ABSTRACT
Background
To characterize the use of nephrotoxic medications in patients with chronic kidney disease (CKD) Stages G3–5 in routine care.
Methods
We studied cohorts of adults with confirmed CKD G3–5 undergoing routine care from 1 January 2016 through 31 December 2018 in two health systems [Stockholm CREAtinine Measurements (SCREAM), Stockholm, Sweden (N = 57 880) and Geisinger, PA, USA (N = 16 255)]. We evaluated the proportion of patients receiving nephrotoxic medications within 1 year overall and by baseline kidney function, ranked main contributors and examined the association between receipt of nephrotoxic medication and age, sex, CKD G-stages comorbidities and provider awareness of the patient's CKD using multivariable logistic regression.
Results
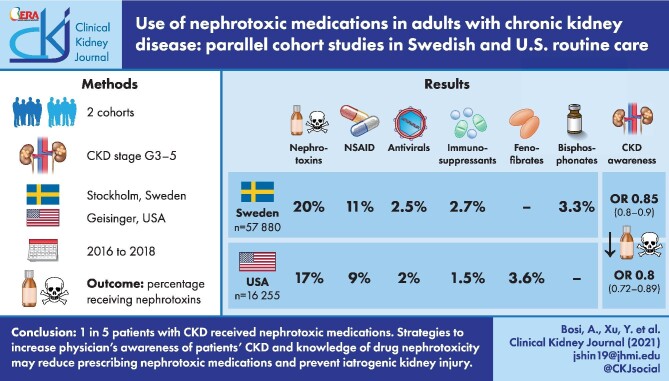
During a 1-year period, 20% (SCREAM) and 17% (Geisinger) of patients with CKD received at least one nephrotoxic medication. Among the top nephrotoxic medications identified in both cohorts were non-steroidal anti-inflammatory drugs (given to 11% and 9% of patients in SCREAM and Geisinger, respectively), antivirals (2.5% and 2.0%) and immunosuppressants (2.7% and 1.5%). Bisphosphonate use was common in SCREAM (3.3%) and fenofibrates in Geisinger (3.6%). Patients <65 years of age, women and those with CKD G3 were at higher risk of receiving nephrotoxic medications in both cohorts. Notably, provider awareness of a patient's CKD was associated with lower odds of nephrotoxic medication use {odds ratios [OR] 0.85[95% confidence interval (CI) 0.80–0.90] in SCREAM and OR 0.80 [95% CI 0.72–0.89] in Geisinger}.
Conclusions
One in five patients with CKD received nephrotoxic medications in two distinct health systems. Strategies to increase physician's awareness of patients’ CKD and knowledge of drug nephrotoxicity may reduce prescribing nephrotoxic medications and prevent iatrogenic kidney injury.
Keywords: chronic kidney disease, estimated glomerular filtration rate, nephrotoxicity
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Preventing adverse drug events is a public health priority. In the USA alone, adverse drug events lead to 2 million hospitalizations annually [1], cause 3.5 million office visits and 1 million emergency department visits [2, 3] and add $3.5 billion to annual healthcare costs [4]. A large proportion of adverse drug events are preventable, such as those resulting from inappropriate use or dosing errors [5].
A number of drugs and drug metabolites, hereby referred to as nephrotoxic, may exert adverse effects on the kidney through one or more common pathogenic mechanisms [6]. For example, nonsteroidal anti-inflammatory drugs (NSAIDs) can cause several types of acute kidney injury (AKI), including hemodynamically mediated AKI and acute interstitial nephritis. Other drugs may elicit nephrotoxicity by damaging tubular cells or causing crystal nephropathy, rhabdomyolysis and thrombotic microangiopathy [6–8]. Sometimes these medications may be indicated if the benefit from using them surpasses the potential harm in the kidney. However, other times these medications may not be indicated and/or could be replaced with non nephrotoxic alternatives.
Individuals with chronic kidney disease (CKD) are particularly susceptible to toxic insults that lead to an accelerated reduction in glomerular filtration rate (GFR). Exposure to ‘nephrotoxic medications’ has been associated with adverse drug events, with more emergency department visits and hospitalizations, longer hospital stays and a higher risk of mortality in persons with CKD [9, 10].
The Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline recommends avoidance or dosage reduction of nephrotoxic medications for persons with CKD Stage ≥ G3 [estimated GFR (eGFR) <60 mL/min/1.73 m2] [11]. However, the uptake of this recommendation in routine clinical settings is unknown. Previous studies have focused on a small selected list of nephrotoxic medications in patients referred to nephrologist care and/or were based on self-reported use of medications, which is subject to recall bias [12–16].
To bridge this knowledge gap, we aimed to quantify the extent of nephrotoxic medication use among patients with CKD ≥G3 followed in the outpatient care setting from two distinct and geographically diverse health systems. We identified those that could potentially be avoided or replaced by non-nephrotoxic alternatives and evaluated explanatory factors, including provider awareness of kidney disease that could inform clinical practice towards safer medication use.
MATERIALS AND METHODS
Data sources
This study includes two parallel cohorts of patients with CKD undergoing routine clinical care. The Swedish cohort is derived from the Stockholm CREAtinine Measurements (SCREAM) project, an extraction of routine care data of all patients undergoing creatinine testing in Region Stockholm. Region Stockholm is the sole provider of universal care to >2 million residents [17]. Healthcare data are cross-linked to several national data sources, with complete information on demographics, dispensed drugs and vital status, with no loss to follow-up. The US cohort was derived from >3 million adults receiving primary care at Geisinger, a large, predominantly rural, integrated healthcare system serving 44 counties in central and northeast Pennsylvania. This study was approved by the Stockholm Ethics Review Board, the Geisinger Medical Center Institutional Review Board and the Johns Hopkins Institutional Review Board.
Study population
In both cohorts we utilized deidentified patient data from outpatient encounters from 2016 to 2018 and included all adult (>18 years) patients with confirmed CKD G3–G5, in accordance with current KDIGO criteria [9]. We required patients with CKD to have at least two outpatient serum/plasma creatinine measurements denoting an eGFR <60 mL/min/1.73 m2 and imposed the gap between the two measurements to be >90 days but <1 year. The date of their second eGFR <60 mL/min/1.73 m2 result was defined as the index date. We excluded participants undergoing maintenance dialysis at baseline and those with a history of any organ transplant, because immunosuppression, although nephrotoxic in some cases, is necessary in those patients. Patients were followed up prospectively for 12 months or until kidney failure (dialysis or kidney transplantation) or death, whichever came first.
Nephrotoxic medications
During the 12-month observation period, we extracted information on outpatient drug prescriptions in Geisinger and information on pharmacy dispensations in SCREAM. Whereas drug prescriptions in Geisinger were identified in medical records, SCREAM ascertained medication use by linkage with the Swedish drug register, a government-run nationwide register with >99% coverage of all prescribed dispensations at Swedish pharmacies, which are connected to each citizen's unique personal identification number [18].
We composed a list of 115 single medications provided in ambulatory care with proven or purported nephrotoxicity (detailed in Supplementary data, Table S1). We created this list based on a literature review, warnings issued by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA) and statements in the US/Swedish pharmacopeias, followed by panel discussion and consensus [6, 9, 12, 14, 19–23]. We excluded ophthalmic, topical and inhaled formulations, as well as single medication dosages provided in nursing homes, and focused exclusively on oral and intravenous ambulatory medications. For simplicity, single medications were grouped into pharmacological classes according to the Anatomical Therapeutic Chemical (ATC) classification system of the World Health Organization [24]. Thus, when we refer to a certain drug class, we refer to the medications considered nephrotoxic (as listed in Supplementary data, Table S1) within that drug class. Whenever a single medication contained more than one nephrotoxic chemical substance in its composition, we computed the pharmacological class of each nephrotoxic chemical substance therein. Thus participants taking combination medications containing two or more nephrotoxic substances contributed to multiple different pharmacological classes.
Covariates
Covariates at baseline included demographics (e.g. sex, age), body mass index (BMI, in Geisinger only), CKD G-stages, comorbidities, type of prescribing unit (i.e. primary healthcare provider or other) and an indicator of providers’ awareness of their patients’ CKD status. We used the index creatinine measurement to estimate GFR with the 2009 Chronic Kidney Disease Epidemiology Collaboration creatinine-based equation [25] and categorized it into G-stages (G3a, 45–59 mL/min/1.73 m2; G3b, 30–44 mL/min/1.73 m2; G4/5, <30 mL/min/1.73 m2) according to KDIGO criteria. In SCREAM, all patients were assumed to be white for GFR estimation, as information on race was not available. BMI was available in Geisinger and categorized into <18.5 kg/m2 (underweight), 18.5–24.9 kg/m2 (normal weight), 25.0–29.9 kg/m2 (overweight) and ≥30.0 kg/m2 (obese). Comorbidities were defined by the presence of relevant diagnostic codes [International Classification of Diseases (ICD) revisions 9 and 10] before the index date and used to calculate the Charlson comorbidity index, excluding the category of kidney disease [26, 27]. We determined the history of AKI by the presence of relevant diagnostic codes (ICD-9 code 584.x and ICD-10 code N17) before the index date. Finally, we created an indicator of providers’ awareness of their patients’ CKD status. In SCREAM, this was defined as the composite of either the presence of a CKD diagnostic code or a nephrology consultation before the index date. In Geisinger, the ICD codes in medical records were automated for reimbursement purposes and did not reflect physicians’ awareness of CKD. We thus used a conservative definition and assessed provider awareness by preceding nephrology consultations only. Finally, for each drug dispensation/prescription, we classified their corresponding prescribing units as primary healthcare and other outpatient healthcare providers.
Statistical analysis
Baseline characteristics were presented as percentage and number for categorical variables and mean with standard deviation (SD) or median with interquartile range (IQR) for continuous variables as appropriate.
We first reported the proportion of individuals receiving at least one nephrotoxic medication during a 1-year follow-up, overall and by CKD G-stages. Next, we calculated the proportion of individuals receiving nephrotoxic medications grouped in pharmacological classes and ranked the order. We next estimated the volume of nephrotoxic medication dispensations/prescriptions out of the total number of nephrotoxic medications identified within 1 year. We further evaluated the volume of dispensations and prescriptions being issued by primary healthcare providers versus other outpatient healthcare providers.
We evaluated whether selected sociodemographic or clinical variables were associated with the use of nephrotoxic medications using multivariable logistic regression models, adjusting for individual's follow-up time. Clinical predictors included age, sex, ethnicity (African American versus non–African American, only in Geisinger), CKD G-stage, Charlson comorbidity index, BMI (only in Geisinger), history of AKI and provider awareness of the patient's CKD. We did not consider single comorbidities as predictors of nephrotoxic medication use because they may be the indications for such medications. As a sensitivity analysis, we evaluated the consistency of clinical predictors among individuals receiving NSAIDs (the most common nephrotoxic medication identified in both cohorts) and among individuals receiving nephrotoxic medications other than NSAIDs.
Because there is still controversy about whether proton pump inhibitors (PPIs) and warfarin exert nephrotoxicity [23, 28], we did not include these medications in our main analysis but evaluated as a sensitivity analysis the number of patients affected should these two drugs be included in our listing. Analyses in Geisinger were performed using SAS, version 9.4 (SAS Institute, Cary, NC, USA), and analyses in SCREAM were performed using R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Cohort characteristics
After applying inclusion and exclusion criteria (flow charts in Supplementary data, Figures S1 and S2), we identified 57 880 patients with confirmed CKD G3–5 attending routine outpatient healthcare in SCREAM. Their mean age was 79 years (SD 11) and mean eGFR was 46 mL/min/1.73 m2 (SD 11). Of these, only 23% had a diagnosis of CKD or had ever visited a nephrologist (Table 1). Likewise, we identified 16 255 patients in Geisinger, who had a mean age of 76 years (SD 11), a mean eGFR of 44 mL/min/1.73 m2 (SD, 11) and 27% with a documented visit to nephrology care (Table 2).
Table 1.
Baseline characteristics of the cohort in SCREAM, overall and by nephrotoxic medication use within 1 year
| Any nephrotoxic medication | |||
|---|---|---|---|
| Characteristics | Overall (N = 57 880) | Never users (n = 46 548) | Ever users (n = 11 332) |
| Age (years), mean (SD) | 79 (11) | 79 (11) | 75 (11) |
| <65 | 9 (5302) | 8 (3785) | 13 (1517) |
| 65–74 | 23 (13 044) | 21 (9649) | 30 (3395) |
| 75–84 | 37 (21 242) | 36 (16 896) | 38 (4346) |
| ≥85 | 32 (18 292) | 35 (16 218) | 18 (2074) |
| Women | 55 (31 600) | 53 (24 636) | 62 (6964) |
| eGFR (mL/min/1.73 m2), mean (SD) | 46 (11) | 46 (11) | 49 (10) |
| 45–59 | 62 (36 121) | 60 (27 926) | 72 (8195) |
| 30–44 | 28 (16 004) | 29 (13 432) | 23 (2572) |
| <30 | 10 (5755) | 11 (5190) | 5 (565) |
| History of acute kidney injury | 7 (4001) | 7 (3418) | 5 (583) |
| Provider awareness of CKD | 23 (13 482) | 25 (11 432) | 18 (2050) |
| Charlson comorbidity indexa, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| 0 | 13 (7415) | 12 (5572) | 16 (1843) |
| 1 | 24 (13 743) | 23 (10 686) | 27 (3057) |
| ≥2 | 63 (36 722) | 65 (30 290) | 57 (6432) |
| Diabetes | 29 (16 845) | 30 (13 819) | 27 (3026) |
| Hypertension | 82 (47 684) | 84 (38 875) | 78 (8809) |
| Cardiovascular disease (coronary heart disease, stroke, peripheral vascular disease) | 39 (22 710) | 42 (19 458) | 29 (3252) |
| Congestive heart failure | 28 (16 277) | 31 (14 200) | 18 (2077) |
| Cancer | 29 (16 758) | 29 (13 515) | 29 (3243) |
Values are presented as % (n) unless stated otherwise.
Charlson comorbidity index is a sum of item scores for comorbid conditions except kidney disease.
Table 2.
Baseline characteristics of the cohort in Geisinger, overall and by nephrotoxic medication use within one year
| Any nephrotoxic medication | |||
|---|---|---|---|
| Characteristics | Overall (N = 16 255) | Never users (n = 13 438) | Ever users (n = 2817) |
| Age (years), mean (SD) | 76 (11) | 77 (11) | 73 (11) |
| <65 | 15 (2512) | 14 (1854) | 23 (658) |
| 65–74 | 29 (4638) | 28 (3698) | 33 (940) |
| 75–84 | 34 (5519) | 35 (4673) | 30 (846) |
| ≥85 | 22 (3586) | 24 (3213) | 13 (373) |
| Women | 59 (9648) | 59 (7889) | 62 (1759) |
| White race | 97 (15 835) | 97 (13 101) | 97 (2734) |
| Body mass index (kg/m2), mean (SD) | 31.2 (7.4) | 31.0 (7.3) | 32.3 (7.8) |
| Underweight (<18.5) | 1 (144) | 1 (122) | 1 (22) |
| Normal (18.5–24.9) | 16 (2565) | 16 (2203) | 13 (362) |
| Overweight (25.0–29.9) | 27 (4309) | 27 (3666) | 23 (643) |
| Obese (≥30) | 44 (7162) | 43 (5758) | 50 (1404) |
| eGFR (mL/min/1.73 m2), mean (SD) | 44 (11) | 44 (11) | 46 (10) |
| 45–59 | 53 (8597) | 51 (6877) | 61 (1720) |
| 30–44 | 35 (5657) | 36 (4781) | 31 (876) |
| <30 | 12 (2001) | 13 (1780) | 8 (221) |
| History of AKI | 22 (3586) | 23 (3038) | 19 (548) |
| Provider awareness of CKD | 27 (4446) | 28 (3792) | 23 (654) |
| Charlson comorbidity indexa, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| 0 | 17 (2700) | 16 (2159) | 19 (541) |
| 1 | 24 (3850) | 24 (3193) | 23 (657) |
| ≥2 | 60 (9705) | 60 (8086) | 57 (1619) |
| Diabetes | 44 (7225) | 44 (5948) | 45 (1277) |
| Hypertension | 90 (14 566) | 90 (12 093) | 88 (2473) |
| Cardiovascular disease (coronary heart disease, stroke, peripheral vascular disease) | 55 (8974) | 56 (7577) | 50 (1397) |
| Congestive heart failure | 27 (4370) | 28 (3795) | 20 (575) |
| Cancer | 23 (3803) | 23 (3157) | 23 (646) |
Values are presented as % (n) unless stated otherwise.
Charlson comorbidity index is a sum of item scores for comorbid conditions except kidney disease.
Receipt of nephrotoxic medications within 1 year
Over the 1 year of follow-up, 20% of patients in SCREAM and 17% of patients in Geisinger received at least one nephrotoxic medication. Compared with patients not receiving nephrotoxic medications, those receiving nephrotoxic medications were younger, more often women and had a higher baseline eGFR in both cohorts. Patients with a history of AKI or provider awareness of their CKD status were less likely to receive nephrotoxic medications.
Main nephrotoxic medications in SCREAM
Table 3 describes and ranks the main drug classes of nephrotoxic medications dispensed, overall and by baseline eGFR category, in SCREAM. The most common nephrotoxic medications dispensed were NSAIDs (dispensed to 11% of participants at least once). Of these, 59% received only one dispensation, 19% received two dispensations and 22% received three or more dispensations within the 1-year observation period. Other nephrotoxic medications dispensed to >1% of individuals were bisphosphonates (3.3%), immunosuppressants (2.7%) and antivirals (2.5%). The use of most drug classes decreased with lower eGFR categories, with the exception of the proportion of nephrotoxic antibiotics, which showed an increasing trend.
Table 3.
Proportion of unique individuals receiving nephrotoxic medications in SCREAM and ranking of most commonly prescribed drug classes, overall and by eGFR category
| Medications | Overall (N = 57 880) | G3a eGFR 45–59 (n = 36 121) | G3b eGFR 30–44 (n = 16 004) | G4/5 eGFR < 30 (n = 5755) | P-value |
|---|---|---|---|---|---|
| Any nephrotoxic medication | 19.6 (11 332) | 22.7 (8195) | 16.0 (2572) | 9.8 (565) | <0.001 |
| Drug class | |||||
| NSAIDs | 11.0 (6382) | 12.9 (4668) | 9.1 (1454) | 4.5 (260) | <0.001 |
| 1 dispensationa, % | 58.9 | 58.4 | 58.7 | 68.1 | <0.001 |
| 2 dispensationsa, % | 18.7 | 18.5 | 19.5 | 16.9 | <0.001 |
| 3 or more dispensationsa, % | 22.4 | 23.1 | 21.8 | 15.0 | <0.001 |
| Bisphosphonates | 3.3 (1923) | 3.9 (1438) | 2.7 (431) | 0.9 (54) | <0.001 |
| Immunosuppressants | 2.7 (1577) | 3.3 (1184) | 1.8 (286) | 1.9 107 | <0.001 |
| Antivirals, systemic use | 2.5 (1444) | 2.9 (1038) | 2 (324) | 1.4 (82) | <0.001 |
| Psycholeptics | 0.8 (479) | 1.0 (370) | 0.5 (87) | 0.4 (22) | <0.001 |
| Intestinal anti-inflammatory drugs | 0.8 (439) | 0.9 (330) | 0.6 (90) | 0.3 (19) | <0.001 |
| Antiprotozoals | 0.5 (278) | 0.5 (174) | 0.4 (68) | 0.6 (36) | 0.17 |
| Antibiotics | 0.2 (128) | 0.2 (63) | 0.2 (39) | 0.5 (26) | <0.001 |
| Othersb | 0.6 (112) | 0.2 (82) | 0.1 (23) | 0.1 (7) | 0.17 |
| Fenofibrates | 0.1 (75) | 0.1 (48) | 0.2 (25) | 0.03 (2) | 0.08 |
| Antimycobacterials | 0.08 (48) | 0.10 (38) | 0.05 (8) | 0.03 (2) | 0.05 |
| Antineoplastic agents | 0.07 (41) | 0.09 (33) | 0.04 (7) | 0.02 (1) | 0.04 |
| Non-NSAIDs analgesics | 0.06 (36) | 0.07 (25) | 0.06 (10) | 0.02 (1) | 0.34 |
| Immunostimulants | 0.02 (11) | 0.03 (10) | 0.01 (1) | 0 | 0.14 |
Values are presented as % (n) unless stated otherwise.
Among NSAID users, proportions receiving one/two/three or more dispensations, overall and by eGFR category.
Medications included in others: isotretinoin, amphotericin B, normal human immunoglobulins, deferasirox and palifermin.
Main nephrotoxic medications in Geisinger
In Geisinger (Table 4), NSAIDs were also the most commonly prescribed nephrotoxic medication (prescribed to 8.7% of participants). Of these, 43% received only one prescription, 20% received two prescriptions and 37% received three or more prescriptions within the 1-year observation period. Other nephrotoxic medications prescribed to >1% of individuals were fenofibrates (3.6%), antivirals (2.0%), immunosuppressants (1.5%), antibiotics (1.5%) and antineoplastic agents (1.4%). The use of some but not all nephrotoxic medication classes decreased with lower eGFR categories. Antibiotics that showed an increasing trend and no decreasing trends were observed with lower eGFR for fenofibrates or antivirals. Among NSAID users, the proportion of one-time NSAID use increased with lower eGFR.
Table 4.
Proportion of unique individuals receiving nephrotoxic medications in Geisinger and ranking of most commonly prescribed drug classes, overall and by eGFR category
| Medications | Overall (N = 16 255) | G3a eGFR 45–59 (n = 8597) | G3b eGFR 30–44 (n = 5657) | G4/5 eGFR < 30 (n = 2001) | P-value |
|---|---|---|---|---|---|
| Any nephrotoxic medications | 17.3 (2817) | 20.0 (1720) | 15.5 (876) | 11.0 (221) | <0.001 |
| Drug class | |||||
| NSAIDs | 8.7 (1409) | 11.2 (963) | 7.1 (403) | 2.1 (43) | <0.001 |
| 1 prescriptiona, % | 42.8 | 40.7 | 46.4 | 55.8 | 0.03 |
| 2 prescriptionsa, % | 19.7 | 20.9 | 16.6 | 20.9 | 0.19 |
| 3 or more prescriptionsa, % | 37.5 | 38.4 | 37.0 | 23.3 | 0.13 |
| Fenofibrates | 3.6 (578) | 3.3 (286) | 3.8 (213) | 4.0 (79) | 0.23 |
| Antivirals, systemic use | 2.0 (331) | 2.2 (190) | 1.9 (106) | 1.7 (35) | 0.24 |
| Immunosuppressants | 1.5 (246) | 2.1 (178) | 1.0 (55) | 0.6 (13) | <0.001 |
| Antibiotics | 1.5 (239) | 1.3 (114) | 1.4 (77) | 2.4 (48) | 0.001 |
| Antineoplastic agents | 1.4 (226) | 2.0 (170) | 0.9 (51) | 0.2 (5) | <0.001 |
| Intestinal anti-inflammatory drugs | 0.6 (102) | 0.6 (54) | 0.7 (37) | 0.5 (11) | 0.88 |
| Bisphosphonates | 0.5 (80) | 0.6 (54) | 0.4 (25) | 0.1 (1) | 0.003 |
| Psycholeptics | 0.3 (53) | 0.4 (32) | 0.3 (18) | 0.2 (3) | 0.29 |
| Antimycobacterials | 0.1 (18) | 0.1 (12) | 0.1 (6) | 0 | 0.24 |
| Immunostimulants | 0.06 (9) | 0.09 (8) | 0.02 (1) | 0 | 0.09 |
| Othersb | 0.06 (9) | 0.07 (6) | 0.04 (2) | 0.05 (1) | 0.69 |
| Antiprotozoals | 0.02 (4) | 0.03 (3) | 0 | 0.05 (1) | 0.32 |
Values are presented as % (n) unless stated otherwise.
Among NSAID users, proportions receiving one/two/three or more dispensations, overall and by eGFR category.
Medications included in others: quinidine, isotretinoin, amphotericin B, normal human immunoglobulins, deferasirox and palifermin.
Volume of nephrotoxic medications
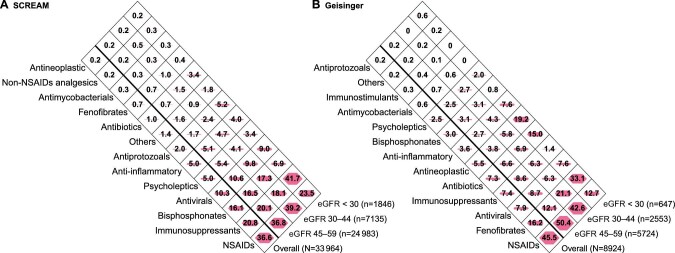
In SCREAM, there were a total of 33 964 nephrotoxic medication dispensations within 1 year in 11 332 patients and NSAIDs represented 36.6% of them (Figure 1, Panel A). Other medications accounting for >10% of total nephrotoxic dispensations were immunosuppressants (20.8%), bisphosphonates (16.1%) and antivirals (10.3%). As many as 41% of all nephrotoxic drug dispensations were done in connection with a primary healthcare encounter (Supplementary data, Table S2). In Geisinger, there were a total of 8924 nephrotoxic medications prescribed in 2817 patients. NSAIDs represented 45.5% of all nephrotoxic drug prescriptions (Figure 1, Panel B), followed by fenofibrates (16.2%). Approximately half of the identified nephrotoxic drug prescriptions (52%) were done in connection with a primary healthcare encounter (Supplementary data, Table S3).
FIGURE 1:
Volume (in percentage) of dispensations (in SCREAM, Panel A) or prescriptions (in Geisinger, Panel B), grouped in drug classes, out of the total number of nephrotoxic medications identified (N = 33 964 dispensations in SCREAM, N = 8924 prescriptions in Geisinger). Shaded polygons are proportional to the total area within each diamond for the indicated percentage. Medications included in the ‘Others’ class are isotretinoin, amphotericin B, normal human immunoglobulins, deferasirox and palifermin for SCREAM (Panel A) and quinidine, isotretinoin, amphotericin B, normal human immunoglobulins, deferasirox and palifermin for Geisinger (Panel B). Differences are due to market availability in each country.
Sensitivity analyses
Including PPIs and warfarin in our listing increased the number of patients receiving nephrotoxic medications in SCREAM to 48%; PPIs ranked as the main prescribed nephrotoxic drug class (provided to 29% of participants; Supplementary data, Table S4) followed by warfarin (provided to 14% of participants). Likewise, in Geisinger, the proportion of patients receiving nephrotoxic medications increased to 56%, with PPIs being prescribed to 38% of participants and warfarin to 17% (Supplementary data, Table S4). These two classes of medications represented the largest volume of prescriptions/dispensations (Supplementary data, Figure S3).
Factors associated with the receipt of nephrotoxic medications
In both cohorts, younger age and female sex were associated with a higher likelihood of nephrotoxic drug receipt (Figure 2). Conversely, more severe CKD and a history of AKI were associated with a lower likelihood of nephrotoxic drug use. Notably, participants with a diagnosis code for CKD or a documented visit to nephrology care were less likely to use nephrotoxic drugs in both cohorts, and this was mainly explained by lower use of NSAIDs, not by lower use of other nephrotoxic drugs (Supplementary data, Table S5). Women were more likely to use NSAIDs as well as other nephrotoxic drugs in both cohorts. Both NSAIDs and other nephrotoxic medications were less frequently used with more severe CKD in SCREAM, but this trend was not observed in Geisinger.
FIGURE 2:
Odds ratios of nephrotoxic medication use in SCREAM (in blue) or Geisinger (in brown) by baseline demographic and clinical characteristics. Charlson comorbidity index is a sum of item scores for comorbid conditions listed in the table except CKD. Covariates included in both cohorts included age, sex, baseline eGFR, history of AKI, Charlson comorbidity index and awareness of CKD; additional covariates in Geisinger include race and BMI.
DISCUSSION
We performed a comprehensive evaluation of the potentially inappropriate use of >100 nephrotoxic medications across the spectrum of CKD G3– in outpatient care of two distinct and geographically diverse health systems. We refer to these nephrotoxic medications as ‘potentially inappropriate’, as we cannot exclude the possibility that the benefit of using such medications exceeds the potential harm in the kidney. We observed that approximately one in five patients received at least one nephrotoxic medication within 1 year. NSAIDs were the most commonly prescribed medications, together with nephrotoxic antivirals and immunosuppressants. Other potentially inappropriate nephrotoxic medications were cohort specific (bisphosphonates in SCREAM, fenofibrates in Geisinger). Nearly half of all nephrotoxic medications were prescribed in connection with primary healthcare consultations. Women were at higher risk of receiving nephrotoxic medications compared with men and patients with a CKD diagnosis or attending nephrology visits were at consistently lower risk of nephrotoxic medication use.
Our study uses data from contemporary cohorts and adds knowledge to previous literature: Ingrasciotta et al. [14] reported that 50% of patients received nephrotoxic medication prescriptions within 1 year prior to or after the first CKD diagnosis in southern Italy between 2006 and 2011. The higher prevalence in this study may be explained by the, in our opinion, incorrect inclusion of medications such as angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers in their listing, based on the summary of product characteristics that states to ‘use with caution’ in persons with CKD because of potential AKI risk. Davis-Ajami et al. [9] reported that 72% of patients with a CKD diagnosis in the 2006–2012 US Medical Expenditure Panel Survey data received nephrotoxic medications; the long ascertainment period (12 years) and the inclusion of PPIs and diuretics in their listing probably also explain the high prevalence reported. Schmidt-Mende et al. [29] evaluated the dispensation of contraindicated medications among older adults (>65 years) accessing healthcare in Stockholm in 2010 with CKD Stages 3–4, which included both nephrotoxic medications and medications that should be avoided because of a high risk of adverse events (e.g. hyperkalemia due to spironolactone in CKD Stage 4). In this context, 9% of older adults with CKD Stage 3 and 38% with CKD Stage 4 received contraindicated drugs. They did not report on nephrotoxic medications separately.
In both cohorts, the most commonly used nephrotoxic medications were NSAIDs, which were given to 9–11% of patients. This proportion is in the lower range of what has been reported by previous studies, with NSAIDs being used by 9–49% of studied subjects [9, 30, 31]. Since over-the-counter use was not evaluated in our study, it is possible that the true NSAID use is higher. For example, in the Atherosclerosis Risk in Communities study, Secora et al. [12] observed that 24% of participants with CKD Stage 3 and 11% of participants with CKD Stages 4–5 self-reported the use of NSAIDs at a study visit. We add to this knowledge by reporting that more than one-third of NSAIDs users had chronic use (three dispensations or more within 1 year). Non-nephrotoxic alternatives, such as acetaminophen, may be preferred in this case.
Other common nephrotoxic medications varied by cohort, possibly reflecting differences in clinical practice or in the perception of risks between countries. In SCREAM, bisphosphonates were commonly dispensed. However, their use is contraindicated or they should be used with caution in patients with CKD, primarily because of a lack of information about their safety and effectiveness in settings of low eGFR. Although nephrotoxicity has traditionally been associated with the less-used injection formulations, a recent study of new oral bisphosphonate users from the UK and Spain observed a modest (15%) increased risk of CKD progression compared to non use [32]. In the USA, consecutive drug safety announcements by the FDA were followed by a significant decline in bisphosphonate use in recent years [33, 34]. Nonnephrotoxic osteoporotic medications, such as denosumab, may be an alternative in some cases. In Geisinger, fenofibrate use was high, in line with the reported dramatic increase in fenofibrate prescribing in recent years [35]. Although alternative lipid-lowering agents are available, we recognize that the exact effect of fenofibrates in kidney function is not clear and recent reports suggest that increases in serum creatinine may be reversible [36], explained by hemodynamic changes rather than actual tubular injury [37, 38]. Finally, we felt that the evidence was not solid enough as to where PPIs or warfarin exert nephrotoxicity [23, 28] and decided not to include these medications in our primary analyses. This being said, a large proportion of participants in both cohorts used these medications and we note that alternative kidney-safer antiacids, like histamine-2 receptor antagonists, or direct oral anticoagulants may be a choice for patients with CKD if/when indicated.
Our results identified populations where more stringent efforts are needed to reduce nephrotoxic medication use. While it was reassuring that in SCREAM almost all nephrotoxic medications were less used with more severe CKD, this decreasing trend was not consistently observed in Geisinger. Individuals with younger age (<65 years) or with CKD Stage G3a were at higher risk of receiving nephrotoxic drugs, which might reflect lesser concern by physicians in prescribing these medications to patients perceived as healthier. Women were more likely than men to receive both NSAIDS and nephrotoxic medications other than NSAIDs. Reasons behind this observation are unknown, but multiple studies have shown differences in drug utilization between women and men in the general population, especially with regard to the use of psychotropic drugs and analgesics [39–41]. Gender modifies the perception of disease, healthcare-seeking behavior, utilization of medical services, interaction with healthcare providers and decision making [42–46]. Our most interesting finding is perhaps that patients identified as having CKD were less likely to receive nephrotoxic medications, suggesting that increased provider awareness may reduce inappropriate nephrotoxic drug use. This is in line with the analysis by Kurani et al. [15] of participants with CKD Stages 3–5 from the 2011–2016 National Health and Nutrition Examination Surveys; they observed higher use of NSAIDs among patients unaware of their CKD compared with those who were aware [15]. We note that they assessed awareness using the patients’ self-reports and the engagement of patients in prescribing and medication management was unknown.
Finally, in both cohorts, about half of all prescriptions of nephrotoxic medications were from primary care physicians. This was also seen in a small Italian study [31] and likely reflects the fact that most outpatient medications are de facto prescribed by primary care providers. However, it also points towards a segment of healthcare where more stringent efforts may be needed to screen and diagnose CKD (to increase both provider and patient awareness) and to alert to the risks of inappropriately prescribing nephrotoxic medications when safer alternative choices are available. Several interventions are available to prevent inappropriate prescribing, including the involvement of pharmacists or the establishment of manual or computer-aided alerts [10]. Chertow et al. [47] evaluated a real-time computerized decision support system for drug prescription for inpatients with CKD; after a 2-month implementation, the frequency of inappropriate drug use decreased from 59% to 35% and the length of hospital stay was also significantly reduced. In Sweden, a clinical decision support system was implemented to support kidney-related drug prescribing (alerting on both nephrotoxicity or the need for dose adjustment to prevent adverse effects) based on the patient's eGFR and integrated in the electronic health record system, which was made available to primary and outpatient specialist care [21]. Likewise, digital applications intended to improve outpatient medication safety are available. Stephanie et al. [48] evaluated a community-based digital intervention involving both patient and clinician and found the 1-year implementation largely reduced the rate and severity of medication discrepancies (proximal cause of medical errors) in high-risk CKD patients.
A strength of our study is ascertainment of the population using persistently low eGFR levels as opposed to CKD diagnoses, which have poor sensitivity. Another strength is the prospective examination of medication claims and dispensations as opposed to self-reports. Lastly, we believe that our analysis of two distinct health systems from two different countries increases generalizability. However, there are also limitations, including the lack of information on whether patients actually filled the prescription (in Geisinger) or purchased over-the-counter medications (in both cohorts). Thus our results may, if any, underestimate the real use of nephrotoxic medications in the CKD population. Second, we used referrals to nephrologists only as a surrogate for the provider's CKD awareness in the Geisinger cohort due to the automatic loading of CKD diagnostic codes without the physician's approval. Because management of moderate CKD may be done in the absence of a nephrologist consultation, this definition probably underestimates the true proportion of CKD patients identified by their physicians. Finally, our findings are representative of clinical practice during 2016–2018 in the two health systems assessed; extrapolation to other systems, countries or periods should be done with caution.
To conclude, we found that the use of nephrotoxic medications (mainly NSAIDs) by patients with CKD G3–5 was common. Strategies to increase the physician's awareness of patients’ CKD status and knowledge of drug nephrotoxicity may reduce nephrotoxic medication prescriptions and prevent iatrogenic kidney injury.
Supplementary Material
Contributor Information
Alessandro Bosi, Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden.
Yunwen Xu, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Alessandro Gasparini, Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden.
Björn Wettermark, Department of Pharmacy, Disciplinary Domain of Medicine and Pharmacy, Uppsala University, Uppsala, Sweden.
Peter Barany, Department of Clinical Science, Intervention and Technology, Karolinska Institute, Stockholm, Sweden.
Rino Bellocco, Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden.
Lesley A Inker, Division of Nephrology, Department of Internal Medicine, Tufts Medical Center, Boston, MA, USA.
Alex R Chang, Division of Nephrology, Geisinger Health System, Danville, PA, USA.
Mara McAdams-DeMarco, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Morgan E Grams, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Jung-Im Shin, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Juan J Carrero, Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden.
FUNDING
Research reported in this publication was supported by the Swedish Research Council [2019-01 059 (J.J.C.) and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health], [R01 DK115534 and R01 DK100446 (M.E.G.) and K01 DK121825 J.-I.S.)]. The funding sources had no role in the design and conduct of the study, analysis or interpretation of the data and preparation or final approval of the manuscript before publication.
CONFLICT OF INTEREST STATEMENT
PB is a member of the CKJ editorial board.
REFERENCES
- 1.Classen DC, Pestotnik SL, Evans RSet al. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1997; 277: 301–306 [PubMed] [Google Scholar]
- 2.Bourgeois FT, Shannon MW, Valim Cet al. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf 2010; 19: 901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budnitz DS, Pollock DA, Weidenbach KNet al. National surveillance of emergency department visits for outpatient adverse drug events. JAMA 2006; 296: 1858–1866 [DOI] [PubMed] [Google Scholar]
- 4.Institute of Medicine . Preventing Medication Errors. Washington, DC: National Academies Press, 2007 [Google Scholar]
- 5.Office of Disease Prevention and Health Promotion . National Action Plan for Adverse Drug Event Prevention. Washington, DC: US Department of Health and Human Services, 2014 [Google Scholar]
- 6.Patel JB, Sapra A. Nephrotoxic medications. http://www.ncbi.nlm.nih.gov/books/NBK553144/ (28 November 2020, date last accessed) [PubMed] [Google Scholar]
- 7.Pannu N, Nadim MK. An overview of drug-induced acute kidney injury. Crit Care Med 2008; 36(4 Suppl): S216–S223 [DOI] [PubMed] [Google Scholar]
- 8.Naughton CA. Drug-induced nephrotoxicity. Am Fam Physician 2008; 78: 743–750 [PubMed] [Google Scholar]
- 9.Davis-Ajami ML, Fink JC, Wu J. Nephrotoxic medication exposure in U.S. adults with predialysis chronic kidney disease: health services utilization and cost outcomes. J Manag Care Spec Pharm 2016; 22: 959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tesfaye WH, Castelino RL, Wimmer BCet al. Inappropriate prescribing in chronic kidney disease: a systematic review of prevalence, associated clinical outcomes and impact of interventions. Int J Clin Pract 2017; 71: e12960. [DOI] [PubMed] [Google Scholar]
- 11.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120: c179–c184 [DOI] [PubMed] [Google Scholar]
- 12.Secora A, Alexander GC, Ballew SHet al. Kidney function, polypharmacy, and potentially inappropriate medication use in a community-based cohort of older adults. Drugs Aging 2018; 35: 735–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mort JR, Aparasu RR. Prescribing potentially inappropriate psychotropic medications to the ambulatory elderly. Arch Intern Med 2000; 160: 2825. [DOI] [PubMed] [Google Scholar]
- 14.Ingrasciotta Y, Sultana J, Giorgianni Fet al. The burden of nephrotoxic drug prescriptions in patients with chronic kidney disease: a retrospective population-based study in southern Italy. PLoS One 2014; 9: e89072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurani S, Jeffery MM, Thorsteinsdottir Bet al. Use of potentially nephrotoxic medications by U.S. adults with chronic kidney disease: NHANES, 2011–2016. J Gen Intern Med 2020; 35: 1092–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okoro RN, Farate VT. The use of nephrotoxic drugs in patients with chronic kidney disease. Int J Clin Pharm 2019; 41: 767–775 [DOI] [PubMed] [Google Scholar]
- 17.Runesson B, Gasparini A, Qureshi ARet al. The Stockholm CREAtinine Measurements (SCREAM) project: protocol overview and regional representativeness. Clin Kidney J 2016; 9: 119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wettermark B, Hammar N, MichaelFored Cet al. The new Swedish prescribed drug register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007; 16: 726–735 [DOI] [PubMed] [Google Scholar]
- 19.Laville SM, Metzger M, Stengel Bet al. Evaluation of the adequacy of drug prescriptions in patients with chronic kidney disease: results from the CKD-REIN cohort. Br J Clin Pharmacol 2018; 84: 2811–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurani S, Jeffery MM, Thorsteinsdottir Bet al. Use of potentially nephrotoxic medications by U.S. adults with chronic kidney disease: NHANES, 2011–2016. J Gen Intern Med 2020; 35: 1092–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shemeikka T, Bastholm-Rahmner P, Elinder C-Get al. A health record integrated clinical decision support system to support prescriptions of pharmaceutical drugs in patients with reduced renal function: design, development and proof of concept. Int J Med Inform 2015; 84: 387–395 [DOI] [PubMed] [Google Scholar]
- 22.Peng Y-C, Lin C-L, Yeh H-Zet al. Association between the use of proton pump inhibitors and the risk of ESRD in renal diseases: a population-based, case-control study. Medicine (Baltimore) 2016; 95: e3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazarus B, Chen Y, Wilson FPet al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016; 176: 238–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization . The anatomical therapeutic chemical classification system with defined daily doses (ATC/DDD). https://www.who.int/classifications/atcddd/en/ (28 November 2020, date last accessed) [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CHet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan H, Sundararajan V, Halfon Pet al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43: 1130–1139 [DOI] [PubMed] [Google Scholar]
- 27.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45: 613–619 [DOI] [PubMed] [Google Scholar]
- 28.Klatte DCF, Gasparini A, Xu Het al. Association between proton pump inhibitor use and risk of progression of chronic kidney disease. Gastroenterology 2017; 153: 702–710 [DOI] [PubMed] [Google Scholar]
- 29.Schmidt-Mende K, Wettermark B, Andersen Met al. Prevalence of renally inappropriate medicines in older people with renal impairment—a cross-sectional register-based study in a large primary care population. Basic Clin Pharmacol Toxicol 2019; 124: 256–265 [DOI] [PubMed] [Google Scholar]
- 30.Davis JS, Lee HY, Kim Jet al. Use of non-steroidal anti-inflammatory drugs in US adults: changes over time and by demographic. Open Heart 2017; 4: e000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbieri MA, Rottura M, Cicala Get al. Chronic kidney disease management in general practice: a focus on inappropriate drugs prescriptions. J Clin Med 2020; 9: 1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson DE, Ali MS, Pallares Net al. Safety of oral bisphosphonates in moderate-to-severe chronic kidney disease: a bi-national cohort analysis. J Bone Miner Res 2021; 36: 820–832 [DOI] [PubMed] [Google Scholar]
- 33.Kim SC, Kim DH, Mogun Het al. Impact of the U.S. Food and Drug Administration's safety-related announcements on the use of bisphosphonates after hip fracture. J Bone Miner Res 2016; 31: 1536–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Titan SM, Laureati P, Sang Yet al. Bisphosphonate utilization across the spectrum of eGFR. Arch Osteoporos 2020; 15: 69. [DOI] [PubMed] [Google Scholar]
- 35.Jackevicius CA. Use of fibrates in the United States and Canada. JAMA 2011; 305: 1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mychaleckyj JC, Craven T, Nayak Uet al. Reversibility of fenofibrate therapy-induced renal function impairment in ACCORD type 2 diabetic participants. Diabetes Care 2012; 35: 1008–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chauhan K, Nadkarni GN, Debnath Net al. The association of fenofibrate with kidney tubular injury in a subgroup of participants in the ACCORD trial. Clin J Am Soc Nephrol 2019; 14: 1521–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frazier R, Mehta R, Cai Xet al. Associations of fenofibrate therapy with incidence and progression of CKD in patients with type 2 diabetes. Kidney Int Rep 2019; 4: 94–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bäckryd E. Gender differences in dispensed analgesics in Sweden during 2006–2015: an observational, nationwide, whole-population study. Int J Womens Health 2018; 10: 55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orlando V, Mucherino S, Guarino Iet al. Gender differences in medication use: a drug utilization study based on real world data. Int J Environ Res Public Health 2020; 17: 3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molnar AO, Bota S, Jeyakumar Net al. Potentially inappropriate prescribing in older adults with advanced chronic kidney disease. PLoS One 2020; 15: e0237868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosen S, Ham B, Mogil JS. Sex differences in neuroimmunity and pain. J Neurosci Res 2017; 95: 500–508. [DOI] [PubMed] [Google Scholar]
- 43.Mauvais-Jarvis F, Bairey Merz N, Barnes PJet al. Sex and gender: modifiers of health, disease, and medicine. Lancet North Am Ed 2020; 396: 565–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deepmala D, Franz L, Aponte Cet al. Identification of provider characteristics influencing prescription of analgesics: a systematic literature review. Pain Pract 2013; 13: 504–513 [DOI] [PubMed] [Google Scholar]
- 45.Carrero JJ, Hecking M, Chesnaye NCet al. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 2018; 14: 151–164 [DOI] [PubMed] [Google Scholar]
- 46.Mauvais-Jarvis F, Berthold HK, Campesi Iet al. Sex- and gender-based pharmacological response to drugs. Pharmacol Rev 2021; 73: 730–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chertow GM, Lee J, Kuperman GJet al. Guided medication dosing for inpatients with renal insufficiency. JAMA 2001; 286: 2839–2844 [DOI] [PubMed] [Google Scholar]
- 48.Ong SW, Jassal SV, Porter ECet al. Digital applications targeting medication safety in ambulatory high-risk CKD patients: randomized controlled clinical trial. Clin J Am Soc Nephrol 2021; 16: 532–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.