Abstract
Healing of articular cartilage is a major clinical challenge as it also lacks a direct vasculature and nerves, and carries a limited number of resident chondrocytes that do not proliferate easily. Damaged articular cartilages are usually replaced by fibrocartilages, which are mechanically and structurally weaker and less resilient. Regenerative medicine involving stem cells is considered to have a definitive potential to overcome the limitations associated with the currently available surgical methods of cartilage repair. Among various stem cell types, mesenchymal stem cells (MSCs) are preferred for clinical applications. These cells can be readily derived from various sources and have the ability to trans-differentiate into various tissue-specific cells, including those of the cartilage by the process of chondrogenesis. Compared to embryonic or induced pluripotent stem cells (iPSCs), no ethical or teratogenic issues are associated with MSCs. These stem cells are being extensively evaluated for the treatment of joint affections and the results appear promising. Unlike human medicine, in veterinary medicine, the literature on stem cell research for cartilage regeneration is limited. This review, therefore, aims to comprehensively discuss the available literature and pinpoint the achievements and limitations associated with the use of MSCs for articular cartilage repair in animal species.
Keywords: Animals, cartilage regenerative medicine, chondrogenesis, clinical studies, mesenchymal stem cell, preclinical studies
1. Introduction
1.1. Cartilage structure and its lack of regenerative potential
The articular cartilage is an opalescent layer of hyaline tissue that furnishes an exceptional resilience and almost frictionless movement to the diarthrodial joints (Mankin 1984). Cartilage is a highly differentiated tissue maintained by a single exiguously distributed cell type, known as the chondrocyte, and is devoid of direct blood vessels, lymphatics or nerves (Kinner et al. 2005; Duarte Campos et al. 2012). Like other tissues, stem cells are also present in the articular cartilage but their role remains to be elucidated (Williams et al. 2010; Pretzel et al. 2011; Nelson et al. 2014). Overall, the cartilage structure is the same in all species and comprises of superficial, radial, and deep zones. The deep zone is separated from the subchondral bone by a wavy tidemark zone. The cartilage matrix mainly comprises water, collagen (imparts tensile strength), and proteoglycans (provide functional resistance to compression) (Maroudas 1979; Pool 2001). The thickness of the articular cartilage varies from one type of joint to another and also with the age of the animal (Athanasiou et al. 1995). The average thickness of the knee cartilage in adult rabbits, sheep, dogs, goats, horses, and humans is 0.3, 0.4–0.5, 0.6–1.3, 0.7–1.5, 1.5–2.0, and 2.2–2.5, respectively (Frisbie et al. 2006).
The articular cartilage has limited healing potential because it is a terminally differentiated tissue lacking a direct connection with the vasculature and innervations. Osteoarthritis (OA), a common cause of joint dysfunction, may be induced either by trauma or auto-immune reactions. Trauma-induced defects in the cartilage may be either of the partial- or full-thickness type. Partial-thickness defects are confined to the cartilage tissue itself while those of full-thickness penetrate the subchondral bone (Hunziker 1999; Gugjoo et al. 2016). Due to absence of the fibrin clot and thus, reparative stem cells, partial-thickness defects do not heal spontaneously. These defects are analogous to fissures or clefts seen in the early stages of OA (Hunziker 1999). Although the full-thickness defects heal spontaneously, they result in a mechanically and structurally weakened fibrous tissue that lacks integration with the native cartilage (Hunziker 1999; Arican et al. 2006; Tiwary et al. 2014).
Auto-immune diseases like rheumatoid arthritis involve a more generalized affection of the joints with progressive cartilage erosion. OA affects about 21.4 and 20.0% of the human (Barbour et al. 2016) and dog (Johnston 1997) population, respectively. In horses, OA is one of the most common causes of lameness. A survey has reported that approximately 33% of the equine patients carry cartilage lesions associated with OA (Rose 1977). As the cartilage is a weight-bearing tissue, its erosion from joints elicits pain and progresses to the loss of joint function. Therefore, it is imperative to develop therapeutic approaches that can regenerate the integrated hyaline tissues for better joint rehabilitation (Hunziker 1999; Gugjoo et al. 2016; Juneau et al. 2016).
1.2. Why mesenchymal stem cell therapy?
Majority of the current treatment options available for cartilage rehabilitation fail to regenerate the cartilage structure. Surgical procedures like induction of microfractures, subchondral bone drilling, lavage and debridement, perichondral arthroplasty, periosteal arthroplasty, autologous osteochondral transplantation, and autogenetic cancellous bone grafts have failed to regenerate the articular cartilage effectively (Tiwary et al. 2014; Gugjoo et al. 2016; Jeuken et al. 2016; Gugjoo et al. 2017; Wang 2017). To address this issue, there is an increasing focus on the study of cartilage in the field of regenerative medicine and different ways of employing various components, including the cells for cartilage regeneration being devised (Kaiser 1992; Ehnert et al. 2009). The cells employed for this purpose are either stem cells or tissue-specific chondrocytes. Chondrocytes are primarily employed for majority of the cellular therapies (approximately 80%) in cartilage regenerative medicine (Fraser et al. 2006). Results of the chondrocyte implantation (ACI) technique are appreciable but its clinical applications are limited due to limited availability of their sources, likelihood of the cells to dedifferentiate into fibroblasts, and degeneration in the pre-damaged cartilage (Punwar and Khan 2011). Additionally, the aging chondrocytes show declining mitotic and synthetic activity and synthesize smaller and less uniform aggrecan molecules bearing less functional link proteins (Adkisson et al. 2001).
Comparatively, although stem cells contribute to only about 15% of the cellular therapies for cartilage regeneration, their involvement is increasing with each passing day (Fraser et al. 2006). Stem cells harvested from numerous sources have the ability to differentiate into different lineages based on the available niche. It is considered to be an all-in-one solution for diverse ailments including those of the cartilage. Among various types of stem cells, the adult multi-potent MSCs mainly contribute to regenerative therapeutics. These cells are readily available from numerous sources, easily harvested and have an ability to differentiate into mesodermal and extra-mesodermal tissues. Furthermore, the teratogenic and ethical issues associated with embryonic stem cell (ESC) and induced pluripotent stem cells (iPSCs) therapy are not encountered with the application of MSCs (Cardoso et al. 2017; Wang et al. 2017; Gugjoo, Amarpal, Sharma, et al. 2019).
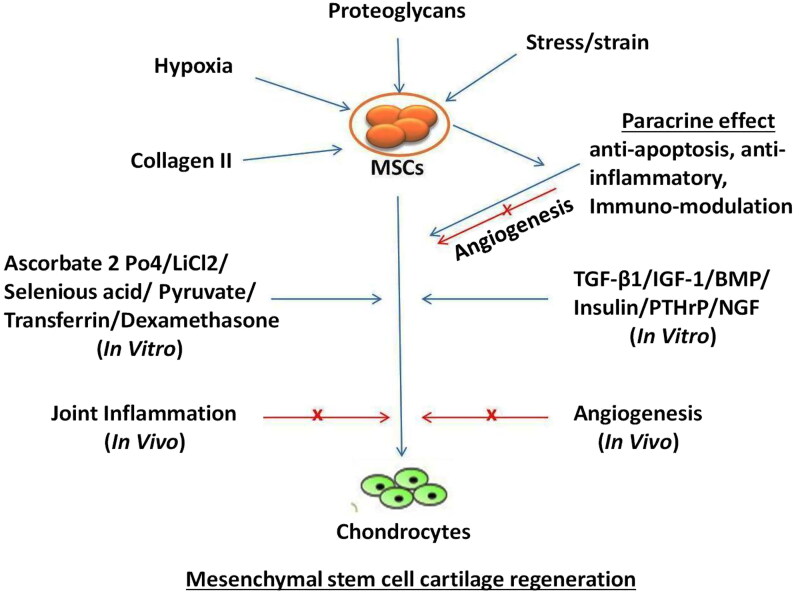
Extensive literature available on the use of MSCs have variably supported their therapeutic potential (Carrade, Affolter, et al. 2011; Carrade, Lame, et al. 2011; Spaas, Oosterlinck, et al. 2012; Spaas, Guest, et al. 2012 ; Iacono et al. 2016; Gugjoo et al. 2017; Kazemi et al. 2017; Kriston-Pál et al. 2017; Feng et al. 2018; Zhang et al. 2018). Terminal differentiation or paracrine action of the MSCs can provide relevant clinical benefits. Initially, it was considered that the MSCs contribute to lesion healing by integrating directly into the tissue. However, this mechanism is considered relatively insignificant compared to their trophic effect (Stewart and Stewart 2011; Gugjoo, Amarpal, Makhdoomi et al. 2019). The trophic action involves the release of a diverse array of cytokines, growth factors, chemokines, and immuno-modulatory proteins (Stewart and Stewart 2011; Gugjoo, Amarpal, Fazili, et al. 2019). This action may be induced by the secretion of proteins or peptides and hormones, transfer of mitochondria through tunneling nanotubes or microvesicles, and/or the transfer of exosomes or microvesicles containing RNA and other molecules (Figure 1) (Spees et al. 2016; Gugjoo, Amarpal, Makhdoomi, et al. 2019).
Figure 1.
In vitro and in vivo mesenchymal stem cell cartilage regeneration. Blue arrows represent facilitation of chondrogenesis; red arrows represent inhibition of chondrogenesis; x represents blocking the pathway.
The characteristic immuno-compromised, immuno-modulatory, and anti-inflammatory features of MSCs make them more appropriate therapeutic agents for OA. The MSCs express MHC-I and variably express MHC-II or T-cell co-stimulatory molecules, particularly those derived from for instance the equine bone marrow (BM), umbilical cord (UC) matrix, and/or UC blood (De Schauwer et al. 2014; Schnabel et al. 2014; Berglund et al. 2017). Equine MSCs express cytokines such as TSG-6 (receptor antagonist of pro-inflammatory cytokine IL-1) and IL1-Ra (anti-inflammatory action and inhibitor of matrix metalloproteinases) that reduce inflammation (Kode et al. 2009). MSCs obtained from different sources decrease lymphocyte proliferation, produce tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), and increase the secretion of prostaglandin (PGE2) and interleukin-6 (IL-6) (Kang et al. 2008; Kode et al. 2009; Peroni and Borjesson 2011; Carrade et al. 2012; Colbath et al. 2017; Yang et al. 2018). Despite these desirable features observed in vitro, these cells cannot be used as definitive therapy in clinical conditions as contrary to their laboratory observations, under in vivo conditions, these cells are uncontrolled. Moreover, these cells do not remain confined to the implantation site but migrate and reach other sites (migration and homing) (Guest et al. 2008; Kode et al. 2009; Stewart & Stewart 2011). Therefore, the results obtained in in vitro studies may not be reproduced exactly as those under in vivo conditions.
The literature cited in this manuscript has been retrieved from various authentic sources such as MEDLINE, PubMed, PubMed Central, and ScienceDirect. Initially, data on general studies relevant to animal MSCs were collected. From the collected material, the literature on chondrogenic studies was sorted. Application of the collected information was aimed at evaluating the chondrogenic potential of MSCs of animal origin both in vitro as well as in vivo. Additionally, our own experience regarding this topic has been shared.
2. In vitro studies on mesenchymal stem cell
MSCs are present in majority of the tissues of an adult individual and are characterized by specialized properties such as self-renewal, multiplication, immuno-modulation, and multi-lineage differentiation (Gugjoo, Amarpal, Makhdoomi, et al. 2019). Isolation followed by in vitro culturing of the stem cells is imperative because they are present in limited concentrations in the tissues. MSCs usually have a limited expansion potential and after a certain number of passages, the cells tend to become senescent as their viabilities and proliferation potentials decrease. Such variability is associated with the tissue source, age of the animal, and techniques employed for their culturing (Corradetti et al. 2013; Xiong et al. 2014). Many variations have also been reported among different breeds. As an illustration, BM-MSCs from the German Shepherd, Labrador, and Golden Retriever dogs tend to undergo senescence rapidly compared to those derived from the Border Collie, Malinois, and Hovawart breeds (Bertolo et al. 2015).
To confirm the presence of MSCs, in vitro cellular and molecular characterization is performed based on the recommendations of the International Society for Cell Therapy (ISCT), which include evaluation of plastic adherence, expression of surface receptors (CD105, CD90, CD73, and CD90) and inability to express CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA-DR molecules. Furthermore, the cells should at least differentiate into the osteogenic, chondrogenic and adipogenic lineages in their respective media (Dominici et al. 2006). Although these recommendations were earlier applicable only for the human MSCs, the same recommendations have also been adopted for the characterization of animal MSCs (De Schauwer et al. 2011; Pascucci et al. 2011; Gugjoo et al. 2015; Hillmann et al. 2016; Broeckx et al. 2019). MSCs generally meet the criteria for plastic adherence and pluripotency, but fail to meet those on surface marker expression. The differences in the expression patterns of their surface markers vary with the type of antibody used, cell sources, and methods employed for culturing (Ranera et al. 2011; Screven et al. 2014).
2.1. In vitro culturing of MSCs for chondrogenesis
In vitro chondrogenesis may be achieved either in 2D or 3D culture systems. However, the efficiency of chondrogenesis tends to be lower in the former system. An in vitro scaffold-based 3D culture system for chondrogenesis is being increasingly studied compared to the commonly studied scaffold-free one. Such a system tends to support cell aggregation, mimic the in vivo environment, improve cell communication, and produce the extracellular matrix (ECM) (Liu et al. 2016; Nam et al. 2018). The scaffold-free 3D cultures which are commonly studied include pellet and micromass culture systems. In the pellet system, cells in the pellet form are entrapped into the secreted ECM, unlike that of the micromass culture. The two systems are variably supported for chondrogenic studies. In general, the efficiency of chondrogenesis is enhanced in the micromass culture technique but the pellet culture is considered to be more useful for clinical applications. This preference to the pellet culture system is due to its enhanced efficiency in generating sufficient chondrocytes compared to that of the micromass culture technique (Nam et al. 2018). Some in vitro studies have demonstrated that the cartilage may be generated by recapitulating various developmental processes of mesenchymal condensation. The cartilage tissue thus secreted may resemble a hyaline tissue, both, in physiological stratification as well as biomechanical features (Nam et al. 2018). For instance, evaluation of the phenotype of chondrogenically differentiated equine BM-MSCs reveals the presence of primary cilia and an intense synthetic and metabolic activity comparable with that of the chondrocyte phenotype, depicting a steady transformation of MSCs into the actual chondrogenic lineage (Luesma et al. 2016). Overall, native chondrocytes are considered superior in their ability to secrete a matrix with better mechanical properties compared to the hydrogel laden BM-MSCs (Mauck et al. 2006).
The initial step involved in the in vitro chondrogenesis of MSCs is the creation of condensed mesenchymal cell bodies (CMBs). These consist of packed MSCs that have increased cell–cell contact but do not undergo any proliferation. This is followed by the process of chondrogenic differentiation utilizing different growth factors (Hall and Miyake 2000; DeLise et al. 2000; Vickers et al. 2010; Bhumiratana et al. 2014). Under in vitro conditions, the CMBs generate tissues comparable to those of the native cartilages on osseous surfaces and also develop mechanically strong, completely integrated interfaces between the cartilages and their tissues (Bhumiratana et al. 2014). Osteogenic differentiation of MSCs primarily takes place by the intra-membranous ossification pathway (Scotti et al. 2010). To create an environment favorable for chondrogenesis, it is imperative to push MSCs down the endochondral ossification pathways involving condensation of MSCs followed by chondrogenic differentiation and formation of the cartilage template, following which, their progress may be restricted before the osteogenic pathways ensue (Kozhemyakina et al. 2015). The chondrogenesis is supported by the expression of Sox9 which represses chondrocyte hypertrophy possibly by inhibiting Runx2 (Zhou et al. 2006), Wnt (Topol et al. 2009), Col10a1, and VEGFA (Hattori et al. 2010; Leung et al. 2011), required for osteogenesis. The upregulation of Sox6 that in turn promotes the Sox9 gene may be promoted by pyruvate dehydrogenase kinase isoform 2 (PDK2) (Wang et al. 2017).
In a previous study, evaluation of the chondrogenic potential of the MSCs was accomplished by the incorporation of dexamethasone and TGF-β1 into the culture (Johnstone et al. 1998). Subsequent successful chondrogenic studies utilized many other growth factors and chemicals including the insulin-like growth factor-1 (IGF1), bone morphogenetic proteins (BMPs), parathyroid hormone-related peptides (PTHrP), insulin, ascorbate-2-phosphate, selenious acid, transferrin, sodium pyruvate, nerve growth factor (NGF), and lithium chloride (Johnstone et al. 1998; Mackay et al. 1998; Yoo et al. 1998; Lee et al. 2000; Sekiya et al. 2001; Zhang et al. 2004; Sekiya et al. 2005; Kim et al. 2008; Pei et al. 2008; Guilak et al. 2010; Lu et al. 2017). Different growth factors used at varying concentrations result in different expressions of these cells. After 14 d of cell culture, TGF-β1 (10 ng/mL) (Zeiter et al. 2009) and TGF-β3 (1, 10, and 100 ng/mL) (Goldman and Barabino 2016) induced the expression of chondrogenic genes in bovine BM-MSCs. Contrarily, the cells may or may not have been affected by BMP-2. A study reported that BMP-2 (50 ng/mL) may not have any effect on the chondrogenesis of bovine MSCs (Zeiter et al. 2009) while in other studies, it was reported that BMP-2 (1, 10, and 100 ng/mL) promotes the differentiation of these cells toward the osteogenic lineage (Goldman and Barabino 2016). A combination of growth factors (BMP-2 + TGF-β1) can also differentiate the MSCs toward the chondrogenic lineage (Branly et al. 2017). This variability in results depicts the role of other culture factors in the differentiation of the MSCs. Growth factors tend to induce differentiation of the MSCs toward a hypertrophic chondrocyte template (type X collagen synthesis) that results in their divergence toward osteogenesis instead of restricting them into following the pathway for chondrogenesis (Gugjoo et al. 2016).
Recently, new techniques are being studied to evaluate anti-angiogenesis of the MSCs transfected with a non-viral endostatin plasmid. This is aimed at regenerating avascular tissues like the cartilage (Sun et al. 2009). Blockade of the vascular endothelial growth factor, one of the significant contributors in the development of osteophytes in OA, prevents chondrocyte hypertrophy of the MSCs in a lab animal model (Matsumoto et al. 2009), and subsequently, prevents progression of the disease. However, such an inhibition does not negatively affect either the viability of the cells or their chondrogenesis (Jeng et al. 2010). Thus, further studies are required to develop techniques that restrict the MSCs to chondrogenesis without progression to osteogenesis.
2.2. Effect of mechanical factors on the in vitro chondrogenesis of MSCs
Mechanical factors may or may not affect the differentiation of MSCs. No effect on the differentiation of bovine MSCs was observed when subjected to hydraulic pressure (0.5–3 MPa for 4 h/d) (Zeiter et al. 2009), whereas shear stress (10 Dyn/cm2) induced the differentiation of these cells toward the chondrogenic or osteogenic lineages (Goldman and Barabino 2016). Adverse effects of inflammatory mediators like IL-1β on the chondrogenesis of bovine MSCs may be prevented by combining the mechanical (electromagnetic fields) and growth factors (TGF-β3) (Ongaro et al. 2015). However, to restrict cells to a specific chondrogenic lineage and prevent the induction of a hypertrophic osteogenic pathway, shear stress may be employed along with a sufficient concentration of TGF-β3 (Goldman and Barabino 2016). Therefore, mechanical loading of the bovine BM-MSCs by rapid chromatin condensation may induce their differentiation toward the chondrogenic lineage. This may be coincident with the upregulation of fibrochondrogenic phenotype marker expression (Heo et al. 2015). An extracorporeal shockwave therapy used for promoting osteogenesis reduces chondrogenesis in the 3-D cultures of BM-MSCs, possibly through the regulation of adenosine release and activation of the A2B receptor (Tan et al. 2017).
Hypoxia, which is normally present in the cartilage environment, induces chondrogenesis of human and sheep MSCs (Zscharnack et al. 2009; Guilak et al. 2010; Ronzière et al. 2010; Bornes et al. 2015). Although hypoxia (5%) lowers the proliferation potential of adipose-derived human MSCs (AD-MSCs), it causes a three-fold increase in the secretion of collagen and proteoglycans (Guilak et al. 2010). At lower oxygen concentrations (2%), the MSCs get arrested during the process of chondrogenesis. They do not undergo hypertrophic maturation despite addition of BMP-1 and BMP-2 (Ronzière et al. 2010). Significantly superior expressions of aggrecan and collagen II mRNAs, GAG quantities and proteoglycan staining are observed in BM-MSCs seeded with collagen and hyaluronic acid under hypoxic conditions when compared to cells in which normoxia is maintained. However, it also tends to increase hypertrophic chondrogenesis that may progress toward osteogenesis (Bornes et al. 2015). Thus, further research is required to study the successful regeneration of the hyaline cartilage without its progression toward the fibrocartilage phenotype and/or osteogenic lineage.
2.3. Effect of native environmental factors
Apart from various exogenous growth factors, numerous in vitro factors that resemble the in vivo microenvironment may favor chondrogenesis. The cartilage-derived ECM favors the chondrogenic differentiation of rat BM stromal cells without the influence of any exogenous growth factor (Yin et al. 2016). Addition of laminarin (beta-(1→3)-D-glucan) reduces the proliferation and chondrogenic differentiation of the MSCs derived from the rat BM-MSCs (Larguech et al. 2017). The addition/replacement of different substrates to the culture media may lead to preferential chondrogenesis. Compared to the cultures maintained under conditions of normoxia, the hypoxic cultures of sheep BM-MSCs seeded with collagen and hyaluronic acid demonstrate superior expressions of aggrecan and collagen II mRNAs, amount of glycosaminoglycans, and proteoglycan staining (Bornes et al. 2015). Variable concentrations (25, 50, and 100%) of synovial fluid support the viability, proliferation, and chondrogenic differentiation of equine BM-MSCs (Boone et al. 2018). When goat BM-MSCs (encapsulated in polyethylene glycol) are cultured in collagen (Collagen I and II)-based and hyaluronic-based extracellular matrices, they are directed to the chondrogenic and osteogenic lineages, respectively (Hwang et al. 2011). 3D cultured synthetic biodegradable scaffolds also direct goat BM-MSCs to the chondrogenic lineage. Chondroitin sulfate promotes the mesenchymal condensation of goat MSCs and thereby upregulates their cartilage-specific genes. Additionally, supplementation of polyethylene glycol to chondroitin sulfate may prevent hypertrophic chondrocyte formation (Varghese et al. 2008). Sheep MSCs, under in vitro conditions (placed on porous calcium polyphosphate along with tri-iodothyronine), secrete cells of the osteochondral tissue. The MSCs tend to form a cartilaginous structure together with the osteogenic tissue. It was found that the osteogenic tissue remained over the calcium polyphosphate, over which, the cartilaginous tissue was formed (Lee et al. 2015). Thus, the microenvironment plays a significant role that enables the cells to exhibit their characteristic properties, to which currently very limited exogenous control can be applied under in vivo conditions.
2.4. Effect of cell source on the in vitro chondrogenesis by MSCs
The cell source may also affect musculoskeletal differentiation, including chondrogenesis (Gugjoo et al. 2017; Gugjoo et al. 2018). Among various cell sources, the BM and adipose tissue (AD)-derived MSCs are commonly utilized for the in vivo clinical studies and trials (Nam et al. 2018). However, cells derived from different sources may have variable differentiation potentials. MSCs derived from the synovium (S-MSCs) enable the formation of a large and heavy cartilage pellet compared to the BM-MSCs, AD-MSCs, periosteal-MSCs (P-MSCs), and muscle-MSCs (M-MSCs) (Shirasawa et al. 2006). The cell concentrations of equine BM are lesser compared to those of the AD tissue (Toupadakis et al. 2010). However, the equine BM-derived cells demonstrate superior activity for differentiation into musculoskeletal tissues (Kisiday et al. 2008; Vidal et al. 2008). Stem cells derived from the equine BM, AD, and tendons proliferate faster than the UC-MSCs under in vitro conditions (Burk et al. 2013; Barberini et al. 2014). It was reported that 80% confluency of BM-MSCs, AD-MSCs, and UC-MSCs was achieved in 11 d, 7.3 ± 1.52 and 15.25 ± 6.65 d, respectively (Barberini et al. 2014). The growth rate of UC-MSCs, however, increases upon addition of higher concentrations (20%) of fetal bovine serum (Toupadakis et al. 2010). The chondrogenic potential of equine BM-MSCs is higher than that of the AD-MSCs (Vidal et al. 2008). Sheep perivascular stem cells (considered as natural ancestors of the MSCs) may have better chondrogenic potential as compared to that of sheep BM-MSCs, as evidenced by increased synthesis of the ECM (Hindle et al. 2016). In one of the studies, it was found that the chondrogenic potential of ovine UC-MSCs was superior to that of the BM-MSCs (Burk et al. 2013). MSCs from fetal membranes are generally considered fast growing with better differentiation properties (Somal et al. 2016). The characteristics of BM-MSCs harvested from the equine sternum and ileum were comparable (Lombana et al. 2015). Contrarily, AD-MSCs of the intra-articular fat pad showed superior chondrogenic potential compared to the non-articular fat-derived MSCs (Stewart 2011). This indicates that the cells harvested from different regions of the body but of same individual may or may not demonstrate similar characteristics. Thus, while instituting the MSC therapy, it is imperative to consider characteristics of the cells based on their source.
2.5. Effect of donor age, health status, and breed on in vitro chondrogenesis of MSCs
The cellular characteristics may also vary with age and health status of the donor. MSCs undergo age-related functional loss with respect to their differentiation potentials and proliferation capacities (Kretlow et al. 2008; Huang et al. 2010; Yu et al. 2011; Peffers et al. 2016). This has implications on the healing potential and health status of the individuals as degeneration of the tissues ensues subsequently with aging (Zaim et al. 2012). A reduction in the potentials for proliferation (40%) and osteogenesis is observed in BM-MSCs of aged dogs (Volk et al. 2012). A higher level of population doubling and expression profile of surface markers (CD73 and CD80) and pluripotency markers (Oct3/4 and Nanog) were observed in the MSCs derived from young dogs compared to those harvested from aged dogs (Lee et al. 2017). Therefore, to obtain better results, young donors may preferentially be selected to harvest MSCs for orthopedic applications. Comparison of equine MSCs derived from the synovial fluid and synovial membranes of diseased joints (osteoarthritic and osteochondrosis dissecans) with those of healthy tissues has shown similar phenotypic and multipotency potentials but the chondrogenic potential of MSCs harvested from healthy tissues is better than those of the former (Fülber et al. 2016). Furthermore, studies on the pathophysiological conditions in different species and their influence on the MSCs are needed to understand the correct approach for employing the cells for our benefit. Additionally, characteristics of the BM-MSCs may vary depending on the breed as well. From the standpoint of chondrogenic differentiation, the BM-MSCs derived from the Labrador, Retriever, and Hovawart dog breeds demonstrated better chondrogenesis compared to those of the Border collie and German shepherd breeds (Bertolo et al. 2015). All these complicacies and variabilities in the features of MSCs pose impediments in the determination of a definitive stem cell therapy.
MSCs, as discussed above, are variably affected by donors, tissue sources, and may even vary within given cell populations. Such variabilities complicate their use in regenerative medicine. As detailed above, these conventional assays are usually applied to measure properties of the MSCs en masse, and hence, fail to control a particular cell population. Extensive variability within clonal MSC populations also exists. This affects their functional differentiation capacities, molecular state biophysical properties, and paracrine effects (McLeod and Mauck 2017). Recently, a study on mice showed that the transcriptomic profile and chromatin accessibility signatures may impart such differences. It was also demonstrated that chromatin accessibility signatures may be more accurate than those of the transcriptomic profiles. The transcription factors associated with the manifestation of these characteristic differences of the cells depending on their sources have been characterized (Ho et al. 2018). Thus, a system needs to be identified that could locate them individually for appropriate clinical applications in tissue engineering and regenerative medicine (McLeod and Mauck 2017).
3. In vivo preclinical experimental models/clinical studies
Numerous in vivo chondrogenic studies involving MSCs have been conducted on almost all the veterinary relevant mammalian species like sheep, goat, dog, and horse, with the exception of bovines, cats, and swine. In the majority of these studies, implantation of the MSCs has yielded good results. However, the majority of the studies were non-uniform with differences in the sources of cells, culture techniques, dosages, passage numbers, implantation methods, growth factors, and type of scaffolds. Mostly, cells derived from either allogeneic or autologous transplants have been used in these studies. Allogeneic MSCs implanted either once or repeatedly have been reported as safe and are not known to induce any hypersensitivity reactions (Vangsness et al. 2014; Vega et al. 2015; Ardanaz et al. 2016). It has been reported that these cells survive even up to 14 weeks after in vivo implantation (Feng et al. 2018). Apart from the allogeneic cells, human xenogenic stem cells too are known to give better results compared to those of the control. This may be attributed to their characteristic immunocompromised feature. However, the use of xenogenic MSCs is currently not recommended in clinical trials. MSCs have anti-inflammatory properties but high-end inflammation usually reduces their ability for chondrogenic differentiation without affecting their phenotypic characteristics and proliferation potential (Ando et al. 2012; Zayed et al. 2016). In equine OA, the limited efficacy of their MSCs may be explained by an increased expression of the adhesion molecule, a decrease in the migration of related genes (Barrachina et al. 2016; Reesink et al. 2017), and the production of glycosaminoglycans (Zayed et al. 2016). Pro-inflammatory cytokines like IL-1β, IL-17, and TNF-α decrease the expression levels of cartilage-specific genes like SOX-9 and TGF-β1, and those encoding aggrecan, collagen II (Kondo et al. 2013; Zayed et al. 2016), and galactin (Reesink et al. 2017) in the MSCs. However, the expression levels in MSCs may decrease depending upon the type of their source. In the presence of inflammatory mediators, a reduction in the expression of aggrecan only is seen in the MSCs derived from the synovial fluid when compared to that of the BM-MSCs (Zayed et al. 2016). The role of inflammation on the pathways related to the stem cell growth and cytokine expression should be further explored to have a better understanding of the limitations in the efficacy of stem cell therapy during inflammatory conditions. Currently, the initial step in cartilage rehabilitation should be aimed at decreasing the inflammation by the adoption of anti-inflammatory drugs. This may be followed by the application of MSCs for better results. The in vivo articular studies conducted in different animals are described below.
3.1. MSC studies in sheep
Numerous studies on the repair of osteochondral ailments using MSCs have been conducted in sheep. Most of the studies (listed in Table 1) have reported the usefulness of MSCs for improving the condition of joint ailments compared to that of the control (Guo et al. 2004; Feitosa et al. 2010; Zscharnack et al. 2010; Al Faqeh et al. 2012; Caminal, Moll, et al. 2014; Caminal, Fonseca, et al. 2014; Song et al. 2014; Garcia et al. 2014; Zorzi et al. 2015; Desando et al. 2016; Whitehouse et al. 2017; Abdalmula et al. 2017; Feng et al. 2018). In addition to cultured MSCs, the BM aspirate too has been reported to improve cartilage healing (Duygulu et al. 2012). However, no obvious improvement compared to the control was observed in a single study upon use of the BM aspirate (Delling, Brehm, Metzger, et al. 2015). MSCs implanted into the joints remain viable and attach themselves to the joint structures (Delling, Brehm, Ludewig, et al. 2015; Feng et al. 2018).
Table 1.
Chondrogenic in vivo preclinical experimental mesenchymal stem cell studies in sheep.
| Model type | Number of animals | Model defect size/study period | Biomaterial used | Cell dose | Evaluation criteria | Overall result | References |
|---|---|---|---|---|---|---|---|
| Medial femoral condyle defect | 28 (n = 16 cell along β-TCP with treated; n = 8 in β-TCP only and n = 4 in control) | 8 mm (diameter) and 4 mm (depth)/24 weeks | Autologous BM-MSCs + beta-tricalcium phosphate (β-TCP) | 3 × 107 | Macroscopic observation, histological, immuno-histochemical, biochemical analysis | Experimental animal defects were resurfaced with hyaline-like tissue. An ideal interface formed between the engineered cartilage, adjacent normal cartilage, and the underlying bone | Guo et al. (2004) |
| Osteonecrosis of femoral head | 8 animals (4 in control group; 2 each in sheep MSC group and human MSC group treated after 8 weeks of induced necrosis) | 10 mL of absolute ethanol induced | Sheep BM-MSCs (transfected) and human dental stem cells | 1 × 106 (each cell type) | Light microscopy | Better bone regeneration in cell treated group animals | Feitosa et al. (2010) |
| Chronic model of medial femoral condyles osteochondral lesions | 10 (40 defects; group I: chondrogenically differentiated MSC/hydrogel constructs; group II: undifferentiated ovine MSC/hydrogel constructs; Group III: cell free hydrogel; group IV: control | 7 mm/ 6 months | Autologous BM-MSCs/collagen I hydrogel constructs | 4 ×105 MSCs mixed with collagen I | Histopathology | Group I had significantly better histologic scores with morphologic characteristics of hyaline cartilage such as columnarization and presence of collagen type II compared to others. However, each group showed variability in results | Zscharnack et al. (2010) |
| Chronic model of anterior cruciate ligament excision | 16; 6 animals in group I (pre-differentiated MSCs and II (undifferentiated MSCs) and 4 (control group) | 6 weeks | Chondrogenically differentiated MSCs or undifferentiated MSCs | 10 × 106 per joint | Gross, histological and clinical observation | Retardation of osteoarthritis in cell treated groups. Non-significant difference in group I and II except for macroscopic observations of meniscus repair. Severe osteoarthritis in control | Al Faqeh et al. (2012) |
| Chronic model full thickness medial femorotibial condyles and meniscal tear | 10 (20 defects 10 studied at 6 months period while other 10 at 12 months period) one of the limbs remained control | 60 mm defect size/6 months or 12 months | BM-MSCs | 1.1 × 107 (6 month period animals) or 1.2 × 107 (12 month period animals) | Radiography, MRI, ultrasound, macroscopic and histological analyses | Regeneration of articular cartilage and meniscus was case-dependent but statistically significant improvement was found in specific macroscopic and histological parameters | Caminal, Moll, et al. (2014) |
| Medial femoro-tibial condyle defect | 9 (18 defects) | 7 mm defect size/4 and 12 months | BM-MSCs alone or seeded on co-polymeric poly-lactide:polyglycolic acid scaffolds either | 3.3 × 106±0.4 × 106 cells | Biomechanical testing, macroscopic and histological analyses | Better macroscopic scores at 4 months in cell treated compared to 12 months evaluation period. Non-significant histopathological scores at 12 months between cell treated and cell free groups | Caminal, Fonseca, et al. (2014) |
| Chronic anterior cruciate ligament transection and medial meniscectomy | 18 (6 animals in each group) Group I: BM-MSCs; group II: bone marrow mononuclear cells; group III: control | 8 weeks | Autologous BM-MSCs | 10 × 106 after 12 weeks of model creation | Macroscopically and histologically, and glycosaminoglycan (GAG) contents, gene expression levels (collagen II, aggrecan and matrix metalloproteinase-13), tumor necrosis factor-α (TNF-α) and transforming growth factor beta | Significantly higher cartilage regeneration and lower proteoglycan loss in group I than group II. Comparable inhibition of PGE2, TNF-α and TGF-β levels in synovial fluid and promotion of higher levels of Aggrecan and Col II in two cell treated groups. Down regulation of MMP-13 also comparable. Both the cell treated groups had significantly better cartilage than control | Song et al. (2014) |
| Full thickness lateral femoral condyle defect | Group I (amniotic membrane); group II (cryopreserved amniotic membrane previously cultivated 12 (4 each group) with BM-MSCs; group III (cryopreserved amniotic membrane alone); group IV (control) | 7 × 5 mm/8 weeks | BM-MSCs and amniotic membrane | 2 × 106 cells and amniotic membrane | Gross and histopathology | Significant difference between treatment and control group. Non-significant differences in treatment groups | Garcia et al. (2014) |
| Partial thickness medial femoral condyle defect | 15 animals/ 30 knees (group I: scaffold plus cell; group II; scaffold only; group III control) | 10 mm/6 months | Xenogenic AD-MSCs and collagen/chitosan scaffold | 1 × 106 along with scaffold | Microscopic and macroscopic analysis | Significantly higher histological scores in cell treated group compared to others | Zorzi et al. (2015) |
| Unilateral medial meniscectomy | 20 (Group I: 6 animals, BM-MSCs + scaffold; Group II: 6 animals BM concentrate + scaffold; group III: 4 animals scaffold treated group IV: 4 animals, control | 12 weeks | BM-MSCs + scaffold (Hyaff®-11) and BM concentrate + scaffold (Hyaff®-11) | 6 × 106 seeded on scaffold | Macroscopy, histology, immunohistochemistry, and micro-computed tomography | BM concentrate better inhibited inflammation in cartilage, meniscus, and synovium. It also improved cartilage healing. subchondral bone thickness decreased in both the cell treated groups | Desando et al. (2016) |
| Meniscal cartilage tear model | 30 animals (3 groups with 10 animals in each group evaluated at 13 weeks and 6 months). Group I: scaffold laden MSCs; group II: scaffold only and group III: suturing only) | 5 × 3 mm/13 weeks and 6 months | BM-MSCs collagen I scaffold | 1 × 106/cm2 | Macroscopy and histopathology | Statistically significant improvement in cell treated compared to control at 13 weeks. But no difference at 6 months period | Whitehouse et al. (2017) |
| Anterior cruciate ligament resection and medial meniscectomy | Group I: AD-MSCs and hyaluronic acid; group II: hyaluronic acid and group III: control) | 14 weeks after treatment | Allogenic AD-MSCs and Hyaluronic acid | 5 × 107 cells at 3 weeks) and low (1 × 107 cells at 6 weeks) | Magnetic resonance imaging (MRI), macroscopy, micro-computed tomography, and cartilage-specific staining | AD-MSCs + hyaluronic acid could efficiently block osteoarthritis progression and promote cartilage regeneration. | Feng et al. (2018) |
The cells were implanted either locally or by peripheral injection. Intravenous implantation of the BM mesenchymal precursor cells in a sheep mono-arthritis model leads to a reduction in their lameness, joint pain, and swelling. The joint examination revealed a decrease in the cartilage erosions, synovial stromal cell activation, and angiogenesis. Additionally, a slight infiltration of the synovial tissues with CD4+ lymphocytes and CD14+ monocytes or macrophages was observed. All these findings were contrary to those observed in the control animals (Abdalmula et al. 2017). Similar results were reported in another model of induced OA (medial meniscectomy and ACL resection) treated with an intra-articular implantation of different doses of allogenic AD-MSCs and HA 6 weeks after the surgery. The implanted cells were effectively viable up to 14 weeks and demonstrated a significant reduction in the concentration of anti-inflammatory factors (TNF‐α and IL‐6) in the joint. It was evident from the improved histological and microCT scores of the healed tissue that the progression of OA was reduced, and instead, cartilage regeneration was promoted (Feng et al. 2018).
Various comparative studies have variably supported an improvement in repair after the application of MSCs. In a comparative study on an OA model, the cartilage regenerative potential and stability of the healed tissue was found to be more in animals treated with the BM-MSCs compared to the BM mononuclear cells, the results of which were better than those observed in the control animals. Treatments using both humoral cells and chemokines inhibited PGE2, TNF-α, and TGF-β levels in the synovial fluid, and promoted an increase in the levels of aggrecan and Col2A1 expression. Furthermore, the MMP-13 expression was downregulated in sheep chondrocytes (Song et al. 2014). Hyaluronan-laden BM concentrate containing MSCs and growth factors, upon effective implantation, prevented OA, and promoted regenerative processes in the cartilage and associated tissues. It is likely that a reduction in the inflammation resulting from both the treatments switched off the fibrotic and hypertrophic processes in the joints (Desando et al. 2016).
Variable results have been reported when an in vivo application of the chondrogenically differentiated MSCs produced in vitro was compared to that of the undifferentiated MSCs. A better healing with in vitro chondrogenic differentiated cells was reported in one of the studies (Zscharnack et al. 2010), while the healing in others was better but comparable (Al Faqeh et al. 2012; Bornes et al. 2018). Comparable histological scores were observed between the two groups when the hypoxia-cultured BM-MSCs (scaffold seeded and chondrogenically primed) were compared to those cultured under conditions of normoxia (scaffold seeded only) (Bornes et al. 2018). The variations may be due to differences in type of model used, cell concentrations, and the period for which follow up was conducted. Cell preservation does not have any significant deleterious effects on the cartilage healing potential of the amniotic-MSCs as comparable healing occurs upon implantation of either fresh or cryopreserved MSCs (Garcia et al. 2014).
It has been reported that, apart from the autologous or allogeneic MSCs, xenogenic AD-MSCs loaded onto the chitosan/collagen scaffold also promote cartilage healing. A higher International Cartilage Repair Society Score (ICRS-1) in animals treated with the cells compared to those of the scaffold-implanted or control animals was reported (Zorzi et al. 2015). Contrary to the above studies, one of the sheep experimental studies has failed to give any positive outcome for the OA conditions compared to the control upon intra-articular implantation of the autologous MSCs. Such observations were recorded using a 0.5 Tesla MRI system after a period of 12 weeks (Delling, Brehm, Metzger, et al. 2015). The poor response observed in this study could be due to the weak joint injury induced with meniscal damage that was incapable of inducing discernible osteoarthritic changes in the control group. Additionally, the concentration of implanted cells may not have been sufficient to address the challenge (Feng et al. 2018).
Interestingly, in all these studies, hyaline regeneration was not evident and the regenerated tissue failed to integrate with the native cartilage or subchondral bone. Additionally, the healed tissues underwent chondroid metaplasia and headed toward osteogenesis (Zscharnack et al. 2010; Caminal, Moll, et al. 2014). Moreover, the cell-treatment may lead to an improvement in the condition in the early period post-application, but later, the healing appears comparable between the treated and non-treated animals (Caminal, Fonseca, et al. 2014; Whitehouse et al. 2017). This may occur due to various reasons like continuous weight-bearing by the affected joint, inhibitory effect of inflammation on the migration and expression of MSCs, and an unclear pathophysiology of the condition. It may be concluded that the application of MSCs may benefit the treatment of OA. However, this cannot be guaranteed. Thus, further studies are required to standardize the MSC therapy for cartilage regeneration.
3.2. MSC studies in goats
Osteochondral studies in goats have mostly favored the use of MSCs, although it is yet to be standardized for optimal tissue regeneration (Table 2) (Murphy et al. 2003; Zhu et al. 2011; Bekkers et al. 2013; Jurgens et al. 2013; Nam et al. 2013; Pei et al. 2014; Zhang et al. 2018). In a medial femoral condyle and trochlear groove defect model, the AD-MSCs and/or stromal vascular fraction (SVF) seeded onto a collagen I/III scaffold resulted in better cartilage healing after 4 months than in animals treated with the acellular collagen I/III scaffold. Improved healing in the form of increased content of collagen type II, glycosaminoglycan, and formation of the hyaline-like cartilage was reported. The elastic modulus of the healed tissue was comparable to that of the native tissue. However, non-significant differences in the healing between the animals treated with AD-MSCs and SVF were observed (Jurgens et al. 2013). Similarly, animals treated with BM-MSCs were found to show better ICRS and O’Driscoll scores as well as cartilage-specific gene expression profiles compared to those of the control (Nam et al. 2013). In a model of medial meniscus excision and anterior cruciate ligament resection, intra-articular implantation of BM-MSCs were found to retard articular cartilage degeneration, subchondral bone sclerosis, and osteophytic remodeling at 12 weeks compared to the control group. However, severe OA was reported at later stages (Murphy et al. 2003). This may be due to the uncontrolled movement of the animals that lead to further degeneration of the joint. Combined use of tissue engineered osteochondral defect and BM-MSCs cultured in a bioreactor resulted in a better repair of the osteochondral defect compared to that of the control (graftless). Such a repair process is potentiated by mechanical stimulation of the graft (Pei et al. 2014).
Table 2.
Chondrogenic in vivo preclinical experimental mesenchymal stem cell studies in goat.
| Model type | Number of animals | Model defect size/study period | Biomaterial used | Cell dose | Evaluation criteria | Overall result | References |
|---|---|---|---|---|---|---|---|
| Chronic model of excision of the medial meniscus and resection of the anterior cruciate ligament | 24 (6 animals cell treated/hyaluronan and 3 animals control for 12 weeks evaluation; 9 animals cell treated/hyaluronan and 6 animals for 26 weeks evaluation) | 12 and 26 weeks | BM-MSCs + hyaluronan | 10 × 106 loaded in hyaluronan | Histochemistry | Marked regeneration of the medial meniscus. Degeneration of the articular cartilage, osteophytic remodeling, and subchondral sclerosis was reduced in cell-treated group compared to control at 12 weeks. However, later the cell treated and control had severe osteoarthritis. | Murphy et al. (2003) |
| Mandibular condyle Osteochondral defect model | 50 (12 in each group) (group I: NELL-1-modified BMMSCs/PLGA, group II: BMMSCs/PLGA; group III: PLGA alone; group IV: control | 3 mm-diameter × 5 mm-depth/6 weeks and 24 weeks | NELL-1 transfect autologous BM-MSCs and poly-lactic-co-polyglycolic acid scaffold | 3 × 106 cells seeded on PLGA scaffold | Macroscopic, histology and immuno histochemistry, microCT | Group I showed Rapid and vigorous healing leading to fibrocartilage formation at 6 weeks. At 24 weeks complete repair of native articular cartilage and subchondral bone at 24 weeks. In group II: repaired completely filled the defect with fibrocartilage at 24 weeks, but the cartilage was less well-organized group I. In group III and IV the defects were poorly repaired, and no cartilage in the group IV or only small portion of cartilage in the group III was formed | Zhu et al. (2011) |
| Full-thickness chondral defect in medial femoral condyles | 8 (16 knees) chondron and cell treatment vs. microfracture | 5 mm/6 months | Chondron (chondrocytes in own matrix) and BM-MSCs | 10% chondron/90% MSC combination at a concentration of 1× 106 cells/mL | Macroscopic and microscopic scoring, biochemical analysis, histological and immunohistochemical analyses | Combination of BM-MSCs and chondrons lead to significantly better microscopic, macroscopic, and biochemical cartilage regeneration compared to microfracture treatment. | Bekkers et al. (2013) |
| Osteochondral defects created in medial condyles and trochlear grooves | 8 (group I: scaffolds seeded with cultured AD-MSCs; group II: scaffolds seeded with SVF cells; group III: acellular scaffolds | 5 × 3 mm/1 and 4 months | Stromal vascular fraction (SVF), AD-MSCs along with collagen type I/III scaffold | 5 × 106 (SVF) and 5 × 105 (AD-MSCs) seeded on scaffold | Macroscopy, immunohistochemistry, biomechanical analysis, microCT analysis, and biochemistry | Cell treated groups had more extensive collagen type II, hyaline-like cartilage, and higher elastic moduli, and their glycosaminoglycan content in the cartilaginous layer that approached native tissue values. In control lesser regenerative effect was seen. No difference in healing was seen between SVF treated and AD-MSC treated animals | Jurgens et al. (2013) |
| Chronic full-thickness chondral defect in medial femoral condyles | 18 (36 defects) group I: bone marrow stimulation and BM-MSCs; group II: bone marrow stimulation; group III: control | 5 mm/6 months | BM-MSCs | 1 × 107 cells after 2 weeks after bone marrow stimulation for three consecutive weeks | Macroscopic, histology, biochemical assays (glycosaminoglycans) and gene expressions (aggrecan, collagen II and Sox9). | Hyaline-like tissue with higher glycosaminoglycans and chondrogenic gene expression in group I compared to group II that had fibrocartilage. Lowest healing in control | Nam et al. (2013) |
| Full-thickness femoral condyle cartilage defects | 6 (microfracture and cell/scaffold groups) | 6.5 mm-diameter/6 and 9 months | Human WJMSCs seeded in an acellular cartilage extracellular matrix (ACECM)-oriented scaffold | 1 × 106 cells seeded on ACECM | Analysis of inflammatory response, Magnetic resonance imaging, Gross morphology, Histology, Immunohistochemical and immunofluorescent staining, Biomechanical testing and Biochemical quantitative analyses | No significant differences between the two groups in immuno-inflammatory parameters. MRI demonstrated higher-quality cartilage and complete subchondral bone at defect sites in the cell treated group at 9 months. Histological revealed extracellular cartilage, cartilage lacuna and collagen type II levels were higher in cell treated group compared to the microfracture, while the cell treated group exhibited a higher elasticity modulus | Zhang et al. (2018) |
In a comparative study conducted on the medial femoral condyle defect model, a combination of 10% chondron (chondrocytes in their own matrix) and BM-MSCs result in better healing than microfracture. Statistically significant microscopic, macroscopic, and biochemical cartilage regeneration was observed in the cell-treated animals compared to that observed in microfracture treatment (Bekkers et al. 2013). A comparative study on the healing of the goat mandibular condyle defect showed that the implantation of Nell-1 (growth factor that targets cells committed to the osteochondral lineage) modified the BM-MSCs/poly lactic-co-glycolic acid (PLGA) and repaired the defect by the induction of fibrocartilage at 6 weeks and with native articular cartilage by the 24th week. Implantation of undifferentiated BM-MSCs/PLGA favored the repair of the defect by fibrocartilage. The cells were found to be viable up to 6 weeks (Zhu et al. 2011). Similarly, xenogenic WJ-MSCs seeded on the ECM of the acellular cartilage lead to better cartilage and subchondral bone repair upon implantation in a femoral condyle defect model compared to that of the microfracture at the 9-month period. Better cartilage repair evidenced in the form of increased production of the ECM, lacunas and collagen type II, and higher mechanical strength (higher elastic modulus) was reported in cell/scaffold-treated animals (Zhang et al. 2018).
Unlike those of the sheep, MSCs in goats may not be able to heal the cartilage in all cases. Additionally, the continued weight-bearing on the affected joint may lead to OA, comparable in both treated as well as the control group (Murphy et al. 2003).
Therefore, the MSCs may potentially be utilized for the repair of osteochondral defects. However, the procedure is yet to be standardized and may be dependent on the cell source, its dosage, passage number of the cells, route of implantation, type of scaffold, and incorporation of the growth factor.
3.3. MSC studies in dogs
Unlike caprines and ovines, stem cell therapy in canines has been instituted both in preclinical experimental models (Table 3) (Mokbel et al. 2011; Yang et al. 2011; Hang et al. 2012; Yun et al. 2016; Kazemi et al. 2017) as well as in clinical cases (Table 4) (Black et al. 2008; Yoon et al. 2012; Vilar et al. 2013; Cuervo et al. 2014; Marx et al. 2014; Vilar et al. 2014; Harman et al. 2016). Barring a single study in which the cells were implanted at acupoints (bladder 54, gall bladder 29, and gall bladder 30), in all others, they have been implanted once locally (Marx et al. 2014). The cells were implanted either alone (Marx et al. 2014; Vilar et al. 2014) or with platelet-rich plasma(PRP)/fibrin (Vilar et al. 2013; Kazemi et al. 2017; Kriston-Pál et al. 2017) or hyaluronic acid (Guercio et al. 2012; Kriston-Pál et al. 2017; Li et al. 2018). The cases were followed-up for 1 month (Marx et al. 2014), 6 months (Vilar et al. 2013; Cuervo et al. 2014), 1 year (Kriston-Pál et al. 2017), and 5 years (Yoon et al. 2012).
Table 3.
Chondrogenic in vivo preclinical experimental mesenchymal stem cell studies in dogs.
| Model type | Number of animals | Model defect size/study period | Biomaterial used | Cell dose | Evaluation criteria | Overall result | References |
|---|---|---|---|---|---|---|---|
| Partial thickness chondral defects of lateral femoral condyle | 32 (8 control; 12 group II; 12 group III) | 3mm diameter and 1 mm depth/8 weeks | Autologous BM-MSCs | 1.4–1.6 × 106 | Morphological, histological, and fluorescence analysis | Significant recovery in cell seeded groups. In group II better results at 8 weeks | Mokbel et al. (2011) |
| Osteochondral defects of medial femoral condyles | Group I: cell + scaffold; group II: control | 4.2 mm diameter and 6 mm depth/3 and 6 months | Chondrogenically-induced BMSCs and scaffold | – | Gross morphology and by histological, biochemical, biomechanical and micro-CT analyses | Statistically significant improvement in gross and histological, and cartilage stiffness in cell/scaffold treated animals compared to control. Comparable Micro-CT analysis of the subchondral bone in two groups. Better results in later period than at early period. | Yang et al. (2011) |
| Osteonecrosis of the femoral head | 24 (54 hip joints) group I: transgenic BM-MSCs; group II: BM-MSCs group III: control | 2mm diameter and 2 mm width/12 weeks | VEGF 165 transgenic bone marrow mesenchymal stem cells or simple BM-MSCs | 2 × 107 | Radiography, single-photon emission Computed tomography, histopathology, histomorphometric analysis and immunofluorescent staining for von Willebrand factor | Better results in group I compared to group II and group III | Hang et al. (2012) |
| Osteochondral defects | 12 (24 (defects) group I: cell treated; group II: control | 6 mm diameter and depth of 5 mm/24 weeks | Autologous BM-MSCs + platelet rich fibrin | 1 × 106 | Macroscopic and histopathology | Group I had statistically significant improved histological features compared to control | Kazemi et al. (2017) |
| Chondral defects of stifle joint | 24 (48 defects) group I: cell + hyaluronic treated; group II hyaluronic acid | 4 mm/28 weeks | BM-MSCs + hyaluronic acid (HA) | 1 × 107 | Macroscopy, magnetic resonance imaging (MRI), histopathology, immunohistochemistry for type II collagen | Group I had statistically significant improvement compared to group II | Li et al. (2018) |
Table 4.
Chondrogenic in vivo clinical mesenchymal stem cell studies in dogs.
| Clinical condition/ailment | Number of animals included | Study period | Study type | Cell source | Cell dose | Evaluation criteria | Overall result | References |
|---|---|---|---|---|---|---|---|---|
| Chronic osteoarthritis of Coxo-femoral joint | 21 | 90 d | Case series (Randomized, double blinded, placebo controlled trial) | Autologous AD-MSC | 5× 106 (20 dogs) and 4.5 × 06 (1 dog) | Orthopedic examination scores, lameness and composite scores and size effect | Statistically significant improvement in cell treated as compared to placebo treated animals | Black et al. (2007) |
| Chronic osteoarthritis of humeroradial joint | 14 | 180 d | Case series (Randomized, double blinded, non-placebo controlled trial) | Autologous AD-MSC | 3–5 × 106 | Orthopedic examination Score and size effect | Statistically significant improvement in cell treated | Black et al. (2008) |
| Chronic osteoarthritis of humeroradial joint | 4 | 30 d | Case series (Uncontrolled study) | Autologous AD-MSC | 3–5 × 106 (laden in Platelet-rich plasma or hyaluronic acid) | Clinical tests like trot pain on palpation and functional improvement in disability | Improvement with time as per owner (no statistical observation | Guercio et al. (2012) |
| Chronic arthritis of the hip joint | 8 | 180 | Autologous AD-MSC | 15 × 106 | Force platform analysis | Significant improvement in cell treated cases | Vilar et al. (2013) | |
| Hip osteoarthritis | 39 | 180 d | Randomized Comparative clinical trial | Autologous AD-MSC vs. PRGF | 30 × 106 | VAS, Bioarth Scale Assessment, Radiography, clinical exam. | Statistically significant improvement in cell treated | Cuervo et al. (2014) |
| Chronic arthritis of the hip joint | 5 | 30 d | – | allogenic AD-MSC | 0.2–0.8 × 106 | physical and orthopedic examinations | Positive outcome but no statistical data | Marx et al. (2014) |
| Chronic arthritis of the hip joint | 10 | 180 d | Case control (Blinded control study) | Autologous AD-MSC | 15 × 106 | X-ray and platform gait analysis | Statistically significant improvement in cell treated | Vilar et al. (2014) |
| Hip, elbow, stifle, or shoulder joints | 93 | 60 d | Case series study (prospective, randomized, masked, and placebo-controlled) | Allogenic AD-MSCs | 12 × 106 (Cryopreserved with 85.1% viability) | Owner client-specific outcome measurement (CSOM) and secondary measures included veterinary pain on manipulation, veterinary global score, and owner global score | Statistically significant clinical improvement in cell treated | Harman et al. (2016) |
| Elbow dysplasia and osteoarthritis | 30 dogs (39 elbow joints) | 1 year | Case series (Uncontrolled study) | Allogenic AD-MSCs + Hyaluronic acid (0.5%) | 12 × 106 ± 3.2 × 106cells | Owner and veterinarian examination, arthroscopy and histopathology | Significant improvement with hyaline regeneration | Kriston-Pál et al. (2017) |
| Hip, knee, radiocarpal intercarpal, elbow and ulnar osteoarthritis | 10 patients | 90 d/4 years (5 animals) | Case reports | Autologous AD-MSCs | 3 × 107 | Physical examination and assessment for lameness, pain on manipulation, range of motion of the joint and functional disability | Statistically significant improvement for lameness at trot and for the range of motion of the joint. Statistically insignificant was detected for lameness at walk, pain on manipulation and functional disability | Dražilov et al. (2018) |
Overall, clinical evaluation has supported the therapeutic outcome of the studies involving the following parameters: pain, visual analog scale, pain on manipulation scale, veterinary global scale, client-specific outcome measurement, quantitative force platform gait analysis, and range of motion. Histological assessment revealed that the healed tissue consisted of mixed fibrocartilage and hyaline that lacked complete integration into the native cartilage (Mokbel et al. 2011; Kazemi et al. 2017). A study in which follow-up arthroscopic evaluation was conducted revealed that the regenerated cartilage was hyaline (Kriston-Pál et al. 2017). In all these studies, the parameters for therapeutic evaluation were variable, with a lack in general consensus. Assessment of OA-associated lameness by pain assessment scales usually lack accuracy and concordance. A more advanced technique of quantitative force platform gait analysis can be used for its clinical evaluation (Vilar et al. 2014).
Various comparative studies have variably shown that repair with MSCs is better compared to that involving other available treatment options. In one of the studies, use of dog AD-MSCs gave better results at 6 months when compared with the results in which the platelet-rich growth factor (PRGF) was used (Cuervo et al. 2014). Chondrogenically induced dog BM-MSCs with a biphasic scaffold tend to show significantly improved gross and histological scores, and stiffness of the healed cartilage in comparison with that of the cell-free scaffold-implanted tissues (Yang et al. 2011). Additionally, the VEGF165 transgenesis of MSCs may further potentiate their reparative effect (Hang et al. 2012).
In addition to the factors mentioned above, the implantation period may also affect the cell-mediated effect. In one of the studies, it was demonstrated that the immediate implantation of cells after defect formation may have better outcomes than those wherein cells were implanted later (one month) (Mokbel et al. 2011). This may be due to the chronicity that may occur in the cartilage defects treated later, although further studies are required to ascertain this. One of the studies showed that the SVF also induces better healing and is comparable to that of the dog AD-MSCs implanted at acupoints (Marx et al. 2014). This could possibly be due to the additive effect of growth factors available in the latter treatment option (Kazemi et al. 2017). However, results obtained with SVF may not be recapitulated every time as its humoral/growth factor constituents are variable.
Thus, numerous factors that influence the results of stem cell therapy need to be studied. This may include application of pre-differentiated MSCs and also the cells that are transfected with the chondrogenic lineage-specific expression. Furthermore, uniform studies involving MSCs need to be conducted and their relation to sources, concentrations, inclusion of growth factors, and scaffolds need to be determined.
3.4. MSC studies in equines
Most equine MSC studies, whether preclinical or clinical, have failed to yield comprehensive cartilage regeneration but showed clinical improvement particularly on the basis of a reduction in the clinical symptoms and the condition of animals returning to work, as listed in Table 5 and Table 6 (Wilke et al. 2007; Frisbie et al. 2009; McIIwraith et al. 2011; Raheja et al. 2011; Spaas, Oosterlinck, et al. 2012; Yamada et al. 2013; Broeckx et al. 2014; Ferris et al. 2014; Broeckx et al. 2019). The healing appeared to be better in MSC-treated cases at the early period but decreased in the later stages (Wilke et al. 2007). Like autologous cells, single time intra-articular implantation of allogeneic BM-MSCs too has failed to elicit immune response (Ardanaz et al. 2016). However, repeated intra-articular implantation elicits adverse reactions against allogenic BM-MSCs (Joswig et al. 2017). In many such studies, an attempt has been made to mimick chronic condition of the OA by implanting cells after a gap of some days/weeks after defect creation (Wilke et al. 2007; Yamada et al. 2013).
Table 5.
Chondrogenic in vivo preclinical experimental mesenchymal stem cell studies in horse.
| Model type | Number of animals | Model defect size/Study period | Biomaterial used | Cell dose | Evaluation criteria | Overall result | References |
|---|---|---|---|---|---|---|---|
| Chronic Full-thickness cartilage defects in femoro-patellar articulations | 6 (12 defects Group I: autogenous fibrin vehicle containing MSCs; group II: autogenous fibrin alone in control joints | 15 mm/1 and 8 months | MSCs loaded into self -polymerizing autogenous fibrin vehicle | MSCs/fibrinogen mixture containing 12 × 106 MSC/mL | Histology, histochemistry, collagen type I and type II, immunohistochemistry, collagen type II in situ hybridization, and matrix biochemical assays | Arthroscopic scores in group I were significantly improved at the 30-d. Biopsy showed MSC-implanted defects contained increased fibrous tissue with several defects containing predominantly type II collagen. At 8 months no significant difference between stem cell-treated and control defects. | Wilke et al. (2007) |
| Middle carpal joint osteochondral defect | 24 8 each group (group I: AD-MSCs; group II: BM-MSC; group III: control | 15 mm/70 d | AD-MSCs and BM-MSCs | 16.3 × 106 (AD-MSCs) and 10.5 × 106 (BM-MSCs) | Clinical outcome, Macroscopy, histopathology, Articular Cartilage Matrix Evaluation | Non-significant differences in healing improvement with cell treatment were seen compared to control except improvement in PGE2 levels. | Frisbie et al. (2009) |
| Full thickness femoral condyle defects followed by microfracture | 10 (20 defects in stifle joint) Group I: BM-MSCs and microfracture; Group II: microfracture | 10 mm/6 and 12 months | MSCs and hyaluronan | 20 × 106 MSCs + 22 mg hyaluronan | Radiography, arthroscopy, Magnetic resonance imaging and gross, histologic, histomorphometric, immunohistochemical, and biochemical examinations | Non-significant evidence of any clinically significant improvement in the joints with BM-MSCs. Arthroscopic and gross evaluation confirmed a significant increase in repair tissue firmness and a trend for better overall repair tissue quality (cumulative score of all arthroscopic and gross grading criteria) in BM-MSC-treated joints. Immuno-histochemical analysis showed significantly greater levels of aggrecan in repair tissue treated with BM-MSC injection. There were no other significant treatment effects. | McIIwraith et al. (2011) |
| Chronic chondral defects in the medial femoral trochlea | 4 (8 defects) group I; MSCs; group II: control | 10 mm/5 months | AD-MSCs | 1.35 × 107 | Macroscopic, histopathological and histochemical evaluations | The use of MSC in the treatment of chondral defects minimized joint inflammation, as confirmed by synovial fluid analysis. The treatment resulted in an improved repair tissue, verified by macroscopic examination, histochemical and histopathological analysis | Yamada et al. (2013) |
| Chondral defect, model | 12 (24 defects) Group I: BM-MSCs + APEF; group II: APEF | 15 mm defect in lateral trochlear ridge/3 and 12 months | BM-MSCs along with autologous platelet enhanced fibrin scaffolds (APEF) | 12 × 106 | Arthroscopy, histological examination, magnetic resonance imaging (MRI), micro-computed tomography (micro-CT), and biomechanical testing | Addition of BM-MSCs to APEF did not enhance cartilage repair and stimulated bone formation in some cartilage defects | Goodrich et al. (2016) |
Table 6.
Chondrogenic in vivo clinical mesenchymal stem cell studies in horse.
| Clinical condition/ailment | Number of animals | Study period | Biomaterial used | Cell dose | Evaluation criteria | Overall result | References |
|---|---|---|---|---|---|---|---|
| Bilateral articular cartilage fissure defects of the medial femoral condyles and concurrent cranial cruciate ligament injury | Single case | 4 and 15 months | Autologous BM-MSCs + fibrin glue | 1 × 107 implanted at 90 d, 3 and 13 months period | Arthroscopic examination and performance records | At 4 months marked cartilage surface smoothing, reduction in the cartilage defect depth. Further, moderate improvement in the cranial cruciate ligament was observed. After 15 months of the initial MSC treatment the horse returned to racing and had comparable race earning to that of pre-injury records | Raheja et al. (2011) |
| Chronic degenerative joint disease of pastern joint | Single case | 4, 8, and 12 weeks and 1 year period | Peripheral blood-derived MSCs | 2.5 × 106 | American Association of Equine Practitioners (AAEP), radiography and pressure plate analysis | Clinical improvement observed and pressure plate analysis showed load rate (LR) symmetry ratio increased considerably in both gaits, indicating an increased speed of loading at the walk as well as at the trot. A clear improvement in peak vertical force (PVF) and vertical impulse (VI) symmetry ratios was evident at the trot, indicating an increased symmetry of the weight-bearing function of the forelimbs. | Spaas et al. (2012) |
| Degenerative joint disease of fetlock | 20 (group I PRP; group 2) MSCs; group 3) MSCs and PRP; or group 4) chondrogenic induced MSCs and PRP | 6 and 12 weeks and 6 and 12 months | Peripheral blood MSCs, platelet-rich plasma (PRP) | 6.7 × 103 MSCs/cm2 cells chondrogenically induced + 200 × 106 platelets | American Association of Equine Practitioners (AAEP) | Group 4 animals generated the highest evolution scores; although it had statistically insignificant difference against group 4 in the early or late evolution score. | Broeckx et al. (2014) |
| Early stage fetlock degenerative joint disease | 75 (50 in treated and 25 in control) | 3, 6, and 18 weeks and 1 year | Chondrogenically induced Allogenic MSCs + allogenic plasma | 2 × 106 cells + ∼85 × 106 platelets | AAEP score system | lameness scores (p<.001), flexion test responses (p<.034), and joint effusion scores (p<.001) | Broeckx et al. (2019) |
The BM-/AD-MSCs could repair equine meniscal tear models by the production of cartilage compared to control group where the defect is either partially repaired or not at all (González-Fernández et al. 2016). In an equine femoropatellar defect model, MSCs seeded in a self-polymerizing autogenous fibrin vehicle have been used with better outcomes at early stages compared to those in which the defects in the fibrin vehicle were treated. In cell-treated animals, arthroscopic scores were significantly improved at 30 d. However, after a longer follow up period (8 months), histological scores of the cell-treated group were comparable to those of the control (Wilke et al. 2007). Hyaluronan-laden BM-MSCs, upon transplantation along with induction of microfractures in an equine induced chondral defect model, could result in better arthroscopic and gross appearances. However, an insignificant improvement in the clinical and histological examinations compared to those of the microfractures was reported after an evaluation period of 12 months. Overall, it was reported that the use of BM-MSCs led to better cartilage quality with an increased aggrecan and tissue firmness (McIIwraith et al. 2011). In another equine chondral defect model (15 mm defect in lateral trochlear ridge), it was shown that the BM-MSCs (12 × 106) along with autologous platelet-enhanced fibrin scaffolds (APEF) does not carry any added advantage over APEF alone at 12-month evaluation. After 3 months, the healed tissue had a cobblestone appearance with a fair to good subchondral integration, and at the later evaluation period, the healed tissue appeared less smooth with less subchondral bone integration (Goodrich et al. 2016). Thus, it may be inferred that, in equines, the MSCs may enhance early matrix synthesis, but without any long-term benefit.
The ability of BM-MSCs in healing the cartilage tissue is considered to be better than that of the AD-SVF. Use of BM-MSCs comparably results in better clinical, biochemical, and histological improvement in osteoarthritic joints at day 70. Moreover, the animals treated with BM-MSCs are better able to reduce the PGE2 levels in the synovial fluid. Unlike BM-MSCs, AD-SVF increases the unwanted concentrations of TNF-α in the synovial fluid (Frisbie et al. 2009). Implantation of the BM aspirate in combination with the microfracture technique yields a cartilage with better macroscopic characteristics and histological and MRI scores in an equine model (15 mm full-thickness lateral trochlear ridge defects) compared to those in which the microfracture technique alone was used for an 8-month evaluation period. Although microfracture (a surgical technique to induce tiny fractures in the subchondral bone) may make the stem cells available, their cell numbers may not be sufficient enough to cause the desired amount of healing (Fortier et al. 2010). In a comparative study, implantation of MSCs along with PRP was shown to improve the functionality and sustainability of damaged fetlock joints from 6 weeks to 6 months as compared to the use of PRP alone. Implantation of chondrogenically differentiated MSCs and PRPs led to the highest short-term clinical evaluation scores right from 6 weeks through 12 weeks and from an evaluation period from 6 to 12 months (Broeckx et al. 2014). In a randomized, multicentered, double-blinded, and placebo-controlled study, intra-articular (fetlock joint) implantation of chondrogenically-induced allogenic MSCs along with the allogenic plasma has led to significant improvement in lameness scores, flexion test responses, and joint effusion scores compared to those of the control group horses. The relevant improvement to clinical therapy was seen as early as 3 weeks and continued till 18 weeks. During the various evaluation periods, significant improvement was observed in the treated cases compared to that of the control cases. However, it is worth mentioning that not all the cases displayed a same level of improvement (Broeckx et al. 2019). In contrast to sheep MSC studies, chondrogenically-differentiated equine MSCs, upon transplantation, have mostly resulted in better clinical outcomes. However, it needs to be validated whether the observed response was due to the addition of plasma or PRP to the MSCs or due to any other reason. The utility of chondrogenically-differentiated allogenic MSCs needs further validation as differentiated cells express MHC-II, and thus, may incite the immune response and get rejected.
Currently, the recommended dosage of stem cell implantation is 2 × 107 in the hyaluronan scaffold (22 mg of Hyvisc (hyaluronate sodium, 3 × 106 Da, Anika Therapeutics, Woburn, MA, ]) (Schnabel et al. 2013), prior to which NSAIDs are administered to reduce the joint flare (Gugjoo, Amarpal, Makhdoomi, et al. 2019). Clinical evaluation of the use of autologous BM-MSCs in 33 horses having joint affections (meniscal, cartilage, or ligamentous damage) revealed that 43% of horses returned to the previous level of work, 33% returned to work, and 24% failed to return to work (Ferris et al. 2014). In a clinical study employing a combination of PRP and chondrogenically-induced MSCs, it was found that the clinical parameters in these animals were better than those treated with PRP and undifferentiated MSCs followed by those of the PRP-treated animals (Broeckx et al. 2014). A single clinical case affected with bilateral articular cartilage fissure defects of the medial femoral condyles and concurrent cranial cruciate ligament injury has been reported, in which multiple improvements were observed by the application of BM-MSCs. The initial cell/fibrin glue mixture was delivered arthroscopically into the articular cartilage defects 90 d after the initial arthroscopic examination followed by two more cell implantations at 5 and 13 months. Evaluation by arthroscopy at 4 months (after the initial MSC treatment) revealed marked cartilage surface smoothing and a reduction in the depth of the cartilage defect. Furthermore, moderate improvement in the cranial cruciate ligament was observed. After 15 months of the initial MSC treatment, the horse returned to racing and had comparable race earning to that of the pre-injury records (Raheja et al. 2011). Similarly, in another case report, MSCs derived from the peripheral blood implanted twice at intervals of 8 weeks led to an improvement in the chronic degenerative disease of the pastern joint (Spaas, Oosterlinck, et al. 2012).
Preclinical experimental models usually provide uniform conditions to understand the effect of the MSCs. However, the results obtained therein may not be recapitulated under clinical conditions. Clinical settings, whether in animals or humans, usually provide uncontrolled studies, since variability is observed in the joint type and lesion (s) including their site and duration of existence. Additionally, age of the patient is also non-uniform. Moreover, cell sources, culture techniques, passage number, cell number, methods of implantation and addition of growth factors, and scaffolds could have a bearing on the outcome. Furthermore, incorporation of other surgical techniques and evaluation criteria need to be conducted uniformly (Gugjoo, Amarpal, Makhdoomi, et al. 2019).
4. Conclusions and future perspectives
Articular cartilage, once damaged, tends to undergo deterioration with each passing day due to its typical location and limited innate healing potential. With limited success of current surgical techniques, incorporation of stem cells in regenerative medical therapy is being extensively studied to enable better cartilage rehabilitation. Among the various stem cells, MSCs, particularly those obtained from adipose tissue and BM, are being studied to evaluate their clinical applications. This technology promises to develop mechanically strong cartilage-to-cartilage interface, and involves mesenchymal condensation into cellular bodies under the influence of growth factors. However, this technique is yet to be validated under in vivo clinical conditions. The clinical application of MSCs has mainly been adopted in dogs and horses, whereas in sheep and goats, MSCs have been mainly studied in preclinical experimental models. The chondrogenesis of MSCs highly varies with respect to the cell sources, culture techniques, passage number, number of implantations required, and incorporation of growth factors and scaffolds; thus, this warrants further studies. In general, better repair is observed upon treatment with MSCs in comparison to that of the control. However, there are various concerns associated with MSC treatment such as lack of typical hyaline tissue regeneration, integration of regenerated tissue matrix with the host native cartilage or subchondral bone, and its comparable effectiveness in all cases. Thus, further studies and experiments need to be conducted before the regenerative medicine involving stem cells can be considered fully effective and utilized clinically.
Acknowledgments
Authors of the manuscript thank and acknowledge their respective Universities/Institutes. Utmost gratitude goes to SERB-DST, GOI for boosting the moral by supporting the research project on stem cell studies.
Funding Statement
This work was supported by Science and Engineering Research Board (SERB-DST), GOI (Grant No. EMR/2017/001484).
Disclosure statement
The authors declare that there is no conflict of interest.
References
- Abdalmula A, Dooley LM, Kaufman C, Washington EA, House JV, Blacklaws BA, Ghosh P, Itescu S, Bailey SR, Kimpton WG.. 2017. Immuno-selected STRO-3+ mesenchymal precursor cells reduce inflammation and improve clinical outcomes in a large animal model of monoarthritis. Stem Cell Res Ther. 8(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkisson HD, Gillis MP, Davis EC, Maloney WHruska KA.. 2001. In vitro generation of scaffold independent neocartilage. Clin Orthop Relat Res. S391:280–294. [DOI] [PubMed] [Google Scholar]
- Al Faqeh H, Nor Hamdan BMY, Chen HC, Aminuddin BS, Ruszymah BHI.. 2012. The potential of intra‐articular injection of chondrogenic‐induced bone marrow stem cells to retard the progression of osteoarthritis in a sheep model. Exp Gerontol. 47(6):458–464. [DOI] [PubMed] [Google Scholar]
- Ando W, Heard BJ, Chung M, Nakamura N, Frank CB, Hart DA.. 2012. Ovine synovial membrane-derived mesenchymal progenitor cells retain the phenotype of the original tissue that was exposed to in-vivo inflammation: evidence for a suppressed chondrogenic differentiation potential of the cells. Inflamm Res. 61(6):599–608. [DOI] [PubMed] [Google Scholar]
- Ardanaz N, Vázquez FJ, Romero A, Remacha AR, Barrachina L, Sanz A, Ranera B, Vitoria A, Albareda J, Prades M, et al. . 2016. Inflammatory response to the administration of mesenchymal stem cells in an equine experimental model: effect of autologous, and single and repeat doses of pooled allogeneic cells in healthy joints. BMC Vet Res. 12(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arican M, Koylu O, Uyaroglu A, Erol M, Çalım KN.. 2006. The effect of (Hylan G-F 20) on bone metabolism in dogs with experimental osteochondral defects. J Turk Vet Surg. 12:20–23. [Google Scholar]
- Athanasiou AK, Agarwal A, Muffoletto A.. 1995. Biomechanical properties of hip cartilage in experimental animal models. Clin Orthop. 316:254–266. [PubMed] [Google Scholar]
- Barberini D, Freitas NP, Magnoni M, Maia L, Listoni A, Heckler M, Sudano M, Golim M, da Cruz Landim-Alvarenga F, Amorim R, et al. . 2014. Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: immuno-phenotypic characterization and differentiation potential. Stem Cell Res Ther. 5(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour KE, Helmick CG, Boring M, Zhang X, Lu H, Holt JB.. 2016. Prevalence of doctor diagnosed arthritis at state and county levels-United States, 2014. Morb Mortal Wkly Rep. 65(19):489–484. [DOI] [PubMed] [Google Scholar]
- Barrachina L, Remacha AR, Romero A, Vázquez FJ, Albareda J, Prades M, Ranera B, Zaragoza P, Martín-Burriel I, Rodellar C.. 2016. Effect of inflammatory environment on equine bone marrow derived mesenchymal stem cells immunogenicity and immunomodulatory properties. Vet Immunol Immunopathol. 171:57–65. [DOI] [PubMed] [Google Scholar]
- Bekkers JEJ, Tsuchida AI, van Rijen MHP, Vonk LA, Dhert WJA, Creemers LB, Saris DBF.. 2013. Single-stage cell-based cartilage regeneration using a combination of chondrons and mesenchymal stromal cells: comparison with microfracture. Am J Sports Med. 41(9):2158–2166. [DOI] [PubMed] [Google Scholar]
- Berglund AK, Fisher MB, Cameron KA, Poole EJ, Schnabel LV.. 2017. Transforming growth factor-β2 downregulates major histocompatibility complex (MHC) I and MHC II surface expression on equine bone marrow-derived mesenchymal stem cells without altering other phenotypic cell surface markers. Front Vet Sci. 4(4):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolo A, Steffen F, Malonzo-Marty C, Stoyanov J.. 2015. Canine mesenchymal stem cell potential and the importance of dog breed: implication for cell-based therapies. Cell Transplant. 24(10):1969–1980. [DOI] [PubMed] [Google Scholar]
- Bhumiratana S, Eton RE, Oungoulian SR, Wan LQ, Ateshian GA, Vunjak-Novakovic G.. 2014. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proc Natl Acad Sci USA. 111(19):6940–6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black LL, Gaynor J, Gahring D, Adams C, Aron D, Harman S, Gingerich DA, Harman R. 2007. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther. 8(4):272–84. [PubMed] [Google Scholar]
- Black LL, Gaynor J, Adams C.. 2008. Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther. 9:192–200. [PubMed] [Google Scholar]
- Boone L, Mumaw J, Thoresen M, Gogal R, Peroni J.. 2018. Viability, proliferation, and chondrogenesis of equine bone marrow derived mesenchymal stromal cells after exposure to varying concentrations of allogeneic synovial fluid in vitro. J Equine Vet Sci. 62:1–7. [Google Scholar]
- Bornes TD, Adesida AB, Jomha NM.. 2018. Articular cartilage repair with mesenchymal stem cells after chondrogenic priming: a pilot study. Tissue Eng Part A. 24(9–10):761–774. [DOI] [PubMed] [Google Scholar]
- Bornes TD, Jomha NM, Mulet-Sierra A, Adesida AB.. 2015. Hypoxic culture of bone marrow-derived mesenchymal stromal stem cells differentially enhances in vitro chondrogenesis within cell-seeded collagen and hyaluronic acid porous scaffolds. Stem Cell Res Ther. 6(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branly T, Bertoni L, Contentin R, Rakic R, Gomez-Leduc T, Desancé M, Hervieu M, Legendre F, Jacquet S, Audigié F, et al. . 2017. Characterization and use of equine bone marrow mesenchymal stem cells in equine cartilage engineering. Study of their hyaline cartilage forming potential when cultured under hypoxia within a biomaterial in the presence of BMP-2 and TGF-ß1. Stem Cell Rev Rep. 13(5):611–630. [DOI] [PubMed] [Google Scholar]
- Broeckx S, Zimmerman M, Crocetti S, Suls M, Mariën T, Ferguson SJ, Chiers K, Duchateau L, Franco-Obregón A, Wuertz K, et al. . 2014. Regenerative therapies for equine degenerative joint disease: a preliminary study. PLoS One. 9(1):e85917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeckx SY, Seys B, Suls M, Vandenberghe A, Mariën T, Adriaensen E, Declercq J, Van Hecke L, Braun G, Hellmann K, et al. . 2019. Equine allogeneic chondrogenic induced mesenchymal stem cells are an effective treatment for degenerative joint disease in horses. Stem Cells Dev. 28(6):410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk J, Ribitsch I, Gittel C, Juelke H, Kasper C, Staszyk C, Brehm W.. 2013. Growth and differentiation characteristics of equine mesenchymal stromal cells derived from different sources. Vet J. 195(1):98–106. [DOI] [PubMed] [Google Scholar]
- Caminal M, Fonseca C, Peris D, Moll X, Rabanal RM, Barrachina J, Codina D, García F, Cairó JJ, Gòdia F, et al. . 2014. Use of a chronic model of articular cartilage and meniscal injury for the assessment of long-term effects after autologous mesenchymal stromal cell treatment in sheep. New Biotechnol. 31(5):492–498. [DOI] [PubMed] [Google Scholar]
- Caminal M, Moll X, Codina D, Rabanal RM, Morist A, Barrachina J, Garcia F, Pla A, Vives J.. 2014. Transitory improvement of articular cartilage characteristics after implantation of polylactide: polyglycolic acid (PLGA) scaffolds seeded with autologous mesenchymal stromal cells in a sheep model of critical-sized chondral defect. Biotechnol Lett. 36(10):2143–2153. [DOI] [PubMed] [Google Scholar]
- Cardoso MT, Pinheiro AO, Vidane AS, Casals JB, de Oliveira VC, Gonçalves NJN, Martins DS, Ambrósio CE.. 2017. Characterization of teratogenic potential and gene expression in canine and feline amniotic membrane-derived stem cells. Reprod Dom Anim. 52(s2):58–64. [DOI] [PubMed] [Google Scholar]
- Carrade DD, Affolter VK, Outerbridge CA, Watson JL, Galuppo LD, Buerchler S, Kumar V, Walker NJ, Borjesson DL.. 2011. Intradermal injections of equine allogeneic umbilical cord-derived mesenchymal stem cells are well tolerated and do not elicit immediate or delayed hypersensitivity reactions. Cytotherapy. 13(10):1180–1192. [DOI] [PubMed] [Google Scholar]
- Carrade DD, Lame MW, Kent MS, Clark KC, Walker NJ, Borjesson DL.. 2012. Comparative analysis of the immunomodulatory properties of equine adult-derived mesenchymal stem cells. Cell Med. 4(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrade DD, Owens SD, Galuppo LD, Vidal MA, Ferraro GL, Librach F, Buerchler S, Friedman MS, Walker NJ, Borjesson DL, et al. . 2011. Clinicopathologic findings following intra-articular injection of autologous and allogeneic placentally derived equine mesenchymal stem cells in horses. Cytotherapy. 13(4):419–430. [DOI] [PubMed] [Google Scholar]
- Colbath AC, Dow SW, Phillips JN, McIlwraith CW, Goodrich LR.. 2017. Autologous and allogeneic equine mesenchymal stem cells exhibit equivalent immunomodulatory properties in vitro. Stem Cells Dev. 26(7):503–511. [DOI] [PubMed] [Google Scholar]
- Corradetti B, Meucci A, Bizzaro D, Cremonesi F, Lange Consiglio A.. 2013. Mesenchymal stem cells from amnion and amniotic fluid in the bovine. Reproduction. 145(4):391–400. [DOI] [PubMed] [Google Scholar]
- Cuervo B, Rubio M, Sopena J, Dominguez J, Vilar J, Morales M, Cugat R, Carrillo J.. 2014. Hip osteoarthritis in dogs: a randomized study using mesenchymal stem cells from adipose tissue and plasma rich in growth factors. Int J Mol Sci. 15(8):13437–13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schauwer C, Goossens K, Piepers S, Hoogewijs MK, Govaere JLJ, Smits K, Meyer E, Van Soom A, Van de Walle GR.. 2014. Characterization and profiling of immunomodulatory genes of equine mesenchymal stromal cells from non-invasive sources. Stem Cell Res Ther. 5(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schauwer C, Meyer E, Van de Walle GR, Van Soom A.. 2011. Markers of stemness in equine mesenchymal stem cells: a plea for uniformity. Theriogenology. 75(8):1431–1443. [DOI] [PubMed] [Google Scholar]
- DeLise AM, Fischer L, Tuan RS.. 2000. Cellular interactions and signalling in cartilage development. Osteoarthritis Cartilage. 8(5):309–334. [DOI] [PubMed] [Google Scholar]
- Delling U, Brehm W, Ludewig E, Winter K, Jülke H.. 2015. Longitudinal evaluation of effects of intraarticular mesenchymal stromal cell administration for the treatment of osteoarthritis in an ovine model. Cell Transplant. 24(11):2391–2407. [DOI] [PubMed] [Google Scholar]
- Delling U, Brehm W, Metzger M, Ludewig E, Winter K, Jülke H.. 2015. In vivo tracking and fate of intra-articularly injected superparamagnetic iron oxide particle-labeled multipotent stromal cells in an ovine model of osteoarthritis. Cell Transplant. 24(11):2379–2390. [DOI] [PubMed] [Google Scholar]
- Desando G, Giavaresi G, Cavallo C, Bartolotti I, Sartoni F, Nicoli Aldini N, Martini L, Parrilli A, Mariani E, Fini M, et al. . 2016. Autologous bone marrow concentrate in a sheep model of osteoarthritis: new perspectives for cartilage and meniscus repair. Tissue Eng Part C. 22(6):608–619. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM.. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherarpy. 8(4):315–317. [DOI] [PubMed] [Google Scholar]
- Dražilov SS, Mrkovački J, Spasovski V, Fazlagić A, Pavlović S, Nikčević G.. 2018. The use of canine mesenchymal stem cells for the autologous treatment of osteoarthritis. Acta Vet Hung. 66(3):76–389. [DOI] [PubMed] [Google Scholar]
- Duarte Campos DF, Drescher W, Rath B, Tingart M, Fischer H.. 2012. Supporting biomaterials for articular cartilage repair. Cartilage. 3(3):205–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duygulu F, Demirel M, Atalan G.. 2012. Effects of intra-articular administration of autologous bone marrow aspirate on healing of full-thickness meniscal tear: an experimental study on sheep. Acta Orthop Traumatol Turc. 46(1):61–67. [DOI] [PubMed] [Google Scholar]
- Ehnert S, Glanemann M, Schmitt A, Vogt S, Shanny N, Nussler NC, Stöckle U, Nussler A.. 2009. The possible use of stem cells in regenerative medicine: dream or reality?” Langenbeck’s. Langenbecks Arch Surg. 394(6):985–997. [DOI] [PubMed] [Google Scholar]
- Feitosa MLT, Fadel L, Beltrão-Braga PCB, Wenceslau CV, Kerkis I, Kerkis A, Birgel Júnior EH, Martins JFP, Martins D. D S, Miglino MA, et al. . 2010. Successful transplant of mesenchymal stem cells in induced osteonecrosis of the ovine femoral head. Preliminary results. Acta Cir Bras. 25(5):416–422. [DOI] [PubMed] [Google Scholar]
- Feng C, Luo X, He N, Xia H, Lv X, Zhang X, Li D, Wang F, He J, Zhang L, et al. . 2018. Efficacy and persistence of allogeneic adipose‐derived mesenchymal stem cells combined with hyaluronic acid in osteoarthritis after intra‐articular injection in a sheep model. Tissue Eng Part A. 24(3–4):219–233. [DOI] [PubMed] [Google Scholar]
- Ferris DJ, Frisbie DD, Kisiday JD, McIlwraith CW, Hague BA, Major MD, Schneider RK, Zubrod CJ, Kawcak CE, Goodrich LR, et al. . 2014. Clinical outcome after intra-articular administration of bone marrow derived mesenchymal stem cells in 33 horses with stifle injury. Vet Surg. 43(3):255–265. [DOI] [PubMed] [Google Scholar]
- Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, Stokol T, Cheetham J, Nixon AJ.. 2010. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 92(10):1927–1937. [DOI] [PubMed] [Google Scholar]
- Fraser JK, Wulur I, Alfonso Z, Hedrick MH.. 2006. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 24(4):150–154. [DOI] [PubMed] [Google Scholar]
- Frisbie DD, Cross MW, McIlwraith CW.. 2006. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human. Vet Comp Orthop Traumatol. 19(03):142–146. [PubMed] [Google Scholar]
- Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW.. 2009. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 27(12):1675–1680. [DOI] [PubMed] [Google Scholar]
- Fülber J, Maria DA, Silva LCLCD, Massoco CO, Agreste F, Baccarin RYA.. 2016. Comparative study of equine mesenchymal stem cells from healthy and injured synovial tissues: an in vitro assessment. Stem Cell Res Ther. 7(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D, Longo U, Vaquero J, Forriol F, Loppini M, Khan W, Denaro V.. 2014. Amniotic membrane transplant for articular cartilage repair: an experimental study in sheep. Curr Stem Cell Res Ther. 10(1):77–83. [DOI] [PubMed] [Google Scholar]
- Goldman SM, Barabino GA.. 2016. Hydrodynamic loading in concomitance with exogenous cytokine stimulation modulates differentiation of bovine mesenchymal stem cells towards osteochondral lineages. BMC Biotechnol. 16(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Fernández ML, Pérez-Castrillo S, Sánchez-Lázaro JA, Prieto-Fernández JG, López-González ME, Lobato-Pérez S, Colaço BJ, Olivera ER, Villar-Suárez V.. 2016. Assessment of regeneration in meniscal lesions by use of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Am J Vet Res. 77(7):779–788. [DOI] [PubMed] [Google Scholar]
- Goodrich LR, Chen AC, Werpy NM, Williams AA, Kisiday JD, Su AW, Cory E, Morley PS, McIlwraith CW, Sah RL, et al. . 2016. Addition of mesenchymal stem cells to autologous platelet-enhanced fibrin scaffolds in chondral defects: does it enhance repair? J Bone Joint Surg. 98(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guercio A, Di Marco P, Casella S, Cannella V, Russotto L, Purpari G, Di Bella S, Piccione G.. 2012. Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. Cell Biol Int. 36(2):189–194. [DOI] [PubMed] [Google Scholar]
- Guest DJ, Ousey JC, Smith M.. 2008. Defining the expression of marker genes in equine mesenchymal stromal cells. Stem Cells Cloning. 1:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugjoo MB, Amarpal Chandra V, Wani MY, Dhama K, Sharma GT.. 2018. Mesenchymal stem cell research in veterinary medicine. Curr Stem Cell Res Ther. 13(8):645–657. [DOI] [PubMed] [Google Scholar]
- Gugjoo MB, Amarpal, Ahmed AA, Aithal HP, Kinjavdekar P, Pawde AM, Kumar GS, Sharma GT.. 2017. Mesenchymal stem cells with IGF-1 and TGF- β1 in laminin gel for osteochondral defects in rabbits. Biomed Pharmacother. 93:1165–1174. [DOI] [PubMed] [Google Scholar]
- Gugjoo MB, Amarpal, Fazili MR, Shah RA, Sharma GT.. 2019. Mesenchymal stem cell: basic research and potential applications in cattle and buffalo. J Cell Physiol. 234(6):8618–8635. [DOI] [PubMed] [Google Scholar]
- Gugjoo MB, Amarpal, Kinjavdekar P, Aithal HP, Ansari MM, Pawde AM, Sharma GT.. 2015. Isolation, culturing and characterization of New Zealand White rabbit mesenchymal stem cells derived from bone marrow. Asian J Anim Vet Adv. 10(10):537–548. [Google Scholar]
- Gugjoo MB, Amarpal, Makhdoomi DM, Sharma GT.. 2019. Equine mesenchymal stem cells: properties, sources, characterization and potential therapeutic applications. J Equine Vet Sci. 72:16–27. [DOI] [PubMed] [Google Scholar]
- Gugjoo MB, Amarpal, Sharma GT, Aithal HP, Kinjavdekar P.. 2016. Cartilage tissue engineering: role of mesenchymal stem cells along with growth factors and scaffolds. Indian J Med Res. 144:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugjoo MB, Amarpal, Sharma GT.. 2019. Mesenchymal stem cell basic research and applications in dog medicine. J Cell Physiol. 234:4140–4153. doi: 10.1002/jcp.28348 [DOI] [PubMed] [Google Scholar]
- Guilak F, Estes BT, Diekman BO, Moutos FT, Gimble JM.. 2010. 2010 Nicolas Andry Award: multipotent adult stem cells from adipose tissue for musculoskeletal tissue engineering. Clin Orthop Relat Res. 468(9):2530–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Wang C, Zhang Y, Xia R, Hu M, Duan C, Zhao Q, Dong L, Lu J, Qing Song Y, et al. . 2004. Repair of large articular cartilage defects with implants of autologous mesenchymal stem cells seeded into beta-tricalcium phosphate in a sheep model. Tissue Eng. 10(11–12):1818–1829. [DOI] [PubMed] [Google Scholar]
- Hall BK, Miyake T.. 2000. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 22(2):138–147. [DOI] [PubMed] [Google Scholar]
- Hang D, Wang Q, Guo C, Chen Z, Yan Z.. 2012. Treatment of osteonecrosis of the femoral head with VEGF165 transgenic bone marrow mesenchymal stem cells in mongrel dogs. Cells Tissues Organs. 195(6):495–506. [DOI] [PubMed] [Google Scholar]
- Harman R, Carlson K, Gaynor J, Gustafson S, Dhupa S, Clement K, Hoelzler M, McCarthy T, Schwartz P, Adams C.. 2016. A prospective, randomized, masked, and placebo-controlled efficacy study of intra-articular allogeneic adipose stem cells for the treatment of osteoarthritis in dogs. Front Vet Sci. 3:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Muller C, Gebhard S, Bauer E, Pausch F, Schlund B, Bosl MR, Hess A, Surmann-Schmitt C, von der Mark H, et al. . 2010. SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development. 137(6):901–911. [DOI] [PubMed] [Google Scholar]
- Heo SJ, Thorpe SD, Driscoll TP, Duncan RL, Lee DA, Mauck RL.. 2015. Biophysical regulation of chromatin architecture instills a mechanical memory in mesenchymal stem cells. Sci Rep. 5:16895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmann A, Ahrberg AB, Brehm W, Heller S, Josten C, Paebst F, Burk J.. 2016. Comparative characterization of human and equine mesenchymal stromal cells: a basis for translational studies in the equine model. Cell Transplant. 25(1):109–124. [DOI] [PubMed] [Google Scholar]
- Hindle P, Baily J, Khan N, Biant LC, Simpson AHR, Péault B.. 2016. The infrapatellar fat pad as a source of perivascular stem cells with increased chondrogenic potential for regenerative medicine. Stem Cells Dev. 25(21):1659–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho YT, Shimbo T, Wijaya E, Ouchi Y, Takaki E, Yamamoto R, Kikuchi Y, Kaneda Y, Tamai K.. 2018. Chromatin accessibility identifies diversity in mesenchymal stem cells from different tissue origins. Sci Rep. 8(1):1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SC, Wu TC, Yu HC, Chen MR, Liu CM, Chiang WS, Lin KM.. 2010. Mechanical strain modulates age-related changes in the proliferation and differentiation of mouse adipose-derived stromal cells. BMC Cell Biol. 11(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker EB. 1999. Biologic repair of articular cartilage. Defect models in experimental animals and matrix requirements. Clin Orthop Relat Res. 367:S135–S146. [PubMed] [Google Scholar]
- Hwang NS, Varghese S, Li H, Elisseeff J.. 2011. Regulation of osteogenic and chondrogenic differentiation of mesenchymal stem cells in PEG-ECM hydrogels. Cell Tissue Res. 344(3):499–509. [DOI] [PubMed] [Google Scholar]
- Iacono E, Lanci A, Merlo B, Ricci F, Pirrone A, Antonelli C, Mariella J, Castagnetti C.. 2016. Effects of amniotic fluid mesenchymal stem cells in carboxymethyl cellulose gel on healing of spontaneous pressure sores: clinical outcome in seven hospitalized neonatal foals. Turk J Biol. 40:484–492. [Google Scholar]
- Jeng L, Olsen BR, Spector M.. 2010. Engineering endostatin-producing cartilaginous constructs for cartilage repair using nonviral transfection of chondrocyte-seeded and mesenchymal-stem-cell-seeded collagen scaffolds. Tissue Eng Part A. 16(10):3011–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeuken R, Roth A, Peters R, van Donkelaar C, Thies J, van Rhijn L, Emans P.. 2016. Polymers in cartilage defect repair of the knee: current status and future prospects. Polymers. 8(6):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SA. 1997. Osteoarthritis. Joint anatomy, physiology, and pathobiology. Vet Clin North Am Small Anim Pract. 27(4):699–723. [DOI] [PubMed] [Google Scholar]
- Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU.. 1998. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 238(1):265–272. [DOI] [PubMed] [Google Scholar]
- Joswig AJ, Mitchell A, Cummings KJ, Levine GJ, Gregory CA, Smith R, Watts AE.. 2017. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res Ther. 8(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneau C, Paine R, Chicas E, Gardner E, Bailey L, McDermott J.. 2016. Current concepts in treatment of patellofemoral osteochondritis dissecans. Int J Sports Phys Ther. 11(6):903–925. [PMC free article] [PubMed] [Google Scholar]
- Jurgens W, Kroeze RJ, Zandieh-Doulabi B, van Dijk A, Renders GA, Smit TH, van Milligen FJ, Ritt MJ, Helder MN.. 2013. One-step surgical procedure for the treatment of osteochondral defects with adipose-derived stem cells in a caprine knee defect: a pilot study. Biores Open Access. 2(4):315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser LR. 1992. The future of multihospital systems. Top Health Care Financ. 18(4):32–45. [PubMed] [Google Scholar]
- Kang JW, Kang KS, Koo HC, Park JR, Choi EW, Park YH.. 2008. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 17(4):681–693. [DOI] [PubMed] [Google Scholar]
- Kazemi D, Shams Asenjan K, Dehdilani N, Parsa H.. 2017. Canine articular cartilage regeneration using mesenchymal stem cells seeded on platelet rich fibrin. Bone Joint Res. 6(2):98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Kim HJ, Im GI.. 2008. PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochem Biophys Res Commun. 373(1):104–108. [DOI] [PubMed] [Google Scholar]
- Kinner B, Capito RM, Spector M.. 2005. Regeneration of articular cartilage. Adv Biochem Eng Biotechnol. 94:91–123. [DOI] [PubMed] [Google Scholar]
- Kisiday JD, Kopesky PW, Evans CH, Grodzinsky AJ, McIlwraith CW, Frisbie DD.. 2008. Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. J Orthop Res. 26(3):322–331. [DOI] [PubMed] [Google Scholar]
- Kode JA, Mukherjee S, Joglekar MV, Hardikar AA.. 2009. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 11(4):377–391. [DOI] [PubMed] [Google Scholar]
- Kondo M, Yamaoka K, Sonomoto K, Fukuyo S, Oshita K, Okada Y, Tanaka Y.. 2013. IL-17 inhibits chondrogenic differentiation of human mesenchymal stem cells. PLoS One. 8(11):e79463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhemyakina E, Lassar AB, Zelzer E.. 2015. A pathway to bone: signalling molecules and transcription factors involved in chondrocyte development and maturation. Development. 142(5):817–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretlow JD, Jin YQ, Liu W, Zhang W, Hong TH, Zhou G, Baggett LS, Mikos AG, Cao Y.. 2008. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 9(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriston-Pál É, Czibula Á, Gyuris Z, Balka G, Seregi A, Sükösd F, Süth M, Kiss-Tóth E, Haracska L, Uher F, et al. 2017. Characterization and therapeutic application of canine adipose mesenchymal stem cells to treat elbow osteoarthritis. Can J Vet Res. 81:73–78. [PMC free article] [PubMed] [Google Scholar]
- Larguech G, Cesaro A, Toumi H, Martinic I, Petoud S, Daniellou R, Lespessailles E.. 2017. Growth inhibition of mesenchymal stem cells by laminarin: impact on chondrocyte differentiation. Anat Physiol. 7:3. [Google Scholar]
- Lee J, Lee KS, Kim CL, Byeon JS, Gu NY, Cho IS, Cha SH.. 2017. Effect of donor age on the proliferation and multipotency of canine adipose-derived mesenchymal stem cells. J Vet Sci. 18(2):141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD.. 2000. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 18(6):675–679. [DOI] [PubMed] [Google Scholar]
- Lee WD, Hurtig MB, Pilliar RM, Stanford WL, Kandel RA.. 2015. Engineering of hyaline cartilage with a calcified zone using bone marrow stromal cells. Osteoarthritis Cartilage. 23(8):1307–1315. [DOI] [PubMed] [Google Scholar]
- Leung VYL, Gao B, Leung KKH, Melhado IG, Wynn SL, Au TYK, Dung NWF, Lau JYB, Mak ACY, Chan D, et al. . 2011. SOX9 governs differentiation stage-specific gene expression in growth plate chondrocytes via direct concomitant transactivation and repression. PLoS Genet. 7(11):e1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Duan X, Fan Z, Chen L, Xing F, Xu Z, Chen Q, Xiang Z.. 2018. Mesenchymal stem cells in combination with hyaluronic acid for articular cartilage defects. Sci Rep. 8(1):9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Liu J, Qi C, Fang Y, Zhang L, Zhuo R, Jiang X.. 2016. Thermosensitive injectable insitu forming carboxymethyl chitin hydrogel for three dimensional cell culture. Acta Biomater. 35:228–237. [DOI] [PubMed] [Google Scholar]
- Lombana KG, Goodrich LR, Phillips JN, Kisiday JD, Ruple-Czerniak A, McIlwraith CW.. 2015. An investigation of equine Mesenchymal stem cell characteristics from Different harvest sites: more similar than not. Front Vet Sci. 2:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Lei D, Jiang T, Yang L, Zheng L, Zhao J.. 2017. Nerve growth factor from Chinese cobra venom stimulates chondrogenic differentiation of mesenchymal stem cells. Cell Death Dis. 8(5):e2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luesma MJ, Cantarero I, Ranera B, Remacha AR, Castiella T, Romero A, Martín I, Rodellar C, Junquera C.. 2016. Primary cilia in chondrogenic differentiation of equine bone marrow mesenchymal stem cells: ultrastructural study. J Equine Vet Sci. 47:47–54. [Google Scholar]
- Lydon H, Getgood A, Henson F.. 2017. Healing of osteochondral defects via endochondral ossification in an ovine model. Cartilage. 10(1):94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF.. 1998. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 4(4):415–428. [DOI] [PubMed] [Google Scholar]
- Mankin HJ. 1984. Synovium and cartilage in health and disease. In: Newton CD, Nunamaker DM, editors. Textbook of small animal orthopaedics. Philadelphia (PA): JB. Lippincott Company; p. 90. [Google Scholar]
- A. Maroudas 1979. Physico-chemical properties of articular cartilage. In: Freeman M, editor. Adult articular cartilage. London: Pitman Medical; p. 215–290. [Google Scholar]
- Marx C, Silveira MD, Selbach I, da Silva AS, Braga L. M G D M, Camassola M, Nardi NB.. 2014. Acupoint injection of autologous stromal vascular fraction and allogeneic adipose-derived stem cells to treat hip dysplasia in dogs. Stem Cells Int. 2014:391274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Cooper GM, Gharaibeh B, Meszaros LB, Li G, Usas A, Fu FH, Huard J.. 2009. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 60(5):1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck RL, Yuan X, Tuan RS.. 2006. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 14(2):179–189. [DOI] [PubMed] [Google Scholar]
- McIIwraith CW, Frisbie DD, Rodkey WG, Kisiday JD, Werpy NM, Kawcak CE, Steadman JR.. 2011. Evaluation of intraarticular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy. 27:1552–1561. [DOI] [PubMed] [Google Scholar]
- McLeod CM, Mauck RL.. 2017. On the origin and impact of mesenchymal stem cell heterogeneity: new insights and emerging tools for single cell analysis. Eur Cell Mater. 34:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokbel A, El-Tookhy O, Shamaa AA, Sabry D, Rashed L, Mostafa A.. 2011. Homing and efficacy of intra-articular injection of autologous mesenchymal stem cells in experimental chondral defects in dogs. Clin Exp Rheumatol. 29:275–284. [PubMed] [Google Scholar]
- Murphy JM, Fink DJ, Hunziker EB, Barry FP.. 2003. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 48(12):3464–3474. [DOI] [PubMed] [Google Scholar]
- Nam H, Karunanithi P, Loo WC, Naveen S, Chen H, Hussin P, Chan L, Kamarul T.. 2013. The effects of staged intra-articular injection of cultured autologous mesenchymal stromal cells on the repair of damaged cartilage: a pilot study in caprine model. Arthritis Res Ther. 15(5):R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam YJ, Rim YA, Lee J, Ju JH.. 2018. Current therapeutic strategies for stem cell-based cartilage regeneration. Stem Cells Int. 2018:8490489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson L, McCarthy HE, Fairclough J, Williams R, Archer CW.. 2014. Evidence of a viable pool of stem cells within human osteoarthritic cartilage. Cartilage. 5(4):203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongaro A, Pellati A, Setti S, Masieri FF, Aquila G, Fini M, Caruso A, De Mattei M.. 2015. Electromagnetic fields counteract IL-1b activity during chondrogenesis of bovine mesenchymal stem cells. J Tissue Eng Regen Med. 9(12):E229–38. [DOI] [PubMed] [Google Scholar]
- Pascucci L, Curina G, Mercati F, Marini C, Dall’Aglio C, Paternesi B, Ceccarelli P.. 2011. Flow cytometric characterization of culture expanded multipotent mesenchymal stromal cells (MSCs) from horse adipose tissue: towards the definition of minimal stemness criteria. Vet Immunol Immunopathol. 144(3–4):499–506. [DOI] [PubMed] [Google Scholar]
- Peffers MJ, Collins J, Fang Y, Goljanek-Whysall K, Rushton M, Loughlin J, Proctor C, Clegg PD.. 2016. Age-related changes in mesenchymal stem cells identified using a multi-omics approach. Europ Cells Mat. 31:136–139. [DOI] [PubMed] [Google Scholar]
- Pei M, He F, Vunjak-Novakovic G.. 2008. Synovium-derived stem cell-based chondrogenesis. Differentiation for cartilage repair: monitoring its success by magnetic resonance imaging and histology. Arthritis Res Ther. 5:R60–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Fan JJ, Zhang XQ, Zhang ZY, Yu M.. 2014. Repairing the osteochondral defect in goat with the tissue-engineered osteochondral graft preconstructed in a double-chamber stirring bioreactor. BioMed Res Int. 2014:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peroni JF, Borjesson DL.. 2011. Anti-inflammatory and immunomodulatory activities of stem cells. Vet Clin North Am Equine Pract. 27(2):351–362. [DOI] [PubMed] [Google Scholar]
- AR. Pool 2001. Cartilage in health and disease. In: Koopman WJ, editor. Arthritis and allied conditions. Philadelphia (PA): Lippincott Williams and Wilkins; p. 226–284. [Google Scholar]
- Pretzel D, Linss S, Rochler S, Endres M, Kaps C, Alsalameh S, Kinne RW.. 2011. Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res Ther. 13(2):R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punwar S, Khan WS.. 2011. Mesenchymal stem cells and articular cartilage repair: clinical studies and future direction. Open Orthop J. 5(1):296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raheja LF, Galuppo LD, Bowers-Lepore J, Dowd JP, Tablin F, Yellowley CE.. 2011. Treatment of bilateral medial femoral condyle articular cartilage fissures in a horse using bone marrow-derived multipotent mesenchymal stromal cells. J Equine Vet Sci. 31(3):147–154. [Google Scholar]
- Ranera B, Lyahyai J, Romero A, Vázquez FJ, Remacha AR, Bernal ML, Zaragoza P, Rodellar C, Martín-Burriel I.. 2011. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet Immunol Immunopathol. 144(1–2):147–154. [DOI] [PubMed] [Google Scholar]
- Reesink HL, Sutton RM, Shurer CR, Peterson RP, Tan JS, Su J, Paszek MJ, Nixon AJ.. 2017. Galectin-1 and galectin-3 expression in equine mesenchymal stromal cells (MSCs), synovial fibroblasts and chondrocytes, and the effect of inflammation on MSC motility. Stem Cell Res Ther. 8(1):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzière MC, Perrier E, Mallein-Gerin F.. 2010. Chondrogenic potential of bone marrow- and adipose tissue-derived adult human mesenchymal stem cells. Biomed Mater Eng. 20(3):145–158. [DOI] [PubMed] [Google Scholar]
- Rose R. 1977. An analysis of conditions causing lameness in the horse. Proceedings of the 54th Annual Meeting of the Australian Veterinary Association. Rome, Italy: Food and Agriculture Organization. p. 99–102. [Google Scholar]
- Schnabel LV, Fortier LA, McIlwraith CW, Nobert KM.. 2013. Therapeutic use of stem cells in horses: which type, how, and when? Vet J. 197(3):570–577. [DOI] [PubMed] [Google Scholar]
- Schnabel LV, Pezzanite LM, Antczak DF, Felippe MJ, Fortier LA.. 2014. Equine bone marrow-derived mesenchymal stromal cells are heterogeneous in MHC class II expression and capable of inciting an immune response in vitro. Stem Cell Res Ther. 5(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti C, Tonnarelli B, Papadimitropoulos A, Scherberich A, Schaeren S, Schauerte A, Lopez-Rios J, Zeller R, Barbero A, Martin I, et al. . 2010. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developing engineering. Proc Nat Acad Sci USA. 107(16):7251–7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screven R, Kenyon E, Myers MJ, Yancy HF, Skasko M, Boxer L, Bigley EC, Borjesson DL, Zhu M.. 2014. Immunophenotype and gene expression profile of mesenchymal stem cells derived from canine adipose tissue and bone marrow. Vet Immunol Immunopathol. 161(1–2):21–31. [DOI] [PubMed] [Google Scholar]
- Sekiya I, Colter DC, Prockop DJ.. 2001. BMP-6 enhances chondrogenesis in a subpopulation of human marrow stromal cells. Biochem Biophys Res Commun. 284(2):411–418. [DOI] [PubMed] [Google Scholar]
- Sekiya I, Larson BL, Vuoristo JT, Reger RL, Prockop DJ.. 2005. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 320(2):269–276. [DOI] [PubMed] [Google Scholar]
- Shirasawa S, Sekiya I, Sakaguchi Y, Yagishita K, Ichinose S, Muneta T.. 2006. In vitro chondrogenesis of human synovium derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. J Cell Biochem. 97(1):84–97. [DOI] [PubMed] [Google Scholar]
- Somal A, Bhat IA, B I, Pandey S, Panda BSK, Thakur N, Sarkar M, Chandra V, Saikumar G, Sharma GT, et al. . 2016. A comparative study of growth kinetics, in vitro differentiation potential and molecular characterization of fetal adnexa derived caprine mesenchymal stem cells. PLoS One. 11(6):e0156821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F, Tang J, Geng R, Hu H, Zhu C, Cui W, Fan W.. 2014. Comparison of the efficacy of bone marrow mononuclear cells and bone mesenchymal stem cells in the treatment of osteoarthritis in a sheep model. Int J Clin Exp Pathol. 7:1415–1426. [PMC free article] [PubMed] [Google Scholar]
- Spaas JH, Guest DJ, Van de Walle GR.. 2012. Tendon regeneration in human and equine athletes: ubi sumus-quo vadimus (where are we and where are we going to)? Sports Med. 42(10):871–890. [DOI] [PubMed] [Google Scholar]
- Spaas JH, Oosterlinck M, Broeckx S, Dumoulin M, Saunders J, Van Soom A, Pille F, Van de Walle GR.. 2012. Treatment of equine degenerative joint disease with autologous peripheral blood-derived mesenchymal stem cells: a case report. Vlaams Diergeneeskund Tijdschr. [Flem Vet Magaz]. 81:11–15. [Google Scholar]
- Spees JL, Lee RH, Gregory CA.. 2016. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 7(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MC, Stewart AA.. 2011. Mesenchymal stem cells: characteristics, sources, mechanisms of action. Vet Clin North Am Equine Pract. 27:243–261. [DOI] [PubMed] [Google Scholar]
- Stewart MC. 2011. Cell-based therapies: current issues and future directions. Vet Clin North Am Equine Pract. 27(2):393–399. [DOI] [PubMed] [Google Scholar]
- Sun XD, Jeng L, Bolliet C, Olsen BR, Spector M.. 2009. Non-viral endostatin plasmid transfection of mesenchymal stem cells via collagen scaffolds. Biomaterials. 30(6):1222–1231. [DOI] [PubMed] [Google Scholar]
- Tan L, Zhao B, Ge FT, Sun DH, Yu T.. 2017. Shockwaves inhibit chondrogenic differentiation of human mesenchymal stem cells in association with adenosine and A2B receptors. Sci Rep. 7:1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwary R, Amarpal, Aithal HP, Kinjavdekar P, Pawde AM, Singh R.. 2014. Effect of IGF-1 and uncultured autologous bone-marrow-derived mononuclear cells on repair of osteochondral defect in rabbits. Cartilage. 5:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol L, Chen W, Song H, Day TF, Yang Y.. 2009. Sox9 inhibits Wnt signaling by promoting beta-catenin phosphorylation in the nucleus . J Biol Chem. 284(5):3323–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toupadakis CA, Wong A, Genetos DC, Cheung WK, Borjesson DL, Ferraro GL, Galuppo LD, Leach JK, Owens SD, Yellowley CE, et al. . 2010. Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical cord tissue. Am J Vet Res. 71(10):1237–1245. [DOI] [PubMed] [Google Scholar]
- Vangsness CT, Farr J, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M.. 2014. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 96(2):90–98. [DOI] [PubMed] [Google Scholar]
- Varghese S, Hwang NS, Canver AC, Theprungsirikul P, Lin DW, Elisseeff J.. 2008. Chondroitin sulphate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 27(1):12–21. [DOI] [PubMed] [Google Scholar]
- Vega A, Martín-Ferrero MA, Del Canto F, Alberca M, García V, Munar A, Orozco L, Soler R, Fuertes JJ, Huguet M, et al. . 2015. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplant. 99(8):1681–1690. [DOI] [PubMed] [Google Scholar]
- Vickers SM, Gotterbarm T, Spector M.. 2010. Cross-linking affects cellular condensation and chondrogenesis in type II collagen-GAG scaffolds seeded with bone marrow-derived mesenchymal stem cells. J Orthop Res. 28(9):1184–1192. [DOI] [PubMed] [Google Scholar]
- Vidal MA, Robinson SO, Lopez MJ, Paulsen DB, Borkhsenious O, Johnson JR, Moore RM, Gimble JM.. 2008. Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet Surg. 37(8):713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar JM, Batista M, Morales M, Santana A, Cuervo B, Rubio M, Cugat R, Sopena J, Carrillo JM.. 2014. Assessment of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells in osteoarthritic dogs using a double blinded force platform analysis. BMC Vet Res. 10(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar JM, Morales M, Santana A, Spinella G, Rubio M, Cuervo B, Cugat R, Carrillo JM.. 2013. Controlled, blinded force platform analysis of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells associated to PRGF-Endoret in osteoarthritic dogs. BMC Vet Res. 9(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk SW, Wang Y, Hankenson KD.. 2012. Effects of donor characteristics and ex vivo expansion on canine mesenchymal stem cell properties: implications for MSC-based therapies. Cell Transplant. 21(10):2189–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. 2017. Shan XB, Qiao YJ PDK2 promotes chondrogenic differentiation of mesenchymal stem cells by upregulation of Sox6 and activation of JNK/MAPK/ERK pathway. Braz J Med Biol Res. 50(2):e5988. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang M, Yuan Z, Ma N, Hao C, Guo W, Zou G, Zhang Y, Chen M, Gao S, Peng J, et al. . 2017. Advances and prospects in stem cells for cartilage regeneration. Stem Cells Int. 2017:4130607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse MR, Howells NR, Parry MC, Austin E, Kafienah W, Brady K, Goodship AE, Eldridge JD, Blom AW, Hollander AP, et al. . 2017. Repair of torn a vascular meniscal cartilage using undifferentiated autologous mesenchymal stem cells: from in vitro optimization to a first-in-human study. Stem Cells Transl Med. 6(4):1237–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke MM, Nydam DV, Nixon AJ.. 2007. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthop Res. 25(7):913–925. [DOI] [PubMed] [Google Scholar]
- Williams R, Khan IM, Richardson K, Nelson L, McCarthy HE, Analbelsi T, Singhrao SK, Dowthwaite GP, Jones RE, Baird DM, et al. . 2010. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS One. 5(10):e13246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Bai C, Wu S.. 2014. Biological characterization of mesenchymal stem cells from bovine umbilical cord. Anim Cells Syst. 1(1):59–67. [Google Scholar]
- Yamada ALM, Carvalho AD, Moroz A, Deffune E, Watanabe M, Hussni C, Rodrigues C, Alves A.. 2013. Mesenchymal stem cell enhances chondral defects healing in horses. Stem Cells Discov. 3(4):218–225. [Google Scholar]
- Yang HM, Song WJ, Li Q, Kim SY, Kim HJ, Ryu MO, Ahn JO, Youn HY.. 2018. Canine mesenchymal stem cells treated with TNF-α and IFN-γ enhance anti-inflammatory effects through the COX-2/PGE2 pathway. Res Vet Sci. 119:19–26. [DOI] [PubMed] [Google Scholar]
- Yang Q, Peng J, Lu SB, Guo QY, Zhao B, Zhang L, Wang AY, Xu WJ, Xia Q, Ma XL, et al. 2011. Evaluation of an extracellular matrix-derived acellular biphasic scaffold/cell construct in the repair of a large articular high-load-bearing osteochondral defect in a canine model. Chin Med J (Engl). 124:3930–3938. [PubMed] [Google Scholar]
- Yin H, Wang Y, Sun Z, Sun X, Xu Y, Li P, Meng H, Yu X, Xiao B, Fan T, et al. . 2016. Induction of mesenchymal stem cell chondrogenic differentiation and functional cartilage microtissue formation for in vivo cartilage regeneration by cartilage extracellular matrix-derived particles. Acta Biomater. 33:96–109. [DOI] [PubMed] [Google Scholar]
- Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, Johnstone B.. 1998. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 80(12):1745–1757. [DOI] [PubMed] [Google Scholar]
- Yoon HY, Lee JH, Jeong SW.. 2012. Long-term follow-up after implantation of autologous adipose tissue derived mesenchymal stem cells to treat a dog with stifle joint osteoarthrosis. J Vet Clin. 29:82–86. [Google Scholar]
- Yu JM, Wu X, Gimble JM, Guan X, Freitas MA, Bunnell BA.. 2011. Age-related changes in mesenchymal stem cells derived from rhesus macaque bone marrow. Aging Cell. 10(1):66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S, Ku SK, Kwon YS.. 2016. Adipose-derived mesenchymal stem cells and platelet-rich plasma synergistically ameliorate the surgical-induced osteoarthritis in Beagle dogs. J Orthop Surg Res. 11(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaim M, Karaman S, Cetin G, Isik S.. 2012. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann Hematol. 91(8):1175–1186. [DOI] [PubMed] [Google Scholar]
- Zayed MN, Schumacher J, Misk N, Dhar MS.. 2016. Effects of pro-inflammatory cytokines on chondrogenesis of equine mesenchymal stromal cells derived from bone marrow or synovial fluid. Vet J. 217:26–32. [DOI] [PubMed] [Google Scholar]
- Zeiter S, Lezuo P, Ito K.. 2009. Effect of TGF-β1, BMP-2 and hydraulic pressure on chondrogenic differentiation of bovine bone marrow mesenchymal stromal cells. Biorheology. 46:45–55. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu S, Guo W, Wang M, Hao C, Gao S, Zhang X, Li X, Chen M, Jing X, et al. 2018. Human umbilical cord Wharton’s Jelly mesenchymal stem cells combined with an acellular cartilage extracellular matrix scaffold improve cartilage repair compared with microfracture in a caprine model. Osteoarthritis Cartilage. 30077(18):S1063–S4584. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang C, Liao W, Li Z, Guo X, Zhao Q, Duan C, Xia R.. 2004. In vitro chondrogenic phenotype differentiation of bone marrow-derived mesenchymal stem cells. J Huazhong Univ Sci Technolog Med Sci. 24:275–378. [DOI] [PubMed] [Google Scholar]
- Zhou G, Zheng Q, Engin F, Munivez E, Chen Y, Sebald E, Krakow D, Lee B.. 2006. Dominance of SOX9 function over RUNX2 during skeletogenesis. Proc Natl Acad Sci USA. 103(50):19004–19009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Zhang B, Man C, Ma Y, Hu J.. 2011. NEL-like molecule-1-modified bone marrow mesenchymal stem cells/poly lactic-co-glycolic acid composite improves repair of large osteochondral defects in mandibular condyle. Osteoarthritis Cartilage. 19(6):743–750. [DOI] [PubMed] [Google Scholar]
- Zorzi A, Amstalden E, Plepis A, Martins V, Ferretti M, Antonioli E, Duarte A, Luzo A, Miranda J.. 2015. Effect of human adipose tissue mesenchymal stem cells on the regeneration of ovine articular cartilage. Int J Mol Sci. 16(11):26813–26831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zscharnack M, Hepp P, Richter R, Aigner T, Schulz R, Somerson J, Josten C, Bader A, Marquass B.. 2010. Repair of chronic osteochondral defects using predifferentiated mesenchymal stem cells in an ovine model. Am J Sports Med. 38(9):1857–1869. [DOI] [PubMed] [Google Scholar]
- Zscharnack M, Poesel C, Galle J, Bader A.. 2009. Low oxygen expansion improves subsequent chondrogenesis of ovine bone-marrow-derived mesenchymal stem cells in collagen type I hydrogel. Cells Tissues Organs. 190(2):81–93. [DOI] [PubMed] [Google Scholar]