Abstract
Background
Eosinophilic myocarditis (EM) is a relatively rare form of myocarditis that could progress to restrictive cardiomyopathy and might be fatal if left untreated. Although myocardial biopsy is considered to be the gold standard for the diagnosis of myocarditis, its use in paediatrics remains controversial and not easily applicable in routine practice.
Case summary
A 10-year-old girl with no prior medical history presented to the emergency department for fever, odynophagia, and gastrointestinal symptoms despite 48 h of antibiotics (Cefaclor). Physical examination revealed diffuse petechiae and abdominal tenderness but was otherwise unremarkable. Her vital signs were normal. She was found to have hypereosinophilia and increased cardiac markers on laboratory testing. Echocardiography showed diffuse left ventricular (LV) myocardial infiltrates, moderate LV dilatation, and mild systolic dysfunction. Bone marrow biopsy confirmed B cell acute lymphoblastic leukaemia. The diagnosis of EM was made. High doses of steroids and chemotherapy were initiated. Cardiac magnetic resonance imaging (MRI) identified eosinophilic infiltrates and sub-endocardial enhancement strongly suggestive of EM. Left ventricular function was slightly decreased. Intra-ventricular micro-thrombi were suspected, and warfarin was started. The outcome was favourable. Leucocyte and eosinophil counts were normalized within a month. At 6 months, cardiac MRI demonstrated a significant decrease in eosinophilic infiltration and micro-thrombi, normalization of LV function, and of sub-endocardial enhancement.
Discussion
This case demonstrates that non-invasive multi-modality imaging along with typical laboratory and clinical findings allow for appropriate diagnosis of EM while avoiding biopsy. It also highlights that an early diagnosis, timely treatment, and rigorous follow-up improve disease progression and outcome.
Keywords: Paediatric, Myocarditis, Eosinophilia, Cardiac magnetic resonance, Restrictive cardiomyopathy, Case report
Learning points.
Echocardiography, including two-dimensional speckle tracking analysis and cardiac magnetic resonance imaging are important tools in patients with severe hypereosinophilia to exclude cardiac injury. These tools allow for appropriate diagnosis, timely management, and follow-up.
In daily practice, the use of endomyocardial biopsy is not always applicable in children, and it may be replaced with non-invasive multi-modality imaging along with clinical and laboratory findings.
Introduction
Myocarditis is an inflammatory disease of the myocardium that results from various infectious and non-infectious conditions diagnosed by established histological, immunological, and immunohistological criteria.1 In children, the outcome is variable depending on the underlying aetiology.1 Eosinophilic myocarditis (EM) is a relatively rare form of myocarditis which could progress to restrictive cardiomyopathy and might be fatal if left untreated. Clinical manifestations are highly variable, and diagnosis is often challenging. The place of endomyocardial biopsy (EMB) in the diagnosis of paediatric EM remains debateable whereas it is the gold standard in adult myocarditis. In children, diagnosis could be made by a combination of clinical and laboratory findings along with multimodal imaging. We hereby report the case of a 10-year-old girl who presented with non-specific characteristics and pronounced eosinophilia. Multimodal imaging allowed for appropriate diagnosis of EM and timely management.
Timeline
| Date | Events |
|---|---|
| 30 April | A 10-year-old girl with no prior medical history presented to the emergency department of our hospital for persistent fever and odynophagia despite 48 h f antibiotics (Cefaclor). She was found to have hypereosinophilia on and increased cardiac markers laboratory testing. |
| 01 May | Transthoracic echocardiography showed diffuse myocardial infiltrates of the left ventricle with moderate dilatation and mild systolic dysfunction. Bone marrow biopsy was performed and confirmed B cell acute lymphoblastic leukaemia (ALL) CD34+ CD19+. The diagnosis of eosinophilic myocarditis secondary to B ALL CD34+ CD19+ was made. High doses of steroids and chemotherapy were initiated. |
| 02 May | Cardiac magnetic resonance imaging (MRI) identified eosinophilic infiltrates and sub-endocardial enhancement of both ventricles strongly suggestive of eosinophilic myocarditis left ventricular (LV) function was slightly decreased. Anticoagulation with warfarin in the presence of micro-thrombi, was initiated |
| 04 June | The outcome was favourable. Leucocyte and eosinophil counts normalized within a month. She was discharged home on anticoagulation and steroids (tapered). Outpatient follow-up appointment was scheduled. |
| 6-month evolution | Cardiac MRI demonstrated significant decrease in eosinophilic infiltration and micro-thrombi, and normalization of LV function. Sub-endocardial enhancement also normalized leaving no sequela |
| 12-month evolution | A two-dimensional strain analysis showed mild posterior and apical segments dysfunction |
Case presentation
A 10-year-old girl with no prior medical history presented to the emergency department of our hospital for persistent fever and odynophagia despite 48 h of antibiotics (Cefaclor). She reported abdominal pain and vomiting with diarrhoea. Physical examination revealed diffuse petechiae and abdominal tenderness but was otherwise unremarkable. Her vital signs were normal for age. Laboratory testing showed important leukocytosis (52.9 g/L) with elevated eosinophil count (53%), blast cells (8%), and platelet count (41 g/L) (Supplementary material online, Figure S1).
Cardiac troponin T (2009 ng/L; Nl < 14 ng/L) and ProBNP (20 722 ng/L; Nl < 300 ng/L) were also elevated (Supplementary material online, Figure S2). The electrocardiogram (ECG) revealed ST-segment changes in V4–V6 derivations (Supplementary material online). Transthoracic echocardiography showed diffuse myocardial infiltrates of the left ventricle (LV) with moderate dilatation and mild mitral insufficiency (Figure 1) (Supplementary material online, Video 1 and 2). Left ventricular systolic dysfunction was identified by the measurement of the LV fractional shortening using M-mode (25% and 30%) and by the calculation of the LV ejection fraction using the Simpson method (43%). In addition, a two-dimensional strain analysis identified a reduced global longitudinal peak strain (average of −13% vs. normal value of −25%) and a basal septal and posterior hypokinesia (Figure 2). Micro-thrombi in the LV were suspected (Figure 3).
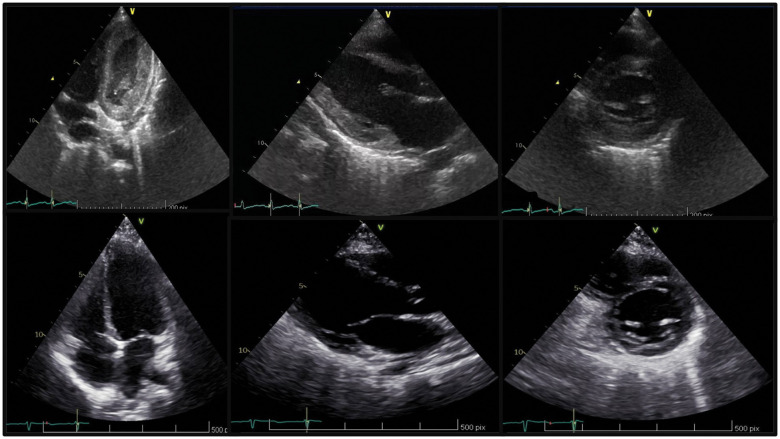
Figure 1.
Transthoracic echocardiography, apical four-chamber view, long-axis view and short-axis view showing thickened left ventricular secondary to myocardial infiltration of eosinophils (A) and the normalization after 1 year (B).
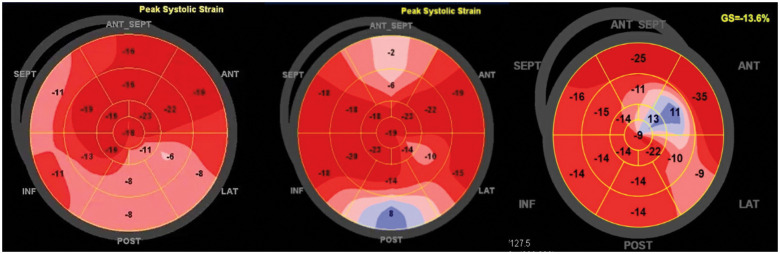
Figure 2.
Two-dimensional speckle tracking bull’s eye patterns of global longitudinal strain illustrating basal, septal, and posterior hypokinesia at the time of diagnosis (A) that recover gradually at 1 month (B) and 12 months (C) with residual abnormal deformation of the apical inferior segment 12 months.
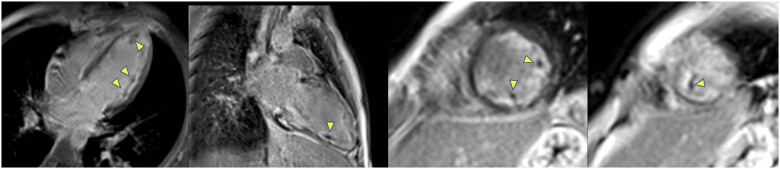
Figure 3.
Late gadolinium enhancement 2D-IR-FE images at the time of diagnosis demonstrates well multiple micro-thrombi (arrows) in the cavity of left ventricular as dark spot of unenhanced signal. The sub-endocardial gadolinium uptake is also clearly visible as a hypersignal by contrast to the intermediate signal of the blood.
Cardiac magnetic resonance imaging (MRI) identified eosinophilic infiltrates and sub-endocardial enhancement of both ventricles strongly suggestive of EM (Figure 4). Left ventricular function was slightly decreased with an ejection fraction of 47% (Figure 5).
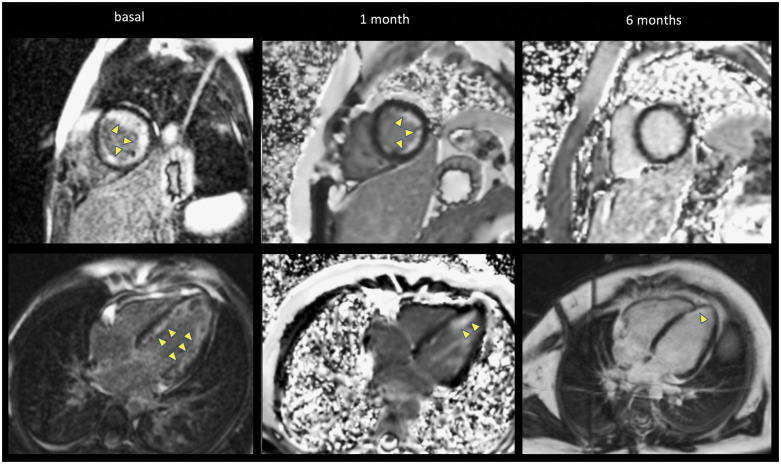
Figure 4.
Late gadolinium enhancement by cardiac magnetic resonance imaging showing the progression of diffuse sub-endocardial enhancement (arrows) of the left ventricular (four-chamber and short-axis views). A large sub-endocardial circumferential signal hyperenhancement is present at the time of diagnosis, decreases at 1 month and resolve at 6 months. Note the different types of magnetic resonance acquisitions present in this figure (2D-IR-FE in the left column, 2D-PSIR-FE in the middle column, and upper right figure and 3D-IR-FE) with the characteristic noise in the background of PSIR images.
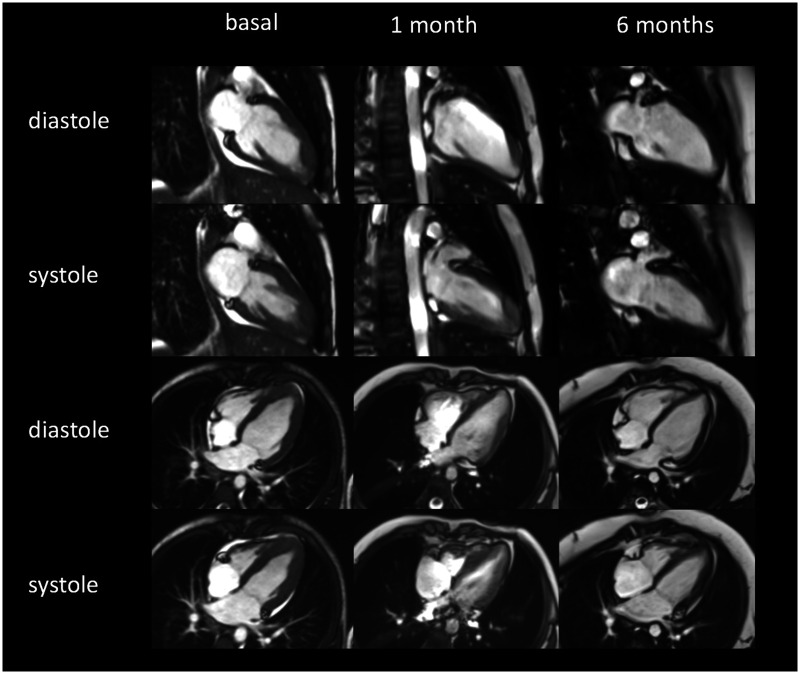
Figure 5.
Two-chamber and four-chamber cine magnetic resonance imaging in diastole and systole demonstrate a decreased cardiac contraction at the time of diagnosis that recover gradually at 1 and 6 months.
Specific tests excluded viral infections and parasitic diseases (schistosomiasis, echinococcosis, or helminthiasis). Hypersensitivity reaction to Cefaclor was possible but less likely because of the extent of hypereosinophilia and other blood abnormalities.
Haematological disorder was strongly suspected. Thus, a bone marrow biopsy was performed and confirmed B cell acute lymphoblastic leukaemia (ALL) CD34+ CD19+.
Treatment with high dose steroids (methylprednisolone 48 mg/day intravenously IV for 1 week) and chemotherapy, in cycles as per protocol, was initiated. IV steroids were then shifted to oral for 20 days (60 mg/m2/day) and tapered afterwards over 8 days. Anticoagulation with warfarin in the presence of micro-thrombi, although controversial, was initiated after several discussions among the team. Her hospital stay was complicated by well tolerated, isolated monomorphic ventricular premature beats which resolved without treatment.
Leucocyte and eosinophil counts normalized within a month (Supplementary material online, Figure S1). Progressive improvement of cardiac function was noticed on follow-up echocardiography (Suppementary material online, Video 3) and cardiac MRI. In addition, cardiac MRI demonstrated a significant decrease in eosinophilic infiltration and micro-thrombi (Figures 4 and 5). Sub-endocardial enhancement also normalized within 6 months, leaving no sequela (Figure 4). However, a two-dimensional strain analysis, repeated at 12 months, showed a mild posterior and apical segments dysfunction that was not noted on echocardiography or on MRI (Figure 2).
Discussion
Myocarditis causes significant morbidity and mortality in children. Depending on the aetiology, the outcome may vary from complete recovery to dilated cardiomyopathy in approximately one-third of cases.2 The incidence is difficult to determine and probably underestimated because of its wide spectrum of clinical presentation. The most common causes of myocarditis are infectious agents, toxins, drugs, metabolic abnormalities, hypersensitivity reactions, and systemic autoimmune diseases.
Among these, EM is a rare complication of eosinophilia. However, it could be severe and might lead to restrictive cardiomyopathy and even death.3–5 The degree of myocardial tissue injuries correlates, among other factors, with eosinophil activation’s duration and extent. Particularly, myocardial involvement is more common when hypereosinophilia is significant (more than 5000 eosinophils/mm3).4
Eosinophils degranulation and release of mediators explain endothelial and myocardial cells damage, necrosis, thrombosis, and eventually endomyocardial fibrosis. Thrombi are more often described at the apex of the left ventricle, similarly to our case. For unknown reasons, this process could stop at different stages which would explain the variety of manifestations (total normalization to severe restrictive cardiomyopathy).3–5 The aetiology of EM could be divided into three main categories: idiopathic, reactive (vasculitis, parasitic infection, autoimmune disease, drugs), and myeloid disorders.6 Malignancies count for around 1%.3,4 Antibiotics, mainly minocycline and beta-lactam, are the most common agents of hypersensitivity EM.3
Clinical signs and symptoms are not correlated with the degree of infiltrates. They are non-specific such as tachypnoea, abdominal pain, and diarrhoea. Some patients will present with chest pain, syncope, and palpitations. The diagnosis should be suspected in the presence of elevated cardiac biomarkers2 and typical ECG abnormalities (ST-T segment abnormalities) which are found in 93–100% of paediatric patients.7 In our reported case, myocarditis was diagnosed based on integrated clinical manifestations, laboratory findings, and non-invasive multi-modality imaging. Thus, EMB was not necessary. Although EMB is recommended in the adult population, it is not routinely done in paediatrics because it is invasive, technically challenging and carries a risk of cardiac perforation (1–5%).8 Literature on the need of biopsy with histological evidence for the diagnosis and thus the treatment of paediatric EM is still scarce. The American Heart Association (AHA), the American College of Cardiology (ACC), and the European Society of Cardiology (ESC) recommend EMB in case of recent, unexplained heart failure associated with a normal-sized or dilated left ventricle and compromised haemodynamic status. They also recommend EMB if heart failure is associated with a dilated left ventricle and new arrhythmia and if heart failure does not respond to usual medical treatment within 1–2 weeks.8
Echocardiography identifies left ventricle systolic dysfunction and myocardium infiltrates.4 A two-dimensional speckle tracking echocardiography is very useful to detect early and minimal systolic dysfunction. Hsiao et al. reported that LV strain and strain rate derived by two-dimensional speckle tracking echocardiography are promising prognostic tools in patients with preserved LV ejection fraction. These measurements also predict deterioration and overall event-free survival.9 Cardiac MRI allows for EM diagnosis with specific images of eosinophilic infiltrates. It also detects intra-ventricular thrombi and myocardial injuries (such as endocardial inflammation, patchy distribution of gadolinium enhancement, or oedema). Finally, cardiac MRI is an important follow-up tool if restrictive cardiomyopathy develops. In our case, MRI allowed appropriate diagnosis and timely management, without the need for invasive procedures such as EMB.
Despite being controversial in the treatment of EM, steroids were used to treat leukaemia and EM because of their strong ability to inhibit eosinophilic cell degranulation, limiting therefore myocardial necrosis.2,4
Conclusion
This case demonstrates that non-invasive multi-modality imaging along with typical laboratory and clinical findings allow for appropriate diagnosis of EM while avoiding biopsy.
It also highlights that an early diagnosis, timely treatment, and rigorous follow-up improve disease progression and outcome.
Lead author biography

Nesrine Farhat studied medicine at Liège University, Belgium. She started her medical training at the University Hospital Liège. She made her fellowship in the paediatric cardiology unit of the University Hospital Liège (CHU) in Belgium and after that in the Children's University Hospital of Geneva (HUG) in Switzerland. Currently, she works as a paediatric cardiologist the Pediatric Cardiology Unit of the University Hospital Liège (CHU) in Belgium.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
References
- 1.Nordet P, Martin I, Gyarfas I, Goodwin J, Thiene G, Olsen E. et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies. Circulation 1996;93:841–842. [DOI] [PubMed] [Google Scholar]
- 2.Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB. et al. ; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648. [DOI] [PubMed] [Google Scholar]
- 3.Brambatti M, Matassini MV, Adler ED, Klingel K, Camici PG, Ammirati E.. Eosinophilic myocarditis: characteristics, treatment, and outcomes. J Am Coll Cardiol 2017;70:2363–2375. [DOI] [PubMed] [Google Scholar]
- 4.Séguéla P-E, Iriart X, Acar P, Montaudon M, Roudaut R, Thambo J-B.. Eosinophilic cardiac disease: molecular, clinical and imaging aspects. Arch Cardiovasc Dis 2015;108:258–268. [DOI] [PubMed] [Google Scholar]
- 5.Habib G, Bucciarelli-Ducci C, Caforio ALP, Cardim N, Charron P, Cosyns B, et al. ; Indian Academy of Echocardiography. Multimodality imaging in restrictive cardiomyopathies: an EACVI expert consensus document in collaboration with the “Working Group on Myocardial and Pericardial Diseases” of the European Society of Cardiology Endorsed by The Indian Academy of Echocardiography. Eur Heart J Cardiovasc Imaging 2017;18:1090–1121. [DOI] [PubMed] [Google Scholar]
- 6.Cheung CC, Constantine M, Ahmadi A, Shiau C, Chen LYC.. Eosinophilic myocarditis. Am J Med Sci 2017;354:486–492. [DOI] [PubMed] [Google Scholar]
- 7.Durani Y, Egan M, Baffa J, Selbst SM, Nager AL.. Pediatric myocarditis: presenting clinical characteristics. Am J Emerg Med 2009;27:942–947. [DOI] [PubMed] [Google Scholar]
- 8.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U. et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol 2007;50:1914–1931. [DOI] [PubMed] [Google Scholar]
- 9.Hsiao J-F, Koshino Y, Bonnichsen CR, Yu Y, Miller FA, Pellikka PA. et al. Speckle tracking echocardiography in acute myocarditis. Int J Cardiovasc Imaging 2013;29:275–284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.