Abstract
Background
Bronchodilators are the mainstay for symptom relief in the management of stable chronic obstructive pulmonary disease (COPD). Aclidinium bromide is a new long‐acting muscarinic antagonist (LAMA) that differs from tiotropium by its higher selectivity for M3 muscarinic receptors with a faster onset of action. However, the duration of action of aclidinium is shorter than for tiotropium. It has been approved as maintenance therapy for stable, moderate to severe COPD, but its efficacy and safety in the management of COPD is uncertain compared to other bronchodilators.
Objectives
To assess the efficacy and safety of aclidinium bromide in stable COPD.
Search methods
We identified randomised controlled trials (RCT) from the Cochrane Airways Group Specialised Register of trials (CAGR), as well as www.clinicaltrials.gov, World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), US Food and Drug Administration (FDA) website and Almirall Clinical Trials Registry and Results. We contacted Forest Laboratories for any unpublished trials and checked the reference lists of identified articles for additional information. The last search was performed on 7 April 2014 for CAGR and 11 April 2014 for other sources.
Selection criteria
Parallel‐group RCTs of aclidinium bromide compared with placebo, long‐acting beta2‐agonists (LABA) or LAMA in adults with stable COPD.
Data collection and analysis
Two review authors independently selected studies, assessed the risk of bias, and extracted data. We sought missing data from the trial authors as well as manufacturers of aclidinium. We used odds ratios (OR) for dichotomous data and mean difference (MD) for continuous data, and reported both with their 95% confidence intervals (CI). We used standard methodological procedures expected by The Cochrane Collaboration. We applied the GRADE approach to summarise results and to assess the overall quality of evidence.
Main results
This review included 12 multicentre RCTs randomly assigning 9547 participants with stable COPD. All the studies were industry‐sponsored and had similar inclusion criteria with relatively good methodological quality. All but one study included in the meta‐analysis were double‐blind and scored low risk of bias. The study duration ranged from four weeks to 52 weeks. Participants were more often males, mainly Caucasians, mean age ranging from 61.7 to 65.6 years, and with a smoking history of 10 or more pack years. They had moderate to severe symptoms at randomisation; the mean post‐bronchodilator forced expiratory volume in one second (FEV1) was between 46% and 57.6% of the predicted normal value, and the mean St George's Respiratory Questionnaire score (SGRQ) ranged from 45.1 to 50.4 when reported.
There was no difference between aclidinium and placebo in all‐cause mortality (low quality) and number of patients with exacerbations requiring a short course of oral steroids or antibiotics, or both (moderate quality). Aclidinium improved quality of life by lowering the SGRQ total score with a mean difference of ‐2.34 (95% CI ‐3.18 to ‐1.51; I2 = 48%, 7 trials, 4442 participants) when compared to placebo. More patients on aclidinium achieved a clinically meaningful improvement of at least four units decrease in SGRQ total score (OR 1.49; 95% CI 1.31 to 1.70; I2 = 34%; number needed to treat (NNT) = 10, 95% CI 8 to 15, high quality evidence) over 12 to 52 weeks than on placebo. Aclidinium also resulted in a significantly greater improvement in pre‐dose FEV1 than placebo with a mean difference of 0.09 L (95% CI 0.08 to 0.10; I2 = 39%, 9 trials, 4963 participants). No trials assessed functional capacity. Aclidinium reduced the number of patients with exacerbations requiring hospitalisation by 4 to 20 fewer per 1000 over 4 to 52 weeks (OR 0.64; 95% CI 0.46 to 0.88; I2 = 0%, 10 trials, 5624 people; NNT = 77, 95% CI 51 to 233, high quality evidence) compared to placebo. There was no difference in non‐fatal serious adverse events (moderate quality evidence) between aclidinium and placebo.
Compared to tiotropium, aclidinium did not demonstrate significant differences for exacerbations requiring oral steroids or antibiotics, or both, exacerbation‐related hospitalisations and non‐fatal serious adverse events (very low quality evidence). Inadequate data prevented the comparison of aclidinium to formoterol or other LABAs.
Authors' conclusions
Aclidinium is associated with improved quality of life and reduced hospitalisations due to severe exacerbations in patients with moderate to severe stable COPD compared to placebo. Overall, aclidinium did not significantly reduce mortality, serious adverse events or exacerbations requiring oral steroids or antibiotics, or both.
Currently, the available data are insufficient and of very low quality in comparisons of the efficacy of aclidinium versus tiotropium. The efficacy of aclidinium versus LABAs cannot be assessed due to inaccurate data. Thus additional trials are recommended to assess the efficacy and safety of aclidinium compared to other LAMAs or LABAs.
Keywords: Aged; Female; Humans; Male; Middle Aged; Adrenergic beta‐2 Receptor Agonists; Adrenergic beta‐2 Receptor Agonists/therapeutic use; Bronchodilator Agents; Bronchodilator Agents/therapeutic use; Disease Progression; Muscarinic Antagonists; Muscarinic Antagonists/therapeutic use; Pulmonary Disease, Chronic Obstructive; Pulmonary Disease, Chronic Obstructive/drug therapy; Randomized Controlled Trials as Topic; Scopolamine Derivatives; Scopolamine Derivatives/therapeutic use; Tiotropium Bromide; Tropanes; Tropanes/therapeutic use
Plain language summary
Effectiveness and safety of inhalers containing the drug aclidinium bromide for managing patients with stable COPD
Review question
We reviewed the evidence on the effectiveness and safety of aclidinium inhalers used by people with chronic obstructive pulmonary disease (COPD).
Background
COPD, also known as 'smoker's lung disease', includes conditions called emphysema and chronic bronchitis where there is airway narrowing that cannot be fully corrected. It is a progressive disease. COPD patients usually have breathing problems and a cough that produces a lot of phlegm. It is diagnosed by international guidelines set by the Global Initiative for Obstructive Lung Disease (GOLD). Symptoms may worsen during flare‐ups. The main aims of treating COPD patients are to relieve symptoms, reduce flare‐ups and improve quality of life. Aclidinium is a new inhaled drug that widens the airways (a bronchodilator). It is delivered by an inhaler called Genuair or Pressair. We wanted to discover whether aclidinium was better or worse than using other inhalers or a dummy inhaler.
Study characteristics
The evidence was current to 7 April 2014. We included 12 studies involving 9547 COPD patients over a period of four to 52 weeks. These studies were sponsored by drug companies and were well designed. Both patients and the people doing the research did not know which treatment the patients were getting; although in one study one treatment was known to both parties. More men than women took part, and they were mostly Caucasians. They were in their 60s and had smoked a lot in their lives. These people had moderate to severe symptoms when they started treatment.
Key results
Aclidinium did not reduce the number of people with flare‐ups that need additional drugs. There was little or no difference in deaths or serious side effects between aclidinium and a dummy inhaler. Aclidinium inhalers improved quality of life more than the dummy inhalers.
People who took aclidinium had fewer hospital admissions due to serious flare‐ups. Based on our results, among 1000 COPD patients using a dummy inhaler over four weeks to one year 37 would have severe flare‐ups needing hospital admission. Only 17 to 33 patients out of 1000 would require hospital admission if they were using aclidinium inhalers. We also set out to compare this new medication with tiotropium, which is already used to treat COPD. There were only two studies for this comparison thus we could not be sure how aclidinium compared to tiotropium. We also could not compare aclidinium with another well known inhaler that contains the drug formoterol because of unreliable data.
Quality of the evidence
For the comparison of aclidinium inhalers and dummy inhalers, we are confident that there are benefits in terms of the number of hospitalisations and patients' quality of life; we are less certain about the numbers of flare‐ups needing additional drugs and serious side effects. We do not have enough information to assess any effect on the number of deaths. We did not have enough information to reliably compare aclidinium with tiotropium or formoterol.
Summary of findings
Summary of findings for the main comparison. Aclidinium bromide compared to placebo for stable chronic obstructive pulmonary disease.
| Aclidinium bromide compared to placebo for stable chronic obstructive pulmonary disease | ||||||
| Patient or population: patients with stable chronic obstructive pulmonary disease Settings: community Intervention: aclidinium bromide Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Aclidinium bromide | |||||
| Mortality (all‐cause) Follow‐up: 6‐52 weeks | 5 per 1000 | 5 per 1000 (2 to 10) | OR 0.92 (0.43 to 1.94) | 5252 (9 studies) | ⊕⊕⊝⊝ low1 | |
| Exacerbations requiring steroids, antibiotics or both Follow‐up: 4‐52 weeks | 137 per 1000 | 122 per 1000 (105 to 141) | OR 0.88 (0.74 to 1.04) | 5624 (10 studies) | ⊕⊕⊕⊝ moderate2,3,4 | |
| Quality of life Number of patients who achieved at least 4 units improvement in SGRQ total score Follow‐up: 12‐52 weeks | 396 per 1000 | 494 per 1000 (462 to 527) | OR 1.49 (1.31 to 1.7) | 4420 (7 studies) | ⊕⊕⊕⊕ high | The mean quality of life (SGRQ total score change from baseline) in the intervention groups was 2.34 lower (3.18 to 1.51 lower); (4442 participants; 7 studies) |
| Functional capacity Six‐minute walking distance | See comment | See comment | Not estimable | 0 (0) | See comment | No study assessed functional capacity |
| Hospital admissions due to exacerbations Follow‐up: 4‐52 weeks | 37 per 1000 | 24 per 1000 (17 to 33) | OR 0.64 (0.46 to 0.88) | 5624 (10 studies) | ⊕⊕⊕⊕ high2,3 | |
| Non‐fatal serious adverse events Follow‐up: 4‐52 weeks | 56 per 1000 | 50 per 1000 (40 to 64) | OR 0.89 (0.7 to 1.14) | 5651 (10 studies) | ⊕⊕⊕⊝ moderate2,3,4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 ‐2 for imprecision: the CI includes the possibility of both appreciable benefit and harm. 2Chanez 2010 failed to report some outcomes of lung function in the published full text but it is unlikely to affect this outcome. 3Chanez 2010 is double blinded for aclidinium and placebo arms though it is open label for tiotropium arm with no study limitation for this comparison. 4 ‐1 for imprecision: the CI includes important benefit and potential harm.
Summary of findings 2. Aclidinium bromide compared to tiotropium for stable chronic obstructive pulmonary disease.
| Aclidinium bromide compared to tiotropium for stable chronic obstructive pulmonary disease | ||||||
| Patient or population: patients with stable chronic obstructive pulmonary disease Settings: community Intervention: aclidinium bromide Comparison: tiotropium | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Tiotropium | Aclidinium bromide | |||||
| Mortality (all‐cause) | See comment | See comment | Not estimable | 329 (1) | See comment | No deaths were reported |
| Exacerbations requiring steroids, antibiotics or both Follow‐up: 4‐6 weeks | 0 per 1000 | 11 per 1000 (0 to 26)1 | OR 2.64 (0.31 to 22.18) | 729 (2 studies) | ⊕⊝⊝⊝ very low2,3,4 | |
| Quality of life St George's Respiratory Questionnaire (SGRQ) score | See comment | See comment | Not estimable | 0 (0) | See comment | No studies measured and reported quality of life for aclidinium and tiotropium |
| Functional capacity Six‐minute walk distance | See comment | See comment | Not estimable | 0 (0) | See comment | No studies measured and reported functional capacity for aclidinium and tiotropium |
| Hospital admissions due to exacerbations Follow‐up: 4‐6 weeks | 4 per 1000 | 2 per 1000 (0 to 18) | OR 0.54 (0.07 to 4.11) | 729 (2 studies) | ⊕⊝⊝⊝ very low2,3,4 | |
| Non‐fatal serious adverse events Follow‐up: 4‐6 weeks | 18 per 1000 | 12 per 1000 (3 to 46) | OR 0.67 (0.17 to 2.65) | 729 (2 studies) | ⊕⊝⊝⊝ very low2,3,4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The corresponding risk for aclidinium bromide was calculated using the risk difference to avoid having zero in both columns.
2 ‐1 for high risk of bias in Chanez 2010 because it was open label for tiotropium arm. 3Chanez 2010 failed to report some outcomes of lung function in the published full text but it is unlikely to affect this outcome. 4 ‐2 for imprecision: the CI includes the possibility of both appreciable benefit or harm.
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is "a common, preventable and treatable disease, characterised by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases" (GOLD 2013). Tobacco smoke is the major risk factor in the pathogenesis of COPD; chemicals, occupational exposures, indoor and outdoor air pollution are also recognised risk factors (GOLD 2013; Hogg 2009; MacNee 2006; TSANZ 2012; WHO 2012).
COPD is the third leading cause of death after heart disease and malignancy in the United States (CDC 2011) and accounts for approximately 30,000 deaths each year in the UK (NICE 2010). It was the fourth leading cause of mortality in 2004, with three million deaths worldwide (WHO 2008). Ninety per cent of deaths from COPD occurred in low and middle‐income countries in 2008 (WHO 2010). The World Health Organization (WHO) has estimated that COPD will become the third leading cause of death worldwide in 2030 due to a projected increase in smoking and environmental pollution (WHO 2012a). Exacerbations and co‐morbidities contribute to the overall severity of COPD in patients (GOLD 2013). Currently available prevalence data do not reflect the actual total burden of COPD because of under reporting, with diagnosis only being made when the disease is clinically apparent (GOLD 2013).
COPD also has a significant economic impact, mainly due to exacerbations. The total annual cost of COPD to the National Health Service (NHS) in the UK is estimated to be over GBP 800 million, for direct healthcare costs (NICE 2011). It accounts for 56% (EUR 38.6 billion) of the total cost of respiratory diseases in the European Union, while the estimated cost in the United States (US) is USD 29.5 billion and USD 20.4 billion, for direct and indirect costs respectively (GOLD 2013).
Acute exacerbations are a major cause of morbidity and mortality in COPD patients and are defined as "an event in the natural course of the disease characterised by a change in the patient's baseline dyspnoea, cough, and/or sputum, that is beyond normal day‐to‐day variations, is acute in onset and may warrant a change in medication in a patient with underlying COPD" (GOLD 2013).
Currently there is no cure for COPD. Apart from smoking cessation and long‐term oxygen therapy in severely hypoxic patients, other therapeutic options do not improve survival (GOLD 2013). Thus, the major goal of medication is to relieve symptoms, reduce the frequency and severity of exacerbations, and improve quality of life (ATS/ERS 2011; Chong 2012; GOLD 2013; Sutherland 2004; TSANZ 2012).
Management of stable COPD is multidisciplinary, with options such as smoking cessation (van der Meer 2012); education (Effing 2009); vaccination for influenza (Poole 2010) and pneumococcal infections (Walters 2010); breathing exercises (Holland 2012); pulmonary rehabilitation (Lacasse 2009); pharmacotherapy with inhaled bronchodilators, inhaled corticosteroids for severe COPD or frequent exacerbations (GOLD 2013; TSANZ 2012; Yang 2012), phosphodiesterase‐4 inhibitors (Chong 2011); long‐term domiciliary oxygen therapy (Cranston 2008); and lung volume reduction surgery (Tiong 2009). Regular long‐term use of oral corticosteroids is not recommended for stable COPD and is associated with an increased risk of systemic side effects (GOLD 2013; Walters 2009). Oral theophylline has a modest bronchodilator effect (Ram 2009) but is less effective than inhaled long‐acting bronchodilators (GOLD 2013). Mucolytic agents show a slight reduction in exacerbations but have no effect on the overall quality of life (Poole 2012). Neither of these medications are routinely recommended for stable COPD (GOLD 2013). Long‐acting bronchodilators, either a long‐acting beta2‐agonist (LABA) (Nannini 2012; Welsh 2011) or a long‐acting muscarinic antagonist (LAMA) (Karner 2012), are the first‐line maintenance therapy for moderate to severe, stable COPD (GOLD 2013; NICE 2010).
Description of the intervention
Aclidinium bromide is a new long‐acting antimuscarinic agent that blocks the action of the neurotransmitter acetylcholine. It was approved by the US Food and Drug Administration (FDA) on 23 July 2012 for use in moderate to severe, stable COPD patients (FDA 2012). It is marketed as Tudorza Pressair by Forest Laboratories and Almirall in the US. It is a dry powder formulation (Sims 2011) and the FDA approved dosage is 400 µg inhaled twice daily. In Europe and the UK it has been launched as Eklira Genuair by Almirall.
It is delivered by a state of the art multidose dry powder inhaler (MDPI), termed Genuair or Pressair, which is preloaded with a one‐month supply of medication. The MDPI is specially designed with a visible dose level indicator with an anti‐double dosing mechanism, multiple feedback mechanisms to indicate successful inhalation, such as an audible click and a slightly sweet taste, as well as an end‐of‐dose lock‐out system to prevent further use after the final dose (Maltais 2012; Sims 2011).
How the intervention might work
Airway obstruction mediated by vagal cholinergic tone is the major reversible contributor to COPD (Jones 2011). Currently there are five known subtypes of muscarinic cholinergic receptors (M1 to M5), of which three (M1, M2 and M3) are present in the bronchial airway smooth muscle (Karakiulakis 2012; Maltais 2012).
Acetylcholine acts on M1 receptors to facilitate further neurotransmission from parasympathetic ganglia, and binds to M3 receptors located on the airway smooth muscle cells to induce bronchoconstriction. M2 receptors mediate feedback inhibition of acetylcholine release at the cholinergic nerve endings (Karakiulakis 2012; Sims 2011; Vogelmeier 2011).
Aclidinium bromide is a LAMA which inhibits the action of acetylcholine at the muscarinic receptors with approximately a six‐fold kinetic selectivity for M3 receptors compared to the M2 subtype, resulting in a more effective bronchodilator action with fewer M2 mediated cardiac side effects (Maltais 2012; Sims 2011). The onset of action of aclidinium bromide (30 minutes) is similar to ipratropium (30 minutes) but faster than tiotropium (80 minutes). The duration of action of aclidinium (t1/2 = 29 hours) is shorter than for tiotropium (t1/2 = 64 hours) but longer than for ipratropium (t1/2 = 8 hours) (Maltais 2012).
These muscarinic receptors are also present in other parts of the body, such as M1 receptors in the central nervous system (CNS); M2 in the heart; M3 in the gastrointestinal tract (GIT), iris and sphincter; and M4 in the neostriatum, whereas the functional role of M5 receptors is unclear (Gavaldà 2010). Thus, the non‐selective blockade of muscarinic receptors has the potential for systemic side effects.
Aclidinium has been shown in preclinical and clinical studies to rapidly hydrolyse in the plasma into two inactive metabolites, with a very short plasma half life of 2.4 minutes, while the plasma half life for ipratropium is 96 minutes and for tiotropium it is more than six hours (Maltais 2012). This low and transient level in the plasma leads to less drug‐drug interaction and contributes to a more favourable safety profile.
Why it is important to do this review
Although a long‐lasting bronchodilator effect and favourable safety profile of aclidinium bromide has been shown in a number of clinical trials (Jones 2011; Jones 2012), the summarised safety and efficacy profile of this agent is lacking when compared to placebo or currently established treatment options such as LABAs or LAMAs. We aimed to fill this gap by performing a systematic review of the findings of all available randomised controlled trials to help clinicians provide evidence‐based, long‐term management of stable COPD.
Objectives
To assess the efficacy and safety of aclidinium bromide in stable COPD.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) with a parallel‐group design comparing aclidinium bromide with placebo or a LABA or LAMA, both open‐label and blinded studies. Since COPD is a progressive disorder which deteriorates with time, we excluded cross‐over trials. We also excluded cluster‐randomised trials to avoid bias.
Types of participants
We included studies involving adults (over 18 years of age) diagnosed with moderate to severe COPD as defined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD 2013), American Thoracic Society (ATS), European Respiratory Society (ERS) (ATS/ERS 2011), Thoracic Society of Australia and New Zealand (TSANZ 2012), UK National Institute for Health and Clinical Excellence (NICE 2010) or the WHO. Participants in the studies had evidence of airway obstruction (post‐bronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio of < 70% and FEV1 < 80% of predicted value) with clinical presentation of dyspnoea, chronic cough or sputum production with or without a history of smoking. We excluded studies which enrolled patients with bronchial asthma, bronchiectasis, cystic fibrosis or other lung diseases.
Types of interventions
Aclidinium bromide versus placebo
Aclidinium bromide versus long‐acting beta2‐agonist (LABA)
Aclidinium bromide versus long‐acting muscarinic antagonist (LAMA)
Types of outcome measures
Primary outcomes
Mortality (all‐cause and respiratory)
Exacerbations requiring a short course of an oral steroid or antibiotic, or both
Quality of life measured by a validated scale, the St George's Respiratory Questionnaire (SGRQ) or Chronic Respiratory Disease Questionnaire (CRQ)
Secondary outcomes
Change in lung function (FEV1, FEV1/FVC)
Functional capacity by six‐minute walking distance
Hospital admissions due to exacerbations or from all causes
Improvement in symptoms measured by the Transitional Dyspnoea Index (TDI)
Adverse events
Non‐fatal serious adverse events
Withdrawals due to lack of efficacy or adverse events
We assessed mortality and exacerbations as primary outcomes since exacerbations are the major cause of morbidity and mortality in COPD patients. We also classified quality of life as a primary outcome since it is one of the most important parameters that can measure both the subjective and objective well‐being of COPD patients, who have to live with this chronic disease. We recorded change in lung function from the baseline, exercise capacity, hospital admissions and symptom improvement as secondary outcomes as these may not directly reflect mortality and morbidity in COPD. For the safety profile of this new intervention (aclidinium bromide), we studied adverse events, non‐fatal serious adverse events and withdrawals from studies as secondary outcome measures.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register of trials (CAGR), which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see Appendix 1 for further details). We searched all records in the CAGR coded as 'COPD' using the following terms:
Aclidinium* or "LAS34273" or "Tudorza" or "Eklira" or "Genuair" or "Pressair" or "LAMA" or "Muscarinic Antagonist*".
We also conducted a search of ClinicalTrials.gov (Appendix 2) and the WHO International Clinical Trials Registry Platform (ICTRP) (WHO ICTRP) for additional trials. We searched all databases from their inception with no restrictions on language of publication. The initial search was conducted in March 2013 and it was updated in April 2014.
Searching other resources
We thoroughly checked the reference lists of all primary studies and review articles for additional references. We contacted corresponding authors of identified trials and asked them to identify other published and unpublished studies. We also contacted manufacturers and experts in the field. We searched the US FDA website (FDA 2012) for details of the clinical trials. In addition, we searched the manufacturers' websites (Forest Pharmaceuticals and Almirall) for additional information on the studies identified through the electronic searches. We had planned to translate studies published in a language other than English.
Data collection and analysis
Selection of studies
Two review authors (HN and SM) independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third review author (ZS) who is an expert in the field. We included the trials meeting the criteria regardless of language or publication status (published, unpublished, in press and in progress). We recorded the excluded studies together with the reasons for exclusion.
Data extraction and management
Two review authors (HN and SM) independently extracted and recorded the data from included studies using standard data extraction forms. The data were cross‐checked. The data extraction included study characteristics: mainly study design, participants, interventions, primary and secondary outcome measures; and the analysis performed in the original studies. Where there were discrepancies, we consulted a third review author (ZS) to resolve the inconsistencies. In the case of insufficient or missing data, we contacted the corresponding authors of the studies for additional information. One of the review authors (HN) entered the data into Review Manager 5 software for analysis and the data were checked by another review author (SM).
Assessment of risk of bias in included studies
Two review authors (HN and SM) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor (ZS). We assessed the risk of bias according to the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
We graded each potential source of bias as high, low or unclear. We recorded these judgements in the 'Risk of bias' tables accompanying the characteristics of each included study and summarised them in the 'Risk of bias' summary figure. We contacted the investigators of the RCTs for the details of procedures involved in the conduct of the trials and the replies were kept for evidence. We had planned to exclude trials with high risk of bias. We used the information from the assessment of risk of bias to carry out stratified analysis.
Measures of treatment effect
Dichotomous data
We analysed dichotomous outcome data (such as mortality, exacerbations and withdrawals) using the Mantel‐Haenszel odds ratio (OR) with 95% confidence interval (CI). We had planned to apply the Peto odds ratio if events were rare.
We also calculated the number needed to treat (NNT) for dichotomous outcomes to reflect the number of patients necessary to obtain a beneficial or harmful outcome with the intervention.
Continuous data
We assessed continuous data variables (such as quality of life, symptoms, lung function and exercise capacity) as fixed‐effect mean differences (MD) with 95% CIs when the same scale was used to measure the outcome in all the included studies. We planned to use the standardised mean difference (SMD) when all studies assessed the same outcome but measured it in different ways. We preferentially applied the MD based on change from baseline over the MD based on absolute values.
Unit of analysis issues
We analysed the participants as the unit of analysis for dichotomous data. For continuous data we used the MD, which was the average change from baseline and not the absolute mean. For outcomes that may occur more than once, such as exacerbations, hospital admissions and adverse events, we analysed the number of participants with one or more events.
Dealing with missing data
We contacted the investigators or study sponsors in order to verify key study characteristics and to obtain missing numerical outcome data, where possible. In cases where missing data were not available despite attempts to obtain the data, we recorded this information in our review. We performed sensitivity analyses to assess the impact of unknown status or assumptions made about missing data on participants who withdrew from the trials on the overall pooled result of the meta‐analysis. We followed the intention‐to‐treat (ITT) principle in the analysis of outcomes from the randomised trials, if appropriate.
Assessment of heterogeneity
We assessed heterogeneity between the trials by checking for poor overlap of the confidence intervals in the forest plot and by applying the Chi2 test, with a 10% level of significance. in each analysis we used the I² statistic to measure the percentage of inconsistency in results due to inter‐trial variability. When we identified substantial heterogeneity, we explored it by pre‐specified subgroup analysis. The level of statistical variation between the trials was considered as high if the I² value was more than 50%.
Assessment of reporting biases
We minimised reporting bias as a result of non‐publication of studies or selective outcome reporting by using a systematic search strategy, contacting study authors and manufacturers, and checking multiple references of the included studies. We also visually inspected funnel plots for asymmetry. If we suspected reporting bias because of the asymmetrical appearance of the funnel plot after exclusion of other reasons for funnel plot asymmetry, we had planned to contact the study authors requesting them to provide any missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we had planned to explore the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Data synthesis
We analysed the data using Review Manager 5. For pooling the outcomes of the studies, we used a fixed‐effect model if the I2 statistic was consistent with homogeneous results. We applied a random‐effects model for data synthesis when heterogeneity was identified (I2 > 50%) and it could not be explained by factors identified in the subgroup analyses. We combined dichotomous outcome variables using a Mantel‐Haenszel OR with 95% CI. For continuous outcome data, we analysed the results as MD with 95% CI. Where treatment effects were reported as MD with 95% CI or exact P value, we had planned to calculate the standard error, enter it with the MD, and combine the results using a fixed‐effect model generic inverse variance (GIV) analysis. We calculated the NNT from the pooled OR and assumed control risk (ACR) using the formula described in Section 12.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We created summary of findings tables using the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and using the GRADEpro software for overall grading of the quality of the evidence. We included the following outcomes.
Mortality (all‐cause and respiratory).
Exacerbations requiring a short course of an oral steroid or antibiotic, or both.
Quality of life.
Functional capacity by the six‐minute walking distance.
Hospital admissions due to exacerbations or all causes.
Non‐fatal serious adverse events.
Subgroup analysis and investigation of heterogeneity
If there was significant heterogeneity, we had planned to perform subgroup analysis in order to explain it. We had planned to carry out the following subgroup analyses.
Dose of aclidinium bromide (e.g. 200 µg; 400 µg).
Frequency of aclidinium bromide (once daily; twice daily).
Duration of treatment period (short‐term (12 weeks or less); long‐term (more than 12 weeks)).
Disease severity at baseline (FEV1 < 50% predicted; FEV1 ≥ 50% predicted).
Concurrent therapy with theophylline (dichotomised as yes or no).
We had planned to include the following outcomes in subgroup analyses.
Exacerbations.
Quality of life.
Change in lung function.
Sensitivity analysis
We assessed the robustness of our analyses by repeating the meta‐analysis after exclusion of studies with high risk of bias and those with unclear methodological data.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification and Characteristics of ongoing studies for complete details.
Results of the search
We conducted the initial search of the Cochrane Airways Group Specialised Register of trials (CAGR) on 14 March 2013 and the search of other sources (WHO ICTRP, Almirall, www.clinicaltrials.gov) on 28 June 2013 with no restriction on language. In January 2014, a second search was done for all resources. A third updated search was conducted on 7 April 2014 for the CAGR and on 11 April 2014 for other sources. We identified a total of 189 records from the CAGR (103 from the first search, 59 from the second, and 27 from the third search) and a total of 107 reports from other sources (40 from WHO ICTRP, 38 from www.clinicaltrials.gov, 24 from Almirall clinical trial registry, five from the reference lists of included studies). After removal of duplicates, we screened the titles and abstracts of 240 records for eligibility and excluded 106 reports. We thoroughly studied the remaining 134 references for further assessment, retrieving full text articles where applicable and contacting manufacturers about unpublished trials. From our search, we excluded a total of 73 references for 35 studies with complete agreement between the authors. Details of studies which failed to meet the inclusion criteria were recorded in 'Characteristics of excluded studies'. One study (NCT01636401) had been completed but the results were not available and it was awaiting classification. Another ongoing study (ASCENT COPD) is expected to be completed by January 2018. We identified a total of 12 studies reported in 59 references that were eligible for inclusion. For details of the search results please see Figure 1. We asked Forest Research Institute if there were any additional study reports or references to studies that they had sponsored, but there was no reply. Two of the included studies (AUGMENT COPD; Sliwinski 2010) were in abstract form and upon request we received the required information for AUGMENT COPD from Almirall. They also provided data for ACLIFORM and NCT01572792. From the correspondence, NCT01572792 data was in the public domain at the American Thoracic Society (ATS) conference in San Diego in May 2014.
1.
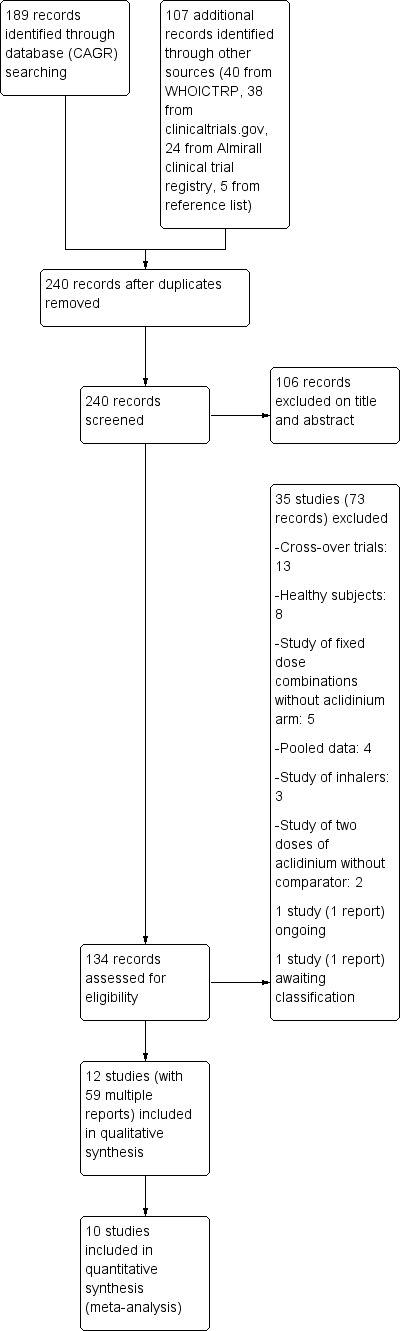
Study flow diagram.
Included studies
See Table 3 for an overview of the included studies.
1. Overview of included studies.
| Duration of study | Number randomised | Severity of participants | Dose of aclidinium | Frequency of aclidinium | |
| ACCLAIM/COPD I | 52 weeks | 843 | Moderate to severe (GOLD) |
200 µg | Once daily |
| ACCLAIM/COPD II | 52 weeks | 804 | Moderate to severe (GOLD) |
200 µg | Once daily |
| ACCORD COPD I | 12 weeks | 561 | Moderate to severe (GOLD) |
200, 400 µg | Twice daily |
| ACCORD COPD II | 12 weeks | 544 | Moderate to severe (GOLD) |
200, 400 µg | Twice daily |
| ACLIFORM | 24 weeks | 1729 | Moderate to severe (GOLD) |
400 µg | Twice daily |
| ATTAIN | 24 weeks | 828 | Moderate to severe (GOLD) |
200, 400 µg | Twice daily |
| AUGMENT COPD | 24 weeks | 1692 | Moderate to severe (GOLD) |
400 µg | Twice daily |
| Beier 2013 | 6 weeks | 414 | Moderate to severe (GOLD) |
400 µg | Twice daily |
| Chanez 2010 | 4 weeks | 464 | Moderate to severe (ATS) |
25, 50, 100, 200, 400 µg |
Once daily |
| Maltais 2011 | 6 weeks | 181 | Moderate to severe (GOLD) |
200 µg | Once daily |
| NCT01572792 | 28 weeks | 921 | Moderate to severe (GOLD) |
400 µg | Twice daily |
| Sliwinski 2010 | 4 weeks | 566 | Moderate to severe | 200 µg | Once daily |
Study design and duration
All trials were randomised, double‐blind, parallel group, multicentre studies. One trial (Chanez 2010) was open label for the tiotropium arm but double blind for the aclidinium and placebo arms. Six trials (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II;ATTAIN; Maltais 2011) studied aclidinium bromide and placebo. In the two trials of Beier 2013 and Chanez 2010, aclidinium bromide was assessed in comparison to both placebo and tiotropium bromide. Four trials (ACLIFORM; AUGMENT COPD; NCT01572792; Sliwinski 2010) studied aclidinium bromide versus placebo and formoterol along with a fixed dose combination of aclidinium and formoterol. NCT01572792 was the 28‐week extension study of AUGMENT COPD; the participants who agreed to participate in the extension study were kept on the same treatment and placebo arms as in the primary study.
The trials were of different study duration, ranging from four to 52 weeks, with a mean of 20.7 weeks. Six of the included studies were of short duration with two trials each having a duration of four weeks (Chanez 2010; Sliwinski 2010), six weeks (Beier 2013; Maltais 2011) and 12 weeks (ACCORD COPD I; ACCORD COPD II). The rest were long duration trials, with two studies of 52 weeks duration (ACCLAIM/COPD I; ACCLAIM/COPD II), one study of 28 weeks duration (NCT01572792) and three studies of 24 weeks duration (ACLIFORM; ATTAIN; AUGMENT COPD).
Setting
Most of the studies were based in the US and Canada (ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; AUGMENT COPD; Maltais 2011; NCT01572792). Other study locations were in Europe (ACCLAIM/COPD I; ACLIFORM; ATTAIN; Beier 2013; Chanez 2010), Australia and New Zealand (ACCLAIM/COPD II; ATTAIN; AUGMENT COPD; NCT01572792), South Africa (ACCLAIM/COPD II; ACLIFORM; ATTAIN) and Korea (ACLIFORM).
Participants
A total of 9547 participants were randomised in 12 eligible studies. The largest trial was ACLIFORM with 1729 participants, whilst Maltais 2011 had the fewest participants with a total of 181. The remaining trials had numbers of participants ranging from 414 to 1692. The participants were current or former cigarette smokers with a smoking history of ≥ 10 pack years who were diagnosed with stable COPD according to the GOLD criteria and with a post‐bronchodilator FEV1/FVC ratio of < 70% and FEV1 < 80% of predicted value (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ACLIFORM; ATTAIN; AUGMENT COPD; Beier 2013; Maltais 2011; NCT01572792). American Thoracic Society (ATS) criteria were used for diagnosis of COPD in patients with a smoking history of ≥ 10 pack years in one other trial (Chanez 2010). No detailed information was available for COPD diagnosis for Sliwinski 2010.
The trials were conducted in adults ≥ 40 years of age, including both male and female patients (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ACLIFORM; ATTAIN; AUGMENT COPD; Beier 2013; Chanez 2010; Maltais 2011; NCT01572792). There was no specific description of age for the participants of Sliwinski 2010. The mean age of the participants ranged from 61.7 to 65.6 years and the majority were males. More than 90% of the participants were Causacians.
Participants had moderate to severe COPD according to the GOLD criteria with FEV1 ≥ 30% and < 80% in 10 studies (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ACLIFORM; ATTAIN; AUGMENT COPD; Beier 2013; Maltais 2011; NCT01572792) and moderate to severe COPD according to the ATS criteria in one trial (Chanez 2010). Moderate to severe COPD patients were also enrolled in Sliwinski 2010, however the specific criteria used for severity assessment were not mentioned. The participants' mean post‐bronchodilator FEV1 was between 46% and 57.6% predicted normal in the trials. Their baseline mean FEV1 was 1.21 L to 1.51 L and the mean St George's Respiratory Questionnaire score (SGRQ) score ranged from 45.1 to 50.4.
Interventions
The participants underwent a two‐week run‐in period to ensure disease stability and washout of disallowed medications in eight trials with full text publications (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ATTAIN; Beier 2013; Chanez 2010; Maltais 2011).
An aclidinium dose of 200 μg was studied in the only or one of the intervention arms in eight trials (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ATTAIN; Chanez 2010; Maltais 2011; Sliwinski 2010). A higher dose of 400 μg was studied in eight trials in one of the treatment arms (ACCORD COPD I; ACCORD COPD II; ACLIFORM; ATTAIN; AUGMENT COPD; Beier 2013; Chanez 2010; NCT01572792). Three studies (ACLIFORM; AUGMENT COPD; NCT01572792) and Sliwinski 2010 studied aclidinium 400 μg and 200 μg, respectively, in comparison to formoterol and placebo together with fixed dose combination arms of aclidinium plus various doses of formoterol. NCT01572792 was the extension study of AUGMENT COPD in which the patients who completed AUGMENT COPD and agreed to participate were kept on the same intervention in a double‐blind fashion for another 28 weeks.
Aclidinium was given once daily in five trials (ACCLAIM/COPD I; ACCLAIM/COPD II; Chanez 2010; Maltais 2011; Sliwinski 2010) while a twice daily dosage was used in the other seven trials (ACCORD COPD I; ACCORD COPD II; ACLIFORM; ATTAIN; AUGMENT COPD; Beier 2013; NCT01572792).
Administration of aclidinium was by inhalation via a novel, multidose dry powder inhaler (Genuair) in 10 trials (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACLIFORM; ATTAIN; AUGMENT COPD; Chanez 2010; Maltais 2011; NCT01572792; Sliwinski 2010), whereas either a Genuair or Pressair inhaler was used to deliver aclidinium in two studies (ACCORD COPD II; Beier 2013).
Tiotropium was delivered by the Handihaler in Beier 2013 and Chanez 2010; the latter was an open label study. Formoterol was studied as one of the interventions in ACLIFORM; AUGMENT COPD; NCT01572792 and Sliwinski 2010 where it was given via a Genuair inhaler, which was not an approved inhaler for formoterol.
Concomitant medications
The participants were permitted to continue inhaled corticosteroids (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ATTAIN; Beier 2013; Chanez 2010; Maltais 2011), systemic corticosteroids (oral or parenteral) at doses equivalent to prednisone ≤ 10 mg/day or 20 mg every other day (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ATTAIN; Beier 2013; Maltais 2011) and oral sustained‐release theophylline (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ATTAIN; Beier 2013; Chanez 2010) provided the administration of these medications was stable for at least four weeks prior to screening; these medications had to be discontinued at least six hours before each study visit. Use of salbutamol or albuterol as rescue medication was also allowed (ACCORD COPD I; ACCORD COPD II; ATTAIN; Beier 2013; Chanez 2010; Maltais 2011). Oxygen therapy for less than 15 hours per day could be continued but not for two hours before study visits (ACCLAIM/COPD I; ACCLAIM/COPD II; ATTAIN; Beier 2013; Maltais 2011). In ACCORD COPD I inhaled anticholinergics and LABAs were specifically mentioned as not allowed during the study period.
Outcomes
The primary outcomes of the included studies were not identical with our review's primary outcomes because most of the individual trials assessed lung function as the primary outcome. Change from baseline in the morning pre‐dose (trough) FEV1 was the primary outcome in eight individual trials (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ACLIFORM; ATTAIN; AUGMENT COPD; Chanez 2010), which was analysed as a secondary outcome in this review. Quality of life measured by the SGRQ, one of the primary outcomes of our review, was studied in the same eight trials (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ACLIFORM; ATTAIN; AUGMENT COPD; Chanez 2010) either as the change from baseline or as the percentage of participants who achieved the minimal clinically important difference (that is ≥ four unit decrease in SGRQ total score). Most of the data for the other primary outcomes of mortality and exacerbations were well reported in the trials and the authors also provided further necessary information regarding the number of patients with exacerbations who required a short course of oral steroids or antibiotics, or both.
None of the included studies assessed functional capacity by the six‐minute walking distance, which was one of the secondary outcomes of our review. Specific data on hospital admissions due to exacerbations were also not mentioned in the published texts. However, the trial investigators provided the required data for this outcome. Other secondary outcomes of this review such as adverse events, non‐fatal serious adverse events and withdrawals were well reported. Data in the format required for the meta‐analysis of some of the secondary outcomes, especially lung function and TDI score, were kindly provided on request.
Funding
Studies were sponsored by Almirall, SA, Barcelona, Spain or Forest Laboratories, Inc, NY, USA.
Excluded studies
We excluded a total of 35 studies with 73 references as they failed to meet the eligibility criteria of our review (see Characteristics of excluded studies for details). Thirteen had a cross‐over study design; eight were phase one studies conducted in healthy participants; five lacked aclidinium as one of the treatment arms; four were reports of pooled data; three assessed the efficacy of and preferences for inhalers; and two studied aclidinium without a comparator.
Risk of bias in included studies
Generally the included studies had good methodological quality with low risk of bias in most of the domains. Detailed assessment of risk of bias across all studies is presented in Characteristics of included studies; and Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
One published study (Beier 2013) provided detailed information on random sequence generation by a computer generated schedule and allocation concealment via an interactive voice‐response system (IVRS). Although not explicitly described in the trial reports, from correspondence all Almirall‐sponsored trials applied a computer generated randomisation schedule which was prepared prior to initiating the trial. This was used to assign a treatment sequence to a randomisation number by the statistics and programming group within Almirall, according to the relevant standard operating procedures. The randomisation was performed in order to avoid any possible bias. The block size was determined in agreement with the clinical trial manager and the statistician and was not to be communicated to the investigators. In all studies, the IVRS (and in some cases an interactive web‐response system (IWRS)) was used to sequentially randomise patients to the intervention arms according to the randomisation ratio defined in each study as well as the block size that was determined by the sponsor (Appendix 3). Since Sliwinski 2010 was available as an abstract only, the information was insufficient to accurately assess the selection bias.
Blinding
All of the included studies had a double‐blind design with blinding of participants, caregivers and investigators. From correspondence, blinding was applicable for all study outcomes. In the placebo‐controlled studies (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II;ACLIFORM; ATTAIN; AUGMENT COPD; Beier 2013;Chanez 2010;Maltais 2011; NCT01572792; Sliwinski 2010) matching placebo was prepared to have the same external appearance with the same composition except for the active ingredient so that the aclidinium bromide and placebo were indistinguishable. In Chanez 2010 the tiotropium arm was open label, though the trial was double‐blinded for the aclidinium and placebo arms, causing a high risk of bias for the comparison with tiotropium but a low risk of bias for the comparison with placebo. For all trials, outcome assessors remained blinded with regard to the treatment assignments throughout the study period. Independent blinded experts and reviewers were assigned for analysing the spirometry data and dyspnoea scores (baseline dyspnoea index (BDI) and TDI). A double‐dummy technique was applied in Beier 2013 to ensure the double‐blinding of the trial in order to minimise bias.
Incomplete outcome data
All eight full text trials (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ATTAIN; Beier 2013; Chanez 2010; Maltais 2011) reported the number of dropouts, along with the reasons, for all the study arms. The number of and reasons for withdrawals for three trials (ACLIFORM; AUGMENT COPD; NCT01572792) were kindly provided on request by the investigators. However, Sliwinski 2010 did not report sufficient information to assess attrition bias. Nine of the included studies were rated as having a low risk of bias, either because the number of dropouts was considered low and was balanced between groups (ACCLAIM/COPD I; ACCORD COPD I; ACCORD COPD II; ACLIFORM; Beier 2013; Chanez 2010), because withdrawal rates were high but evenly distributed across study arms (NCT01572792), or because withdrawal rates were regarded as acceptable given the methods of imputation reported in the published articles (ACCLAIM/COPD II; ATTAIN). Efficacy analyses and safety outcomes were performed on the intention‐to‐treat population which consisted of all randomised patients who received at least one dose of study medication and who had a baseline and at least one post‐baseline FEV1 assessment (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ATTAIN; Beier 2013; Chanez 2010; Maltais 2011). The last observation carried forward approach was used to impute missing data (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ATTAIN; Chanez 2010). The remaining three trials were rated as unclear because of uneven dropouts and with no clear information on the methods of imputation (AUGMENT COPD; Maltais 2011) or because of unavailable data for dropouts (Sliwinski 2010).
Selective reporting
Seven published trials reported all the outcomes documented in the methodology section of the published manuscripts without any apparent bias (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ATTAIN; Beier 2013; Maltais 2011). The pre‐specified outcomes of the three trials (ACLIFORM; AUGMENT COPD; NCT01572792) were supplied on request, with no detectable reporting bias. There were two unreported outcomes, namely trough FVC and peak expiratory flow rate (PEFR), in the Chanez 2010 trial though these outcome measures were specified in the methodology. Limited information prevented full assessment of reporting bias for Sliwinski 2010.
Other potential sources of bias
The studies were sponsored and funded by manufacturers of aclidinium, Almirall, SA, Barcelona, Spain and Forest Laboratories, Inc, NY, USA, and some of the authors received financial support from the same, all of which were declared with no potential source of bias. Sliwinski 2010 was published as an abstract in 2010 but as of 2014 has failed to be published as full text, thus publication bias could not be ruled out. In ACCORD COPD II the baseline mean FEV1 was 1.40 L for the aclidinium 200 μg arm, 1.25 L for the aclidinium 400 μg arm and 1.46 L for the placebo arm. This relative imbalance in baseline lung function was taken into consideration in performing the meta‐analysis and judged as not causing a significant high risk of bias.
Effects of interventions
We included data from 10 studies for quantitative synthesis (meta‐analysis) in the comparison of aclidinium bromide versus placebo (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ACLIFORM; ATTAIN; AUGMENT COPD; Beier 2013; Chanez 2010; Maltais 2011). Two studies (Beier 2013; Chanez 2010) assessed tiotropium as well and these data were pooled for the comparison of aclidinium bromide versus LAMA. Four trials (ACLIFORM; AUGMENT COPD; NCT01572792; Sliwinski 2010) included both aclidinium and formoterol as intervention arms, however formoterol was given via the Genuair inhaler in these studies thus the data were considered inappropriate for comparison of aclidinium bromide versus LABA.
1. Aclidinum bromide versus placebo
Primary outcomes
Mortality (all‐cause)
The number of deaths was reported in nine studies involving a total of 5252 participants (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ACLIFORM; ATTAIN; AUGMENT COPD; Beier 2013; Maltais 2011). Overall, there was no statistically significant difference in the number of deaths between the aclidinium and placebo groups (OR 0.92; 95% CI 0.43 to 1.94, low quality evidence; Table 1). Five patients out of 1000 (95% CI 2 to 10) patients receiving aclidinium died over 6 to 52 weeks, which was similar to the placebo group. Subgroup analysis of aclidinium once daily and twice daily showed an OR of 0.63 (95% CI 0.25 to 1.60; 3 trials, 1828 participants) and an OR of 1.69 (95% CI 0.46 to 6.21; 6 trials, 3424 participants) respectively (Analysis 1.1). There was no significant difference between the subgroups.
1.1. Analysis.
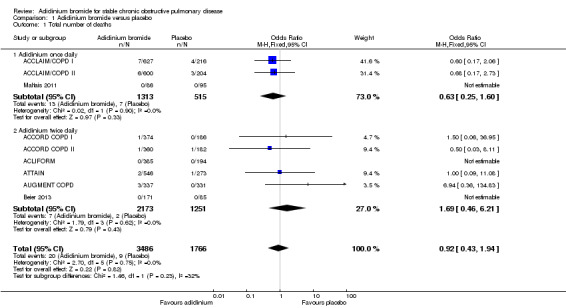
Comparison 1 Aclidinium bromide versus placebo, Outcome 1 Total number of deaths.
Exacerbations requiring a short course of an oral steroid or antibiotic, or both
Overall, data from 10 trials involving 5624 participants were pooled for patients experiencing at least one COPD exacerbation requiring a short course of oral steroids or antibiotics, or both (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ACLIFORM; ATTAIN; AUGMENT COPD; Beier 2013; Chanez 2010; Maltais 2011). The exact data for the trials which did not specifically mention the number of moderate exacerbations requiring oral steroids, antibiotics or both were kindly supplied by the sponsors. Aclidinium demonstrated a non‐significant reduction in moderate exacerbations compared to placebo (OR 0.88; 95% CI 0.74 to 1.04, moderate quality evidence). In patients on aclidinium, 122 people out of 1000 (95% CI 105 to 141) had exacerbations over 4 to 52 weeks, compared to 137 out of 1000 for patients on placebo (Table 1). In the subgroup analysis there was no significant difference between once daily (OR 0.93; 95% CI 0.73 to 1.20; 4 trials, 2201 participants) and twice daily aclidinium (OR 0.83; 95% CI 0.66 to 1.05; 6 trials, 3423 participants; test for subgroup differences: P = 0.51, Analysis 1.2).
1.2. Analysis.

Comparison 1 Aclidinium bromide versus placebo, Outcome 2 Number of patients with exacerbations requiring steroids, antibiotics or both.
Quality of life
Quality of life was assessed by the SGRQ in seven studies (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ACLIFORM; ATTAIN; AUGMENT COPD), either as the change from the baseline mean value or as the percentage of patients who achieved the minimal clinically important difference in SGRQ total score of ≥ four units reduction. Some of the data, in the format necessary for pooling, were kindly provided by the sponsors. Meta‐analysis of both measurements showed a statistically significant improvement with aclidinium bromide in comparison to placebo. Overall, aclidinium decreased the SGRQ total score by a mean difference of ‐2.34 units compared with placebo (95% CI ‐3.18 to ‐1.51; 7 trials, 4442 participants). A significant reduction in SGRQ total score was observed for both aclidinium once daily (MD ‐1.96; 95% CI ‐3.47 to ‐0.45; 2 trials, 1560 participants) and twice daily (MD ‐2.51; 95% CI ‐3.50 to ‐1.51; 5 trials, 2882 participants) with no significant difference between subgroups (test for subgroup differences: P = 0.55, Analysis 1.3).
1.3. Analysis.
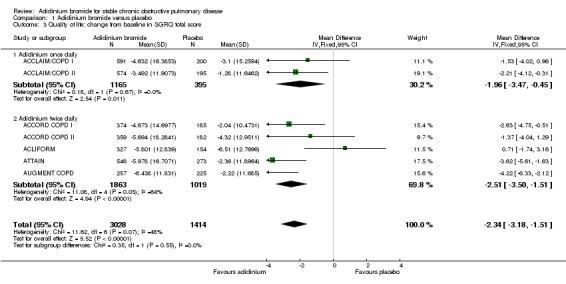
Comparison 1 Aclidinium bromide versus placebo, Outcome 3 Quality of life: change from baseline in SGRQ total score.
More patients on aclidinium reported a clinically significant improvement (a fall of at least four units in SGRQ total score) in quality of life than in the placebo group, which was of statistical significance (OR 1.49; 95% CI 1.31 to 1.70; 7 trials, 4420 participants; Analysis 1.4). A total of 494 per 1000 patients on aclidinium (95% CI 462 to 527) compared to 396 out of 1000 patients on placebo achieved a clinically important improvement in SGRQ score, the quality of evidence being rated as high (Table 1). In absolute terms, 98 more per 1000 (from 66 more to 131 more) patients on aclidinium experienced clinically meaningful improvements in quality of life than on placebo over 12 to 52 weeks. For every 10 people treated with aclidinium instead of placebo, one additional person was estimated to achieve this clinically important improvement in quality of life (NNT = 10; 95% CI 8 to 15). Both twice daily (OR 1.55; 95% CI 1.32 to 1.81; 5 trials, 2860 participants) and once daily aclidinium (OR 1.36; 95% CI 1.08 to 1.73; 2 trials, 1560 participants) demonstrated significant improvement with no statistical difference in the subgroup analysis (test for subgroup differences: P = 0.38; Figure 3).
1.4. Analysis.
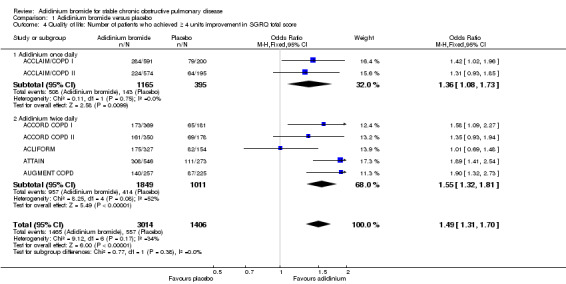
Comparison 1 Aclidinium bromide versus placebo, Outcome 4 Quality of life: Number of patients who achieved ≥ 4 units improvement in SGRQ total score.
3.
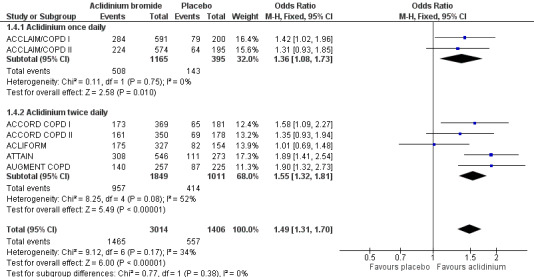
Forest plot of comparison: 1 Aclidinium bromide versus placebo, outcome: 1.4 Quality of life: Number of patients who achieved ≥ 4 units improvement in SGRQ total score.
Secondary outcomes
Lung function
Nine trials studied changes from baseline in trough and peak FEV1, and trough and peak FVC (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ACLIFORM; ATTAIN; AUGMENT COPD; Beier 2013; Maltais 2011) and seven studies reported the change from baseline in normalised FEV1 area under the curve in the first 12 hours (FEV1 AUC0‐12) (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ACLIFORM; ATTAIN; AUGMENT COPD). Most of the trials reported the data for these outcomes as the difference in aclidinium versus placebo values, but the required data for each intervention arm and placebo arm for our meta‐analysis were kindly provided by Almirall. These were pooled as the change from baseline to the end of the study.
The predose FEV1 for participants taking aclidinium was increased by 0.09 L (or 90 mL) at the end of the trials compared with participants using placebo inhalers (95% CI 0.08 to 0.10; 9 trials, 4963 participants). A greater improvement in the trough FEV1 was noted with twice daily dosing (MD 0.10; 95% CI 0.09 to 0.12; 6 trials, 3164 participants) compared to once daily (MD 0.07; 95% CI 0.05 to 0.09; 3 trials, 1799 participants; test for subgroup differences: P = 0.02, Analysis 1.5).
1.5. Analysis.
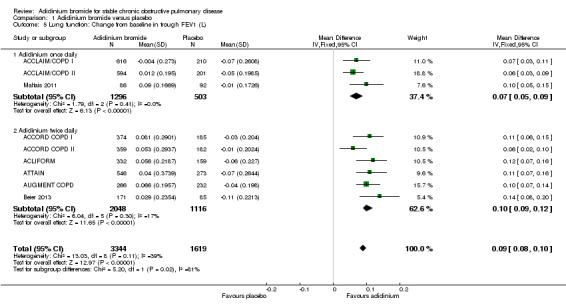
Comparison 1 Aclidinium bromide versus placebo, Outcome 5 Lung function: Change from baseline in trough FEV1 (L).
The meta‐analysis for peak FEV1 change from baseline, using the random‐effects model due to significant heterogeneity (I2 = 56%), yielded an overall MD of 0.17 L (95% CI 0.15 to 0.20; 9 trials, 4962 participants). No difference in the pooled MD was observed between twice daily (MD 0.17; 95% CI 0.15 to 0.19; 6 trials, 3160 participants) and once daily aclidinium (MD 0.19; 95% CI 0.12 to 0.25; 3 trials, 1802 participants; test for subgroup differences: P = 0.62, Analysis 1.6).
1.6. Analysis.

Comparison 1 Aclidinium bromide versus placebo, Outcome 6 Lung function: Change from baseline in peak FEV1 (L).
Aclidinium resulted in a statistically significant improvement of normalised FEV1 AUC0‐12 from baseline with a pooled MD of 0.13 L, or 130 mL, compared to placebo (95% CI 0.10 to 0.16; 7 trials, 1237 participants). The pooled MD for twice daily (MD 0.13; 95% CI 0.10 to 0.17; 5 trials, 1106 participants) and once daily aclidinium (MD 0.13; 95% CI 0.08 to 0.19; 2 trials, 131 participants) were similar (Analysis 1.7).
1.7. Analysis.
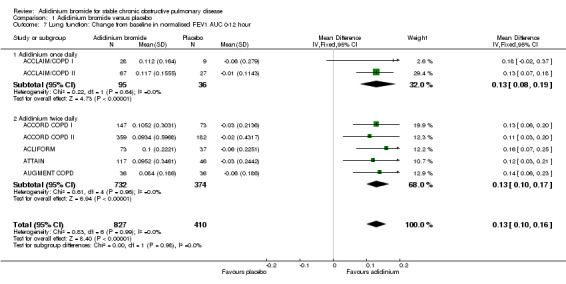
Comparison 1 Aclidinium bromide versus placebo, Outcome 7 Lung function: Change from baseline in normalised FEV1 AUC 0‐12 hour.
The mean change in baseline trough FVC was 0.16 L greater with aclidinium than with placebo (95% CI 0.14 to 0.18; 9 trials, 4963 participants). There was no difference between twice daily (MD 0.17; 95% CI 0.14 to 0.20; 6 trials, 3164 participants) and once daily aclidinium (MD 0.14; 95% CI 0.10 to 0.18; 3 trials, 1799 participants; test for subgroup differences: P = 0.26, Analysis 1.8).
1.8. Analysis.
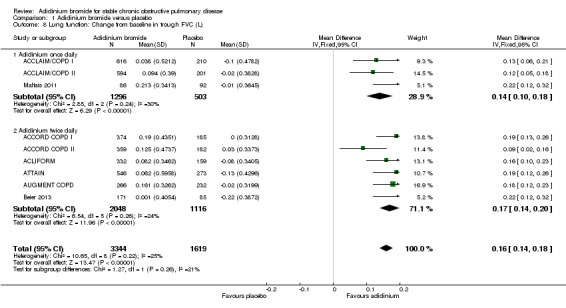
Comparison 1 Aclidinium bromide versus placebo, Outcome 8 Lung function: Change from baseline in trough FVC (L).
The improvement in peak FVC from baseline was also significantly greater in patients on aclidinium compared to placebo with a pooled MD of 0.27 L (95% CI 0.23 to 0.31; 9 trials, 4962 participants) in the meta‐analysis using a random‐effects model as the heterogeneity was high (I2 = 56%). Subgroup analysis demonstrated no significant difference between twice daily (MD 0.25; 95% CI 0.22 to 0.28; 6 trials, 3160 participants) and once daily aclidinium (MD 0.33; 95% CI 0.23 to 0.42; 3 trials, 1802 participants; test for subgroup differences: P = 0.13, Analysis 1.9).
1.9. Analysis.

Comparison 1 Aclidinium bromide versus placebo, Outcome 9 Lung function: Change from baseline in peak FVC (L).
Functional capacity
None of the individual studies measured functional capacity.
Hospital admissions due to exacerbations
The published reports of the included studies did not specifically mention hospital admissions due to either exacerbations or any cause. However, data for hospital admissions due to exacerbations, that is severe COPD exacerbations, were obtained for 10 studies (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ACLIFORM; ATTAIN; AUGMENT COPD; Beier 2013; Chanez 2010; Maltais 2011) from the study sponsors.
There were fewer patients on aclidinium who suffered one or more exacerbation(s) leading to hospitalisation than on placebo over 4 to 52 weeks (OR 0.64; 95% CI 0.46 to 0.88; 10 studies, 5624 participants) (Figure 4). Twenty four patients per 1000 (95% CI 17 to 33) on aclidinium suffered from at least one severe COPD exacerbation requiring hospital admission compared to 37 per 1000 on placebo, the quality of evidence being classified as high (Table 1). In absolute terms, aclidinium resulted in 13 fewer patients with exacerbation‐related hospitalisations per 1000 (4 to 20 fewer) than placebo. It was estimated that for every 77 patients treated with aclidinium instead of placebo, one additional person was free from a severe COPD exacerbation necessitating hospitalisation (NNT = 77; 95% CI 51 to 233). Subgroup analysis showed that the difference between twice daily (OR 0.59; 95% CI 0.35 to 1.01; 6 trials, 3423 participants) and once daily aclidinium (OR 0.67; 95% CI 0.45 to 0.99; 4 trials, 2201 participants) was not statistically significant (test for subgroup differences: P = 0.73, Analysis 1.10).
4.
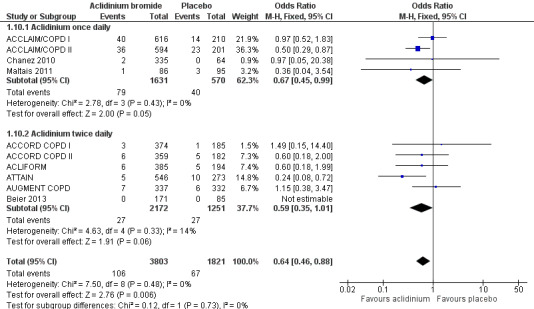
Forest plot of comparison: 1 Aclidinium bromide versus placebo, outcome: 1.10 Number of patients with hospital admissions due to COPD exacerbation.
1.10. Analysis.
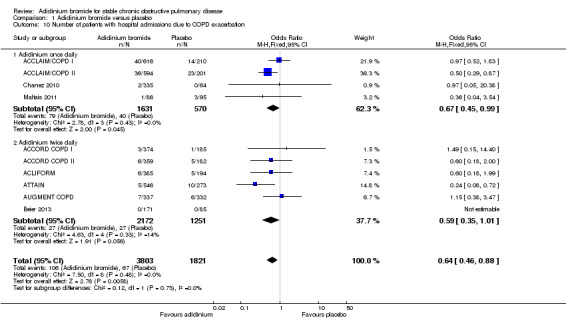
Comparison 1 Aclidinium bromide versus placebo, Outcome 10 Number of patients with hospital admissions due to COPD exacerbation.
Improvement in symptoms
Changes in symptom of dyspnoea were assessed in eight studies using the transitional dyspnoea index (TDI) score (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ACLIFORM; ATTAIN; AUGMENT COPD; Maltais 2011) and reported as either the change in mean value from baseline or as percentage of participants who achieved the minimal clinically important difference in TDI focal score of ≥ one unit increment.
Patients on aclidinium reported a MD of 0.84 units improvement in TDI compared with placebo (95% CI 0.50 to 1.18; 8 trials, 4490 participants) using a random‐effects model as the heterogeneity was high (I2 = 68%). This heterogeneity was caused by ACCORD COPD II in which the patients on aclidinium had a relatively lower baseline FEV1 with more severe disease (GOLD stage III) than in the placebo arm. Repeating the analysis with the exclusion of this particular study resulted in a MD of 0.95 units (95% CI 0.72 to 1.19) without heterogeneity (I2 = 0%). Both once daily (MD 1.08; 95% CI 0.46 to 1.71; 3 trials, 1597 participants) and twice daily aclidinium (MD 0.72; 95% CI 0.33 to 1.11; 5 trials, 2893 participants) demonstrated an improvement in TDI focal score with no statistical difference in the subgroup analysis (test for subgroup differences: P = 0.33, Analysis 1.11).
1.11. Analysis.

Comparison 1 Aclidinium bromide versus placebo, Outcome 11 Improvement in symptoms: Change from baseline in TDI focal score.
In terms of percentage of COPD patients achieving ≥ one unit improvement in TDI focal score for dyspnoea, more patients on aclidinium attained this minimal clinically important difference than for those on placebo (OR 1.73; 95% CI 1.52 to 1.98; 8 trials, 4289 participants; I2 = 0%). A similar improvement was noted for both once daily (OR 1.75; 95% CI 1.39 to 2.20; 3 trials, 1589 participants) and twice daily aclidinium (OR 1.72; 95% CI 1.47 to 2.03; 5 trials, 2700 participants; test for subgroup differences: P = 0.92, Analysis 1.12).
1.12. Analysis.
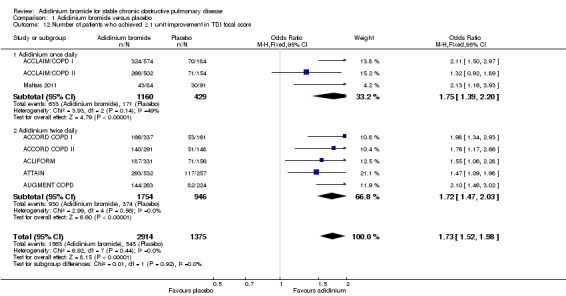
Comparison 1 Aclidinium bromide versus placebo, Outcome 12 Number of patients who achieved ≥ 1 unit improvement in TDI focal score.
Non‐fatal serious adverse events
Ten studies (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ACLIFORM; ATTAIN; AUGMENT COPD; Beier 2013; Chanez 2010; Maltais 2011) reported this outcome with participants as the level of analysis (that is the number of people who had non‐fatal serious adverse events as opposed to the number of adverse events in total). When the findings of these studies were pooled, no difference was observed between aclidinium and placebo (OR 0.89; 95% CI 0.70 to 1.14; 10 trials, 5651 participants) (Figure 5). Among 1000 patients, 50 receiving aclidinium (95% CI 40 to 64) and 56 on placebo developed non‐fatal serious adverse events, with moderate quality of evidence (Table 1). This result appeared to be independent of dosing (twice daily OR 0.95; 95% CI 0.68 to 1.34; 6 trials, 3424 participants; once daily OR 0.83; 95% CI 0.58 to 1.18; 4 trials, 2227 participants; test for subgroup differences: P = 0.57, Analysis 1.13).
5.
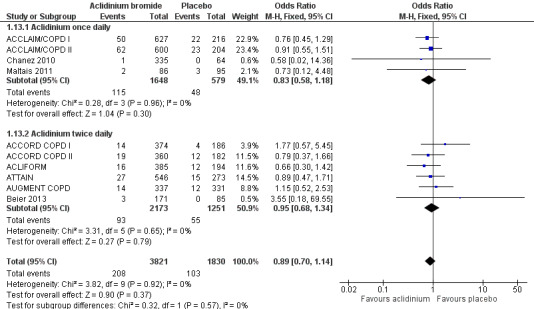
Forest plot of comparison: 1 Aclidinium bromide versus placebo, outcome: 1.13 Non‐fatal serious adverse events.
1.13. Analysis.
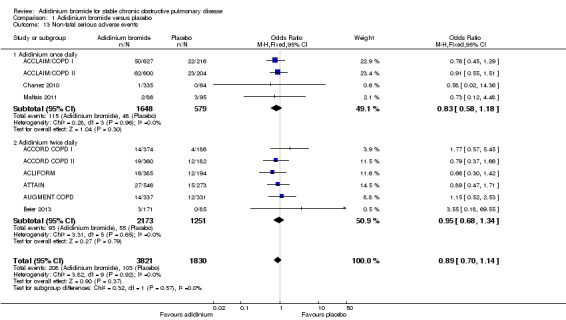
Comparison 1 Aclidinium bromide versus placebo, Outcome 13 Non‐fatal serious adverse events.
Withdrawals
Withdrawals due to either lack of efficacy or adverse events were provided in 10 studies (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ACLIFORM; ATTAIN; Beier 2013; Chanez 2010; Maltais 2011; NCT01572792).
There was a statistically and clinically significant reduction in withdrawals due to lack of efficacy with aclidinium compared to placebo (OR 0.31; 95% CI 0.23 to 0.43; 10 trials, 5672 participants). The effect estimates were similar for twice daily (OR 0.32; 95% CI 0.20 to 0.51; 6 trials, 3445 participants) and once daily aclidinium (OR 0.31; 95% CI 0.20 to 0.47; 4 trials, 2227 participants; test for subgroup differences: P = 0.91, Analysis 1.14).
1.14. Analysis.
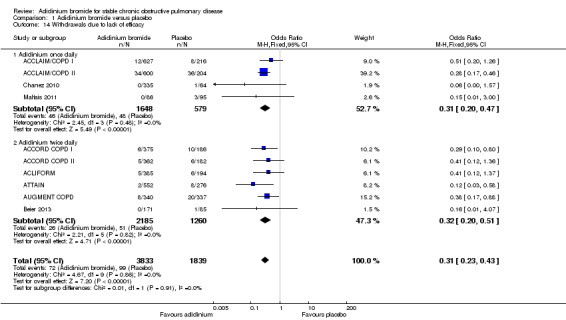
Comparison 1 Aclidinium bromide versus placebo, Outcome 14 Withdrawals due to lack of efficacy.
Overall, aclidinium resulted in a non‐significant reduction in withdrawals due to adverse events compared with placebo (OR 0.76; 95% CI 0.57 to 1.01; 10 trials, 5672 participants). No significant difference was observed for once daily dosing (OR 0.65; 95% CI 0.42 to 1.00; 4 trials, 2227 participants) and twice daily dosage regimens (OR 0.84; 95% CI 0.59 to 1.21; 6 trials, 3445 participants; test for subgroup differences: P = 0.36, Analysis 1.15).
1.15. Analysis.
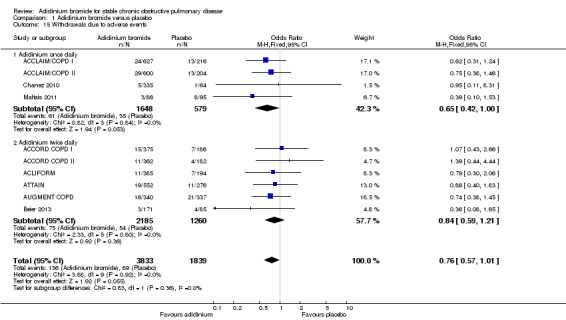
Comparison 1 Aclidinium bromide versus placebo, Outcome 15 Withdrawals due to adverse events.
2. Aclidinum bromide versus long‐acting muscarinic antagonist
Primary outcomes
There were no deaths reported for both the aclidinium and tiotropium arms in a total of 329 patients in Beier 2013 (Analysis 2.1).
2.1. Analysis.

Comparison 2 Aclidinium bromide versus long‐acting muscarinic antagonist, Outcome 1 Total number of deaths.
Two studies assessed exacerbations requiring a short course of oral steroids or antibiotics, or both, in 729 participants (Beier 2013 ; Chanez 2010). Aclidinium was associated with a higher number of exacerbations compared to tiotropium but this was not statistically significant (OR 2.64; 95% CI 0.31 to 22.18) (Analysis 2.2). There were no patients with moderate exacerbations in the tiotropium arm compared to five of 506 participants in the aclidinium arm. However, the quality of evidence was very low because of the high risk of bias in Chanez 2010, which was open label for the tiotropium arm and had very serious imprecision of the results (Table 2).
2.2. Analysis.
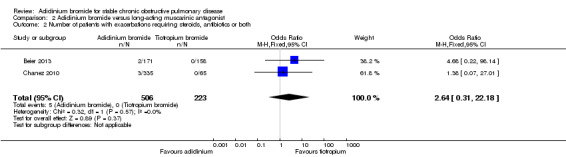
Comparison 2 Aclidinium bromide versus long‐acting muscarinic antagonist, Outcome 2 Number of patients with exacerbations requiring steroids, antibiotics or both.
None of the studies measured quality of life for aclidinium and tiotropium.
Secondary outcomes
Only one trial provided data for aclidinium and tiotropium on lung function (Beier 2013). Aclidinium was associated with a greater improvement in trough FEV1 (MD 0.04; 95% CI ‐0.01 to 0.09; 1 trial, 329 participants) (Analysis 2.3), peak FEV1 (MD 0.01; 95% CI ‐0.04 to 0.06; 1 trial, 329 participants) (Analysis 2.4), trough FVC (MD 0.08; 95% CI ‐0.01 to 0.17; 1 trial, 329 participants) (Analysis 2.5) and peak FVC (MD 0.04; 95% CI ‐0.05 to 0.13; 1 trial, 329 participants) (Analysis 2.6) than tiotropium, however none were statistically significant.
2.3. Analysis.
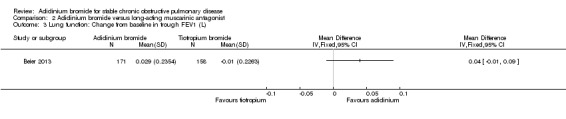
Comparison 2 Aclidinium bromide versus long‐acting muscarinic antagonist, Outcome 3 Lung function: Change from baseline in trough FEV1 (L).
2.4. Analysis.
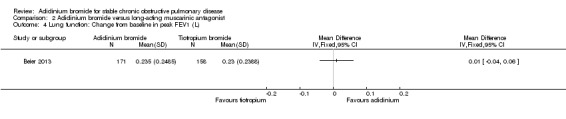
Comparison 2 Aclidinium bromide versus long‐acting muscarinic antagonist, Outcome 4 Lung function: Change from baseline in peak FEV1 (L).
2.5. Analysis.
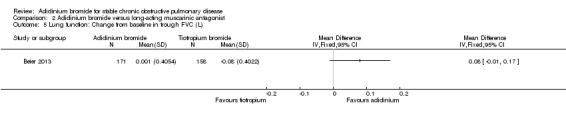
Comparison 2 Aclidinium bromide versus long‐acting muscarinic antagonist, Outcome 5 Lung function: Change from baseline in trough FVC (L).
2.6. Analysis.

Comparison 2 Aclidinium bromide versus long‐acting muscarinic antagonist, Outcome 6 Lung function: Change from baseline in peak FVC (L).
Functional capacity was not assessed in the two studies included in this comparison.
Aclidinium reduced the number of patients with hospitalisations due to COPD exacerbations compared to tiotropium but the difference was not statistically significant (OR 0.54; 95% CI 0.07 to 4.11; 2 trials, 729 participants) (Analysis 2.7). Two patients per 1000 (95% CI 0 to 18) on aclidinium versus four patients per 1000 on tiotropium were admitted to hospital for severe COPD exacerbations, but this was very low level evidence (Table 2). The wide CI included the possibility of no difference.
2.7. Analysis.
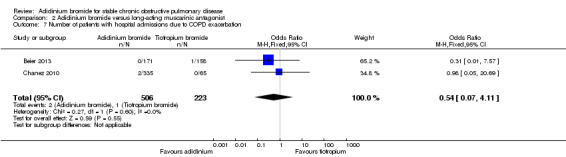
Comparison 2 Aclidinium bromide versus long‐acting muscarinic antagonist, Outcome 7 Number of patients with hospital admissions due to COPD exacerbation.
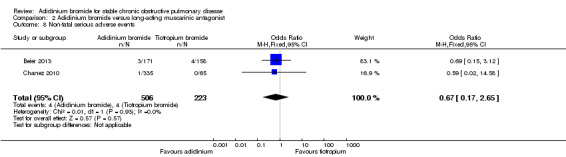
Data from the two trials (Beier 2013 ; Chanez 2010) were combined for non‐fatal serious adverse events. Aclidinium demonstrated a non‐significant reduction in non‐fatal serious adverse events compared with tiotropium (OR 0.67; 95% CI 0.17 to 2.65; 2 trials, 729 participants) (Analysis 2.8). In a total of 1000 patients, 12 on aclidinium (95% CI 3 to 46) and 18 on tiotropium experienced non‐fatal serious adverse events over a period of four to six weeks, with a very low quality of evidence as the CIs were wide.
2.8. Analysis.
Comparison 2 Aclidinium bromide versus long‐acting muscarinic antagonist, Outcome 8 Non‐fatal serious adverse events.
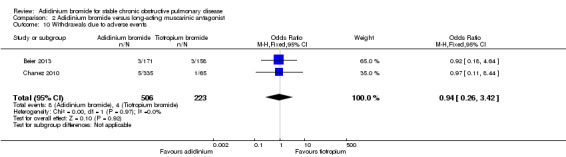
Both Beier 2013 and Chanez 2010 reported withdrawals due to lack of efficacy or adverse events. There were no withdrawals due to lack of efficacy for both aclidinium and tiotropium in these two studies (Analysis 2.9). No significant difference existed between aclidinium and tiotropium for withdrawals due to adverse events (OR 0.94; 95% CI 0.26 to 3.42; 2 trials, 729 participants) (Analysis 2.10).
2.9. Analysis.
Comparison 2 Aclidinium bromide versus long‐acting muscarinic antagonist, Outcome 9 Withdrawals due to lack of efficacy.
2.10. Analysis.
Comparison 2 Aclidinium bromide versus long‐acting muscarinic antagonist, Outcome 10 Withdrawals due to adverse events.
3. Aclidinum bromide versus long‐acting beta2‐agonist
Inadequate and inaccurate data limited this comparison as formoterol was given via the Genuair inhaler in the trials, which was not an approved inhaler for formoterol (ACLIFORM; AUGMENT COPD; NCT01572792; Sliwinski 2010).
4. Adverse events
Adverse events with aclidinium were reported in a total of 10 studies (ACCLAIM/COPD I; ACCLAIM/COPD II; ACCORD COPD I; ACCORD COPD II; ACLIFORM; ATTAIN; AUGMENT COPD; Beier 2013; Chanez 2010; Maltais 2011). We have presented the adverse event data from both comparisons with placebo and tiotropium in section 4 of Data and analyses.
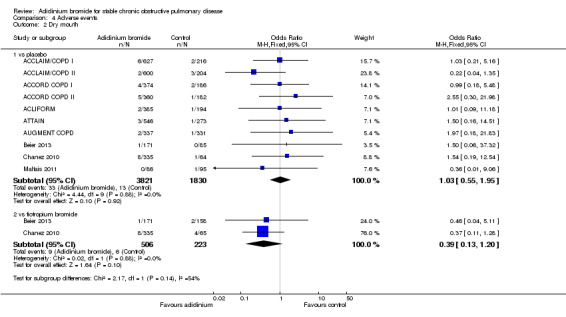
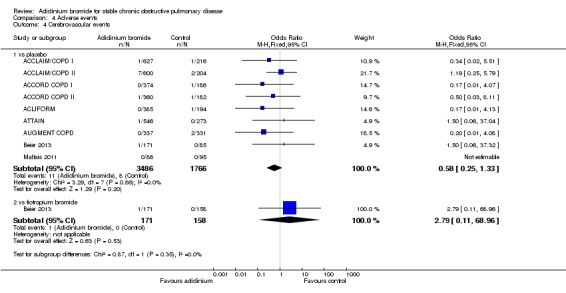
A lower incidence of cardiac events (Analysis 4.1) was more prominent with aclidinium compared to tiotropium and placebo, but both were statistically non‐significant. There was no significant difference between aclidinium and placebo or tiotropium for the anticholinergic side effect of dry mouth (Analysis 4.2). Constipation was non‐significantly more frequent with aclidinium compared to both placebo and tiotropium (Analysis 4.3). Aclidinium non‐significantly decreased cerebrovascular events (Analysis 4.4) compared to placebo (OR 0.58; 95% CI 0.25 to 1.33; 9 trials, 5252 participants). However, in Beier 2013 cerebrovascular events were more frequent with aclidinium compared to tiotropium but the CIs were very wide and the difference was not statistically significant (OR 2.79; 95% CI 0.11 to 68.96; 1 trial, 329 participants).
4.1. Analysis.
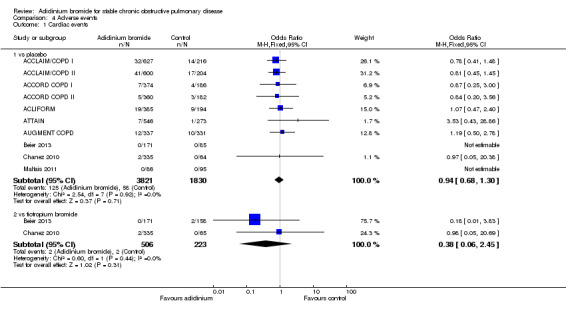
Comparison 4 Adverse events, Outcome 1 Cardiac events.
4.2. Analysis.
Comparison 4 Adverse events, Outcome 2 Dry mouth.
4.3. Analysis.

Comparison 4 Adverse events, Outcome 3 Constipation.
4.4. Analysis.
Comparison 4 Adverse events, Outcome 4 Cerebrovascular events.
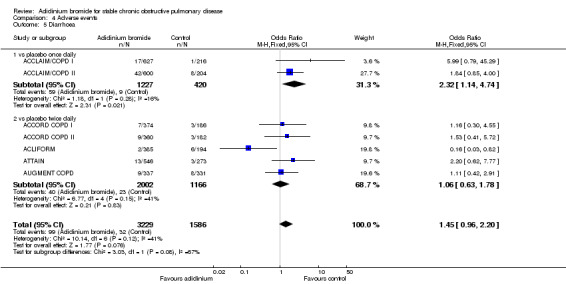
Diarrhoea was found to be significantly increased with aclidinium (once daily therapy) compared to placebo (OR 2.32; 95% CI 1.14 to 4.74; 2 trials, 1647 participants). However, no statistical difference was observed between once daily and twice daily aclidinium (OR 1.06; 95% CI 0.63 to 1.78; 5 trials, 3168 participants; test for subgroup differences: P = 0.08, Analysis 4.5).
4.5. Analysis.
Comparison 4 Adverse events, Outcome 5 Diarrhoea.
Other reported adverse events such as nasopharyngitis, headache, cough, hypertension, respiratory tract infections, urinary tract infections, fatigue, dizziness, dyspnoea, arthralgia, back pain and oropharyngeal pain showed no significant difference between aclidinium and placebo or tiotropium in the pooled analysis.
Discussion
Summary of main results
We calculated summary estimates of the effects of aclidinium on clinical outcomes in comparison to placebo and tiotropium. Aclidinium improved quality of life and reduced exacerbation‐related hospitalisations compared to placebo. Aclidinium significantly lowered the SGRQ total score by 2.34 units (from 3.18 to 1.51 lower), although this mean improvement did not reach the accepted threshold of four units for a clinically important difference. However, more patients on aclidinium achieved a minimal clinically important difference of at least four units decrease in the SGRQ total score (462 to 527 per 1000) than those on placebo (396 per 1000). A total of 10 patients need to be treated with aclidinium to attain one additional person with a four unit improvement in SGRQ total score. Similarly, aclidinium significantly reduced the number of patients with exacerbation‐related hospital admissions compared to placebo (17 to 33 per 1000 versus 37 per 1000), which would correspond to approximately 77 patients having to be treated with aclidinium to prevent one additional exacerbation‐related hospitalisation. However, aclidinium therapy failed to demonstrate a significant reduction in the number of patients experiencing an exacerbation that required an oral steroid or antibiotic, or both. In terms of safety, no significant difference between aclidinium and placebo was observed in all‐cause mortality or non‐fatal serious adverse events. All reported deaths in the trials were not related to aclidinium therapy.
For the secondary outcomes, improvements in symptom scales and spirometric indices appeared clinically significant with aclidinium compared to placebo. Patients treated with aclidinium experienced clinically significant improvements in dyspnoea with a TDI focal score change from baseline of 0.50 to 1.18 units. Exclusion of the data from ACCORD COPD II with its baseline imbalance in COPD severity resulted in a larger increase in the TDI focal score of 0.72 to 1.19 units, without significant heterogeneity. The proportion of patients on aclidinium who exceeded the minimum clinically important difference of one unit in TDI focal score was higher than with placebo. Lung function measurements of trough and peak FEV1, trough and peak FVC and normalised FEV1 area under the curve in the first 12 hours (FEV1 AUC0‐12) were significantly higher with aclidinium compared to placebo. A significantly lower number of participants withdrew from studies due to lack of efficacy in the aclidinium group compared to the placebo group. Similarly, fewer withdrawals due to adverse events were observed among patients on aclidinium than on placebo, but the differences were not statistically significant. We could not show a difference in most of the efficacy outcomes related to dosing of aclidinium, except for trough FEV1 where the twice daily dosage demonstrated a relatively superior improvement compared with the once daily regimen.
Evaluation of the effects of aclidinium in relation to LABAs was unsuccessful due to a lack of trials of good design and inaccurate data.
For the comparison of aclidinium and LAMAs, we were able to pool the data from two studies (Beier 2013; Chanez 2010) which included tiotropium as one of the intervention arms. Based on the currently available, limited data, aclidinium did not differ significantly from tiotropium in terms of exacerbations requiring oral steroids or antibiotics, or both, exacerbation‐related hospitalisations and non‐fatal serious adverse events. There were no reported cases of deaths or withdrawals due to lack of efficacy for both aclidinium and tiotropium. Withdrawals due to adverse events were similar with aclidinium in comparison to tiotropium. Patients treated with aclidinium had greater improvements in the spirometric indices of trough FEV1, peak FEV1, trough FVC and peak FVC than with tiotropium. However, only Beier 2013 contributed to the lung function outcomes and the evidence for the other outcomes were of very low quality, thus reducing our confidence in the conclusions. Therefore, we strongly recommend future trials comparing the effects of aclidinium and tiotropium to strengthen our confidence in conclusions about the efficacy of this novel LAMA in relation to established LAMAs.
There was no statistically significant difference between the number of participants suffering from non‐serious adverse events with aclidinium compared to placebo and tiotropium. Concern about a possible cardiovascular risk with aclidinium was not reinforced in our review as the risk did not differ from placebo or tiotropium. This was also in accordance with the greater kinetic selectivity of aclidinium on M3 over M2 receptors (Maltais 2012; Sims 2011). Interestingly, patients on once daily aclidinium developed diarrhoea more often than with placebo but this was not of clinical importance. However, further trials are needed in order to allow valid conclusions with regard to this. The numbers of anticholinergic side effects such as dry mouth and constipation were slightly higher with aclidinium than placebo but they were not of clinical or statistical significance. Other adverse events were comparable between aclidinium and placebo or tiotropium.
Overall completeness and applicability of evidence
There were substantial numbers of trials investigating aclidinium and placebo for this review, but the number of trials assessing aclidinium compared to tiotropium or formoterol were inadequate. Four of the included trials did investigate formoterol as one of the treatment arms (ACLIFORM; AUGMENT COPD; NCT01572792; Sliwinski 2010) yet, from discussion with the study sponsors, both aclidinium and formoterol were delivered in Genuair inhalers to maintain double‐blindness. While aclidinium delivered in the Genuair is an approved drug, this is not the case for formoterol. Thus the data for formoterol were considered unsuitable to be included in our analysis. However, in two trials with tiotropium (Beier 2013; Chanez 2010) the tiotropium was administered in its approved HandiHaler. In Beier 2013, two Genuair inhalers loaded with either aclidinium or placebo and one HandiHaler loaded with either tiotropium or placebo were supplied. To maintain blinding, patients were instructed to use both inhalers each morning and the Genuair only each evening. However, tiotropium delivered via the HandiHaler was open label for Chanez 2010. This study limitation decreased the quality of evidence for the treatment effects of this comparison. In this review, subgroup analysis was performed for dosing of aclidinium, once daily versus twice daily. Most of the once daily trials studied aclidinium 200 μg whereas twice daily trials studied mainly aclidinium 400 μg; some of the twice daily trials investigated both aclidinium 200 μg and 400 μg. These differences in the total daily dose among the trials might have impacted on the summary estimates of subgroups but with minimal alterations in the overall effect estimates. The results from this review indicate an improvement in the mean health‐related quality of life and a reduction in exacerbations requiring hospital admission for stable COPD patients on aclidinium compared to placebo. The mean improvement for these outcomes was statistically significant but was relatively small in relation to the minimum clinically important difference. However, there was a significant number of patients who had a clinically relevant improvement. The evidence that this review provides strengthens and supports the efficacy of aclidinium compared to placebo for use in patients with stable COPD. However, the lack of trials prevents a comprehensive assessment of the evidence on the efficacy of aclidinium compared to tiotropium or formoterol, or other LABAs or LAMAs. No significant increase in deaths, non‐fatal serious adverse events and other adverse events compared with placebo make aclidinium a relatively safe medication for use as maintenance therapy by patients with moderate to severe stable COPD.
Quality of the evidence
The studies included in this review were generally of good to excellent methodological quality and the single conference abstract, which was of unknown quality, did not contribute any data to the analysis. The inclusion and exclusion criteria for the trials were almost identical. The results were unlikely to be compromised by performance (selection and allocation) or detection biases as all trials were industry‐sponsored, in accordance with the pre‐specified protocol, and double‐blind; except for Chanez 2010 where the tiotropium arm was open label. Selective reporting was considered to be at low risk with the exception of Chanez 2010, where some of the lung function outcomes were not reported but none were outcomes of our review. The percentage of withdrawals in the included trials ranged from 6.3% to 42.2% for long‐term studies of more than12 weeks, whereas short‐term studies of 12 weeks or less had relatively lower withdrawal rates of 2.5% to 19.9%. However, most of the participants who completed treatment remained for follow‐up, the percentage of participants lost to follow‐up in the included trials being 0% to 3.4%. Missing data were imputed using the last observation carried forward approach in the individual studies. Several studies did not report on exacerbations requiring hospital admission or a short course of oral steroids or antibiotics, or both, and lung function data in a way that could be included in the review, however all these data were supplied by Almirall. All studies utilised intention‐to‐treat analysis. Visual inspection of the funnel plot for exacerbations with aclidinium and placebo did not suggest publication bias (Figure 6).
6.

Funnel plot of comparison: 1 Aclidinium bromide versus placebo, outcome: 1.2 Number of patients with exacerbations requiring steroids, antibiotics or both.
In particular, the quality of the evidence ranged from very low to high for the outcomes of this review. High quality evidence reinforced the validity of findings on quality of life and hospitalisations due to exacerbations, for aclidinium in comparison to placebo, while the evidence for mortality was low quality; the evidence on exacerbations requiring a short course of oral steroids or antibiotics, or both, and non‐fatal serious adverse events was rated as moderate. However, the evidence for the majority of outcomes for aclidinium versus tiotropium was of very low quality. We believe that additional information from future studies might change our confidence in these results and we will continue to update this review in an attempt to maximise the evidence to inform future clinical practice.
Potential biases in the review process
We made every effort to obtain grey literature in order to minimise the impact of publication bias. In addition to the Cochrane Airways Group's systematic electronic search, we performed a comprehensive search of other sources (for example searching drug company databases, clinical trial registration sites, and checking reference lists) for the identification of potentially relevant published and unpublished studies, with no language restrictions. The manufacturer was very helpful in providing additional information on completed published trials as well as completed but unpublished trials. Publication bias, therefore, was less likely, as supported by the funnel plot for the exacerbation data. Two review authors independently conducted trial selection and data extraction. We contacted authors for missing and incomplete data. To maximise the accuracy of our review, we requested the exact values of the data required in our meta‐analysis directly from the drug companies. We did not use data from estimations based on figures or other available indirect data. The manufacturer (Almirall) was accommodating in supplying information about study designs, details of trials and missing data for several of the studies. Possible limitations of the meta‐analysis include double‐counting of patients from overlapping publications and trials. We avoided this potential concern of double counting by including only the results of the first period (24 weeks) of the primary study (AUGMENT COPD) instead of the data at the end (one year) of the extension study (NCT01572792) as the extension period was not a truly randomised comparison and had substantial withdrawals. Clinical characteristics of the patients recruited into the trials and the disease severity as measured by baseline spirometry were similar between trials except for one trial (ACCORD COPD II) in which there was a higher proportion of patients with lower baseline mean FEV1 in the aclidinium 400 µg arm (1.25 L) than in the placebo arm (1.46 L). Thus, we repeated our analysis of all outcome measures with the exclusion of this study to detect any alterations in the summary estimates of the effects of aclidinium. However, the clinical homogeneity of the majority of the trials yielded statistical homogeneity for many outcome measures across the trials, except for symptom improvement measured by change from baseline in mean TDI focal score where the effect estimate was significantly altered by the data from this study. Another potential source of bias could be concomitant medications, however medications had to be used at a stable dose for at least four weeks and were stopped at least six hours before each study visit in the primary trials, making any possible interaction with aclidinium less likely.
Agreements and disagreements with other studies or reviews
There are a number of published reviews on aclidinium in the current literature.
Jones 2013 reported the efficacy of twice daily aclidinium and included the three studies ACCORD COPD I; ACCORD COPD II and ATTAIN. Pooled data from ACCORD COPD I and ATTAIN showed a statistically significant improvement in lung function (trough FEV1 and peak FEV1) and quality of life, which is in agreement with the results of our review. It, however, mentioned that aclidinium significantly reduced the frequency of exacerbations, which our review failed to prove. Pooled data from the two studies (ACCORD COPD I; ATTAIN) in that review showed a significant reduction in the rate of moderate to severe exacerbations for the aclidinium 400 μg dose when compared with placebo (0.31 versus 0.44; rate ratio 0.71; P = 0.01). Our review separately analysed moderate exacerbations that required a short course of oral steroids or antibiotics, or both, and severe exacerbations requiring hospital admissions. We also included trials of other doses of aclidinium, with a total of 10 studies in our meta‐analysis. We found that aclidinium reduced severe exacerbations necessitating hospitalisation but not moderate exacerbations requiring oral steroids or antibiotics, or both.
A significant improvement in lung function with aclidinium over placebo in moderate to severe COPD was also reported in a meeting abstract by D'Urzo 2013a. This was derived from a pooled analysis of the ACCORD COPD I; ACCORD COPD II and ATTAIN trials. Aclidinium produced consistent improvements in both trough FEV1 (100 mL, P < 0.0001) and peak FEV1 (172 mL, P < 0.0001) from baseline to week 12 compared to placebo, which was in accordance with our findings.
Other available evidence comes from Karabis 2013, which analysed the comparative efficacy of aclidinium versus glycopyrronium and tiotropium using network meta‐analysis. Twenty‐one studies were included, with three studies on aclidinium (ACCORD COPD I; ACCORD COPD II; ATTAIN). The authors concluded that aclidinium was comparable to tiotropium and glycopyrronium regarding trough FEV1 and TDI score improvement. For the SGRQ score, aclidinium resulted in a larger improvement than tiotropium 5 µg but comparable improvement to tiotropium 18 µg and glycopyrronium. The quality of the evidence for these conclusions, which were based on indirect comparison by network meta‐analysis through statistical inference, is questionable. We also determined that improvements in trough FEV1 with aclidinium were not statistically different from tiotropium. However, no trials in our review directly compared aclidinium to tiotropium in terms of SGRQ or TDI score.
In another review by Suppli 2012, 10 trials, both parallel‐group and cross‐over studies, were included after a systematic search. However, there was no meta‐analysis and the conclusions drawn were based solely on the individual trial reports for a particular outcome. Thus, we did not consider the conclusions from that review to be suitable for comparison with our findings. Similarly, the other published reviews (Alagha 2011; Alagha 2014; Maltais 2012; Sims 2011; Woods 2013) were descriptive without pooling of the data making their findings inappropriate for comparison.
D'Urzo 2013b performed a pooled analysis of the anticholinergic adverse events from three trials. Gelb 2013 and D'Urzo 2013 had a duration of 52 weeks and the open label continuation phase of ACCORD COPD II was for 40 weeks. The highest incidence rates of adverse events with long‐term aclidinium therapy were urinary tract infections (2.9%), oropharyngeal pain (1.8%) and constipation (1.5%). Our review also demonstrated that the anticholinergic adverse events associated with aclidinium were similar to those associated with placebo or tiotropium.
The major concern with the prolonged use of inhaled aclidinium bromide, a long‐acting anticholinergic, is the possible cardiovascular risk. In our analysis cardiac events were not increased with aclidinium compared to placebo, and were even non‐significantly fewer with aclidinium than with tiotropium. Donohue 2013 analysed the major adverse cardiovascular events (MACE) with aclidinium 400 µg of cardiovascular (CV) death, non‐fatal myocardial infarction (MI) and non‐fatal stroke by pooling the results of two double‐blind trials with a duration of 52 weeks (D'Urzo 2013; Gelb 2013) and one open label study over 40 weeks (the continuation phase of ACCORD COPD II or LAS‐MD‐38 part B). The MACE composite scores with aclidinium for double‐blind and open label studies were low (1.4% and 1.6% respectively). Similarly, Ferguson 2013 pooled the CV events from ACCORD COPD I; ACCORD COPD II and ATTAIN trials and reported that the MACE composite scores were equal for aclidinium and placebo (0.3%). A double‐blind, randomised, placebo‐controlled parallel‐group phase IV trial (ASCENT COPD) that is ongoing is assessing the cardiovascular safety of long‐term aclidinium therapy and is scheduled to be completed by January 2018.
Authors' conclusions
Implications for practice.
Inhaled aclidinium bromide use in stable COPD is associated with better health‐related quality of life and fewer hospitalisations due to severe exacerbations than with placebo. The results from this review indicate a larger percentage of patients attaining the minimal clinically important difference of at least four units change and a small mean improvement in health‐related quality of life for patients on aclidinium therapy compared to placebo. Aclidinium does not significantly lower the number of patients with exacerbations requiring a short course of oral steroids or antibiotics or both compared to placebo. Significant improvements in spirometric indices of trough and peak FEV1, and trough and peak FVC were seen with aclidinium compared with placebo. Adverse events, non‐fatal serious adverse events and mortality did not differ significantly between aclidinium and placebo. Currently available data for aclidinium in comparison to tiotropium were insufficient for coming to a valid conclusion. Inappropriate drug delivery of formoterol limits our effort to determine the relative efficacy and safety of aclidinium compared to LABAs. We did not conduct a cost‐effectiveness analysis so we cannot comment on implications for resource allocations.
Implications for research.
Additional long‐term studies are required to establish the risks and benefits of aclidinium compared to LAMAs and LABAs. Pharmacoeconomic analyses would be helpful to assist healthcare providers in making decisions about the cost‐effectiveness of aclidinium compared to other long‐acting bronchodilators such as LABA and LAMA. In future COPD trials, strategies using specific approved inhalers for formoterol, tiotropium and aclidinium while maintaining the procedure of blinding should be implemented to have accurate data for the individual interventions.
Feedback
Approriateness of decision to pool exacerbation and hospitalization outcomes, 13 March 2015
Summary
We agree with the review authors' statement regarding lack of information to reliably compare aclidinium and tiotropium, but we have some concerns regarding whether it was appropriate to meta‐analyze pooled data for COPD exacerbation and hospitalisation outcomes for aclidinium vs tiotropium.
1) The short duration (4 to 6 weeks) of the two included studies (Chanez 2010 and Beier 2013) may not reflect an adequate time period for assessment of COPD exacerbation or hospitalisation rates and we cannot project how the patients in Chanez 2010 and Beier 2013 would have fared in terms of exacerbations beyond the 4 to 6 week study periods. Of note, Cochrane Reviews of tiotropium vs placebo [1] tiotropium vs ipratropium [2] and tiotropium vs long‐acting beta‐agonists [3] included only studies of at least 12 weeks duration. We do not believe the short study periods in this review captures enough COPD exacerbations to perform clinically meaningful comparisons, and that the meta‐analyses should not have been done.
2) In Chanez 2010, a total of eight COPD exacerbations were reported in the published paper across the five different aclidinium dosage arms. However, only three exacerbations were included in Analysis 2.2 and two hospitalizations in the aclidinium arms were included in Analysis 2.7. The published study reported only total COPD exacerbations, and the events were not categorized according to the definitions in this review and we are unable to replicate the meta‐analyses. To help readers interpret the findings, we would have liked clarification regarding how the events were categorized and which dosage arms these events occurred in.
3) In Beier 2013, the published paper describes COPD exacerbations occurring in 2.4% of patients, suggesting approximately 10 events in total. The study also describes two COPD exacerbations resulting in withdrawal in each of the treatment groups, but it is unclear whether these events were included in final analysis of the study or in either Analysis 2.2 or 2.7, leading to difficulties interpreting this analysis. Given the short study duration, the quality of the data (assessed as "very low" in the review) and the lack of clarity regarding data extraction, we feel that the included meta‐analyses do not provide appropriate context for clinical decision making. While the odds ratios and confidence intervals are reported as not clinically significant, including them in this review may lead readers to inappropriately hypothesize that there is no difference between aclidinium and tiotropium, or that aclidinium may be associated with more moderate COPD exacerbations and fewer hospitalizations than tiotropium. However, the current pooled estimates have such wide confidence intervals that it could include a clinically meaningful increase or decrease in COPD exacerbations or hospitalizations with aclidinium compared to tiotropium.
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
References:
1. Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2014, Issue 7. Art. No.: CD009285. DOI: 10.1002/14651858.CD009285.pub3
2. Cheyne L, Irvin‐Sellers MJ, White J. Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2013, Issue 9. Art. No.: CD009552. DOI: 10.1002/14651858.CD009552.pub2
3. Chong J, Karner C, Poole P. Tiotropium versus long‐acting beta‐agonists for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 9. Art. No.: CD009157. DOI: 10.1002/14651858.CD009157.pub2.
Reply
Thank you very much for your interest and comments regarding our Cochrane review on “Aclidinium bromide for stable chronic obstructive pulmonary disease”.
1) For comparison of aclidinium and tiotropium we only found two short trials (Chanez 2010 and Beier 2013). According to the published protocol, both studies met our inclusion criteria, in which there was no predefined minimum trial duration. As practicing physicians, we have also seen cases of exacerbations occurring very frequently in our practice even within a short period after discharge from hospital. We do agree with your statement regarding the clinical meaningfulness of analyses 2.2 and 2.7, the results of which are not reliable for projection beyond 4 to 6 weeks. The readers might also inappropriately infer that aclidinium may be associated with more moderate exacerbations (analysis 2.2) but fewer hospitalizations (analysis 2.7) than tiotropium as you mentioned, based on the direction of the treatment effect, but both of these are non‐significant. The aim of Cochrane Reviews is to provide unbiased evidence to the readers; therefore we could not omit these analyses solely due to statistical imprecision. Due to imprecision and lack of blinding in the tiotropium arm in Chanez 2010, the quality of evidence for these comparisons was rated as "very low" meaning we are very uncertain about the estimate. We have included our reasons for down‐grading the quality of evidence for these analyses in the summary of findings table 2 and also in the discussion under the "overall completeness and applicability of evidence" section. We will be updating this review at regular intervals and if new trials comparing aclidinium with tiotropium become available in the future, we will update these analyses and hope to provide better evidence.
2 and 3)
Regarding the discrepancies of the number of events used for meta‐analyses and those in the published papers, most trials did not report mild, moderate or severe exacerbations separately. We specified in our protocol that we would analyse moderate exacerbations requiring short course of oral steroids and/or antibiotics and severe exacerbations needing hospitalisation separately. Dr Esther Garcia Gil (head of late stage development respiratory) and Christina Serra and Rosa Segarra (clinical trial managers) from Almirall helped us in providing the necessary data. Thus, the values you found in the review are provided by Almirall as the data from published articles are not appropriate for inclusion in the analyses. We describe how we obtained this information under the “effects of intervention” section. The data was checked by two review authors, the Almirall personnel and by the statistical editor of the Airways group. Thus, we hope readers will be able to appreciate why the data entered for analyses cannot be identified in the published articles.
We do hope that these will clarify your doubts and if you have further queries and comments, we are happy to respond.
Best regards,
Han Ni
MBBS, MMedSc (Internal Medicine), MRCP, FCCP
Contact author
Contributors
Julian Lee, BSc. (Pharm), Lower Mainland Pharmacy Services, Vancouver, Canada (julian.lee1@fraserhealth.ca)
Erin Ready, BSc. (Pharm), Lower Mainland Pharmacy Services, Vancouver, Canada
Aaron Tejani, PharmD, Therapeutics Initiative (UBC), Vancouver, Canada (aaron.tejani@ti.ubc.ca)
What's new
| Date | Event | Description |
|---|---|---|
| 1 April 2015 | Feedback has been incorporated | Feedback and rebuttal added. |
Acknowledgements
We would like to thank the editors and staff of the Cochrane Airways Group and The Cochrane Collaboration for their utmost help and support, especially Dr Emma J Welsh, Ms Elizabeth Stovold and Ms Emma Jackson for their feedback, suggestions, advice and help for this review. We would like to acknowledge editor Ian Yang for his valuable advice and comments on this review. We thank the trial authors who helped us with additional data or information. Our utmost gratitude goes to Dr Esther Garcia Gil, head of late stage development respiratory; Christina Serra and Rosa Segarra, clinical trial managers, from Almirall who helped in clarifying issues and supplying additional information and data about the studies sponsored by them. Special thanks go to Dr Aung Win Thein, Associate Professor at Melaka‐Manipal Medical College for his feedback on the protocol. We would like to express our thanks to Professor Jacqueline Ho and other personnel from the Malaysian Cochrane Network and the Julius Centre, University of Malaya for their utmost help in providing training on Cochrane systematic reviews. Lastly, we are grateful to Professor Prathap Tharyan, Director of the South Asian Cochrane Center and Network for his invaluable guidance for this review.
Ian Yang was the Editor for this review and commented critically on the review.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (The Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy for ClinicalTrials.gov
Search terms: "aclidinium" OR "aclidinium bromide" OR "LAMA" OR "muscarinic antagonists" OR "LAS34273"
Condition: COPD or Chronic Obstructive Pulmonary Disease
Study type: interventional studies
Appendix 3. Details of Almirall randomisation processes
The procedures for randomising Almirall sponsored studies have been detailed in correspondence between Esther Garcia Gil, Head of Late Stage Development Respiratory ,and HN, the details of which are given below.
Responses to your specific questions: 1. Randomisation process: prior to initiating the trial, a computer generated randomisation schedule was prepared to assign a treatment sequence to a randomisation number by the Statistics/Programming group within Almirall, according to the relevant Standard Operating Procedure (SOP). The randomisation was performed in order to avoid any possible bias due to the order of the IMP administrations. The block size was determined in agreement with the Clinical Trial Manager and the Statistician and was not to be communicated to the investigators. In all studies, we used IVRS (and in some cases IWRS) to sequentially randomise patients to the intervention arms according to the randomisation ratio defined in each study as well as the block size determined by the sponsor as mentioned above. 2. Blinding: in all our studies, blinding was applicable for all study outcomes. In the placebo‐controlled studies, matching placebo of aclidinium bromide had the same external appearance with the same composition, except for the active ingredient. In regards to the study that included tiotropium, in order to minimise bias, a double‐dummy technique to ensure the double blind of the trial was applied.
Data and analyses
Comparison 1. Aclidinium bromide versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total number of deaths | 9 | 5252 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.43, 1.94] |
| 1.1 Aclidinium once daily | 3 | 1828 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.25, 1.60] |
| 1.2 Aclidinium twice daily | 6 | 3424 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.46, 6.21] |
| 2 Number of patients with exacerbations requiring steroids, antibiotics or both | 10 | 5624 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.04] |
| 2.1 Aclidinium once daily | 4 | 2201 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.73, 1.20] |
| 2.2 Aclidinium twice daily | 6 | 3423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.66, 1.05] |
| 3 Quality of life: change from baseline in SGRQ total score | 7 | 4442 | Mean Difference (IV, Fixed, 95% CI) | ‐2.34 [‐3.18, ‐1.51] |
| 3.1 Aclidinium once daily | 2 | 1560 | Mean Difference (IV, Fixed, 95% CI) | ‐1.96 [‐3.47, ‐0.45] |
| 3.2 Aclidinium twice daily | 5 | 2882 | Mean Difference (IV, Fixed, 95% CI) | ‐2.51 [‐3.50, ‐1.51] |
| 4 Quality of life: Number of patients who achieved ≥ 4 units improvement in SGRQ total score | 7 | 4420 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.31, 1.70] |
| 4.1 Aclidinium once daily | 2 | 1560 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.08, 1.73] |
| 4.2 Aclidinium twice daily | 5 | 2860 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.32, 1.81] |
| 5 Lung function: Change from baseline in trough FEV1 (L) | 9 | 4963 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [0.08, 0.10] |
| 5.1 Aclidinium once daily | 3 | 1799 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [0.05, 0.09] |
| 5.2 Aclidinium twice daily | 6 | 3164 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [0.09, 0.12] |
| 6 Lung function: Change from baseline in peak FEV1 (L) | 9 | 4962 | Mean Difference (IV, Random, 95% CI) | 0.17 [0.15, 0.20] |
| 6.1 Aclidinium once daily | 3 | 1802 | Mean Difference (IV, Random, 95% CI) | 0.19 [0.12, 0.25] |
| 6.2 Aclidinium twice daily | 6 | 3160 | Mean Difference (IV, Random, 95% CI) | 0.17 [0.15, 0.19] |
| 7 Lung function: Change from baseline in normalised FEV1 AUC 0‐12 hour | 7 | 1237 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.10, 0.16] |
| 7.1 Aclidinium once daily | 2 | 131 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.08, 0.19] |
| 7.2 Aclidinium twice daily | 5 | 1106 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.10, 0.17] |
| 8 Lung function: Change from baseline in trough FVC (L) | 9 | 4963 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [0.14, 0.18] |
| 8.1 Aclidinium once daily | 3 | 1799 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [0.10, 0.18] |
| 8.2 Aclidinium twice daily | 6 | 3164 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [0.14, 0.20] |
| 9 Lung function: Change from baseline in peak FVC (L) | 9 | 4962 | Mean Difference (IV, Random, 95% CI) | 0.27 [0.23, 0.31] |
| 9.1 Aclidinium once daily | 3 | 1802 | Mean Difference (IV, Random, 95% CI) | 0.33 [0.23, 0.42] |
| 9.2 Aclidinium twice daily | 6 | 3160 | Mean Difference (IV, Random, 95% CI) | 0.25 [0.22, 0.28] |
| 10 Number of patients with hospital admissions due to COPD exacerbation | 10 | 5624 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.46, 0.88] |
| 10.1 Aclidinium once daily | 4 | 2201 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.45, 0.99] |
| 10.2 Aclidinium twice daily | 6 | 3423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.35, 1.01] |
| 11 Improvement in symptoms: Change from baseline in TDI focal score | 8 | 4490 | Mean Difference (IV, Random, 95% CI) | 0.84 [0.50, 1.18] |
| 11.1 Aclidinium once daily | 3 | 1597 | Mean Difference (IV, Random, 95% CI) | 1.08 [0.46, 1.71] |
| 11.2 Aclidinium twice daily | 5 | 2893 | Mean Difference (IV, Random, 95% CI) | 0.72 [0.33, 1.11] |
| 12 Number of patients who achieved ≥ 1 unit improvement in TDI focal score | 8 | 4289 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.52, 1.98] |
| 12.1 Aclidinium once daily | 3 | 1589 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.39, 2.20] |
| 12.2 Aclidinium twice daily | 5 | 2700 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.47, 2.03] |
| 13 Non‐fatal serious adverse events | 10 | 5651 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.14] |
| 13.1 Aclidinium once daily | 4 | 2227 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.58, 1.18] |
| 13.2 Aclidinium twice daily | 6 | 3424 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.68, 1.34] |
| 14 Withdrawals due to lack of efficacy | 10 | 5672 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.23, 0.43] |
| 14.1 Aclidinium once daily | 4 | 2227 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.20, 0.47] |
| 14.2 Aclidinium twice daily | 6 | 3445 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.20, 0.51] |
| 15 Withdrawals due to adverse events | 10 | 5672 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.57, 1.01] |
| 15.1 Aclidinium once daily | 4 | 2227 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.42, 1.00] |
| 15.2 Aclidinium twice daily | 6 | 3445 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.59, 1.21] |
Comparison 2. Aclidinium bromide versus long‐acting muscarinic antagonist.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total number of deaths | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Number of patients with exacerbations requiring steroids, antibiotics or both | 2 | 729 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.64 [0.31, 22.18] |
| 3 Lung function: Change from baseline in trough FEV1 (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Lung function: Change from baseline in peak FEV1 (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Lung function: Change from baseline in trough FVC (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Lung function: Change from baseline in peak FVC (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Number of patients with hospital admissions due to COPD exacerbation | 2 | 729 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.07, 4.11] |
| 8 Non‐fatal serious adverse events | 2 | 729 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.17, 2.65] |
| 9 Withdrawals due to lack of efficacy | 2 | 729 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Withdrawals due to adverse events | 2 | 729 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.26, 3.42] |
Comparison 4. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiac events | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 vs placebo | 10 | 5651 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.30] |
| 1.2 vs tiotropium bromide | 2 | 729 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.06, 2.45] |
| 2 Dry mouth | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 vs placebo | 10 | 5651 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.55, 1.95] |
| 2.2 vs tiotropium bromide | 2 | 729 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.13, 1.20] |
| 3 Constipation | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 vs placebo | 7 | 4252 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.59, 1.99] |
| 3.2 vs tiotropium bromide | 1 | 329 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.79 [0.11, 68.96] |
| 4 Cerebrovascular events | 9 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 vs placebo | 9 | 5252 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.25, 1.33] |
| 4.2 vs tiotropium bromide | 1 | 329 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.79 [0.11, 68.96] |
| 5 Diarrhoea | 7 | 4815 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.96, 2.20] |
| 5.1 vs placebo once daily | 2 | 1647 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.32 [1.14, 4.74] |
| 5.2 vs placebo twice daily | 5 | 3168 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.63, 1.78] |
| 6 Nasopharyngitis | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 vs placebo | 8 | 4710 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.95, 1.52] |
| 6.2 vs tiotropium bromide | 1 | 329 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.50, 3.29] |
| 7 Headache | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 vs placebo | 10 | 5651 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.98, 1.50] |
| 7.2 vs tiotropium bromide | 2 | 729 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.33, 1.60] |
| 8 Cough | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 vs placebo | 10 | 5651 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.78, 1.55] |
| 8.2 vs tiotropium bromide | 2 | 729 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.16, 1.56] |
| 9 Hypertension | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 vs placebo | 7 | 4654 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.54, 1.22] |
| 9.2 vs tiotropium bromide | 1 | 400 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.07, 27.01] |
| 10 Respiratory tract infections | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 vs placebo | 6 | 3474 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.77, 1.34] |
| 10.2 vs tiotropium bromide | 1 | 400 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.58 [0.14, 46.44] |
| 11 Urinary tract infections | 9 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 vs placebo | 9 | 5395 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.67, 1.55] |
| 11.2 vs tiotropium bromide | 1 | 400 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.05, 20.69] |
| 12 Fatigue | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 vs placebo | 7 | 4395 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.31, 1.03] |
| 12.2 vs tiotropium bromide | 1 | 400 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.06] |
| 13 Dizziness | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 vs placebo | 6 | 3853 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.54, 1.49] |
| 13.2 vs tiotropium bromide | 1 | 400 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.06, 5.65] |
| 14 Dyspnoea | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 vs placebo | 7 | 4177 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.44, 1.10] |
| 15 Arthralgia | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 vs placebo | 6 | 4273 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.98, 2.78] |
| 16 Back pain | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 vs placebo | 7 | 4454 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.74, 1.39] |
| 17 Oropharyngeal pain | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 vs placebo | 6 | 3635 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.66, 1.64] |
4.6. Analysis.
Comparison 4 Adverse events, Outcome 6 Nasopharyngitis.
4.7. Analysis.
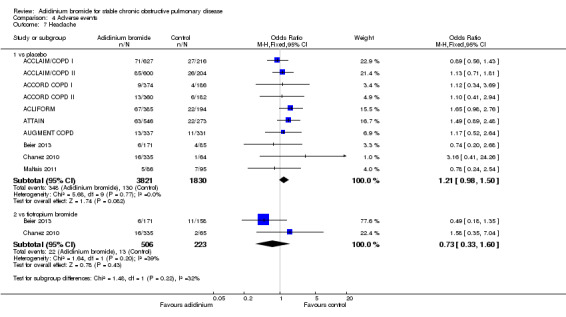
Comparison 4 Adverse events, Outcome 7 Headache.
4.8. Analysis.
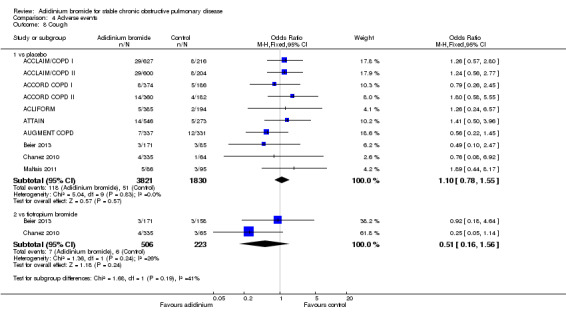
Comparison 4 Adverse events, Outcome 8 Cough.
4.9. Analysis.
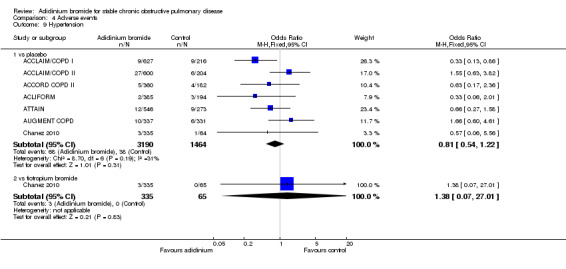
Comparison 4 Adverse events, Outcome 9 Hypertension.
4.10. Analysis.
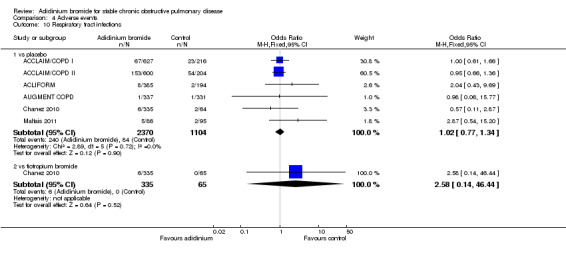
Comparison 4 Adverse events, Outcome 10 Respiratory tract infections.
4.11. Analysis.
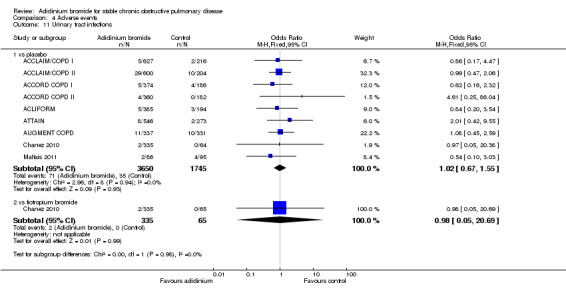
Comparison 4 Adverse events, Outcome 11 Urinary tract infections.
4.12. Analysis.
Comparison 4 Adverse events, Outcome 12 Fatigue.
4.13. Analysis.

Comparison 4 Adverse events, Outcome 13 Dizziness.
4.14. Analysis.
Comparison 4 Adverse events, Outcome 14 Dyspnoea.
4.15. Analysis.
Comparison 4 Adverse events, Outcome 15 Arthralgia.
4.16. Analysis.
Comparison 4 Adverse events, Outcome 16 Back pain.
4.17. Analysis.
Comparison 4 Adverse events, Outcome 17 Oropharyngeal pain.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ACCLAIM/COPD I.
| Methods | Study design: double‐blind, randomised, placebo‐controlled, parallel‐group, phase III study Study duration: 52 weeks Run‐in: 14 days Setting: multicentre trial Number of study centres and location: 132 centres in 16 European countries (one in Andorra, five in Austria, four in Belgium, 10 in Bulgaria, eight in the Czech Republic, three in Denmark, nine in France, 10 in Germany, eight in Hungary, six in Italy, four in Netherlands, nine in Poland, nine in Romania, 25 in Russia, eight in Spain, 13 in the UK) Date of study: August 2006 to May 2008 Randomisation: yes Blinding: double‐blind (subject, investigator) Withdrawals: stated |
|
| Participants | Number screened: 1313 Number randomised: 843 Number in treatment group: 627 (inhaled aclidinium 200 μg once daily) Number in control group: 216 Number of withdrawals (treatment/control): 89/47 Number completing trial (treatment/control): 538/169 Mean age (years) (treatment/control): 62.6/61.9 Gender (male/female): 488/139 (treatment), 175/41 (control) Caucasian (%) (treatment/control): 100/99.5 Inclusion criteria: male and non‐pregnant, non‐lactating female patients aged ≥ 40 years with a diagnosis of COPD according to GOLD criteria, with a post‐bronchodilator FEV1/FVC ratio of ≤ 70% and FEV1 < 80% of the predicted value. The predose FEV1 at randomisation was within 80‐120% of the pre‐bronchodilator FEV1 at screening. Current or previous cigarette smokers with a smoking history of ≥ 10 pack‐years Exclusion criteria: history or current diagnosis of asthma, allergic rhinitis or atopy; blood eosinophil count > 600 cell/mm3; respiratory tract infection or COPD exacerbation within six weeks prior to screening or during the run‐in period; hospitalisation for an acute COPD exacerbation within three months prior to screening; use of long‐term oxygen therapy; clinically significant respiratory diseases other than COPD; unstable cardiac conditions Baseline characteristics of treatment/control groups: comparable | |
| Interventions | Intervention: aclidinium 200 μg once daily via the Genuair inhaler Comparison: matching placebo once daily via the Genuair inhaler As‐needed therapy: inhaled salbutamol was permitted on as‐needed basis, but had to be discontinued six hours prior to and during a study visit Concomitant medications: inhaled corticosteroids or oral sustained‐release theophyllines; oral or parenteral corticosteroids at maximal doses equivalent to 10 mg/day of prednisone or 20 mg every other day; oxygen therapy (less than 15 hours per day) were allowed if the dosage had been stable for at least four weeks prior to screening Definition of COPD exacerbations: an increase in COPD symptoms over at least two consecutive days, associated with increased use of bronchodilators (mild exacerbation), treatment with antibiotics and/or systemic corticosteroids (moderate exacerbation) or leading to hospitalisation (severe exacerbation) |
|
| Outcomes |
Primary outcomes: trough FEV1 at weeks 12 and 28 Secondary outcomes: number of patients who achieved a clinically relevant improvement in health‐related quality of life at 52 weeks, as measured by a ≥ four units decrease from baseline on the SGRQ total score; and time to first moderate or severe COPD exacerbation Time points: spirometry was conducted according to American Thoracic Society (ATS)/European Respiratory Society (ERS) recommendations at one hour pre‐dose and immediately before dosing during study visits on day one (baseline); day two; week one; every month up to week 20; and thereafter every two months until week 52. Measurements were also performed at 0.25, 0.5, 1, 2 and 3 hours post‐dose on day one; and at 0.5, 1, 2 and 3 hours post‐dose at weeks 1, 4, 8, 12, 28, 44 and 52. Health status and dyspnoea were evaluated pre‐dose on day one (baseline) and at weeks 12, 28, 44 and 52 using the St George’s Respiratory Questionnaire (SGRQ; self administered) and Baseline/Transitional Dyspnoea Index (BDI/TDI; administered by an independent reviewer) |
|
| Notes | Full text publication Source of funding: Almirall, SA, Barcelona, Spain, and Forest Laboratories, Inc, NY, USA Study number: ClinicalTrials.gov NCT00363896 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from correspondence): "a computer generated randomisation schedule was prepared to assign a treatment sequence to a randomisation number. The block size was determined in agreement with the clinical trial manager and the statistician and was not to be communicated to the investigators" |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "IVRS (or IWRS in some cases) was used to sequentially randomise patients to the intervention arms according to the randomisation ratio and the block size determined as mentioned above" |
| Blinding of participants and personnel (performance bias) Aclidinium versus placebo | Low risk | Double‐blind (participant and investigator) Quote (from correspondence): "matching placebo of aclidinium bromide had the same external appearance with the same composition, except for the active ingredient. Blinding was applicable for all study outcomes" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from report): "spirometry data were electronically transmitted to a data‐management centre where an independent, blinded, spirometric expert reviewed the acceptability and repeatability of the data. Dyspnoea was evaluated using baseline/transitional dyspnoea index (BDI/TDI) administered by an independent reviewer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: number of withdrawals and reasons were clearly mentioned for both intervention and placebo arms. Withdrawal rates were relatively similar between groups (aclidinium 14.2% and placebo 21.8%). All efficacy analyses and safety summaries were performed on the intent‐to‐treat population, comprising all randomised patients who received at least one dose of study medication and who had a baseline and at least one post‐baseline trough FEV1 measurement Quote (from report): "missing data were imputed using a last observation carried forward approach" |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol was not available, but the published reports included all pre‐specified outcomes |
| Other bias | Low risk | Comment: no apparent source of bias was observed |
ACCLAIM/COPD II.
| Methods | Study design: double‐blind, randomised, placebo‐controlled, parallel‐group, phase III study Study duration: 52 weeks Run‐in: 14 days Setting: multicentre trial Number of study centres and location:119 sites in seven countries (72 sites in the United States, 13 sites in Argentina, 13 sites in Australia, seven sites in Canada, two sites in Mexico, three sites in New Zealand and nine sites in South Africa) Date of study: August 2006 to June 2008 Randomisation: yes Blinding: double‐blind (subject, investigator) Withdrawals: stated |
|
| Participants | Number screened: 1456 Number randomised: 804 Number in treatment group: 600 (inhaled aclidinium 200 μg once daily) Number in control group: 204 Number of withdrawals (treatment/control): 154/86 Number completing trial (treatment/control): 446/118 Mean age (years) (treatment/control): 65.1/65.2 Gender (male/female): 383/217 (treatment), 124/80 (control) Caucasian (%) (treatment/control): 92/92.7 Inclusion criteria: male and non‐pregnant, non‐lactating female patients aged ≥ 40 years with a diagnosis of COPD according to GOLD criteria, with a post‐bronchodilator FEV1/FVC ratio of ≤ 70% and FEV1 < 80% of the predicted value. The pre‐dose FEV1 at randomisation within 80‐120% of the pre‐bronchodilator FEV1 at screening. Current or previous cigarette smokers with a smoking history of ≥ 10 pack‐years Exclusion criteria: history or current diagnosis of asthma, allergic rhinitis or atopy; blood eosinophil count > 600 cell/mm3; respiratory tract infection or COPD exacerbation within six weeks prior to screening or during the run‐in period; hospitalisation for an acute COPD exacerbation within three months prior to screening; use of long‐term oxygen therapy; clinically significant respiratory diseases other than COPD; unstable cardiac conditions Baseline characteristics of treatment/control groups: comparable | |
| Interventions | Intervention: aclidinium 200 μg once daily via the Genuair inhaler Comparison: matching placebo once daily via the Genuair inhaler As‐needed therapy: inhaled salbutamol was permitted on as‐needed basis, but had to be discontinued six hours prior to and during a study visit Concomitant medications: inhaled corticosteroids or oral sustained‐release theophyllines; oral or parenteral corticosteroids at maximal doses equivalent to 10 mg/day of prednisone or 20 mg every other day; oxygen therapy (less than 15 hours per day) were allowed if the dosage had been stable for at least four weeks prior to screening Definition of COPD exacerbations: an increase in COPD symptoms over at least two consecutive days, associated with increased use of bronchodilators (mild exacerbation), treatment with antibiotics and/or systemic corticosteroids (moderate exacerbation) or leading to hospitalisation (severe exacerbation) |
|
| Outcomes |
Primary outcomes: trough FEV1 at weeks 12 and 28 Secondary outcomes: number of patients who achieved a clinically relevant improvement in health‐related quality of life at 52 weeks, as measured by a ≥ four units decrease from baseline on the SGRQ total score; and time to first moderate or severe COPD exacerbation Time points: spirometry was conducted according to American Thoracic Society (ATS)/European Respiratory Society (ERS) recommendations at one hour pre‐dose and immediately before dosing during study visits on day one (baseline); day two; week one; every month up to week 20; and thereafter every two months until week 52. Measurements were also performed at 0.25, 0.5, 1, 2 and 3 hours post‐dose on day one; and at 0.5, 1, 2 and 3 hours post‐dose at weeks 1, 4, 8, 12, 28, 44 and 52. Health status and dyspnoea were evaluated pre‐dose on day one (baseline) and at weeks 12, 28, 44 and 52 using the St George’s Respiratory Questionnaire (SGRQ; self administered) and Baseline/Transitional Dyspnoea Index (BDI/TDI; administered by an independent reviewer) |
|
| Notes | Full text publication Source of funding: Almirall, SA, Barcelona, Spain, and Forest Laboratories, Inc, NY, USA Study number: ClinicalTrials.gov NCT00358436 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from correspondence): "a computer generated randomisation schedule was prepared to assign a treatment sequence to a randomisation number. The block size was determined in agreement with the clinical trial manager and the statistician and was not to be communicated to the investigators" |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "IVRS (or IWRS in some cases) was used to sequentially randomise patients to the intervention arms according to the randomisation ratio and the block size determined as mentioned above" |
| Blinding of participants and personnel (performance bias) Aclidinium versus placebo | Low risk | Double‐blind (subject and investigator) Quote (from correspondence): "matching placebo of aclidinium bromide had the same external appearance with the same composition, except for the active ingredient. Blinding was applicable for all study outcomes" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from report): "spirometry data were electronically transmitted to a data‐management centre where an independent, blinded, spirometric expert reviewed the acceptability and repeatability of the data. Dyspnoea was evaluated using baseline/transitional dyspnoea index (BDI/TDI) administered by an independent reviewer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: dropout was higher in the placebo group (aclidinium 25.7% and placebo 42.2%) but efficacy analyses and safety summaries were performed on the intent‐to‐treat population, comprising all randomised patients who received at least one dose of study medication and who had a baseline and at least one post‐baseline trough FEV1 measurement Quote (from report): "missing data were imputed using a last observation carried forward approach" |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol was not available, but the published reports included all pre‐specified outcomes |
| Other bias | Low risk | Comment: no apparent source of bias was observed |
ACCORD COPD I.
| Methods | Study design: double‐blind, randomised, placebo‐controlled, parallel‐group, phase III study Study duration: 12 weeks Run‐in: two weeks Follow‐up: two weeks by phone contact/study visit Setting: multicentre trial Number of study centres and location: 106 study sites (100 in the United States and six additional sites in Canada) Date of study: April 2009 to July 2009 Randomisation: yes Blinding: double‐blind (subject, caregiver, investigator, outcomes assessor) Withdrawals: stated |
|
| Participants | Number screened: 1062
Number randomised: 561
Number in treatment group: 185 (Inhaled aclidinium 200 μg twice daily), 190 (Inhaled aclidinium 400 μg twice daily)
Number in control group: 186
Number of withdrawals (treatment/control): 33 (200 μg), 24 (400 μg)/37
Number completing trial (treatment/control): 152 (200 μg), 166 (400 μg)/149
Mean age (years): 63.1 (200 μg), 64.9 (400 μg), 65.1 (control)
Gender (male/female): 101/83 (200 μg), 100/90 (400 μg), 96/90 (control) Caucasian (%): 91.8 (200 μg), 95.3 (400 μg), 94.1 (control) Inclusion criteria: male and female patients ≥ 40 years of age, current or former cigarette smokers with a smoking history of ≥ 10 pack‐years and diagnosed with moderate‐to‐severe COPD (post‐bronchodilator FEV1/FVC < 70% and FEV1 ≥ 30% but < 80% of predicted) Exclusion criteria: other significant respiratory conditions (including asthma), respiratory infection or COPD exacerbation ≤ six weeks prescreening (≤ three months if it resulted in hospitalisation), clinically significant cardiovascular conditions including myocardial infarction during the previous six months, unstable arrhythmia, Bazett‐corrected QTc > 470 msec and medical conditions where anticholinergic drugs are contraindicated Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Intervention: inhaled aclidinium 200 μg twice daily, inhaled aclidinium 400 μg twice daily at the same time in the morning (between 8:00 and 10:00 AM) and evening (between 8:00 and 10:00 PM) via a multiple‐dose dry powder inhaler (Genuair) Comparison: inhaled placebo twice daily via Genuair inhaler As‐needed therapy: albuterol as rescue medication but had to be discontinued ≥ six hours before each study visit Concomitant medications: inhaled corticosteroids (ICS), systemic corticosteroids equivalent to ≤ 10mg/day of prednisone or 20 mg every other day, and theophylline if treatment was stable for ≥ four weeks prior to screening. Theophylline and ICS were discontinued the morning before each study visit. Inhaled anticholinergics and LABAs were prohibited throughout the study Definition of COPD exacerbation: an increase in COPD symptoms ≥ two consecutive days resulting in medical intervention and categorised as mild (increased use of rescue medication), moderate (treatment with antibiotics and/or systemic corticosteroids), or severe (hospitalisation) |
|
| Outcomes |
Primary outcomes: change in morning pre‐dose (trough) FEV1 (the average of two pre‐dose FEV1 values) from baseline to week 12 Secondary outcomes: change in peak FEV1 (the highest value observed within three hours post‐morning dose) from baseline to week 12 Other outcomes: changes from baseline on day one (peak FEV1 only), weeks 1, 4, and 8 (trough and peak FEV1) and week 12 (AUC0‐3/3h FEV1, trough, peak, and AUC0‐3/3h FVC, and trough IC), changes from baseline at weeks 4, 8 and 12 in SGRQ and TDI (including percentage of subjects with a clinically meaningful improvement (decrease of ≥ four points for SGRQ or increase of ≥ one unit for TDI)), changes from baseline at week 12 in COPD Nighttime Symptoms Questionnaire and Daily Sleep Diary scores, rescue medication use over 12 weeks, and COPD exacerbation rate |
|
| Notes | Full text publication Source of funding: Forest Research Institute, Inc. Study number: ClinicalTrials.gov NCT00891462 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from correspondence): "a computer generated randomisation schedule was prepared to assign a treatment sequence to a randomisation number. The block size was determined in agreement with the clinical trial manager and the statistician and was not to be communicated to the investigators" |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "IVRS (or IWRS in some cases) was used to sequentially randomise patients to the intervention arms according to the randomisation ratio and the block size determined as mentioned above" |
| Blinding of participants and personnel (performance bias) Aclidinium versus placebo | Low risk | Double‐blind (subject, caregiver and investigator) Quote (from correspondence): "matching placebo of aclidinium bromide had the same external appearance with the same composition, except for the active ingredient. Blinding was applicable for all study outcomes" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind including blinding of outcomes assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: withdrawals were relatively low and balanced across the groups with similar reasons (aclidinium 200 μg 17.8%, aclidinium 400 μg 12.6% and placebo 19.9%). All efficacy analyses and safety outcomes were performed on the intent‐to‐treat population, comprising all randomised patients who received at least one dose of study medication and who had a baseline and at least one post‐baseline trough FEV1 measurement Quote (from report): "missing values were imputed using the last‐observation‐carried‐forward approach" |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol was not available, but the published reports included all pre‐specified outcomes |
| Other bias | Low risk | Comment: no apparent source of bias was observed |
ACCORD COPD II.
| Methods | Study design: randomised, double‐blind, placebo‐controlled, parallel group, phase III study Study duration: 12 weeks Run‐in: two weeks Follow‐up: phone call or visit two weeks after the last study treatment dose Setting: multicentre trial Number of study centres and location: 103 study centres (101 in the United States and two in Canada) Date of study: December 2009 to September 2010 Randomisation: yes Blinding: double blind (subject, caregiver, investigator, outcomes assessor) Withdrawals: stated |
|
| Participants | Number screened: 1236
Number randomised: 544
Number in treatment group: 184 (Inhaled aclidinium 200 μg twice daily), 178 (Inhaled aclidinium 400 μg twice daily)
Number in control group:182
Number of withdrawals (treatment/control): 29 (200 μg), 30 (400 μg)/31
Number completing trial (treatment/control): 155 (200 μg), 148 (400 μg)/151
Mean age (years): 63.4 (200 μg), 63.2 (400 μg), 61.7 (control)
Gender (male/female): 100/82 (200 μg), 99/84 (400 μg), 89/88 (control) Caucasian (%): 89.1 (200 μg), 90.4 (400 μg), 92.3 (control) Inclusion criteria: male and female patients ≥ 40 years old, current or former cigarette smokers with a smoking history of ≥ 10 pack‐years and diagnosed with stable moderate‐to‐severe COPD according to GOLD guidelines (post‐bronchodilator FEV1/FVC < 70% and FEV1 ≥ 30% and < 80% of predicted) Exclusion criteria: COPD exacerbation requiring hospitalisation ≤ three months before screening, respiratory tract infection or COPD exacerbation ≤ six weeks before screening, other clinically significant respiratory condition including asthma, clinically significant cardiovascular conditions including myocardial infarction ≤ six months or newly diagnosed arrhythmia ≤ three months before screening, history of hypersensitivity reaction or contraindications to inhaled anticholinergics Baseline characteristics of treatment/control groups: percentage of severe (GOLD stage III) patients: 46.4% (aclidinium 200 μg), 54.2% (aclidinium 400 μg), 36.8% (placebo) baseline mean FEV1 (L): 1.40 (aclidinium 200 μg), 1.25 (aclidinium 400 μg), 1.46 (placebo) |
|
| Interventions | Intervention: inhaled aclidinium 200 μg twice daily, inhaled aclidinium 400 μg twice daily via a multiple‐dose dry powder inhaler (Genuair/Pressair) Comparison: inhaled placebo twice daily As‐needed therapy: albuterol (salbutamol) was permitted as rescue medication but was discontinued ≥ six hours before study visits Concomitant medications: theophylline, inhaled corticosteroids (ICS), oral or parenteral corticosteroids equivalent to ≤ 10mg/day of prednisone or 20 mg every other day were allowed if treatment was stable for ≥ four weeks before screening. These medications were discontinued ≥ six hours before each study visit Other short/long acting anticholinergics and LABAs were prohibited throughout the study |
|
| Outcomes |
Primary outcomes: change in morning pre‐dose (trough) FEV1 from baseline to week 12 Secondary outcomes: change in peak FEV1 (maximum FEV1 reading observed ≤ three hours post‐morning dose) from baseline to week 12 Other outcomes: changes from baseline in morning trough and peak FEV1 at day one (peak FEV1 only) and weeks 1, 4, and 8. Changes from baseline in FEV1 at 0.5‐3 hour post‐dose and area under the concentration‐time curve from 0 to 3 hour normalized over 3 hour (AUC0‐3/3h), FVC (trough, peak), and IC (trough, three hours post‐dose) at day one (except for trough) and weeks 1, 4, 8 and 12. Changes from baseline in SGRQ score at weeks 4, 8 and 12 and TDI at week 12, percentage of patients with a minimal clinically important differences from baseline in SGRQ (decrease of ≥ four points) or TDI (increase of ≥ one unit) at study end |
|
| Notes | Full text publication Source of funding: Almirall, SA, Barcelona, Spain, and Forest Laboratories, Inc, NY, USA Study number: ClinicalTrials.gov NCT01045161 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from correspondence): "a computer generated randomisation schedule was prepared to assign a treatment sequence to a randomisation number. The block size was determined in agreement with the clinical trial manager and the statistician and was not to be communicated to the investigators" |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "IVRS (or IWRS in some cases) was used to sequentially randomise patients to the intervention arms according to the randomisation ratio and the block size determined as mentioned above" |
| Blinding of participants and personnel (performance bias) Aclidinium versus placebo | Low risk | Double‐blind (subject, caregiver and investigator) Quote (from correspondence): "matching placebo of aclidinium bromide had the same external appearance with the same composition, except for the active ingredient. Blinding was applicable for all study outcomes" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind including blinding of outcomes assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: number of withdrawals were low and even across the groups with similar reasons (aclidinium 200 μg 15.8%, aclidinium 400 μg 16.9% and placebo 17%). Efficacy analyses and safety summaries were based on the intent‐to‐treat population, defined as all randomised patients who received at least one dose of double blind study medication and who had a baseline and at least one post‐baseline trough FEV1 assessment. Quote (from report): "missing data were imputed by the last‐observation‐carried‐forward approach" |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol was not available, but the published report included all pre‐specified outcomes |
| Other bias | Low risk | Comment: no apparent source of bias was observed Relatively higher percentage of severe COPD patients were recruited in aclidinium 400 μg arm than placebo, however sensitivity analysis by exclusion of this study data had no significant change on the overall effect estimates |
ACLIFORM.
| Methods | Study design: randomised, double‐blind, placebo‐controlled, parallel group, phase III study Study duration: 24 weeks Setting: multicentre trial Number of study centres and location: 197 study sites in 22 countries (two sites in Austria, two in Belgium, five in Bulgaria, two in Croatia, 12 in Czech Republic, four in Denmark, five in Finland, seven in France, 28 in Germany, 15 in Hungary, four in Italy, eight in Repulbic of Korea, seven in Netherlands, 21 in Poland, 12 in Romania, five in Russia, seven in Slovakia, nine in South Africa, seven in Spain, five in Sweden, 14 in Ukraine, 16 in the United Kingdom) Date of study: October 2011 to January 2013 Randomisation: yes Blinding: double‐blind (subject, investigator) |
|
| Participants | Number randomised: 1729 Number in treatment group: 381 (aclidinium 400 μg plus formoterol 6 μg), 385 (aclidinium 400 μg plus formoterol 12 μg), 385 (aclidinium 400 μg monotherapy) Number in control group: 384 (formoterol 12 μg) or 194 (placebo) Number of withdrawals : 40 (aclidinium 400 μg plus formoterol 6 μg), 34 (aclidinium 400 μg plus formoterol 12 μg), 50 (aclidinium 400 μg monotherapy), 45 (formoterol 12 μg) or 34 (placebo) Number completing trial : 341 (aclidinium 400 μg plus formoterol 6 μg), 351 (aclidinium 400 μg plus formoterol 12 μg), 335 (aclidinium 400 μg monotherapy), 339 (formoterol 12 μg) or 160 (placebo) Mean age (years): 62.9 (aclidinium 400 μg plus formoterol 6 μg), 62.7 (aclidinium 400 μg plus formoterol 12 μg), 63.1 (aclidinium 400 μg monotherapy), 63.4 (formoterol 12 μg) or 64.2 (placebo) Gender (male/female): 259/122 (aclidinium 400 μg plus formoterol 6 μg), 261/124 (aclidinium 400 μg plus formoterol 12 μg), 256/129 (aclidinium 400 μg monotherapy), 255/129 (formoterol 12 μg) or 138/56 (placebo) Caucasian (%): 96.1 (aclidinium 400 μg plus formoterol 6 μg), 95.3 (aclidinium 400 μg plus formoterol 12 μg), 94.3 (aclidinium 400 μg monotherapy), 94.3 (formoterol 12 μg) or 94.3 (placebo) Inclusion criteria: male or non‐pregnant, non‐lactating female ≥ 40 years, current or ex‐smokers with a cigarette smoking history of at least 10 pack‐years, diagnosed with stable moderate to severe COPD as defined by the GOLD at the screening visit, able to perform repeatable pulmonary function testing for FEV1 according to "ATS/ERS" 2005 criteria at screening visit Exclusion criteria: asthma, any respiratory tract infection or COPD exacerbation in the six weeks before screening visit, hospitalisation for an acute COPD exacerbation within three months prior to the screening visit, clinically significant respiratory conditions including active tuberculosis, interstitial lung disease or massive pulmonary thromboembolic disease, pulmonary resection or lung volume reduction surgery within 12 months prior to screening visit, history of lung transplantation, bronchiectasis, alpha1‐antitrypsin deficiency, chronic use of oxygen therapy greater than or equal to 15 hours/day, clinically significant cardiovascular conditions, hospitalisation within 12 months prior to screening visit for heart failure functional classes III according to the New York Heart Association, corrected QT interval "QTc" > 470 msec at screening visit |
|
| Interventions | Intervention: inhaled aclidinium/formoterol fixed dose combination (FDC) high dose twice daily, inhaled aclidinium/formoterol FDC low dose twice daily, inhaled aclidinium 400 μg twice daily Comparison: inhaled formoterol 12 μg twice daily, inhaled dose‐matched placebo twice daily |
|
| Outcomes |
Primary outcomes: change from baseline in morning pre‐dose (trough) FEV1 and one hour post‐morning dose FEV1 at week 24 Secondary outcomes: change from baseline in Transition Dyspnoea Index (TDI) score and St. George's Respiratory Questionnaire (SGRQ) total score at week 24 |
|
| Notes | Source of support: Almirall SA, Barcelona, Spain, and Forest Laboratories, Inc, New York, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from correspondence): "a computer generated randomisation schedule was prepared to assign a treatment sequence to a randomisation number. The block size was determined in agreement with the clinical trial manager and the statistician and was not to be communicated to the investigators" |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "IVRS (or IWRS in some cases) was used to sequentially randomise patients to the intervention arms according to the randomisation ratio and the block size determined as mentioned above" |
| Blinding of participants and personnel (performance bias) Aclidinium versus placebo | Low risk | Double‐blind (subject and investigator) Quote (from correspondence): "matching placebo of aclidinium bromide had the same external appearance with the same composition, except for the active ingredient. Blinding was applicable for all study outcomes" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: withdrawal rates were somewhat higher in the placebo group but overall low in all groups (aclidinium 13%, placebo 17.5%, formoterol 11.7%, fixed dose combination with formoterol 6 μg 10.5%, fixed dose combination with formoterol 12 μg 8.8%) and the reasons were provided upon request |
| Selective reporting (reporting bias) | Low risk | Comment: no published report available, but results for all specified outcomes were supplied on request |
| Other bias | Low risk | Comment: no apparent source of bias was observed |
ATTAIN.
| Methods | Study design: double‐blind, randomised, placebo‐controlled, parallel‐group, phase III study Study duration: 24 weeks Run‐in: two weeks Setting: multicentre trial Number of study centres and location: 103 sites in 11 countries (10 sites in the Czech Republic, five in France, 17 in Germany, 13 in Hungary, three in Italy, one in Peru, 21 in Poland, 10 in the Russian Federation, five in Spain, 13 in South Africa and five in the Ukraine) Date of study: October 2009 to November 2010 Randomisation: yes Blinding: double‐blind (subject, investigator) Withdrawals: stated |
|
| Participants | Number screened: 1061
Number randomised: 828 Numer included in statistical analysis: 819 Number in treatment group: 280 (Inhaled aclidinium 200 μg twice daily), 272 (Inhaled aclidinium 400 μg twice daily) Number in control group: 276 Number of withdrawals (treatment/control): 24 (200 μg), 17 (400 μg)/41 Number completing trial (treatment/control): 253 (200 μg), 252 (400 μg)/232 Mean age (years): 62.3 (200 μg), 62.9 (400 μg), 62.0 (control) Gender (male/female): 181/96 (200 μg), 182/87 (400 μg), 189/84 (control) Caucasian (%): 95.0 (200 μg), 95.5 (400 μg), 95.2 (control) Inclusion criteria: male and female patients aged ≥ 40 yrs, current or former cigarette smokers with a smoking history of ≥ 10 pack‐years with a diagnosis of COPD according to GOLD criteria (post‐bronchodilator FEV1/FVC ratio of < 70% and FEV1 < 80% of the predicted value) Exclusion criteria: history or current diagnosis of asthma; respiratory tract infection or COPD exacerbation within six weeks (three months if hospitalisation) before screening or during the run‐in period; clinically relevant respiratory conditions other than COPD; unstable cardiac conditions, including myocardial infarction, within the previous six months; and contraindications to the use of anticholinergic drugs Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Intervention: inhaled aclidinium 200 μg twice daily or 400 μg twice daily via a multiple‐dose dry powder inhaler (Genuair) Comparison: placebo twice daily via Genuair inhaler As‐needed therapy: inhaled salbutamol was permitted but was discontinued six hours before and during study visits Concomitant medications: inhaled corticosteroids or oral sustained‐release theophyllines; systemic corticosteroids at doses equivalent to 10 mg per day of prednisone or 20 mg every other day; and oxygen therapy (< 15 hours per day) if their administration had been stable for ≥ four weeks before screening Definition of COPD exacerbation: increase in COPD symptoms over at least two consecutive days, resulting in the increased use of short‐acting bronchodilators and/or inhaled corticosteroids (mild exacerbation), treatment with antibiotics and/or systemic corticosteroids (moderate exacerbation), or hospitalisation (severe exacerbation) |
|
| Outcomes |
Primary outcomes: change from baseline in morning pre‐dose (trough) FEV1 at week 12 and 24 Secondary outcomes: change from baseline in peak FEV1 (highest FEV1 value observed within three hours of morning dosing) at week 12 and 24, percentages of patients achieving clinically significant improvements in SGRQ total score and TDI focal score at week 24 Time points: standardised spirometric measurements (FEV1, FVC and inspiratory capacity) before the morning dose on day one (baseline) and during visits at weeks 1, 4, 8, 12, 18 and 24. FEV1 and FVC measurements were obtained at 0.5, 1, 2 and 3 hour post‐dose and inspiratory capacity measurements at three hour post‐dose on day one and weeks 1, 4, 12 and 24. Health status was evaluated pre‐dose at baseline and weeks 4, 12 and 24 using the St George’s Respiratory Questionnaire (SGRQ). Dyspnoea was assessed at baseline using the Baseline Dyspnoea Index (BDI) and changes were measured using the Transitional Dyspnoea Index (TDI) at weeks 4, 12 and 24 |
|
| Notes | Full text publication Source of funding: Almirall, SA, Barcelona, Spain, and Forest Laboratories, Inc, NY, USA Study number: ClinicalTrials.gov NCT01001494 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from correspondence): "a computer generated randomisation schedule was prepared to assign a treatment sequence to a randomisation number. The block size was determined in agreement with the clinical trial manager and the statistician and was not to be communicated to the investigators" |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "IVRS (or IWRS in some cases) was used to sequentially randomise patients to the intervention arms according to the randomisation ratio and the block size determined as mentioned above" |
| Blinding of participants and personnel (performance bias) Aclidinium versus placebo | Low risk | Double‐blind (subject and investigator) Quote (from report): "all study centres had identical spirometry equipment, detailed study manual and training" Quote (from correspondence): "matching placebo of aclidinium bromide had the same external appearance with the same composition, except for the active ingredient. Blinding was applicable for all study outcomes" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind Quote (from report): "dyspnoea was assessed at baseline using the Baseline Dyspnoea Index (BDI) and changes were measured using the Transitional Dyspnoea Index (TDI) at weeks 4, 12 and 24. The BDI and TDI were administered by an independent reviewer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: overall dropout was low in all groups though the number of withdrawals were slightly higher in the placebo group (aclidinium 200 μg 8.6%, aclidinium 400 μg 6.3% and placebo 14.9%). Efficacy analyses and safety outcomes were performed on the intention‐to‐treat (ITT) population, defined as all randomised patients who took one or more dose of study medication and had a baseline and one or more post‐baseline FEV1 assessment Quote (from report): "missing data were imputed using last observation carried forward (LOCF). For spirometry data, linear interpolation and time‐matched LOCF were applied" |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol was not available, but the published reports included all pre‐specified outcomes |
| Other bias | Low risk | Comment: no apparent source of bias was observed |
AUGMENT COPD.
| Methods | Study design: randomised, double‐blind, placebo‐controlled, parallel group, phase III study Study duration: 24 weeks Run‐in: present, duration not mentioned Follow‐up: two weeks Setting: multicentre trial Number of study centres and location: 222 study sites (193 sites in the United States, 11 in Australia, 10 in Canada and eight in New Zealand) Date of study: September 2011 to March 2013 Randomisation: yes Blinding: double‐blind (subject, caregiver, investigator, outcomes assessor) Withdrawals: available on request (though published only as abstract) |
|
| Participants | Number randomised: 1692 Number in treatment group: 338 (aclidinium 400 μg plus formoterol 6 μg), 338 (aclidinium 400 μg plus formoterol 12 μg), 340 (aclidinium 400 μg monotherapy) Number in control group: 339 (formoterol 12 μg) or 337 (placebo) Number of withdrawals : 62 (aclidinium 400 μg plus formoterol 6 μg), 66 (aclidinium 400 μg plus formoterol 12 μg), 72 (aclidinium 400 μg monotherapy), 69 (formoterol 12 μg) or 101 (placebo) Number completing trial : 276 (aclidinium 400 μg plus formoterol 6 μg), 272 (aclidinium 400 μg plus formoterol 12 μg), 268 (aclidinium 400 μg monotherapy), 270 (formoterol 12 μg) or 236 (placebo) Mean age (years): 63.9 (aclidinium 400 μg plus formoterol 6 μg), 64.2 (aclidinium 400 μg plus formoterol 12 μg), 64.4 (aclidinium 400 μg monotherapy), 63.7 (formoterol 12 μg) or 63.5 (placebo) Gender (male/female): 187/151 (aclidinium 400 μg plus formoterol 6 μg), 168/170 (aclidinium 400 μg plus formoterol 12 μg), 188/152 (aclidinium 400 μg monotherapy), 169/170 (formoterol 12 μg) or 175/162 (placebo) Caucasian (%): 92.8 (aclidinium 400 μg plus formoterol 6 μg), 91 (aclidinium 400 μg plus formoterol 12 μg), 93.2 (aclidinium 400 μg monotherapy), 93.7 (formoterol 12 μg) or 95.5 (placebo) Inclusion criteria: male and female patients aged ≥ 40 yrs diagnosed with stable, moderate to severe COPD as defined by the GOLD criteria and stable airway obstruction, current or former cigarette smokers with a smoking history of at least 10 pack‐years Exclusion criteria: hospitalisation for acute COPD exacerbation within three months prior to the first visit, any respiratory tract infection or COPD exacerbation in the six weeks before first visit, respiratory conditions other than COPD, asthma, chronic use of oxygen therapy greater than or equal to 15 hours/day, clinically significant cardiovascular conditions, history of hypersensitivity reaction to inhaled anticholinergics |
|
| Interventions | Intervention: inhaled fixed dose combination of aclidinium 400 μg plus formoterol 6 μg or 12 μg twice daily, inhaled aclidinium 400 μg twice daily Comparison: inhaled formoterol 12 μg twice daily, inhaled dose‐matched placebo twice daily |
|
| Outcomes |
Primary outcomes: change from baseline in morning pre‐dose (trough) FEV1 and one hour post‐dose FEV1 at week 24 Secondary outcomes: change from baseline in St. George's Respiratory Questionnaire (SGRQ) total score and improvement in Transition Dyspnoea Index (TDI) score at week 24 |
|
| Notes | This trial was available as abstract only Source of support: Almirall SA, Barcelona, Spain, and Forest Laboratories, Inc, New York, USA Study number: ClinicalTrials.gov NCT01437397 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from correspondence): "a computer generated randomisation schedule was prepared to assign a treatment sequence to a randomisation number. The block size was determined in agreement with the clinical trial manager and the statistician and was not to be communicated to the investigators" |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "IVRS (or IWRS in some cases) was used to sequentially randomise patients to the intervention arms according to the randomisation ratio and the block size determined as mentioned above" |
| Blinding of participants and personnel (performance bias) Aclidinium versus placebo | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: number of withdrawals and the reasons were provided upon request. Dropout was relatively higher for placebo group (aclidinium 21.2%; placebo 30%, formoterol 20.4%, fixed dose combination with formoterol 6 μg 18.3%; fixed dose combination with formoterol 12 μg 19.5%). No clear information on what method of imputation was used for each outcome |
| Selective reporting (reporting bias) | Low risk | Comment: no published report available, but results for all specified outcomes were supplied on request |
| Other bias | Low risk | Comment: no apparent source of bias was observed |
Beier 2013.
| Methods | Study design: randomised, double‐blind, double‐dummy, placebo‐ and active comparator‐controlled, parallel‐group, phase IIIb study Study duration: six weeks Run‐in: two to three weeks Setting: multicentre trial Number of study centres and location: 49 study sites in four countries (three sites in the Czech Republic, 23 in Germany, eight in Hungary and 15 in Poland) Date of study: October 2011 to March 2012 Randomisation: yes Blinding: double‐blind (subject, investigator) Withdrawals: stated |
|
| Participants | Number screened: 485 Number randomised: 414 Number in treatment group: 171 (aclidinium 400 μg twice daily), 158 (tiotropium 18 μg once daily) Number in control group: 85 Number of withdrawals (treatment/control): 5 (aclidinium 400 μg twice daily), 4 (tiotropium 18 μg once daily)/5 Number completing trial (treatment/control): 166 (aclidinium 400 μg twice daily), 154 (tiotropium 18 μg once daily)/80 Mean age (years): 61.8 (aclidinium 400 μg twice daily), 62.8 (tiotropium 18 μg once daily), 62.2 (placebo) Gender (male/female): 114/57(aclidinium 400 μg twice daily), 116/42 (tiotropium 18 μg once daily), 48/37 (placebo) Caucasian (%): 100 (aclidinium 400 μg twice daily), 100 (tiotropium 18 μg once daily), 98.8 (placebo) Inclusion criteria: patients aged ≥ 40 years with a clinical diagnosis of stable moderate‐to‐severe COPD (post‐bronchodilator FEV1/FVC < 70%, and FEV1 ≥ 30% and < 80%), either current or former cigarette smokers (smoking history of ≥ 10 pack‐years) Exclusion criteria: history or current diagnosis of asthma or other clinically significant respiratory or cardiovascular conditions, any respiratory tract infection or COPD exacerbation ≤ six weeks before screening (≤ three months if hospitalisation), contraindications and hypersensitivity to muscarinic antagonists and inability to use the study inhalers properly Baseline characteristics of treatment/control groups: comparable | |
| Interventions | Intervention: aclidinium bromide 400 μg twice daily in the morning (9:00 ± 1 hour) and evening (21:00 ± 1 hour) via the Genuair/Pressair multidose dry powder inhaler, tiotropium 18 μg once daily in the morning (9:00 ± 1 hour) via the HandiHaler Comparison: matched placebo As‐needed therapy: inhaled salbutamol 100 μg/puff was permitted except ≤ six hours before each visit Concomitant medications: stable use of oral sustained‐release theophylline (not other methylxanthines), inhaled corticosteroids and oral or parenteral corticosteroids (prednisone ≤ 10 mg/day or 20 mg/every other day, or equivalent) were permitted except ≤ six hours before each visit. Oxygen therapy (except ≤ two hours before each visit) was also allowed |
|
| Outcomes |
Primary outcomes: change from baseline in normalized FEV1 area under the curve over the 24‐hour period post‐morning dose (AUC0–24) at week six Secondary outcomes: change from baseline in normalized FEV1 AUC over the nighttime period (AUC12–24) at week six Other outcomes: changes from baseline in normalized FEV1 AUC for the 12‐hour period post‐morning treatment (AUC0–12), morning predose (trough) and peak FEV1 and FVC Time points: spirometry was conducted according to American Thoracic Society (ATS)/European Respiratory Society (ERS) recommendations over 24 hours following morning treatment on day one and at week six. Three manoeuvres were performed at each time point. Additional measurements (up to a total of eight tests) were made if the first three were not acceptable |
|
| Notes | Full text publication Source of funding: Almirall, SA, Barcelona, Spain, and Forest Laboratories, Inc, New York, USA Study number: ClinicalTrials.gov NCT01462929 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from report): "random sequence generation was by computer‐generated schedule" Quote (from correspondence): "a computer generated randomisation schedule was prepared to assign a treatment sequence to a randomisation number. The block size was determined in agreement with the clinical trial manager and the statistician and was not to be communicated to the investigators" |
| Allocation concealment (selection bias) | Low risk | Quote (from report): "allocation via an interactive voice‐response system" Quote (from correspondence): "IVRS (or IWRS in some cases) was used to sequentially randomise patients to the intervention arms according to the randomisation ratio and the block size determined as mentioned above" |
| Blinding of participants and personnel (performance bias) Aclidinium versus placebo | Low risk | Double‐blind (subject and investigator), double‐dummy Quote (from report): "patients and study personnel remained blinded to treatment allocation throughout the study" Quote (from correspondence): "matching placebo of aclidinium bromide had the same external appearance with the same composition, except for the active ingredient. Blinding was applicable for all study outcomes" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: number of withdrawals and reasons were clearly mentioned for intervention and placebo arms Withdrawals were low and balanced across the groups (aclidinium 2.9%, tiotropium 2.5%, placebo 5.9%). Efficacy analyses and safety summaries were based on the intent‐to‐treat population, which included all randomised patients who received at least one dose of study medication and who had a baseline and at least one post‐baseline trough FEV1 value Quote (from report): "no patients were lost to follow‐up" |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol was not available, but the published reports included all pre‐specified outcomes |
| Other bias | Low risk | Comment: no apparent source of bias was observed |
Chanez 2010.
| Methods | Study design: randomised, parallel‐group, double‐blind (open‐label for patients randomised to tiotropium), phase IIb study Study duration: four weeks Run‐in: two weeks Setting: multicentre trial Number of study centres and location: 42 centres in Europe and Russia Randomisation: yes Blinding: double‐blind (open label for patients randomised to tiotropium) Withdrawals: stated |
|
| Participants | Number screened: 694 Number randomised: 464 Number in treatment group: 66 (aclidinium 25 μg), 65 (aclidinium 50 μg), 70 (aclidinium 100 μg), 67 (aclidinium 200 μg), 67 (aclidinium 400 μg), 65 (tiotropium 18 μg) Number in control group: 64 Number of withdrawals (treatment/control): 4 (aclidinium 25 μg), 3 (aclidinium 50 μg), 3 (aclidinium 100 μg), 3 (aclidinium 200 μg), 3 (aclidinium 400 μg), 4 (tiotropium 18 μg)/3 Number completing trial (treatment/control): 62 (aclidinium 25 μg), 62 (aclidinium 50 μg), 67 (aclidinium 100 μg), 64 (aclidinium 200 μg), or 64 (aclidinium 400 μg), 61 (tiotropium 18 μg)/61 Mean age (years): 60.4 (aclidinium 25 μg), 61.1 (aclidinium 50 μg), 61.6 (aclidinium 100 μg), 62.1 (aclidinium 200 μg), 62.0 (aclidinium 400 μg), 62.2 (tiotropium 18 μg), 61.2 (placebo) Gender (male/female): 49/16 (aclidinium 25 μg), 47/18 (aclidinium 50 μg), 54/15 (aclidinium 100 μg), 57/9 (aclidinium 200 μg), 53/14 (aclidinium 400 μg), 56/8 (tiotropium 18 μg), 55/9 (placebo) Ethnicity: not stated Inclusion criteria: male and female patients ≥ 40 years with a diagnosis of stable moderate to severe COPD according to American Thoracic Society criteria with a smoking history of ≥ 10 pack‐years, and FEV1 in the range of 30–65% of predicted normal (Quanjer predicted normal value) at the screening visit; pre‐dose FEV1 at the randomisation visit within 20% of the screening visit value. The ratio between FEV1 and FVC was required to be ≤ 70% at both the screening visit and randomisation visit Exclusion criteria: history of or current asthma, allergic rhinitis, or atopy; reversibility to inhaled salbutamol 400 mg > 20% of pre‐dose value; blood eosinophil count > 400 cells/mm3; respiratory tract infection or COPD exacerbation within one month (or hospitalisation within three months) of the screening visit; clinically significant or relevant cardiovascular conditions, laboratory tests, or electrocardiogram (ECG) parameters; QTc interval > 450 ms; history of narrow‐angle glaucoma, symptomatic prostatic hypertrophy, or bladder neck obstruction Baseline characteristics of treatment/control groups: comparable | |
| Interventions | Intervention: inhaled aclidinium 25 μg, 50 μg, 100 μg, 200 μg, or 400 μg (via multidose dry‐powder inhaler, Genuair) or tiotropium 18 μg (via Handi‐Haler) once daily in the morning between 08.00 and 12.00 Comparison: matching placebo once daily As‐needed therapy: salbutamol 100 mg/puff was allowed as rescue medication, and was discontinued for eight hours prior to any visit Concomitant medications: inhaled corticosteroids, oral sustained release theophyllines (suspended at least 48 hours before each study visit), antihistamines, nedocromil, and ketotifen was permitted, provided the stable dose was administered prior to randomisation. The morning dose of these medications was delayed until the completion of spirometry measurements at each visit. Any other medication used for the treatment of COPD was withdrawn prior to the start of the study, and medications with pro‐arrhythmic effects or that affect heart rate or QTc were not permitted |
|
| Outcomes |
Primary outcomes: trough FEV1 (the mean value of the three highest readings assessed at 22, 23, and 24 hour following the trial drug administration) on day 29 for aclidinium (all doses) versus placebo Secondary outcomes: trough FEV1 on days 2, 8, and 15; trough FVC (the mean value of the three highest readings assessed at 22, 23, and 24 hour following the trial drug administration) on days 2, 8, 15, and 29; change from baseline in trough and peak FEV1 and FVC; change from baseline in total and component (symptoms, activity, and impact) scores of the St. George’s Respiratory Questionnaire (SGRQ); improvement in Transition Dyspnoea Index (TDI); number of days with COPD symptoms; change from baseline in average morning and evening peak expiratory flow rate (PEFR); and use of rescue medication Time points: Two spirometry measurements at one hour interval at the screening visit and before randomisation on day one, and the averaged values provided the screening and baseline values. Efficacy spirometry measurements were taken at 0.5, 1, 2, 3, 4, 5, and 6 hours after the first and last dose (days 1 and 29 respectively) in addition to 22, 23, and 24 hours after the first dose and at each study visit (i.e. after the 7th, 14th, and 28th day of drug administration). Three acceptable readings were taken for each measurement at each time point according to the ATS/ERS recommendations on spirometric assessments |
|
| Notes | Full text publication Source of funding: Almirall, Barcelona, Spain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from correspondence): "a computer generated randomisation schedule was prepared to assign a treatment sequence to a randomisation number. The block size was determined in agreement with the clinical trial manager and the statistician and was not to be communicated to the investigators" |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "IVRS (or IWRS in some cases) was used to sequentially randomise patients to the intervention arms according to the randomisation ratio and the block size determined as mentioned above" |
| Blinding of participants and personnel (performance bias) Aclidinium versus placebo | Unclear risk | Quote: "double blind for aclidinium and placebo arms but open label for patients randomised to tiotropium arm" Comment: high risk of bias for comparison with tiotropium but low risk of bias for comparison with placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind for aclidinium and placebo arms but open label for patients randomised to tiotropium arm" Comment: high risk of bias for comparison with tiotropium but low risk of bias for comparison with placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: number of withdrawals were low and comparable across the study arms (aclidinium 25 μg 6.1%, aclidinium 50 μg 4.6%, aclidinium 100 μg 4.3%, aclidinium 200 μg 4.5%, aclidinium 400 μg 4.5%, tiotropium 6.2%, placebo 4.7%). Efficacy data are reported for the intention‐to‐treat (ITT) population, defined as all randomised patients who received at least one dose of study medication and who had a baseline and at least one post‐baseline efficacy assessment. The safety population comprised of all randomised patients who received at least one dose of study medication. Quote (from report): "missing spirometry data were handled as follows: for discontinuations because of lack of efficacy, the least favourable value prior to discontinuation was imputed; for other discontinuations, the last value carried forward approach was used. When only some values were missing from a test day, linear interpolation was used to estimate a value missing between two valid measurements. If values were missing for an entire visit or at the beginning or the end of an assessment period (i.e. 0.5, 6, 22, or 24 hour), time‐matched values of the previous available visit were used" |
| Selective reporting (reporting bias) | High risk | Comment: trough FVC on days 2,8,15 and 29; and change from baseline in average morning and evening peak expiratory flow rate (PEFR) were mentioned as secondary end points of the trial, but no data on these outcomes was reported in the published results |
| Other bias | Low risk | Comment: no apparent source of bias was observed |
Maltais 2011.
| Methods | Study design: randomised, double‐blind, parallel group, phase III study Study duration: six weeks Run‐in: two weeks Setting: multicentre trial Number of study centres and location: 52 study sites (42 sites in the United States and 10 additional sites in Canada) Date of study: July 2007 to October 2007 Randomisation: yes Blinding: double‐blind (subject, investigator, outcomes assessor) Withdrawals: stated |
|
| Participants | Number screened: 587 Number randomised: 181 Number in treatment group: 86 Number in control group: 95 Number of withdrawals (treatment/control): 5/17 Number completing trial (treatment/control): 81/78 Mean age (years) (treatment/control): 64.0/65.6 Gender (male/female): 52/34 (treatment), 53/42 (control) Caucasian (%) (treatment/control): 96.5/96.8 Inclusion criteria: males and females ≥ 40 years, current and ex‐smokers with a smoking history ≥ 10 pack‐years, clinical diagnosis of moderate to severe stable COPD (post‐bronchodilator FEV1/FVC < 70% and FEV1 ≥ 30% and < 80% predicted), functional residual capacity (FRC) ≥ 120% predicted at screening, and Baseline Dyspnoea Index (BDI) focal score ≤ seven Exclusion criteria: previous hospitalisation for an acute COPD exacerbation ≤ three months pre‐screening, or respiratory tract infection or COPD exacerbation six weeks pre‐screening, history of asthma, allergic rhinitis or atopy, contraindications to clinical exercise testing according to the American Thoracic Society (2003), cycled ≥ 20 min during constant work‐rate exercise tests during run‐in or participated in current or previous COPD rehabilitation programs ≤ six weeks pre‐randomisation Baseline characteristics of treatment/control groups: comparable | |
| Interventions | Intervention: inhaled aclidinium 200 μg once‐daily via a multidose dry powder inhaler (Genuair) Comparison: placebo once‐daily As‐needed therapy: levalbuterol (US) or salbutamol (Canada) was allowed ≥ six hours before each visit Concomitant medications: inhaled, oral or parenteral corticosteroids at doses ≤ 10 mg/day or 20 mg every other day were allowed if use was stable ≥ four weeks before screening. No other COPD medications were allowed. Oxygen therapy was allowed ≤ 15 hours/day but not within two hours of study visits |
|
| Outcomes |
Primary outcomes: change in exercise tolerance from baseline to week six Secondary outcomes: changes in exercise tolerance from baseline to day one (randomisation) and week three, and changes in trough FEV1, IC, FRC, IC/TLC from baseline to day one, week three and week six Other outcomes: changes from baseline in exercise measures of Inspiratory capacity (IC) and breathing pattern, dyspnoea and leg discomfort Time points: pulmonary function tests and cycle ergometry performed at study visits (screening, run‐in, randomisation, weeks three and six) |
|
| Notes | Full text publication Source of funding: Forest Laboratories, Inc. and Almirall, SA Study number: ClinicalTrials.gov NCT00500318 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from correspondence): "a computer generated randomisation schedule was prepared to assign a treatment sequence to a randomisation number. The block size was determined in agreement with the clinical trial manager and the statistician and was not to be communicated to the investigators" |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "IVRS (or IWRS in some cases) was used to sequentially randomise patients to the intervention arms according to the randomisation ratio and the block size determined as mentioned above" |
| Blinding of participants and personnel (performance bias) Aclidinium versus placebo | Low risk | Double‐blind (subject and investigator) Quote (from correspondence): "matching placebo of aclidinium bromide had the same external appearance with the same composition, except for the active ingredient. Blinding was applicable for all study outcomes" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind including blinding of outcomes assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: dropout was three times higher for placebo arm (aclidinium 5.8%, placebo 17.9%). Analyses of efficacy endpoints and safety outcomes were performed on the intent‐to‐treat (ITT) population, defined as patients who received one dose of study drug and with a baseline value and one post‐baseline assessment of exercise tolerance. However, no clear information on the method of imputation for the missing data |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol was not available, but the published reports included all pre‐specified outcomes |
| Other bias | Low risk | Comment: no apparent source of bias was observed |
NCT01572792.
| Methods | Study design: randomised, double‐blind, placebo‐controlled, parallel group, phase III, extension study of AUGMENT COPD (NCT01437397) Study duration: 28 weeks Follow‐up: four weeks Setting: multicentre trial Number of study centres and location: 208 study sites (179 sites in the United States, 11 in Australia, 10 in Canada and eight in New Zealand) Date of study: April 2012 to June 2013 Randomisation: yes Blinding: double‐blind (subject, caregiver, investigator, outcomes assessor) |
|
| Participants | Number randomised: 921 Number in treatment group: 205 (aclidinium 400 μg plus formoterol 6 μg), 184 (aclidinium 400 μg plus formoterol 12 μg), 194 (aclidinium 400 μg monotherapy) Number in control group: 192 (formoterol 12 μg) or 146 (placebo) Number of withdrawals: 26 (aclidinium 400 μg plus formoterol 6 μg), 29 (aclidinium 400 μg plus formoterol 12 μg), 29 (aclidinium 400 μg monotherapy), 32 (formoterol 12 μg) or 25 (placebo) Number completing trial: 179 (aclidinium 400 μg plus formoterol 6 μg), 155 (aclidinium 400 μg plus formoterol 12 μg), 165 (aclidinium 400 μg monotherapy), 160 (formoterol 12 μg) or 121 (placebo) The demographics remain the same as AUGMENT COPD, patients were kept on the same treatment arm Inclusion criteria: patients who completed the treatment phase of the lead‐in study, LAC‐MD‐31 (AUGMENT COPD) with no medical contraindication; compliance with LAC‐MD‐31 study procedures and IP dosing; agreed to participate in this six‐month extension study Exclusion criteria: no specific exclusion criteria |
|
| Interventions | Intervention: inhaled aclidinium/formoterol FDC high dose twice daily, inhaled aclidinium/formoterol FDC low dose twice daily, inhaled aclidinium 400 μg twice daily, Comparison: inhaled formoterol 12 μg twice daily, inhaled dose‐matched placebo twice daily |
|
| Outcomes | Primary outcomes: safety and tolerability, adverse events, clinical laboratory parameters, vital sign measurement, and electrocardiogram parameters at week 28 | |
| Notes | Source of support: Almirall SA, Barcelona, Spain, and Forest Laboratories, Inc, New York, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from correspondence): "a computer generated randomisation schedule was prepared to assign a treatment sequence to a randomisation number. The block size was determined in agreement with the clinical trial manager and the statistician and was not to be communicated to the investigators" |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "IVRS (or IWRS in some cases) was used to sequentially randomise patients to the intervention arms according to the randomisation ratio and the block size determined as mentioned above" |
| Blinding of participants and personnel (performance bias) Aclidinium versus placebo | Low risk | Double blind (subject, caregiver and investigator) Quote (from correspondence): "matching placebo of aclidinium bromide had the same external appearance with the same composition, except for the active ingredient. Blinding was applicable for all study outcomes" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind including blinding of outcomes assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: dropouts and the reasons were provided upon request. Withdrawal rates at the end of one year were high but relatively even across the groups (aclidinium 29.7%; placebo 37.4%, formoterol 29.8%, fixed dose combination with formoterol 6 μg 26.0%; fixed dose combination with formoterol 12 μg 28.1%) |
| Selective reporting (reporting bias) | Low risk | Comment: no published report available, but results for all specified outcomes were supplied on request |
| Other bias | Low risk | Comment: no apparent source of bias was observed |
Sliwinski 2010.
| Methods | Study design: randomised, double‐blind, placebo‐controlled, parallel group study Study duration: four weeks Randomisation: yes, method not stated Blinding: double‐blind |
|
| Participants | Number randomised: 566 Number in treatment group: 121 (aclidinium 200 μg plus formoterol 6 μg), 120 (aclidinium 200 μg plus formoterol 12 μg), 125 (aclidinium 200 μg plus formoterol 18 μg), 76 (aclidinium 200 μg monotherapy) Number in control group: 65 (formoterol 12 μg) or 59 (placebo) Inclusion criteria: moderate to severe COPD Exclusion criteria: not stated | |
| Interventions | Intervention: aclidinium 200 μg plus formoterol 6 μg, 12 μg or 18 μg or monotherapy with aclidinium 200 μg once daily via the Genuair, multidose dry powder inhaler Comparison: formoterol 12 μg or placebo once daily via the Genuair inhaler |
|
| Outcomes |
Primary outcome: change from baseline in normalised FEV1 area under the curve over 12 hours (AUC 0‐12h) at week four Other outcomes: safety (outcomes were not mentioned specifically) |
|
| Notes | This trial was available as abstract only Source of support: Almirall SA, Barcelona, Spain, and Forest Laboratories, Inc, New York, USA Study number: EUCTR2007‐004435‐30‐CZ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information on methods of randomisation though it is a randomised trial |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details were provided on allocation concealment |
| Blinding of participants and personnel (performance bias) Aclidinium versus placebo | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: insufficient information on withdrawals and the reasons in the abstract |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information regarding study end points and pre‐specified outcomes |
| Other bias | High risk | Comment: incomplete information for proper assessment. Publication bias cannot be ruled out as no published full text article was available though the abstract had been published since 2010 |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| D'Urzo 2013 | Extension study of ACCORD COPD I, which assessed two doses of aclidinium without comparator |
| D'Urzo 2013a | Pooled subgroup analysis of three trials |
| D'Urzo 2013b | Pooled analysis of three trials |
| de Miquel 2008 | Healthy subjects |
| Donohue 2013 | Pooled analysis of three trials |
| EUCTR2007‐000010‐36‐DE | Cross‐over trial |
| EUCTR2007‐003648‐31‐DE | Cross‐over trial |
| Ferguson 2013 | Pooled analysis of ACCORD COPD I, ACCORD COPD II and ATTAIN trials |
| Flach 2010 | Healthy subjects |
| Fuhr 2012 | Cross‐over trial |
| Gelb 2013 | Study of two doses of aclidinium without comparator |
| Jansat 2009 | Healthy subjects |
| Jansat 2009a | Healthy subjects |
| Joos 2010 | Cross‐over trial |
| Kerwin 2013 | Cross‐over trial |
| Lasseter 2008 | Healthy subjects |
| Lasseter 2012 | Healthy subjects |
| Magnussen 2009 | Assess the efficacy of Genuair inhaler |
| Magnussen 2010 | Cross‐over trial |
| NCT00435760 | Cross‐over trial |
| NCT00626522 | Study of three different doses of aclidinium bromide/formoterol combination versus placebo |
| NCT00706914 | Study of aclidinium bromide and formoterol fumarate fixed‐dose combination (FDC) versus formoterol fumarate |
| NCT01078623 | Cross‐over trial |
| NCT01437540 | Study of aclidinium bromide/formoterol fumarate combination versus formoterol fumarate |
| NCT01551888 | Study of aclidinium/formoterol fixed dose combination versus formoterol |
| NCT01908140 | Study of aclidinium bromide/formoterol fumarate combination versus salmeterol/fluticasone |
| NCT01915784 | Study on preference of inhalers |
| NCT02038829 | Cross‐over trial |
| NCT02039050 | Cross‐over trial |
| Ortiz 2010 | Healthy subjects |
| Schelfhout 2010 | Healthy subjects |
| Singh 2012 | Cross‐over trial |
| van der Palen 2013 | Preference study of Genuair versus HandiHaler inhalers |
| Vestbo 2010 | Cross‐over trial |
| Watz 2013 | Cross‐over trial |
Characteristics of studies awaiting assessment [ordered by study ID]
NCT01636401.
| Methods | Study design: placebo controlled, phase III study Study duration: 12 weeks Study centre and location: Seoul National University Hospital, Republic of Korea Date of study: August 2012 to May 2013 |
| Participants | Inclusion criteria: male or female ≥ 40 years, current or former smokers with a cigarette smoking history of at least 10 pack‐years, diagnosed with stable, moderate to severe COPD as defined by the GOLD (post‐bronchodilator FEV1/FVC ratio < 70% and FEV1 ≥ 30% to < 80% of the predicted value) Exclusion criteria: history of or current asthma, hospitalisation for an acute COPD exacerbation within three months prior to the first visit, any respiratory tract infection or COPD exacerbation in the six weeks before first visit, clinically significant respiratory conditions other than COPD |
| Interventions | Intervention: inhaled aclidinium bromide 400 μg twice daily Comparison: matching placebo twice daily |
| Outcomes |
Primary outcome: change from baseline in morning predose (trough) FEV1 at week 12 Secondary outcome: change from baseline in peak FEV1 at week 12 |
| Notes | Source of support: Daewoong Pharmaceutical Co Ltd |
Characteristics of ongoing studies [ordered by study ID]
ASCENT COPD.
| Trial name or title | Double‐blind, randomised, placebo‐controlled, parallel‐group, phase IV study to evaluate the effect of aclidinium bromide on long‐term cardiovascular safety and COPD exacerbations in patients with moderate to very severe COPD (ASCENT COPD) |
| Methods | Study design: randomised, double‐blind, placebo‐controlled, parallel‐group, phase IV study Study duration: 36 months Setting: multicentre trial Number of study centres and locations: 158 centres (152 in the United States and six in Canada) |
| Participants | Estimated enrolment: 4000 Inclusion criteria: male and females of ≥ 40 years of age; current or former smokers with at least 10 pack years of smoking; diagnosed with stable, moderate to very severe COPD according to GOLD criteria 2011 (post‐bronchodilator FEV1 < 70% predicted and FEV1/FVC ratio < 70% at first visit with at least one of the following criteria: a. documented cerebrovascular disease b. documented coronary artery disease c. documented peripheral vascular disease or history of claudication d. at least two artherothrombotic risk factors Exclusion criteria: significant diseases other than COPD or cardiovascular disease; unstable or life threatening cardiovascular disease or COPD; co‐morbid lung diseases; current treatment with a combination of LAMA and LABA/ICS therapy; planned lung transplant or lung volume reduction surgery; malignancies (except treated basal cell and squamous cell (skin) carcinoma); respiratory infection or COPD exacerbation within four weeks prior to screening; Uncontrolled infection from human immunodeficiency virus (HIV) and/or active hepatitis; drug or alcohol abuse within the past 12 months |
| Interventions | Intervention: inhaled aclidinium bromide 400 μg twice daily via a multi‐dose dry‐powder inhaler Comparison: dose matched placebo twice daily via a multi‐dose dry‐powder inhaler |
| Outcomes |
Primary outcomes: Time to first major adverse cardiovascular event (up to 36 months) Rate of moderate or severe COPD exacerbations per patient per year during the first year of treatment Secondary outcomes: Rate of hospitalisations due to COPD exacerbation per patient per year during the first year of treatment Time to first major adverse cardiovascular event (MACE) or other serious cardiovascular events of interest (up to 36 months) |
| Starting date | October 2013 |
| Contact information | Sandra Beaird, 1‐800‐678‐1605 ext 66297, FRXClinTrials@frx.com |
| Notes | Estimated primary completion date: January 2018 Source of support: Forest Laboratories, Inc, New York, USA |
Differences between protocol and review
All the references that were identified were in the English language and we did not require translations. We did not perform analyses using the Peto odds ratio because there were no rare events. We did not calculate standardised mean difference as no data from differing metric scales were combined. We analysed the outcome data for quality of life by the SGRQ total score and improvement in symptoms by the TDI focal score as mean changes from baseline (continuous data) as well as the percentage of patients who achieved a minimal clinically important difference in these scores (dichotomous data). Subgroup analysis for the dose of aclidinium, duration of therapy, baseline severity of COPD and concurrent therapy with theophylline were not conducted as planned.
Contributions of authors
HN and ZS wrote the protocol with suggestions and input on the methods from SM. HN and SM performed the search of additional resources, screened the search results and retrieved full text articles. HN and SM selected studies for inclusion. HN contacted the trial authors and manufacturer of aclidinium (Almirall) for unpublished data. HN and SM independently performed risk of bias assessment of included studies and extracted data. When any difference arose between HN and SM, ZS was consulted. HN performed data entry, which was checked by SM. HN performed data analysis with statistical expertise and advice from SM. HN drafted the manuscript with statistical input from SM and clinical input from ZS. All authors revised and agreed on the full review manuscript prior to submission for editorial review.
Sources of support
Internal sources
No sources of support supplied
External sources
Cochrane Airways Group, UK.
Declarations of interest
The authors have no connection with any organisations which could have caused a conflict of interest. We are doing this systematic review for academic purposes.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
ACCLAIM/COPD I {published and unpublished data}
- EUCTR2005‐005101‐39‐AT. A 52‐week randomised, double‐blind, parallel group, placebo controlled, multicentre clinical trial, to assess the efficacy and safety of 200 µg of the anticholinergic LAS 34273 compared to placebo, both administered once daily by inhalation, in the maintenance treatment of patients with moderate to severe, stable chronic obstructive pulmonary disease. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2005‐005101‐39 (accessed 1 May 2014).
- Jones PW, Agusti A, Chanez P, Magnussen H, Fabbri L, Maroni J, et al. A phase III study evaluating aclidinium bromide, a novel long‐acting antimuscarinic in patients with COPD: ACCLAIM/COPD 1 [Abstract]. American Thoracic Society International Conference; May 15‐20; San Diego. 2009:A6180 [Poster #207].
- Jones PW, Agusti A, Chanez P, Magnussen H, Fabbri L, Maroni J, et al. Efficacy and safety of aclidinium bromide, a novel long‐acting muscarinic antagonist, in patients with moderate to severe COPD [Abstract]. European Respiratory Society Annual Congress; Sep 12‐16; Vienna. 2009:[P2022].
- Jones PW, Chanez P, Agusti A, Magnussen H, Fabbri L, Caracta C, et al. A phase III study evaluating aclidinium bromide, a novel long‐acting antimuscarinic, in patients with COPD: ACCLAIM/COPD I [Abstract]. Thorax 2009;64 Suppl IV:A168 [P213]. [Google Scholar]
- Jones PW, Rennard SI, Agusti A, Chanez P, Magnussen H, Fabbri L, et al. Efficacy and safety of once‐daily aclidinium in chronic obstructive pulmonary disease. Respiratory Research 2011;12:55. [PUBMED: 21518460] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00363896. A trial assessing LAS34273 in moderate to severe stable chronic obstructive pulmonary disease (COPD). http://www.clinicaltrials.gov/ct2/show/study/NCT00363896 (accessed 1 May 2014).
ACCLAIM/COPD II {published and unpublished data}
- Jones PW, Rennard SI, Agusti A, Chanez P, Magnussen H, Fabbri L, et al. Efficacy and safety of once‐daily aclidinium in chronic obstructive pulmonary disease. Respiratory Research 2011;12:55. [PUBMED: 21518460] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00358436. Efficacy and safety of LAS 34273 in patients with moderate to severe stable chronic obstructive pulmonary disease (COPD). http://www.clinicaltrials.gov/ct2/show/study/NCT00358436 (accessed 1 May 2014).
- Rennard S, Donohue J, Bateman E, Gross N, Garcia Gil E, Caracta C. ACCLAIM/COPD II: efficacy and safety of aclidinium bromide; a novel long‐acting muscarinic antagonist in COPD patients, a phase III study [Abstract]. European Respiratory Society Annual Congress Sep 12‐16; Vienna. 2009:[E4351].
- Rennard S, Donohue J, Bateman E, Gross N, Garcia Gil E, Caracta C. Efficacy and safety of the novel, long‐acting antimuscarinic, aclidinium bromide, in COPD patients in a phase III study: ACCLAIM/COPD II [Abstract]. American Thoracic Society International Conference; May 15‐20; San Diego. 2009:A6178 [Poster #205].
ACCORD COPD I {published and unpublished data}
- D'Urzo A, Make BJ, Kerwin EM, Rekeda L, Sanz MT, Caracta C, et al. Safety and tolerability of twice daily aclidinium bromide in COPD patients: ACCORD COPD I [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A1614. [Google Scholar]
- D’Urzo A, Make B, Kerwin E, Rekeda L, Garcia Gil E, Caracta C, et al. ACCORD COPD I: Safety and tolerability of twice daily aclidinium bromide in COPD patients [Abstract]. European Respiratory Society Annual Congress; Sep 24‐28; Amsterdam. 2011; Vol. 38 (55):729s [P3998].
- Gelb A, Donohue J, D’Urzo A, Rekeda L, Garcia Gil E, Lateiner J. ACCORD COPD I: Twice‐daily aclidinium bromide improves quality of life and dyspnea in COPD patients [Abstract]. European Respiratory Society Annual Congress; Sep 24‐28; Amsterdam 2011;38(55):149s [P876]. [Google Scholar]
- Gelb AF, Donohue JF, D'Urzo A, Rekeda L, Jarreta D, Lateiner J. Improvements in quality of life and dyspnea in COPD patients with twice‐daily aclidinium [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A1616. [Google Scholar]
- Kerwin E, D'Urzo A, Gelb A, Lakkis H, Garcia Gil E, Caracta C. Twice‐daily aclidinium bromide in COPD patients: Efficacy and safety results from ACCORD COPD I [Abstract]. European Respiratory Society Annual Congress; Sep 18‐22; Barcelona. 2010:[P1235].
- Kerwin E, D’Urzo A, Gelb A, Lakkis H, Garcia Gil E, Caracta C. Efficacy and safety of twice‐daily aclidinium bromide in patients with COPD: results from ACCORD COPD 1 [Abstract]. Chest 2010;138(4):469A. [Google Scholar]
- Kerwin E, Rennard S, Gelb A, Rekeda L, Garcia Gil E, Caracta C. ACCORD COPD I: Improvements in nighttime symptoms and rescue medication use in COPD with twice‐daily aclidinium bromide [Abstract]. European Respiratory Society Annual Congress; Sep 24‐28; Amsterdam. 2011; Vol. 38, issue 55:149s [P873].
- Kerwin EM, D'Urzo AD, Gelb AF, Lakkis H, Garcia Gil E, Caracta CF. Efficacy and safety of a 12‐week treatment with twice‐daily aclidinium bromide in COPD patients (ACCORD COPD I). COPD 2012;9(2):90‐101. [PUBMED: 22320148] [DOI] [PubMed] [Google Scholar]
- Kerwin EM, Rennard SI, Gelb AF, Rekeda L, Garcia Gil E, Caracta C. Twice‐daily aclidinium bromide in COPD patients: nighttime symptoms and rescue medication use in ACCORD COPD I [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A1592. [Google Scholar]
- NCT00891462. Efficacy and safety of aclidinium bromide for treatment of moderate to severe chronic obstructive pulmonary disease (COPD) (LAS‐MD‐33). http://www.clinicaltrials.gov/ct2/show/study/NCT00891462 (accessed 1 May 2014).
ACCORD COPD II {published and unpublished data}
- NCT01045161. To assess the long‐term safety, efficacy and tolerability of inhaled aclidinium bromide in the treatment of moderate‐to‐severe chronic obstructive pulmonary disease (COPD) (LAS‐MD‐38). http://clinicaltrials.gov/ct2/show/study/NCT01045161 (accessed 1 May 2014).
- Rennard SI, Scanlon PD, Ferguson GT, Rekeda L, Maurer BT, Garcia Gil E, et al. ACCORD COPD II: A randomised clinical trial to evaluate the 12‐week efficacy and safety of twice‐daily aclidinium bromide in chronic obstructive pulmonary disease patients. Clinical Drug Investigation 2013;33(12):893‐904. [DOI] [PubMed] [Google Scholar]
ACLIFORM {published and unpublished data}
- EUCTR2011‐001524‐38‐GB. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed‐dose combinations compared with individual components and placebo when administered to patients with stable chronic obstructive pulmonary disease. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2011‐001524‐38 (accessed 1 May 2014).
- NCT01462942. Long‐term efficacy and safety of aclidinium/formoterol fixed‐dose combination. http://clinicaltrials.gov/show/NCT01462942 (accessed 1 May 2014).
- Singh D, Jones P, Bateman E, Korn S, Serra C, Molins E, et al. Evaluation of the efficacy and safety of two doses of aclidinium and formoterol in fixed‐dose combination in patients with COPD: the ACLIFORM study. Chest 2014;145:375A. [DOI] [PMC free article] [PubMed] [Google Scholar]
ATTAIN {published and unpublished data}
- Agusti A, Jones PW, Bateman E, Singh D, Lamarca R, Miquel G, et al. Improvement in symptoms and rescue medication use with aclidinium bromide in patients with chronic obstructive pulmonary disease: results from ATTAIN [Abstract]. European Respiratory Society Annual Congress; Sep 24‐28; Amsterdam. 2011; Vol. 38, issue 55:149s [P874].
- Bateman ED, Singh D, Jones PW, Agusti A, Lamarca R, Miquel G, et al. The ATTAIN study: safety and tolerability of aclidinium bromide in chronic obstructive pulmonary disease [Abstract]. European Respiratory Society Annual Congress; Sep 24‐28; Amsterdam. 2011; Vol. 38, issue 55:730s [P4005].
- EUCTR2009‐011600‐27‐CZ. Efficacy and safety of aclidinium bromide at two dose levels vs placebo when administered to patients with moderate to severe chronic obstructive pulmonary disease (COPD). https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2009‐011600‐27.
- Jones P, Agusti A, Bateman E, Singh D, Lamarca R, Miquel G, et al. Aclidinium bromide in patients with chronic obstructive pulmonary disease (COPD): Reduction in exacerbations as defined by health‐care utilisation and the EXACT diary card [Abstract]. Chest 2011;140(4):529A. [Google Scholar]
- Jones P, Agusti A, Bateman E, Singh D, Lamarca R, Miquel G, et al. Aclidinium bromide in patients with chronic obstructive pulmonary disease: Improvement in symptoms and health status in the ATTAIN Study [Abstract]. Chest 2011;140(4):547A. [Google Scholar]
- Jones P, Bateman E, Singh D, Agusti A, Lamarca R, Miquel G, et al. ATTAIN: Efficacy and safety of twice‐daily aclidinium bromide in chronic obstructive pulmonary disease (COPD) [Abstract]. Chest 2011;140(4):975A. [Google Scholar]
- Jones PW, Agusti A, Bateman ED, Singh D, Lamarca R, Miquel G, et al. Aclidinium bromide in patients with chronic obstructive pulmonary disease: Efficacy and safety results from ATTAIN [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A6350. [Google Scholar]
- Jones PW, Agusti A, Bateman ED, Singh D, Lamarca R, Miquel G, et al. Aclidinium bromide in patients with chronic obstructive pulmonary disease: Improvement in health status in ATTAIN [Abstract]. European Respiratory Society Annual Congress, Amsterdam, The Netherlands, September 24‐28 2011;38(55):150s [P877]. [Google Scholar]
- Jones PW, Chuecos F, Lamarca R, Singh D, Agusti A, Bateman ED, et al. Unreported exacerbations of chronic obstructive pulmonary disease are associated with a reduction in health status: results from the ATTAIN study. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting abstracts):A6072. [Google Scholar]
- Jones PW, Singh D, Agusti A, Bateman ED, Lamarca R, Miquel G, et al. Aclidinium bromide reduces COPD exacerbations as defined by healthcare utilisation and EXACT: results from ATTAIN [Abstract]. European Respiratory Society Annual Congress, Vienna, Austria, September 1‐5. 2012; Vol. 40, issue Suppl 56:9s [195].
- Jones PW, Singh D, Bateman ED, Agusti A, Lamarca R, Miquel G, et al. Efficacy and safety of twice‐daily aclidinium bromide in COPD patients: the ATTAIN study. European Respiratory Journal 2012;40(4):830‐6. [PUBMED: 22441743] [DOI] [PubMed] [Google Scholar]
- NCT01001494. Efficacy and safety of aclidinium bromide at two dose levels versus placebo administered in chronic obstructive pulmonary disease (COPD) patients. http://www.clinicaltrials.gov/ct2/show/study/NCT01001494 (accessed 1 May 2014).
- Singh D, Bateman ED, Jones PW, Agusti A, Lamarca R, Miquel G, et al. The ATTAIN study: bronchodilatory effect of aclidinium bromide in chronic obstructive pulmonary disease (COPD) [Abstract]. European Respiratory Society Annual Congress; Sep 24‐28; Amsterdam. 2011; Vol. 38, issue 55:149s [P875].
- Singh D, Jones PW, Bateman ED, Agusti A, Lamarca R, Miquel G, et al. ATTAIN: Twice‐daily aclidinium bromide in patients with moderate to severe chronic obstructive pulmonary disease [Abstract]. Thorax 2011;66 Suppl 4:A171 [P255]. [Google Scholar]
AUGMENT COPD {published and unpublished data}
- D'Urzo A, Mergel V, Leselbaum A, Caracta C. Efficacy and safety of fixed‐dose combination aclidinium bromide/formoterol fumarate in patients with COPD: results from the AUGMENT COPD trial. Chest 2013;144(4):1025A. [Google Scholar]
- D'Urzo A, Rennard S, Mergel V, Garcia Gil E, Leselbaum A, Caracta C. The AUGMENT COPD trial: efficacy and safety of a fixed‐dose combination of aclidinium bromide and formoterol fumarate in COPD patients. Chest 2014;145:426A. [Google Scholar]
- NCT01437397. Efficacy, safety and tolerability of aclidinium bromide/formoterol fumarate compared with formoterol fumarate in patients with moderate to severe chronic obstructive pulmonary disease (COPD) (LAC). http://clinicaltrials.gov/ct2/show/study/NCT01437397 (accessed 1 May 2014).
Beier 2013 {published and unpublished data}
- Beier J, Kirsten AM, Mroz R, Segarra R, Chuecos F, Caracta C, et al. Efficacy and safety of aclidinium bromide compared with tiotropium and placebo in patients with moderate‐to‐severe COPD: a phase IIIb study. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier J, Kirsten AM, Mroz R, Segarra R, Chuecos F, Caracta C, et al. Efficacy of aclidinium bromide compared with tiotropium and placebo in patients with moderate to severe COPD: a phase IIIb study [Abstract]. Thorax 2012;67 Suppl 2:A26 [S51]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier J, Kirsten AM, Mroz R, Segarra R, Chuecos F, Caracta C, et al. Improvements in COPD symptoms and rescue medication use with aclidinium bromide compared with tiotropium and placebo: a phase IIIb study. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A4276. [Google Scholar]
- Beier J, Kirsten AM, Mruz R, Segarra R, Chuecos F, Caracta C, et al. Efficacy and safety of aclidinium bromide compared with placebo and tiotropium in patients with moderate‐to‐severe chronic obstructive pulmonary disease: results from a six‐week, randomised, controlled phase IIIb study. COPD 2013;10(4):511‐22. [PUBMED: 23819698] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier J, Kirsten AM, Mróz R, Segarra R, Chuecos F, Caracta C, et al. Efficacy and safety of aclidinium bromide vs placebo and tiotropium in COPD: a phase IIIb study [Abstract]. European Respiratory Society Annual Congress; Sep 7‐11; Barcelona. 2013; Vol. 42 Suppl 57:4s [185]. [DOI] [PMC free article] [PubMed]
- Beier J, Kirsten AM, Mróz R, Segarra R, Chuecos F, Caracta C, et al. Improvement in COPD symptoms with aclidinium bromide vs placebo and tiotropium: A phase IIIb study [Abstract]. European Respiratory Society Annual Congress; Sept 7‐11; Barcelona. 2013; Vol. 42 Suppl 57:4s [184].
- EUCTR2011‐000834‐12‐DE. A multiple dose, double‐blind, double‐dummy, placebo controlled, parallel clinical trial to assess the efficacy and safety of twice daily inhaled aclidinium bromide 400 µg compared to placebo and to tiotropium bromide in patients with stable moderate to severe chronic obstructive pulmonary disease (COPD). https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2011‐000834‐12 (accessed 1 May 2014).
- NCT01462929. Efficacy and safety of aclidinium bromide 400 μg compared to placebo and to tiotropium bromide in patients with stable moderate to severe chronic obstructive pulmonary disease (COPD). http://www.clinicaltrials.gov/ct2/show/study/NCT01462929 (accessed 1 May 2014).
Chanez 2010 {published and unpublished data}
- Chanez P, Burge PS, Dahl R, Creemers J, Chuchalin A, Lamarca R, et al. Aclidinium bromide provides long‐acting bronchodilation in patients with COPD. Pulmonary Pharmacology and Therapeutics 2010;23(1):15‐21. [PUBMED: 19683590] [DOI] [PubMed] [Google Scholar]
- Chanez P, Burge S, Dahl R, Creemers J, Lamarca R, Garcia GE. Once‐daily administration of aclidinium bromide, a novel, long‐acting anticholinergic: a phase II, dose‐finding study [Abstract]. American Thoracic Society International Conference; May 16‐21; Toronto. 2008:[A286].
- Charez P, Burge S, Creemers J, Lamarca R, Garcia GE. Once daily administration of aclidinium bromide a novel long acting anticholinergic a phase II dose finding study [Abstract]. European Respiratory Society Annual Congress; Oct 4‐8; Berlin. 2008:[2736].
Maltais 2011 {published and unpublished data}
- Casaburi R, Maltais F, Celli B, Porszasz J, Garcia Gil E, Caracta C. Aclidinium bromide improves exercise endurance and decreases exertional dyspnoea in patients with COPD [Abstract]. European Respiratory Society Annual Congress, Barcelona, Spain, September 18‐22. 2010:[5558].
- Celli B, Maltais F, Casaburi R, Porszasz J, Garcia Gil E, Caracta C. Aclidinium bromide improves resting lung function in patients with moderate to severe COPD [Abstract]. European Respiratory Society Annual Congress, Barcelona, Spain, September 18‐22. 2010:[P1183].
- Maltais F, Celli B, Casaburi R, Porszasz J, Jarreta D, Seoane B, et al. Aclidinium bromide improves exercise endurance and lung hyperinflation in patients with moderate to severe COPD. Respiratory Medicine 2011;105(4):580‐7. [PUBMED: 21183326] [DOI] [PubMed] [Google Scholar]
- Maltais F, Celli B, Porszasz J, Casaburi R, Garcia GE, Caracta C. Aclidinium bromide improves exercise endurance, dyspnea and inspiratory capacity in patients with moderate to severe COPD [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A4428. [Google Scholar]
- NCT00500318. A study of exercise endurance and lung hyperinflation in patients with moderate to severe chronic obstructive pulmonary disease (COPD). http://www.clinicaltrials.gov/ct2/show/study/NCT00500318 (accessed 1 May 2014).
NCT01572792 {unpublished data only}
- NCT01572792. Efficacy, safety and tolerability of two fixed dose combinations of aclidinium bromide/formoterol fumarate, aclidinium bromide, formoterol fumarate and placebo for 28‐weeks treatment in patients with moderate to severe stable chronic obstructive pulmonary disease (COPD). http://clinicaltrials.gov/ct2/show/NCT01572792 (accessed 1 May 2014).
Sliwinski 2010 {published and unpublished data}
- EUCTR2007‐004435‐30‐CZ. A randomised, four‐week, placebo‐controlled, double‐blind, six arm parallel group, dose‐finding clinical trial, to assess the efficacy and safety of three different doses of formoterol (6, 12 & 18µg) combined with the inhaled anticholinergic aclidinium bromide 200 µg, aclidinium bromide 200 µg monotherapy and formoterol 12 µg monotherapy all administered once daily by inhalation via Almirall inhaler in patients with stable moderate to severe chronic obstructive pulmonary disease. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2007‐004435‐30 (accessed 1 May 2014).
- Sliwinski P, Perng D‐W, Chuchalin A, Jones PW. Efficacy and safety of once‐daily aclidinium bromide 200 µg in combination with formoterol in patients with COPD [Abstract]. Thorax 2010;65 Suppl 4:P137. [Google Scholar]
References to studies excluded from this review
D'Urzo 2013 {published and unpublished data}
- D'Urzo A, Kerwin E, Donohue J, Rennard S, Gelb A, Lakkis H, et al. Effects of twice‐daily aclidinium bromide in COPD patients: A long‐term extension of ACCORD‐COPD I [Abstract]. European Respiratory Society Annual Congress, Sep 1‐5; Vienna. 2012; Vol. 40, issue Suppl 56:528s [P2890].
- D'Urzo A, Kerwin E, Rennard S, He T, Garcia Gil E, Caracta C. Improvements in lung function with twice‐daily aclidinium bromide: results of a long‐term, phase 3 trial in patients with chronic obstructive pulmonary disease [Abstract]. Chest 2012;142(4_Meeting abstracts):740A. [Google Scholar]
- D'Urzo A, Kerwin E, Rennard S, He T, Garcia Gil E, Caracta C. One‐year extension study of ACCORD COPD I: safety and efficacy of two doses of twice‐daily aclidinium bromide in patients with COPD. COPD 2013;10(4):500‐10. [PUBMED: 23679347] [DOI] [PubMed] [Google Scholar]
- D'Urzo A, Kerwin EM, Donohue JF, Rennard SI, Gelb AF, Lakkis H. Long‐term extension study of ACCORD COPD I: effects of two doses of twice‐daily aclidinium bromide in COPD patients [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A2913. [Google Scholar]
- Gelb A, D'Urzo A, Tashkin D, Zhong X, Gil EG, Caracta C. Effects of aclidinium bromide in patients with chronic obstructive pulmonary disease: clinically significant improvements in health status in two one‐year studies. Chest 2012;142(4_Meeting abstracts):691A. [Google Scholar]
- NCT00970268. Long‐term extension study of the safety, tolerability, and efficacy of aclidinium bromide in patients with moderate to severe chronic obstructive pulmonary disease (COPD) (LAS‐MD‐36). http://www.clinicaltrials.gov/ct2/show/study/NCT00970268 (accessed 1 May 2014).
D'Urzo 2013a {unpublished data only}
- D'Urzo A, Jones P, Ferguson G, Rekeda L, Gil EG, Caracta C. Aclidinium bromide improves lung function in a wide range of patients with moderate to severe COPD: Pooled subgroup analysis of the ACCORD COPD I and II and ATTAIN trials. Chest 2013;144(4_Meeting abstracts):746A. [Google Scholar]
D'Urzo 2013b {unpublished data only}
- D'Urzo A, Rennard S, Jones P, Rekeda L, Gil EG, Caracta C. Exposure‐adjusted anticholinergic adverse events following long‐term treatment with aclidinium bromide in patients with COPD. Chest 2013;144(4_Meeting abstracts):717A. [Google Scholar]
de Miquel 2008 {unpublished data only}
- Miquel G, Schrodter A, Miletzki B, Gurniak M, Serra C, Jansat JM. Low systematic exposure to aclidinium bromide, a novel long‐acting anticholinergic, after multiple doses [Abstract]. American Thoracic Society International Conference; May 16‐21; Toronto. 2008:Poster #F53.
Donohue 2013 {unpublished data only}
- Donohue J, Tashkin D, Ferguson G, Kowey P, Rekeda L, Shrestha P, et al. Long‐term cardiovascular safety of aclidinium bromide in patients with COPD. Chest 2013;144(4_Meeting abstracts):716A. [Google Scholar]
EUCTR2007‐000010‐36‐DE {unpublished data only}
- A multiple dose, double‐blind, double‐dummy, three period cross‐over, placebo controlled clinical trial to assess the efficacy and safety of once daily inhaled aclidinium bromide 200 µg given either in the morning or in the evening in patients with stable moderate to severe chronic obstructive pulmonary disease (COPD). https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2007‐000010‐36 (accessed 1 May 2014).
EUCTR2007‐003648‐31‐DE {unpublished data only}
- A phase IIa, randomised, multicentre, evaluator‐blinded, four‐way crossover clinical trial to study the pharmacokinetics, safety, tolerability and effects on lung function of one day treatment of formoterol 12 µg once daily delivered by two different dry powder inhalers (Aerolizer® and Almirall inhaler), of the fixed dose combination formoterol 12 µg + aclidinium bromide 200 µg once daily delivered by Almirall inhaler, and of formoterol 12 µg twice daily delivered by Aerolizer®, in moderate to severe chronic obstructive pulmonary disease patients. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2007‐003648‐31 (accessed 1 May 2014).
Ferguson 2013 {unpublished data only}
- Ferguson G, Kerwin E, Singh D, Kowey P, Rekeda L, Shrestha P, et al. Cardiovascular safety of aclidinium bromide in COPD: pooled results from three placebo‐controlled studies. Chest 2013;144(4_Meeting abstracts):715A. [Google Scholar]
Flach 2010 {unpublished data only}
- Flach S, Jansat JM, Ho J, Garcia GE, Caracta C, Ortiz S. Metabolism and excretion of aclidinium bromide following intravenous administration of [14C] aclidinium bromide in healthy subjects [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A4463. [Google Scholar]
Fuhr 2012 {published and unpublished data}
- Fuhr R, Magnussen H, Ribera A, Kirsten A, Falques M, Caracta C, et al. Efficacy and safety of twice‐daily aclidinium bromide compared with tiotropium and placebo in patients with moderate to severe COPD [Abstract]. European Respiratory Society Annual Congress; 2010 September 18‐22; Barcelona, Spain. 2010:[P1236].
- Fuhr R, Magnussen H, Ribera A, Kirsten A‐M, Falques M, Caracta C, et al. Efficacy and safety of twice‐daily aclidinium bromide 400 µg compared with placebo and tiotropium 18 µg once daily in moderate to severe COPD patients [Abstract]. Chest 2010;138(4):465A. [DOI] [PubMed] [Google Scholar]
- Fuhr R, Magnussen H, Sarem K, Llovera AR, Kirsten AM, Falques M, et al. Efficacy of aclidinium bromide 400 µg twice daily compared with placebo and tiotropium in patients with moderate to severe COPD. Chest 2012;141(3):745‐52. [PUBMED: 21903737] [DOI] [PubMed] [Google Scholar]
- NCT00868231. Efficacy of aclidinium bromide administered in chronic obstructive pulmonary disease (COPD) patients. http://www.clinicaltrials.gov/ct2/show/NCT00868231 (accessed 1 May 2014).
Gelb 2013 {published and unpublished data}
- Gelb A, D'Urzo A, Tashkin D, Zhong X, Gil EG, Caracta C. Effects of aclidinium bromide in patients with chronic obstructive pulmonary disease: clinically significant improvements in health status in two 1‐year studies. Chest 2012;142(4_Meeting abstracts):691A. [Google Scholar]
- Gelb A, Tashkin D, Make B, Zhong X, Garcia Gil E, Caracta C. Long‐term safety and efficacy of twice‐daily aclidinium bromide in patients with COPD. Respiratory Medicine 2013;107(12):1957‐65. [DOI] [PubMed] [Google Scholar]
- Gelb A, Tashkin D, Make B, Zhong X, Garcia Gil E, Caracta C. Long‐term safety of twice‐daily aclidinium bromide in COPD patients: a one‐year, double‐blind study [Abstract]. European Respiratory Society Annual Congress; Sep 1‐5; Vienna. 2012; Vol. 40 (Suppl 56):376s [P2118].
- Gelb AF, Make BJ, Tashkin DP, Zhong X, Garcia GE, Caracta C. Long‐term efficacy and safety of twice‐daily aclidinium bromide in COPD patients: a one‐year Study [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185 (Meeting Abstracts):A2256. [Google Scholar]
- NCT01044459. Long‐term safety, tolerability and efficacy of aclidinium bromide in patients with moderate to severe chronic obstructive pulmonary disease (COPD) (LAS‐MD‐35). http://www.clinicaltrials.gov/ct2/show/study/NCT01044459 (accessed 1 May 2014).
- Tashkin D, Gelb A, Make B, Zhong X, Garcia Gil E, Caracta C. Long‐term efficacy of twice‐daily aclidinium bromide in COPD patients: a one‐year study [Abstract]. European Respiratory Society Annual Congress; Sep 1‐5; Vienna. 2012; Vol. 40 (Suppl 56):528s [P2893].
Jansat 2009 {published data only}
- Jansat JM, Lamarca R, Garcia Gil E, Ferrer P. Safety and pharmacokinetics of single doses of aclidinium bromide, a novel long‐acting, inhaled antimuscarinic, in healthy subjects. International Journal of Clinical Pharmacology and Therapeutics 2009;47(7):460‐8. [PUBMED: 19640353] [DOI] [PubMed] [Google Scholar]
Jansat 2009a {published data only}
- Jansat JM, Lamarca R, Miquel G, Schrodter A, Miletzki B, Gurniak M. Safety and pharmacokinetics of multiple doses of aclidinium bromide, a novel long‐acting muscarinic antagonist for the treatment of chronic obstructive pulmonary disease, in healthy participants. Journal of Clinical Pharmacology 2009;49(10):1239‐46. [PUBMED: 19592595] [DOI] [PubMed] [Google Scholar]
Joos 2010 {published and unpublished data}
- Joos GF, Schelfhout VJ, Pauwels RA, Kanniess F, Magnussen H, Lamarca R, et al. Bronchodilatory effects of aclidinium bromide, a long‐acting muscarinic antagonist, in COPD patients. Respiratory Medicine 2010;104(6):865‐72. [PUBMED: 20044242] [DOI] [PubMed] [Google Scholar]
- Joss G, Schelflout V, Kanniess F, Ludwig‐Segpiel A, Garcia Gil E, Massana Montejo E. Bronchodilator effects of aclidinium bromide a novel long‐acting anticholinergic in COPD patients a phase II study [Abstract]. European Respiratory Journal 2007;30 Suppl 51:210s [1299]. [Google Scholar]
Kerwin 2013 {unpublished data only}
- EUCTR2009‐015901‐38‐DE. Efficacy, safety and tolerability of two fixed‐dose combinations of aclidinium bromide with two doses of formoterol fumarate compared with aclidinium bromide, formoterol fumarate and placebo all administered twice daily in stable, moderate to severe chronic obstructive pulmonary disease patients. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2009‐015901‐38 (accessed 1 May 2014).
- Kerwin E, Lapidus R, Leselbaum A, Ortiz S, Rowe P, Caracta C. Dose‐ranging study of two fixed‐dose combinations of twice‐daily aclidinium bromide plus formoterol in patients with moderate to severe COPD. Chest 2013;144(4_Meeting Abstracts):747A. [Google Scholar]
- NCT01049360. Efficacy and safety study of two fixed‐dose combinations of aclidinium bromide with formoterol fumarate compared with aclidinium bromide, formoterol fumarate and placebo (LAC‐MD‐27). http://clinicaltrials.gov/ct2/show/NCT01049360 (accessed 1 May 2014).
Lasseter 2008 {unpublished data only}
- Lasseter KC, Aubets J, Chuecos F, Garcia GE. Aclidinium bromide, a long‐acting antimuscarinic, does not affect QT interval in healthy subjects. Journal of Clinical Pharmacology 2011;51(6):923‐32. [DOI] [PubMed] [Google Scholar]
- Lasseter KC, Aubets J, Garcia GE. Aclidinium bromide a novel long acting anticholinergic does not affect QT interval in healthy subjects [Abstract]. American Thoracic Society International Conference; May 16‐21; Toronto. 2008:A655[#F54].
Lasseter 2012 {published and unpublished data}
- Lasseter K, Dilzer S, Jansat JM, Garcia GE, Caracta CF, Ortiz S. Safety and pharmacokinetics of multiple doses of aclidinium bromide administered twice daily in healthy volunteers. Pulmonary Pharmacology and Therapeutics 2012;25(2):193‐9. [PUBMED: 22366196] [DOI] [PubMed] [Google Scholar]
- Lasseter K, Dilzer S, Jansat JM, Garcia Gil E, Caracta C, Ortiz S. Safety and pharmacokinetics of multiple doses of aclidinium bromide administered twice daily in healthy volunteers [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A1615. [DOI] [PubMed] [Google Scholar]
Magnussen 2009 {published data only}
- Magnussen H, Watz H, Zimmermann I, Macht S, Greguletz R, Falques M, et al. Peak inspiratory flow through the Genuair inhaler in patients with moderate or severe COPD. Respiratory Medicine 2009;103(12):1832‐7. [PUBMED: 19651504] [DOI] [PubMed] [Google Scholar]
Magnussen 2010 {unpublished data only}
- EUCTR2008‐006886‐10‐DE. A multiple dose, double blind, double‐dummy, two‐week three way cross‐over, placebo‐controlled clinical trial to assess the efficacy and safety of twice daily inhaled aclidinium bromide 400 µg compared to placebo and to an active comparator in patients with stable moderate to severe chronic obstructive pulmonary disease (COPD). https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2008‐006886‐10 (accessed 1 May 2014).
- Magnussen H, Ribera LA, Kirsten AM, Falques M, Caracta C, Garcia GE. Efficacy and safety of aclidinium bromide 400 {micro}g twice daily compared with placebo and tiotropium in patients with moderate to severe COPD [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A4440. [Google Scholar]
NCT00435760 {unpublished data only}
- EUCTR2005‐005804‐17‐NL. A single dose, double‐blind, double‐dummy, three period cross‐over, placebo controlled clinical trial to assess the rate of onset of action of inhaled LAS 34273 200 µg compared to placebo and tiotropium 18 µg in patients with chronic obstructive pulmonary disease (COPD). http://apps.who.int/trialsearch/Trial.aspx?TrialID=EUCTR2005‐005804‐17‐NL or https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2005‐005804‐17.
- NCT00435760. Clinical trial to assess rate of onset of bronchodilator action in severe stable chronic obstructive pulmonary disease (COPD) patients. http://clinicaltrials.gov/ct2/show/study/NCT00435760 (accessed 2 May 2014).
NCT00626522 {unpublished data only}
- NCT00626522. Aclidinium/formoterol fixed combination dose finding study. http://clinicaltrials.gov/ct2/show/NCT00626522 (accessed 2 May 2014).
NCT00706914 {unpublished data only}
- NCT00706914. Comparison of aclidinium bromide and formoterol fumarate in patients with moderate to severe chronic obstructive pulmonary disease (COPD). http://clinicaltrials.gov/ct2/show/NCT00706914 (accessed 2 May 2014).
NCT01078623 {unpublished data only}
- NCT01078623. Efficacy and safety of two fixed dose combinations of aclidinium bromide with formoterol fumarate (ALIGHT‐COPD). http://clinicaltrials.gov/show/NCT01078623 (accessed 2 May 2014).
NCT01437540 {published and unpublished data}
- Make B, Donohue J, Zhong X, Leselbaum A, Caracta C. Long‐term safety of a fixed‐dose combination of aclidinium bromide/formoterol fumarate in patients with stable moderate to severe COPD. Chest 2014;145:386A. [Google Scholar]
- NCT01437540. Safety and tolerability of aclidinium bromide/formoterol fumarate compared with formoterol fumarate in patients with moderate to severe chronic obstructive pulmonary disease (LAC). http://clinicaltrials.gov/ct2/show/study/NCT01437540 (accessed 2 May 2014).
NCT01551888 {unpublished data only}
- NCT01551888. Pharmacokinetic, safety and tolerability study of aclidinium/formoterol fixed dose combination and formoterol in patients with moderate to severe chronic obstructive pulmonary disease (COPD). http://clinicaltrials.gov/ct2/show/NCT01551888 (accessed 2 May 2014).
NCT01908140 {unpublished data only}
- EUCTR2013‐000116‐14‐HU. A randomised, double‐blind, double‐dummy, active‐controlled study evaluating the efficacy, safety and tolerability of twice‐daily aclidinium bromide /formoterol fumarate compared with twice‐daily salmeterol/fluticasone propionate for 24‐weeks treatment in symptomatic patients with chronic obstructive pulmonary disease (COPD). https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2013‐000116‐14 (accessed 2 May 2014).
- NCT01908140. Study of aclidinium bromide/formoterol fumarate compared with salmeterol/fluticasone propionate in patients with chronic obstructive pulmonary disease (COPD). http://clinicaltrials.gov/ct2/show/NCT01908140 (accessed 2 May 2014).
NCT01915784 {unpublished data only}
- NCT01915784. Preference, satisfaction and ease of use of Genuair® (Pressair™) and Breezhaler® (Neohaler™) inhalers in patients with COPD. http://clinicaltrials.gov/ct2/show/NCT01915784 (accessed 2 May 2014).
NCT02038829 {unpublished data only}
- NCT02038829. A dose‐range finding study of SUN‐101 in subjects with moderate to severe COPD (GOLDEN 6). http://clinicaltrials.gov/ct2/show/NCT02038829 (accessed 2 May 2014).
NCT02039050 {unpublished data only}
- NCT02039050. Evaluation of long‐acting muscarinic antagonists in COPD (MAN04). http://clinicaltrials.gov/ct2/show/NCT02039050 (accessed 2 May 2014).
Ortiz 2010 {unpublished data only}
- Ortiz S, Flach S, Caracta C, Garcia GE, Jansat JM. Safety and tolerability of aclidinium bromide administered intravenously and absolute bioavailability of inhaled aclidinium bromide in healthy subjects [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A4464. [Google Scholar]
- Ortiz S, Flach S, Caracta C, Garcia Gil E, Jansat J. Absolute bioavailability of inhaled aclidinium bromide and safety and tolerability of aclidinium bromide administered intravenously in healthy subjects [Abstract]. European Respiratory Society Annual Congress; Sep 18‐22; Barcelona. 2010:[P1180].
Schelfhout 2010 {published and unpublished data}
- Schelfhout VJ, Ferrer P, Jansat JM, Peris F, Gil EG, Pauwels RA, et al. Activity of aclidinium bromide, a new long‐acting muscarinic antagonist: a phase I study. British Journal of Clinical Pharmacology 2010;69(5):458‐64. [PUBMED: 20573081] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelfhout VJ, Joos GF, Ferrer P, Kannies F, Luria X, Richter K, et al. Activity of LAS34273, a new long acting anticholinergic antagonist, in COPD patients [abstract]. American Thoracic Society 99th International Conference; May 16‐21; Seattle. 2003; Vol. B024 Poster 44.
- Schelfhout VJ, Joos GF, Garcia Gil E, Montejo EM. Bronchodilator/broncho‐protective effects of aclidinium bromide, a novel long‐acting anticholinergic:a phase I study. European Respiratory Journal 2007;30 Suppl 51:356S. [Google Scholar]
Singh 2012 {published and unpublished data}
- Efficacy and safety of three doses of aclidinium bromide compared to placebo and to an active comparator in chronic obstructive pulmonary disease (COPD) patients. http://www.clinicaltrials.gov/ct2/show/NCT01120093 (accessed 2 May 2014).
- EUCTR2009‐017380‐42‐DE. Efficacy and safety of three doses of aclidinium bromide compared to placebo and to an active comparator all administered twice daily by inhalation in patients with stable moderate and severe chronic obstructive pulmonary disease (COPD). https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2009‐017380‐42 (accessed 2 May 2014).
- Singh D, Magnussen H, Kirsten A, Mindt S, Caracta C, Seoane B, et al. A randomised, placebo‐ and active‐controlled dose‐finding study of aclidinium bromide administered twice a day in COPD patients. Pulmonary Pharmacology and Therapeutics 2012;25(3):248‐53. [PUBMED: 22497752] [DOI] [PubMed] [Google Scholar]
- Singh D, Magnussen H, Kirsten A, Mindt S, Caracta C, Seoane B, et al. Corrigendum to " A randomised, placebo‐ and active‐controlled dose‐finding study of aclidinium bromide administered twice a day in COPD patients" [Pulm Pharmacol Ther 25 (3) (2012) 248‐53]. Pulmonary Pharmacology and Therapeutics 2013;26(2):305. [DOI] [PubMed] [Google Scholar]
- Singh D, Magnussen H, Kirsten A, Mindt‐Pruefert S, Caracta C, Jarreta D, et al. Aclidinium bromide: a phase IIB, dose‐finding study [Abstract]. Thorax 2011;66 Suppl 4:A172 [P256]. [Google Scholar]
van der Palen 2013 {published and unpublished data}
- NCT01385696. Study evaluating preference, satisfaction and ease of use of inhalers in chronic obstructive pulmonary disease (COPD) diagnosed patients. http://clinicaltrials.gov/ct2/show/NCT01385696 (accessed 2 May 2014).
- Palen J, Ginko T, Kroker A, Valk P, Goosens M, Padulles L, et al. Preference, satisfaction and errors with two dry powder inhalers in patients with COPD. Expert Opinion on Drug Delivery 2013;10(8):1023‐31. [DOI] [PubMed] [Google Scholar]
- Palen J, Ginko T, Kroker A, Valk P, GoosensM, Padulles L, et al. Preference, satisfaction and critical errors with Genuair® and HandiHaler® in patients with COPD. European Respiratory Journal 2012;40 Suppl 56:389s. [Google Scholar]
Vestbo 2010 {published and unpublished data}
- Vestbo J, Vogelmeier C, Creemers J, Falques M, Ribera A, Gil EG. Onset of effect of aclidinium, a novel, long‐acting muscarinic antagonist, in patients with COPD. COPD 2010;7(5):331‐6. [PUBMED: 20854047] [DOI] [PubMed] [Google Scholar]
- Vestbo J, Vogelmeier C, Creemers J, Ribera A, Garcia Gil E. Fast onset of effect of aclidinium bromide, a novel long‐acting muscarinic antagonist, in patients with COPD [Abstract]. Thorax 2009;64 Suppl IV:A167 [P212]. [DOI] [PubMed] [Google Scholar]
- Vestbo J, Vogelmeier C, Creemers J, Ribera A, Garcia Gil E. Rate of onset of action of aclidinium bromide, a novel, long‐acting, muscarinic antagonist [Abstract]. European Respiratory Society Annual Congress; Sep 12‐16; Vienna. 2009:[E4362].
Watz 2013 {unpublished data only}
- Beeh KM, Watz H, Magnussen H, Puente‐Maestu L, Jarreta D, Caracta C, et al. Aclidinium bromide improves exercise endurance and dynamic hyperinflation and decreases exertional dyspnoea in patients with moderate‐to‐severe COPD. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A2430. [Google Scholar]
- Beeh KM, Watz H, Magnussen H, Puente‐Maestu L, Jarreta D, Caracta C, et al. Effects of aclidinium bromide on exercise endurance, dynamic hyperinflation, physical activity and exertional dyspnoea in patients with moderate to severe COPD [Abstract]. European Respiratory Society Annual Congress, Sep 7‐11; Barcelona. 2013; Vol. 42 Suppl 57:636s [3035].
- EUCTR2011‐002665‐38‐DE. A multiple dose, randomised, double‐blind, placebo controlled, two period crossover clinical trial to assess the effect of aclidinium bromide 400 µg bid on exercise endurance in patients with stable moderate to severe chronic obstructive pulmonary disease (COPD). https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2011‐002665‐38 (accessed 2 May 2014).
- NCT01471171. Efficacy and safety of aclidinium bromide 400 µg BID (twice a day) compared to placebo in patients with stable moderate to severe chronic obstructive pulmonary disease (COPD). http://clinicaltrials.gov/show/NCT01471171 (accessed 2 May 2014).
- Watz H, Beeh KM, Magnussen H, Teres L, Jarreta D, Caracta C, et al. Aclidinium bromide improves static lung function and hyperinflation in patients with moderate‐to‐severe COPD. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A2431. [Google Scholar]
- Watz H, Beeh KM, Magnussen H, Theresa L, Jarreta D, Caracta C, et al. Effect of aclidinium bromide on static lung function and hyperinflation in patients with moderate to severe COPD [Abstract]. European Respiratory Society Annual Congress; Sep 7‐11; Barcelona. 2013; Vol. 42 Suppl 57:981s [4633].
References to studies awaiting assessment
NCT01636401 {unpublished data only}
- NCT01636401. Efficacy and safety of 400 μg twice daily of aclidinium bromide vs. placebo when administered to patients with moderate to severe chronic obstructive pulmonary disease (COPD). http://clinicaltrials.gov/ct2/show/NCT01636401 (accessed 1 May 2014).
References to ongoing studies
ASCENT COPD {unpublished data only}
- NCT01966107. Evaluate the effect of aclidinium bromide on long‐term cardiovascular safety and COPD exacerbations in patients with moderate to very severe COPD (ASCENT COPD). http://clinicaltrials.gov/ct2/show/NCT01966107 (accessed 1 May 2014).
Additional references
Alagha 2011
- Alagha K, Bourdin A, Tummino C, Chanez P. An update on the efficacy and safety of aclidinium bromide in patients with COPD. Therapeutic Advances in Respiratory Disease 2011;5(1):19‐28. [PUBMED: 20884687] [DOI] [PubMed] [Google Scholar]
Alagha 2014
- Alagha K, Palot A, Sofalvi T, Pahus L, Gouitaa M, Tummino C, et al. Long‐acting muscarinic receptor antagonists for the treatment of chronic airway diseases. Therapeutic Advances in Chronic Disease 2014; Vol. 5, issue 2:85‐98. [PUBMED: 24587893] [DOI] [PMC free article] [PubMed]
Almirall
- Almirall. Clinical trial results. http://www.almirall.com/webcorp2/cda/ImD_04_03.jsp?fMarc= (accessed 11 April 2014).
ATS/ERS 2011
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Annals of Internal Medicine 2011;155(3):179‐91. [DOI] [PubMed] [Google Scholar]
CDC 2011
- Hoyert DL, Xu J. Deaths: preliminary data for 2011 (National Vital Statistics Reports). http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_06.pdf (accessed 25 December 2012). [PubMed]
Chong 2011
- Chong J, Poole P, Leung B, Black PN. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD002309.pub2] [DOI] [PubMed] [Google Scholar]
Chong 2012
- Chong J, Karner C, Poole P. Tiotropium versus long‐acting beta‐agonists for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD009157.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cranston 2008
- Cranston JM, Crockett A, Moss J, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD001744.pub2] [DOI] [Google Scholar]
Effing 2009
- Effing T, Monninkhof EEM, Valk PPDLPM, Zielhuis GGA, Walters EH, Palen JJ, et al. Self‐management education for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD002990.pub2] [DOI] [PubMed] [Google Scholar]
FDA 2012
- United States Food, Drug Administration (FDA). Drug approval package; Tudorza Pressair (aclidinium bromide) inhalation powder. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202450Orig1s000TOC.cfm (accessed 25 December 2012).
Forest Pharmaceuticals
- Forest Pharmaceuticals, Inc. Subsidiary of Forest Laboratories, Inc. http://www.forestpharm.com/ (accessed 25 December 2012).
Gavaldà 2010
- Gavaldà A, Garcia‐Gil E. Aclidinium bromide, a novel long‐acting muscarinic antagonist (LAMA). New drugs and targets for asthma and COPD. Progress in Respiratory Research 2010;39:33‐8. [Google Scholar]
GOLD 2013
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2013. Global strategy for diagnosis, management and prevention of COPD. http://www.goldcopd.org/guidelines‐global‐strategy‐for‐diagnosis‐management.html (accessed 14 February 2013).
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. www.cochrane‐handbook.org.
Hogg 2009
- Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annual Review of Pathology: Mechanisms of Disease 2009;4:435‐59. [DOI] [PubMed] [Google Scholar]
Holland 2012
- Holland AE, Hill CJ, Jones AY, McDonald CF. Breathing exercises for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD008250.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2011
- Jones PW, Rennard SI, Agusti A, Chanez P, Magnussen H, Fabbri L, et al. Efficacy and safety of once‐daily aclidinium in chronic obstructive pulmonary disease. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3098801/ (accessed 25 December 2012). [DOI: 10.1186/1465-9921-12-55] [DOI] [PMC free article] [PubMed]
Jones 2012
- Jones PW, Singh D, Bateman ED, Agusti A, Lamarca R, Miquel G, et al. Efficacy and safety of twice‐daily aclidinium bromide in COPD patients: the ATTAIN study. European Respiratory Journal 2012;40(4):830‐6. [PUBMED: 22441743] [DOI] [PubMed] [Google Scholar]
Jones 2013
- Jones P. Aclidinium bromide twice daily for the treatment of chronic obstructive pulmonary disease: a review. Advances in Therapy 2013;30(4):354‐68. [PUBMED: 23553509] [DOI] [PubMed] [Google Scholar]
Karabis 2013
- Karabis A, Lindner L, Mocarski M, Huisman E, Greening A. Comparative efficacy of aclidinium versus glycopyrronium and tiotropium, as maintenance treatment of moderate to severe COPD patients: a systematic review and network meta‐analysis. International Journal of Chronic Obstructive Pulmonary Disease 2013;8:405‐23. [PUBMED: 24043936] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karakiulakis 2012
- Karakiulakis G, Roth M. Muscarinic receptors and their antagonists in COPD: anti‐inflammatory and anti‐remodeling effects. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3512336/ (accessed 25 December 2012). [DOI: 10.1155/2012/409580] [DOI] [PMC free article] [PubMed]
Karner 2012
- Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009285.pub2] [DOI] [PubMed] [Google Scholar]
Lacasse 2009
- Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD003793.pub2] [DOI] [PubMed] [Google Scholar]
MacNee 2006
- MacNee W. ABC of chronic obstructive pulmonary disease: pathology, pathogenesis, and pathophysiology. BMJ 2006;332:1202‐4. [Google Scholar]
Maltais 2012
- Maltais F, Milot J. The potential for aclidinium bromide, a new anticholinergic, in the management of chronic obstructive pulmonary disease. Therapeutic Advances in Respiratory Disease 2012;6(6):345‐61. [PUBMED: 23075544] [DOI] [PubMed] [Google Scholar]
Nannini 2012
- Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long‐acting beta2‐agonist in one inhaler versus long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD006829.pub2] [DOI] [Google Scholar]
NICE 2010
- National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. London: National Clinical Guideline Centre. http://guidance.nice.org.uk/CG101/Guidance/pdf/English (accessed 25 December 2012).
NICE 2011
- National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease, costing report, implementing NICE guidance. http://guidance.nice.org.uk/CG101/CostingReport/pdf/English (accessed 25 December 2012).
Poole 2010
- Poole P, Chacko EE, Wood‐Baker R, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD002733.pub2] [DOI] [Google Scholar]
Poole 2012
- Poole P, Black PN, Cates CJ. Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD001287.pub4] [DOI] [PubMed] [Google Scholar]
Ram 2009
- Ram FSF, Jones P, Jardim J, Castro AA, Atallah ÁN, Lacasse Y, et al. Oral theophylline for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003902] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Sims 2011
- Sims MW, Panettieri RA Jr. Profile of aclidinium bromide in the treatment of chronic obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease 2011;6:457‐66. [PUBMED: 22003291] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suppli 2012
- Suppli Ulrik C. Aclidinium bromide: clinical benefit in patients with moderate to severe COPD. The Open Respiratory Medicine Journal 2012;6:150‐4. [PUBMED: 23264836] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sutherland 2004
- Sutherland ER, Cherniack RM. Management of chronic obstructive pulmonary disease. New England Journal of Medicine 2004;350(26):2689‐97. [PUBMED: 15215485] [DOI] [PubMed] [Google Scholar]
Tiong 2009
- Tiong LU, Gibson PG, Hensley MJ, Hepworth R, Lasserson TJ, Smith B, et al. Lung volume reduction surgery for diffuse emphysema. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD001001.pub2] [DOI] [PubMed] [Google Scholar]
TSANZ 2012
- Abramson M, Crockett AJ, Dabscheck E, Frith PA, George J, Glasgow N, et al. on behalf of Lung Foundation Australia and the Thoracic Society of Australia and New Zealand. The COPD‐X Plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease. Version 2.35 2013. http://www.copdx.org.au/ (accessed 14 February 2014).
van der Meer 2012
- Meer RM, Wagena E, Ostelo RWJG, Jacobs AJE, Schayck CP. Smoking cessation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD002999] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vogelmeier 2011
- Vogelmeier C, Banerji D. NVA237, a long‐acting muscarinic antagonist, as an emerging therapy for chronic obstructive pulmonary disease. Therapeutic Advances in Respiratory Disease 2011;5(3):163‐73. [PUBMED: 21511677] [DOI] [PubMed] [Google Scholar]
Walters 2009
- Walters JAE, Walters EH, Wood‐Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD005374] [DOI] [PubMed] [Google Scholar]
Walters 2010
- Walters JAE, Smith S, Poole P, Granger RH, Wood‐Baker R. Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD001390.pub2] [DOI] [PubMed] [Google Scholar]
Welsh 2011
- Welsh EJ, Cates CJ, Poole P. Combination inhaled steroid and long‐acting beta2‐agonist versus tiotropium for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD007891.pub2] [DOI] [Google Scholar]
WHO 2008
- World Health Organization. The global burden of disease: 2004 update. http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf (accessed 25 December 2012).
WHO 2010
- World Health Organization. Global status report on noncommunicable diseases 2010. http://www.who.int/nmh/publications/ncd_report2010/en/ (accessed 25 December 2012).
WHO 2012
- World Health Organization. Chronic obstructive pulmonary disease. http://www.who.int/respiratory/copd/en (accessed 25 December 2012).
WHO 2012a
- World Health Organization. COPD predicted to be third leading cause of death in 2030. http://www.who.int/respiratory/copd/World_Health_Statistics_2008/en/index.html# (accessed 25 December 2012).
WHO ICTRP
- World Health Organization. International Clinical Trials Registry Platform (ICTRP) search portal. http://apps.who.int/trialsearch/ (accessed 2 May 2014).
Woods 2013
- Woods JA, Nealy KL, Barrons RW. Aclidinium bromide: an alternative long‐acting inhaled anticholinergic in the management of chronic obstructive pulmonary disease. The Annals of Pharmacotherapy 2013;47(7‐8):1017‐28. [PUBMED: 23737515] [DOI] [PubMed] [Google Scholar]
Yang 2012
- Yang IA, Clarke MS, Sim EHA, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD002991.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


