Abstract
Background
Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) is significantly associated with increased mortality. The current study aimed to investigate the predictive ability of the prognostic nutritional index (PNI) in 30-day mortality among AECOPD patients admitted to the ICU.
Material/Methods
Clinical data were extracted from the Medical Information Mart for Intensive Care-III (MIMIC-III) database. Patients were divided into 3 groups according to the tertiles of PNI. Cox proportional hazard regressions were performed to assess the association between PNI and 30-day mortality. Subgroup analyses were performed to identify the consistency of the association. Receiver operator characteristic (ROC) curve analysis was performed to evaluate the predictive accuracy among PNI, serum albumin, neutrophil-to-lymphocyte (NLR), and platelet-to-lymphocyte ratio (PLR).
Results
A total of 494 AECOPD patients were included in this study. The mean age was 70.8±10.4 years old. Kaplan-Meier analysis showed ongoing divergence in rates of mortality among tertiles (p<0.001). After adjusting for confounders, high PNI tertile was an independent favorable predictor of 30-day mortality (HR=0.39; 95% CI, 0.19–0.80; p=0.011) compared to low tertile reference. Subgroup analysis showed that the predictive ability of PNI was especially suitable for patients aged >70 years and with mechanical ventilation. The cut-off value of PNI was 31.8 with sensitivity 62.3% and specificity 64.1%. The area under the ROC of PNI (0.642, 95% CI, 0.560 to 0.717) was better than that of serum albumin, NLR, and PLR.
Conclusions
PNI could serve as a simple and reliable prognostic biomarker for AECOPD patients in the ICU.
Keywords: Medical Informatics; Nutrition Assessment; Pulmonary Disease, Chronic Obstructive
Background
Chronic obstructive pulmonary disease (COPD) is a common chronic airway inflammation featuring persistent respiratory symptoms and airflow limitation [1]. It is a global epidemic respiratory disease and affects millions of people worldwide, estimated to become one of the major causes of death by 2040 [2]. Acute exacerbation of COPD (AECOPD) is significantly associated with poor outcome of patients with COPD. When AECOPD occurs, it is necessary to transfer the patient to the Intensive Care Unit (ICU) due to its high mortality [3]. Malnutrition is also common among patients with COPD, occurring in 10–45% of such patients [4]. Malnutrition significantly increases the risk of exacerbation, length of hospital stay, and costs [5]. In terms of the severity of AECOPD, early risk evaluation is critical, so it is important to find a biomarker that can reflect both the inflammation and nutritional status of the patients.
The prognostic nutritional index (PNI), a new prognostic score, is calculated by serum albumin concentration and peripheral total lymphocyte count. PNI was initially developed to evaluate preoperative nutritional conditions and surgical complications in patients with gastrointestinal cancers [6]. Recently, it has been described as an accurate and independent prognostic predictor in human cancers [7–10], chronic kidney and heart disease [11,12], and autoimmune disease [13,14].
However, to the best of our knowledge, no study has evaluated the predictive value of PNI in AECOPD patients. The aim of this study was to investigate the relationship between PNI and the prognosis of AECOPD patients in the ICU from the Medical Information Mart for Intensive Care-III (MIMIC-III) database and to make comparisons with serum albumin alone, neutrophil-to-lymphocyte (NLR) ratio, and platelet-to-lymphocyte (PLR) ratio.
Material and Methods
Data Source
We extracted the data of this retrospective study from the MIMIC-III version 1.4 (MIMIC-III v1.4) database. MIMIC-III is a large, open, and public database, containing more than 50 000 patients admitted to the ICU at Beth Israel Deaconess Medical Center from 2001 to 2012 [15]. Before getting access to the database, the “Protecting Human Research Participants” course of the National Institutes of Health must be completed. The establishment and use of this database were approved by the Institutional Review Boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. No informed consent was required since all the data were de-identified.
Study Population and Data Extraction
Patients with the diagnosis of AECOPD were selected from the database. The definition of AECOPD was determined using the AECOPD ICD-9-CM code 491.21. For patients with multiple hospitalizations, only the first hospitalization was enrolled. The exclusion criteria included: age<18 years, length of ICU stay <48 h, or missing data >10%.
Data were extracted from the MIMIC-III database through Structured Query Language [16]. Only the initial baseline characteristics and laboratory results after admission to ICU were used in analysis. The following variables were collected: age, sex, length of ICU and hospital stay, white blood cell (WBC) count, neutrophil count, lymphocyte count, platelet count, hemoglobin, serum albumin, alanine transaminase (ALT), aspartate transaminase (AST), pH, partial pressure of oxygen (PO2), partial pressure of carbon dioxide (PCO2), bicarbonate, serum sodium, serum potassium, serum calcium, serum creatinine (Scr), and serum blood urea nitrogen (BUN). Vital signs consisted of body temperature, mean atrial pressure (MAP), heart rate, and respiratory rate. The comorbidities included hypertension, diabetes mellitus (DM), coronary heart disease (CHD), chronic renal disease (CKD), and malignancy. Clinical severity scales, including Sequential Organ Failure Assessment (SOFA) score and Simplified Acute Physiology Score II (SAPS II), were also extracted. For missing variables, predictive mean matching was used to impute numeric features, and logistic regression was used for binary variables. PNI value was calculated with the following equation: 10× serum albumin (g/dL)+0.005× total lymphocyte count (mm3) [6].
The primary outcome was all-cause 30-day mortality. Secondary outcomes included length of ICU stay, length of hospital stay, mechanical ventilation (MV) rate, and renal replacement therapy (RRT) rate.
Statistical Analysis
Baseline characteristics of all patients were stratified by PNI tertiles. Continuous variables are presented as mean±standard deviation (SD) for normal distribution or medians and interquartile range (IQR) for skewed distribution. Comparisons were made by one-way ANOVA or Kruskal-Wallis H test, respectively. Categorical data are expressed as frequency (percentage) and were compared by chi-square test or Fisher’s exact test, as appropriate. Comparisons of cumulative events rate were conducted by log-rank test or Kaplan-Meier survival curve. The Cox proportional hazards model was used to obtain the hazard ratios (HRs) and it corresponding 95% confidence intervals (CIs) between the tertiles of PNI and 30-day mortality. We examined the proportional hazard assumption by testing the statistical significance of interactions between follow-up time and the tertiles of PNI, and found the assumption was correct. The crude model was not adjusted for any variable. Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, comorbidities (hypertension, DM, CAD, CKD, malignancy), laboratory tests (WBC, hemoglobin, platelet, albumin, ALT, Scr, pH, PO2, PCO2, bicarbonate, serum sodium, serum potassium, NLR, PLR), vital signs (MAP), and severity scales (SAPS II and SOFA). Additionally, subgroup analyses were performed to assess the possible modifications of the association between PNI and endpoints. Accuracies of PNI, serum albumin, NLR, and PLR in predicting 30-day mortality were evaluated by receiver operator characteristic (ROC) curves, and the optimal cut-off values were calculated by Youden index. All statistical data were analyzed using SPSS software (v21.0; IBM, Armonk, NY). A 2-tailed P<0.05 was considered statistically significant.
Results
Baseline Characteristics
According to the inclusion criteria, a total of 494 patients were finally included in our study. The flow chart of data selection is shown in Figure 1. The baseline characteristics of the study population are shown in Table 1. The mean age of the population was 70.8±10.4 years old. Among the included patients, 248 (50.2%) were male and 246 (49.8%) were female. There were 164, 165, and 165 patients in low tertile group (PNI ≤30.2), middle tertile group (PNI 30.2–36.2), and high tertile group (PNI >36.2), respectively. Patients in the low tertile group tended to have lower levels of lymphocyte count, hemoglobin, albumin, and MAP and had higher levels of neutrophil count, BUN, SAPSII, NLR, and PLR.
Figure 1.

Study flow chart.
Table 1.
Baseline and clinical characteristics of the study population.
| Characteristics | Low tertile (≤30.2, n=164) | Middle tertile (30.2–36.2, n=165) | High tertile (>36.2, n=165) | P value |
|---|---|---|---|---|
| Age, years (mean±SD) | 71.4±9.8 | 71.8±10.3 | 69.1±10.9 | 0.039 |
| Sex, n (%) | ||||
| Male | 84 (51.2%) | 79 (47.9%) | 85 (51.5%) | 0.776 |
| Female | 80 (48.8%) | 86 (52.1%) | 80 (48.5%) | 0.776 |
| Biochemistry, median (IQR) | ||||
| WBC count (103/μL) | 11.3 (8.5, 15.7) | 11.2 (7.9, 15.9) | 10.7 (7.8, 15.0) | 0.377 |
| Neutrophil count (103/μL) | 9.8 (7.0, 13.7) | 9.5 (6.1, 14.0) | 8.1 (5.7, 12.7) | <0.001 |
| Lymphocyte count (103/μL) | 0.7 (0.3, 1.2) | 1.0 (0.5, 1.7) | 1.2 (0.6, 1.9) | <0.001 |
| Platelet count (103/μL) | 229 (162, 307) | 245 (175, 311) | 238 (186, 300) | 0.508 |
| Hemoglobin (g/dL) | 10.4 (9.3, 11.5) | 10.9 (9.6, 12.4) | 11.3 (9.9, 12.7) | <0.001 |
| Serum albumin (g/dL) | 2.4 (2.63, 2.8) | 3.2 (3.1, 3.3) | 3.8 (3.6, 4.0) | <0.001 |
| Serum sodium (mmol/L) | 137 (134, 141) | 138 (134, 141) | 138.0 (135, 140) | 0.958 |
| Serum potassium (mmol/L) | 4.1 (3.5, 4.6) | 4.3 (3.7, 4.7) | 4.2 (3.8, 4.7) | 0.085 |
| Serum calcium (mmol/L) | 1.11 (1.06, 1.08) | 1.14 (1.06, 1.20) | 1.15 (1.09, 1.18) | 0.270 |
| pH | 7.33 (7.26, 7.41) | 7.33 (7.23, 7.39) | 7.34 (7.25, 7.39) | 0.434 |
| PO2 (mmHg) | 105 (72, 207) | 101.0 (74, 183) | 105.0 (74, 169) | 0.882 |
| PCO2 (mmHg) | 53 (43, 67) | 55.0 (46, 71) | 55.0 (45, 79) | 0.112 |
| Bicarbonate (mmol/L) | 28 (24, 32) | 28 (25,33) | 29 (25, 34) | 0.124 |
| ALT (IU/L) | 22 (15, 44)) | 26 (16, 41) | 21 (15, 35) | 0.208 |
| AST (IU/L) | 28 (17, 46) | 29 (19, 48) | 23 (17, 38) | 0.044 |
| Creatinine (mg/dL) | 1.0 (0.7, 1.4) | 1.0 (0.7, 1.3) | 1.0 (0.75, 1.4) | 0.790 |
| BUN (mmol/L) | 28 (20, 41) | 24 (17, 37) | 23 (18, 34) | 0.045 |
| Vital signs, median (IQR) | ||||
| Temperature (°C) | 36.7 (36.3, 37.1) | 36.7 (36.3, 37.1) | 36.7 (36.3, 37.0) | 0.533 |
| MAP (mmHg) | 74 (68, 84) | 79 (71, 85) | 78 (73, 86) | 0.011 |
| Heart rate (min−1) | 92 (81, 101) | 88 (77, 97) | 88 (80, 99) | 0.136 |
| Respiratory rate (min−1) | 20 (17, 23) | 21 (17, 23) | 20 (18, 23) | 0.873 |
| Inflammatory indicators, median (IQR) | ||||
| NLR | 14.7 (7.4, 29.3) | 8.9 (5.4, 14.3) | 6.6 (3.9, 14.2) | <0.001 |
| PLR | 311.4 (175.6, 621.1) | 230.6 (119.5, 461.1) | 198.6 (122.3, 355.4) | <0.001 |
| Comorbidities, n (%) | ||||
| Hypertension | 75 (45.7%) | 80 (48.5%) | 91 (55.2%) | 0.214 |
| DM | 42 (25.6%) | 38 (23.0%) | 57 (34.5%) | 0.051 |
| CHD | 37 (22.6%) | 46 (27.9%) | 51 (30.9%) | 0.226 |
| CKD | 28 (17.1%) | 27 (16.4%) | 28 (17.0%) | 0.983 |
| Malignancy | 41 (25.0%) | 47 (28.5%) | 43 (26.1%) | 0.764 |
| Scoring system, median (IQR) | ||||
| SAPSII | 40 (33, 49) | 40 (33, 48) | 37 (29, 45) | 0.033 |
| SOFA | 5 (3, 7) | 4 (2, 6) | 4 (2, 6) | 0.057 |
Normally distributed data are presented as the mean±SD, non-normally distributed data are presented as median (IQR) and categorical variables are presented as n (%). WBC – white blood cell; BUN – blood urea nitrogen; ALT – alanine transaminase; AST – aspartate transaminase; MAP – mean atrial pressure; NLR – neutrophil-to-lymphocyte ratio; PLR – platelet-to-lymphocyte ratio; DM – diabetes mellitus; CAD – coronary heart disease; CKD – chronic kidney disease; SAPSII – simplified acute physiology score II; SOFA – sequential organ failure assessment.
Comparisons of Outcomes Among 3 Tertiles
Regarding clinical outcomes (Table 2), 30-day mortality was significantly higher in the low tertile group (24.4%, p<0.001) when compared with the other 2 groups. Durations of ICU stay and hospital stay were also significantly longer in the low tertile group (6.9 days, IQR, 3.9–13.8 and 12.6 days, IQR, 7.4–18.9; both P<0.001). There were no significant differences in mechanical ventilation rate and RRT rate among the 3 groups.
Table 2.
Clinical outcomes between study cohorts.
| Outcomes | Low tertile (≤30.2, n=164) | Middle tertile (30.2–36.2, n=165) | High tertile (>36.2, n=165) | P value |
|---|---|---|---|---|
| 30-day mortality | 40 (24.4%) | 22 (13.3%) | 15 (9.1%) | <0.001 |
| Hospital mortality | 41 (25.0%) | 22 (13.3%) | 15 (9.1%) | <0.001 |
| Length of ICU stay, days | 6.9 (3.9, 13.8) | 5.0 (3.0, 8.6) | 4.0 (2.9, 7.2) | <0.001 |
| Length of hospital stay, days | 12.6 (7.4, 18.9) | 10.4 (6.2, 15.0) | 8.5 (5.6, 13.0) | <0.001 |
| MV rate | 124 (75.6%) | 121 (73.3%) | 115 (69.7) | 0.477 |
| RRT rate | 3 (1.8%) | 3 (1.8%) | 2 (1.2%) | 0.879 |
Values were expressed as median (IQR) or n (%). MV – mechanical ventilation; RRT – renal replacement therapy.
Associations Between PNI and 30-Day Mortality
The 30-day Kaplan-Meier survival curves showed that patients in the low tertile group had a significantly lower mean survival time (25.4, 95% CI, 24.1–26.8; P<0.001) compared with patients in the middle tertile group (27.1, 95% CI, 25.8–28.2) and high tertile group (27.9, 95% CI, 27.0–29.0) (Figure 2). The results of univariate and multivariate Cox proportional regression models assessing the relationship between PNI and 30-day mortality are shown in Table 3. In the crude model, the HRs of death significantly improved as the tertiles of PNI upgraded. The HRs for the middle tertile (HR=0.54; 95% CI, 0.32 to 0.92; P=0.023) and high tertile (HR=0.37; 95% CI, 0.21 to 0.68; P<0.001) were significantly lower compared to low tertile reference. After adjustment for age and sex in model 1, the middle tertile (HR=0.50; 95% CI, 0.29 to 0.85; P=0.011) and high tertile (HR=0.41; 95% CI, 0.23 to 0.75; P=0.004) still had significant favorable results for 30-day mortality. Further adjusting for age, sex, comorbidities, laboratory tests, and severity scales in model 2 did not affect the relationship between high tertile and 30-day mortality (HR=0.39; 95% CI, 0.19 to 0.80; P=0.011).
Figure 2.
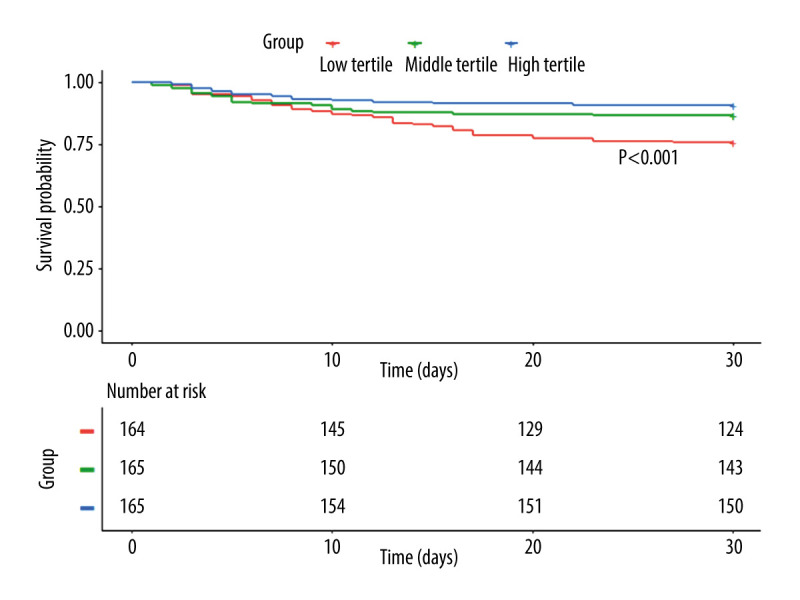
Kaplan-Meier curves of PNI for cumulative 30-day survival.
Table 3.
Unadjusted and adjusted Cox proportional regression model of PNI and 30-day mortality.
| 30-day mortality | Crude model | P value | Model 1 | P value | Model 2 | P value |
|---|---|---|---|---|---|---|
| HR (95% CIs) | HR (95% CIs) | HR (95% CIs) | ||||
| Low tertile | 1.0 (ref.) | / | 1.0 (ref.) | / | 1.0 (ref.) | / |
| Middle tertile | 0.54 (0.32, 0.92) | 0.023 | 0.50 (0.29, 0.85) | 0.011 | 0.57 (0.31, 1.05) | 0.070 |
| High tertile | 0.37 (0.21, 0.68) | <0.001 | 0.41 (0.23, 0.75) | 0.004 | 0.39 (0.19, 0.80) | 0.011 |
Values were expressed as hazard ratios (95% confidence intervals). Model 1 was adjusted for age and sex. Model 2 was adjusted for age, gender, hypertension, diabetes mellitus, coronary heart disease, chronic kidney disease, malignancy, white blood cell count, hemoglobin, platelet count, albumin, alanine transaminase, serum creatinine, serum sodium, serum potassium, pH, PO2, PCO2, bicarbonate, simplified acute physiology score II, and sequential organ failure assessment.
Subgroup Analysis
Considering the influence of age, sex, mechanical ventilation, and SAPSII on the prognosis of AECOPD, we performed subgroup analysis to identify the consistency of association between PNI and 30-day mortality in AECOPD patients. Cox proportional hazards models showed that the upgraded tertiles of PNI were significantly associated with favorable outcome on 30-day mortality in all the subgroups except in patients aged ≤70 years and without mechanical ventilation (Figure 3).
Figure 3.
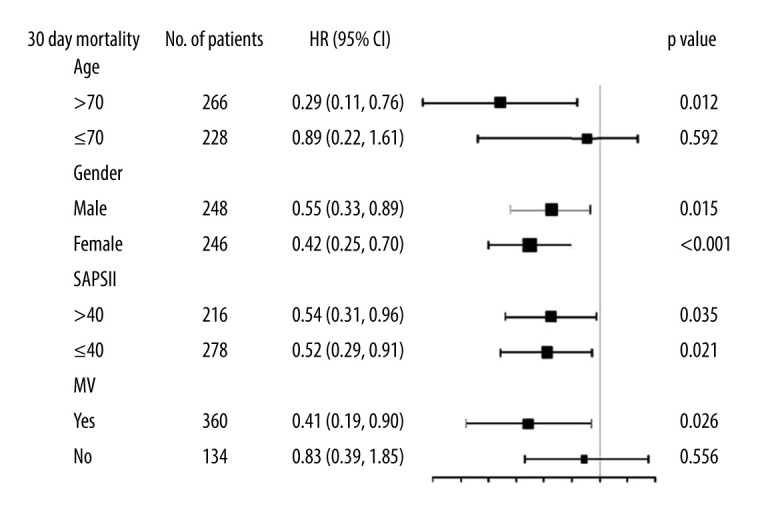
Subgroup analysis of adjusted hazard ratio (HR) per tertile increase of prognostic nutritional index (PNI) for 30-day mortality (adjusted according to model 2). SAPS II – Simplified Acute Physiology Score II; MV – mechanical ventilation.
ROC Curves of PNI, Serum Albumin, NLR, and PLR
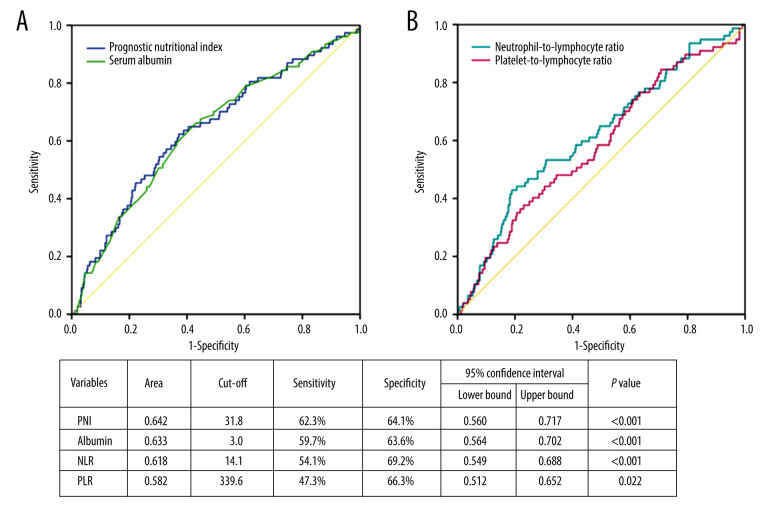
ROC curves analysis was performed to assess the potential prognostic values of PNI, serum albumin, NLR, and PLR in AECOPD patients. The comparisons of accuracy were analyzed by area under ROC (AUROC) curves. As shown in Figure 4, PNI had higher AUROC (0.642, 95% CI, 0.560 to 0.717; P<0.001) in the prediction of 30-day mortality when compared with serum albumin alone (0.633, 95% CI, 0.564, 0.702; P<0.001), NLR (0.618, 95% CI, 0.549 to 0.688; P<0.001) and PLR (0.582, 95% CI, 0.512 to 0.652; P=0.022). The optimal cut-off value for PNI was 31.8 with sensitivity 62.3% and specificity 64.1%.
Figure 4.
Receiver operator characteristic (ROC) curves of prognostic nutritional index (PNI) and serum albumin for 30-day mortality (A). ROC curves of neutrophil-to-lymphocyte (NLR) ratio and platelet-to-lymphocyte ratio (PLR) for 30-day mortality (B). Area under ROC curves and cut-off values were listed in the table.
Discussion
AECOPD is associated with worsening chronic inflammation, worsening respiratory function, and increased risk of death [17]. In addition to inflammation, malnutrition is also common in patients with COPD and often results in worse outcomes for these patients. Despite its importance, malnutrition is usually overlooked during the management of COPD patients. PNI has become a promising prognostic biomarker to reflect both inflammation and nutritional status. As far as we are aware, no study has investigated the association between PNI and disease prognosis in AECOPD. In the present single-center retrospective study, we found that patients in the low tertile group were older and had significantly higher NLR, PLR, and SAPSII score. In addition, the low tertile group also had significantly higher 30-day mortality and longer ICU and hospital stays. Moreover, PNI was an independent predictor for 30-day mortality after univariate and multivariate Cox proportional regression analysis. With the tertiles of PNI upgraded, the HRs of death significantly improved. Low PNI value is due to hypoalbuminemia or lymphocytopenia.
Firstly, several studies have shown that hypoalbuminemia was associated with increased mortality in patients with COPD [18–20]. Albumin is synthesized by the liver and its levels decline with inadequate nutritional intake; therefore, it is one of the tests used for assessment of malnutrition in patients with COPD. Malnutrition often leads to treatment failure and can be associated with a poor prognosis [21]. Besides, serum albumin level has been considered as part of the acute-phase protein response due to its quick consumption in the short term [22]. Therefore, a decreased albumin concentration may reflect a deterioration of clinical status or persistent inflammation during AECOPD [19].
Secondly, lymphocyte count indicates the immune function of patients. Yamaya et al found that the proportions of patients with lymphocytopenia in an exacerbation mortality group were higher than that in an exacerbation survival group [23]. Malnutrition involving albumin deficiency can also decrease cell-mediated immunity, such as lymphocyte proliferation, and deteriorate the immune defense system [24].
NLR and PLR in peripheral blood have been increasingly investigated as a simple systemic inflammatory biomarker. NLR has been shown to be significantly elevated in AECOPD patients compared with stable COPD patients [25–27]. PLR was also higher in AECOPD patients compared to stable COPD patients [28,29]. Bycomparisons of the area under ROC curves, we found that PNI (AUROC 0.642, 95% CI, 0.560 to 0.717, P<0.001) was better than NLR and PLR to predict 30-day mortality. The predictive accuracy of PNI was also better than serum albumin alone. The optimal PNI cut-off value was 31.8 with sensitivity 62.3% and specificity 64.1%. Subgroup analysis found that PNI was not significantly associated with prognosis among patients aged ≤70 years and without mechanical ventilation, which may partly explain the unsatisfactory accuracy. Although the sensitivity and specificity were not relatively high, given the convenience of use, PNI may still have clinical utility.
There were some limitations of this study. First, it was a single-center retrospective observational study and the biases inherent in this type of study should not be ignored. Secondly, the data were extracted from the MIMIC-III database, so selection bias could not be avoided. Thirdly, the predictive value of PNI among obese patients was not analyzed due to missing data on height and weight. Last but not least, the AUROC of PNI did not have satisfactory accuracy. Prospective studies are needed to validate these results, and nomogram logistic analysis would helpful to quantify the contribution to the prognosis of AECOPD.
Conclusions
Given its convenience, reliability, and simplicity of use before ICU admission, PNI could serve as a promising prognostic biomarker for AECOPD patients in the ICU.
Abbreviation
- AECOPD
acute exacerbation of chronic obstructive pulmonary disease
- AST
aspartate transaminase
- BUN
blood urea nitrogen
- CI
confidence interval
- CKD
chronic renal disease
- DM
diabetes mellitus
- HR
hazard ratio
- IQR
interquartile range
- MAP
mean atrial pressure
- MIMIC
Medical Information Mart for Intensive Care
- MV
mechanical ventilation
- NLR
neutrophil-to-lymphocyte
- PCO2
partial pressure of carbon dioxide
- PLR
platelet-to-lymphocyte ratio
- PNI
prognostic nutritional index
- PO2
partial pressure of oxygen
- ROC
receiver operator characteristic
- Scr
serum creatinine
- WBC
white blood cell
- RRT
renal replacement therapy
- SAPSII
Simplified Acute Physiology Score II
- SD
standard deviation
- SOFA
Sequential Organ Failure Assessment
Footnotes
Conflict of interest: None of the authors has any potential conflicts of interest related to this article to declare, and the results of this report have been produced, analyzed, and interpreted without any outside participation
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–82. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 2.Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. 2018;392(10159):2052–90. doi: 10.1016/S0140-6736(18)31694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–31. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins PF, Elia M, Stratton RJ. Nutritional support and functional capacity in chronic obstructive pulmonary disease: A systematic review and meta-analysis. Respirology. 2013;18(4):616–29. doi: 10.1111/resp.12070. [DOI] [PubMed] [Google Scholar]
- 5.Hoong JM, Ferguson M, Hukins C, Collins PF. Economic and operational burden associated with malnutrition in chronic obstructive pulmonary disease. Clin Nutr. 2017;36(4):1105–9. doi: 10.1016/j.clnu.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–5. [Japanese] [PubMed] [Google Scholar]
- 7.Abe A, Kurita K, Hayashi H, et al. Correlation between prognostic nutritional index and occlusal status in gastric cancer. Oral Dis. 2020;26(2):465–72. doi: 10.1111/odi.13242. [DOI] [PubMed] [Google Scholar]
- 8.Ucar G. The prognostic value of the prognostic nutritional index in patients with metastatic colorectal cancer. J Gastrointest Surg. 2020;16(5):e179–84. doi: 10.1111/ajco.13328. [DOI] [PubMed] [Google Scholar]
- 9.Yasar HA, Bir Yucel K, Arslan C, et al. The relationship between prognostic nutritional index and treatment response in patients with metastatic renal cell cancer. 2020;26(5):1110–16. doi: 10.1177/1078155219883004. [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Hu X, Xiao L, et al. Prognostic nutritional index and systemic immune-inflammation index predict the prognosis of patients with HCC. J Oncol Pharm Pract. 2021;25(2):421–27. doi: 10.1007/s11605-019-04492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Tao Y, Wang Z, Lu J. Evaluation of nutritional status and prognostic impact assessed by the prognostic nutritional index in children with chronic kidney disease. Medicine (Baltimore) 2019;98(34):e16713. doi: 10.1097/MD.0000000000016713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zencirkiran Agus H, Kahraman S. Prognostic nutritional index predicts one-year outcome in heart failure with preserved ejection fraction. Acta Cardiol. 2020;75(5):450–55. doi: 10.1080/00015385.2019.1661139. [DOI] [PubMed] [Google Scholar]
- 13.Ahn SS, Jung SM, Song JJ, et al. Prognostic nutritional index is correlated with disease activity in patients with systemic lupus erythematosus. Lupus. 2018;27(10):1697–705. doi: 10.1177/0961203318787058. [DOI] [PubMed] [Google Scholar]
- 14.Correa-Rodríguez M, Pocovi-Gerardino G. The prognostic nutritional index and nutritional risk index are associated with disease activity in patients with systemic lupus erythematosus. 2019;11(3):638. doi: 10.3390/nu11030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. doi: 10.1038/sdata.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jamison DC. Structured Query Language (SQL) fundamentals. Curr Protoc Bioinformatics. 2003;Chapter 9(Unit9.2) doi: 10.1002/0471250953.bi0902s00. [DOI] [PubMed] [Google Scholar]
- 17.Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364(12):1093–103. doi: 10.1056/NEJMoa1008378. [DOI] [PubMed] [Google Scholar]
- 18.Connors AF, Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) Am J Respir Crit Care Med. 1996;154(4 Pt 1):959–67. doi: 10.1164/ajrccm.154.4.8887592. [DOI] [PubMed] [Google Scholar]
- 19.Gunen H, Hacievliyagil SS, Kosar F, et al. Factors affecting survival of hospitalised patients with COPD. Eur Respir J. 2005;26(2):234–41. doi: 10.1183/09031936.05.00024804. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Stavem K, Dahl FA, et al. Factors associated with a prolonged length of stay after acute exacerbation of chronic obstructive pulmonary disease (AECOPD) Int J Chron Obstruct Pulmon Dis. 2014;9:99–105. doi: 10.2147/COPD.S51467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallin R, Gudmundsson G, Suppli Ulrik C, et al. Nutritional status and long-term mortality in hospitalised patients with chronic obstructive pulmonary disease (COPD) Respir Med. 2007;101(9):1954–60. doi: 10.1016/j.rmed.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Chen R, Xing L, You C, Ou X. Prediction of prognosis in chronic obstructive pulmonary disease patients with respiratory failure: A comparison of three nutritional assessment methods. Eur J Intern Med. 2018;57:70–75. doi: 10.1016/j.ejim.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Yamaya M, Usami O, Nakayama S, et al. Malnutrition, airflow limitation and severe emphysema are risks for exacerbation of chronic obstructive pulmonary disease in Japanese subjects: A retrospective single-center study. Int J Chron Obstruct Pulmon Dis. 2020;15:857–68. doi: 10.2147/COPD.S238457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lesourd BM. Nutrition and immunity in the elderly: Modification of immune responses with nutritional treatments. Am J Clin Nutr. 1997;66(2):478s–84s. doi: 10.1093/ajcn/66.2.478S. [DOI] [PubMed] [Google Scholar]
- 25.Günay E, Sarınç Ulaşlı S, Akar O, et al. Neutrophil-to-lymphocyte ratio in chronic obstructive pulmonary disease: A retrospective study. Inflammation. 2014;37(2):374–80. doi: 10.1007/s10753-013-9749-1. [DOI] [PubMed] [Google Scholar]
- 26.Lee H, Um SJ, Kim YS, et al. Association of the neutrophil-to-lymphocyte ratio with lung function and exacerbations in patients with chronic obstructive pulmonary disease. PLoS One. 2016;11(6):e0156511. doi: 10.1371/journal.pone.0156511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SJ, Lee HR, Lee TW, et al. Usefulness of neutrophil to lymphocyte ratio in patients with chronic obstructive pulmonary disease: A prospective observational study. Korean J Intern Med. 2016;31(5):891–98. doi: 10.3904/kjim.2015.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao C, Liu X, Tang Z. Prognostic role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio for hospital mortality in patients with AECOPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2285–90. doi: 10.2147/COPD.S141760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuo H, Xie X, Peng J, et al. Predictive value of novel inflammation-based biomarkers for pulmonary hypertension in the acute exacerbation of chronic obstructive pulmonary disease. Anal Cell Pathol (Amst) 2019;2019 doi: 10.1155/2019/5189165. 5189165. [DOI] [PMC free article] [PubMed] [Google Scholar]