Abstract
Manganese (Mn2+ as MnSO4 &/or MnCl2) is a common and essential element for maintaining life in plants and animals and is found in soil, fresh waters and marine waters; however, over exposure is toxic to organisms. MnSO4 is added to soil for agricultural purposes and people are exposed to Mn2+ in the mining industry. Hypermanganesemia in mammals is associated with neurological issues mimicking Parkinson’s disease (PD) and appears to target dopaminergic neural circuits. However, it also seems that hypermanganesemia can affect many aspects of health besides dopaminergic synapses. We examined the effect on development, behavior, survival, cardiac function, and glutamatergic synaptic transmission in the Drosophila melanogaster. In addition, we examined the effect of Mn2+ on a sensory proprioceptive organ and nerve conduction in a marine crustacean and synaptic transmission at glutamatergic neuromuscular junctions of freshwater crayfish. A dose-response effect of higher Mn2+ retards development, survival and cardiac function in larval Drosophila and survival in larvae and adults. MnSO4 as well as MnCl2 blocks stretch activated responses in primary proprioceptive neurons in a dose-response manner. Mn2+ blocks glutamatergic synaptic transmission in Drosophila as well as crayfish via presynaptic action. This study is relevant in demonstrating the effects of Mn2+ on various physiological functions in order to learn more about acute and long-term consequences Mn2+ exposure.
Keywords: Drosophila, crayfish, crab, sensory, neuromuscular junction, survival, cardiac, manganese
Graphical Abstract
Introduction
Given that manganese (Mn2+) is found in many plants and animals that are consumed by humans as food, it is essential to understand the effects of consuming high levels of Mn. It is well established as an essential element required for photosynthetic abilities in plants as well as cellular function in mammals. For example, Mn acts as a cofactor for aiding in the function of various essential enzymes (i.e., superoxide dismutase) in organisms. However, Mn is also toxic at high levels. Thus, it is of interest to understand the threshold at which subtle effects in physiological processes become compromised. It is difficult to determine what physiological symptoms may manifest when chronically exposed to low levels of manganese but examining acute actions at high levels may provide some insight to what the potential effects might be. Mn is found in seaweed (130 to 735 mg/kg dry weight), formula milk consumed by developing infants (above recommended daily limits; Frisbie et al., 2019), marine and freshwater fish (<0.2 to 19 mg/kg dry weight) and can bioaccumulate in blueberries as well is found on plants from application of agricultural sprays in the range of 2000–4000 mg/kg (Howe, et al., 2004). Hypermanganesemia in mammals can be toxic and is associated with neurological issues mimicking Parkinson’s disease (PD). In fact, the neural injury from Mn affects the same locations within the central nervous system known to be dysfunctional in PD. Manganese has been shown to accumulate in these areas (Calne et al., 1994; Eriksson et al., 1987; Erikson, 2004a,b; Brenneman et al., 1999; Nagatomo et al., 1999; Newland, 1999; Pal et al., 1999; Baek et al., 2003; Nelson et al., 2018). Such findings have led, in part, to suggesting that Mn selectively targets dopaminergic neurons. However, it is not known what the effect(s) are of Mn on neurons which use other types of neurotransmitters or exactly how Mn2+ is offsetting the dopaminergic neural circuit in the mammalian brain. Bonilla-Ramirez et al., (2011) addressed whether Mn was accumulating in the dopaminergic regions of the adult brain of D. melanogaster and found that there indeed was an accumulation and that there was a dose-dependent effect on the adults being able to locomote in a climbing assay. Additionally, acute and long-term feeding of MnSO4 decreased survival of the adult Drosophila likely due to damage in the dopaminergic regions within the CNS.
Nelson et al., (2018) reported on studies conducted with bivalve mollusks where the cilia beating is controlled by dopaminergic and serotonergic innervation. Low concentration of Mn2+ at 50 μM reduced the dopaminergic effects on the cilia beating but a higher concentration of Mn2+ blocked the effect with application of DA. This implied that low concentrations reduced the neuron’s function, potentially by a decrease in DA release but that high concentrations blocked the postsynaptic DA response (Catapane et al., 1978; Aiello, 1990; Carroll and Catapane, 2007; Martin et al., 2008; Nelson et al., 2010, 2018). Nelson et al. (2018) investigated the mechanism in which Mn2+ altered the postsynaptic response. This previous study focused to address if Mn2+ blocked the DA receptors as well as the downstream effects of adenylyl cyclase for the G protein coupled metabotropic receptors (GPCR) of DA. It appeared that Mn2+ resulted in a reduced sensitivity to DA by likely desensitizing the DA receptors and not acutely altering adenylyl cyclase (Nelson et al., 2018). The notion in the effect of Mn exposure is that oxidative stress and altered mitochondrial function occurs (Bowman et al., 2011; Nelson et al., 2018), resulting in the neurons becoming dysfunctional. Such cellular mechanisms would likely be affected in all neurons and other cell types as well. There are many potential actions on neurons by affecting channels and screening electrical potential on membranes. To gain an overview in the effects of Mn2+ on various physiological functions, we conducted experiments in a few model systems in different animals on development, survival, cardiac, sensory, motor, and glutamate synaptic responses.
Larval D. melanogaster have a relatively simple neural circuit to analyze metal toxicity effects on locomotive behaviors. Body wall crawling, and mouth hook movements are readily assessed behaviors to examine the dietary actions of consuming Mn2+ (Badre and Cooper, 2008; Dasari et al., 2007). Thus, to gain some insight into the effects of dietary Mn2+, these larval behaviors were examined in this study. As expected, if the neural circuits for eating and locomotion are severely affected in adults and larvae, one would expect manganese to also affect development and survival. Thus, the general effects on the pupation rate as well as survival of larvae and adults with manganese-tainted food was surveyed herein.
The direct effect of Mn2+ on sensory neurons is lacking in literature. Since Mn2+ can block voltage-gated calcium channels, then it may be possible that Mn2+ can block stretch-activated channels (SACs). We examined the effects of Mn2+ exposure on primary sensory neurons activated by SACs in proprioceptive neurons of a model crab chordotonal organ (Alexandrowicz, 1972; Cooper, 2008; Hartman and Boettiger, 1967; Whitear, 1938).
To address whether Mn2+ has an effect beyond that on dopaminergic synapses, evoked and quantal responses of glutamatergic synapses were also observed when acutely exposed to Mn2+. The spontaneous quantal events of larval D. melanogaster and crayfish neuromuscular junctions (NMJs) were used to assess the acute actions on the model glutamatergic synapse (Titlow and Cooper, 2018). If there remains the presence of spontaneous quantal events of the same general magnitude before and during exposure to Mn2+ while evoked responses are retarded, then these effects would indicate a presynaptic action; however, if both evoked and spontaneous quantal events were gradually declining in amplitude with exposure, then it would be suggestive that the glutamate receptors are affected by Mn2+.
In rodent models, Mn2+ exposure (1–8 mM) was found to inhibit myocardial contraction and electrical function (Jiang and Zheng, 2005). Thus, we examined the acute effect on the heart rate in larval D. melanogaster within in situ preparations. When the in situ larval hearts are bathed in a large amount of saline, leaving them isolated from any endogenous hormonal actions, then the direct effects of exogenously applied compounds can be assessed independently of endogenous compounds of the hemolymph. The heart rate is rapidly altered with release of dopamine, serotonin or octopamine by the larval CNS, (Malloy et al., 2017) which is not an issue with saline flushed in situ preparation of the larval heart. Since the D. melanogaster model is increasingly becoming a model to address cardiac function and pacemaker activity, it serves as a rapid approach to screen the effect of Mn2+ on cardiac function. Since the larval heart tube is not innervated until the transition from larvae to pupa, the direct action on the heart is able to be addressed independently of neural innervation (Johnstone and Cooper, 2006). The heart rate in larval D. melanogaster is very sensitive to extracellular Ca2+ levels, pH, and other modulators, so the change in heart rate can be used as a sensitive index for the effects of compounds that alter these functions (de Castro et al., 2014, Desai-Shah et al., 2010; Majeed et al., 2013, 2014; Titlow et al., 2013). Thus, it was assumed that there would likely be an acute effect by Mn2+ on the heart rate in larval Drosophila due to the responsiveness to Ca2+ in bathing media.
The common forms of Mn in nature are found in the oxidation state of +2 (e.g., manganese chloride [MnCl2]), +4 (e.g., manganese dioxide [MnO2]), and +7 (e.g., potassium permanganate [KMnO4]) (Howe, et al., 2004). Both MnSO4 and MnCl2 are both readily soluble in water and readily dissociate in water. Since cytotoxicity varies between MnSO4 and MnCl2 in some studies (Marreilha dos Santos et al., 2008), we examined the two compounds in some studies herein. The differences in the physiological responses between the compounds appear to be due to differences in the rate of transport into different tissues (Erikson et al., 2004). In the current study, some of the physiological assays were performed with MnSO4 and MnCl2 either being dissolved in food or in the saline applied to exposed tissues.
Surveying several physiological systems in various animals will provide a general understanding of the effect of Mn2+. This knowledge can then be applied to understand the effects on organisms potentially exposed to Mn2+ through industrial mining or agricultural use of MnSO4 in folate sprays intended to benefit plant health. Mn2+ is noted to be found in various tissues of the edible blue crab in marine environments (Callinectes sapidus) (Zotti et al., 2016) and edible crayfish of freshwater systems. Given that MnSO4 is used in agriculture to aid plant health, it is also found on food sources for insects not only in the soil but also in and on plants (Chen et al., 2020; Jhanji et al., 2014). Increasing manganese concentrations also seems to be linked to idiopathic blindness in the American lobster, a condition affecting roughly half of the species population (Ochs et al., 2020). Additionally, commonly eaten crabs in the wetlands of China were found to contain high amounts of manganese that deemed them to be inedible (Zhang et al., 2019). Manganese can be taken up across the gills of crustaceans and is found in the hemolymph (7.35 μg/g wet weight) as well as the midgut of hermit crab (1596 μg/g dw) and in crayfish (374 μg/g dw). In skeletal muscle of crayfish it has been found to be as high as 87 μg/g and in the central nervous system in lobster as high as 193 μg/g. Baden and Eriksson (2006) and Zhang et al., (2019) reviewed the bioaccumulation in various tissues of crustaceans under different conditions. A detailed review of accumulation of Mn2+ in tissues of eatable lobster was reviewed by Ochs et al., (2020). Certain edible termites have also been found to contain astonishingly high amounts of Mn2+, some species containing enough that minimal consumption could exceed the upper limits for intake of Mn2+ in humans (Verspoor et al, 2020).
Because of the Mn2+ exposure on various populations in the ecosystem examining the effects on D. melanogaster can provide an index (Mohandas et al., 2017) of the potential effects on other insect species in the environment. As it is well established, the effects of agents on non-humans are relevant to understanding the potential actions of that agent in humans. To uncover the potential effects of compounds on physiological functions, a higher concentration than that which a system is normally exposed to can unmask the more subtle effects of the compound present at a lower level over longer period of time. Since Mn is of higher concentration in some infant formula products, there is some interest in understanding the potential effects of Mn2+ on neuronal function of human infants fed formula milk in comparison to those fed human breast milk (Frisbie et al., 2019). Thus, with all of these potential avenues for Mn exposure, it is crucial to understand more about the effects of manganese in various other organisms in order to better predict the potential effects on humans.
Methods
Animals
Drosophila melanogaster, Canton S (CS) flies were used in all behavioral and physiological assays. This strain has been isogenic in the lab for several years and was originally obtained from Bloomington Drosophila Stock Center (BDSC). All animals were maintained in vials partially filled with a cornmeal-agar-dextrose-yeast medium. Blue crabs (C. sapidus) were obtained from a local supermarket in Lexington, KY, USA which were delivered from a distribution center in Atlanta, GA, USA. They were bought and maintained in a seawater aquarium for several days prior to use in order to assess their health. The crabs were adults and in the range of 10–15 cm in carapace width (from point to point). All crabs used were alive and were very active upon autotomizing a leg for experimentation. Red Swamp Crayfish (Procambarus clarkii) were obtained from a distribution center in Atlanta, GA, USA and delivered to, and bought from, a local supermarket in Lexington, KY, USA. Throughout the study, midsized crayfish measuring 6–10 cm in body length and 12.5–25 g in body weight were used. Each animal was housed in individual standardized plastic containers with dry fish food exchanged weekly and aerated water (20–21 °C).
Survival and developmental studies of Drosophila melanogaster
To address the effect of Mn2+ on life cycle alterations, the time to pupation from 1st instar larva was measured when exposed to varying dosages of MnCl2 and MnSO4 treatments in tainted food. Ten 1st instars were placed on the food located at the bottom of the glass vials. The vials were used to allow for an aerated and moist environment at 21oC. The larvae were then left to develop into pupa and each day, the number of pupae were counted for each condition. After 7 days, the food was removed and the vial was examined for dead larvae. Each concentration (2.5 mM, 30 mM and 100 mM) of MnCl2 and MnSO4 was performed in triplicate.
Assessing the survival of adults involved the collection of adults within 3 days after enclosing from pupa. The adults were placed in plastic vials containing the tainted food with MnCl2 or MnSO4. The survival of the adults was monitored daily for 21 days. The conditions were replicated in 6 trials for each concentration of MnCl2 and MnSO4 (5 mM, 15 mM and 30 mM). Each trial started with 20 adults. Each experimental group and control group, also referred to as a saline group, had 120 adults in total.
Behavioral assays
Locomotion of larvae was assessed by recording body wall contractions per minute with the use of a dissecting microscope. The 2nd instar larvae were placed in food containing a concentration of either 15mM or 30 mM MnCl2 or MnSO4. This was made by combining 1 gram of corn meal food, estimated as 1 mL in volume, and 1 mL of water to produce 2 mL The MnCl2 and MnSO4 was dissolved in the 1 mL of water at double the concentration and then was mixed with the food to provide the final concentrations. After 24 hours, the 3rd instars were placed on apple-juice agar (1% agar) Petri dishes (about 8.5–9 cm diameter). The larvae were left inside the dish for one minute to acclimate to the environment. Then body wall movements (BWMs) were counted for one minute via a microscope in a lightly illuminated environment at room temperature (21–22oC).
Next, the larvae used for body wall movements were used to assess larval feeding behavior after being transferred to a small Petri dish (5.5 cm diameter) that contained a yeast solution (a few dried yeast granules were mixed with water). The larvae were left for one minute, and then the mouth hook movements (MHMs) were counted for one minute.
Heart rate measures in larval Drosophila
A detailed description, in video format, of exposing the larval heart in early 3rd instars is provided in Cooper et al., (2009). In brief, the larvae were dissected ventrally and pinned on four corners to expose the heart tube. The preparation was then bathed in physiological saline (deCastro et al., 2014). A modified HL3 saline was used to maintain the in situ hearts and body wall muscles (NaCl 70 mM, KCl 5 mM, MgCl2·6H2O 20 mM, NaHCO3 10 mM, Trehalose 5 mM, sucrose 115 mM, BES 25 mM, and CaCl2·2H2O 1 mM, pH 7.1; Stewart et al., 1994). In the experimental groups, varying concentrations of MnCl2 or MnSO4 was dissolved in this saline. The pH of the saline was maintained at 7.1. Each concentration of MnCl2 and MnSO4 (2.5 mM, 15 mM and 30 mM) was examined for 8 to 10 larvae. The heart rate was measured in a 30 second period and was converted to beats per minute for graphical analysis. The rate was taken in the caudal region of the heart tube (Figure 1). The heart rate was first obtained during the initial saline exposure before the bathing solution was exchanged to one containing a compound. Two minutes after the new solution containing an experimental compound was added, the heart rate was obtained again. This was followed by flushing out the compounds with fresh saline three times and then waiting 2 minutes before recording data of the heart rate after the Mn2+ was removed.
Figure 1:
The filleted larva preparation used for measurement of heart rate when exposed to MnSO4 and MnCl2. Heartbeats were counted by manual inspection through a dissecting microscope before and after switching to the compound of interest. Heart rates were measured from the caudal end of the preparation near the point where the two tracheal tubes bifurcate.
Crab proprioceptive neurons
Similar dissection procedures and electrophysiological measures as those thoroughly described in text and video format by Majeed et al. (2013) were used. In brief, the animal was induced to autotomize the first or second walking leg by lightly pinching with pliers at the base of the leg. The propodite-dactylopodite (Pd) chordotonal organ spans the last segment of the leg and was exposed by cutting a window of the cuticle on both sides of the leg (in the propodite segment). The leg was then pinned in a Sylgard-lined dish and covered with crab saline. The Pd nerve was then exposed and pulled into a suction electrode for recording. During the experiment, the dactyl was moved from a flexed position to an open position in a 1 s time frame, held for 10 s, and then moved back to the starting position (see Figure 1A in McCubbin et al., 2020). An insect dissecting pin was used as a reference for the maximum displacement range, and each displacement was marked on the computer recording file. The crab saline used during recordings of the sensory nerves consisted of (in mM): 470 NaCl, 7.9 KCl,15.0 CaCl2·2H2O, 6.98 MgCl2·6H2O, 11.0 dextrose,5 HEPES acid and 5 HEPES base adjusted to pH 7.4. Each concentration of MnCl2 and MnSO4 (1 mM, 15 mM, 30 mM and 100 mM) was examined for 5 to 6 preparations. As a control for osmolarity differences, preparations exposed to 1 M sucrose made in standard crab saline were also studied. The numbers of spikes recorded over the first 10 seconds from the start of the joint displacement were used as an index in the neural activity. Preparations were also examined with 1 M mannitol to examine the effect on the neural activity of the Pd organ.
To measure the compound action potential, the Pd nerve was dissected away from the main leg nerve and from the Pd organ to the autotomy plane in the proximal region of the leg (Figure 3A). The proximal half of the shell of the leg was removed after the nerve was pulled to the side of the leg. The proximal end of the nerve was pulled into a suction electrode to record the neural activity from the proprioceptive sensory cells as well as to evoke a compound action potential (CAP) when the stimulating electrode induced the neurons to produce action potentials (Figure 3B). A region of the distal part of the nerve was pulled into a suction electrode to evoke stimulation in an en passant manner of the nerve. The voltage of the stimulating electrode was increased to produce a maximum amplitude and duration and then the voltage was slightly reduced so the CAP amplitude would be below the maximum response. Thus, any increase in the CAP would be able to be detected upon exposure to Mn2+. Both ends of the nerve, which were placed in the suction electrodes, were packed with clear petroleum jelly to seal the nerve in the electrode and increase resistance around the nerve to the electrode to obtain the largest extracellular signals. A ground wire was placed in the bathing media.
Figure 3:
Recording the evoked compound action potential (CAP) of the Pd nerve in a crab first walking leg. The Pd nerve is dissected out of the leg from the Pd organ to the autotomy plane of the leg. The proximal end is used to record the Pd activity and induced CAP in different bathing media. The distal end of the nerve close to the Pd organ was pulled into a suction electrode to induce CAPs in the Pd nerve. The preparation is pinned to a Sylard lined dish to hold the dissected leg in place. A ground wire is placed in the bathing media.
Neuromuscular junctions of larval Drosophila and crayfish
The effect of Mn2+ on the membrane potential, evoked excitatory junction potentials (EJPs), and spontaneous quantal events (mEJPs) were examined for brief exposures of saline containing Mn2+. This was followed by flushing of the preparation with standard fly saline to determine if the preparations were still viable after exposure to Mn2+.
Third instar larval D. melanogaster were dissected in physiological saline. The segmental nerves were cut and sucked into a suction electrode, which was filled with saline and stimulated. The segmental nerves were stimulated at 0.5 Hz (S88 Stimulator, Astro-Med, Inc., Grass Co., WestWarwick, RI, USA). To monitor the transmembrane potentials of the body wall muscle (m6) of 3rd instar larvae, a sharp intracellular electrode (30 to 40 M resistance) filled with 3M KCl impaled the fiber (Figure 4). An Axoclamp 2B (Molecular Devices, Sunnyvale, CA, USA) amplifier and 1 X LU head stage was used. Fly saline used was the same as detailed above for the heartrate monitoring.
Figure 4:
A schematic diagram of the recording arrangement of a 3rd instar larva for obtaining evoked and spontaneous synaptic excitatory junction potentials from m6 muscle fiber.
The opener muscle of the crayfish has been historically reviewed for studying synaptic transmission (Cooper and Cooper, 2009). The use of the most distal muscle fibers in the preparation provides a reference for consistency among preparations (Cooper et al., 1995). The dissection to expose and selectively stimulate the excitatory motor neuron of the opener muscle is described in textual and video format (Cooper and Cooper, 2009). In brief, the excitatory neuron was isolated from the inhibitor neuron and was then stimulated in the meropodite segment. The stimulation paradigm consisted of providing a train of pulses at 60 Hz with10 s between trains (Figure 5). Responses were recorded with an AxoClamp 2B (Axon Instruments, USA), converted with a PowerLab, 4SP (ADInstruments, USA), and analyzed with LabChart 7.0 (ADInstruments, Colorado Springs, CO, USA) on a computer at a 10 or 20 kHz sampling rate. Dissected preparations were maintained in crayfish saline, a modified Van Harreveld’s solution (in mmol/L: 205 NaCl; 5.3 KCl; 13.5CaCl2·2H2O; 2.45 MgCl2·6H2O; 10 glucose; 0.5 HEPES adjusted to pH 7.4).
Figure 5:
The opener muscle of the crayfish walking leg with facilitated synaptic responses in the excitatory junction potentials from the distal muscle fibers. The synaptic responses depicted show the facilitation which occurs over 25 stimuli. The arrows indicate the stimuli given to the nerve.
Statistical Methods
In general, when normality was to be assumed, Shapiro-Wilks tests were used to validate the assumptions in order to determine the use of a t-test, a paired t-test or a Wilcoxon Signed Rank Test. An ANOVA was used when necessary, along with Bonferroni post-hoc T-test
For the survival analysis in D. melanogaster, Weibull regression was used to model the survival curves with the factors being solution type (Control, MnCl2, MnSO4) and strength of solution (0, 5mM, 15mM and 30mM). ANOVA was then used to determine if a significant difference existed between the factors. If a factor was considered statistically significant, P<0.05, a Bonferroni correction was used to determine the pairwise post-hoc differences in the factors.
For the behavioral studies, ANOVA was used to determine the difference between the 5 treatments (Control, 15 mM and 30 mM of both MnCl2 and MnSO4). If a significant difference was detected, a Bonferroni post-hoc T-test was conducted to determine which treatments were statistically different.
For the heart rate experiment, the data consisted of an initial saline treatment followed by application of either a saline, MnSO4 or MnCl2 treatment and then another saline application. The original data was not normally distributed (Shapiro-Wilks, P<0.05). Therefore, new variables were created with a pre and a post variable creation. Pre is defined as the initial saline treatment minus the application of either saline or Mn solution and post was defined as the initial saline treatment minus the terminal saline treatment. The transformed data did pass the Shapiro-Wilks test (P>0.05). With the two new variables, a 3-way repeated measures ANOVA evaluating the effects of compound (MnSO4 vs MnCl2), dose level (2.5mM, 15mM and 30mM) along with time (pre and post) was conducted using the difference in heart rate. Since we found a significant two-way interaction, we followed up the initial repeated measures ANOVA with a one-way ANOVA with application, treatment, and time held constant to determine the difference in the doses. This p-value was also adjusted using the Bonferroni paradigm. Once a significant result was found, all post-hoc analysis was done with the Bonferroni test.
For the crab neuron and NMJ studies, either a paired t-test (if Shapiro-Wilks test passed) or a Wilcoxon Signed Rank test was used to compare various sensitivities.
A significance level of 0.05 was used in all studies unless the Bonferroni T-test was used, in which case the significance level was adjusted accordingly.
Results
Survival and developmental studies of Drosophila melanogaster
Larval development and survival study were conducted in triplicate with ten 1st instars, providing a total of 30 individuals for each condition. Within a 6 hour window from hatching, the 1st instars were placed in food tainted with NaCl, MnSO4 and MnCl2 at various concentrations (0.0025, 0.015, 0.03 and 0.1 M). The most significant effect was for the higher concentrations of 0.03 and 0.01 M MnSO4 and MnCl2 as this was toxic for the 1st instar. In these conditions, there were no 2nd instars observed. When exposed to a 0.015 M concentration of both MnSO4 and MnCl2, there was a high percent of death. The 0.015 M MnCl2 was toxic at 1st instar stage since only 1 out of 30 larvae produced a pupa and went on to emerge as an adult. The 0.015 M MnSO4 was not conducive for full survival of all 30 larvae as 15 out of the 30 survived to pupa and adults. In these conditions, there were a total of eight 2nd instars present in three vials and the rest of the dead larva (seven) appeared to be 1st instar but degraded as seen for the vials at 0.03 and 0.01 M concentrations.
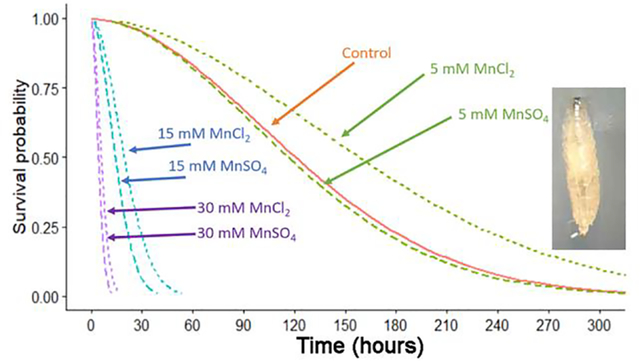
The survival of a different set of adults were examined for survival when exposed to food also tainted with MnSO4 and MnCl2. The young adults did not show a dose-dependent effect with the low dose (5 mM) of either compound when compared to controls (Figure 6); however, the 15 mM and 30 mM showed a greater percentage of deaths over time (Figure 6). The 30 mM of MnSO4 showed a steep decline in survival after 5 days and by 8 days the adults had died. In the same time period, there were a few still surviving in 30 mM MnCl2 but the majority were deceased. By 14 days, all the MnCl2 at 30 mM were dead and >90 % of the 15 mM MnSO4 had died. We found a significant difference between at least two of the solutions (ANOVA, p<0.001). Two sets of curves were statistically different from each other, the Control and MnCl2 curves (Bonferroni T-test, p<0.001) and the Control and MnSO4 curves (Bonferroni T-test, p<0.001). However, MnCl2 and MnSO4 were not significantly different (Bonferroni post-hoc, p>0.05). Similarly, we found at least 2 of the strengths of the solutions were different from each other (ANOVA p<0.001). In fact, the 15mM and 30mM solutions were significantly different from the control as well as different from 5mM (all 4 Bonferroni T-tests, p<0.001). 15mM and 30mM were also different from each other (Bonferroni T-test, p<0.001). Lastly, the control and 5mM solutions were not significantly different (Bonferroni T-test, p>0.05).
Figure 6:
Survival of adults exposed to food tainted with MnSO4 or MnCl2 as compared to controls. Controls were fed standard cornmeal food while others had food tainted with 5, 15 or 30 mM MnSO4 or MnCl2. Twenty adults were placed into each condition and observed daily.
Behavioral assays
After only 24 hours of consuming the food tainted with Mn2+, there were obvious differences in the behaviors associated with consuming 30 mM MnSO4 or MnCl2 (Figure 7). The lower concentration of 15 mM did not show as large of differences. Specifically, the mouth hook movements were significantly different from controls for both MnSO4 and MnCl2 at both concentrations of 15 mM and 30 mM. Also, the mouth hook movements from the 15 mM and 30 mM exposure were significantly different from each other (ANOVA, N>20; p<0.05, Bonferroni T-test)
Figure 7:
Larval behaviors after 24 hours of exposure to tainted food of 15 or 30 mM MnSO4 or MnCl2 as compared to standard media. The mouth hook movements per minute were significantly reduced with both 15 and 30 mM of MnSO4 or MnCl2. The larvae were transferred to a dilute solution of yeast mixed with distilled water for the assay. Crawling behavior, as indexed in body wall contractions per minute, indicated that being exposed to food of 30 mM for MnSO4 or MnCl2 reduced the rate. However only the 15 mM MnSO4 resulted in a significantly lower rate than controls. The rates are expressed as a mean +/− SEM and significance is p<0.05 ANOVA, N>20 larvae for each condition.
The statistical differences in the body wall movements were not as pronounced as the mouth hook movements (Figure 7). The 15 mM MnCl2 did not produce a significant difference from the controls but did when compared to 30 mM MnCl2. The effect of 15 mM MnSO4 was statistical different from controls and both MnSO4 and MnCl2 at 30 mM. The effects of 15 mM and 30 mM MnCl2 were not statistically different from each other. Likewise, the effects of 30 mM of MnSO4 or MnCl2 were not statistically different from each other. (ANOVA, N>20; p>0.05)
Heart rate measures in larval Drosophila
The hearts of the larvae were very sensitive to the exposure of Mn2+ from either MnSO4 or MnCl2 as even the lowest concentration of 2.5 mM influenced the heart rate (Figure 8). We found a significant two-way interaction between dosage and time (pre vs post) (3-way repeated measures ANOVA, p< 0.002). Therefore, we needed to focus on the treatment applications separately.
Figure 8:
The effect of MnSO4 or MnCl2 exposure on heart rate of in situ hearts in 3rd instar larvae. The early 3rd instar larvae is dissected and filleted in order to expose the heart tube to the bathing saline and flush away any endogenous compounds. The heart rate is counted in saline and then the media is changed to saline or to a concentration of either MnSO4 or MnCl2, and this is followed by flushing the preparation with fresh saline without any Mn2+ and the heart rate is counted again. At all concentrations of MnSO4 or MnCl2, the rate dropped significantly upon exposure and the extent in the decreased rate is concentration-dependent. The heart rate did not recover even after 2 minutes following flushing with fresh saline for the 30 mM concentrations for either MnSO4 or MnCl2. There was no significant effect for saline exchanged with saline only. Each line represents an individual larva, and each larva was only examined once.
For the MnCl2 treatment, there was a statistical difference between post measurements (initial – terminal saline) between the 3 dose levels (ANOVA, p=0.024). There was a statistical difference between the 2.5mM and 30mM doses (Bonferroni T-test, p=0.0011) and a lesser significant difference between the 2.5mM and 15mM doses (Bonferroni T-test, p=0.0358). However, there was not statistical difference between pre - measurements, initial saline to treatment application, (ANOVA, p>0.05) between the 3 dose levels.
For the MnSO4 treatment, there were statistical differences between both the pre- and post- measurements between the 3 dose levels (ANOVA, pre p=0.004, ANOVA post <0.0001). For the post measurements, the lowest dose and highest dose were significantly different from each other (Bonferroni T-test, p<0.001) and the lowest dose and middle dose were also significantly different (Bonferroni T-test, p<0.001). Finally, for the pre-measurements, there was only a marginal significance with a Bonferroni T-test with 2.5mM versus 15mM doses (p=0.079).
A percent difference in the heart rate provides a normalized view from the initial differences that can be used for comparisons (Figure 9). The paradigm in exposing the in situ hearts to the compounds allowed comparisons in the heart rate from the initial saline exposure to the compound (Saline to Mn) and the rate in the initial saline to after flushing the Mn2+ bath and returning to the saline without Mn2+ (Mn to Saline) (see Figure 8). Upon flushing the Mn2+ removing saline from the preparations, the heart rate did not return to the initial values, but a semi-recovery was best at 2.5 mM in comparison to the higher concentrations. The 30 mM exposure, for either MnSO4 or MnCl2, affected the heart so substantially that the heart rate was not able to recover in the time observed (2 minutes after flushing away the Mn2+ containing bath).
Figure 9:
Percent differences in heart rate from saline to exposure of MnSO4 or MnCl2. The percent differences are compared for each condition to the initial rate in saline for each individual larva. The “Mn to saline” is the condition of flushing away of Mn to fresh saline and the percent difference is from the initial saline condition.
Crab proprioceptive neurons
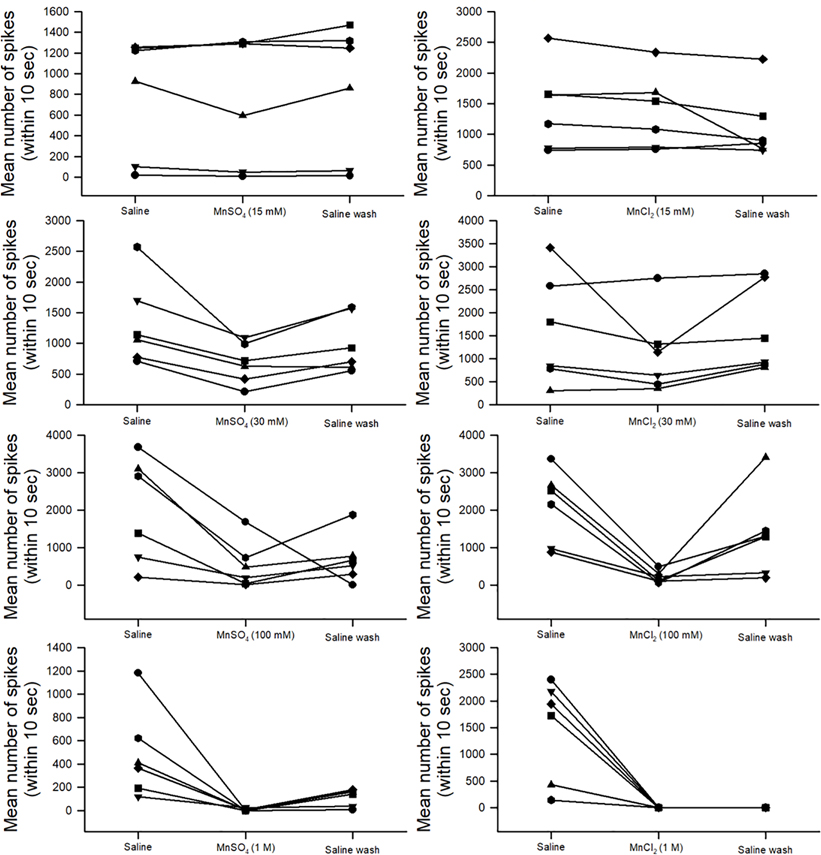
The sensory neurons associated with the crab Pd chordotonal organ showed sensitivity to Mn2+ presented as either MnSO4 or MnCl2 at the high concentration of 100 mM with the depression of neural activity (Wilcoxon Signed Rank test, p<0.05). The 30 mM MnSO4 significantly depressed activity (Wilcoxon Signed Rank test, p<0.05), but the 30 mM MnCl2 did not show significant differences as only 3 of the 6 preparations decreased in average in the number of spikes during the limb displacements while exposed. The 15 mM exposure for either the MnSO4 or MnCl2 did not show significant differences in the number of spikes (Wilcoxon Signed Rank test p>0.05, Figure 10). A high concentration of 1 M for MnSO4 or MnCl2 drastically decreased the activity of the Pd nerve and the activity did not return with exchanging the bath back to fresh saline.
Figure 10:
Activity of the Pd nerve with displacement of the Pd joint from the flexed position to an extended position. The mean number of spikes (averaged of three displacements in each bathing media) for 10 seconds with the 1st second moving the joint from a flex position to an extended position for the remaining 9 seconds (static position). Six preparations were used for each concentration (15, 30, 100 mM and 1M) of MnSO4 or MnCl2. Each line is an individual preparation which was used only once.
Since there was a large variability among preparations for exchanging the bath to low concentrations of Mn2+, it was of interest to know if this also occurred with only exchanging the bath with saline as a sham control (Figure 11A). On average, the percent change in activity with the initial saline to the following saline media exchange increased but was not statistically significant (Figure 11B; Shapiro-Wilks p>0.05, paired t-test, N=6).
Figure 11:
Activity of the Pd nerve with displacement of the Pd joint from the flexed position to an extended position with repeated saline-only bath exchanges. (A) Six preparations were used while measuring the mean number of spikes (average of three displacements for each time the bathing media was exchanged) over 10 seconds with the 1st second being the movement of the joint from a flexed position to an extended position, and the remaining 9 seconds consisting of the extended static position. Each line is an individual preparation which was used only once. (B) The mean percent change from the initial saline exposure to the following bath exchange is depicted along with each individual preparation as dots.
To examine if the 1 M Mn2+ blocked the electrical condition of action potentials in the Pd nerve, evoked nerve activity was induced in order to monitor CAPs. To address the effect of Mn2+, two concentrations were examined (100 mM and 1 M Mn2+). The 100 mM MnSO4 shut down the Pd organ activity, but it was still able to conduct experimentally stimulus-induced nerve impulses (CAPs) along the nerve (Figure 12). In this procedure, the leg is pinned in a static position and the SACs are stimulated in the Pd strand in order to give rise to the spikes seen in Figure 13A. Every 2 seconds, the nerve was electrically stimulated to generate a compound action potential (CAP) as noted by stimulations (i.e. Stim) in Figures 12A1, B1 and C1. Note the CAPs induced are shown in Figures 12A2, B2 and C2 for the same conditions. The CAPS still can be induced while the static position sensitive neurons have stopped firing. This is most likely due to the Mn2+ blocking the SAC within the sensory endings.
Figure 12:
The activity of the stretch-activated channels in the Pd organ and electrical conduction along the Pd nerve. (A1) The activity of the Pd nerve is induced by static-position-sensitive neurons firing while the joint is pinned in a given position (flexed). While recording the activity in the Pd nerve, a stimulation (Stim) is given every 2 seconds by the second suction electrode to induce compound action potentials (CAPs). The CAP from such stimulation is shown in A2. The stimulus artifact (SA) is seen followed by the CAP in the rectangular box. (B1) Exposure to 100 mM MnSO4 decreased the stretch-activated channels (SACs) induced activity in the Pd nerve, but not the ability of the nerve to conduct experimentally induced CAPs (B2). (C1) Return of the sensory response from the SACs appears with the flushing away of Mn2+, but the CAPs are still present (C2).
Figure 13:
The effects of high concentration of MnCl2 or mannitol on the activity of the Pd organ during joint movement and induction of compound action potential (CAP). (A) The activity before and during exposure to 1 M MnCl2. The recording was continuous while adding the MnCl2, which resulted in the activity of the Pd organ to stop immediately. The two large deflections in the trace are due to swishing the bathing media over the nerve. (B) The activity during the movement and hold paradigm before (B1) and during 1 M mannitol exposure (B2). In separate experiments, the CAP was induced before (C1) and during 1 M mannitol exposure (C2). The dotted circle outlines the CAP while the stimulus artifact (SA) is the electrical event in the media from the stimulation.
The activity of the Pd organ rapidly decreased when the joint was held in a static position or during joint displacement when exposed to 1 M Mn2+ (Figure 13A). The CAP was not able to be induced in the nerve during 1 M MnCl2 exposure. Considering that a high concentration of 1 M could alter the nerve’s biophysical properties, an equally high concentration of 1 M mannitol was used to examine the activity of the nerve and the evoked CAPs during the joint displacement regime. The activity of the Pd organ during joint movement and while held in an extended position was still robust while exposed to 1 M mannitol (Figure 13B1 and B2). The shape of the CAP was also very similar before and after exposure to 1 M mannitol (Figure 13C1 and C2). Thus, the high concentration of 1 M MnCl2 is not just due to the concentration. In addition, nerve activity with the same displacement regime was used before and during exposure to 1 M sucrose for six preparations. The percent change in the number of spikes in saline and exposure to 1 M sucrose did not produce a significant change (Figure 14; Shapiro-Wilks, p>0.05, paired t-test, N=6, p=0.216).
Figure 14:
The mean percent change in the number of spikes before and during exposure to 1 M sucrose with displacement of the Pd joint for 10 seconds. There was no significant difference in the activity due to exposure of sucrose.
Neuromuscular junctions of larval Drosophila and crayfish
For assessing synaptic transmission at the neuromuscular junction of the larval D. melanogaster and crayfish, only MnSO4 at 2.5 mM was used because low concentrations of manganese still had a drastic effect in blocking the evoked responses. The intracellular recordings of the body wall muscle (m6) in the larval D. melanogaster have many observable spontaneous quantal events. Note that the evoked excitatory junction potentials (EJPs) rapidly depressed upon exposure and slowly regained some of the amplitude upon removal of the Mn2+ (Figure 15A). However, the full amplitude of the EJPs did not return with flushing fresh saline over the preparation repeatedly. Since the quantal events are present while the evoked responses were blocked, this would indicate that the postsynaptic glutamate receptors on the muscle were not blocked by Mn2+ (Figure 15B). This same trend, as depicted in Figure 15, was observed in 6 out of 6 preparations (Wilcoxon Signed Rank test p<0.05).
Figure 15:
The effect of exposure to MnSO4 (2.5 mM) to synaptic transmission at the larval D. melanogaster neuromuscular junction. (A) Evoked excitatory junction potentials (EJPs) (0.5 Hz stimulation rate) reduced rapidly upon exposure to MnSO4 and slowly recovers upon washing away the MnSO4. (B) The spontaneous quantal events are still present while evoked EJPs are depressed.
The NMJs on the opener muscle of the crayfish, like the NMJs of larval D. melanogaster, were significantly depressed when eliciting evoked EJPs. Additionally, the removal of the Mn2+ partially recovered the amplitude of the EJPs. The occurrence of spontaneous quantal events on the crayfish muscle of the opener muscle was not as frequently observed as for the larval D. melanogaster preparation. However, in the presence of Mn2+, the quantal responses were still present while evoked responses were depressed. This also indicates that the postsynaptic glutamate receptors on the crayfish muscle were not blocked by Mn2+. This same trend, as depicted in Figure 16, was observed in 6 out of 6 preparations for the crayfish NMJ (Wilcoxon Signed Rank test p<0.05).
Figure 16:
The effect of exposure to MnSO4 (2.5 mM) on synaptic transmission at the neuromuscular junction of the opener muscle in the walking leg of crayfish. Evoked excitatory junction potentials (EJPs) (60 Hz stimulation rate for 30 pulses) are reduced rapidly upon exposure to MnSO4 and partially recover after washing away the MnSO4.
Discussion
In this study, we have shown that Mn2+ from MnCl2 or MnSO4 can have profound effects in a dose-dependent manner on development, survival of larvae and adults, behaviors, and cardiac function of D. melanogaster. In addition, Mn2+ depresses synaptic transmission at the D. melanogaster and crayfish NMJs with the proposed mechanism being the blockage of evoked presynaptic transmission. The presence of spontaneous quantal events indicates that the sensitivity of the postsynaptic glutamate receptors is not altered while evoked EJPs are blocked. The proprioceptive sensory input in the crab leg is reduced by Mn2+.The most sensitive physiological disturbances by Mn2+ are on cardiac function and synaptic transmission which are possibly due to blocking voltage-gated Ca2+ channels. The acute 24-hour dietary supplement of Mn2+ depressed body wall and mouth hook movements, which may well be due to effects within the CNS as well as at the NMJs producing the movements.
It would be expected that development and survival would be impacted with acute high concentration of exposure, since low concentrations depressed cardiac function and synaptic transmission at NMJs. Lower concentrations over longer periods of time could potentially allow for compensatory mechanisms to rebuff the effects of a more subtle actions. It is of interest to learn the long-term effects of sublethal doses on neural development and other physiological functions is in larvae. D. melanogaster adults were examined by Bonilla-Ramirez et al., (2011) at 0.5 and 1.0 mM for longer term effects. Adults showed declining climbing abilities over time at these lower doses.
Toxicity studies by feeding MnCl2 or MnSO4 have been used to investigate the effects of exposure to Mn; however, the concentration directed on tissues is more precisely addressed by direct exposure. In this study, larvae were dissected to directly expose the heart to the bathing saline with known concentrations of Mn2+. There were not any large notable differences between delivering Mn2+ via MnCl2 or MnSO4. This is expected as both are salts, which would readily dissociate in water. Thus, if there were differences in the effects, it would be due to the Cl− or SO42− anions. One consideration is the osmolarity changes due to adding MnCl2 or MnSO4 to saline or to the food. Small changes in osmolarity in the saline used for the D. melanogaster heart and NMJs of only 2.5 mM do not cause alterations in function. The BES (a pH buffer) compound as high as 25 mM has been added to the D. melanogaster standard saline for long term studies of hours without decreasing function in neural activity or cardiac function (deCastro et al., 2014, 2019). Synaptic transmission at the crayfish NMJ will generally increase with higher osmolarity (Delaney et al., 1991). If the synaptic vesicle pools would be depleted by prolonged 300 mOSM treatment, then there would be synaptic depression, but one would first observe a large burst of synaptic activity. The effect of osmolarity is also consistent for frog NMJs (Kita & Van der Kloot, 1977; Tanabe & Kijima, 1988; Van der Kloot & Molg, 1994). Thus, the depression in evoked synaptic transmission at the D. melanogaster and crayfish NMJs is not due to the slight increase osmolarity from the 2.5 mM of MnSO4. The crab saline is very high in osmolarity (~1,041 osmolarity), which is common for saline used with marine invertebrates. Increasing osmolarity with a 100 mM MnCl2 or MnSO4 solution may have some impact on the SACs and would most likely result in the activity declining, since it would raise osmolarity outside the neurons and result in shrinkage. However, we have exposed the Pd organ to 1 M sucrose and 1 M mannitol in normal crab saline without any significant changes to neural activity within the time frame used for these studies. We have noted in another on-going study that if Ca2+ and Mg2+ is removed from the saline and then sucrose is added to account for the osmolarity change, there is a depression in overall neural activity of the Pd nerve. This depression does not occur if Ca2+ and Mg2+ is present in the saline along with sucrose as in this study (Parker et al., 2021). For the developing larvae, consuming MnCl2 or MnSO4 in the diet may have additional effects in relation to increasing osmolarity or even the Cl− or SO42− anions. As a control, NaCl at the same concentrations as MnCl2 or MnSO4 were used; however, using the same concentration is not equivalent to the osmolarity in solution. The increased Na+ could also have an impact itself with transport mechanics within the gastrointestinal tract due to co-transports with Na+ ions. The effects of NaCl in the diet did not cause any alteration in time to pupation or survival of the larvae from 2.5 mM to 100 mM.
The effects at the glutamatergic NMJs of the larval D. melanogaster and crayfish at a low concentration (2.5 mM) illustrate that Mn2+ is not specific to dopaminergic synapses. Oddly, it appears Mn2+ exposure enhances dopamine release from dopaminergic neurons in rodent models (Lin et al., 2020) where it depressed synaptic transmission at the glutamatergic NMJs. Blocking the Ca2+ channels with pharmacological agents prevents the Mn2+-induced transmitter release in the dopaminergic neurons in which Lin et al (2020) suggests is due to Mn2+ fluxing through Ca2+ channels. Mn2+ has been noted to drastically depress evoked synaptic transmission in frog cholinergic NMJs (70 μM; Meiri and Rahamimoff, 1972) and block central glutamatergic synapses in the frog at 1 mM (Hackett, 1976).
Since MnSO4 is used at high levels in agricultural sprays, (2000–4000 mg/kg in blueberries; Korcak, 1988) and as a nutrient in soil, it is of interest to know what levels might run off into streams, lakes, marine environments, as well as bioaccumulated in the food chain (Eriksson 2000a). If Mn2+ can be accumulated in a mother’s diet as well as formula milk, the subtle effects on long-term exposure during fetal and infant develop need to be addressed (Howe, et al., 2004; Eriksson 2000b). Taking a comparative approach among different animals and systems has provided an overview of the potential effects of Mn2+ in the environment on invertebrates in general.
In summary, this study demonstrated that Mn2+ obtained as either MnSO4 or MnCl2 has pronounced effects on D. melanogaster larval survival. Concentrations higher than 10 mM in the diet were toxic for the 1st instar of larval D. melanogaster. For adult D. melanogaster, a 15 mM concentration in tainted food resulted in low survival rates and 30 mM was toxic. Additionally, the crawling and eating behaviors of larval D. melanogaster indicated that they are not healthy after 24 hours of consuming 15 mM tainted food. Direct exposure of the larval heart to saline containing 2.5 mM rapidly decreases the heart rate and can even stop the heart from beating. Even after flushing the Mn2+-containing saline away, the heart rate did not recover. Proprioceptive sensory neurons in the marine crab decreased in function with acute exposure at 15 mM while nerves were still able to conduct electrical signals. This indicated that the stretch-activated channels in the sensory ending were likely targeted by Mn2+. Synaptic transmission at NMJ was rapidly blocked in both larval D. melanogaster and the freshwater crayfish with saline at 2.5 mM Mn2+ while spontaneous quantal responses were still observed. This indicated a presynaptic block of evoked transmission which is likely due to the blocking of voltage gated Ca2+ channels.
In future studies, the sensitivity to exogenously applied Mn2+ directly on in situ exposed sensory–central nervous system–motor circuit can be compared to the behavior of animals fed Mn2+. It would be of interest to know if fetal and embryonic tissue also accumulate Mn2+ in various animal models from insects to humans.
Funding: Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103436 (H.T.). and Howard Hughes Medical Institute (#52008116) awarded to the Univ. KY (V.M. Cassone, PI) for development of StemCats undergraduate research experiences. Chellgren Endowed Funding (R.L.C.).
Figure 2:
The first or second walking leg of the crab was used to expose the Pd organ and associated nerve. (A) The joint was initially bent at 90 degrees and then extended out to 180 degrees within 1 second and held for at least another 9 seconds. (B) The entire 10 seconds was then used for analysis in the number of spikes that occurred while being bathed in different solutions.
Table 1:
Larval development and survival study for NaCl, MnSO4 and MnCl2 at 0.0025, 0.015, 0.03 and 0.1 M.
| Food: NaCl (mM) | MnSO4 | MnCl2 | Days | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5 | 15 | 30 | 100 | 2.5 | 15 | 30 | 100 | 2.5 | 15 | 30 | 100 | |
|
| ||||||||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| 18 | 17 | 15 | 17 | 18 | 6 | 0 | 0 | 18 | 1 | 0 | 0 | 4 |
| 30 | 30 | 30 | 29 | 30 | 15 | 0 | 0 | 30 | 1 | 0 | 0 | 5 |
| 30 | 30 | 30 | 29 | 30 | 15 | 0 | 0 | 30 | 1 | 0 | 0 | 6 |
| 30 | 30 | 30 | 29 | 30 | 15 | 0 | 0 | 30 | 1 | 0 | 0 | 7 |
| 30 | 30 | 30 | 29 | 30 | 15 | 0 | 0 | 30 | 1 | 0 | 0 | 8 |
| 30 | 30 | 30 | 29 | 30 | 15 | 0 | 0 | 30 | 1 | 0 | 0 | 9 |
| No Adults | No Adults | No Adults | No Adults | No Adults | No Adults | No pupa | No pupa | No Adults | No Adults | No pupa | No pupa | 10 |
| 1 | 2 | 6 | 0 | 4 | 0 | - | - | 0 | 0 | - | - | 11 |
| 17 | 20 | 19 | 15 | 20 | 15 | - | - | 16 | 0 | - | - | 12 |
| 30 | 30 | 30 | 30 | 30 | 15 | - | - | 30 | 1 | - | - | 13 |
Larvae were placed in food with the compounds from early 1st instar. The number of pupae formed are recorded. The number of adults which emerged from the pupae are also recorded over the days from placing 1st instars in the food.
Highlights.
Exposure to Mn2+ slows development and decreases survival of Drosophila larvae
Mn2+ depresses cardiac function in larval Drosophila
Synaptic transmission at neuromuscular junctions in Drosophila and crayfish is depressed by Mn2+
Proprioceptive function in crab limbs is compromised by Mn2+ in a dose-dependent manner
Footnotes
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiello EL 1990. Nervous control of gill ciliary activity in Mytilus edulis. Stefano GB (Ed.), Neurobiology of Mytilus edulis, vol. 10, Manchester University Press, Manchester: (1990), pp. 189–208. [Google Scholar]
- Alexandrowicz JS, 1972. The comparative anatomy of leg propriocetors in some decapod Crustacea. J. Mar. Biol. Assoc. U.K. 52(3), 605–634. [Google Scholar]
- Baden SP, Eriksson SP, 2006. Role, routes and effects of manganese in crustaceans. Oceanography and Marine Biol. 44, 61–83. [Google Scholar]
- Badre NH, Cooper RL, 2008. Reduced calcium channel function in Drosophila disrupts associative learning in larva, and behavior in adults. Inter. J. Zool. Res. 4(3), 152–164. [Google Scholar]
- Baek SY, Lee MJ, Jung HS, Kim HJ, Lee CR, Yoo C, Lee JH, Lee H, Yoon CS, Kim YH, Park J, Kim W, Jeon BS, Kim Y, 2003. Effect of manganese exposure on MPTP neurotoxicities. Neurotoxicol. 24, 657–665. [DOI] [PubMed] [Google Scholar]
- Bonilla-Ramirez L, Jimenez-Del-Rio M, Velez-Pardo C, 2011. Acute and chronic metal exposure impairs locomotion activity in Drosophila melanogaster: A model to study Parkinsonism. Biometals. 24(6),1045–1057. [DOI] [PubMed] [Google Scholar]
- Bowman AB, Kwakye GF, Hernandez EH, Aschner M, 2011. Role of manganese in neurodegenerative diseases. J. Trace Elem. Med. Biol 25, 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman KA, Cattley RC, Ali SF, Dorman DC, 1999. Manganese induced developmental neurotoxicity in the CD rat: is oxidative damage a mechanism of action? Neurotoxicol. 20, 477–488. [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W, 1994. Manganism and idiopathic parkinsonism: Similarities and differences. Neurol. 44, 1583–1586. [DOI] [PubMed] [Google Scholar]
- Carroll MA, Catapane EJ, 2007. The nervous system control of lateral ciliary activity of the gill of the bivalve mollusc, Crassostrea virginica. Comp. Biochem. Physiol. A 148, 445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catapane EJ, Stefano GB, Aiello E, 1978. Pharmacological study of the reciprocal dual innervation of the lateral ciliated gill epithelium by the CNS of Mytilus edulis (Bivalvia). J. Exp. Biol. 74, 101–113. [Google Scholar]
- Chen H, Yang J, Deng X, Lei Y, Xie S, Guo S, Ren R, Li J, Zhang Z, Xu T, 2020. Foliar-sprayed manganese sulfate improves flavonoid content in grape berry skin of Cabernet Sauvignon (Vitis vinifera L.) growing on alkaline soil and wine chromatic characteristics. Food Chem. 314, 126182. [DOI] [PubMed] [Google Scholar]
- Cooper RL, 2008. Proprioceptive neurons of chordotonal organs in the crab, Cancer magister dana (Decapoda, Brachyura). Crustaceana 81, 447–475. [Google Scholar]
- Cooper AS, Cooper RL, 2009. Historical view and physiology demonstration at the NMJ of the crayfish opener muscle. J. Vis. Exp. 33, e1595, doi: 10.3791/1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RL, Marin L, Atwood HL, 1995. Synaptic differentiation of a single motor neuron: conjoint definition of transmitter release, presynaptic calcium signals, and ultrastructure. J. Neurosci.15, 4209–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AS, Rymond KE, Ward MA, Bocook EL, Cooper RL, 2009. Monitoring heart function in larval Drosophila melanogaster for physiological studies. J. Vis. Exp. 33, e1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S, Viele K, Turner AC, Cooper RL, 2007. Influence of p-CPA and MDMA on physiology, development and behavior in Drosophila melanogaster. Eur. J. Neurosci. 26, 424–438 [DOI] [PubMed] [Google Scholar]
- de Castro C, Titlow J, Majeed ZR, Cooper RL, 2014. Analysis of various physiological salines for heart rate, CNS function, and synaptic transmission at neuromuscular junctions in Drosophila melanogaster larvae. J. Comp. Physiol. A 200, 83–92. [DOI] [PubMed] [Google Scholar]
- de Castro C, Titlow JS, Majeed ZR, Malloy C, King KE, Cooper RL, 2019. Chemical and mechanical factors required for maintaining cardiac rhythm in Drosophila melanogaster larva. J. Entomol.16(2), 62–73. [Google Scholar]
- Delaney K, Tank DW, Zucker RS, 1991. Presynaptic calcium and serotonin-mediated enhancement of transmitter release at crayfish neuromuscular junction J. Neurosci. 11 (9), 2631–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai-Shah M, Papoy AR, Ward M, Cooper RL, 2010. Roles of the SERCA, PMCA and NCX in calcium regulation in the Drosophila larval heart. The Open Physiol. J. 3, 16–36. [Google Scholar]
- Eriksson SP, 2000a. Temporal variations of manganese in the haemolymph and tissues of the Norway lobster, Nephrops norvegicus (L.). Aquatic Toxicol. 48(2–3), 297–307. [DOI] [PubMed] [Google Scholar]
- Eriksson SP, 2000b. Variations of manganese in the eggs of the Norway lobster, Nephrops norvegicus (L.). Aquatic Toxicol. 48(2–3), 291–295. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Dobson AW, Dorman DC, Aschner M, 2004a. Manganese exposure and induced oxidative stress in the rat brain. Sci. Total Environ. 334–335, 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson H, Magiste K, Plantin LO, Fonnum F, Hedstrtm K, Theodorsson G, Norheim E, Kristensson K, Stalberg E, Heilbronn E, 1987. Effects of manganese oxide on monkeys as revealed by a combined neurochemical, histological and neurophysiological evaluation. Arch. Toxicol. 61, 46–52. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Syversen T, Steinnes E, Aschner M, 2004. Globus pallidus: a target brain region for divalent metal accumulation associated with dietary iron deficiency. J. Nutr. Biochem 15(6), 335–41. [DOI] [PubMed] [Google Scholar]
- Frisbie SH, Mitchell EJ, Roudeau S, Domart F, Carmona A, Ortega R, 2019. Manganese levels in infant formula and young child nutritional beverages in the United States and France: Comparison to breast milk and regulations. PLoS One. 14(11), e0223636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett JT, 1976. Selective antagonism of frog cerebellar synaptic transmission by manganese and cobalt ions. Brain Res. 114(1), 47–52. [DOI] [PubMed] [Google Scholar]
- Hartman HB, Boettiger EG, 1967. The functional organization of the propys-dactylus organ in Cancer irroratus Say. Comp. Biochem. Physiol. 22, 651–663. [Google Scholar]
- Howe PD, Malcolm HM, Dobson S, 2004. World Health Organization & International Programme on Chemical Safety. Manganese and its compounds: Environmental aspects. World Health Organization. https://apps.who.int/iris/handle/10665/42992 [Google Scholar]
- Jhanji S, Sadana US, Shankar A, Shukla AK, 2014. Manganese influx and its utilization efficiency in wheat. Indian J. Exp. Biol. 52(6), 650–657. [PubMed] [Google Scholar]
- Jiang Y, Zheng W, 2005. Cardiovascular toxicities upon manganese exposure. Cardiovasc. Toxicol. 5(4), 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone AFM, Cooper RL, 2006. Direct innervation of the Drosophila melanogaster larval aorta. Brain Res. 1083, 159–163. [DOI] [PubMed] [Google Scholar]
- Kita H, Van der Kloot W, 1977. Time course and magnitude of effects of changes in tonicity on acetylcholine release at frog neuromuscular junction. J. Neurophysiol. 40, 212–224. [DOI] [PubMed] [Google Scholar]
- Korcak RF, 1988. Response of blueberry species to excessive manganese. J. Amer. Soc. Horticult. Sci. 113(2),189–193. [Google Scholar]
- Lin M, Colon-Perez LM, Sambo DO, Miller DR, Lebowitz JJ, Jimenez-Rondan F, Cousins RJ, Horenstein N, Aydemir TB, Febo M, Khoshbouei H, 2020. Mechanism of manganese dysregulation of dopamine neuronal activity. J. Neurosci. 40(30), 5871–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed ZR, Stacy A, Cooper RL, 2014. Pharmacological identification of serotonin receptor subtypes on Drosophila larval heart. J, Comp. Physiol. B. 184(2), 205–219. [DOI] [PubMed] [Google Scholar]
- Majeed ZR, Titlow J, Hartman HB, Cooper RL, 2013. Proprioception and tension receptors in crab limbs: Student laboratory exercises. J. Vis. Exp. 80, e51050, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy C, Sifers J, Mikos A, Samadi A, Omar A, Hermanns C, Cooper RL, 2017. Using optogenetics to assess neuroendocrine modulation of heart rate in Drosophila melanogaster larvae. J. Comp. Physiol. A. 203(10), 791–806. [DOI] [PubMed] [Google Scholar]
- Marreilha dos Santos AP, Santos D, Au C, Milatovic D, Aschner M, Batoréu MC, 2008. Antioxidants prevent the cytotoxicity of manganese in RBE4 cells. Brain Res. 1236, 200–205. [DOI] [PubMed] [Google Scholar]
- Martin K, Huggins T, King C, Carroll MA, Catapane EJ, 2008. The neurotoxic effects of manganese on the dopaminergic innervation of the gill of the bivalve mollusc, Crassostrea virginica. Comp. Biochem. Physiol. C 148, 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubbin S, Jeoung A, Waterbury C, and Cooper RL, 2020. Pharmacological profiling of stretch activated channels in proprioceptive neuron. Comp. Biochem. Physiol. C 233,108765. [DOI] [PubMed] [Google Scholar]
- Meiri U, Rahamimoff R, 1972. Neuromuscular transmission: inhibition by manganese ions. Science. 176(4032), 308–309. [DOI] [PubMed] [Google Scholar]
- Mohandas G, Rao SV, Muralidhara Rajini PS, 2017. Whey protein isolate enrichment attenuates manganese-induced oxidative stress and neurotoxicity in Drosophila melanogaster: Relevance to Parkinson’s disease. Biomed. Pharmacother. 95, 1596–1606. [DOI] [PubMed] [Google Scholar]
- Nagatomo S, Umehara F, Hanada K, Nobuhara Y, Takenaga S, Arimura K, et al. , 1999. Manganese intoxication during total parenteral nutrition: Report of two cases and review of the literature. J. Neurol. Sci, 162, 102–105. [DOI] [PubMed] [Google Scholar]
- Nelson M, Adams T, Ojo C, Carroll MA, Catapane EJ, 2018. Manganese toxicity is targeting an early step in the dopamine signal transduction pathway that controls lateral cilia activity in the bivalve mollusc Crassostrea virginica. Comp. Biochem. Physiol. C 213, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M, Huggins T, Licorish R, Carroll MA, Catapane EJ, 2010. Effects of p-aminosalicylic acid on the neurotoxicity of manganese on the dopaminergic innervation of the cilia of the lateral cells of the gill of the bivalve mollusc, Crassostrea virginica. Comp. Biochem. Physiol. C 151, 264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland MC, 1999. Animal models of manganese’s neurotoxicity. Neurotoxicol, 20, 415–432. [PubMed] [Google Scholar]
- Ochs AT, Shields JD, Rice GW, Unger MA, 2020. Acute and long-term manganese exposure and subsequent accumulation in relation to idiopathic blindness in the American lobster, Homarus americanus. VIMS Articles. 1835. https://scholarworks.wm.edu/vimsarticles/1835 [DOI] [PubMed] [Google Scholar]
- Pal PK, Samii A, Calne DB, 1999. Manganese neurotoxicity: A review of clinical features. Neurotoxicol. 20, 227–238. [PubMed] [Google Scholar]
- Parker N, Tanner H, Cooper RL, 2021. The effect of calcium on mechanosensation and neuronal activity in proprioceptive neurons. Soc. Neurosci. Annual Mtg. Nov. 13–17, 2021. Chicago, IL, USA. [Google Scholar]
- Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF 1994. Improved stability of Drosophila larval neuromuscular preparation in haemolymph-like physiological solutions. J. Comp. Physiol. A. 175, 179–191. [DOI] [PubMed] [Google Scholar]
- Tanabe N, Kijima H, 1988. Transmitter release at frog end-plate loaded with a Ca2+-chelator, BAPTA: hypertonicity and erythrosin B augment the release independently of internal Ca2+. Neurosci. Let. 92, 52–57. [DOI] [PubMed] [Google Scholar]
- Titlow JS, Cooper RL, 2018. Glutamatergic synthesis, recycling, and receptor pharmacology at Drosophila and crustacean neuromuscular junctions. In: Parrot S, Denoroy L (eds) Biochemical Approaches for Glutamatergic Neurotransmission. Neuromethods, vol 130. Humana Press, New York, NY. 10.1007/978-1-4939-7228-9_9 [DOI] [Google Scholar]
- Titlow JS, Rufer J, King K, Cooper RL, 2013. Pharmacological analysis of dopamine modulation in the Drosophila melanogaster larval heart. Physiol. Rep. 1(2), e00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W, Molgo J, 1994. Quantal acetylcholine release at the vertebrate neuromuscular junction. Physiol. Rev. 74, 899–991. [DOI] [PubMed] [Google Scholar]
- Verspoor RL, Soglo M, Adeoti R, Djouaka R, Edwards S, Fristedt R, Langton M, Moriana R, Osborne M, Parr CL, Powell K, Hurst GDD, Landberg R, 2020, Mineral analysis reveals extreme manganese concentrations in wild harvested and commercially available edible termites. Sci. Rep. 10(1), 6146. doi: 10.1038/s41598-020-63157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitear M, 1938. Chordotonal organs in Crustacea. Nature. 187, 522–523. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Fang Z, Li J, Sui T, Lin L, Xu X, 2019, Copper, zinc, manganese, cadmium and chromium in crabs from the mangrove wetlands in Qi’ao Island, South China: Levels, bioaccumulation and dietary exposure. Watershed Ecology and the Environ.1, 26–32. 10.1016/j.wsee.2019.09.001. [DOI] [Google Scholar]
- Zotti M, Coco LD, Pascali SA, Migoni D, Vizzini S, Mancinelli G, Fanizzi FP, 2016. Comparative analysis of the proximate and elemental composition of the blue crab Callinectes sapidus, the warty crab Eriphia verrucosa, and the edible crab Cancer pagurus. Heliyon. 2(2), e00075. [DOI] [PMC free article] [PubMed] [Google Scholar]