Abstract
The in vitro activities of two new ketolides, HMR 3647 and HMR 3004, were tested by the agar dilution method against 280 strains of gram-positive bacteria with different antibiotic susceptibility profiles, including Staphylococcus aureus, Enterococcus faecalis, Enterococcus faecium, Streptococcus spp. (group A streptococci, group B streptococci, Streptococcus pneumoniae, and alpha-hemolytic streptococci). Seventeen erythromycin-susceptible (Ems), methicillin-susceptible S. aureus strains were found to have HMR 3647 and HMR 3004 MICs 4- to 16-fold lower than those of erythromycin (MIC at which 50% of isolates were inhibited [MIC50] [HMR 3647 and HMR 3004], 0.03 μg/ml; range, 0.03 to 0.06 μg/ml; MIC50 [erythromycin], 0.25 μg/ml; range, 0.25 to 0.5 μg/ml). All methicillin-resistant S. aureus strains tested were resistant to erythromycin and had HMR 3647 and HMR 3004 MICs of >64 μg/ml. The ketolides were slightly more active against E. faecalis than against E. faecium, and MICs for individual strains varied with erythromycin susceptibility. The MIC50s of HMR 3647 and HMR 3004 against Ems enterococci (MIC ≤ 0.5 μg/ml) and those enterococcal isolates with erythromycin MICs of 1 to 16 μg/ml were 0.015 μg/ml. E. faecalis strains that had erythromycin MICs of 128 to >512 μg/ml showed HMR 3647 MICs in the range of 0.03 to 16 μg/ml and HMR 3004 MICs in the range of 0.03 to 64 μg/ml. In the group of E. faecium strains for which MICs of erythromycin were ≥512 μg/ml, MICs of both ketolides were in the range of 1 to 64 μg/ml, with almost all isolates showing ketolide MICs of ≤16 μg/ml. The ketolides were also more active than erythromycin against group A streptococci, group B streptococci, S. pneumoniae, rhodococci, leuconostocs, pediococci, lactobacilli, and diphtheroids. Time-kill studies showed bactericidal activity against one strain of S. aureus among the four strains tested. The increased activity of ketolides against gram-positive bacteria suggests that further study of these agents for possible efficacy against infections caused by these bacteria is warranted.
Infection with gram-positive bacteria is increasingly important for two main reasons. First, the incidence of infection with gram-positive bacteria has increased over the last decade (4, 16, 17, 20). Second, bacteria such as staphylococci, enterococci, and Streptococcus pneumoniae have developed resistance to conventional antibiotics that have been used for decades. New antimicrobial agents with broader ranges of activity and/or greater potency against these organisms are needed. HMR 3647 (RU-66647) and HMR 3004 (RU-64004) are new antibiotics belonging to a novel class of antibiotics, the ketolides. The parent compound of the ketolides is erythromycin A, which is a 14-ring macrolide. The ketolides possess a 3-keto group in the macrolactone ring instead of the l-cladinose moiety of erythromycin A (1). HMR 3647 has a carbamate group linked to an imidazolium and pyridinium nucleus at C11–12 (5). HMR 3004 (RU-64004) has a quinoline side chain linked to the 11–12 position of the 3-keto-6-methoxy erythromycin A skeleton (RU-006) (2). The mechanism of action of HMR 3647 and HMR 3004 appears to be similar to that of erythromycin A in that the drugs bind to the 50S ribosomal subunit leading to inhibition of protein synthesis in bacteria (6, 7). HMR 3647 has been shown to have antimicrobial activity similar to clindamycin (7). Previously published data indicate that HMR 3647 is more potent than other macrolide-lincosamide-streptogramin B (MLSB) compounds against vancomycin-resistant enterococci and other gram-positive bacteria, but no direct comparison of activities of the two agents against enterococci was made (5, 7). Information regarding kill kinetics of HMR 3647 against enterococci and staphylococci is also limited (3).
In this study, we compared the in vitro activities of HMR 3647 and HMR 3004 with those of erythromycin, clindamycin, and quinupristin-dalfopristin against gram-positive bacteria with different susceptibility profiles, for instance, gentamicin-resistant enterococci, beta-lactamase-producing enterococci, and vancomycin-resistant enterococci. Time-kill studies were performed to determine if these compounds had bactericidal activities against methicillin-susceptible Staphylococcus aureus (MSSA), methicillin-resistant S. aureus (MRSA), and vancomycin-susceptible and vancomycin-resistant enterococci.
MATERIALS AND METHODS
Antibiotics.
HMR 3647 and HMR 3004 were provided by Roussel UCLAF, Romainville, France; clindamycin was obtained from Upjohn Laboratories, Kalamazoo, Mich.; erythromycin was obtained from Sigma, St. Louis, Mo.; and quinupristin-dalfopristin was obtained from Rhône-Poulenc Rorer, Antony Cedex, France. Reconstitution, storage, and dilution of these antibiotics were performed according to the manufacturer’s recommendations.
Bacterial strains.
Two hundred eighty isolates of gram-positive bacteria, including MSSA, MRSA, Streptococcus species, Enterococcus species, Rhodococcus species, Pediococcus species, and Leuconostoc species, were tested. These bacteria either were collected from clinical specimens of various sources or were community fecal isolates. They were collected from the United States, Chile, Argentina, Belgium, and Thailand between 1980 and 1997; duplicate isolates from single patients were excluded, and many of the enterococci had been previously classified as distinct strains by pulsed-field gel electrophoresis or PCR (10, 12, 19). The streptococcal isolates included group A streptococci, group B streptococci, alpha-hemolytic streptococci, and S. pneumoniae. Some of the group A streptococci and S. pneumoniae were provided by Kenneth V. I. Rolston, Anderson Cancer Center, Houston, Tex. These bacteria were chosen based on varied antibiotic susceptibility profiles. The enterococcal isolates consisted of beta-lactamase-producing strains, isolates with high-level resistance to gentamicin, and vancomycin-resistant (Vmr) strains. The genotypes of 31 Enterococcus faecium isolates in the Vmr group were vanA, and 13 were vanB. Among Vmr Enterococcus faecalis isolates, four were vanA genotype and nine were vanB genotype.
Susceptibility testing.
MICs were determined by the agar dilution method according to National Committee for Clinical Laboratory Standards (NCCLS) guidelines for antimicrobial susceptibility testing (15). The agar was prepared with Mueller-Hinton (MH) agar II (Becton Dickinson and Company, Cockeysville, Md.). Escherichia coli ATCC 25922, E. faecalis ATCC 29212, Pseudomonas aeruginosa ATCC 27853, S. aureus ATCC 29213, and S. pneumoniae ATCC 49619 were used as control strains. The susceptibility breakpoints of erythromycin were defined according to NCCLS guidelines; erythromycin MICs of ≥8 μg/ml were considered resistant, and MICs of ≤0.5 μg/ml were considered susceptible for nonstreptococcal species. For Streptococcus spp., erythromycin MICs of ≥1 μg/ml and ≤0.25 μg/ml were considered resistant and susceptible, respectively (14). Isolates for which MICs were between these two values were grouped as resistant isolates for analysis of ketolide MICs according to erythromycin susceptibility. Time-kill studies were performed according to the methods described by Moellering et al. and NCCLS guidelines (11, 13). The experiments were performed with inocula of 105 CFU/ml and a concentration of two to four times the MIC of an antibiotic to determine if there was any bactericidal activity of HMR 3647 and HMR 3004 compared to other antibiotics. Twenty-five microliters of bacterium-antibiotic mixture was taken from the flask for culture on a brain heart infusion (BHI) agar plate at 0, 4, and 24 h in duplicate. Antibiotic carryover effect was removed by centrifuging 1-ml aliquots of cells from the flask at 24 h and washing once with 0.9% NaCl. Serial 10-fold dilutions were made, and 25 μl of each dilution was put on a BHI agar plate in duplicate. A decrease in CFU by ≥3 log10 CFU/ml was defined as the cutoff point for bactericidal effects.
RESULTS AND DISCUSSION
The MICs at which 50% of isolates are inhibited (MIC50s), MIC90s, ranges of MICs, and percentages of isolates inhibited by each antibiotic at a concentration of ≤0.5 μg/ml are shown in Table 1. Among the 19 MSSA, two isolates were resistant to erythromycin, with MICs of >512 μg/ml. One of these showed an inducible MLSB (iMLSB) phenotype, as determined by placement of an erythromycin disk adjacent to a clindamycin disk on an agar plate (8). Another isolate was resistant to erythromycin and clindamycin, with an erythromycin MIC of >512 μg/ml and a clindamycin MIC of >256 μg/ml (constitutive MLSB phenotype). The iMLSB isolate had HMR 3647 and HMR 3004 MICs of 0.03 μg/ml; in contrast, the isolate which was highly resistant to both erythromycin and clindamycin had HMR 3647 and HMR 3004 MICs of >32 and 8 μg/ml, respectively. All erythromycin-susceptible MSSA were inhibited by HMR 3647 at a concentration of 0.03 to 0.06 μg/ml and by HMR 3004 at a concentration of 0.03 to 0.12 μg/ml, which were lower than the MICs of erythromycin by 8- to 16-fold. Both ketolides were also more potent than clindamycin and quinupristin-dalfopristin against MSSA, showing MICs lower than those of clindamycin and quinupristin-dalfopristin by 2- to 4-fold and 8- to 32-fold, respectively. MICs of HMR 3647 for MSSA were in the same range as MICs of HMR 3004. Time-kill studies were performed against three isolates of MSSA for HMR 3647 and four isolates for HMR 3004. One of these isolates was the iMLSB strain, and three were erythromycin susceptible. Although the macrolides are considered bacteriostatic agents, one isolate was very susceptible to HMR 3647, showing a decrease of CFU by 3.83 log10 CFU/ml at 24 h. This isolate was also killed by HMR 3004, having a decrease in CFU of 3.37 log10 CFU/ml at 24 h. We obtained the same result in a repeat experiment. Another isolate showed a decrease of 3 log10 CFU/ml when tested against HMR 3004, while a decrease of 2.54 log10 CFU/ml with HMR 3647 was seen. Both ketolides showed bacteriostatic effects against the remaining isolates (Table 2 and Fig. 1). In general, the ketolides had similar activities against MSSA and were superior to the compared drugs. All MRSA in our study were highly resistant to erythromycin and clindamycin and are likely constitutive MLSB producers, as MICs for each isolate were >512 μg/ml and >256 μg/ml, respectively. They were not inhibited by either ketolide at a concentration of ≤64 μg/ml.
TABLE 1.
MIC50s and MIC90s of HMR 3004, HMR 3647, and other drugs
| Strain type (n) | Drug | MIC (μg/ml)
|
% Inhibited by ≤0.5 μg/ml | ||
|---|---|---|---|---|---|
| 50% | 90% | Range | |||
| MSSA, total (19) | HMR 3004 | 0.06 | 0.12 | 0.03–8 | 95 |
| HMR 3647 | 0.03 | 0.06 | 0.03–>32 | 95 | |
| Erythromycin | 0.25 | >512 | 0.25–>512 | 89 | |
| Clindamycin | 0.12 | >256 | 0.12–>256 | 84 | |
| Quinupristin-dalfopristin | 0.5 | 1 | 0.25–1 | 89 | |
| MSSA Ems (17) | HMR 3004 | 0.06 | 0.06 | 0.03–0.12 | 100 |
| HMR 3647 | 0.03 | 0.06 | 0.03–0.06 | 100 | |
| Erythromycin | 0.25 | 0.25 | 0.25–0.5 | 100 | |
| Clindamycin | 0.12 | 1 | 0.12–>256 | 88 | |
| Quinupristin-dalfopristin | 0.5 | 1 | 0.25–1 | 88 | |
| MSSA Emr (2) | HMR 3004 | 0.03–8 | |||
| HMR 3647 | 0.03–>32 | ||||
| Erythromycin | >512 | ||||
| Clindamycin | 0.12 | ||||
| Quinupristin-dalfopristin | 0.25–0.5 | ||||
| MRSA Emr (15) | HMR 3004 | >64 | >64 | >64 | 0 |
| HMR 3647 | >64 | >64 | 32–>64 | 0 | |
| Erythromycin | >512 | >512 | >512 | 0 | |
| Clindamycin | >256 | >256 | >256 | 0 | |
| Quinupristin-dalfopristin | 1 | 1 | 0.5–1 | 13 | |
| E. faecalis Vms, total (82) | HMR 3004 | 0.5 | 8 | 0.007–64 | 50 |
| HMR 3647 | 0.25 | 4 | 0.015–8 | 56 | |
| Erythromycin | >512 | >512 | 0.06–>512 | 21 | |
| Clindamycin | >256 | >256 | 16–>256 | 0 | |
| Quinupristin-dalfopristin | 16 | 64 | 2–64 | 0 | |
| E. faecalis Vms Ems (17) | HMR 3004 | 0.015 | 0.015 | 0.015 | 100 |
| HMR 3647 | 0.015 | 0.015 | 0.015–0.03 | 100 | |
| Erythromycin | 0.06 | 0.5 | 0.06–0.5 | 100 | |
| Clindamycin | 32 | 32 | 16–32 | 0 | |
| Quinupristin-dalfopristin | 16 | 16 | 2–16 | 0 | |
| E. faecalis Vms Emr (65) | HMR 3004 | 2 | 16 | 0.007–64 | 37 |
| HMR 3647 | 1 | 4 | 0.015–8 | 45 | |
| Erythromycin | >512 | >512 | 1–>512 | 0 | |
| Clindamycin | >256 | >256 | 16–>256 | 0 | |
| Quinupristin-dalfopristin | 32 | 64 | 2–>64 | 0 | |
| E. faecalis Vmr (13) | HMR 3004 | 8 | 16 | 0.015–16 | 15 |
| HMR 3647 | 4 | 16 | 0.015–16 | 15 | |
| Erythromycin | >512 | >512 | 0.25–>512 | 8 | |
| Clindamycin | >256 | >256 | 16–>256 | 0 | |
| Quinupristin-dalfopristin | 32 | 64 | 4–>64 | 0 | |
| E. faecium Vms, total (25) | HMR 3004 | 4 | 16 | 0.015–16 | 40 |
| HMR 3647 | 4 | 16 | 0.015–16 | 40 | |
| Erythromycin | >512 | >512 | 0.12–>512 | 8 | |
| Clindamycin | >256 | >256 | 0.12–>256 | 16 | |
| Quinupristin-dalfopristin | 1 | 8 | 0.5–16 | 8 | |
| E. faecium Vms Ems (2) | HMR 3004 | 0.015 | |||
| HMR 3647 | 0.015–0.03 | ||||
| Erythromycin | 0.12–0.5 | ||||
| Clindamycin | 0.12–16 | ||||
| Quinupristin-dalfopristin | 0.5–8 | ||||
| E. faecium Vms Emr (23) | HMR 3004 | 8 | 16 | 0.015–16 | 35 |
| HMR 3647 | 4 | 16 | 0.015–16 | 35 | |
| Erythromycin | >512 | >512 | 1–>512 | 0 | |
| Clindamycin | >256 | >256 | 0.12–>256 | 13 | |
| Quinupristin-dalfopristin | 1 | 8 | 0.5–16 | 4 | |
| E. faecium Vmr Emr (44) | HMR 3004 | 8 | 16 | 0.007–16 | 16 |
| HMR 3647 | 8 | 16 | 0.015–16 | 16 | |
| Erythromycin | >512 | >512 | 2–>512 | 0 | |
| Clindamycin | >256 | >256 | 0.06–>256 | 11 | |
| Quinupristin-dalfopristin | 1 | 8 | 0.5–16 | 9 | |
| Group A streptococci (16) | HMR 3004 | 0.03 | 0.25 | 0.003–64 | 94 |
| HMR 3647 | 0.06 | 0.12 | 0.007–>64 | 94 | |
| Erythromycin | 0.25 | 0.5 | 0.03–>512 | 94 | |
| Clindamycin | 0.12 | 0.25 | 0.06–>256 | 94 | |
| Quinupristin-dalfopristin | 0.5 | 1 | 0.12–2 | 63 | |
| Group B streptococci (10) | HMR 3004 | 0.03 | 0.03 | 0.03 | 100 |
| HMR 3647 | 0.03 | 0.06 | 0.03–0.06 | 100 | |
| Erythromycin | 0.12 | 0.25 | 0.12–0.25 | 100 | |
| Clindamycin | 0.12 | 0.12 | 0.12 | 100 | |
| Quinupristin-dalfopristin | 2 | 4 | 1–4 | 0 | |
| S. pneumoniae (14) | HMR 3004 | 0.03 | 0.12 | 0.003–0.25 | 100 |
| HMR 3647 | 0.03 | 0.5 | 0.003–0.5 | 100 | |
| Erythromycin | 0.25 | 4 | 0.03–8 | 64 | |
| Clindamycin | 0.06 | 0.12 | 0.015–0.25 | 100 | |
| Quinupristin-dalfopristin | 0.5 | 2 | 0.25–2 | 50 | |
| Alpha-hemolytic streptococci (10) | HMR 3004 | 0.03 | 2 | 0.015–2 | 60 |
| HMR 3647 | 0.03 | 0.5 | 0.015–0.5 | 100 | |
| Erythromycin | 0.5 | 512 | 0.06–>512 | 50 | |
| Clindamycin | 0.06 | 256 | 0.06–256 | 80 | |
| Quinupristin-dalfopristin | 2 | 4 | 1–4 | 0 | |
| Rhodococcus spp. (14) | HMR 3004 | 2 | 4 | 0.03–4 | 21 |
| HMR 3647 | 2 | 16 | 0.06–16 | 21 | |
| Erythromycin | 4 | 16 | 0.25–32 | 21 | |
| Clindamycin | 128 | >256 | 0.25–>256 | 7 | |
| Quinupristin-dalfopristin | 64 | 128 | 2–128 | 0 | |
| Leuconostoc spp. (7) | HMR 3004 | 0.015–2 | |||
| HMR 3647 | 0.03 | ||||
| Erythromycin | 0.12–>512 | ||||
| Clindamycin | 0.015–0.06 | ||||
| Quinupristin-dalfopristin | 2–4 | ||||
| Pediococcus spp. (6) | HMR 3004 | 0.015–0.03 | |||
| HMR 3647 | 0.03 | ||||
| Erythromycin | 0.12 | ||||
| Clindamycin | 0.03–0.25 | ||||
| Quinupristin-dalfopristin | 4–16 | ||||
| Diphtheroids (2) | HMR 3004 | 0.06 | |||
| HMR 3647 | 0.25 | ||||
| Erythromycin | 0.5–>512 | ||||
| Clindamycin | 1–8 | ||||
| Quinupristin-dalfopristin | 2–32 | ||||
| Lactobacillus spp. (3) | HMR 3004 | 0.015–0.03 | |||
| HMR 3647 | <0.003–0.03 | ||||
| Erythromycin | 0.25 | ||||
| Clindamycin | 0.03–0.12 | ||||
| Quinupristin-dalfopristin | 2–4 | ||||
TABLE 2.
Comparative killing of enterococcal and staphylococcal isolates by HMR 3004 and HMR 3647 in time-kill studies
| Strain | MIC (μg/ml)a
|
Change from baseline (log10 CFU/ml) for indicated drug atb:
|
Drug | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HMR 3004 | HMR 3647 | Ampicillin | Nafcillin | Erythromycin | Quinupristin-dalfopristin | 4 h | 24 h | ||
| E. faecalis HG1100 | 0.015 | 0.015 | 2.0 | 8 | 1 | 64 | +0.37 | +0.05 | HMR 3004 |
| (0.06) | (8.0) | +1.18 | −1.99 | Ampicillin | |||||
| E. faecalis BE44 | 0.015 | 0.015 | 1.0 | 8 | 0.25 | 16 | +0.04 | −0.30 | HMR 3004 |
| (0.06) | (0.06) | (4.0) | −0.11 | −0.2 | HMR 3647 | ||||
| −2.53 | −2.58 | Ampicillin | |||||||
| E. faecium TX2466 | 0.007 | 0.015 | 512 | >256 | 2 | 0.5 | +0.59 | −0.57 | HMR 3004 |
| (0.03) | (0.06) | (2.0) | −0.52 | −1.29 | HMR 3647 | ||||
| −0.66 | −3.3 | Quinupristin-dalfopristin | |||||||
| MSSA JT9 | 0.06 | 0.06 | 2 | 0.25 | 0.25 | 1 | −1.41 | −3.37 | HMR 3004 |
| (0.25) | (0.25) | (1.0) | (1.0) | −1.47 | −3.83 | HMR 3647 | |||
| −1.45 | −1.07 | Nafcillin | |||||||
| −1.71 | −3.30 | Erythromycin | |||||||
| MSSA JT22c | 0.06 | 0.03 | NTd | 0.25 | 0.25 | 0.5 | −0.51 | −0.65 | HMR 3004 |
| (0.25) | (0.12) | (1.0) | (1.0) | −0.26 | −0.89 | HMR 3647 | |||
| −0.5 | −1.39 | Erythromycin | |||||||
| −0.52 | −0.41 | Nafcillin | |||||||
| −0.53 | −1.23 | Clindamycin | |||||||
| MSSA JT10 | 0.06 | 0.06 | NT | 0.25 | 0.25 | NT | −1.3 | −3.96 | HMR 3004 |
| (0.25) | (0.25) | (1.0) | (1.0) | −1.19 | −2.54 | HMR 3647 | |||
| −0.58 | −1.28 | Erythromycin | |||||||
| −0.79 | +0.18 | Nafcillin | |||||||
| MSSA JT4 | 0.03 | 0.03 | NT | 0.25 | >512 | 512 | −1.09 | −1.85 | HMR 3004 |
| (0.12) | (0.12) | (1.0) | −1.25 | −1.07 | HMR 3647 | ||||
| −0.86 | +1.04 | Nafcillin | |||||||
Values in parentheses are concentrations used for time-kill experiments.
Starting inoculum was approximately 5 log10 CFU.
MIC of clindamycin for MSSA JT22 was 0.12 μg/ml, and the concentration used in the time-kill experiment was 0.5 μg/ml.
NT, not tested.
FIG. 1.
Time-kill curves of HMR 3647 against S. aureus isolates. Concentrations of HMR 3647 for the time-kill of MSSA JT9, MSSA JT22, and MSSA JT10 (4× MICs) were 0.25, 0.12, and 0.25 μg/ml, respectively.
Because we did not see a bactericidal effect of nafcillin, a potent antistaphylococcal antibiotic, against any of the MSSA tested, the MICs of nafcillin for those isolates were determined by broth microdilution in MH broth according to NCCLS guidelines (15). The MICs in BHI broth were determined simultaneously to simulate the conditions in time-kill studies. For MSSA JT9, the MIC of nafcillin in BHI broth was 0.12 μg/ml. For the remaining isolates, the MIC was 0.25 μg/ml. In MH broth, the MIC for all isolates was 0.25 μg/ml. Then, another time-kill experiment was performed in duplicate, with nafcillin (1 μg/ml) in BHI broth. The changes in colony counts from baseline determined at 24 h of incubation were −0.5 to −1.0 CFU/ml, which were in the same range as the original experiment.
Of 82 vancomycin-susceptible (Vms) E. faecalis isolates, 17 were susceptible to erythromycin, 14 had erythromycin MICs in the intermediate range (1 to 4 μg/ml), and 51 were resistant to erythromycin. All erythromycin-susceptible isolates were inhibited by the ketolides at concentrations of 0.007 to 0.03 μg/ml, which were 4- to 64-fold lower than the MICs of erythromycin. All 14 isolates with intermediate susceptibility to erythromycin had HMR 3647 MICs of 0.015 μg/ml and HMR 3004 MICs of 0.007 to 0.06 μg/ml, except for one isolate which showed an HMR 3004 MIC of 2 μg/ml. For erythromycin-resistant (Emr) isolates, erythromycin MICs were in the range of 128 to >512 μg/ml, while HMR 3647 MICs ranged from 0.03 to 8 μg/ml and HMR 3004 MICs ranged from 0.03 to 64 μg/ml. The MIC50 and MIC90 of HMR 3647 for Emr isolates were 2 and 8 μg/ml, respectively; the MIC50 and MIC90 of HMR 3004 were 4 and 64 μg/ml, respectively. MICs of the two ketolides for beta-lactamase-producing and gentamicin-resistant E. faecalis showed the same trend as for isolates that do not have these properties.
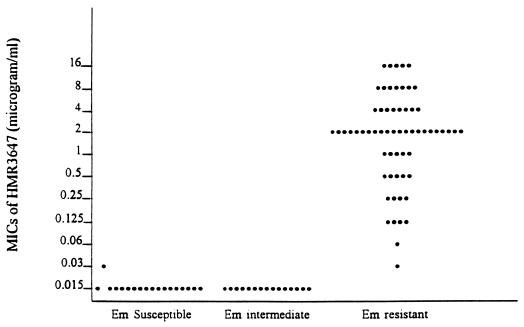
One of 13 Vmr E. faecalis isolates was susceptible to erythromycin, which showed a MIC of 0.25 μg/ml; this isolate had an HMR 3647 MIC of 0.015 μg/ml and an HMR 3004 MIC of 0.03 μg/ml. Another isolate had an erythromycin MIC of 1 μg/ml; for this isolate, MICs of both ketolides were 0.015 μg/ml. The remaining 11 isolates were erythromycin resistant, with MICs of erythromycin ranging from 256 to >512 μg/ml; MICs of both ketolides ranged from 2 to 16 μg/ml. The relationship of ketolide and erythromycin MICs for all E. faecalis isolates is shown in Fig. 2.
FIG. 2.
MICs of HMR 3647 for E. faecalis in relation to erythromycin (Em) susceptibility. The MICs for erythromycin-susceptible isolates were ≤0.5 μg/ml, those for erythromycin-intermediate isolates were 1 to 4 μg/ml, and those for erythromycin-resistant isolates were 128 to >512 μg/ml. For no isolates were erythromycin MICs 8 to 64 μg/ml.
A similar trend was found when the MICs of ketolides against E. faecium were compared to those of erythromycin. Overall, 69 isolates of E. faecium (25 Vms, 44 Vmr) were tested. None of the Vmr E. faecium isolates were susceptible to erythromycin. Three Vms isolates and six Vmr isolates had MICs of erythromycin in the intermediate range (1 to 4 μg/ml). MICs of HMR 3647 against these isolates were 0.015 to 0.12 μg/ml, with only one isolate showing a MIC of 0.12 μg/ml. MICs of HMR 3004 were 0.007 to 0.5 μg/ml, with most isolates showing MICs of 0.015 μg/ml. Three isolates of Vms and one isolate of Vmr E. faecium with moderate-level resistance to erythromycin (erythromycin MICs, 8 to 16 μg/ml) were inhibited by both ketolides at the concentrations 0.015 to 0.03 μg/ml. Two erythromycin-resistant (MICs, 16 μg/ml) Vms isolates were susceptible to clindamycin, and five Vmr isolates showed intermediate erythromycin MICs but were susceptible to clindamycin, suggestive of the iMLSB phenotype. These isolates were all inhibited by ketolides at concentrations of 0.015 to 0.06 μg/ml. Among the 25 Vms E. faecium isolates, 40% were inhibited by both ketolides at a concentration of ≤0.03 μg/ml. MICs of HMR 3647 for the two isolates of Vms E. faecium that were susceptible to erythromycin were 0.015 and 0.03 μg/ml, lower than the erythromycin MIC by 4- to 32-fold; MICs of HMR 3004 for these two isolates were 0.015 μg/ml, lower than the erythromycin MICs by 8- to 32-fold. Subgroup analysis of both Vmr and Vms E. faecium isolates revealed a pattern similar to that seen with E. faecalis. That is, isolates which showed erythromycin MICs of ≥512 μg/ml had ketolide MICs ranging from 1 to 64 μg/ml, with a MIC90 of 16 μg/ml for both ketolides. On the other hand, isolates which had erythromycin MICs of ≤16 μg/ml had ketolide MICs ranging from 0.007 to 0.5 μg/ml.
Among the 13 Vmr E. faecalis isolates, 4 vanA and 9 vanB isolates showed MICs of HMR 3647 in the range of 0.015 to 8 and 0.015 to 16 μg/ml, respectively. MICs of HMR 3004 for vanA and vanB E. faecalis isolates were the same as those of HMR 3647. Among the 44 Vmr E. faecium isolates, 31 vanA and 13 vanB isolates showed a MIC50 and MIC90 of HMR 3647 of 8 and 16 μg/ml (range, 0.015 to 64 μg/ml, with only one isolate of the vanB genotype showing an HMR 3647 MIC of 64 μg/ml). The MICs of HMR 3004 for vanA and vanB E. faecium isolates were approximately the same as those of HMR 3647.
Time-kill studies of HMR 3647 against one erythromycin-susceptible and one erythromycin-resistant E. faecalis isolate revealed a minimal to modest decrease in CFU at 24 h (1.99 and 0.2 log10 CFU/ml [Table 2]). Similar results were observed with HMR 3004, with changes in CFU of 0.05 and −0.30 log10 at 24 h. Against one Vmr E. faecium isolate, both compounds also showed a bacteriostatic effect. There was a decrease of 3.3 log10 CFU/ml for this isolate when tested with quinupristin-dalfopristin. No time-kill study of quinupristin-dalfopristin against E. faecalis was performed, because this species is usually resistant to quinupristin-dalfopristin (9).
One of the 16 group A streptococci was resistant to ketolides, erythromycin, and clindamycin but susceptible to quinupristin-dalfopristin, with a MIC of quinupristin-dalfopristin of 0.25 μg/ml. MICs of HMR 3647, HMR 3004, erythromycin, and clindamycin for this isolate were 64, >64, >512, and >256 μg/ml, respectively, indicative of the constitutive MLSB phenotype. Almost all (15 of 16, 94%) were susceptible to ketolides, erythromycin, clindamycin, and quinupristin-dalfopristin. For the susceptible isolates, MICs of HMR 3647 were 0.007 to 0.12 μg/ml, lower than those of erythromycin by 2- to 8-fold; the MICs of HMR 3004 were 0.003 to 0.25 μg/ml, lower than those of erythromycin by 2- to 16-fold.
All group B streptococci were inhibited by ≤0.03 μg/ml of ketolides, erythromycin, and clindamycin, but none were inhibited by this concentration of quinupristin-dalfopristin (Table 1). Ketolides were approximately four- to eightfold more potent than erythromycin against group B streptococci.
All S. pneumoniae isolates had ketolide MICs lower than those of erythromycin by 4- to 16-fold. The majority of S. pneumoniae isolates in our study (9 of 14) were susceptible to erythromycin, with MICs of HMR 3647 and HMR 3004 ranging from 0.003 to 0.03 μg/ml. The remaining five isolates had erythromycin MICs of 2 to 8 μg/ml. These five isolates were susceptible to clindamycin (MICs, 0.015 to 0.25 μg/ml) and likely express either an efflux system (15) or the inducible MLSB phenotype. The MICs of HMR 3647 for these five isolates ranged from 0.003 to 0.5 μg/ml.
HMR 3647 was the most active agent among the antibiotics tested against alpha-hemolytic streptococci. All 10 isolates were inhibited by HMR 3647 at a concentration of ≤0.5 μg/ml, with a MIC50 of 0.03 μg/ml. With the breakpoint of 0.5 μg/ml, 60, 50, and 80% of isolates were susceptible to HMR 3004, erythromycin, and clindamycin, respectively. The Ems isolates were all inhibited by HMR 3647 and HMR 3004 at concentrations of 0.015 to 0.03 μg/ml. Five of 10 isolates were resistant to quinupristin-dalfopristin and showed MICs of 4 μg/ml, four showed MICs of 2 μg/ml, and one showed a MIC of 1 μg/ml.
Twenty-one percent of Rhodococcus species in this study were susceptible to erythromycin. These isolates had HMR 3647 MICs lower than those of erythromycin by two- to fourfold. MICs of HMR 3004 were lower than those of erythromycin by four- to eightfold. Erythromycin-resistant isolates had ketolide MICs in the range of 2 to 16 μg/ml. All Leuconostoc spp. were inhibited by HMR 3647 at a concentration of 0.03 μg/ml, while four of seven had HMR 3004 MICs of 2 μg/ml and three of seven had HMR 3004 MICs of 0.015 to 0.03 μg/ml. MICs of HMR 3647 were lower than those of erythromycin by four- to eightfold. HMR 3647 and HMR 3004 showed similar activities against pediococci, diphtheroids, and lactobacilli. Both compounds were more active than erythromycin, showing lower MICs by 4- to 64-fold.
Conclusions.
In summary, HMR 3647 and HMR 3004 have similar activities against gram-positive bacteria. Both compounds were more potent than erythromycin against almost all species, except rhodococci, for which these three agents were similar. The MICs of ketolides tended to vary with the MIC of erythromycin. Isolates which showed high erythromycin MICs were more likely to have increased ketolide MICs as well. Strains with the inducible MLSB phenotype of S. aureus were susceptible to ketolides, while constitutive MLSB strains were resistant to ketolides. Some erythromycin-resistant enterococci were inhibited by low concentrations of ketolides. The five S. pneumoniae strains with reduced susceptibility to erythromycin were susceptible to ketolides at a concentration of ≤0.5 μg/ml. Ketolides showed bactericidal activities against some S. aureus isolates, as determined by time-kill studies. Therefore, ketolides might be useful agents in the treatment of gram-positive bacterial infections when the infecting organisms are susceptible to these compounds. Further in vivo studies of these compounds appear warranted.
ACKNOWLEDGMENTS
This work was supported by a grant from Roussel UCLAF.
We thank Upjohn Laboratories and Rhône-Poulenc Rorer for antibiotics and Kenneth V. I. Rolston for pneumococcal strains.
REFERENCES
- 1.Agouridas C, Benedetti Y, Denis A, Fromentin C, Gouin D’Ambrieres S, Le Martret O, Chantot J F. Abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. Ketolides: a new distinct semi-synthetic class of macrolides, abstr. F164; p. 227. [Google Scholar]
- 2.Agouridas C, Benedetti Y, Denis A, Le Martret O, Chantot J F. Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Ketolides: a new distinct class of macrolide antibacterials—synthesis and structural characteristics of RU 004, abstr. F157; p. 140. [Google Scholar]
- 3.Boswell F J, Andrews J M, Wise R. Pharmacodynamic properties of HMR 3647, a novel ketolide, on respiratory pathogens, enterococci and Bacteroides fragilis demonstrated by studies of time-kill kinetics and postantibiotic effect. J Antimicrob Chemother. 1998;41:149–153. doi: 10.1093/jac/41.2.149. [DOI] [PubMed] [Google Scholar]
- 4.Cormican M G, Jones R N. Emerging resistance to antimicrobial agents in gram-positive bacteria. Drugs. 1996;51(Suppl.):6–12. doi: 10.2165/00003495-199600511-00004. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein E J C, Citron D M, Gerado S H, Hudspeth M, Merriam C V. Activities of HMR 3004 (RU 64004) and HMR 3647 (RU 66647) compared to those of erythromycin, clarithromycin, roxithromycin, and eight other antimicrobial agents against unusual aerobic and anaerobic human and animal bite pathogens isolated from skin and soft tissue infections in humans. Antimicrob Agents Chemother. 1998;42:1127–1132. doi: 10.1128/aac.42.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamjian C, Biedenbach D J, Jones R N. In vitro evaluation of a novel ketolide antimicrobial agent, RU-64004. Antimicrob Agents Chemother. 1997;41:454–459. doi: 10.1128/aac.41.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones R N, Biedenbach D J. Antimicrobial activity of RU-66647, a new ketolide. Diagn Microbiol Infect Dis. 1997;27:7–12. doi: 10.1016/s0732-8893(96)00181-2. [DOI] [PubMed] [Google Scholar]
- 8.Leclercq R, Courvalin P. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother. 1991;35:1267–1272. doi: 10.1128/aac.35.7.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Low D E. Quinupristin/dalfopristin: spectrum of activity, pharmacokinetics, and initial clinical experience. Microb Drug Resist. 1995;1:223–234. doi: 10.1089/mdr.1995.1.223. [DOI] [PubMed] [Google Scholar]
- 10.Malathum K, Singh K V, Weinstock G M, Murray B E. Repetitive sequence-based PCR versus pulsed-field gel electrophoresis for typing of Enterococcus faecalis at the subspecies level. J Clin Microbiol. 1998;36:211–215. doi: 10.1128/jcm.36.1.211-215.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moellering R C, Jr, Wennersten C, Weinberg A N. Studies on antibiotic synergism against enterococci. I. Bacteriologic studies. J Lab Clin Med. 1971;77:821–828. [PubMed] [Google Scholar]
- 12.Murray B E, Singh K V, Heath J D, Sharma B R, Weinstock G M. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol. 1990;28:2059–2063. doi: 10.1128/jcm.28.9.2059-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. Methods for determining bactericidal activity of antimicrobial agents. NCCLS document M26-T. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility test, 6th ed. Approved standard M2-A6. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 16.Pittet D, Wenzel R P. Nosocomial bloodstream infections: secular trends in rates, mortality, and contribution to hospital deaths. Arch Intern Med. 1995;155:1177–1184. doi: 10.1001/archinte.155.11.1177. [DOI] [PubMed] [Google Scholar]
- 17.Schaberg D R, Culver D H, Gaynes R P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;91(Suppl. 3B):72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 18.Sutcliffe J, Tait-Kamradt A, Wondrack L. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob Agents Chemother. 1996;40:1817–1824. doi: 10.1128/aac.40.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomayko J F, Murray B E. Analysis of Enterococcus faecalis isolates from intercontinental sources by multilocus enzyme electrophoresis and pulsed-field gel electrophoresis. J Clin Microbiol. 1995;33:2903–2907. doi: 10.1128/jcm.33.11.2903-2907.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinstein M P, Towns M L, Quartey S M, Mirrett S, Reimer L G, Parmigiani G, Reller L B. The clinical significance of positive blood culture in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24:584–602. doi: 10.1093/clind/24.4.584. [DOI] [PubMed] [Google Scholar]