Abstract
The pathology of skin involvement in systemic sclerosis (or scleroderma) was first described in detail in 1892. In this article, we trace the history of cutaneous scleroderma and the evolution of thinking of scholars who have addressed this topic. We focus on skin histopathologic abnormalities and both clinical and laboratory techniques proposed for quantifying skin thickening and mobility. We examine the development of the simple bedside physical examination method of Dr Gerald Rodnan, first published in the 1970s and subsequently modified by others in the early 1990s (modified Rodnan skin score). This method has been found to be the only completely validated technique for assessing skin thickness in systemic sclerosis. Now nearly 50 years later, the modified Rodnan skin thickness scoring system remains the gold standard for use in both systemic sclerosis clinical trials and observational studies.
Keywords: Scleroderma, systemic sclerosis, history, skin thickness score, modified Rodnan skin score
Systemic sclerosis (SSc), originally termed “progressive” systemic sclerosis, became recognized as a clinical entity in the mid-19th century. Some years later, the question was raised whether this was a skin disease that occasionally coincidentally affected internal organs, often fatally, or was a distinctive systemic disorder because of its unusual cutaneous involvement. In a 117-page treatise on scleroderma, Wolters wrote in 1892
According to all observations, scleroderma appears not to be a disease which endangers life directly and, therefore the prognosis in and of itself may be considered not unfavorable … Death usually results from the most varied diseases which are not directly related to the process, such as heart failure, pulmonary phthisis, emphysema, anemia, diarrhea, gastroenteritis, pleurisy, Bright’s disease. 1
Research on sclerodermatous skin thickening can be categorized roughly into two approaches: its histopathology and its mobility. The former has been advanced by improvements in histologic staining and by electron microscopy and the latter by the development of various methods of instrumentation. Their goals have been ascertainment of diagnosis and disease subsetting and, more recently, better assessment of the effects of medications.
In this review, we chronicle the history of published observations regarding the reasons for increased skin thickness and the variety of methods which have been proposed to measure skin thickness.
Skin histopathology
The first description of the pathologic features of scleroderma skin was published in 1861, prior to the invention of histologic stains, from an autopsy. The skin was noted to be “markedly thickened by proliferation of connective tissue.” 2
The first skin biopsy description was reported by Kohn (Kaposi) in 1869. He concluded that there was “thickening of the lymph in the interstices of the skin.” 3
The German dermatopathologist Unna in 1894 wrote that the cutaneous abnormality is “mainly hypertrophy of pre-existing collagen bundles which affects all parts of the dermis” and believed that the changes in systemic sclerosis could not be distinguished from those in localized scleroderma. 4
A 1957 report from the Mayo Clinic was based on experience with more than 200 cases of cutaneous involvement of scleroderma and various localized dermatoses. The authors emphasized the findings of edema, homogenization, fibrosis, and sclerosis of the collagen fibers, as well as negative tests for mucin. 5 The presence of excessive mucin deposition is characteristic of several disorders which mimic SSc, specifically scleredema and scleromyxedema.
Fleischmajer et al. 6 and Hayes and Rodnan 7 reported both light and electron microscopic (EM) changes in skin biopsies from SSc patients. The most consistent finding on EM was normal-appearing dermal connective tissue but replacement of fat by abnormal (hyalinized) connective tissue in subcutaneous areas.
Another contributor to skin thickening, particularly in the diffuse cutaneous variant, is infiltration of mononuclear cells, especially T lymphocytes. The numbers of T cells have been shown to be correlated with the degree of skin thickening in biopsied sites. 8
Skin mobility
In 1912 Schade, a German investigator, devised an instrument to study the effects of edema on superficial tissues “which registers the degree of indentation following the application of a weight on the skin surface and the skin changes taking place after the subsequent removal of the weight.” This instrument was re-discovered and re-described in 1949 by two American researchers, Kirk and Kvorning, 9 who applied it to evaluate age-related elastic properties of skin and subcutis. In 1951, Olmsted et al. 10 at the Cleveland Clinic coined the name “pinchmeter” for this device and found that results differed depending on the site where it was applied. None of these reports included cases of SSc.
Sodeman, a dermatologist, and Burch, a cardiologist, measured venous resistance to water infusion with a manometer and a needle inserted subcutaneously in affected areas. This work was reported in 1938. 11 Resistance in scleroderma skin varied widely but generally was greater than that in the skin of normal individuals.
There is a precursor of the scleroderma “skin score” in the 1961 publication by Bachman. 12 The ability to be pinched on five arm and three lower leg sites bilaterally (total 16) was measured in recumbency with a pen and tape measure repeatedly over 22 months on eight SSc patients. Bachman used the term “skin mobility score” for the computed distance in millimeters of the maximal compression and minimal stretch at each site. While inter-observer consistency among four examiners was rather poor, the repeatability over time by any one observer was acceptable.
In 1969 Black, 13 a British radiologist, sought to evaluate skin thickness of the radial aspect of the forearm. A standard pressure was applied with a wood block and an x-ray beam was directed parallel to the stretched area. Normal women younger than age 65 consistently had thinner skin than age-matched men. Findings in disease states were not reported. In a publication in the following year, Black et al. 14 described a method which most nearly resembles the technique published by Rodnan nearly a decade later (see below). Five-millimeter skin biopsies were taken from the extensor aspect of the forearm of 13 women who had both cutaneous and visceral evidence of SSc. The biopsies were chemically rather than mechanically “defatted” and their hydroxyproline content was measured. Then the collagen content of these biopsies was compared with that of 62 biopsies from normal subjects. The authors concluded that “Measurement of dermal thickness confirmed the impression that the skin is in fact thinner (in SSc) than normal and supports the view that binding of the skin to deeper structures explains many of the physical signs.” This paradoxical finding of thin rather than thick skin may have been because the SSc patients were biopsied in a late (atrophic) stage of their illness (data on disease duration not reported).
Other techniques for quantification of skin thickening
After the pinchmeter was devised in 1951, numerous instruments were used experimentally to assess various qualities of human skin. Most have in common that they test resistance to stretching of skin.
The first of several ultrasonic methods was reported by Cole et al. 15 in scleredema, focused on the backs of nine patients compared to 12 normal controls. Five years later, other investigators 16 used this same technique to compare the findings on an affected forearm of eight SSc patients using the radiographic method of Black described above. They concluded that the results are correlated and, if the artifact introduced in the radiographic method is eliminated, ultrasound is more reliable. Many ultrasound studies have subsequently been reported. Two systematic reviews have been completed during the past 5 years.17,18 The selected studies are partially overlapping. Inter and intra-rater reproducibility were excellent, but they were assessed in only a few reports. 18 Both reviews noted the highly heterogeneous methods and lack of construct and criterion validity evaluations. More data are needed regarding standardization of technique, definition of skin thickness, and responsiveness to change.
Kalis et al., 19 with several other French investigators, used a “twistometer” which measured the extensibility at the test site of both the dermis and epidermis with a twisting motion. They correlated the extensibility results with A-scan ultrasound and showed that there was increased thickness in the center of an affected cutaneous area compared to its periphery.
Brennan et al. 20 in 1992 described a “manikin method” based on visually “estimating the actual percentage of skin involvement at nine (cutaneous) sites” with a manikin as a guide for determining the extent of affected areas. Jimenez et al. 21 prospectively followed 18 diffuse scleroderma patients for a mean of 4.3 years using this technique. Not surprisingly, this method proved inferior in inter-observer agreement as well as in assessing clinical changes.
Falanga and Bucalo 22 in 1993 introduced the clinical application of the “durometer,” an industrial instrument for the convenient measurement of the hardness of non-metallic materials. It is a spring-loaded timing device which records resistance to compression of the relaxed skin by a standard weight against time in seconds. Kissin et al. 23 found that its results correlate well with the modified Rodnan skin score.
Bluestone et al. 24 tested a suction cup method, on the dorsum of the right hand only, in normal individuals and SSc patients in 1970. The indirect measure (proxy) of skin thickness was the amount of distortion produced in response to a series of pre-determined negative pressures applied to the skin surface. Changes over time with improvement of skin thickening were noted. In 2001, Balou et al. 25 described another suction system, but this complicated method has not been pursued since.
Background of the Rodnan method of skin thickness measurement
Dr Gerald Rodnan became interested in scleroderma as an Arthritis Institute Fellow in the Public Health Service. He was Chief of the Division of Rheumatology and Clinical Immunology at the University of Pittsburgh from 1955 until his premature death in 1983 at the age of 56 (Figure 1). In 1963, Rodnan 26 published the clinical and laboratory features of a series of 100 SSc patients.
Figure 1.

Gerald P. Rodnan, MD, 1980.
He spent hours with each new patient and recorded in exquisite detail the history, physical examination findings, and laboratory features pertinent to SSc. His notes were initially kept on 4 × 6 inch file cards, which he updated at the time of return visits.
In the early 1960s, Rodnan explored methods to quantify the extent and severity of skin thickening. He wanted to create a simple, bedside method which could be learned by both primary care physicians and rheumatologists. He divided the total skin surface into multiple areas and experimented with grading skin thickness in these sites from 0 (normal skin) to 4 (severe skin thickness). An example is shown in Figure 2, which illustrates his findings in a patient examined in 1970 who had skin thickening affecting the fingers (2+), forearms (1+), and face (2+) and joint contractures affecting the fingers and toes.
Figure 2.
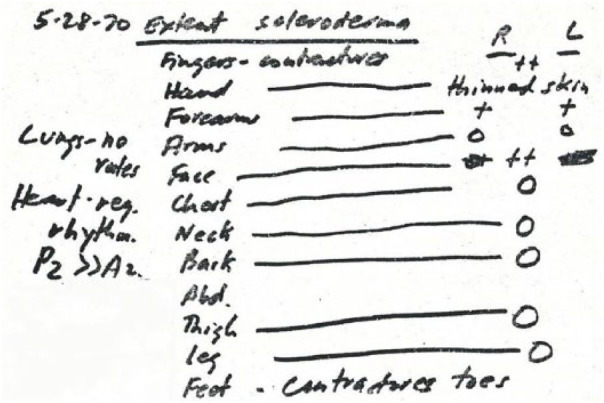
Dr. Rodnan patient record from 1970 illustrating a precursor of the Rodnan skin thickness scoring method first published in 1979.
Rodnan recognized that, to be valid, skin physical examination findings in SSc should be closely correlated with a more objective measure of skin thickness. For this reason, he prospectively performed 7-mm-diameter “core” skin punch biopsies from the dorsum of the forearm. He then shaved off the underlying subcutaneous fat using a dissecting microscope so that the core included only epidermis and dermis. The skin cores were dried to eliminate their water content (edema) and weighed. The result was a core which essentially represented the depth (thickness) of the forearm skin.
The key publication on these data was in 1979. 27 Rodnan and coworkers demonstrated a strong correlation between the physical examination forearm skin score and the weight of the simultaneous corresponding biopsy (Figure 3).
Figure 3.
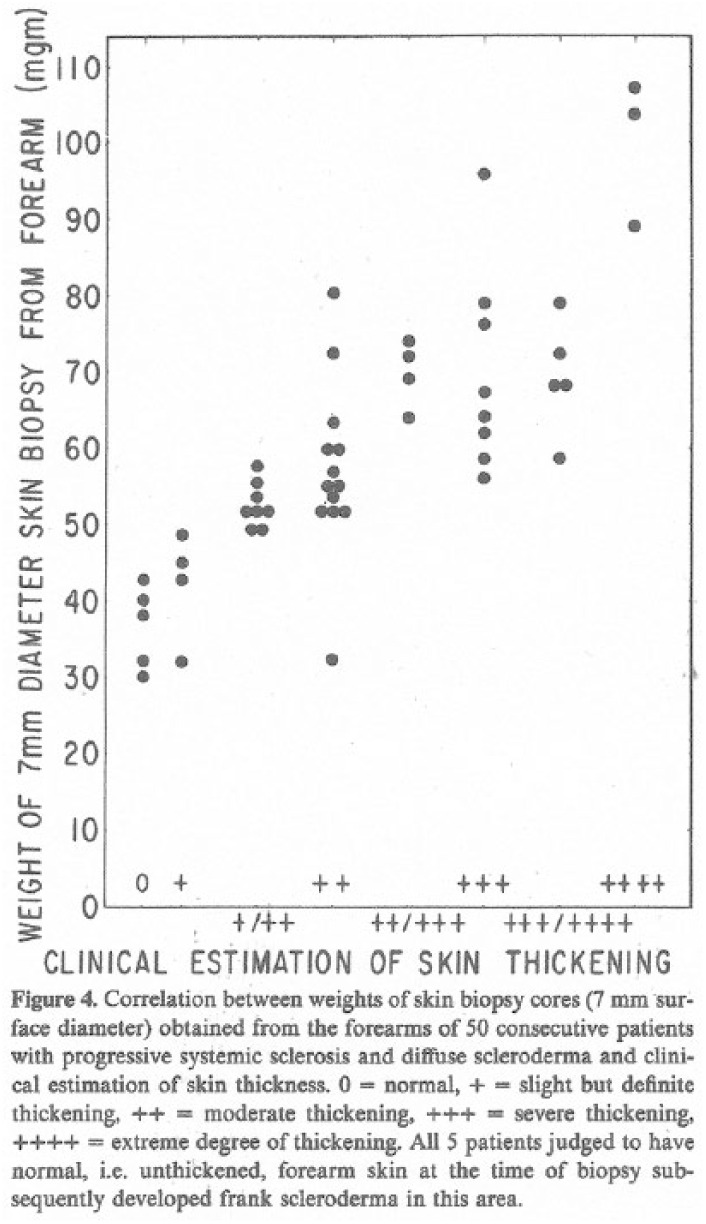
Correlation of forearm skin thickening and corresponding skin biopsy weight (from reference 27).
The first formal publication of the Rodnan skin scoring method was a podium presentation by the Pittsburgh group at the 1980 ARA annual meeting in Washington, DC. 28 The proposal was 0–4+ physical examination scoring of 26 cutaneous areas for a maximum total skin score (TSS) of 104. In this presentation, the authors also illustrated the natural history of skin thickness over time in patients with dcSSc and limited cutaneous (lc) SSc, which was then termed CREST syndrome. In essence, they showed that the TSS in lcSSc is low and remains so, while the TSS in dcSSc increases rapidly, reaching a peak in 1–2 years, and declines thereafter.
The initial report utilizing the Rodnan TSS in a clinical trial was a 1982 publication on d-penicillamine. 29 In this uncontrolled, non-randomized study, the Pittsburgh group compared the natural history of skin thickness in early dcSSc patients treated and not treated with d-penicillamine. The Pittsburgh Scleroderma Database contains first visit and longitudinal TSS data on over 1500 SSc patients personally examined by Rodnan.
In the 1980s, several alternative methods for measuring skin thickness in SSc were proposed. Kahaleh et al. 30 in 1986 published a 0–3+ point method based on palpation in 22 regions (maximum score 66). Agreement between two physician examiners who independently examined 20 patients was low in individual cutaneous sites (15%), regardless of the severity of skin thickening. In 1990, Clements et al. 31 described a different scoring system. On palpation, 10 surface areas were graded from unaffected (normal or 0) to severely “hidebound” (3+) for a maximum score of 30.
The modified Rodnan skin thickness score
There was general agreement in the scleroderma research community in the early 1990s that, of the several methods of assessing skin thickness, the Rodnan method was superior. However, there was concern that some of the proposed cutaneous sites were difficult to measure (toes, neck), thick in normal persons (back) or not easily comparable (breasts in women vs men).
The transition from the original Rodnan scoring system took place in two steps. The first was the change in individual cutaneous sites scored from 0–4+ to 0–3+, originally proposed by Clements et al. 31 The second was the reduction of the number of sites measured from 26 to 17, recommended by Brennan et al. 20 Thereafter, the 17-site, 0–3+ scoring system was termed the “modified Rodnan skin thickness (mRss)” method. In 1995, Pope et al. 32 compared the original Rodnan 26-site, 0–4+ method with the Clements et al.’s 10-site, 0–3+ method and concluded that fewer sites measured “may be more efficient than the traditional Rodnan score.”
In the early 1990s, Clements et al. performed two important studies concerning inter-observer variability and reproducibility of the mRss. In two clinics, each patient’s skin thickness was evaluated by six to seven examiners in a blinded fashion. 33 Good reproducibility between observers was found (standard deviation consistently about 5 units). In a separate publication, both inter-observer (accuracy) and intraobserver (reproducibility) variability were determined to be as reliable as several joint tenderness methods widely used for rheumatoid arthritis. 34
This and other methods of evaluating skin thickness in clinical trials were carefully examined in 2003 by the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) group, which concluded that the modified Rodnan scoring system is the only completely validated method. 35 Recording of the mRss currently used in numerous SSc patient care and research centers, and widely accepted in clinical trials, is illustrated in Figure 4.
Figure 4.
Standard recording of skin thickness in SSc using the modified Rodnan skin thickness scoring method.
Current status of the mRss
Clinical researchers, clinical trialists, and pharmaceutical companies and regulators, for example, the US Food and Drug Administration, have widely accepted the modified Rodnan score as the “gold standard” skin evaluation method in SSc. In 2012, a meta-analysis was performed including 629 SSc patients previously published in seven multicenter clinical trials in diffuse cutaneous SSc. 36 Six of the seven trials used the mRss as the primary outcome measure.
In consideration of its widespread use, a proposal has been recently made to standardize training in performance of the modified Rodnan technique, with both initial certification and re-certification after a period of time, which would apply to all examiners participating in clinical trials in SSc. 37 This and a previous publication contain detailed text descriptions of the examination method and both include illustrations of the various grades of skin thickening. 38
Skin thickening is not a perfect mirror of the natural history of SSc. In dcSSc, an accelerated skin thickness progression rate (STPR) in the first several years of disease 39 and a similar measure, the skin thickness “trajectory,” 40 both correlate with early mortality and morbidity (subsequent internal organ involvement). Although improvement in skin thickening in dcSSc typically ensues during 3–5 years of disease, organ system fibrosis and dysfunction may nevertheless progress. And in lcSSc, it is widely recognized that despite long-standing minimal skin thickening (fingers only), severe and fatal disease complications such as pulmonary arterial hypertension, and small intestinal involvement with malabsorption may develop.
Conclusion
Despite its limitations, the mRss has stood the test of time, becoming and remaining one of the most valuable outcome measures for both clinical trials and observational studies in systemic sclerosis. Its development and endurance are tributes to the wisdom, practicality, and persistence of Dr Gerald Rodnan, who pioneered this concept over 60 years ago.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Wolters M. Beitrag zur Kenntniss der Sclerodermie [Contribution to the understanding of scleroderma]. Arch Derm Syph 1892; 24: 659–738, 943–981. Quote 967. [Google Scholar]
- 2. Foerster A. Zur pathologischen Anatomie des Scleroma der Haut by Erwachsenen [On the pathologic anatomy of scleroma of the skin in adults]. Würzburger Med Ztschr 1861; 2: 294–300. [Google Scholar]
- 3. Kaposi M. Pathologie und Therapie der Hautkrankheiten [Pathology and therapy of skin diseases]. 4th ed. Vienna, Austria: Urban & Schwarzenberg, 1893, pp. 642–652. [Google Scholar]
- 4. Unna PG. Die Histopathologie der Hautkrankheiten [The histopathology of skin diseases]. Berlin: Hirschwald A., 1894, pp. 28–1132. [Google Scholar]
- 5. O’Leary PA, Montgomery H, Ragsdale WE. Dermatohistopathology of various types of scleroderma. Arch Derm 1957; 75: 78–87. [DOI] [PubMed] [Google Scholar]
- 6. Fleischmajer R, Damiano V, Nedwich A. Alteration of subcutaneous tissue in systemic scleroderma. Arch Dermatol 1972; 105: 59. [PubMed] [Google Scholar]
- 7. Hayes RL, Rodnan GP. The ultrastructure of skin in progressive systemic sclerosis (scleroderma). Am J Path 1971; 63: 433–440. [PMC free article] [PubMed] [Google Scholar]
- 8. Roumm AR, Whiteside TL, Medsger TA, et al. Lymphocytes in the skin of patients with progressive systemic sclerosis: quantification, subtyping, and clinical correlations. Arthritis Rheum 1984; 27: 645–653. [DOI] [PubMed] [Google Scholar]
- 9. Kirk E, Kvorning SA. Quantitative measurement of the elastic properties of the skin and subcutaneous tissue in young and old individuals. J Gerontology 1949; 4: 273–284. [DOI] [PubMed] [Google Scholar]
- 10. Olmsted F, Page IH, Corcoran AC. A device for objective clinical measurement of cutaneous elasticity: a “Pinchmeter.” Am J Med Sci 1951; 222: 73–75. [DOI] [PubMed] [Google Scholar]
- 11. Sodeman WA, Burch GE. Tissue pressure: an objective method of following skin changes in scleroderma. Am Heart J 1938; 17: 21–26. [Google Scholar]
- 12. Bachman DM. Quantitating skin mobility in scleroderma. Arch Dermatol 1961; 83: 598–605. [DOI] [PubMed] [Google Scholar]
- 13. Black MM. A modified radiographic method for measuring skin thickness. Brit J Derm 1969; 81: 661–667. [DOI] [PubMed] [Google Scholar]
- 14. Black MM, Bottoms M, Shuster S. Skin collagen content and thickness in systemic sclerosis. Brit J Derm 1970; 83: 552–555. [DOI] [PubMed] [Google Scholar]
- 15. Cole GW, Handler SJ, Burnett K. The ultrasound evaluation of skin thickness in scleroderma. J Clin Ultrasound 1981; 9: 501–503. [DOI] [PubMed] [Google Scholar]
- 16. Myers SL, Cohen JS, Sheets PW, et al. B-mode ultrasound evaluation of skin thickness in progressive systemic sclerosis. J Rheumatol 1986; 13: 577–580. [PubMed] [Google Scholar]
- 17. Ch’ng SS, Roddy J, Keen HI. A systematic review of ultrasonography as an outcome measure of skin involvement in systemic sclerosis. Internat J Rheum Dis 2013; 16: 264–272. [DOI] [PubMed] [Google Scholar]
- 18. Santiago T, Santiago M, Ruaro B, et al. Ultrasonography for the assessment of skin in systemic sclerosis: a systematic review. Arthritis Care Res. Epub ahead of print 21 May 2018. DOI: 10.1002/acr.23597 [DOI] [PubMed] [Google Scholar]
- 19. Kalis B, DeRigal J, Leonard F, et al. In vivo study of scleroderma by non-invasive techniques. Brit J Derm 1990; 122: 785–787. [DOI] [PubMed] [Google Scholar]
- 20. Brennan P, Silman A, Black C, et al. Reliability of skin involvement measures in scleroderma. Brit J Rheumatol 1992; 31: 467–470. [DOI] [PubMed] [Google Scholar]
- 21. Jimenez SA, Andrews RP, Myers AR. Chapter 67. Treatment of rapidly progressive systemic sclerosis with D-penicillamine: a prospective study. In: Black CM, Myers AR. (eds) Current topics in rheumatology: systemic sclerosis (scleroderma). New York: Gower Medical Publishing Limited, 1985, pp. 387–393. [Google Scholar]
- 22. Falanga V, Bucalo B. Use of a durometer to assess skin hardness. J Amer Acad Derm 1993; 29: 47–51. [DOI] [PubMed] [Google Scholar]
- 23. Kissin EY, Schiller AM, Gelbard EB, et al. Durometry for the assessment of skin disease in systemic sclerosis. Arthritis Rheum 2006; 55: 603–609. [DOI] [PubMed] [Google Scholar]
- 24. Bluestone R, Grahame R, Holloway V, et al. Treatment of systemic sclerosis with D-penicillamine. Ann Rheum Dis 1970; 29: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Balou SP, Mackiewicz A, Lyskiewicz A, et al. Direct quantitation of skin elasticity in systemic sclerosis. J Rheumatol 1990; 17: 790–797. [PubMed] [Google Scholar]
- 26. Rodnan GP. The natural history of progressive systemic sclerosis (diffuse scleroderma). Bull Rheum Dis 1963; 13: 305–308. [PubMed] [Google Scholar]
- 27. Rodnan GP, Lipinski E, Luksick J. Skin thickness and collagen content in progressive systemic sclerosis (scleroderma) and localized scleroderma. Arthritis Rheum 1979; 22: 130–140. [DOI] [PubMed] [Google Scholar]
- 28. Medsger TA, Steen VD, Ziegler G, et al. The natural history of skin involvement in progressive systemic sclerosis. Arthritis Rheum 1980; 23: 720–721. [Google Scholar]
- 29. Steen VD, Medsger TA, Rodnan GP. d-Penicillamine therapy in progressive systemic sclerosis (scleroderma). Ann Intern Med 1982; 97: 652–659. [DOI] [PubMed] [Google Scholar]
- 30. Kahaleh MB, Sultany GL, Smith JE. A modified scleroderma skin scoring method. Clin Exper Rheum 1986; 4: 367–369. [PubMed] [Google Scholar]
- 31. Clements PJ, Lachenbruch PA, Ng SW, et al. Skin score: a semiquantitative measure of cutaneous involvement that improves prediction of prognosis in systemic sclerosis. Arthritis Rheum 1990; 33: 1256–1263. [DOI] [PubMed] [Google Scholar]
- 32. Pope JE, Baron M, Bellamy N, et al. Variability of skin scores and clinical measurements in scleroderma. J Rheumatol 1995; 22: 1271–1276. [PubMed] [Google Scholar]
- 33. Clements PJ, Lachenbruch PA, Seibold JR, et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies. J Rheumatol 1993; 20: 1892–1896. [PubMed] [Google Scholar]
- 34. Clements PJ, Lachenbruch PA, Seibold JR, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol 1995; 22: 1281–1285. [PubMed] [Google Scholar]
- 35. Merkel PA, Clements PJ, Reveille JD, et al. Current status of outcome measure development in systemic sclerosis. Report from OMERACT 6. J Rheumatol 2003; 30: 1631–1664. [PubMed] [Google Scholar]
- 36. Merkel PA, Silliman NP, Clements PJ, et al. Patterns and predictors of change in outcome measures in clinical trials in scleroderma. Arthritis Rheum 2012; 64: 3420–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khanna D, Furst DE, Clements PJ, et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J Scleroderma Relat Disord 2017; 2: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clements PJ, Medsger TA, Feghali CA. Chapter 7. Cutaneous involvement in systemic sclerosis. In: Clements PJ, Furst DE. (eds) Systemic sclerosis. 2nd ed. Philadelphia, PA: Lippincott, Williams & Wilkins, 2004, pp. 129–150. [Google Scholar]
- 39. Domsic RT, Rodriguez-Reyna T, Lucas M, et al. Skin thickness progression rate: a predictor of mortality and early internal organ involvement in diffuse scleroderma. Ann Rheum Dis 2011; 70: 104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shand L, Lunt M, Nihtyanova S, et al. Relationship between change in skin score and disease outcome in diffuse systemic sclerosis: application of a latent linear trajectory model. Arthritis Rheum 2007; 56: 2422–2431. [DOI] [PubMed] [Google Scholar]



