Abstract
Background
In western medicine, cervical ectropion is considered as a normal finding that does not require treatment despite the red and inflamed appearance of the cervix. However, cervical ectropion is considered as one of the most common types of chronic cervicitis in China. Topical treatments for cervical ectropion, including microwave tissue coagulation, are widely used in many hospitals in China they are considered likely to reduce the chances of development of abnormal metaplasia and infection.
Objectives
To compare the efficacy and potential side effects of microwave tissue coagulation with other interventions or no intervention in the treatment of cervical ectropion.
Search methods
We searched The Cochrane Library, MEDLINE, EMBASE, Chinese Biomedical Literature Database (CBM), Chinese Medical Current Contents (CMCC), CAJ Full‐text Database and the Chinese Scientific Journals Database. We also searched for related literature on the Internet with search engines such as Google and searched the reference lists of articles and handsearched relevant Chinese journals. The search of these databases was conducted from inception to May 2009.
Selection criteria
Only authentic randomised controlled trials (RCTs) were considered for inclusion.
Data collection and analysis
Two review authors independently interviewed the original authors of claimed RCTs published in China and then assessed the risk of bias of three RCTs and extracted data.
Main results
No studies were found that met the participant inclusion criteria of the protocol. One hundred and thirty‐three studies were reported as RCTs, but only only three studies were identified as authentic RCTs. However, it was not possible to confirm that the participants were symptomatic prior to treatment. and the methodological quality of the three RCTs was generally low. No trial compared treatment to no intervention and most trials didn't assess relief of symptoms or quality of life and satisfaction, which are very important to women.
Authors' conclusions
There were no RCTs in symptomatic women with cervical ectropion comparing microwave therapy with another treatment or no treatment. Although microwave therapy improved the appearance of the cervix compared with both laser and interferon‐alpha suppository therapy, any other benefit for the women was not clear.
Plain language summary
Microwave therapy for cervical ectropion
In western countries, cervical ectropion or erosion caused by the movement of columnar epithelium onto the vaginal portion of the cervix is considered a normal physiological process not requiring intervention. In China, however, cervical ectropion is considered as one of the most common types of chronic cervicitis and is often treated. One of the topical treatments widely used is microwave tissue coagulation (MTC). From Chinese databases, the authors identified 131 potential randomised controlled clinical trials (RCTs) that compared microwave therapy to no treatment or other treatments. Only three of these studies involving a total of 540 participants were identified as RCTs. Two studies (420 participants) compared MTC versus laser, and one study (120 participants) compared MTC with an interferon‐alpha suppository. The follow‐up period of the RCTs was adequate. None of the studies assessed relief of symptoms or quality of life and satisfaction, which are very important to women. The authors of the review considered that they could not answer the review question about the appropriate use of microwave therapy as only these three low quality RCTs were found. Although the trials showed improved appearance of the cervix with microwave treatment compared with the control therapy, it was not possible to ascertain whether the women were symptomatic before treatment. The review protocol also required participants to be symptomatic with mucopurulent discharge or to have contact bleeding before treatment. At present, intervention for inflammatory cervical ectropion is still controversial.
Background
Description of the condition
The term cervical ectropion (CE) has replaced that of cervical erosion. It is also sometimes referred to as the transformation zone of the cervix. A cervical ectropion occurs when, due to hormonal changes, the columnar epithelium moves onto the vaginal portion of the cervix. A cervical ectropion appears red and may have an inflamed appearance due to the columnar epithelium being thinner than squamous epithelium. This makes the underlying blood vessels more apparent. Along with the red appearance, the columnar epithelium may also secrete mucus which, if abundant, causes a vaginal discharge (Chang 1991). In western countries, cervical ectropion is considered as a physiological process not requiring treatment. In China, however, cervical ectropion is considered as one of the most common types of chronic cervicitis and it is thought that severe cervical ectropion may cause infertility (Dalgic 2001). As a result, treatment for cervical ectropion is widely practiced in hospitals in China. It is reported that 20% to 50% of Chinese women of reproductive age have cervical ectropion (Zhang 1995; Cai 1997; Peng 2002; Xiao 2003; Zhang 2003).
In the Chinese medical literature, the occurrence of cervical ectropion is not considered to be caused solely by estrogenic hormonal changes; inflammation and trauma have also been implicated in its etiology (Yue 2000). Inflammatory ectropion presents as a defect or degeneration and necrosis of cervical squamous epithelium combined with infiltration of large numbers of inflammatory cells as evidenced using histopathology. On a smear, superficial, intermediate and basement cells can be found with degenerated and necrotic cells, mild dyskaryotic cells and large numbers of neutrophils; small numbers of monocytes, lymphocytes, plasmocytes and macrophages can also be seen (Tang 1995).
Cervical ectropion has been associated with both the combined oral contraceptive pill and chlamydia trachomatis (Critchlow 1995). Changing the method of contraception may be considered for those women who are unable to accept the increase in physiological discharge associated with cervical ectropion. Screening for chlamydia and other sexually transmitted infections should be considered for those women who are at risk. Ectropion has also been found in 5% to 25% of women presenting with postcoital bleeding (Selo‐Ojeme 2004). These women need appropriate follow up with a cervical smear and colposcopy.
Clinical macroscopic examination describes three grades (I, II and III) based on size, the severity of the lesion and the rate of growth of columnar epithelium. These are described as simple pattern, granular pattern and mammilla pattern (Yue 2005). Macroscopically it may be difficult to distinguish cervical ectropion from cervical intraepithelial neoplasia (CIN), cancer in situ. Thus a cervical smear for cytological examination should be routinely performed to exclude dysplasia. In addition, colposcopy and a biopsy may also be needed for histopathologic examination.
Description of the intervention
Microwave therapy uses electromagnetic waves at a frequency of up to 2.45 billion Hz. The biophysical effect at the target site is through both an internal heating effect and a non‐heating effect. Unlike the external heating of other physical treatments, microwave tissue coagulation (MTC) involves internal heating using human tissue as the heat source (Guan 2004).
Treatments are only performed after excluding malignancy and specific or non‐specific cervical or vaginal infections. The purpose of treatment is to induce necrosis and exfoliation of the columnar epithelium at the affected site and allow repair by neoformative squamous epithelium. This, in turn, is considered likely to reduce the chances of development of abnormal metaplasia and infection.
How the intervention might work
When the microwave electrode touches the topical lesion, a microwave magnetic field forms in the tissue and the small‐scope high temperature produced instantly, which induces degeneration, coagulation and necrosis of the topical tissue protein. New squamous cells then migrate from peripheral tissue and repair the wound. The general non‐heating effect of microwave induces dilation of superficial blood vessels, acceleration of blood flow and enhancement of tissue metabolism, which in turn promote the elimination of inflammatory products. There is, therefore, a synergistic action within the therapy (Guan 2004).
Why it is important to do this review
Presently, the rationale for use of microwave therapy for asymptomatic cervical ectropion is unclear.
Although this systematic review was first published in the Cochrane Database of Systematic Review in 2007, many methods, including microwave treatment, are still used to treat cervical ectropion in China. It is necessary to evaluate these interventions.
Objectives
To compare the efficacy and the potential side effects of microwave tissue coagulation with other interventions or no intervention in the treatment of cervical ectropion.
Methods
Criteria for considering studies for this review
Types of studies
Only authentic randomised controlled trials (RCTs) were included in the review.
Types of participants
Women aged 18 to 60 years with cervical ectropion who were symptomatic with mucopurulent vaginal discharge or contact bleeding, or both. The women were diagnosed by vaginal examination and graded by ectropion size and tissue pattern.
Diagnostic criteria
Macroscopic examination to discriminate between acute and chronic cervicitis.
Three grades were based on ectropion size: grade I, covering one third of the cervix; grade II, from one third to two thirds; grade III, over two thirds of the cervix. Three patterns were defined based on the severity of the lesion and the growth of columnar epithelium: simple pattern, granular pattern and mammilla pattern. A simple pattern was where the surface of the ectropion was planus and covered by simple columnar epithelium; the granular pattern was where the surface was not smooth and the glandular epithelium was hyperplasmic, accompanied by hyperplasmic mesenchyma; the mammilla pattern was where the surface was more severely uneven.
Exclusion criteria
Asymptomatic ectropion.
Women who were pregnant or post‐menopausal
Women with cervical intraepithelial neoplasia (CIN1, 2, 3), cancer in situ, or early cervical cancer diagnosed through a Papanicolaou test, histological examination or colposcopy.
Women with vaginal or cervical infection (vulvovaginal candidiasis, bacterial vaginosis, trichomonas vaginitis, chlamydial or gonococcal infection).
Types of interventions
Microwave tissue coagulation (MTC) versus no treatment or another treatment such as laser coagulation, infrared light, an interferon‐alpha suppository (Aoping suppository), the combination of MTC with an interferon‐alpha suppository, electrocautery or cryotherapy.
Types of outcome measures
Primary outcomes
1) Relief of symptoms ‐ vaginal discharge, contact bleeding as assessed by the woman or health professional, or both. 2) Quality of life or satisfaction. 3) Appearance of the cervix.
Secondary outcomes
Assessment criterion of curative effect
1) Healing well: cervix had become smooth and glossy, ectropion had disappeared or lesion on cervical surface had become squamous metaplasia with the new squamous epithelium coloured on iodine testing. 2) Not healing well: reduction in the grade of cervix ectropion or no change after treatment.
Additional outcome measure
1) The rate of bleeding during operation.
Follow‐up period
For measurement of cervical ectropion, a follow‐up period of 8 to 12 weeks was considered adequate. For symptoms of vaginal discharge and contact bleeding (and quality of life, satisfaction), a follow‐up period of more than three months was considered adequate.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Menstrual Disorders and Subfertility Group Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 2) , MEDLINE (January 1966 to May 2009), EMBASE (January 1974 to May 2009), Chinese Biomedical Literature Database (CBM) (January 1978 to May 2009), The Chinese Medical Current Contents (CMCC) (January 1994 to May 2009), CAJ Full‐text Database (CNKI) (January 1994 to May 2009), and Chinese Scientific Journals Database (VIP) (January 1989 to May 2009). No language restrictions were made. We used the search strategies detailed in Appendix 1; Appendix 2; and Appendix 3.
Searching other resources
We also searched for related literature on the Internet with search engines such as Google (30 May 2009), the reference lists of articles and handsearched relevant Chinese journals that were most likely to publish trials on cervical ectropion. We made attempts to contact some experts and manufacturers but did not identify any related studies in this way.
Data collection and analysis
Selection of studies
We developed a standardized data extraction form and performed a pilot to ensure consistency and completeness. Two review authors (LYL, ZP) independently selected the potential studies for inclusion. Two review authors telephone interviewed the authors of claimed RCTs and considered trials for inclusion using a specially designed questionnaire (see Additional Table 1).
1. Questions used to identify an authentic RCT.
| Process | Questions |
| Question 1 | Introduce myself and purpose: How do you do? I was a student of Lanzhou University, was doing a review about microwave tissue coagulation in the treatment of cervical ectropion. Purpose of my study was to compare the effects of variety randomisation methods. I had searched out a paper that published in (time, journal) written by you. Could you please tell me what method to be used in this trial? |
| Question 2 | If the subject cannot describe the method clearly, change the question like this: could you please tell me when a new participant enrolled, how did you decide which group the participant should be allocated to? |
| Question 3 | If there was any problem about the first author, the second author or others should be interviewed. |
| Question 4 | Next two questions aim to understand the category of support source for investigated study, I selected one of them or both: (1) Was your study funded by government or any other source? (2) Was your study for new drug development? |
| Question 5 | Judgment should be made immediately for whether it was a real RCT or not. If it be judged as real RCT, next questions aim to understand the status of allocation concealment: (1) Do you know allocation concealment? If so, please clarify. (2) Did you use any method to mask allocation sequence? If any, please clarify. |
| Question 6 | Next question aims to understand the validity of blinding particularly pay attention to whether the detector was blinded or not: Please tell me who were blinded in your trial? |
| Question 7 | We said "thank you" to author. |
| Question 8 | Finally, We needed to judge whether the subject knew well the trial principle or not: if anyone persisted in the method of randomisation as correct but actually wrong or ineligible, it should be judged didn't know; if anyone claimed that "I knew we performed not good enough" or "it was impossible to perform a completely correct RCT" and so on, it should be judged as knew well about the principle of trial but violated knowingly claimed the non‐RCT as RCT. |
| Question 9 | Put all of record in the form. |
RCTs: randomised controlled trials
Data extraction and management
It was planned for two review authors (TJ, MB) to independently enter data into a data extraction form. Discrepancies would be resolved by a third review author (LYL). We obtained missing data from authors wherever possible. Data were to be checked and entered into RevMan by one review author (LYL). However, in the event there were no eligible studies.
Assessment of risk of bias in included studies
We assessed the risk of bias of each trial in terms of adequate sequence generation, allocation concealment, blinding, that incomplete outcome data were addressed, the trial was free of selective reporting and free of other biases. These were classified as 'Yes' for low risk of bias; 'No' for high risk of bias; or 'Unclear' for moderate risk of bias, according to the guidelines of the Cochrane Handbook of Systematic Reviews of Interventions 5.0.1 and described by Wu 2007. Sensitivity analyses were to be undertaken on the basis of whether the quality factors were 'Yes', 'No' or 'Unclear'. Differences were to be resolved by discussion among the review authors.
The following characteristics were assessed.
Sequence generation
(1) Yes, adequate sequence generation was reported using one of the following approaches: random number tables, computer‐generated random numbers. (2) No, other methods of allocation used that appeared to be biased.
(3) Unclear, did not specify one of the adequate methods outlined in (1) but mentioned 'random'.
Allocation sequence concealment
(1) Yes, adequate measures to conceal allocations with the key being that the person who generated the allocation sequence did not recruit or allocate the participants, such as central randomisation; serially‐numbered, opaque, sealed envelopes; or another description that contained convincing elements of concealment. (2) No, inadequately concealed allocation where the description reported an approach that did not fall into one of the categories in (1). Did not conceal allocation.
(3) Unclear, unclearly concealed trials in which the author did not report an allocation concealment approach at all.
Blinding
(1) Blinding of participants (Yes, No or Unclear). (2) Blinding of caregivers (Yes, No or Unclear). (3) Blinding of outcome assessment (Yes, No or Unclear).
Incomplete outcome data addressed
(1) Trials where an intention‐to‐treat analysis was possible and few participants were lost to follow up. (2) Trials which reported exclusions, as listed in (1), but exclusions were less than 10%. (3) No reporting on exclusions, exclusions of at least 10%, or wide differences in exclusions between groups.
Free of selective reporting
(1) Yes, if all the important outcomes (the primary outcomes stated in the review) were reported or if the trial protocol was available and all of the trial's pre‐specified (primary and secondary) outcomes that were of interest to this review were reported.
(2) No, if not all the pre‐specified outcomes were reported, the primary outcomes were changed, or if some of the important outcomes were incompletely reported.
(3) Unclear, if there is insufficient information to assess whether the risk of selective outcome reporting was present.
Free of other bias
Source of funding:
(1) Yes, if the trial was unfunded;
(2) No, if the trial was funded;
(3) Unclear, if the source of funding was not clear.
Baseline imbalance:
(1) Yes, if there was no baseline imbalance in important characteristics;
(2) No, if there was a baseline imbalance due to chance or due to imbalanced exclusion after randomisation;
(3) Unclear, if the baseline characteristics were not reported.
Measures of treatment effect
For dichotomous data, two‐by‐two tables were generated for each trial and the data expressed as a risk ratio (RR) with 95% confidence intervals (CI), for example cure rate and rate of bleeding during operation.
Unit of analysis issues
The included trials were to have a simple parallel group design where participants were individually randomised to treatment and control groups.
Dealing with missing data
We contacted the original authors to request missing data. Then we would discuss why data may be missing.
Assessment of heterogeneity
We were to assess statistical heterogeneity (variation) between the results of different studies included in the meta‐analysis by inspecting the scatter in the data points on the graph and the overlap in their confidence intervals. The Chi2 test was used with significance set at a P value less than 0.1 and the I2 statistic to estimate the total variation across studies due to heterogeneity, where I2 less than 25% was considered as low‐level heterogeneity, 25% to 50% as moderate level and higher than 50% as high‐level heterogeneity (Higgins 2003).
Assessment of reporting biases
Potential publication bias was not tested by a funnel plot because there were not sufficient trials.
Data synthesis
It was planned to analyse abstracted data following the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009) and to use RevMan software to complete the data analysis. The results of individual studies would be pooled only if they were clinically similar with respect to the participants, interventions and clinical criteria for defining outcomes.
Published graphs were to display the results using the fixed‐effect approach unless there was statistical heterogeneity, in which case the random‐effects model was to be used. However, unless the results are robust to both models, they would need to be treated with caution. The results may be converted to absolute measures, such as the number needed to treat (NNT). Continuous data were to be expressed as mean differences (MD) and 95% confidence intervals. Meta‐analytic methods for continuous data assume that the underlying distribution of the measurements is normal. Results were also given as reported in the publication, for example as median and range with non‐parametric tests of significance.
Subgroup analysis and investigation of heterogeneity
It was planned to perform subgroup analysis according to different interventions and the grades of cervical ectropion.
Sensitivity analysis
We planned to conduct a sensitivity analysis and to explore the effect of including only trials with adequate methodology.
Results
Description of studies
Results of the search
No English language studies were identified in The Cochrane Library, MEDLINE or EMBASE. One hundred and thirty‐three potentially eligible Chinese trials were identified using the described search strategy and inclusion criteria. We tried our best to contact the authors of claimed RCTs to get missing information about randomisation and other data. No unpublished studies, grey literature or ongoing trials were found (see Figure 1).
1.
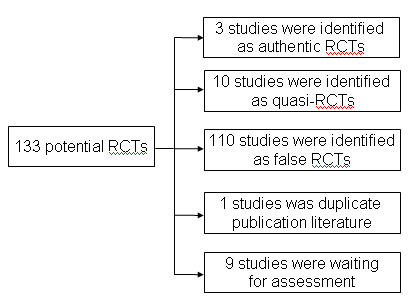
Flow chart of the study excluded process
Included studies
No studies were available for review.
Excluded studies
One hundred and twenty‐four studies were excluded (Characteristics of excluded studies). Ten studies were identified as quasi‐randomised controlled trials from the description of the authors; 105 were identified as non‐RCTs and excluded. The others were excluded as there was no documentation on whether the women who had cervical ectropion were symptomatic. One was a duplicate publication. Nine studies were not assessed because we were unable to access the full‐text versions or failed to contact the authors. Finally, only three studies were identified as true randomised controlled trials. The three trials were single centre and had a parallel group design. However, it was not possible to ascertain whether any of these three randomised studies met the participant inclusion or exclusion criteria as there was no documentation as to whether the women who had cervical ectropion were symptomatic. We have presented the findings of these three RCTs in Additional tables (see Additional Table 2; Table 3; Table 4).
2. Characteristics of the first RCT.
| Study | Song 1994 |
| Methods | Randomization method not stated.
Parallel, single centre, single blinded study.
Number of women randomised: n = 300;
Number of women analysed: n = 300.
Units of comparison: individuals.
Source of funding: unclear. RCT duration: from Jan 1991 to May 1992 |
| Participants | Inclusion criteria: women with cervical ectropion. Macroscopic examination: discriminating acute with chronic cervicitis. Age: MTC group (n = 154): 19 to 57 years (mean age 33); Laser group (n = 146): 28 to 54 years (mean age 35). Average gravidity: MTC group: 2.2; laser group: 1.9. Average parity: MTC group: 1.0; laser group: 1.0. Grade II: MTC group: 32; laser group: 43; Grade III: MTC group: 122; laser group: 103. Symptom: unclear. Exclusion criteria: excluded women with cervical intraepithelial neoplasia (CIN1, 2, 3), cancer in situ or early cervical cancer which were diagnosed through Papanicolaou test and/or histological examination or colposcopy. Excluded women with vaginal or cervical infection. Source of participants: medical outpatients. |
| Interventions | Treatment: MTC; Control: CO2 laser. Timing of treatment: during 3 to 7 days after menstrual period (once). |
| Outcomes | Efficacy: cure rate (according to appearance of cervix). Adverse effect: bleeding during operation, bellyache during operation, bleeding post‐operation, topical infection bleeding post‐operation. The follow‐up period: 8‐12 weeks. |
| Note | Location: China Setting: Department of Gynaecology and Obstetrics of Chaoyang Hospital Funding: unclear |
MTC: microwave tissue coagulation
CIN: cervical intraepithelial neoplasia
3. Characteristics of the second RCT.
| Study | Wang 2003 |
| Methods | Randomisation: random digits table;
Parallel, single centre, no blinding.
Number of women randomised: n = 120;
Number of women analysed: n = 120.
Units of comparison: individuals.
Source of funding: unclear. RCT duration: from Aug 2001 to Oct 2001. |
| Participants | Inclusion criteria: women with cervical ectropion. Age: MTC group (n = 60): unclear; Laser group (n = 60): unclear. Grade I: MTC group: 8; laser group: 9; Grade II: MTC group: 27; laser group: 28; Grade III: MTC group: 25; laser group: 23; Simple pattern: MTC group: 42; laser group: 43; Granular pattern or Mammilla pattern: MTC group: 18; laser group: 17. Symptom: unclear. Exclusion criteria: Excluded women with cervical intraepithelial neoplasia (CIN1, 2, 3), cancer in situ or early cervical cancer. Excluded women with vaginal or cervical infection. Source of participants: not stated. |
| Interventions | Treatment: MTC; Control: CO2 laser. Timing of treatment: during 3 to 7 days after menstrual period (once). |
| Outcomes | Efficacy: Cure rate (According to appearance of cervix); Adverse effect: vaginal discharge post‐treatment. The follow‐up period: 2,3,4,6,8,12 weeks. |
| Note | Location: China. Setting: Shiyan Maternal and Child Health Hospital, Hubei. Funding: unclear. |
MTC: microwave tissue coagulation
CIN: cervical intraepithelial neoplasia
4. Characteristics of the third RCT.
| Study | Xu 2004 |
| Methods | Randomisation: random digits table; Parallel, single centre, no blinding. Number of women randomised: n = 120; Number of women analysed: n = 120. Units of comparison: individuals. Source of funding: Unclear. RCT duration: from Jun 2000 to May 2002. |
| Participants | Inclusion criteria: women with cervical ectropion. Papanicolaou test: Grade I or II. Age: 23 to 50 years (mean age 33). Grade I: MTC group: 15; Laser group: 13; Grade II: MTC group: 27; Laser group: 28; Grade III: MTC group: 19; Laser group: 18. Symptom: unclear. Exclusion criteria: not reported. Source of participants: medical outpatient. |
| Interventions | Treatment: MTC (once); Control: interferon‐alpha suppository (aoping suppository): 6000 U once/two days; duration: 6‐10 days. (Repeat it after next menstrual period). Timing of treatment: during 3 to 7 days after menstrual period. |
| Outcomes | Efficacy: cure rate (according to appearance of cervix); Adverse effect: vaginal discharge post‐treatment. The follow‐up period: 8,to 12 weeks. |
| Note | Location: China Setting: Department of Gynaecology and Obstetrics of The First Hospital of Nanping, Fujian. Funding: unclear |
MTC: microwave tissue coagulation
CIN: cervical intraepithelial neoplasia
Risk of bias in included studies
No studies were available for review. We assessed the risk of bias of the excluded RCTs.
Allocation
None
Blinding
None
Incomplete outcome data
None
Selective reporting
None
Other potential sources of bias
None
Effects of interventions
No studies were found that met the participant inclusion criteria of the protocol. Although three studies were identified as authentic RCTs, from 131 potential RCTs, it was not possible to confirm that the participants were symptomatic prior to treatment.
Discussion
Summary of main results
In China, cervical ectropion is considered as one of the most common types of chronic cervicitis and requires treatment. Three randomised studies involving 540 participants were found. Two studies (Song 1994; Xu 2004) compared MTC versus laser (420 participants) and one study (Wang 2003) compared MTC versus interferon‐alpha suppository. Relief of symptoms, quality of life and satisfaction were not reported in any of the three studies. The study results suggested that microwave therapy gave similar or greater improvements in the appearance of the cervix over laser or interferon suppository use. One study reported the rate of bleeding during operation (Song 1994). The difference was statistically significant (RR 0.15, 95% CI 0.06 to 0.37; 300 participants, one trial) between the MTC and laser groups. Other adverse effects such as bleeding and topical infection after operation were also reported in Song 1994. No differences were found between the two groups. The other study (Xu 2004) reported a statistically significant difference for the duration of vaginal discharge post‐treatment (mean difference ‐3.30, 95% CI ‐5.27 to ‐1.33; 120 participants, one trial).
Wang 2003 reported that bleeding after operation occurred in a few participants with no difference between MTC and an interferon‐alpha suppository (120 participants, one trial).
However, none of these trials employed assessor blinding, which raises the possibility of assessor bias. In addition, there were no data to clarify whether these women were symptomatic before treatment or received any benefit from the treatment in addition to improved cervical appearance. No study compared microwave therapy with no treatment. This is important as the rationale for use of this therapy for cervical ectropion is not clear.
Overall completeness and applicability of evidence
Up to now, no clearly applicable evidence has been found to show any benefit from MTC in the treatment of cervical ectropion.
Quality of the evidence
The information on quality issues for the three RCTs was poorly reported. Although we were finally able to access this information, from the original authors, the risk of recall bias is high. There were also varying standards of classification for the grade and subtype of cervical ectropion. Consideration of sample sizes was not reported, which may lead to a low‐test power. We found that most Chinese authors misunderstood the concept of randomisation. One hundred and thirty‐three studies were reported as RCTs, however only three were authentic RCTs (2.3%, 3/131). Some of studies were experience collections or case collections and most of them were written retrospectively.
The quality of the three RCTs was poor (see Additional Table 5).
5. Quality table.
| Study ID | Song 1994 | Xu 2004 | Wang 2003 |
| Design | Parallel single‐centre. | Parallel single‐centre. | Parallel single‐centre. |
| Adequate sequence generation? | Yes. | Yes(random number table). | Yes(random number table). |
| Allocation concealment? | Yes(envelopes). | No. | No. |
| Blinding? | Yes (Single: participants). | No. | No. |
| Incomplete outcome data addressed? | Unclear. | Unclear. | Unclear. |
| Free of selective reporting? | Unclear. | Unclear. | Unclear. |
| Free of other bias? | Unclear. | Unclear. | Unclear. |
| Power calculation | Unclear. | Unclear. | Unclear. |
There was not enough information in the trial reports to determine whether the allocation sequence was adequately concealed. We contacted the authors by telephone. Two trials used random number tables (Wang 2003; Xu 2004). One trial used adequate concealment (envelopes) prior to allocation (Song 1994). Concealment was not used in the other two trials (Wang 2003; Xu 2004).
No trial mentioned blinding. By interviewing the authors it was identified that only one trial was single blind, for the participants (Song 1994). The assessors were not blinded in any trial.
As follow up and withdrawal rates were not reported in all trials, and no participant 'Flow diagram' was available, it is unclear whether all the participants were followed up. As a result, we judged the losses to follow up and withdrawal from the trials by the data presented.
The three trials did not report on 'relief of symptoms' or 'quality of life or satisfaction', which were primary outcomes for this review. We were unable to obtain any information about the protocol for these trials. They were judged ;Unclear' due to insufficient information.
No trial mentioned other potential sources of bias, and the source of funding was not clear.
Potential biases in the review process
We failed to find any unpublished or ongoing trials. This may have led to potential bias.
Agreements and disagreements with other studies or reviews
This review was originally published two years ago and there has not been any other systematic review about this intervention. It is not clear whether the methods, including microwave treatment, have any benefit for women. However, women with cervical ectropion continue to have treatment with various topical treatments. Many of these studies are published in Chinese journals and suggest effectiveness but none of them meet the requirements of a randomised controlled clinical trial.
Authors' conclusions
Implications for practice.
Although the appearance of the cervix can be improved with microwave and other therapies it is not clear whether this has any benefit for the women. Women presenting with increased vaginal discharge from an ectropion can be advised that this is physiological, following swabs to rule out sexually transmitted infections, bacterial vaginosis and candidiasis. Postcoital bleeding, with an associated ectropion, requires follow up with cervical cytology and colposcopy to exclude cervical cancer or its precursors.
Implications for research.
Further studies should be undertaken and they need to consider the rationale for treating cervical ectropion, including primary outcomes that are important for women. Only symptomatic women with cervical ectropion should be included and their characteristics clearly reported. Treatment needs to be compared with no treatment. Correct randomisation is required for allocating the participants along with allocation concealment, long‐term follow up, blind assessment of the results, presentation of complete outcome data, clarification of other potential sources of bias and more careful and detailed monitoring of adverse effects. Reporting on the quality issues should be improved according to Consolidated Standards of Reporting Trials (CONSORT) Statement (Schulz 2010).
What's new
| Date | Event | Description |
|---|---|---|
| 20 September 2010 | Amended | Contact details updated. |
| 20 March 2010 | Review declared as stable | The conclusions of this review are believed to be well established, therefore there is no plan to update this review in the future. |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 4, 2007
| Date | Event | Description |
|---|---|---|
| 31 May 2009 | New search has been performed | Converted to new review format. |
| 3 August 2007 | New citation required but conclusions have not changed | conclusions not changed |
Acknowledgements
The authors wish to kindly thank the Menstrual Disorders and Subfertility Cochrane Group for extensive support in the preparation of this review. Thanks to Prof Guanjian Liu, Ms Haiyan Feng and Marian Showell for their help in writing the review.
Appendices
Appendix 1. MEDLINE search strategy
1 exp uterine cervical erosion/ or exp uterine cervicitis/.
2 (cervi$ adj2 erosion$).tw
3 cervicitis.tw.
4 (cervi$ adj2 ectop$).tw.
5 (cervi$ adj2 ectrop$).tw.
6 columnar ectop$.tw.
7 (cervi$ adj2 infect$).tw.
8 or/1‐7
9 exp Microwaves/
10 Microwave$.tw.
11 ultrahigh frequency wave$.tw.
12 radio wave$.tw.
13 ehf wave$.tw.
14 or/9‐13
15 8 and 14
16 randomised controlled trial.pt.
17 controlled clinical trial.pt.
18 randomized.ab.
19 placebo.tw.
20 clinical trials as topic.sh.
21 randomly.ab.
22 trial.ti.
23 (crossover or cross‐over or cross over).tw.
24 or/16‐23
25 (animals not (humans and animals)).sh.
26 24 not 25
27 26 and 15
Appendix 2. EMBASE search strategy
1 exp uterine cervicitis/ or exp uterine cervix erosion/
2 (cervi$ adj2 erosion$).tw.
3 cervicitis.tw.
4 (cervi$ adj2 ectop$).tw.
5 (cervi$ adj2 ectrop$).tw.
6 columnar ectop$.tw.
7 (cervi$ adj2 infect$).tw.
8 or/1‐7
9 exp Microwave Radiation/
10 Microwave$.tw.
11 ultrahigh frequency wave$.tw.
12 radio wave$.tw.
13 ehf wave$.tw.
14 or/9‐13
15 8 and 14
16 Clinical Trial/
17 Randomized Controlled Trial/
18 exp randomisation/
19 Single Blind Procedure/
20 Double Blind Procedure/
21 Crossover Procedure/
22 Placebo/
23 Randomi?ed controlled trial$.tw.
24 Rct.tw.
25 random allocation.tw.
26 randomly allocated.tw.
27 allocated randomly.tw.
28 (allocated adj2 random).tw.
29 Single blind$.tw.
30 Double blind$.tw.
31 ((treble or triple) adj blind$).tw.
32 placebo$.tw.
33 prospective study/
34 or/16‐33
35 case study/
36 case report.tw.
37 abstract report/ or letter/
38 or/35‐37
39 34 not 38
40 39 and 15
Appendix 3. The Cochrane Library search strategy
1 exp uterine cervical erosion/ or exp uterine cervicitis/
2 (cervi$ adj2 erosion$).tw.
3 cervicitis.tw.
4 (cervi$ adj2 ectop$).tw.
5 (cervi$ adj2 ectrop$).tw.
6 columnar ectop$.tw.
7 (cervi$ adj2 infect$).tw.
8 or/1‐7
9 exp Microwaves/
10 Microwave$.tw.
11 ultrahigh frequency wave$.tw.
12 radio wave$.tw.
13 ehf wave$.tw.
14 or/9‐13
15 8 and 14
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ban 2004 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Cao 2006 | Non‐RCT (according to looking up study in detail) |
| Chen 1999 | Non‐RCT (according to looking up study in detail) |
| Chen 2002a | Non‐RCT (according to looking up study in detail) |
| Chen 2003 | Non‐RCT (according to looking up study in detail) |
| Chen 2006a | Non‐RCT (according to looking up study in detail) |
| Chen 2006b | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Chen 2007 | Non‐RCT (according to looking up study in detail) |
| Chen 2008 | There was no documentation as to whether the women who had cervical ectropion were symptomatic |
| Cheng 2005 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Cheng 2006a | Non‐RCT (according to looking up study in detail) |
| Dai 2002 | Non‐RCT (through call to the author, we found it was a pseudo randomised controlled trial) |
| Dai 2004 | Non‐RCT (according to looking up study in detail) |
| Dai 2008 | Non‐RCT (according to looking up study in detail) |
| Dang 2003 | Non‐RCT (through call to the author, we found it was a pseudo randomised controlled trial) |
| Fan 2003 | Non‐RCT (according to looking up study in detail) |
| Fan 2005 | Non‐RCT (according to looking up study in detail) |
| Feng 2008 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Gan 2006 | Non‐RCT (according to looking up study in detail) |
| Gao 2005 | Non‐RCT (according to looking up study in detail) |
| Gu 2005a | Non‐RCT (through called for the author, we found it was a pseudo randomised controlled trial) |
| Gu 2005b | Non‐RCT (according to looking up study in detail) |
| Guan 2001 | Non‐RCT (through call to the author, we found it was a pseudo randomised controlled trial) |
| Guo 1997 | Retrospective clinical trial (according to looking up study in detail) |
| Guo 2003 | Non‐RCT (through call to the author, we found it was a pseudo randomised controlled trial) |
| Guo 2008 | There was no documentation as to whether the women who had cervical ectropion were symptomatic |
| Han 2007 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Hao 2006 | Quasi‐randomised controlled trial (through call to the author) |
| He 2000 | Non‐RCT (through call to the author, we found it was a pseudo‐ randomised controlled trial) |
| He 2009 | There was no documentation as to whether the women who had cervical ectropion were symptomatic |
| Hu 1996 | Non‐RCT (through call to the author, we found it was a pseudo‐ randomised controlled trial) |
| Hu 2002 | Retrospective clinical trial (through call to the author) |
| Hu 2007 | Non‐RCT (according to looking up study in detail) |
| Hu 2008 | Non‐RCT (according to looking up study in detail) |
| Huang 1997 | Non‐RCT (through call to the author, we found it was a pseudo‐ randomised controlled trial) |
| Huang 2000 | Retrospective clinical trial (according to looking up study in detail) |
| Ji 2003 | Non‐RCT (through call to the author, we found it was a pseudo‐ randomised controlled trial) |
| Jiang 2001 | Non‐RCT (through call to the author, we found it was a pseudo‐ randomised controlled trial) |
| Lei 2007 | Non‐RCT (according to looking up study in detail) |
| Li 2002 | Non‐RCT (through call to the author, we found it was a pseudo‐ randomised controlled trial) |
| Li 2003 | Non‐RCT (according to looking up study in detail) |
| Lin 2004 | Non‐RCT (through call to the author, we found it was a pseudo‐ randomised controlled trial) |
| Liu 2001a | Quasi‐randomised controlled trial (through call to the author) |
| Liu 2001b | Quasi‐randomised controlled trial (through call to the author) |
| Liu 2002 | Quasi‐randomised controlled trial (through call to the author) |
| Liu 2003a | Non‐RCT (through call to the author, we found it was a pseudo randomised controlled trial) |
| Liu 2003b | Non‐RCT (through call to the author, we found it was a pseudo randomised controlled trial) |
| Liu 2003c | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Liu 2004 | Non‐RCT (according to looking up study in detail) |
| Liu 2005 | Retrospective clinical trial (according to looking up study in detail) |
| Liu 2006 | Non‐RCT (according to looking up study in detail) |
| Long 2003 | Quasi‐randomised controlled trial (Although called for the author) |
| Lu 2004 | Non‐RCT (Through called for the author, we found it was a pseudo randomised controlled trial) |
| Luo 2004 | Non‐RCT (Through called for the author, we found it was a pseudo randomised controlled trial) |
| Luo 2007 | Non‐RCT (according to looking up study in detail) |
| Lv 2004a | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Lv 2004b | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Ma 2001 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Mo 2004 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Pan 1999 | Non‐RCT (according to looking up study in detail) |
| Qin 2001 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Ran 2004 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Ruan 2007 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Shan 2002 | Quasi‐randomised controlled trial (through call to the author) |
| Shao 2003 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Shu 2006 | Non‐RCT (according to looking up study in detail) |
| Song 1994 | RCT ( There was no documentation as to whether the women who had cervical ectropion were symptomatic) |
| Sun 2006a | Non‐RCT (according to looking up study in detail) |
| Sun 2006b | Non‐RCT (according to looking up study in detail) |
| Wang 1996 | Non‐RCT (Through called for the author, we found it was a pseudo randomised controlled trial) |
| Wang 1997 | Retrospective clinical trial (Through called for the author) |
| Wang 2001 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Wang 2002 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Wang 2002c | Non‐RCT (we found it was a pseudo‐randomised controlled trial) |
| Wang 2003 | RCT ( There was no documentation as to whether the women who had cervical ectropion were symptomatic.) |
| Wang 2006a | Non‐RCT (according to looking up study in detail) |
| Wang 2006b | Non‐RCT (according to looking up study in detail) |
| Wang 2008 | Non‐RCT (according to looking up study in detail) |
| Wei 2008 | There was no documentation as to whether the women who had cervical ectropion were symptomatic |
| Wen 2002 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Wen 2003 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Wu 1996 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Wu 2003 | Retrospective clinical trial (through call to the author) |
| Wu 2004 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial)l) |
| Wu 2007 | There was no documentation as to whether the women who had cervical ectropion were symptomatic |
| Xie 1999 | Quasi‐randomised controlled trial (Through called for the author) |
| Xie 2003 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Xie 2006 | Non‐RCT (according to looking up study in detail) |
| Xie 2007 | Non‐RCT (according to looking up study in detail) |
| Xu 2000 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Xu 2004 | RCT ( there was no documentation as to whether the women who had cervical ectropion were symptomatic.) |
| Xu 2005 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Xu 2007a | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Xu 2007b | Non‐RCT (according to looking up study in detail) |
| Xue 2002 | Non‐RCT (we found it was a pseudo‐randomised controlled trial) |
| Xue 2004 | Non‐RCT (according to looking up study in detail) |
| Yang 1997 | Retrospective clinical trial (according to looking up study in detail) |
| Yang 2001 | Non‐RCT ( According to looking up study in detail) |
| Yang 2007 | Non‐RCT (according to looking up study in detail) |
| Yang 2008a | Non‐RCT (according to looking up study in detail) |
| Yang 2008b | Non‐RCT (according to looking up study in detail) |
| Ye 2005 | Non‐RCT (Through called for the author, we found it was a pseudo randomised controlled trial) |
| You 2004 | Quasi‐randomised controlled trial (Through called for the author) |
| Yu 2003 | Non‐RCT (according to looking up study in detail) |
| Yu 2004 | Retrospective clinical trial (through call to the author) |
| Yu 2008 | Non‐RCT (according to looking up study in detail) |
| Zhan 2001 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Zhang 1999 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Zhang 2001 | Retrospective clinical trial (according to looking up study in detail) |
| Zhang 2002 | Retrospective clinical trial (through call to the author) |
| Zhang 2005a | Non‐RCT (Through called for the author, we found it was a pseudo randomised controlled trial) |
| Zhang 2005b | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Zhang 2005c | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Zhang 2006a | Duplicate publication literature (It was the same study with "Zhang 2006b") |
| Zhang 2006b | Quasi‐randomised controlled trial (Although called for the author) |
| Zhang 2007 | Non‐RCT (according to looking up study in detail) |
| Zhao 1997 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Zhao 2003 | Quasi‐randomised controlled trial (through call to the author) |
| Zhao 2007 | Non‐RCT (according to looking up study in detail) |
| Zhao 2008 | Non‐RCT (according to looking up study in detail) |
| Zhong 2005 | Non‐RCT (according to looking up study in detail) |
| Zhou 2002 | Non‐RCT (Through called for the author, we found it was a pseudo randomised controlled trial) |
| Zhu 1997 | Non‐RCT (through call to the author, we found it was a pseudo‐randomised controlled trial) |
| Zhu 2007 | Non‐RCT (according to looking up study in detail) |
Characteristics of studies awaiting assessment [ordered by study ID]
Chen 2001.
| Methods | RCT duration: not yet known Randomisation method not stated Blinding: not yet known Number of women randomised: n = 204 Number of women analysed: n = 204 Unit of comparison: individuals. |
| Participants | Inclusion criteria: women with cervical erosion without cervical cancer and infusorium, neisseria gonorrhoeae vaginitis and mould infection Microwave group (n = 106): Grade I: 22; Grade II: 58; Grade III: 26 Age: 32y; No.of pregnancy: 2.2; No. of births: 1.2 Electrocautery group (n = 98): Grade I: 28; Grade II: 50; Grade III: 20 Age: 30y; No. of pregnancy: 3.1; No. of births: 1.6 Source of participants: medical outpatients. |
| Interventions | Treatment: microwave tissue coagulation (MTC)
Control: electrocautery. Time of treatments: during 3 to 7 days after menstrual period. |
| Outcomes | Efficacy: cure rate (according to appearance of cervix) Adverse reaction: bleeding during operation, bellyache pain during operation, more bleeding during decrustation than menstruation The follow‐up period: 8 to 12 weeks |
| Notes | Location: China Setting: Department of Gynaecology and Obstetrics of Nanyou Hospital, Zhanjiang City and No. 422 Liberation Army Hospital Funding: unclear Failed to contact the author, and it is unclear whether the trial is an authentic RCT. |
Chen 2002b.
| Methods | RCT duration: not yet known Randomisation method not stated Blinding: not yet known Number of women randomised: n = 360 Number of women analysed: n = 360 Unit of comparison: individuals. |
| Participants | Inclusion criteria: women with cervical erosion without cervical cancer and infusorium and mould infection Interferon suppository group: n = 120 Microwave group: n = 120 Interferon suppository plus microwave group: n = 120 Grade I. interferon suppository group: 24; microwave group: 24; interferon suppository plus microwave group: 24 Grade II. interferon suppository group: 63; microwave group: 63; interferon suppository plus microwave group: 63 Grade III. interferon suppository group: 33; microwave group: 33; interferon suppository plus microwave group: 33 Source of participants: medical outpatients. |
| Interventions | 1. Interferon suppository 2. Microwave 3. Interferon suppository plus microwave |
| Outcomes | Efficacy: cure rate (according to appearance of cervix) The time of vaginal fluid and the status of vaginal bleeding The follow‐up period: 8 weeks |
| Notes | Location: China Setting: Department of Gynaecology and Obstetrics, Nanjing first hospital affiliated to Nanjing Medical University Funding: unclear Failed to contact the author, and it is unclear whether the trial is an authentic RCT. |
Chen 2004.
| Methods | RCT duration 5 years from Jan 2000 to Dec 2005 Randomisation method not stated Single centre Blinding: not yet known Number of women randomised: n = 188 Number of women analysed: n = 188 Unit of comparison: individuals. |
| Participants | Inclusion criteria: women with severe cervical erosion without cervical cancer and infusorium, mould infection and venereal disease Microwave group: n = 98 Interferon suppository plus microwave group: n = 90 Exclusion criteria: excluded women with cervical cancer, diagnosed through Papanicolaou test. Excluded women with infusorium, mould infection and venereal disease Source of participants: medical outpatients. |
| Interventions | 1. Microwave group: 45W (power) 2. Interferon suppository plus microwave group: after microwave, interferon suppository one pill once every other day, course of treatment for 15 days |
| Outcomes | Efficacy: Cure rate (according to appearance of cervix) The follow‐up period: not yet known |
| Notes | Location: China Setting: Department of Gynaecology and Obstetrics of Mudanjiang Railway Central Hospital in Heilongjiang Funding: unclear Failed to contact the author, and it is unclear whether the trial is an authentic RCT. |
Cheng 2000.
| Methods | Randomisation method: not yet known Single centre Blinding: not yet known. |
| Participants | Inclusion criteria: women with severe cervical erosion |
| Interventions | 1. Treatment: microwave group 2. Control: not yet known |
| Outcomes | Efficacy: cure rate (according to appearance of cervix) The follow‐up period: not yet known |
| Notes | Location: China Setting: Department of Gynaecology and Obstetrics of Yiwu Traditional Chinese Medicine Hospital Funding: unclear Failed to find the full text. |
He 1995.
| Methods | RCT duration: not yet known Randomisation method: not yet known Single centre Blinding: not yet known Number of women randomised: n = 260 Number of women analysed: n = 260 Unit of comparison: individuals. |
| Participants | Inclusion criteria: women with cervical erosion Microwave group: n = 130 Electrocautery group: n = 130 Source of participants: medical outpatients. |
| Interventions | 1. Microwave group 2. Electrocautery group |
| Outcomes | Efficacy: cure rate (according to appearance of cervix) The follow‐up period: not yet known |
| Notes | Location: China Setting: The Third People's Hospital of Xiaoshan City, Zhejiang province Funding: unclear Failed to contact the author, and it is unclear whether the trial is an authentic RCT. |
Li 2004.
| Methods | Randomisation method: not yet known Single centre Blinding: not yet known. |
| Participants | Inclusion criteria: women with severe cervical erosion |
| Interventions | 1. Treatment: microwave group 2. Control: not yet known |
| Outcomes | Efficacy: cure rate The follow‐up period: not yet known |
| Notes | Location: China Setting: Women and Children's Hospital of Jinzhou City Funding: unclear Failed to find the full text. |
Shi 2000.
| Methods | Randomisation method: not yet known Single centre Blinding: not yet known. |
| Participants | Inclusion criteria: women with severe cervical erosion |
| Interventions | 1.Treatment: microwave group 2. Control: not yet known |
| Outcomes | Efficacy: cure rate (according to appearance of cervix) The follow‐up period: not yet known |
| Notes | Location: China Setting: Women and Children's Hospital of Nantong City Funding: unclear Failed to find the full text. |
Sun 2001.
| Methods | RCT duration 2.5 years from Jul 1997 to Dec 1999 Randomisation method not stated Single centre Blinding: not yet known Number of women randomised: n = 150 Number of women analysed: n = 150 Unit of comparison: individuals. |
| Participants | Inclusion criteria: women with cervical erosion without cervical cancer and infusorium, neisseria gonorrhoeae vaginitis and mould infection Microwave group (n = 106): Grade I: 18; Grade II: 47; Grade III: 25 Age: 32y (20 to 40) Electrocautery group (n = 90): Grade I: 12; Grade II: 31; Grade III: 17 Age: 33y (19 to 56) Source of participants: medical outpatients. |
| Interventions | 1. Microwave group 2. Electrocautery group The follow‐up period: 8 and 36 months. Once a week after treatment, once 4 weeks, once 8 weeks, then once 3 months for going on 1 year. And then once half yearly. |
| Outcomes | Efficacy: cure rate (according to appearance of cervix) The time of vaginal fluid and the status of vaginal bleeding Bleeding during operation and after operation, more bleeding during decrustation than menstruation |
| Notes | Location: China Setting: The first hospital affiliated to Qiqihaer Medical College of Heilongjiang Funding: unclear Failed to contact the author, and it is unclear whether the trial is an authentic RCT. |
Yang 2004.
| Methods | Randomisation method: not yet known Single centre Blinding: not yet known |
| Participants | Inclusion criteria: women with severe cervical erosion |
| Interventions | 1. Treatment: microwave group 2. Control: not yet known |
| Outcomes | Efficacy: cure rate The follow‐up period: not yet known |
| Notes | Location: China Setting: Department of Gynaecology and Obstetrics of Shuangya People's Hospital Funding: unclear Failed to find the full text. |
Differences between protocol and review
Ya Li Liu is the first author.
Contributions of authors
Yali Liu and Taixiang Wu were responsible for protocol and review development, Kehu Yang was responsible for organising the research team and review development. Data Collection for the review was carried out independently by LYL,YK, ZP and TJY. Entering data into RevMan: LYL, TJ, MB. Analysis of data: WT, LYL,YH, LJ. Interpretation of data: WT, LYL, YH. Writing the protocol and review: LYL, YK. Providing general advice on the protocol and review: WT, HR. Helen Roberts was the English and western medical clinical contributor
Sources of support
Internal sources
No sources of support found, Not specified.
External sources
No sources of support supplied
Declarations of interest
None known
Stable (no update expected for reasons given in 'What's new')
References
References to studies excluded from this review
Ban 2004 {published data only}
- Ban HM. A analysis of 298 cases of cervical ectropion with combined therapy of microwave tissue coagulation (MTC) and interferon suppository. Clinical Medicine 2004;24(7):67‐8. [Google Scholar]
Cao 2006 {published data only}
- Cao ZM. Observation on therapeutic effect of 168 cases of cervical ectropion by combined interferon suppository with microwave tissue coagulation (MTC). Public Medical Forum Magazine(B) 2006;10(11):969‐70. [Google Scholar]
Chen 1999 {published data only}
- Chen RH, Li CP. A comparable study of microwave tissue coagulation (MTC) and cryotherapy in treatment of cervical ectropion. Journal of Baotou Medical College 1999;15(3):45‐6. [Google Scholar]
Chen 2002a {published data only}
- Chen P. Clinical observation of cervical ectropion by microwave tissue coagulation (MTC) and interferon suppository. Journal of Hebei 2002;8(12):1059‐60. [Google Scholar]
Chen 2003 {published data only}
- Chen XJ. Observation therapeutic effect of midrange, severe degree cervical ectropion with combined therapy of microwave tissue coagulation (MTC) and interferon suppository. Journal of Xianning College (Medical Sciences) 2003;17(5):346‐7. [Google Scholar]
Chen 2006a {published data only}
- Chen HF. Clinical analysis of 320 cases of cervical ectropion by combined therapy of interferon suppository and microwave tissue coagulation(MTC). Modern Preventive Medicine 2006;33(4):622. [Google Scholar]
Chen 2006b {published data only}
- Chen JE. Clinical observation of cervical ectropion by microwave tissue coagulation (MTC) and interferon suppository. Journal of Qiqihar College 2006;27(11):1305‐6. [Google Scholar]
Chen 2007 {published data only}
- Chen Q. Observation therapeutic effect of 135 cases of cervical ectropion with combined therapy of microwave tissue coagulation(MTC) and interferon suppository. Chinese Health Care 2007;15(17):52‐3. [Google Scholar]
Chen 2008 {published data only}
- Chen L. Observation therapeutic effect of cervical ectropion with microwave tissue coagulation (MTC) and interferon suppository. Modern Journal of Integrated Traditional Chinese and Western Medicine 2008;17(29):4544‐5. [Google Scholar]
Cheng 2005 {published data only}
- Cheng XX. Observation therapeutic effect of cervical ectropion with microwave tissue coagulation (MTC) and interferon suppository. Chinese Medical Research and Clinic 2005;3(10):52‐3. [Google Scholar]
Cheng 2006a {published data only}
- Cheng LY. Clinical observation on therapeutic effect of 110 cases of cervical ectropion with combined therapy of microwave tissue coagulation(MTC) and interferon suppository. Chinese Journal of Clinical Healthcare 2006;9(3):872. [Google Scholar]
Dai 2002 {published data only}
- Dai KL, Guan YJ, Guan YZ. Clinical observation of 106 cases of cervical ectropion in treatment by combined microwave tissue coagulation (MTC) with interferon suppository. Journal of Chinese Practical Medicine 2002;4(1):71. [Google Scholar]
Dai 2004 {published data only}
- Dai Q. Observation therapeutic effect of cervical ectropion with combined therapy of microwave tissue coagulation (MTC) and interferon‐alpha suppository. Zhejiang Clinical Medical Journal 2004;6(3):201. [Google Scholar]
Dai 2008 {published data only}
- Dai JD. Clinical observation therapeutic effect of severe degree cervical ectropion with three kinds of physical therapies. Jiangxi Medicine Journal 2008;43(6):574‐6. [Google Scholar]
Dang 2003 {published data only}
- Dang CY. Observation therapeutic effect of cervicitis with CO2 laser and microwave tissue coagulation (MTC). Laser Journal 2003;24(5):12. [Google Scholar]
Fan 2003 {published data only}
- Fan WL. Observation therapeutic effect of cervical ectropion with combined therapy of microwave tissue coagulation (MTC) and interferon suppository. Chinese Journal of Misdiagnostics 2003;3(11):1670‐1. [Google Scholar]
Fan 2005 {published data only}
- Fan LL. Fan LL. Clinical observation of 64 cases of cervical ectropion in treatment by combined microwave tissue coagulation (MTC) and interferon suppository. Fujian Medical Journal 2005;27(3):32‐4. [Google Scholar]
Feng 2008 {published data only}
- Feng JC. Observation therapeutic effect of 135 cases of cervical ectropion with combined therapy of microwave tissue coagulation (MTC) and interferon suppository. Journal of Shanxi College of Traditional Chinese Medicine 2008;9(3):23. [Google Scholar]
Gan 2006 {published data only}
- Gan YQ, Gan CX. Clinical observation of cervical ectropion in treatment by combined microwave tissue coagulation(MTC) with interferon suppository. Modem Hospital 2006;6(9):59‐60. [Google Scholar]
Gao 2005 {published data only}
- Gao QX, Li FE, Fu GR. Clinical observation of cervical ectropion by microwave tissue coagulation (MTC) and interferon suppository. Journal of Yulin College 2005;15(5):95‐6. [Google Scholar]
Gu 2005a {published data only}
- Gu XF, Zhou J, Jiang L, Jiang WB, Zhou Q, Wang CY, et al. Observation on therapeutic effect of cervical ectropion with combined therapy of microwave tissue coagulation (MTC) and interferon suppository. Chinese Practical Journal of Rural Doctor 2005;12(2):25‐6. [Google Scholar]
Gu 2005b {published data only}
- Gu XH. A clinical observation on cervical ectropion with microwave tissue coagulation (MTC) and interferon suppository. Modern Journal of Integrated Traditional Chinese and Western Medicine 2005;14(12):1575‐6. [Google Scholar]
Guan 2001 {published data only}
- Guan XR, Zhang YH, Li JJ. Observation on therapeutic effect of cervical ectropion with microwave tissue coagulation (MTC) and electrocoagulation. Chinese Clinical New Medicine 2001;1(1):28‐9. [Google Scholar]
Guo 1997 {published data only}
- Guo YQ. A comparable study of microwave tissue coagulation (MTC) and infrared ray in treatment of cervical ectropion. Jiangsu Journal of Preventive Medicine 1997;‐(2):50. [Google Scholar]
Guo 2003 {published data only}
- Guo YN, Li H. Clinical understanding of cervical ectropion by interferon suppository. The Journal of Practical Medicine 2003;19(5):479. [Google Scholar]
Guo 2008 {published data only}
- Guo HJ. Observation therapeutic effect of cervical ectropion with interferon suppository. Journal of Taishan Medical College 2008;8(3):225. [Google Scholar]
Han 2007 {published data only}
- Han ZF. Clinical understand of 143 cases of cervical ectropion with microwave tissue coagulation (MTC) and interferon suppository. Journal of Community Medicine 2007;5(9):31. [Google Scholar]
Hao 2006 {published data only}
- Hao FY, Wei L, Zhang WQ, Yao JW, Wu X. Clinical observation of cervical ectropion with microwave tissue coagulation (MTC), carbon dioxide laser and LEEP. Shanxi Medicine Journal 2006;35(6):552‐4. [Google Scholar]
He 2000 {published data only}
- He YN, Lin MY, Lin J. An analysis on therapeutic effect of cervical ectropion by CO2 laser, infrared ray and microwave tissue coagulation (MTC). Strait Journal of Preventive Medicine 2000;6(6):66. [Google Scholar]
He 2009 {published data only}
- He YZ, Guo ZA. Observation therapeutic effect of 200 cases of cervical ectropion with microwave tissue coagulation (MTC) and interferon suppository. North China Coal Medical College 2009;11(3):373‐4. [Google Scholar]
Hu 1996 {published data only}
- Hu L. A comparable study of microwave tissue coagulation (MTC) and laser in treatment of cervical ectropion. Journal of Nanjing College Population Programme Management 1996;‐(2):68‐9. [Google Scholar]
Hu 2002 {published data only}
- Hu HQ. Clinical analysis of 212 cases of severe degree cervical ectropion with combined therapy of microwave tissue coagulation (MTC) and interferon suppository. Journal of Nanjing Military Medical College 2002;24(4):279‐80. [Google Scholar]
Hu 2007 {published data only}
- Hu MR, Peng B. A study of cervical ectropion with combined therapy of microwave tissue coagulation(MTC) and interferon suppository. Journal of Xianning College (Medical Sciences) 2007;21(2):141‐2. [Google Scholar]
Hu 2008 {published data only}
- Hu D, Li P. Observation therapeutic effect of midrange, severe degree cervical ectropion with microwave tissue coagulation (MTC) and interferon suppository. Chinese Journal of Misdiagnostics 2008;8(6):1367. [Google Scholar]
Huang 1997 {published data only}
- Huang JB, Zhu QM, Xu J. A comparable observation of microwave tissue coagulation (MTC), laser and cryotherapy in treatment of cervical ectropion. The Journal of Medical Theory and Practice 1997;9(10):469‐70. [Google Scholar]
Huang 2000 {published data only}
- Huang KL, Zhang YY, Guo HX. A study of microwave tissue coagulation (MTC) in treatment of 126 cases of cervical ectropion. Journal of Navy Medicine 2000;21(3):238‐9. [Google Scholar]
Ji 2003 {published data only}
- Ji F, Chen ZP, Fei MJ, Shen GD. Clinical observation of laser treatment for cervicitis. Modern Diagnosis Treatment 2003;5:315. [Google Scholar]
Jiang 2001 {published data only}
- Jiang XY, Wen Q, Li GL. Clinical analysis of 93 cases of travellers cervical ectropion with microwave tissue coagulation (MTC). Chinese Journal of Frontier Health and Quarantine 2001;24(3):191‐2. [Google Scholar]
Lei 2007 {published data only}
- Lei YJ. Cervical ectropion in treatment by microwave tissue coagulation(MTC). China Modern Doctor 2007;45(1):16‐7. [Google Scholar]
Li 2002 {published data only}
- Li J, Yue L, Ma R, Duan YM. Clinical observation of 79 cases of cervical ectropion in treatment by microwave tissue coagulation (MTC). Chinese Journal of Synthetic Medicine 2002;3(6):537. [Google Scholar]
Li 2003 {published data only}
- Li J, Chen XY. Clinical observation of 100 cases of cervical ectropion in treatment by combined microwave tissue coagulation (MTC) and interferon suppository. Journal of Huaihai Medicine 2003;21(5):367‐8. [Google Scholar]
Lin 2004 {published data only}
- Lin DH. Clinical analysis on therapeutic effect of 198 cases of cervical ectropion with combined therapy of microwave tissue coagulation (MTC) and interferon‐alpha suppository. Liaoning Medical Journal 2004;18(6):306‐7. [Google Scholar]
Liu 2001a {published data only}
- Liu HY. A comparable observation of microwave tissue coagulation (MTC), laser and drug in treatment of cervical ectropion. Chinese Journal of Coal Industry Medicine 2001;4(6):437. [Google Scholar]
Liu 2001b {published data only}
- Liu Y, Li JM, Tang XL. Clinical observation on therapeutic effect of cervical ectropion with microwave tissue coagulation (MTC). Journal of Chengdu Military Command Hospital 2001;3(1):10‐1. [Google Scholar]
Liu 2002 {published data only}
- Liu LP, Yu HT. Observation of 186 cases of cervical ectropion on therapeutic effect by combined microwave tissue coagulation (MTC) with interferon suppository. Heilongjiang Medical Journal 2002;26(3):189‐90. [Google Scholar]
Liu 2003a {published data only}
- Liu KX, Shi HY, Xu XH. Observation on therapeutic effect of chronic cervicitis with combined therapy of microwave tissue coagulation (MTC) and interferon suppository. Journal of Baotou Medical College 2003;19(2):120‐1. [Google Scholar]
Liu 2003b {published data only}
- Liu EY. Clinical observation of cervical ectropion on treatment by combined microwave tissue coagulation (MTC) with interferon suppository. Journal of Qiqihar Medical College 2003;24(1):29‐30. [Google Scholar]
Liu 2003c {published data only}
- Liu YJ, Li SQ. Observation on therapeutic effect of severe degree cervical ectropion with microwave tissue coagulation (MTC) and CO2 laser. Chinese Primary Health Care 2003;17(7):78‐9. [Google Scholar]
Liu 2004 {published data only}
- Liu J, Wang JL. A comparable study of microwave tissue coagulation (MTC) and infrared ray in treatment of cervical ectropion. Chinese Journal of Misdiagnostics 2004;4(2):266‐7. [Google Scholar]
Liu 2005 {published data only}
- Liu FQ. Clinical analysis of cervical ectropion with combined therapy of microwave tissue coagulation (MTC) and interferon suppository. Central Plains Medical Journal 2005;32(13):11‐2. [Google Scholar]
Liu 2006 {published data only}
- Liu MC, Zeng AQ. Clinical analysis of 86 cases of cervical ectropion with combined therapy of microwave tissue coagulation(MTC) and interferon suppository. China Medical Herald 2006;3(33):63‐4. [Google Scholar]
Long 2003 {published data only}
- Long JH. Observation therapeutic effect of severe degree cervical ectropion with combined therapy of Microwave tissue coagulation (MTC) and interferon suppository. Medical Journal West China 2003;1(4):322‐3. [Google Scholar]
Lu 2004 {published data only}
- Lu YQ, Liao YX. A comparable study of cervical ectropion by three therapeutic methods. Journal of Guangxi Medical University 2004;21(3):433‐4. [Google Scholar]
Luo 2004 {published data only}
- Luo HY. Observation on therapeutic effect of cervical ectropion by combined microwave tissue coagulation (MTC) with interferon suppository. Journal of North Sichuan Medical College 2004;19(3):44‐5. [Google Scholar]
Luo 2007 {published data only}
- Luo J. A study on cervical ectropion with microwave tissue coagulation(MTC) and interferon. Central Plains Medical Journal 2007;34(23):97. [Google Scholar]
Lv 2004a {published data only}
- Lv P, Liang HM, Wang F. 128 cases of cervical ectropion in treatment by combined microwave tissue coagulation (MTC) with interferon suppository. Hebei Medical Journal 2004;26(11):882‐3. [Google Scholar]
Lv 2004b {published data only}
- Lv XQ, Wang NN. Observation on therapeutic effect of cervical ectropion by interferon suppository. Journal of Chinese Medicine Research 2004;4(5):415‐6. [Google Scholar]
Ma 2001 {published data only}
- Ma SF, Ma SH, Wu XZ. Cervical ectropion in treatment by microwave tissue coagulation (MTC). Nei Mongolia Medical Journal 2001;33(3):262. [Google Scholar]
Mo 2004 {published data only}
- Mo PX. Clinical observation of cervical ectropion in treatment by combined microwave tissue coagulation (MTC) with interferon suppository. Journal of Medical Forum 2004;25(21):37‐40. [Google Scholar]
Pan 1999 {published data only}
- Pan GL. A comparable study of microwave tissue coagulation (MTC) and carbon dioxide laser in treatment of 200 cases of cervical ectropion. Modern Rehabilitation 1999;3(10):1271. [Google Scholar]
Qin 2001 {published data only}
- Qin CL, Chen CJ, Zhang WH. 360 cases of cervical ectropion in treatment by microwave tissue coagulation (MTC). Henan Medical Information 2001;9(18):26‐7. [Google Scholar]
Ran 2004 {published data only}
- Ran XH. Observation on therapeutic effect of 120 cases cervical ectropion with microwave tissue coagulation(MTC). Modern Journal of Integrated Traditional Chinese and Western Medicine 2004;13(10):1335. [Google Scholar]
Ruan 2007 {published data only}
- Ruan LQ. Observation therapeutic effect of severe degree of cervical ectropion with microwave tissue coagulation (MTC) and interferon suppository. North China Coal Medical College 2007;9(5):690‐1. [Google Scholar]
Shan 2002 {published data only}
- Shan YZ, Huang WH, Jiang XH, Wang YY. Clinical observation of severe degree cervical ectropion with combined therapy of microwave tissue coagulation (MTC) and interferon suppository. Journal of Practical Obstetrics and Gynecology 2002;18(4):254. [Google Scholar]
Shao 2003 {published data only}
- Shao CH, Lv CT. Clinical study of 128 cases of cervical ectropion in treatment by combined microwave tissue coagulation (MTC) with interferon suppository. Clinical Medicine 2003;23(3):38. [Google Scholar]
Shu 2006 {published data only}
- Shu XF. Observation therapeutic effect of cervical ectropion with combined therapy of microwave tissue coagulation(MTC) and Aoping suppository. Maternal and Child Health Care of China 2006;21(19):2728‐9. [Google Scholar]
Song 1994 {published data only}
- Song XH, Yu XM. A comparable study of microwave tissue coagulation (MTC) and CO2 laser in treatment of cervical ectropion. China Journal Obstetrics and Gynecology 1994;29(1):19‐22. [PubMed] [Google Scholar]
Sun 2006a {published data only}
- Sun Y. A comparable observation of microwave tissue coagulation(MTC) and infrared ray in treatment of cervical ectropion. The Journal of Medical Theory and Practice 2006;19(6):786. [Google Scholar]
Sun 2006b {published data only}
- Sun WN. A analysis on therapeutic effect of cervical ectropion by microwave tissue coagulation(MTC) and infrared ray. Chinese Health Care 2006;14(16):72. [Google Scholar]
Wang 1996 {published data only}
- Wang HZ, Li RZ, Yang YZ, Wu RF. Observation on therapeutic effect of 237 cases of cervical ectropion with microwave tissue coagulation (MTC) and CO2 laser. Journal of Hebei Medical University 1996;17(6):342‐3. [Google Scholar]
Wang 1997 {published data only}
- Wang XZ, Zhu XF. Clinical analysis of 94 cases of cervical ectropion by microwave tissue coagulation (MTC). Acta Medicinae Sinica 1997;10(1):50‐1. [Google Scholar]
Wang 2001 {published data only}
- Wang ZM, Liu YD. Clinical observation therapeutic effect of severe degree cervical ectropion with combined therapy of microwave tissue coagulation (MTC) and interferon suppository. Journal of Practical Obstetrics and Gynecology 2001;17(3):182. [Google Scholar]
Wang 2002 {published data only}
- Wang BD. Clinical analysis of 112 cases of cervical ectropion by combined therapy of microwave tissue coagulation (MTC) and interferon‐alpha suppository. Journal of Practical Obstetrics and Gynecology 2002;18(3):188. [Google Scholar]
Wang 2002c {published data only}
- Wang X. Clinical observation of 240 cases of cervical ectropion in treatment by microwave tissue coagulation (MTC). Chinese Journal of Synthetical Medicine 2002;3(8):25. [Google Scholar]
Wang 2003 {published data only}
- Wang JE. Clinical observation of 120 cases of cervical ectropion in treatment by combined microwave tissue coagulation (MTC) with interferon suppository. China New Medicine 2003;‐(1):49‐50. [Google Scholar]
Wang 2006a {published data only}
- Wang Y. Hou XH. A study of microwave tissue coagulation (MTC) and cryotherapy in treatment of cervical ectropion. Public Medical Forum Magazine 2006;10(12):1081‐2. [Google Scholar]
Wang 2006b {published data only}
- Wang GH, Wang ML, Wang SL, Wu XY, Ren JG. Observation on Curative Effect with Four Kinds of Physical Therapies for Cervical Ectropion. Journal of Practical Medical Techniques 2006;13(14):2390‐1. [Google Scholar]
Wang 2008 {published data only}
- Wang Y. Observation therapeutic effect of cervical ectropion with microwave tissue coagulation (MTC) and interferon suppository. Zhejiang Clinical Medical Journal 2008;10(5):676‐8. [Google Scholar]
Wei 2008 {published data only}
- Wei ZJ. A comparable study of microwave tissue coagulation(MTC) and CO2 laser in treatment of cervical ectropion. Journal of Community Medicine 2008;6(11):63. [Google Scholar]
Wen 2002 {published data only}
- Wen K, Fei WR. Clinical study of 325 cases of cervical ectropion in treatment by interferon suppository. Journal of Practical Obstetrics and Gynecology 2002;18(2):119. [Google Scholar]
Wen 2003 {published data only}
- Wen W, Wu M. A comparable study of three therapeutic methods on cervical ectropion. Chinese Journal of Family Planning 2003;‐(5):309‐10. [Google Scholar]
Wu 1996 {published data only}
- Wu LY, Liu GH, Hui BX, Zou WR. A study of microwave tissue coagulation (MTC) and electrocoagulation (EC) in treatment of cervical ectropion. Academiae Medicinae Suzhou 1996;16(2):351. [Google Scholar]
Wu 2003 {published data only}
- Wu SY, Wang JY, Guo XY. Clinical analysis of 51 cases of cervical ectropion by microwave tissue coagulation (MTC). Sichuan Medical Journal 2003;24(3):319‐20. [Google Scholar]
Wu 2004 {published data only}
- Wu Y, Zhang L. Observation therapeutic effect of 101 cases of cervical ectropion with combined microwave tissue coagulation (MTC) with interferon‐alpha suppository. China Pharmacist 2004;7(7):546‐7. [Google Scholar]
Wu 2007 {published data only}
- He YZ, Guo ZA. Observation therapeutic effect of cervical ectropion with combined therapy of microwave tissue coagulation(MTC) and infrared ray. The Journal of Medical Theory and Practice 2007;20(12):1438‐9. [Google Scholar]
Xie 1999 {published data only}
- Xie YH, Li XH, Jia YN. A comparable observation of microwave tissue coagulation (MTC) and infrared ray in treatment of cervical ectropion. People's Military Surgeon 1999;42(11):662‐3. [Google Scholar]
Xie 2003 {published data only}
- Xie QR, Liu SJ. Clinical analysis of 118 cases of cervical ectropion with combined therapy of microwave tissue coagulation (MTC) and interferon‐alpha suppository. Chinese Journal of Coal Industry Medicine 2003;6(10):964‐5. [Google Scholar]
Xie 2006 {published data only}
- Xie HX. Observation on therapeutic effect of cervical ectropion by combined interferon suppository with microwave tissue coagulation(MTC). The Journal of Medical Theory and Practice 2006;19(3):315‐6. [Google Scholar]
Xie 2007 {published data only}
- Xie D. Clinical observation on the effect of microwave combined with interferon suppository on cervical ectropion. China Tropical Medicine Journal 2007;7(2):241. [Google Scholar]
Xu 2000 {published data only}
- Xu XY. A analysis of 126 cases of cervical ectropion with interferon‐alpha suppository. Journal of Practical Obstetrics and Gynecology 2000;16(5):244. [Google Scholar]
Xu 2004 {published data only}
- Xu LF, Wang XF. 60 cases of cervical ectropion in treatment by microwave tissue coagulation (MTC). Fujian Medical Journal 2004;26(6):97‐8. [Google Scholar]
Xu 2005 {published data only}
- Xu W. Clinical therapeutic effect of 115 cases of severe degree cervical ectropion with microwave tissue coagulation (MTC) and interferon suppository. Chinese Journal of Practice Medicine 2005;4(10):1034‐5. [Google Scholar]
Xu 2007a {published data only}
- Xu ZQ, Hou LW, Zu Y, Tong JS, Yu HY. Pathological analysis of cervical ectropion by interferon suppository and microwave tissue coagulation (MTC). Maternal and Child Health Care of China 2007;22(33):4785‐6. [Google Scholar]
Xu 2007b {published data only}
- Xu LL. Observation therapeutic effect of cervical ectropion with combined therapy of microwave tissue coagulation(MTC) and interferon suppository. Modern Journal of Integrated Traditional Chinese and Western Medicine 2007;16(7):488,588. [Google Scholar]
Xue 2002 {published data only}
- Xue F, Cai XY, Cao SP. Clinical observation on chronic cervicitis with microwave tissue coagulation (MTC). Chinese Journal of Synthetical Medicine 2002;3(6):537. [Google Scholar]
Xue 2004 {published data only}
- Xue ZL. Observation therapeutic effect of cervical ectropion with combined therapy of microwave tissue coagulation (MTC) and interferon suppository. Journal of Chinese Practical Medicine 2004;6(9):112. [Google Scholar]
Yang 1997 {published data only}
- Yang XL. A comparable study of microwave tissue coagulation (MTC) and laser in treatment of cervicitis. Journal of Guangdong Medical College 1997;15(4):357‐8. [Google Scholar]
Yang 2001 {published data only}
- Yang ZY, Zhou GL, Li Z, Rao ZQ, Chen J. Clinical observation on therapeutic effect of cervical ectropion with combined therapy of microwave tissue coagulation (MTC) and interferon suppository. Chinese Journal of Family Planning 2001;5:299‐300. [Google Scholar]
Yang 2007 {published data only}
- Yang XL. A analysis on therapeutic effect of cervical ectropion by infrared rays. China Practical Medicine 2007;2(1):22‐3. [Google Scholar]
Yang 2008a {published data only}
- Yang GY. Observation therapeutic effect of midrange, severe degree cervical ectropion with microwave tissue coagulation (MTC) and interferon suppository. Maternal and Child Health Care of China 2008;23(9):1310‐1. [Google Scholar]
Yang 2008b {published data only}
- Yang LJ. Clinical understand of cervical ectropion with microwave tissue coagulation (MTC) and interferon suppository. Journal of Laparoscopic Surgery 2008;13(6):475‐6. [Google Scholar]
Ye 2005 {published data only}
- Ye LL, Zhou QH, Chen LQ. Clinical analysis on infrared rays therapy and microwave therapy for 200 cases of cervical ectropion. Modern Diagnosis Treatment 2005;16(1):23‐4. [Google Scholar]
You 2004 {published data only}
- You HR, Dai SJ, Peng GP. Observation on therapeutic effect of severe degree cervical ectropion with high frequency electric wave knife. Sichuan Medical Journal 2004;25(6):699‐700. [Google Scholar]
Yu 2003 {published data only}
- Yu NZ. Clinical observation on therapeutic effect of 120 cases of midrange, severe degree cervical ectropion with combined therapy of microwave tissue coagulation (MTC) and interferon suppository. Fujian Medical Journal 2003;25(2):93. [Google Scholar]
Yu 2004 {published data only}
- Yu CG, Wang AL, Li CX, Lv CH. 252 cases of cervicitis with combined therapy of Microwave tissue coagulation (MTC) and interferon suppository. Shanxi Medical Journal 2004;33(11):1044‐5. [Google Scholar]
Yu 2008 {published data only}
- Yu YL. Clinical study of 656 cases of cervical erosion in treatment by microwave tissue coagulation (MTC) and CO2 laser. Medical Journal of Chinese People's Health 2008;20(19):2277. [Google Scholar]
Zhan 2001 {published data only}
- Zhan MM. A comparable study of microwave tissue coagulation (MTC) and Pome ray in treatment of cervical ectropion. Zhejiang Journal of Traumatic Surgery 2001;6(5):339. [Google Scholar]
Zhang 1999 {published data only}
- Zhang GF, Fu XH, Zhang QL. A comparable study of microwave tissue coagulation (MTC) and laser in treatment of cervical ectropion. Journal of Henan Medical University. Journal of Henan Medical University 1999;34(4):60‐1. [Google Scholar]
Zhang 2001 {published data only}
- Zhang AM. Comparative observation of cervical ectropion by microwave tissue coagulation (MTC) and electrocoagulation (EC). Journal of Heze Medical College 2001;13(3):56‐7. [Google Scholar]
Zhang 2002 {published data only}
- Zhang YH, Huang JZ. Clinical observation of 66 cases of cervical ectropion in treatment by microwave tissue coagulation(MTC). Shanxi Clinical Medicine Journal 2002;11(4):284‐5. [Google Scholar]
Zhang 2005a {published data only}
- Zhang YC. A comparable study of infrared light and microwave tissue coagulation (MTC) in treatment of 300 cases of cervical ectropion. Chinese Medical Research & Clinical 2005;3(8):46‐7. [Google Scholar]
Zhang 2005b {published data only}
- Zhang Y. Clinical observation of cervical ectropion in treatment by microwave tissue coagulation (MTC) and interferon suppository. Chinese Medical Journal of Metallurgical Industry 2005;22(3):296. [Google Scholar]
Zhang 2005c {published data only}
- Zhang XY. A comparable study of microwave tissue coagulation (MTC) and interferon‐alpha suppository in treatment of cervical ectropion. Journal of Handan Medical College 2005;18(6):547‐8. [Google Scholar]
Zhang 2006a {published data only}
- Zhang Y, Li JL, Wang XY. A comparable study of microwave tissue coagulation (MTC), CO2 and LEEP in treatment of cervical ectropion. Sichuan Medical Journal 2006;27(8):859‐60. [Google Scholar]
Zhang 2006b {published data only}
- Zhang Y, Zhao Y. A comparable study of microwave tissue coagulation (MTC), CO2 laser and LEEP in treatment of cervical ectropion. Chinese Journal of Obstetrics & Gynecology and Pediatrics 2006;2(4):228‐9. [Google Scholar]
Zhang 2007 {published data only}
- Zhang BN. Observation therapeutic effect of cervical ectropion with combined therapy of microwave tissue coagulation(MTC) and interferon suppository. Shanxi Medicine Journal 2007;36(8):601‐2. [Google Scholar]
Zhao 1997 {published data only}
- Zhao ML, Zou SX, Li FJ. Observation on therapeutic effect of 108 cases of cervical ectropion with microwave tissue coagulation (MTC). Acta Academiae Medicinae Qingdao Universitatis 1997;‐(2):183. [Google Scholar]
Zhao 2003 {published data only}
- Zhao XD, Liang XH. Observation on therapeutic effect of severe degree cervical ectropion with combined therapy of microwave tissue coagulation (MTC) and interferon suppository. Youjiang Medical Journal 2003;31(4):352‐3. [Google Scholar]
Zhao 2007 {published data only}
- Zhao Q, Bian AP. Clinical analysis on therapeutic effect of cervical ectropion with combined therapy of microwave tissue coagulation(MTC) and interferon suppository. Journal of Medical Forum 2007;28(3):54‐5. [Google Scholar]
Zhao 2008 {published data only}
- Zhao Y. Observation therapeutic effect of cervical ectropion with microwave tissue coagulation (MTC) and interferon suppository. Chinese Journal of Modern Drug Application 2008;2(13):62‐3. [Google Scholar]
Zhong 2005 {published data only}
- Zhong WH. A study of combine microwave tissue coagulation (MTC) with interferon suppository in treatment of severe degree cervical ectropion. Journal of Medical Forum 2005;26(3):43,6. [Google Scholar]
Zhou 2002 {published data only}
- Zhou GZ. Observation on therapeutic effect of 100 cases of cervical ectropion with microwave tissue coagulation (MTC). Youjiang Medical Journal 2002;30(5):403. [Google Scholar]
Zhu 1997 {published data only}
- Zhu ZZ, Teng ZR. A study of microwave tissue coagulation (MTC) in treatment of cervical ectropion. Acta Universitatis Medicinalis Secondae Shanghai 1997;17(2):136‐7. [Google Scholar]
Zhu 2007 {published data only}
- Zhu YZ, Liu SY, Liu SQ. Clinical study of 112 cases of cervical ectropion with microwave tissue coagulation(MTC) and interferon suppository. China Practical Medicine 2007;2(1):33‐4. [Google Scholar]
References to studies awaiting assessment
Chen 2001 {published data only}
- Chen M, Li HW. A study of microwave tissue coagulation (MTC) and electrocoagulation (EC) in the treatment of cervical ectropion. Academic Journal of Guangzhou Medical College 2001;29(2):54‐5,62. [Google Scholar]
Chen 2002b {published data only}
- Chen Y, Bao CY. Clinical study of 360 cases of cervical ectropion in treatment by interferon suppository and microwave tissue coagulation (MTC). Henan Medical Information 2002;10(24):1‐2. [Google Scholar]
Chen 2004 {published data only}
- Chen GL. Clinical observation on treating severe cervical ectropion. Chinese Journal of the Practical Chinese with Modern Medicine 2004;4(17):3749‐50. [Google Scholar]
Cheng 2000 {published data only}
- Cheng FR. Clinical observation on therapeutic effect of cervical ectropion with microwave tissue coagulation (MTC). Chinese Journal of Medical Writing 2000;7(11):1220. [Google Scholar]
He 1995 {published data only}
- He ZY. A comparable study of microwave tissue coagulation (MTC) and cryotherapy in treatment of cervical ectropion. Journal of Proceeding of Medicine 1995;9(2):14‐5. [Google Scholar]
Li 2004 {published data only}
- Li J. Clinical observation on therapeutic effect of 206 cases of cervical ectropion with microwave tissue coagulation (MTC). Chinese Journal of Clinical Pharmacy 2004;2(4):72‐3. [Google Scholar]
Shi 2000 {published data only}
- Shi YQ. Clinical observation on cervical ectropion with microwave tissue coagulation (MTC). Chinese Journal of Medical Writing 2000;7(24):2823‐4. [Google Scholar]
Sun 2001 {published data only}
- Sun YC, Liu CY, Yang QH. A comparable study of microwave tissue coagulation (MTC) and electrocoagulation in treatment of cervical ectropion. Practical Journal of Rural Doctor 2001;8(6):23,6. [Google Scholar]
Yang 2004 {published data only}
- Yang L. Clinical observation on therapeutic effect of cervical ectropion with microwave therapy apparatus. Chinese Journal of Synthetical Medicine 2004;5(12):1126. [Google Scholar]
Additional references
Cai 1997
- Cai CX. An epidemic investigation on cervix ectropion for women staff in the city of Linhai. Modern Preventive Medicine 1997;24(3):317‐8, 21. [Google Scholar]
Chang 1991
- Chang AR. 'Erosion' of the uterine cervix; an anachronism. The Australian & New Zealand Journal of Obstetrics & Gynaecology 1991;31(4):358‐62. [DOI] [PubMed] [Google Scholar]
Critchlow 1995
- Critchlow C, Wolner‐Hanssen P, Eschenbach D, Kiviat NB, Koutsky LA, Stevens CE, et al. Determinants of cervical ectopia and of cervicitis: Age, oral contraception, specific cervical infection, smoking, and douching. American Journal Obstetrics and Gynecology 1995;173:534‐43. [DOI] [PubMed] [Google Scholar]
Dalgic 2001
- Dalgic H, Kuscu NK. Laser therapy in chronic cervicitis. Archives of Gynecology and Obstetrics 2001;265(2):64‐6. [DOI] [PubMed] [Google Scholar]
Guan 2004
- Guan Z. Minimally invasive obstetrics. Beijing: People's Military Medical Press, 2004:342‐3. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration. John Wiley & Sons, Ltd, 2008.
Peng 2002
- Peng XF, Wang XY, Cheng LH. 2735 cases of the cervical ectropion epidemiology factors analysis. International Medicine and Health Guidance News 2002;8:80‐1. [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 Statement: updated guidelines for reporting parallel grouprandomised trials. Trials 2010;11(1):32[Epub ahead of print]. [Google Scholar]
Selo‐Ojeme 2004
- Selo‐Ojeme DO, Dayoub N, Patel A. A clinico‐pathological study of postcoital bleeding. Archives of Gynecology and Obstetrics 2004;270:34‐6. [DOI] [PubMed] [Google Scholar]
Tang 1995
- Tang SE, Zhou XM. The cytopathologic diagnosis of cervical lesions and uterocervical carcinomas. Chinese Journal Obstetrics Gynecology 1995;30(1):10‐4. [PubMed] [Google Scholar]
Wu 2007
- Wu TX, Liu GJ. The concepts, design, practice and report of allocation concealment and blinding. Chinese Journal of Evidence‐Based Medicine 2007;7(3):222‐5. [Google Scholar]
Xiao 2003
- Xiao LZ, Dai KW, Li L. Research on epidemiological feature of cervical ectropion among the married women in urban area. Chinese Primary Health Care 2003;17(1):42‐3. [Google Scholar]
Yue 2000
- Yue Jie. Gynecology and obstetrics. V. Beijing: People's Medical Publishing Houses, 2000. [Google Scholar]
Yue 2005
- Yue J. Gynecology and obstetrics. VI. Beijing: People's Medical Publishing Houses, 2005:265‐7. [Google Scholar]
Zhang 1995
- Zhang PZ. An analysis of epidemiological factors for 4284 cases of women with cervix ectropion. Journal of Lanzhou Medical College 1995;21(2):85‐6. [Google Scholar]
Zhang 2003
- Zhang XY. 1131 cases with the cervical ectropion epidemiology factors analysis. Chinese Journal of Epidemiology 2003;24(6):486. [Google Scholar]


