Abstract
Introduction
Analysis of fibrinolytic disorders is challenging and may potentially lead to underdiagnosis of patients with an increased bleeding tendency.
Aim
To compare clinical characteristics, laboratory measurements, and treatment modalities in a monocenter cohort of patients in whom fibrinolytic studies were performed.
Methods
Retrospective study of patients in whom fibrinolytic studies were performed between January 2016 and February 2020 in the Hemophilia Treatment Center, Nijmegen‐Eindhoven‐Maastricht, the Netherlands. Plasminogen activator inhibitor type 1 (PAI‐1) antigen and activity level, α2‐antiplasmin activity, tissue plasminogen activator, and euglobulin clot lysis time (ECLT) before and after venous compression were determined in all patients. Data of bleeding assessment tool (BAT) score, clinical characteristics, results of primary and secondary hemostasis assays, and general treatment plans were collected.
Results
In total, 160 patients were included: 97 (61%) without and 63 (39%) with a laboratory‐based fibrinolytic disorder. Mean BAT score did not differ between the groups (9.3 vs 9.8, respectively). The presumptive fibrinolytic disorders were distributed as follows: 34 patients had an increased ECLT ratio or low baseline ECLT, 25 patients had low PAI‐1 antigen and activity level, and four patients had both. The majority of these patients were treated with tranexamic acid monotherapy (60%) with only 40% additional treatment options, whereas 80% of patients without a presumptive fibrinolytic disorder had multiple treatment modalities.
Discussion
Analysis of fibrinolytic disorders in selected patients has a high diagnostic yield. General incorporation of fibrinolytic analysis in the diagnostic workup of patients with bleeding of unknown cause can improve diagnosis and management of their bleeding episodes.
Keywords: bleeding of unknown cause, fibrin clot lysis time, fibrinolysis, plasminogen activator inhibitor 1, tissue plasminogen activator, tranexamic acid
Essentials.
Fibrinolytic disorders can be a cause in patients with extensive bleeding.
Retrospective cohort study of preselected patients in whom fibrinolytic testing was performed.
Fibrinolytic analysis revealed that 39% of patients had a presumptive fibrinolytic disorder.
Implementation of fibrinolytic analysis led to a personalized treatment plan for the patients.
1. INTRODUCTION
The diagnostic analysis of patients who experience recurrent mild to moderate bleeding symptoms is challenging. 1 , 2 , 3 Measurement of primary and secondary hemostasis is standardized and protocolized. Still, in a large proportion of these patients, a definitive diagnosis cannot be determined. 4 , 5 A fibrinolytic defect could be causative, but this is usually not examined because of the nonstandardized and complex diagnostic analysis, especially with respect to fibrinolysis. 6 , 7
Fibrinolysis consists of two consecutive steps. First, plasmin is formed by cleavage of plasminogen by plasminogen activators, such tissue‐type plasminogen activator (tPA) and urokinase‐type plasminogen activator. Second, fibrin is degraded by plasmin to fibrin degradation products. 8 , 9 , 10 , 11 Fibrin has an important role in its own degradation by providing binding sites for plasminogen activators. 12 Blockage of these binding sides by soluble lysine analogues, like tranexamic acid (TXA), leads to inhibition of fibrinolysis. 6 The process of fibrinolysis is balanced by inhibitors like thrombin activatable fibrinolysis inhibitor, which is activated by thrombin when a clot is formed, 13 plasminogen activator inhibitor type 1 (PAI‐1) and α2‐antiplasmin (α2‐AP). 9 PAI‐1 inhibits tPA and urokinase‐type plasminogen activator, whereas α2‐AP directly binds to plasmin, making it ineffective. Hyperfibrinolysis usually leads to a mild to moderate bleeding phenotype, with mainly mucosal bleeding, such as heavy menstrual bleeding, (late) postoperative bleeding, and postpartum bleeding. 6 , 7 PAI‐1 deficiency and α2‐AP cause a deficiency of fibrinolysis inhibitors, resulting in an increased plasmin activity. 7 , 14 , 15 , 16 , 17 , 18 , 19
Only a few previous studies have investigated the role of fibrinolysis in patients with mild to moderate bleeding symptoms, but these showed conflicting results. 2 , 20 , 21 , 22 , 23 , 24 , 25 , 26 Analysis of fibrinolytic disorders is challenging and in most centers not performed, resulting in a significant number of patients with bleeding of unknown cause. The most encountered diagnostic challenge is that assays are not standardized, and definitions of fibrinolytic disorders are lacking. In our hemophilia treatment center (HTC), we analyze fibrinolysis in patients with a high chance of having a fibrinolytic disorder. The objective of this study is to describe the characteristics of patients in which fibrinolysis was analyzed and to evaluate the clinical and laboratory differences between patients with and without a presumptive fibrinolytic disorder. The results provide practical insight in the clinical implication of performing fibrinolytic assays and the possibilities of adjusting treatment accordingly. Because no definition of a fibrinolytic disorder is issued by the SSC of the ISTH, we will use “presumptive fibrinolytic disorder” as the term for when patients had an aberrant result of fibrinolytic assays.
2. PATIENTS AND METHODS
2.1. Patients
All consecutive patients in whom fibrinolytic studies were performed between January 2016 and February 2020 in the HTC of the Radboud University Medical Center, Nijmegen, the Netherlands, were included in this retrospective cohort study. Patients were analyzed for the presence of fibrinolytic disorders after discussion in a multidisciplinary team meeting with at least two hematologists specialized in coagulation, a pediatric hematologist, a coagulation laboratory specialist, and a clinical chemist. Indications for undergoing fibrinolytic studies are: (1) Tosetto bleeding assessment toll (BAT) score ≥10 and no definite diagnosis after performing primary and secondary coagulation assays (referred to as bleeding of unknown cause patients); (2) bleeding history specific for fibrinolytic disorders irrespective of BAT score (i.e., bleeding after initial coagulation after an invasive procedure, severe mucocutaneous bleeding); (3) positive family history for a fibrinolytic disorder (independent of BAT score); and (4) known bleeding disorder with extensive bleeding despite adequate targeted treatment (i.e., factor levels according to guidelines) irrespective of the BAT score. Patients who were tested but could not be classified to one of these categories were included in category 5: fibrinolytic studies not indicated.
Only patients who did not give informed consent to use medical information for research purposes in the electronic patient file were excluded. This study did not need approval of the medical ethical committee because only medical records were collected of patients who gave digital informed consent for anonymous usage of their data. The research was executed according to the Declaration of Helsinki. The study is reported according to the STROBE statement, 27 see Appendix S1.
For each patient demographics, bleeding score determined with the Tosetto BAT score, 28 , 29 general treatment plan, relevant medical history, and medication interfering with hemostasis (e.g., anticoagulant therapy, nonsteroidal anti‐inflammatory drugs, antiplatelet therapy) were recorded by the treating physician. First‐tier screening coagulation assays and second‐tier, confirmatory assays, were performed in all referred patients, as described before. 3 All laboratory results were obtained from the laboratory database and, together with the clinical patient data, stored in an anonymized database for analysis.
2.2. Fibrinolytic studies
2.2.1. Sample collection
Plasma collection for fibrinolytic studies was performed between 9:00 and 9:30 a.m. because tPA and PAI‐1 antigen and activity level have a diurnal variation. 30 Patients were in a fasting state and were not allowed to drink caffeine‐containing beverages 24 h before the test. Blood was drawn by venipuncture in 3.2% buffered sodium citrate siliconized blood collecting tubes (Becton Dickenson, Plymouth, UK) after 20 min of rest in a seated position for PAI‐1 antigen and activity, euglobulin clot lysis time (ECLT) before, and α2‐AP activity determination. Then, samples were collected after 10 min of compression with an inflated blood pressure cuff at 10 mmHg above diastolic pressure, immediately after release of pressure for ECLT after compression and tPA determination. 31 All the collected samples were directly put on ice and kept at 4°C.
2.2.2. PAI‐1 antigen and activity level
Samples collected before venous compression were used for PAI‐1 antigen and activity determination. Citrated blood was centrifuged for 15 min at 4200 g at 4°C. Plasma was collected and transferred to new tubes, which were centrifuged for 15 min at 17,000 g at 4°C to remove all platelets because platelets contain PAI‐1. Afterward, the Zymutest PAI‐1 antigen assay and Zymutest PAI‐1 activity assay (both Hyphen Biomed, Neuville‐sur‐Oise, France) were performed according to the manufacturer's instructions and measured at the FLUOstar microplate reader (BMG Labtech, Ortenburg, Germany). PAI‐1 antigen and activity level reference ranges were determined in 40 healthy individuals and validated in a separate cohort of 41 healthy volunteers. This yielded a reference range of 3.4–39.0 ng/ml for PAI‐1 antigen level, with a lower limit of quantification of <2.5 ng/ml. For PAI‐1 activity level, the reference range is 0–10.7 ng/ml. The lower limit of qualification was determined to be <1.0 ng/ml. Therefore, samples with a PAI‐1 activity level of ≤1.0 ng/ml in combination with a PAI‐1 antigen level <3.4 ng/ml (the lower limit of normal) are diagnosed as low PAI‐1.
2.2.3. Euglobulin clot lysis time
Blood samples collected before and after venous compression were centrifuged twice for 15 min at 4200 g at 4°C. After centrifuging, 2.5 ml of citrated plasma was transferred to a plastic tube and 22 ml cold distilled water and 1.9 ml 0.25% acetic acid were added. The solution was incubated for 30 min on ice and afterwards centrifuged for 10 min at 3500 g at 4°C, the supernatant discarded. The pellets were resuspended with 2.5 ml of cold veronal buffer (5.9 g barbital‐sodium, 7.3 g sodium chloride, 21 ml 1 M HCl in 800 ml distilled water, pH adjusted to 7.35) until fully resolved. Because the test was performed in duplicate, 1 ml of suspension was transferred to another tube and to each tube 30 µl thrombin (Nodia, Boom, Belgium; final concentration, 200 U/ml) was added. The tubes were transferred to a water bath of 37°C and time was recorded for lysis of the formed clot up until 300 min by visual inspection of the clot at regular time intervals (first 18 min every 2 min, up until the hour, every 5 min, and thereafter every 15 min) by a specialized laboratory technician. The duplicates were used to cross reference the resolution time (i.e., to see if both clots were lysed). The ratio between ECLT before and after venous compression was calculated. Reference range was determined in 27 healthy controls and was based on the 95% confidence interval (CI), which was 1.2–5.7. This was thereafter validated in an independent group of 47 healthy volunteers. An ECLT ratio of >5.7 indicates hyperfibrinolysis. Additionally, a baseline ECLT of <116 min (the lower limit in healthy controls) was also classified as hyperfibrinolysis.
2.2.4. tPA activity level
For the analysis of tPA activity, 2 ml of citrated blood after venous compression was transferred in a tube containing 2 ml refrigerated TPA acetate buffer (concentration 200 mM, pH 3.9). It was centrifuged for 15 min at 4200 g at 4°C and afterwards 10 µl 1 M HCL was added per 150 µl citrated plasma. tPA activity level was measured with the Zymuphen tPA activity assay (Hyphen Biomed) according to the manufacturer's instructions and measured at the FLUOstar microplate reader (BMG Labtech). The reference range was determined to be 0.7–7.4 IU/ml with a lower limit of quantification of <0.5 IU/ml.
2.2.5. Alpha‐2‐antiplasmin level
α2‐AP activity level was determined in citrated plasma with the chromogenic Stachrom antiplasmin assay at the STA Evolution (both Diagnostic Stago, Asnières sur Seine, France) according to the manufacturer's instructions. Reference values are 80% to 120%.
2.3. Statistical analysis
All parameters are reported as median (interquartile range) unless stated otherwise. We used descriptive statistics for patient characteristics. Patients with a presumptive fibrinolytic disorder (patients with ECLT abnormalities [an ECLT ratio >5.7 or baseline ECLT <116 min]) or low PAI‐1 (PAI‐1 activity level ≤1.0 ng/ml and PAI‐1 antigen level <3.4 ng/ml) were compared with patients without fibrinolytic disorder (i.e., those with normal assay results). Student's t‐test and Mann‐Whitney test were used accordingly. Frequencies were compared with the χ2 test. Spearman correlation was used to determine correlations. Statistical analyses were performed with Prism GraphPad, version 9. All p values are two‐sided and a p value <0.05 was considered statistically significant.
3. RESULTS
3.1. Patients
All 164 patients in whom fibrinolysis analysis was performed between January 2016 and February 2020 were identified based on the centrally collected data of the laboratory of hemostasis. Two patients did not give informed consent, one patient was tested twice (with the same results), and in one patient, only PAI‐1 antigen level was determined, resulting in 160 unique patients that were included in the study. Of those, 97 (61%) did not have a fibrinolytic disorder, whereas 63 patients (39%) did. The patients with a presumptive fibrinolytic disorder were subdivided in 33 patients with an increased ECLT ratio and one with only a low baseline ECLT (taken together as 34 patients and referred to as “increased ECLT ratio”), 25 patients with a low PAI‐1 antigen and activity level, and an additional four patients had both an increased ECLT ratio and low PAI‐1 antigen and activity level. No patient had an α2‐AP deficiency. The patient flow diagram is shown in Figure 1.
FIGURE 1.
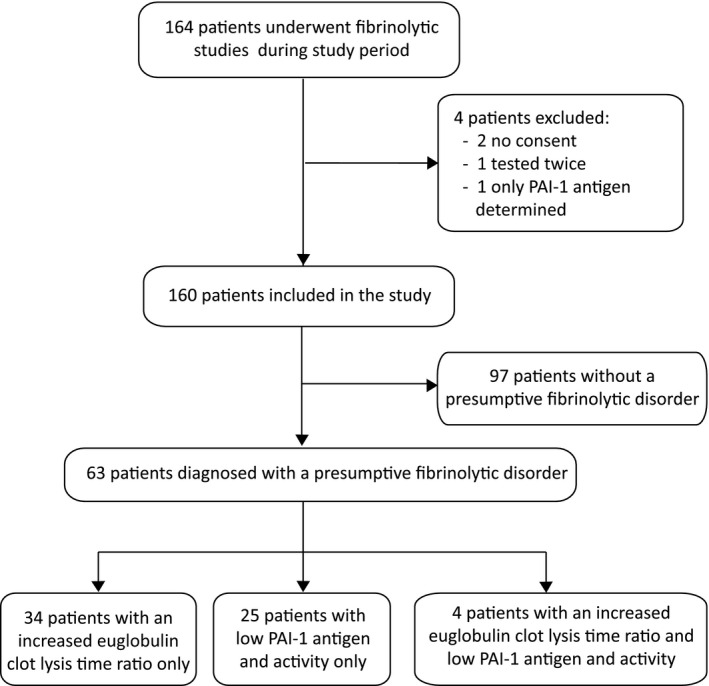
Flow diagram of patients included. Increased euglobulin clot lysis time (ECLT) ratio was defined as a ratio >5.7 or patients with a short ECLT at baseline (<116 min). Low PAI‐1 is defined as a PAI‐1 activity level ≤1.0 ng/ml in combination with a PAI‐1 antigen level <3.4 ng/ml. PAI‐1, plasminogen activator inhibitor type 1
Patient demographics and clinical characteristics are shown in Table 1. Median age of the patients did not differ between the groups (49 years [range 17–76]) for patients without a fibrinolytic disorder versus 38 years (15–78) for patients with a presumptive fibrinolytic disorder. Females were most prevalent in both groups (91% vs 87%) and blood groups were evenly distributed between patient categories. The main reason for fibrinolytic testing was a BAT score ≥10 in both groups (49% vs 59%). However, in the group of patients without a fibrinolytic disorder, more patients were tested although it was not indicated (29% vs 11%, p < 0.01). Information about ethnicity was not available, and it is unknown if fibrinolysis is affected by ethnicity.
TABLE 1.
Patient demographics and clinical characteristics
| All patients (n = 160) | No fibrinolytic disorder (n = 97) | Presumptive fibrinolytic disorder (n = 63) | p value* | |
|---|---|---|---|---|
| Age, median, y (extremes) | 45 (15–78) | 49 (17–76) | 38 (15–78) | 0.29 |
| Female, n (%) | 143 (89) a | 88 (91) | 55 (87) a | 0.60 |
| Reason for fibrinolytic studies, n (%) | ||||
| 1. BAT score ≥10 | 84 (52) | 47 (49) | 37 (59) | 0.14 |
| 2. Bleeding history typical for fibrinolytic disorders | 25 (16) | 13 (13) | 12 (19) | 0.31 |
| 3. Positive family history | 5 (3) | 3 (3) | 2 (3) | 1.00 |
| 4. Bleeding despite adequate coagulation measures | 11 (7) | 6 (6) | 5 (8) | 0.62 |
| 5. Not indicated | 35 (22) | 28 (29) | 7 (11) | <0.01 |
| Blood group, n (%) | ||||
| Unknown | 23 (14) | 14 (14) | 9 (14) | 1.00 |
| 0 (null) | 78 (49) | 46 (48) | 32 (51) | 0.75 |
| A/B/AB (non‐O) | 59 (37) | 37 (38) | 22 (35) | 0.74 |
| BAT score, mean (SD) | 9.5 (3.5) | 9.3 (3.6) | 9.8 (3.5) | 0.45 |
| Epistaxis | 0.6 (0.8) | 0.5 (0.7) | 0.6 (0.9) | 0.60 |
| Cutaneous | 0.8 (0.5) | 0.8 (0.5) | 0.9 (0.6) | 0.41 |
| Minor wounds | 0.8 (0.7) | 0.8 (0.7) | 0.8 (0.8) | 0.90 |
| Oral cavity | 0.5 (0.7) | 0.3 (0.6) | 0.6 (0.8) | 0.03 |
| GI bleeding | 0.4 (0.7) | 0.3 (0.5) | 0.5 (0.9) | 0.54 |
| Tooth extraction | 0.9 (1.4) | 0.8 (1.4) | 1.1 (1.5) | 0.18 |
| Surgery | 2.1 (1.6) | 2.2 (1.6) | 1.8 (1.5) | 0.10 |
| Menorrhagia a | 2.1 (1.3) | 2.3 (1.2) | 2.2 (1.2) | 0.79 |
| Postpartum hemorrhage a | 1.1 (1.4) | 1.2 (1.4) | 1.2 (1.5) | 0.82 |
| Muscle hematomas | 0.1 (0.6) | 0.2 (0.6) | 0.1 (0.6) | 0.51 |
| Hemarthroses | 0.1 (0.5) | 0.1 (0.6) | 0.1 (0.4) | 0.83 |
| CNS bleeding | 0.1 (0.6) | 0.1 (0.6) | 0.1 (0.5) | 0.91 |
| Use of anticoagulants, n (%) | 6 (4) | 4 (4) | 2 (3) | 1.00 |
| Use of antiplatelet drugs, n (%) | 4 (3) | 4 (4) | ‐ | 0.11 |
| Use of NSAIDs, n (%) | 1 (1) | ‐ | 1 (2) | 0.39 |
Abbreviations: BAT, bleeding assessment tool; CNS, central nervous system; GI, gastrointestinal; NSAID, nonsteroidal anti‐inflammatory drug.
*p value between patients without and with a presumptive fibrinolytic disorder.
Bleeding scores of male patients were described as missing data, except for the male patient who was in a transgender trajectory, originating from female.
The BAT score of the patients did not differ significantly between the two groups with a mean (SD) of 9.3 (3.6) versus 9.8 (3.5), respectively (Figure 2 and Table 1). Moreover, no difference in BAT score was observed between the different fibrinolytic disorder groups (increased ECLT ratio 9.9 [3.2], low PAI‐1 antigen and activity level 9.4 [4.0], and both disorders 11 [4.1], Figure 2). Subcategories of the BAT score did not differ either, except for oral cavity bleeding (p < 0.01; Table 1 and Figure S1A). There were no differences in subcategories of the BAT score between the different presumptive fibrinolytic disorders (Figure S1B and Table S2).
FIGURE 2.
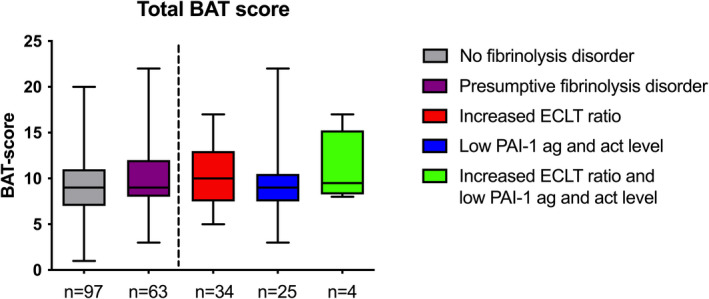
BAT score of patients included in the study, divided according to the results of fibrinolysis studies. Total BAT score of the patients without (gray) and with a presumptive fibrinolytic disorder (purple). Right, the patients with a fibrinolytic disorder are subdivided in patients with an increased euglobulin clot lysis time (ECLT) ratio or short baseline ECLT (red), patients with a low PAI‐1 antigen and activity level (ag and act; blue), and patients with both a high ECLT ratio and low PAI‐1 antigen and activity level (green). Box represents median with interquartile range, whiskers indicate range. BAT, bleeding assessment tool; PAI‐1, plasminogen activator inhibitor type 1
3.2. Results of fibrinolysis studies
The results of the fibrinolysis analyses are described in Table 2 for the different groups. The fibrinolytic results per indication for testing in Table 3. When indications for testing were adhered to, 40% to 50% of the tests were positive for a presumptive fibrinolytic disorder, whereas 80% was negative when fibrinolytic analysis was not indicated.
TABLE 2.
Results of fibrinolytic studies
| Parametera | Reference values | All patients (n = 160) | No fibrinolytic disorder (n = 97) | Presumptive fibrinolytic disorder group (n = 63)b | Increased ECLT ratio (n = 34)c | Low PAI‐1 ag and act (n = 25)c | Increased ECLT ratio and low PAI‐1 ag and act (n = 4)c |
|---|---|---|---|---|---|---|---|
| ECLT before, min | >116 | 270 (170‐>300) | >300 (257‐>300) | 170 (137–227) | 217 (147–282) | 165 (130–180) | 155 (140–170) |
| ECLT after, min | >18 | 78 (33–150) | 125 (78–259) | 29 (22–47) | 23 (19–30) | 55 (40–80) | 20 (18–25) |
| ECLT ratio | <5.7 | 2.9 (1.8–5.5) | 2.2 (1.2–3.3) | 6.1 (2.9–8.8) | 8.5 (6.3–11.3) | 2.9 (2.2–3.7) | 7.5 (6.1–8.3) |
| tPA, IU/ml | 0.7–7.4 | 2.0 (1.2–3.1) | 1.5 (1.0–2.2) | 3.1 (2.0–6.1) | 6.0 (3.1–9.4) | 2.2 (1.4 −2.5) | 5.8 (5.5–5.8) |
| PAI‐1 antigen, ng/ml | 3.4–39.0 | 7.9 (4.4–14.2) | 12.0 (7.4–18.5) | 3.5 (<2.5–7.2) | 6.8 (4.9–12.0) | <2.5 (<2.5–2.7) | <2.5 (<2.5–3.0) |
| PAI‐1 activity, ng/ml | <10.4 | 1.1 (<1.0–2.6) | 1.8 (1.0–4.2) | <1.0 (<1.0–1.0) | 1.0 (<1.0–1.7) | <1.0 (<1.0‐<1.0) | <1.0 (<1.0–<1.0) |
| α‐2‐antiplasmin activity, % | 80–120 | 108 (102–115) | 110 (102–118) | 107 (100–112) | 108 (102–113) | 103 (98–111) | 112 (95–118) |
Abbreviations: ECLT, euglobulin clot lysis time; tPA, tissue‐plasminogen activator; PAI‐1, plasminogen activator inhibitor‐1.
All numbers are given as median (interquartile range).
These include patients with an increased ECLT ratio and low PAI‐1 antigen and activity level, causing the range to include normal values.
Subdivision of patients with a presumed fibrinolytic disorder in which the ECLT ratio increased if ≥5.7 or baseline ECLT <116 min, low PAI‐1 if PAI‐1 antigen ≤3.4 ng/ml and PAI‐1 activity ≤1.0 ng/ml, α‐2 antiplasmin decreased if activity level <80%.
TABLE 3.
Fibrinolytic study results according to diagnostic category
| Number (%) | BAT scorea | ECLT before, min | ECLT after, min | ECLT ratio | tPA in ng/ml | PAI‐1 antigen, ng/ml | PAI‐1 activity, ng/ml | |
|---|---|---|---|---|---|---|---|---|
| 1. Bleeding of unknown cause (BAT score ≥10), n = 84 | ||||||||
| No FD | 47 (56) | 12.1 ± 2.6 | >300 (295–>300) | 154 (103–206) | 1.9 (1.2–2.5) | 1.4 (1.0–2.3) | 13.6 (8.3–21.2) | 2.6 (1.3–4.6) |
| PFD | 37 (44) | 12.0 ± 2.7 | 177 (153–227) | 27 (21–46) | 6.3 (3.6–9.5) | 5.5 (2.2–6.9) | 4.9 (2.8–8.0) | <1.0 (<1.0–1.3) |
| 2. Bleeding history specific for fibrinolytic disorder, n = 25 | ||||||||
| No FD | 13 (52) | 7.0 ± 1.4 | >300 (212–>300) | 157 (91–229) | 1.9 (1.5–3.4) | 2.2 (1.5–2.7) | 12.3 (8.5–22.3) | 1.3 (<1.0–2.9) |
| PFD | 12 (48) | 5.5 ± 0.7 | 181 (147–214) | 22 (28–39) | 6.9 (4.8–9.1) | 3.3 (2.9–3.8) | 3.6 (2.9–4.3) | <1.0 (<1.0‐<1.0) |
| 3. Family history for fibrinolytic disorders, n = 5 | ||||||||
| No FD | 3 (60) | 6.8 ± 2.0 | 299 (250‐>300) | 102 (67–149) | 2.9 (1.8–3.4) | 1.5 (1.1–1.9) | 8.3 (7.5–17.0) | 1.1 (<1.0–2.1) |
| PFD | 2 (40) | 7.0 ± 1.9 | 172 (142–189) | 33 (26–60) | 4.2 (2.9–7.0) | 2.2 (1.7–3.8) | <2.5 (<2.5–4.3) | <1.0 (<1.0‐<1.0) |
| 4. Bleeding despite adequate coagulation measures, n = 11 | ||||||||
| No FD | 6 (55) | 7.3 ± 1.0 | >300 (>300‐>300) | 131 (120–201) | 2.3 (1.6–2.5) | 1.5 (1.3–2.0) | 11.4 (8.3–13.7) | 1.8 (1.0–4.3) |
| PFD | 5 (45) | 6.7 ± 1.5 | 215 (130–258) | 32 (29–34) | 6.7 (4.4.−8.8) | 2.4 (2.2–3.1) | 4.3 (3.0–10.5) | 1.0 (<1.0–1.1) |
| 5. Fibrinolytic analysis not indicated, n = 35 | ||||||||
| No FD | 28 (80) | 6.6 ± 2.1 | >300 (179‐>300) | 101 (58–236) | 2.3 (1.3–4.0) | 1.6 (1.1–2.4) | 9.1 (6.3–16.2) | 1.6 (<1.0–2.9) |
| PFD | 7 (20) | 7.6 ± 1.8 | 152 (140–193) | 35 (30–58) | 4.6 (2.9–6.3) | 2.9 (2.1–3.0) | <2.5 (<2.5–3.0) | <1.0 (<1.0‐<1.0) |
Abbreviations: BAT score, bleeding assessment tool score; BUC, bleeding of unknown cause; ECLT, euglobulin clot lysis time; no FD, no fibrinolytic disorder; PAI‐1, plasminogen activator inhibitor‐1; PFD, presumptive fibrinolytic disorder; tPA, tissue‐plasminogen activator.
All numbers are given as median (interquartile range), except for BAT score, which is expressed as mean ± standard deviation.
A clear difference was observed in ECLT before venous compression between the patient groups. This was >300 min (257–>300) in patients without a fibrinolytic disorder, but markedly lower in patients with a presumptive fibrinolytic disorder (170 min [137–227], Table 2). Of the latter group, patients with a low PAI‐1 antigen and activity level had an ECLT time before compression of 165 min (130–180), indicating an already existing hyperfibrinolysis in patients with a low PAI‐1 antigen and activity level but still in the normal range.
The increased ECLT ratio (Figure 3A) was associated with a higher tPA activity level after venous compression (Figure 3B and Table 2). The tPA activity level was 1.5 IU/ml (1.0–2.2) in patients without a fibrinolytic disorder and 3.1 IU/ml (2.0–6.1) in patients with a presumptive fibrinolytic disorder (p < 0.00). Furthermore, patients with an elevated ECLT ratio had a higher tPA activity level after compression (6.0 IU/ml [3.1–9.4]) compared with patients with low PAI‐1 antigen and activity level (2.2 IU/ml [1.4–2.5], p < 0.00). tPA activity level after venous compression showed a strong correlation with ECLT ratio (r = 0.77, 95% CI 0.70‐0.83, p < 0.00; Figure S2A). This correlation was due to a strong correlation between tPA and ECLT after compression (r = −0.75, 95% CI −0.81 to ‐0.67, p < 0.00; Figure 2B), which was strongest in patients with a presumptive fibrinolytic disorder (r = −0.79, 95% CI −0.87 to ‐0.68, p < 0.00; Figure S2C), compared with patients without fibrinolytic disorder (r = −0.59, 95% CI −0.71 to ‐0.44, p < 0.00; Figure S2D).
FIGURE 3.
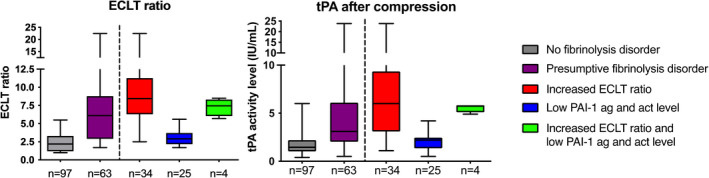
Results of euglobulin clot lysis time ratio and tissue plasminogen activity (tPA) after compression. (a) Result of the euglobulin clot lysis time (ECLT) ratio in the group without (gray) and with a presumptive fibrinolytic disorder (purple). To the right of the line, ECLT ratio in patients with an increased ECLT ratio or short baseline ECLT (red), patients with a low PAI‐1 antigen and activity level (blue), and patients with both an increased ECLT ratio and low PAI‐1 antigen and activity level (green). (b) tPA after venous compression in patients without (gray) and with a presumptive fibrinolytic disorder (purple). To the right of the dotted line, tPA in patients with only an increased ECLT ratio or short baseline ECLT (red), patients with a low PAI‐1 antigen and activity level (blue), and patients with both an increased ECLT ratio and low PAI‐1 antigen and activity level (green). In panels a and b, box represents median with interquartile range, whiskers indicate range. PAI‐1, plasminogen activator inhibitor type 1
PAI‐1 antigen and activity levels were clearly higher in patients without a fibrinolytic disorder (PAI‐1 antigen 12.0 ng/ml [7.4–18.5]; PAI‐1 activity level 1.8 ng/ml [1.0–4.2]) than in patients with a presumptive fibrinolytic disorder (PAI‐1 antigen 3.5 ng/ml [<2.5–7.2]; PAI‐1 activity level <1.0 ng/ml [<1.0–1.0]; Table 2 and Figure 4). The correlation between PAI‐1 antigen and PAI‐1 activity is shown in Figure S3. In patients without a fibrinolytic disorder there was a strong negative correlation between PAI‐1 antigen level and ECLT ratio (r = −0.65, 95% CI −0.75 to −0.51, p < 0.00, Figure S2E), whereas patients with a presumptive fibrinolytic disorder showed a strong positive correlation (r = 0.75, 95% CI 0.61–0.84, p < 0.00, Figure S2F).
FIGURE 4.
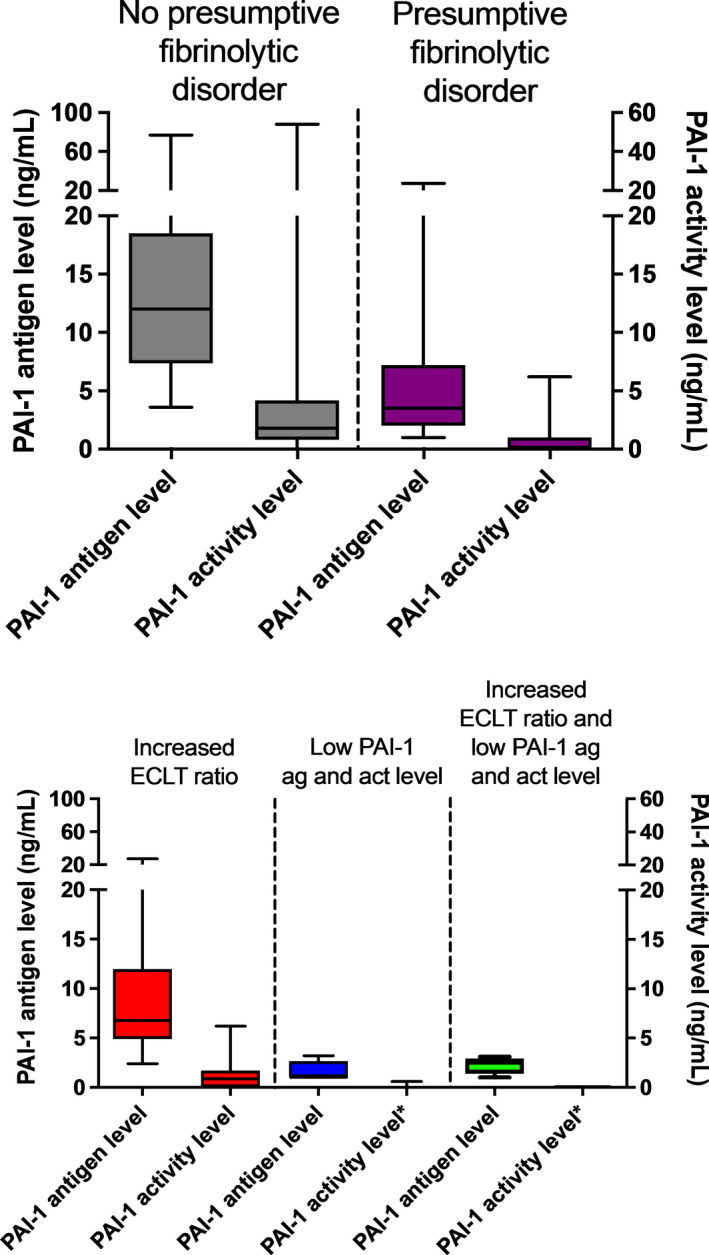
Results of PAI‐1 antigen and activity level. (a) PAI‐1 antigen level (on left y‐axis) and PAI‐1 activity level (on right y‐axis) in patients without (gray) and with a presumptive fibrinolytic disorder (purple). (b) PAI‐1 antigen level (left y‐axis) and PAI‐1 activity level (right y‐axis) in patients with only a high ECLT ratio (red), patients with a low PAI‐1 antigen and activity level (blue), and patients with both a high ECLT ratio and low PAI‐1 antigen and activity level (green). Box represents median with interquartile range, whiskers indicate range. *Low PAI‐1 was defined as a PAI‐1 activity level ≤1.0 ng/ml; therefore, almost no values are shown. Three patients had a PAI‐1 activity level of 0.5 ng/ml. ECLT, euglobulin clot lysis time; PAI‐1, plasminogen activator inhibitor type 1
α2‐AP concentration did not differ between the groups (110% [102–118] vs 107% [100–112], Table 2). We did not find any patients with a α2‐AP deficiency in this cohort.
Previous reports have suggested a potential role for the ABO blood group system in fibrinolysis 32 ; therefore, the results of fibrinolytic analysis were compared between blood groups (O vs A/B/AB [non‐O]; Figure S4). A significant higher ECLT ratio in patients with blood group O (3.4 [1.9–6.0]) was found if compared with non‐O (2.4 [1.3–4.1], p < 0.01), Figure S4A. Moreover, a similar effect was seen for only tPA. Patients with blood group O had higher tPA levels (2.4 IU/ml [1.4–4.2]) compared with patients with non‐O blood groups (1.6 IU/ml [1.0–2.5]), p< 0.01, respectively, Figure S4B). PAI‐1 antigen and activity did not differ between blood groups (data not shown). The percentage of patients with certain blood groups did not differ between patients with or without a presumptive fibrinolytic disorder (Table 1).
The results of the other coagulations assays (platelet function assays, presence of platelet receptors, activation markers, von Willebrand factor (VWF) activity and antigen levels, and individual factor activity levels) that were performed in the included patients are shown in Table S2. No clear differences in additional hemostatic diagnosis were seen between the group with or without a presumptive fibrinolytic disorder.
3.3. General treatment plans
Finally, general treatment plans were compared between patients, as shown in Table 4. In our HTC, a general treatment plan is composed for patients with a sex‐specific elevated BAT score and is dependent on the abnormalities found in coagulation analysis. Fourteen patients (14%) without a fibrinolytic disorder had no treatment plan, mostly because the BAT score was low (patients with a positive family history for bleeding disorders or not indicated testing). All patients (100%) with a presumptive fibrinolytic disorder had a treatment plan with TXA versus 80 (82%) of the patients without a fibrinolytic disorder. Only six patients (6%) without a fibrinolytic disorder had no additional hemostatic agents in their plan, mostly because of a low BAT score, whereas 38 (60%) of the patients with a presumptive fibrinolytic disorder had no additional hemostatic agents. Of the patients with a presumptive fibrinolysis disorder, additional hemostatic agents were adjusted to other hemostatic abnormalities (e.g., D‐amino D‐arginine vasopressin [DDAVP] in patients with also low VWF activity, and platelet transfusion in patients with a thrombocytopathy). Only six patients (10%) with a presumptive fibrinolytic disorder had additional hemostatic agents in their treatment plan without specific hemostatic abnormalities.
TABLE 4.
General treatment plans
| No fibrinolytic disorder (n = 97) | Presumptive fibrinolytic disorder (n = 63) | |
|---|---|---|
| No treatment plan, n (%) | 14 (14) | — |
| TXA, n (%) | 80 (82) | 63 (100) |
| No TXA, n (%) | 3 (3) | — |
| Additional hemostatic agents | ||
| No additional hemostatic agent, n (%) | 6 (6) | 38 (60) |
| DDAVP, n (%) | 38 (39) | 7 (11) |
| DDAVP with | ||
| Platelet transfusion, n (%) | 17 (17) | 9 (15) |
| von Willebrand factor concentrate, n (%) | 5 (5) | 1 (2) |
| Factor VIII concentrate, n (%) | 2 (2) | 1 (2) |
| von Willebrand factor and factor VIII concentrate, n (%) | 2 (2) | — |
| Other (without DDAVP) | ||
| Plasma, n (%) | 1 (1) | 1 (2) |
| rFVIIa, n (%) | 2 (2) | 1 (2) |
| von Willebrand factor, n (%) | 3 (3) | 2 (3) |
| von Willebrand factor and factor VIII, n (%) | 1 (1) | — |
| Platelet transfusion, n (%) | 5 (5) | 2 (3) |
| Fibrinogen, n (%) | — | 1 (2) |
Abbreviations: DDAVP, D‐amino D‐arginine vasopressin; rFVIIa, recombinant activated factor VII; TXA, tranexamic acid.
4. DISCUSSION
In this retrospective cohort study, we have shown that a large proportion (39%) of patients with an increased bleeding tendency who were tested for fibrinolytic abnormalities had a presumptive fibrinolytic disorder. Most of these patients had an increased ECLT ratio and, to a lesser extent, a low PAI‐1 antigen and activity level. Interestingly, patients who were tested according to the prespecified indications had a higher chance (40%–48%) of having a presumptive fibrinolytic disorder, whereas this was only 20% in patients in whom testing was not indicated (see Table 3). This shows that analysis of fibrinolytic abnormalities is most efficient when it is done according to prespecified indications, like a high BAT score bleeding diathesis, a typical bleeding history, a family history for fibrinolytic disorders, and excessive bleeding in patients with a coagulation disorder despite adequate supplementation therapy.
The patients included in our study had a high bleeding tendency, as illustrated by a mean BAT score of 9.8 and 9.3 in patients with and without a presumptive fibrinolytic disorder, respectively. This is explained by the selection criteria for undergoing fibrinolytic testing. In contrast, in most other studies investigating prevalence of fibrinolytic disorders in patients with bleeding of unknown origin all patients with a bleeding tendency were tested, despite the BAT score. 2 , 20 , 21 , 24 The mean BAT score in those studies was 4–5, 20 , 21 and because a large proportion in those studies were women, a BAT score ≤6 can be considered as normal. 28 In our study, had undergone fibrinolytic testing because of either a high BAT score, family or typical bleeding history, or excessive bleeding with adequate supplementation therapy.
The ECLT can be used to analyze the fibrinolytic system in patients with a bleeding diathesis. 33 The ECLT reflects the fibrinolysis capacity in vivo because of the venous compression, incorporating the contribution of endothelium, platelets and red blood cells (ABO blood group). 32 , 34 Our results indicate that patients with blood group O have an enhanced fibrinolytic capacity because of a higher activity level of tPA reflected by a higher ECLT ratio compared with patients without blood group O. Blood groups were evenly distributed over patients with and without a fibrinolytic disorder (Table 1). When patients with and without a presumptive fibrinolytic disorder were analyzed separately, differences in ECLT ratio and tPA between blood group remained intact (data not shown). However, the ECLT is not available as a commercial test, but our results show the importance of the ECLT. The reference ranges were determined in two cohorts of healthy volunteers without bleeding symptoms (27 and 47 volunteers). Furthermore, in one patient, the ECLT was performed twice, with a comparable ECLT and both times diagnosed as low PAI‐1. Only five patients in this current cohort had a short baseline ECLT, whereas a total of 37 patients were diagnosed with a presumptive fibrinolytic disorder based on an increased ratio of the ECLT before and after venous compression, demonstrating the added value of this ratio compared with baseline ECLT alone. Because most HTCs do not perform fibrinolysis testing, this results in a significant proportion of patients diagnosed with bleeding of unknown origin while they might actually have a fibrinolytic disorder. Also, even patients with a known coagulation defect can have a fibrinolytic disorder. For example, a mild hemophilia A patient who had a bleeding 7 days after surgery, despite adequate factor VIII activity levels, appeared to have a long ECLT ratio. His treatment plan was updated with an extended treatment duration of TXA in case of bleeding or surgery.
In this study, we observed a strong correlation between ECLT ratio and tPA, which is in accordance with previous studies. 35 This relationship is expected because tPA is the main activator of fibrinolysis and venous compression releases tPA from the endothelium. Furthermore, others described a correlation between free PAI‐1 antigen level and ECLT. 36 , 37 In patients with a PAI‐1 deficiency a shortening of the ECLT was found compared with patients with a normal PAI‐1 level. 18 , 19 , 38 This was also seen in our patients where those with a low PAI‐1 had a lower baseline ECLT. Furthermore, ECLT ratio and PAI‐1 antigen showed a moderate correlation. Whereas in patients with a presumptive fibrinolytic disorder it showed a strong positive correlation, the correlation was negative in patients without a fibrinolytic disorder. This positive correlation was mainly driven by patients with an increased ECLT ratio, whereas in patients with low PAI‐1 the correlation was weak or absent. This may indicate that the balance between PAI‐1 and tPA is shifted in patients with an increased ECLT ratio. This observation was previously shown in a cohort where ECLT shortening and tPA increase after exercise was largely dependent on the basal PAI‐1 level. 39
The limited diagnostic yield of current hemostatic assays underscores the need for better diagnostic algorithms. The introduction of global assays of hemostasis can improve diagnostic screening. 40 This global assay ideally should measure both thrombin generation and plasmin generation because plasmin generation reflects fibrinolysis. An example of such an assay is the Nijmegen Hemostasis Assay, 41 which we have previously performed in patients with a PAI‐1 deficiency and plasminogen deficiency. This assay was able to distinguish these patient categories based on differences in thrombin and plasmin generation profiles. 42 However, this assay is unable to detect patients with hyperfibrinolysis based on tPA excess because of added tPA in the Nijmegen Hemostasis Assay. 41 Therefore, for further research, it would be of interest to compare the results of different fibrinolytic assays and additional replication of our data should be performed before these assays can be used in regular diagnostics.
All patients with a presumptive fibrinolytic disorder had TXA as the first step in treatment for bleeding episodes. For 60% of the patients, this was the only treatment used. The majority of patients with a presumptive fibrinolytic disorder who did have additional treatment modalities besides TXA had another hemostatic diagnosis like a low‐normal VWF, or such a high BAT score or severe bleeding tendency that the clinician preferred to extend the general treatment plan with specific hemostatic products in case of a life‐threatening bleed (which was the case in 10% of the patients with a fibrinolytic disorder). For patients without a fibrinolytic disorder, 80% had additional treatment modalities in their plan, mostly DDAVP and platelet transfusions. Additional hemostatic treatment is associated with higher costs for medication and blood products and can lead to adverse events like hyponatremia in case of DDAVP and transfusion reactions to platelet transfusions. The optimization of general treatment plans by diagnosing a presumptive fibrinolytic disorder could prevent exposure to these costly treatments, with associated risk of adverse events and without the rationale of being effective in these specific cases. However, the efficiency and safety of individualization of treatment plans in patients with a presumptive fibrinolytic disorder in our HTC is not analyzed and a prospective study should be performed before implementing this strategy to other HTCs.
Our study has several limitations. First, this is a retrospective study that analyzed the results of patients in whom fibrinolytic testing was performed. This introduces selection bias, and it might be possible that we have missed patients with a presumptive fibrinolytic disorder. Some patients were diagnosed with a presumptive fibrinolytic disorder but diagnostic testing was not indicated. However, because of the retrospective nature of this study, depending on the extent of reporting of the treating physician, patients could be misclassified as “not indicated,” whereas some could have a typical bleeding history for fibrinolytic disorders. Also, some patients had a BAT score <10 but with two severe bleeding events, causing the physician wanting to exclude all causes for a bleeding diathesis. Indeed, the patients who had a presumptive fibrinolytic disorder were mainly young patients with a relative high BAT score for the number of hemostatic challenges. Furthermore, most patients in our study were women. Because they often have higher BAT scores from menorrhagia and postpartum hemorrhage, 7 which are also hallmarks of a fibrinolytic disorder, they were more frequently tested for the presence of a fibrinolytic disorder.
Another limitation is the limited generalizability of the ECLT ratio to detect fibrinolytic disorders. This assay was validated in our hospital with two groups of healthy volunteers. Preferably, the reference ranges should be validated in a larger cohort before these assays are implemented widely. However, the other fibrinolysis tests (PAI‐1 antigen and activity, tPA and α2‐AP activity level) were performed with commercially available kits. In other studies, a different definition of a PAI‐1 deficiency (e.g., only low PAI‐1 activity, low tPA‐PAI‐1 complex) was used, which makes it impossible to compare such studies. 22 , 23 , 24
In this study, we used the Tosetto BAT score instead of the more widely used ISTH BAT score. Even though diagnostic performance of the BAT scores is approximately equal, 43 it makes our results more difficult to compare with other cohorts. However, none of the BAT scores is validated for patients with a possible fibrinolytic disorder. Last, the patients included in the study were selected based on a high BAT score, family history, and typical bleeding history, indicating that these results cannot be generalized to other cohorts.
In conclusion, our study shows that analysis of fibrinolytic disorders in selected patients with a high suspicion for fibrinolytic disorder (e.g., bleeding of unknown cause patients with a BAT score ≥10, a fibrinolysis specific bleeding history) has a high diagnostic yield, but these results cannot be generalized to other patient groups. In future studies, the added value of performing global hemostatic assays like plasmin generation in diagnosing patients with a presumptive fibrinolytic disorder should be established. Based on the results of fibrinolysis studies and combined with standard coagulation assays, treatment of patients with a presumptive fibrinolytic disorder could be optimized. A personalized treatment plan based on aberrant coagulation assays could provide a more targeted management. Therefore, analysis of fibrinolytic disorders in patients with a bleeding tendency unexplained by routine hemostatic testing can optimize treatment for this patient group.
RELATIONSHIP DISCLOSURE
W.L.v.H. received unrestricted grants from Bayer, Shire, Novo Nordisk, and CSL Behring and is the cofounder and chief science officer of Enzyre BV, a Radboudumc spinoff company. The remaining authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Lars L.F.G. Valke provided clinical care for the patients, designed the study, collected and analyzed the data, and wrote the manuscript. Laurens Nieuwenhuizen and Britta A.P. Laros‐van Gorkom provided clinical care for the patients. Nicole M.A. Blijlevens critically revised the manuscript. Danielle Meijer and Waander L. van Heerde oversaw diagnostic testing and wrote the manuscript. Saskia E.M. Schols provided clinical care for the patients and wrote the manuscript. All authors critically revised the manuscript and gave final approval for publication.
Supporting information
Supplementary Material
Valke LLFG, Meijer D, Nieuwenhuizen L, et al. Fibrinolytic assays in bleeding of unknown cause: Improvement in diagnostic yield. Res Pract Thromb Haemost. 2022;6:e12681. doi: 10.1002/rth2.12681
Handling Editor: Dr Johnny Mahlangu
REFERENCES
- 1. Quiroga T, Mezzano D. Is my patient a bleeder? A diagnostic framework for mild bleeding disorders. Hematology. 2012;2012:466‐474. doi: 10.1182/asheducation-2012.1.466 [DOI] [PubMed] [Google Scholar]
- 2. Gebhart J, Hofer S, Panzer S, et al. High proportion of patients with bleeding of unknown cause in persons with a mild‐to‐moderate bleeding tendency: Results from the Vienna Bleeding Biobank (VIBB). Haemophilia. 2018;24:405‐413. doi: 10.1111/hae.13422 [DOI] [PubMed] [Google Scholar]
- 3. Zegers SAM, Smit Y, Saes JL, et al. Diagnostic work up of patients with increased bleeding tendency. Haemophilia. 2020;26:269‐277. doi: 10.1111/hae.13922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boender J, Kruip MJ, Leebeek FW. A diagnostic approach to mild bleeding disorders. J Thromb Haemost. 2016;14:1507‐1516. doi: 10.1111/jth.13368 [DOI] [PubMed] [Google Scholar]
- 5. Rodeghiero F, Pabinger I, Ragni M, et al. Fundamentals for a systematic approach to mild and moderate inherited bleeding disorders: An EHA consensus report. Hemasphere. 2019;3:e286. doi: 10.1097/HS9.0000000000000286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kolev K, Longstaff C. Bleeding related to disturbed fibrinolysis. Br J Haematol. 2016;175:12‐23. doi: 10.1111/bjh.14255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saes JL, Schols SEM, van Heerde WL, Nijziel MR. Hemorrhagic disorders of fibrinolysis: a clinical review. J Thromb Haemost. 2018;16(8):1498‐1509. doi: 10.1111/jth.14160 [DOI] [PubMed] [Google Scholar]
- 8. Cesarman‐Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. Br J Haematol. 2005;129:307‐321. doi: 10.1111/j.1365-2141.2005.05444.x [DOI] [PubMed] [Google Scholar]
- 9. Longstaff C, Kolev K. Basic mechanisms and regulation of fibrinolysis. J Thromb Haemost. 2015;13(Suppl 1):S98‐105. doi: 10.1111/jth.12935 [DOI] [PubMed] [Google Scholar]
- 10. Rijken DC, Lijnen HR. New insights into the molecular mechanisms of the fibrinolytic system. J Thromb Haemost. 2009;7:4‐13. doi: 10.1111/j.1538-7836.2008.03220.x [DOI] [PubMed] [Google Scholar]
- 11. Lijnen HR. Elements of the fibrinolytic system. Ann N Y Acad Sci. 2001;936:226‐236. doi: 10.1111/j.1749-6632.2001.tb03511.x [DOI] [PubMed] [Google Scholar]
- 12. Silva MM, Thelwell C, Williams SC, Longstaff C. Regulation of fibrinolysis by C‐terminal lysines operates through plasminogen and plasmin but not tissue‐type plasminogen activator. J Thromb Haemost. 2012;10:2354‐2360. doi: 10.1111/j.1538-7836.2012.04925.x [DOI] [PubMed] [Google Scholar]
- 13. Foley JH, Kim PY, Mutch NJ, Gils A. Insights into thrombin activatable fibrinolysis inhibitor function and regulation. J Thromb Haemost. 2013;11(Suppl 1):306‐315. doi: 10.1111/jth.12216 [DOI] [PubMed] [Google Scholar]
- 14. Fay WP, Parker AC, Condrey LR, Shapiro AD. Human plasminogen activator inhibitor‐1 (PAI‐1) deficiency: characterization of a large kindred with a null mutation in the PAI‐1 gene. Blood. 1997;90:204‐208. [PubMed] [Google Scholar]
- 15. Fay WP, Shapiro AD, Shih JL, Schleef RR, Ginsburg D. Brief report: complete deficiency of plasminogen‐activator inhibitor type 1 due to a frame‐shift mutation. N Engl J Med. 1992;327:1729‐1733. doi: 10.1056/NEJM199212103272406 [DOI] [PubMed] [Google Scholar]
- 16. Leebeek FW, Stibbe J, Knot EA, Kluft C, Gomes MJ, Beudeker M. Mild haemostatic problems associated with congenital heterozygous alpha 2‐antiplasmin deficiency. Thromb Haemost. 1988;59:96‐100. [PubMed] [Google Scholar]
- 17. Mehta R, Shapiro AD. Plasminogen activator inhibitor type 1 deficiency. Haemophilia. 2008;14:1255‐1260. doi: 10.1111/j.1365-2516.2008.01834.x [DOI] [PubMed] [Google Scholar]
- 18. Iwaki T, Nagahashi K, Takano K, et al. Mutation in a highly conserved glycine residue in strand 5B of plasminogen activator inhibitor 1 causes polymerisation. Thromb Haemost. 2017;117:860‐869. doi: 10.1160/TH16-07-0572 [DOI] [PubMed] [Google Scholar]
- 19. Iwaki T, Tanaka A, Miyawaki Y, et al. Life‐threatening hemorrhage and prolonged wound healing are remarkable phenotypes manifested by complete plasminogen activator inhibitor‐1 deficiency in humans. J Thromb Haemost. 2011;9:1200‐1206. doi: 10.1111/j.1538-7836.2011.04288.x [DOI] [PubMed] [Google Scholar]
- 20. Gebhart J, Kepa S, Hofer S, et al. Fibrinolysis in patients with a mild‐to‐moderate bleeding tendency of unknown cause. Ann Hematol. 2017;96:489‐495. doi: 10.1007/s00277-016-2893-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hofer S, Ay C, Rejto J, et al. Thrombin‐generating potential, plasma clot formation, and clot lysis are impaired in patients with bleeding of unknown cause. J Thromb Haemost. 2019;17:1478‐1488. doi: 10.1111/jth.14529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agren A, Kolmert T, Wiman B, Schulman S. Low PAI‐1 activity in relation to the risk for perioperative bleeding complications in transurethral resection of the prostate. Thromb Res. 2007;119:715‐721. doi: 10.1016/j.thromres.2006.06.014 [DOI] [PubMed] [Google Scholar]
- 23. Agren A, Wiman B, Schulman S. Laboratory evidence of hyperfibrinolysis in association with low plasminogen activator inhibitor type 1 activity. Blood Coagul Fibrinolysis. 2007;18:657‐660. doi: 10.1097/MBC.0b013e3282dded21 [DOI] [PubMed] [Google Scholar]
- 24. Agren A, Wiman B, Stiller V, et al. Evaluation of low PAI‐1 activity as a risk factor for hemorrhagic diathesis. J Thromb Haemost. 2006;4:201‐208. doi: 10.1111/j.1538-7836.2005.01709.x [DOI] [PubMed] [Google Scholar]
- 25. Wiewel‐Verschueren S, Knol HM, Lisman T, et al. No increased systemic fibrinolysis in women with heavy menstrual bleeding. J Thromb Haemost. 2014;12:1488‐1493. doi: 10.1111/jth.12645 [DOI] [PubMed] [Google Scholar]
- 26. Szczepaniak P, Zabczyk M, Undas A. Increased plasma clot permeability and susceptibility to lysis are associated with heavy menstrual bleeding of unknown cause: a case‐control study. PLoS One. 2015;10:e0125069. doi: 10.1371/journal.pone.0125069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453‐1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 28. Tosetto A, Rodeghiero F, Castaman G, et al. A quantitative analysis of bleeding symptoms in type 1 von Willebrand disease: results from a multicenter European study (MCMDM‐1 VWD). J Thromb Haemost. 2006;4:766‐773. doi: 10.1111/j.1538-7836.2006.01847.x [DOI] [PubMed] [Google Scholar]
- 29. Bowman M, Mundell G, Grabell J, et al. Generation and validation of the condensed MCMDM‐1VWD bleeding questionnaire for von Willebrand disease. J Thromb Haemost. 2008;6:2062‐2066. doi: 10.1111/j.1538-7836.2008.03182.x [DOI] [PubMed] [Google Scholar]
- 30. Angleton P, Chandler WL, Schmer G. Diurnal variation of tissue‐type plasminogen activator and its rapid inhibitor (PAI‐1). Circulation. 1989;79:101‐106. doi: 10.1161/01.cir.79.1.101 [DOI] [PubMed] [Google Scholar]
- 31. Cooper PCPFE. Euglobulin clot lysis time. In: Jespersen JBRM, Haverkate F, editors. ECAT Assay procedures a manual of laboratory techniques. Springer; 1992. p. 125‐129. [Google Scholar]
- 32. Colonia VJ, Roisenberg I. Investigation of associations between ABO blood groups and coagulation, fibrinolysis, total lipids, cholesterol, and triglycerides. Hum Genet. 1979;48:221‐230. doi: 10.1007/BF00286907 [DOI] [PubMed] [Google Scholar]
- 33. Ilich A, Noubouossie DF, Henderson M, et al. Development and application of global assays of hyper‐ and hypofibrinolysis. Res Pract Thromb Haemost. 2020;4:46‐53. doi: 10.1002/rth2.12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weisel JW, Litvinov RI. Red blood cells: the forgotten player in hemostasis and thrombosis. J Thromb Haemost. 2019;17:271‐282. doi: 10.1111/jth.14360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Urano T, Nishikawa T, Nagai N, Takada Y, Takada A. Amounts of tPA and PAI‐1 in the euglobulin fraction obtained at different pH: their relation to the euglobulin clot lysis time. Thromb Res. 1997;88:75‐80. doi: 10.1016/s0049-3848(97)00193-x [DOI] [PubMed] [Google Scholar]
- 36. Urano T, Sakakibara K, Rydzewski A, Urano S, Takada Y, Takada A. Relationships between euglobulin clot lysis time and the plasma levels of tissue plasminogen activator and plasminogen activator inhibitor 1. Thromb Haemost. 1990;63:82‐86. [PubMed] [Google Scholar]
- 37. Urano T, Sumiyoshi K, Pietraszek MH, Takada Y, Takada A. PAI‐1 plays an important role in the expression of t‐PA activity in the euglobulin clot lysis by controlling the concentration of free t‐PA. Thromb Haemost. 1991;66:474‐478. [PubMed] [Google Scholar]
- 38. Minowa H, Takahashi Y, Tanaka T, et al. Four cases of bleeding diathesis in children due to congenital plasminogen activator inhibitor‐1 deficiency. Haemostasis. 1999;29:286‐291. doi: 10.1159/000022514 [DOI] [PubMed] [Google Scholar]
- 39. Urano T, Suzuki Y, Arakida M, Kanamori M, Takada A. The expression of exercise‐induced tPA activity in blood is regulated by the basal level of PAI‐1. Thromb Haemost. 2001;85:751‐752. [PubMed] [Google Scholar]
- 40. van Geffen M, van Heerde WL. Global haemostasis assays, from bench to bedside. Thromb Res. 2012;129:681‐687. doi: 10.1016/j.thromres.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 41. van Geffen M, Loof A, Lap P, et al. A novel hemostasis assay for the simultaneous measurement of coagulation and fibrinolysis. Hematology. 2011;16:327‐336. doi: 10.1179/102453311X13085644680348 [DOI] [PubMed] [Google Scholar]
- 42. Saes JL, Schols SEM, Betbadal KF, et al. Thrombin and plasmin generation in patients with plasminogen or plasminogen activator inhibitor type 1 deficiency. Haemophilia. 2019;25:1073‐1082. doi: 10.1111/hae.13842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moenen F, Nelemans PJ, Schols SEM, Schouten HC, Henskens YMC, Beckers EAM. The diagnostic accuracy of bleeding assessment tools for the identification of patients with mild bleeding disorders: a systematic review. Haemophilia. 2018;24:525‐535. doi: 10.1111/hae.13486 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


