritis Rheum 1990; 33: 1822–1828. [DOI] [PubMed] [Google Scholar]
- 78. Hashimoto C, Steitz JA. Sequential association of nucleolar 7-2 RNA with two different autoantigens. J Biol Chem 1983; 258: 1379–1382. [PubMed] [Google Scholar]
- 79. Reddy R, Tan EM, Henning D, et al. Detection of a nucleolar 7-2 ribonucleoprotein and a cytoplasmic 8-2 ribonucleoprotein with autoantibodies from patients with scleroderma. J Biol Chem 1983; 258: 1383–1386. [PubMed] [Google Scholar]
- 80. Van Eenennaam H, Vogelzangs JH, Lugtenberg D, et al. Identity of the RNase MRP- and RNase P-associated Th/To autoantigen. Arthritis Rheum 2002; 46: 3266–3272. [DOI] [PubMed] [Google Scholar]
- 81. Kuwana M, Kimura K, Hirakata M, et al. Differences in autoantibody response to Th/To between systemic sclerosis and other autoimmune diseases. Ann Rheum Dis 2002; 61: 842–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yamane K, Ihn H, Kubo M, et al. Antibodies to Th/To ribonucleoprotein in patients with localized scleroderma. Rheumatology (Oxford) 2001; 40: 683–686. [DOI] [PubMed] [Google Scholar]
- 83. Cepeda EJ, Reveille JD. Autoantibodies in systemic sclerosis and fibrosing syndromes: clinical indications and relevance. Curr Opin Rheumatol 2004; 16: 723–732. [DOI] [PubMed] [Google Scholar]
- 84. Van Eenennaam H, Vogelzangs JH, Bisschops L, et al. Autoantibodies against small nucleolar ribonucleoprotein complexes and their clinical associations. Clin Exp Immunol 2002; 130: 532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sharp GC, Irvin WS, Tan EM, et al. Mixed connective tissue disease—an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am J Med 1972; 52: 148–159. [DOI] [PubMed] [Google Scholar]
- 86. Burdt MA, Hoffman RW, Deutscher SL, et al. Long-term outcome in mixed connective tissue disease: longitudinal clinical and serologic findings. Arthritis Rheum 1999; 42: 899–909. [DOI] [PubMed] [Google Scholar]
- 87. Jacobsen S, Halberg P, Ullman S, et al. Clinical features and serum antinuclear antibodies in 230 Danish patients with systemic sclerosis. Br J Rheumatol 1998; 37: 39–45. [DOI] [PubMed] [Google Scholar]
- 88. Lundberg I, Hedfors E. Clinical course of patients with anti-RNP antibodies. A prospective study of 32 patients. J Rheumatol 1991; 18: 1511–1519. [PubMed] [Google Scholar]
- 89. Ricken D. Progressive scleroderma and dermatomyositis. Verh Dtsch Ges Inn Med 1977; 83: 751–756. [PubMed] [Google Scholar]
- 90. Brouwer R, Vree Egberts WT, Hengstman GJ, et al. Autoantibodies directed to novel components of the PM/Scl complex, the human exosome. Arthritis Res Ther 2002; 4: 134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Genth E, Mierau R, Genetzky P, et al. Immunogenetic associations of scleroderma-related antinuclear antibodies. Arthritis Rheum 1990; 33: 657–665. [DOI] [PubMed] [Google Scholar]
- 92. Reimer G, Scheer U, Peters JM, et al. Immunolocalization and partial characterization of a nucleolar autoantigen (PM-Scl) associated with polymyositis/scleroderma overlap syndromes. J Immunol 1986; 137: 3802–3808. [PubMed] [Google Scholar]
- 93. Reichlin M, Maddison PJ, Targoff I, et al. Antibodies to a nuclear/nucleolar antigen in patients with polymyositis overlap syndromes. J Clin Immunol 1984; 4: 40–44. [DOI] [PubMed] [Google Scholar]
- 94. Marguerie C, Bunn CC, Copier J, et al. The clinical and immunogenetic features of patients with autoantibodies to the nucleolar antigen PM-Scl. Medicine (Baltimore) 1992; 71: 327–336. [DOI] [PubMed] [Google Scholar]
- 95. Mimori T, Akizuki M, Yamagata H, et al. Characterization of a high molecular weight acidic nuclear protein recognized by autoantibodies in sera from patients with polymyositis-scleroderma overlap. J Clin Invest 1981; 68: 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Takeda Y, Dynan WS. Autoantibodies against DNA double-strand break repair proteins. Front Biosci 2001; 6: D1412–D1422. [DOI] [PubMed] [Google Scholar]
- 97. Franceschini F, Cavazzana I, Generali D, et al. Anti-Ku antibodies in connective tissue diseases: clinical and serological evaluation of 14 patients. J Rheumatol 2002; 29: 1393–1397. [PubMed] [Google Scholar]
- 98. Rodriguez-Sanchez JL, Gelpi C, Juarez C, et al. Anti-NOR 90. A new autoantibody in scleroderma that recognizes a 90-kDa component of the nucleolus-organizing region of chromatin. J Immunol 1987; 139: 2579–2584. [PubMed] [Google Scholar]
- 99. Chan EK, Imai H, Hamel JC, et al. Human autoantibody to RNA polymerase I transcription factor hUBF. Molecular identity of nucleolus organizer region autoantigen NOR-90 and ribosomal RNA transcription upstream binding factor. J Exp Med 1991; 174: 1239–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Imai H, Ochs RL, Kiyosawa K, et al. Nucleolar antigens and autoantibodies in hepatocellular carcinoma and other malignancies. Am J Pathol 1992; 140: 859–870. [PMC free article] [PubMed] [Google Scholar]
- 101. Dagher JH, Scheer U, Voit R, et al. Autoantibodies to NOR 90/hUBF: longterm clinical and serological followup in a patient with limited systemic sclerosis suggests an antigen driven immune response. J Rheumatol 2002; 29: 1543–1547. [PubMed] [Google Scholar]
- 102. Fujii T, Mimori T, Akizuki M. Detection of autoantibodies to nucleolar transcription factor NOR 90/hUBF in sera of patients with rheumatic diseases, by recombinant autoantigen-based assays. Arthritis Rheum 1996; 39: 1313–1318. [DOI] [PubMed] [Google Scholar]
- 103. Ben-Chetrit E, Chan EK, Sullivan KF, et al. A 52-kD protein is a novel component of the SS-A/Ro antigenic particle. J Exp Med 1988; 167: 1560–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ben-Chetrit E, Fox RI, Tan EM. Dissociation of immune responses to the SS-A (Ro) 52-kd and 60-kd polypeptides in systemic lupus erythematosus and Sjogren’s syndrome. Arthritis Rheum 1990; 33: 349–355. [DOI] [PubMed] [Google Scholar]
- 105. Yoshimi R, Ishigatsubo Y, Ozato K. Autoantigen TRIM21/Ro52 as a possible target for treatment of systemic lupus erythematosus. Int J Rheumatol 2012; 2012: 718237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hudson M, Pope J, Mahler M, et al. Clinical significance of antibodies to Ro52/TRIM21 in systemic sclerosis. Arthritis Res Ther 2012; 14: R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wodkowski M, Hudson M, Proudman S, et al. Monospecific anti-Ro52/TRIM21 antibodies in a tri-nation cohort of 1574 systemic sclerosis subjects: evidence of an association with interstitial lung disease and worse survival. Clin Exp Rheumatol 2015; 33(4 Suppl. 91): S131–S135. [PubMed] [Google Scholar]
- 108. Lischwe MA, Smetana K, Olson MO, et al. Proteins C23 and B23 are the major nucleolar silver staining proteins. Life Sci 1979; 25: 701–708. [DOI] [PubMed] [Google Scholar]
- 109. Savkur RS, Olson MO. Preferential cleavage in pre-ribosomal RNA byprotein B23 endoribonuclease. Nucleic Acids Res 1998; 26: 4508–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ulanet DB, Wigley FM, Gelber AC, et al. Autoantibodies against B23, a nucleolar phosphoprotein, occur in scleroderma and are associated with pulmonary hypertension. Arthritis Rheum 2003; 49: 85–92. [DOI] [PubMed] [Google Scholar]
- 111. Moroi Y, Murata I, Takeuchi A, et al. Human anticentriole autoantibody in patients with scleroderma and Raynaud’s phenomenon. Clin Immunol Immunopathol 1983; 29: 381–390. [DOI] [PubMed] [Google Scholar]
- 112. Hamaguchi Y, Matsushita T, Hasegawa M, et al. High incidence of pulmonary arterial hypertension in systemic sclerosis patients with anti-centriole autoantibodies. Mod Rheumatol 2015; 25: 798–801. [DOI] [PubMed] [Google Scholar]
- 113. Salazar GA, Assassi S, Wigley F, et al. Antinuclear antibody-negative systemic sclerosis. Semin Arthritis Rheum 2015; 44: 680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Abraham DJ, Krieg T, Distler J, et al. Overview of pathogenesis of systemic sclerosis. Rheumatology (Oxford) 2009; 48(Suppl. 3): iii3–iii7. [DOI] [PubMed] [Google Scholar]
- 115. Kayser C, Fritzler MJ. Autoantibodies in systemic sclerosis: unanswered questions. Front Immunol 2015; 6: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Berger M, Steen VD. Role of anti-receptor autoantibodies in pathophysiology of scleroderma. Autoimmun Rev 2017; 16: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 117. Salojin KV, Le Tonqueze M, Saraux A, et al. Antiendothelial cell antibodies: useful markers of systemic sclerosis. Am J Med 1997; 102: 178–185. [DOI] [PubMed] [Google Scholar]
- 118. Negi VS, Tripathy NK, Misra R, et al. Antiendothelial cell antibodies in scleroderma correlate with severe digital ischemia and pulmonary arterial hypertension. J Rheumatol 1998; 25: 462–466. [PubMed] [Google Scholar]
- 119. Wusirika R, Ferri C, Marin M, et al. The assessment of anti-endothelial cell antibodies in scleroderma-associated pulmonary fibrosis. A study of indirect immunofluorescent and western blot analysis in 49 patients with scleroderma. Am J Clin Pathol 2003; 120: 596–606. [DOI] [PubMed] [Google Scholar]
- 120. Ahmed SS, Tan FK, Arnett FC, et al. Induction of apoptosis and fibrillin 1 expression in human dermal endothelial cells by scleroderma sera containing anti-endothelial cell antibodies. Arthritis Rheum 2006; 54: 2250–2262. [DOI] [PubMed] [Google Scholar]
- 121. Arends SJ, Damoiseaux JG, Duijvestijn AM, et al. Functional implications of IgG anti-endothelial cell antibodies in pulmonary arterial hypertension. Autoimmunity 2013; 46: 463–470. [DOI] [PubMed] [Google Scholar]
- 122. Sato S, Hayakawa I, Hasegawa M, et al. Function blocking autoantibodies against matrix metalloptoteinase-1 in patients with systemic sclerosis. J Invest Dermatol 2003; 120: 542–547. [DOI] [PubMed] [Google Scholar]
- 123. Nishijima C, Hayakawa I, Matsushita T, et al. Autoantibody against matrix metalloproteinase-3 in patients with systemic sclerosis. Clin Exp Immunol 2004; 138: 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Baroni SS, Santillo M, Bevilacqua F, et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med 2006; 354: 2667–2676. [DOI] [PubMed] [Google Scholar]
- 125. Loizos N, Lariccia L, Weiner J, et al. Lack of detection of agonist activity by antibodies to platelet-derived growth factor receptor alpha in a subset of normal and systemic sclerosis patient sera. Arthritis Rheum 2009; 60: 1145–1151. [DOI] [PubMed] [Google Scholar]
- 126. Svegliati S, Cancello R, Sambo P, et al. Platelet-derived growth factor and reactive oxygen species (ROS) regulate Ras protein levels in primary human fibroblasts via ERK1/2. Amplification of ROS and Ras in systemic sclerosis fibroblasts. J Biol Chem 2005; 280: 36474–36482. [DOI] [PubMed] [Google Scholar]
- 127. Moroncini G, Grieco A, Nacci G, et al. Epitope specificity determines pathogenicity and detectability of anti-platelet-derived growth factor receptor α autoantibodies in systemic sclerosis. Arthritis Rheum 2015; 67: 1891–1903. [DOI] [PubMed] [Google Scholar]
- 128. Luchetti MM, Moroncini G, Jose Escamez M, et al. Induction of scleroderma fibrosis in skin-humanized mice by administration of anti-platelet-derived growth factor receptor agonistic autoantibodies. Arthritis Rheum 2016; 68: 2263–2273. [DOI] [PubMed] [Google Scholar]
- 129. Classen JF, Henrohn D, Rorsman F, et al. Lack of evidence of stimulatory autoantibodies to platelet-derived growth factor receptor in patients with systemic sclerosis. Arthritis Rheum 2009; 60: 1137–1144. [DOI] [PubMed] [Google Scholar]