Abstract
Secondary Raynaud’s phenomenon is the most common manifestation of systemic sclerosis, affecting more than 99% of systemic sclerosis patients, and a major cause of morbidity. Frequent and prolonged secondary Raynaud’s phenomenon attacks not only cause severe discomfort and pain but also ischemic acral tissue damage. In addition to vasoactive drugs, carbon dioxide (CO2) hand bath and hot water bath are potential non-pharmacological treatment options which can be self-administered by affected patients at any time. In order to compare the efficacy of these two physical measures, this randomized, clinical study evaluated the effects of a single CO2 hand bath in patients with systemic sclerosis and secondary Raynaud’s phenomenon and a healthy control group versus a single hot water hand bath on acral perfusion in systemic sclerosis by Doppler ultrasonography. None of the patients had currently digital ulcers, a vasoactive medication or a concomitant vascular disease. CO2 immersion induced an acute hemodynamic response, whereas hot water immersion had no significant effect on acral perfusion in systemic sclerosis.
Keywords: Systemic sclerosis, carbon dioxide, hot water, Doppler ultrasonography
Introduction
Systemic sclerosis (SSc) frequently manifests as secondary Raynaud’s phenomenon (sRP). sRP’s main symptoms are pain and digital ulcers. SSc is characterized by small digital arteries being constricted by overproduction of collagen, concentric intimal hyperplasia, and dysregulation of vasoconstrictive versus dilating molecular mechanisms. 1
The physical medicine regimen for sRP in SSc2,3 includes carbon dioxide (CO2) hand immersion to increase distal digital blood flow. The treatment of peripheral vascular disorders has a long history of immersing the body into CO2-enriched water based on the vasodilative effects of CO2. CO2 baths contribute to reactivation of capillary perfusion in patients with peripheral arterial occlusive disease. 4 Serial application in primary and secondary Raynaud’s syndrome has the potential to improve tolerance to cold. 5
In a pilot study that we previously presented, Doppler ultrasound with measurements of the resistance index (RI) of digital arteries6–8 was used to quantify treatment effects of CO2 hand immersion. These measurements served to supplement our previous work on synovial perfusion in wrist arthritis after local cryotherapy. 9 We demonstrated an acute hemodynamic response as well as a considerable short-term improvement of acral perfusion after a single carbon dioxide hand immersion in SSc. 10
Thus, the aim of this study was to evaluate the effect of a single CO2 hand immersion in SSc and a healthy control group versus a single hot water immersion on acral perfusion in SSc.
Patients and methods
A total of 24 patients with SSc (11 with limited cutaneous and 13 with diffuse cutaneous SSc) fulfilling the American College of Rheumatology criteria and sRP were assigned to two groups by computer-generated randomization: 12 patients received a CO2 hand immersion and 12 patients a hot water hand immersion (Figure 1). In addition, 12 healthy age-matched controls received a single CO2 hand immersion. Exclusion criteria included the presence of vasoactive medication, concomitant vascular disease, and digital ulcers. Baseline characteristics are presented in Table 1.
Figure 1.
SSc patient performing a CO2 hand bath.
Table 1.
Baseline characteristics of the SSc patients.
| SSc + CO2 hand immersion | SSc + hot water hand immersion | |
|---|---|---|
| Age (mean ± SD) (years) | 57 ± 11 | 58 ± 10 |
| Male | n = 2 | n = 2 |
| Female | n = 10 | n = 10 |
| SSc | ||
| Limited cutaneous form | n = 6 | n = 5 |
| Diffuse cutaneous form | n = 6 | n = 7 |
| Disease duration mean ± SD (years) | 6 ± 3 | 6 ± 4 |
| Raynaud’s phenomenon (RP) | n = 12 | n = 12 |
| Time since onset of RP (mean ± SD) (years) | 6 ± 1 | 5 ± 4 |
| History of digital ulcers | n = 3 | n = 4 |
| Abnormal nailfold capillaries | n = 12 | n = 12 |
| Anti-Scl-70 antibody positivity | n = 12 | n = 12 |
SSc: systemic sclerosis; SD: standard deviation.
All our examinations comply with the Helsinki Declaration. Approval was granted by the ethics committee of the Faculty of Medicine of the Justus Liebig University Giessen, Germany. We obtained written informed consent from each patient.
The following method has been extensively described in our pilot study. 10 Initially, a baseline ultrasound examination of the digital artery on ulnar side of the right index finger was performed. We used a Logiq 7 Pro ultrasound machine (General Electric Medical Systems, Milwaukee, Wisconsin, USA) with a wide-band linear transducer (4.7–13 MHz). After the ultrasound examination, standard hand immersion was performed 15 min using either a hot water hand immersion (40°C–42°C) or a Bastian® CO2 hand immersion (2 g/L CO2, 35°C; Bastian-Werk GmbH, Munich, Germany) for 15 min by a physiotherapist. The physician conducting the ultrasound examinations was blinded to treatment groups’ allocation. RI calculations and further measurements were conducted using the internal software of the ultrasound machine at baseline (before immersion), directly after immersion (T0), 10 (T10), and 20 (T20) minutes after immersion (Figure 1) in the ultrasound examination room next door to the treatment room. Thus, the physician remained blinded to the treatment. We used a spectral Doppler curve to calculate the RI values with the following formula: RI = (S − D)/S, in which S is the peak systolic and D the end-diastolic velocity. An increased RI thus corresponds to increased vascular resistance and reduced arterial blood flow. For statistical analysis, we used the Wilcoxon signed rank test.
Results
The mean RI at baseline as well as the RI values during treatment was significantly higher in both SSc groups than in the healthy controls (p < 0.01 for each time point; see Table 2). However, the mean RI at baseline was not different between the two SSc groups. With respect to the aim of the study, the achieved treatment-induced reduction in RI was significant (p < 0.01) in SSc directly after CO2 immersion, with a lasting tendency (not significant) 10 and 20 min after the CO2 immersion (Figure 2). In contrast, SSc patients receiving hot water immersion and the healthy controls with CO2 immersion showed no significant changes of the RI at all time points.
Table 2.
Results (mean ± SD) of the resistance index (RI) measurements.
| SSc + CO2 hand immersion | SSc + hot water hand immersion | Controls + CO2 hand immersion | |
|---|---|---|---|
| RI at baseline | 0.83 ± 0.07 | 0.8 ± 0.08 | 0.7 ± 0.09 |
| RI at T0 (immediately after immersion) | 0.76 ± 0.08** | 0.8 ± 0.08 | 0.7 ± 0.08 |
| RI at T10 (10 min after immersion) | 0.77 ± 0.09 | 0.81 ± 0.08 | 0.7 ± 0.07 |
| RI at T20 (20 min after immersion) | 0.79 ± 0.08 | 0.81 ± 0.07 | 0.7 ± 0.08 |
SSc: systemic sclerosis; SD: standard deviation.
p < 0.01 (Wilcoxon signed rank test) T0 versus baseline.
Figure 2.
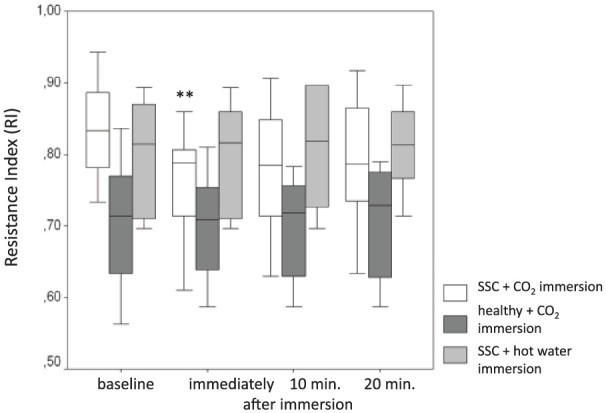
Changes in the resistance index in response to CO2 and hot water hand immersion, respectively.
Discussion
We attribute the higher baseline RI values in SSc to disease-related structural changes in the vasculature. Reduced vasodilatative capabilities are probably the cause for higher levels compared to the control group over. CO2 ameliorated the functional alterations which led to a more significant decrease in the RI compared to hot water immersion and to a healthy control with CO2 immersion.
Impressively, even a single CO2 immersion showed a significant short-term improvement of acral perfusion immediately after bathing, with a tendency of a reduced RI (not significant) after 10 and 20 min. Based on these results of a single CO2 hand immersion, we suggest that regular application of this treatment modality may lower the RI consecutively and thus facilitate improvement of acral perfusion in the long term by as part of a stimulus-response adaptation of the applied physical therapy modality. A recent small follow-up study underlined this hypothesis. 11
We showed hand immersion as an easy-to-use and inexpensive, simple way for SSc patients to improve distal digital blood flow. There were no notable adverse effects. CO2 hand immersion does not require any special equipment or experience. It can be used by physiotherapists on a daily basis and by patients for the treatment of sRP. It is also suitable to combine with drug-based vasodilatation therapies.
To clarify the question of whether a hot water bath induces the same hemodynamic effects as the CO2 hand immersion, the temperature of the water bath was chosen above the indifferent temperature range. Although the higher temperature did not show any effects, the different temperature is a limiting factor.
In summary, this study confirmed the results of our pilot study about CO2 effects on acral perfusion. Furthermore, we have shown for the first time that hot water immersion has no significant effect on acral perfusion in SSc and the capability of Doppler ultrasound to visualize the respective treatment effects.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Uwe Lange  https://orcid.org/0000-0001-7904-0619
https://orcid.org/0000-0001-7904-0619
References
- 1. Müller-Ladner U, Distler O, Ibba-Manneschi L, et al. Mechanisms of vascular damage in systemic sclerosis. Autoimmunity 2009; 42(7): 587–595. [DOI] [PubMed] [Google Scholar]
- 2. Meyer MF, Daigeler A, Lehnhardt M, et al. Therapeutic management of acral manifestations of systemic sclerosis. Med Klin 2017; 102: 209–218. [DOI] [PubMed] [Google Scholar]
- 3. Lange U, Uhlemann C, Berg W, et al. Physical medicine in rheumatology—differential indicative prescription in collagen diseases and vasculitides. Akt Rheumatol 2007; 32: 281–286. [Google Scholar]
- 4. Hartmann BR, Bassenge E, Hartmann M. Effects of serial percutaneus application of carbon dioxide in intermittent claudicatio: results of a controlled trial. Angiology 1997; 48: 957–963. [DOI] [PubMed] [Google Scholar]
- 5. Schmidt J, Monnet P, Normand BB, et al. Microcirculatory and clinical effects of serial percutaneous application of carbon dioxide in primary and secondary Raynaud’s phenomenon. Vasa 2005; 34: 93–100. [DOI] [PubMed] [Google Scholar]
- 6. Keberle M, Tony HP, Jahns R, et al. Assessment of microvascular changes in Raynaud’s phenomenon and connective tissue disease using colour Doppler ultrasound. Rheumatology 2000; 39(11): 1206–1213. [DOI] [PubMed] [Google Scholar]
- 7. Keberle M, Tony HP, Hau M, et al. Colour Doppler ultrasound of the nailbed: an objective tool for monitoring responses to vasodilatory treatment of connective tissue disorders? Rheumatology 2001; 40(8): 954–955. [DOI] [PubMed] [Google Scholar]
- 8. Bregenzer N, Distler O, Meyringer R, et al. Doppler ultrasound identifies increased resistive indices in SSc. Ann Rheum Dis 2004; 63(1): 109–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strunk J, Strube K, Klingenberger P, et al. Two- and three-dimensional Doppler sonographic evaluation of the effect of local cryotherapy on synovial perfusion in wrist arthritis. Rheumatology 2006; 45(5): 637–640. [DOI] [PubMed] [Google Scholar]
- 10. Müller-Eschner M, Albrecht K, Tarner IH, et al. Acute haemodynamic response to carbon dioxide hand immersion in patients with systemic sclerosis evaluated by Doppler ultrasonography. Clin Exp Rheumatol 2013; 31(2 Suppl. 76): 184–185. [PubMed] [Google Scholar]
- 11. Lange U, Bogensperger S, Dischereit G. Physical medicine in scleroderma-what’s new? Phys Med Rehab Kuror 2018; 28: 245. [Google Scholar]


