Abstract
BACKGROUND:
Eliminating persistent racial/ethnic disparities in maternal mortality and morbidity is a public health priority. National strategies to improve maternal outcomes are increasingly focused on quality improvement collaboratives. However, the effectiveness of quality collaboratives for reducing racial disparities in maternity care is understudied.
OBJECTIVE:
To evaluate the impact of a hemorrhage quality-improvement collaborative on racial disparities in severe maternal morbidity from hemorrhage.
STUDY DESIGN:
We conducted a cross-sectional study from 2011 to 2016 among 99 hospitals that participated in a hemorrhage quality improvement collaborative in California. The focus of the quality collaborative was to implement the national maternal hemorrhage safety bundle consisting of 17 evidence-based recommendations for practice and care processes known to improve outcomes. This analysis included 54,311 women from the baseline period (January 2011 through December 2014) and 19,165 women from the postintervention period (October 2015 through December 2016) with a diagnosis of obstetric hemorrhage during delivery hospitalization. We examined whether racial/ethnic-specific severe maternal morbidity rates in these women with obstetric hemorrhage were reduced from the baseline to the postintervention period. In addition, we conducted Poisson Generalized Estimating Equation models to estimate relative risks and 95% confidence intervals for severe maternal morbidity comparing each racial/ethnic group with white.
RESULTS:
During the baseline period, the rate of severe maternal morbidity among women with hemorrhage was 22.1% (12,002/54,311) with the greatest rate observed among black women (28.6%, 973/3404), and the lowest among white women (19.8%, 3124/15,775). The overall rate fell to 18.5% (3553/19,165) in the postintervention period. Both black and white mothers benefited from the intervention, but the benefit among black women exceeded that of white women (9.0% vs 2.1% absolute rate reduction). The baseline risk of severe maternal morbidity was 1.34 times greater among black mothers compared with white mothers (relative risk, 1.34; 95% confidence interval, 1.27–1.42), and it was reduced to 1.22 (1.05–1.40) in the postintervention period. Sociodemographic and clinical factors explained a part of the black–white differences. After controlling for these factors, the black–white relative risk was 1.22 (95% confidence interval, 1.15–1.30) at baseline and narrowed to 1.07 (1.92–1.24) in the postintervention period. Results were similar when excluding severe maternal morbidity cases with transfusion alone. After accounting for maternal risk factors, the black–white relative risk for severe maternal morbidity excluding transfusion alone was reduced from a baseline of 1.33 (95% confidence interval, 1.16–1.52) to 0.99 (0.76–1.29) in the postintervention period. The most important clinical risk factor for disparate black rates for both severe maternal morbidity and severe maternal morbidity excluding transfusion alone was cesarean delivery, potentially providing another opportunity for quality improvement.
CONCLUSION:
A large-scale quality improvement collaborative reduced rates of severe maternal morbidity due to hemorrhage in all races and reduced the performance gap between black and white women. Improving access to highly effective treatments has the potential to decrease disparities for care-sensitive acute hospital-focused morbidities.
Keywords: hemorrhage, maternal morbidity, maternal safety, perinatal quality collaboratives, quality improvement, racial/ethnic disparities
Persistent racial/ethnic disparities in maternal mortality and morbidity exist in the United States.1–3 Black women continue to be 3–4 times more likely than white women to die during childbirth.2 Severe maternal morbidity (SMM) is a composite measure developed by the Centers for Disease Control and Prevention that includes diagnosis and procedure codes reflecting major complications in childbirth, such as pulmonary edema, renal failure, disseminated intravascular coagulation, hysterectomy, and transfusion.4 SMM is 50–100 times more common than maternal death, affecting nearly 60,000 women each year in the United States.5,6 The risk of SMM for black women is twice that of white women, even after adjusting for sociodemographic factors and comorbidities.7 It is estimated that more than one half of cases of maternal mortality and morbidity are preventable8–11 and could be sensitive to quality of care provided at delivery.12,13 Hemorrhage is the most common major complication of childbirth,14–17 the most preventable cause of maternal mortality,8 and by far the most frequent cause of SMM.18
Quality-improvement (QI)interventions may reduce disparities only if they improve quality of care and simultaneously reduce the performance gap between racial/ethnic groups.19 Disparity reduction requires that vulnerable groups with a history of worse outcomes either receive a greater degree of benefit from the quality intervention or have greater access to the intervention. Otherwise, improvement efforts may compound existing disparities by preferentially advantaging white populations.20–23 Unfortunately, little is known about the impact of QI interventions on racial disparities in maternal outcomes.24
Our previous work25 demonstrated that a large-scale QI collaborative resulted in a significant reduction (>20%) in hemorrhage-related SMM following the implementation of a national hemorrhage safety bundle,18 whereas a comparison set of hospitals, not implementing the hemorrhage safety bundle, remained unchanged. In this report, we examine whether the QI collaborative would be able to reduce the gap between black and white rates of SMM from hemorrhage.
Materials and Methods
A multihospital quality collaborative focused on improving outcomes from obstetric hemorrhage was offered to all California hosptials.25 Ninety-nine hospitals averaging 250,000 annual births choose to participate. The collaborative, led by the California Maternal Quality Care Collaborative (CMQCC), began in January 2015 with intensive activities lasting for 18 months. This report includes 6 additional months of collaborative data not presented in an earlier report25 and an analysis of results by race and ethnicity.
The emphasis of the quality collaborative was to implement the national maternal hemorrhage safety bundle consisting of 17 evidence-based recommendations for practice and care processes known to improve outcomes (Table 1).18 The implementation strategy was an adaptation of the Institute for Health Care Improvement collaborative model creating a community of learning, including 2 participant face-to-face meetings, and monthly check-in calls. Hospitals were organized into small teams of 6–8 hospitals led by physician and nurse mentors who provided QI coaching.26 This involved monthly team support and advice for the assessment of barriers and improvement strategies. Baseline outcome data were collected for 48 months from January 2011 through December 2014. The postintervention period was from October 2015 to December 2016. We compared baseline outcome measures with those collected in the postintervention period to examine the effect of the intervention on SMM among the racial/ethnic groups.
TABLE 1.
California Partnership for Maternal Safety Collaborative: hemorrhage safety bundle elementsa
| Readiness domain |
| Hemorrhage cart/including instructions cards for intrauterine balloons and compression stitches |
| Immediate access to hemorrhage medications (kit or equivalent) |
| Hemorrhage response team established (anesthesia, blood bank, advanced gynecological surgery, and other services) |
| Massive transfusion protocol established |
| Emergency release protocol established for O-negative and uncross-matched units of red blood cells |
| Protocol established for those who refuse blood products |
| Unit education to hemorrhage protocols |
| Regular unit-based drills with debriefs for obstetric hemorrhage |
| Recognition and prevention domain |
| Assessment of hemorrhage risk (prenatal, admission, and other) |
| Measurement of cumulative blood loss (formal and as quantitative as possible) |
| Active management of third stage of labor (standard protocol for oxytocin at birth) |
| Response domain |
| Use of unit-standard, stage-based obstetric hemorrhage emergency management plan with checklists |
| Support program for patients, families, and staff for all major obstetric hemorrhages |
| Reporting and systems learning domain |
| Establish culture of huddles to plan for high-risk patients |
| Post-event debriefing to quickly assess what went well and what could have been improved |
| Multidisciplinary reviews of all serious hemorrhages for system issues |
| Monitor outcomes and progress in process measures in perinatal quality improvement committee |
There were 977,968 deliveries in the 4-year baseline period and 314,750 deliveries in the postintervention period, representing one half of all births in California. Obstetric hemorrhage was identified in approximately 6% of women in both time periods (56,865 at baseline and 20,278 during the postintervention period). Obstetric hemorrhage was defined as patients with International Statistical Classification of Diseases, Ninth and Tenth Revision diagnosis codes for antepartum or postpartum hemorrhage, placenta previa, and abruption placentae. Hemorrhage often is undercoded, which can be largely corrected by the addition of procedure codes for transfusion, given the very low rate of transfusion for other indications (codes are provided in Supplemental Table 1).
Discharge diagnosis and procedure codes were obtained from the CMQCC California Maternal Data Center. CMQCC uses a modified form of a previously published probabilistic algorithm to link maternal and newborn hospital discharge records with birth certificates.27 Linkage rates routinely exceed 98%. The baseline hemorrhage population (56,865) in this study was slightly lower than reported in the initial study25 (57,320) after excluding 455 (0.8%) because of nonlinkage to birth certificates resulting in missing sociodemographic factors. Birth certificates were received from the California Department of Public Health 45 days after the end of each month. Discharge files were received from the Office of Statewide Health Planning and Development on a semiannual basis (delayed by 6–9 months). Institutional review board approval was obtained from Stanford University as the study host and the California Committee for the Protection of Human Subjects for the use of the state data sets.
Self-reported race and ethnicity were obtained from birth certificates. All women were categorized into 1 of 3 ethnic groups (Hispanic, non-Hispanic, and unknown/missing), and 1 of 7 racial groups (white, black, Asian, Pacific Islander, American Indian, other, and unknown). We collapsed the “Pacific Islander” and “American Indian” groups with the “other” category and created a 5-category race/ethnicity measure: Hispanic, non-Hispanic white (white), non-Hispanic black (black), Asian, and others. In total, 4.8% of women had an unknown or missing race or ethnicity and were removed from the analysis. The final sample for analysis consisted of 54,311 women in the baseline and 19,165 women in the postintervention period.
The main outcome measure was the rate of SMM among women diagnosed with hemorrhage. Corresponding International Statistical Classification of Diseases codes for SMM are listed on the Centers for Disease Control and Prevention website.4 Transfusion is the most common morbidity for SMM. To identify the effect on other morbidities, we also evaluated rates of SMM excluding transfusion-only cases. As transfusion is also part of the definition for hemorrhage (widely used in all state collaboratives in the AIM project and in the prior report),25 the addition of SMM without transfusion in the numerator provides additional perspective.
We considered the following risk factors for SMM from obstetric hemorrhage: mother’s sociodemographic characteristics (maternal age, education, parity, and insurance status), clinical factors (number of prenatal visits, pre-pregnancy body mass index, multiple pregnancy, chronic hypertension, gestational diabetes, previous cesarean delivery, labor induction, preterm birth), and method of delivery. All of these factors may contribute to racial inequalities in SMM among women with obstetric hemorrhage.
Statistical analysis
We used the χ2 test to examine whether the distributions of maternal social demographic and clinical factors are different between race/ethnic groups and whether they are different in maternal cohorts in the baseline and postintervention period. We then assessed the risk of SMM among women with obstetric hemorrhage by study period and by race/ethnicity. Specifically, we constructed Poisson generalized estimating equation models with sandwich error estimation to estimate relative risks (RRs) and 95% confidence intervals (CIs) for SMM. A estimating equation is a population-average model that accounts for within-hospital nonindependence of observations. We calculated relative risk for SMM by race/ethnicity using white women as the reference, within each study period.
We constructed an initial unadjusted model and a series of adjusted models. The initial unadjusted model included study period (baseline vs postintervention), race/ethnicity, and their interaction term. We then developed risk-adjusted models by adding maternal sociodemographic and clinical factors. We first adjusted for each covariate separately and compared the effect estimates between the unadjusted model and the single-covariate adjusted model. We then constructed a fully adjusted model by adding all covariates in the following sequence: (1) sociodemographic factors, (2) clinical factors except for the method of delivery, and (3) method of delivery. We added method of delivery separately from the other clinical factors to the model because all the other characteristics could also affect the delivery method and thus further influence SMM. Lastly, we performed sensitivity analysis by excluding each covariate one at a time from the fully adjusted model and evaluated the changes of the effect estimates. These analytical models were applied for both SMM and SMM excluding transfusion-only cases. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, NC).
Results
Participating hospitals were diverse in size, ownership, neonatal intensive care level, the volume of deliveries, patient payer mix, and geography and were representative of the state as a whole (Table 2). Among the 54,311 women with obstetric hemorrhage at baseline, 42% were Hispanic, 29% were white, 15% were Asian, 5% were black, and 7% were other race/ethnicity. Racial/ethnic distribution in the postintervention period was similar to the baseline period. Maternal sociodemographic and clinical factors distributed differently across racial/ethnic groups (Table 3).
TABLE 2.
Maternal characteristics of Hemorrhage Quality Collaborative Participants and California delivery population, baseline period: 2011–2014
| Collaborative participants | All California | |
|---|---|---|
| n (%) | n (%) | |
| Patient characteristics | ||
| Number of deliveries | 977,968 | 1,888,173 |
| Race/ethnicity | ||
| White | 309,837 (32.7) | 539,396 (29.2) |
| Black | 55,548 (5.9) | 106,530 (5.8) |
| Asian | 129,833 (13.7) | 216,504 (11.7) |
| Hispanic | 406,626 (42.9) | 912,675 (49.3) |
| Other | 45,142 (4.8) | 74,678 (4.0) |
| Missing | 30,982 | 38,390 |
| Age at delivery, y | ||
| <18 | 15,384 (1.6) | 37,752 (2.0) |
| 18–25 | 246,082 (25.2) | 547,769 (29.0) |
| 26–35 | 554,126 (56.7) | 1018273 (53.9) |
| ≥36 | 162,376 (16.6) | 284,379 (15.1) |
| Education | ||
| Some high school or less | 133,389 (14.4) | 356,203 (19.6) |
| High school/GED | 206,235 (22.3) | 461,488 (25.5) |
| Some college | 262,083 (28.4) | 477,368 (26.3) |
| College grad or more | 322,211 (34.9) | 518,204 (28.6) |
| Missing | 54,050 | 74,910 |
| Insurance | ||
| Medicaid/other government-sponsored | 364,071 (37.2) | 913,050 (48.4) |
| Private insurance | 584,349 (59.8) | 916,782 (48.6) |
| Self-pay/no insurance | 29,526 (3.0) | 58,310 (3.1) |
| Missing | 22 | 31 |
| Nulliparous | 410,607 (42.0) | 752,589 (39.9) |
| Prepregnancy BMI | ||
| <18.5 | 19,265 (2.1) | 38,587 (2.2) |
| 18.5–25 | 410,011 (44.9) | 767,776 (42.8) |
| 25–30 | 254,980 (27.9) | 512,402 (28.6) |
| 30–35 | 130,985 (14.3) | 271,290 (15.1) |
| 35–40 | 58,705 (6.4) | 121,518 (6.8) |
| ≥40 | 39,347 (4.3) | 80,951 (4.5) |
| Missing | 64,675 | 95,649 |
| Prenatal care | ||
| First trimester | 825,239 (86.0) | 1548609 (83.6) |
| Second trimester | 106,875 (11.1) | 239,552 (12.9) |
| Third trimester | 23,143 (2.4) | 54,128 (2.9) |
| No care | 4,202 (0.4) | 9,912 (0.5) |
| Missing | 18,509 | 35,972 |
| Chronic hypertension | 17,012 (1.7) | 31,024 (1.6) |
| Diabetes/gestational diabetes | 106,227 (10.9) | 187,960 (10.0) |
| Multiple gestation | 17,946 (1.8) | 31,058 (1.6) |
| Labor induction | 150,786 (15.4) | 286,710 (15.2) |
| Preterm birth (<37 wk gestation) | 76,153 (7.8) | 144,972 (7.7) |
| Previous cesarean delivery | 165,297 (16.9) | 331,037 (17.5) |
| Method of delivery: cesarean | 316,141 (32.3) | 622,465 (33.0) |
| Hospital characteristics | ||
| Number of hospitals | 99 | 238 |
| Teaching hospitals | 15 (15.2) | 30 (12.6) |
| AAP neonatal level of care | ||
| Level I | 18 (18.2) | 72 (30.3) |
| Level II | 29 (29.3) | 57 (23.9) |
| Level III | 42 (42.4) | 92 (38.7) |
| Level IV | 10 (10.1) | 17 (7.1) |
| Geographic region | ||
| Central-South Coast | 42 (42.4) | 92 (38.7) |
| Central-North Coast and Northeastern | 44 (44.4) | 96 (40.3) |
| Central Valley, Southern Inland | 13 (13.1) | 50 (21.0) |
| Rural or urban-suburban | ||
| Urban-Suburban | 94 (94.9) | 205 (86.1) |
| Rural | 5 (5.1) | 33 (13.9) |
| Average annual delivery volume (live births) | ||
| <1000 | 15 (15.2) | 72 (30.3) |
| 1000–2499 | 55 (55.6) | 117 (49.2) |
| ≥3000 | 29 (29.3) | 49 (20.6) |
| Hospital ownership | ||
| University, city, county | 11 (11.1) | 39 (16.4) |
| Integrated health system | 29 (29.3) | 29 (12.2) |
| Private nonprofit | 48 (48.5) | 128 (53.8) |
| Private investor | 11 (11.1) | 42 (17.6) |
AAP, American Academy of Pediatrics; BMI, body mass index; GED, General Educational Development.
TABLE 3.
Distribution of maternal sociodemographic and clinical factors among women with obstetric hemorrhage, by race/ethnicity and study period
| Study period | Race/ethnicity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All women | White | Black | Asian | Hispanic | Other | |||||||
| Baseline | Postintervention | Baseline | Postintervention | Baseline | Postintervention | Baseline | Postintervention | Baseline | Postintervention | Baseline | Postintervention | |
| Number of women with hemorrhage | 54,311 | 19,165 | 15,775 | 5401 | 3404 | 1114 | 8180 | 3195 | 23,051 | 8161 | 3901 | 1294 |
| Characteristics | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Age at delivery, y | ||||||||||||
| <18 | 906 (1.7) | 200 (1.0) | 76 (0.5) | 15 (0.3) | 91 (2.7) | 14 (1.3) | 13 (0.2) | 4 (0.1) | 702 (3.0) | 164 (2.0) | 24 (0.6) | 3 (0.2) |
| 18–25 | 12,774 (23.5) | 3958 (20.7) | 2700 (17.1) | 707 (13.1) | 1156 (34.0) | 320 (28.7) | 613 (7.5) | 171 (5.4) | 7737 (33.6) | 2579 (31.6) | 568 (14.6) | 181 (14.0) |
| 26–35 | 30,259 (55.7) | 11,001 (57.4) | 9674 (61.3) | 3454 (64.0) | 1622 (47.6) | 557 (50.0) | 5244 (64.1) | 2085 (65.3) | 11,391 (49.4) | 4143 (50.8) | 2328 (59.7) | 762 (58.9) |
| ≥36 | 10,372 (19.1) | 4006 (20.9) | 3325 (21.1) | 1225 (22.7) | 535 (15.7) | 223 (20.0) | 2310 (28.2) | 935 (29.3) | 3221 (14.0) | 1275 (15.6) | 981 (25.1) | 348 (26.9) |
| Education | ||||||||||||
| Some high school or less | 7486 (14.2) | 2005 (10.8) | 582 (3.8) | 132 (2.5) | 326 (10.0) | 85 (8.0) | 239 (3.0) | 79 (2.6) | 6203 (27.7) | 1682 (21.4) | 136 (3.6) | 27 (2.1) |
| High school/GED | 11,484 (21.8) | 3956 (21.3) | 2433 (15.8) | 749 (14.3) | 954 (29.1) | 296 (27.8) | 849 (10.8) | 306 (9.9) | 6732 (30.1) | 2414 (30.7) | 516 (13.6) | 191 (15.2) |
| Some college | 14,399 (27.3) | 5123 (27.6) | 4181 (27.2) | 1341 (25.5) | 1306 (39.9) | 415 (39.0) | 1399 (17.8) | 489 (15.9) | 6208 (27.7) | 2443 (31.0) | 1305 (34.5) | 435 (34.5) |
| College grad or more | 19,353 (36.7) | 7446 (40.2) | 8194 (53.2) | 3027 (57.7) | 687 (21.0) | 269 (25.3) | 5389 (68.4) | 2211 (71.7) | 3252 (14.5) | 1332 (16.9) | 1831 (48.3) | 607 (48.2) |
| Missing | 1589 | 635 | 385 | 152 | 131 | 49 | 304 | 110 | 656 | 290 | 113 | 34 |
| Insurance | ||||||||||||
| Medi-Cal/other government-sponsored | 18,697 (34.4) | 6670 (34.8) | 3079 (19.5) | 1046 (19.4) | 174 (49.2) | 573 (51.4) | 1246 (15.2) | 532 (16.7) | 11,949 (51.8) | 4229 (51.8) | 749 (19.2) | 290 (22.4) |
| Private insurance | 34,310 (63.2) | 11,987 (62.5) | 12,544 (79.5) | 4301 (79.6) | 1,673 (49.1) | 528 (47.4) | 6355 (77.7) | 2376 (74.4) | 10,630 (46.1) | 3790 (46.4) | 3108 (79.7) | 992 (76.7) |
| Self-pay/no insurance | 1303 (2.4) | 508 (2.7) | 151 (1.0) | 54 (1.0) | 57 (1.7) | 13 (1.2) | 579 (7.1) | 287 (9.0) | 472 (2.0) | 142 (1.7) | 44 (1.1) | 12 (0.9) |
| Missing | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Parity | ||||||||||||
| Multiparous | 28,613 (52.7) | 10,101 (52.7) | 7260 (46.0) | 2523 (46.7) | 1972 (57.9) | 668 (60.0) | 3725 (45.5) | 1498 (46.9) | 13,470 (58.4) | 4711 (57.7) | 2186 (56.0) | 701 (54.2) |
| Nulliparous | 25,698 (47.3) | 9064 (47.3) | 8515 (54.0) | 2878 (53.3) | 1432 (42.1) | 446 (40.0) | 4455 (54.5) | 1697 (53.1) | 9581 (41.6) | 3450 (42.3) | 1715 (44.0) | 593 (45.8) |
| Prepregnancy BMI | ||||||||||||
| <18.5 | 1079 (2.1) | 372 (2.0) | 297 (2.0) | 93 (1.8) | 73 (2.3) | 18 (1.7) | 356 (4.8) | 156 (5.1) | 286 (1.3) | 88 (1.1) | 67 (1.9) | 17 (1.4) |
| 18.5–25 | 22,140(43.8) | 7597 (41.1) | 7692 (51.7) | 2570 (48.9) | 1015 (32.4) | 334 (31.0) | 4722 (64.3) | 1919 (62.6) | 7051 (32.7) | 2287 (29.1) | 1660 (45.9) | 487 (39.1) |
| 25–30 | 14,347 (28.4) | 5336 (28.9) | 3837 (25.8) | 1442 (27.5) | 887 (28.3) | 295 (27.3) | 1636 (22.3) | 703 (22.9) | 6919 (32.1) | 2500 (31.9) | 1068 (29.5) | 396 (31.8) |
| 30–35 | 7485 (14.8) | 2953 (16.0) | 1689 (11.4) | 660 (12.6) | 573 (18.3) | 190 (17.6) | 477 (6.5) | 218 (7.1) | 4267 (19.8) | 1691 (21.5) | 479 (13.2) | 194 (15.6) |
| 35–40 | 3287 (6.5) | 1317 (7.1) | 791 (5.3) | 283 (5.4) | 290 (9.3) | 125 (11.6) | 116 (1.6) | 55 (1.8) | 1872 (8.7) | 764 (9.7) | 218 (6.0) | 90 (7.2) |
| ≥40 | 2202 (4.4) | 914 (4.9) | 559 (3.8) | 205 (3.9) | 297 (9.5) | 117 (10.8) | 38 (0.5) | 13 (0.4) | 1182 (5.5) | 517 (6.6) | 126 (3.5) | 62 (5.0) |
| Missing | 3,771 | 676 | 910 | 148 | 269 | 35 | 835 | 131 | 1,474 | 314 | 283 | 48 |
| Prenatal care | ||||||||||||
| First trimester | 45,997 (86.2) | 16,391 (86.9) | 13,854 (89.5) | 4778 (89.9) | 2733 (82.6) | 923 (85.1) | 7245 (90.7) | 2839 (90.0) | 18,871 (83.1) | 6728 (83.7) | 3294 (85.3) | 1123 (87.7) |
| Second trimester | 5781 (10.8) | 1902 (10.1) | 1262 (8.2) | 407 (7.7) | 422 (12.8) | 123 (11.3) | 622 (7.8) | 254 (8.0) | 3022 (13.3) | 994 (12.4) | 453 (11.7) | 124 (9.7) |
| Third trimester | 1192 (2.2) | 435 (2.3) | 249 (1.6) | 91 (1.7) | 103 (3.1) | 30 (2.8) | 105 (1.3) | 61 (1.9) | 642 (2.8) | 233 (2.9) | 93 (2.4) | 20 (1.6) |
| No care | 381 (0.7) | 143 (0.8) | 114 (0.7) | 37 (0.7) | 50 (1.5) | 9 (0.8) | 18 (0.2) | 2 (0.1) | 176 (0.8) | 82 (1.0) | 23 (0.6) | 13 (1.0) |
| Missing | 960 | 294 | 296 | 88 | 96 | 29 | 190 | 39 | 340 | 124 | 38 | 14 |
| Chronic hypertension | ||||||||||||
| No | 52,981 (97.6) | 18,389 (96.0) | 15,424 (97.8) | 5215 (96.6) | 3218 (94.5) | 1025 (92.0) | 8046 (98.4) | 3118 (97.6) | 22,555 (97.8) | 7845 (96.1) | 3738 (95.8) | 1186 (91.7) |
| Yes | 1330 (2.4) | 776 (4.0) | 351 (2.2) | 186 (3.4) | 186 (5.5) | 89 (8.0) | 134 (1.6) | 77 (2.4) | 496 (2.2) | 316 (3.9) | 163 (4.2) | 108 (8.3) |
| Gestational diabetes | ||||||||||||
| No | 47,210 (86.9) | 16,511 (86.2) | 14,449 (91.6) | 4928 (91.2) | 3047 (89.5) | 985 (88.4) | 6802 (83.2) | 2626 (82.2) | 19,885 (86.3) | 6962 (85.3) | 3027 (77.6) | 1010 (78.1) |
| Yes | 7101 (13.1) | 2654 (13.8) | 1326 (8.4) | 473 (8.8) | 357 (10.5) | 129 (11.6) | 1378 (16.8) | 569 (17.8) | 3166 (13.7) | 1199 (14.7) | 874 (22.4) | 284 (21.9) |
| Multiple gestation | ||||||||||||
| No | 51,974 (95.7) | 18,377 (95.9) | 14,847 (94.1) | 5108 (94.6) | 3246 (95.4) | 1075 (96.5) | 7798 (95.3) | 3042 (95.2) | 22,290 (96.7) | 7904 (96.9) | 3793 (97.2) | 1248 (96.4) |
| Yes | 2337 (4.3) | 788 (4.1) | 928 (5.9) | 293 (5.4) | 158 (4.6) | 39 (3.5) | 382 (4.7) | 153 (4.8) | 761 (3.3) | 257 (3.1) | 108 (2.8) | 46 (3.6) |
| Labor induction | ||||||||||||
| No | 44,015 (81.0) | 15,291 (79.8) | 12,385 (78.5) | 4184 (77.5) | 2736 (80.4) | 888 (79.7) | 6703 (81.9) | 2595 (81.2) | 19,044 (82.6) | 6559 (80.4) | 3147 (80.7) | 1065 (82.3) |
| Yes | 10,296 (19.0) | 3874 (20.2) | 3390 (21.5) | 1,217 (22.5) | 668 (19.6) | 226 (20.3) | 1477 (18.1) | 600 (18.8) | 4007 (17.4) | 1602 (19.6) | 754 (19.3) | 229 (17.7) |
| Preterm birth | ||||||||||||
| No | 44,077 (81.2) | 15,803 (82.5) | 12,855 (81.5) | 4461 (82.6) | 2509 (73.8) | 851 (76.5) | 6832 (83.6) | 2718 (85.1) | 18,709 (81.2) | 6711 (82.3) | 3172 (81.4) | 1062 (82.1) |
| Yes | 10,206 (18.8) | 3352 (17.5) | 2914 (18.5) | 937 (17.4) | 889 (26.2) | 262 (23.5) | 1344 (16.4) | 476 (14.9) | 4334 (18.8) | 1445 (17.7) | 725 (18.6) | 232 (17.9) |
| Missing | 28 | 10 | 6 | 3 | 6 | 1 | 4 | 1 | 8 | 5 | 4 | 0 |
| Previous cesarean delivery | ||||||||||||
| No | 46,179 (85.0) | 16,218 (84.6) | 13,843 (87.8) | 4705 (87.1) | 2719 (79.9) | 868 (77.9) | 7045 (86.1) | 2698 (84.4) | 19,310 (83.8) | 6854 (84.0) | 3262 (83.6) | 1093 (84.5) |
| Yes | 8132 (15.0) | 2947 (15.4) | 1932 (12.2) | 696 (12.9) | 685 (20.1) | 246 (22.1) | 1135 (13.9) | 497 (15.6) | 3741 (16.2) | 1307 (16.0) | 639 (16.4) | 201 (15.5) |
| Method of delivery | ||||||||||||
| Vaginal | 32,717 (60.2) | 11,816 (61.7) | 9798 (62.1) | 3418 (63.3) | 1729 (50.8) | 566 (50.8) | 4702 (57.5) | 1881 (58.9) | 14,191 (61.6) | 5143 (63.0) | 2297 (58.9) | 808 (62.4) |
| Cesarean | 21,594 (39.8) | 7349 (38.3) | 5977 (37.9) | 1983 (36.7) | 1675 (49.2) | 548 (49.2) | 3478 (42.5) | 1314 (41.1) | 8860 (38.4) | 3018 (37.0) | 1604 (41.1) | 486 (37.6) |
BMI, body mass index; GED, General Educational Development.
Overall reduction of SMM
Figure 1 shows the trend and the control chart of the quarterly SMM rate among all women with obstetric hemorrhage from 2011–2016. The total SMM rate in the baseline period was relatively stable (Figure 1, A). It dropped continuously after the initiation of the QI collaborative, and there was evidence of special cause of variation during the intervention and the postintervention period as illustrated by 4 consecutive points below the baseline lower control limit (3 standard deviations) and 8 consecutive points below the baseline average. The mean of the quarterly total SMM rate fell from 22.0% in the baseline to 18.6% in the postintervention period. The rate of SMM after excluding cases with transfusion alone also improved (Figure 1, B), with the rate reduced from 6.9% in the baseline to 5.6% in the postintervention period.
FIGURE 1. Control chart of quarterly rates for SMM, 2011–2016.
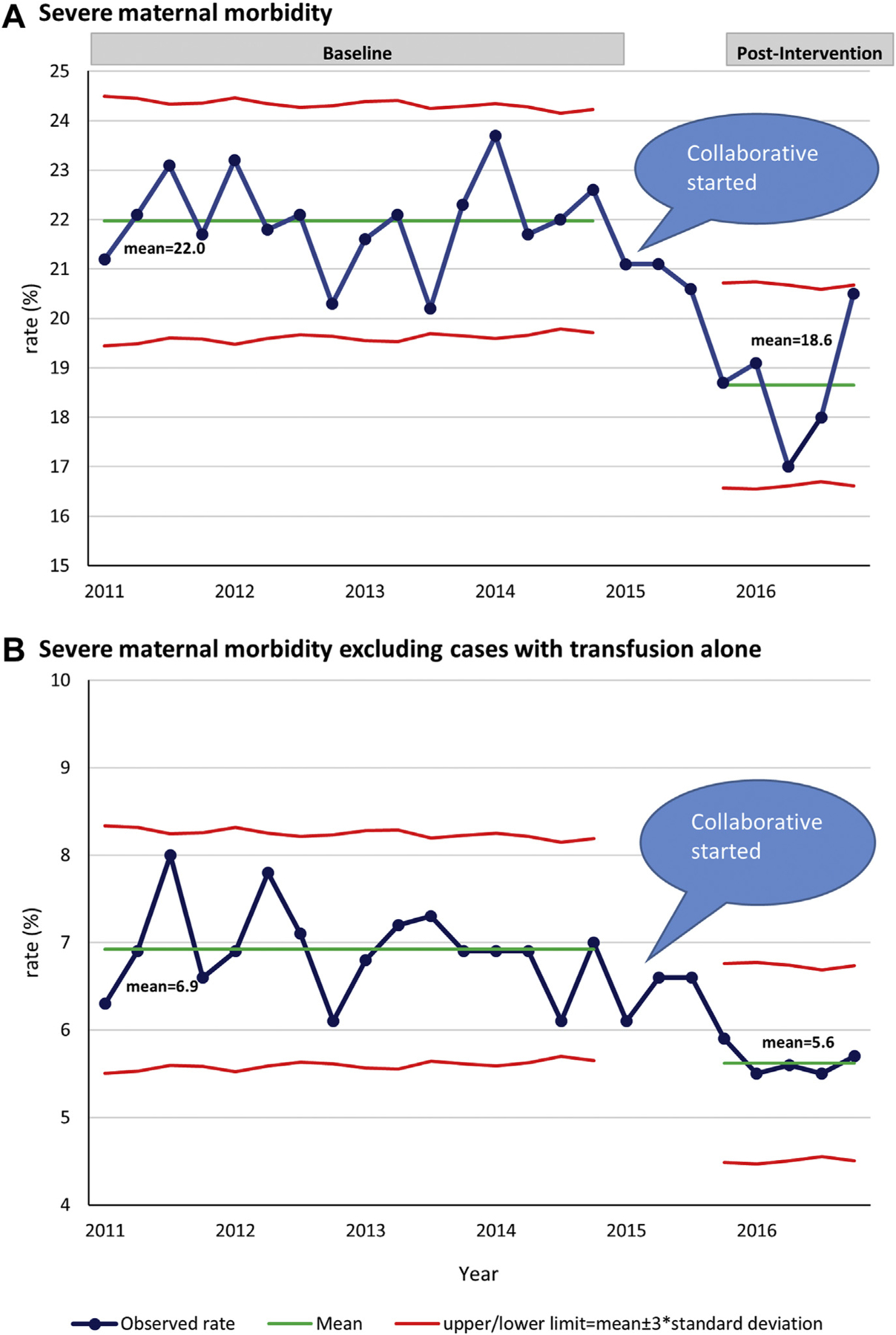
A, SMM. B, SMM excluding cases with transfusion alone, 2011–2016.
SMM, severe maternal morbidity.
The reduction of SMM rate was observed in every racial/ethnic group, with absolute risk reductions ranged from 1.0% (20.5% baseline to 19.5% postintervention) in Asian women to 9.0% (28.6% baseline to 19.6% postintervention) in black women (Figure 2, A). Black women in the postintervention period were 23% less likely to have SMM (adjusted RR, 0.76; 95% CI, 0.65–0.89) as compared with those at baseline after adjusting for maternal sociodemographic and clinical factors. The rate of SMM after excluding transfusion-only cases also reduced in every racial/ethnic group (Figure 2, B). Although black women experienced the largest reduction (2.5% absolute rate reduction), their rate was still the greatest among all racial/ethnic groups in the postintervention period (6.9% vs 5.1–5.5%).
FIGURE 2. Rates of SMM.
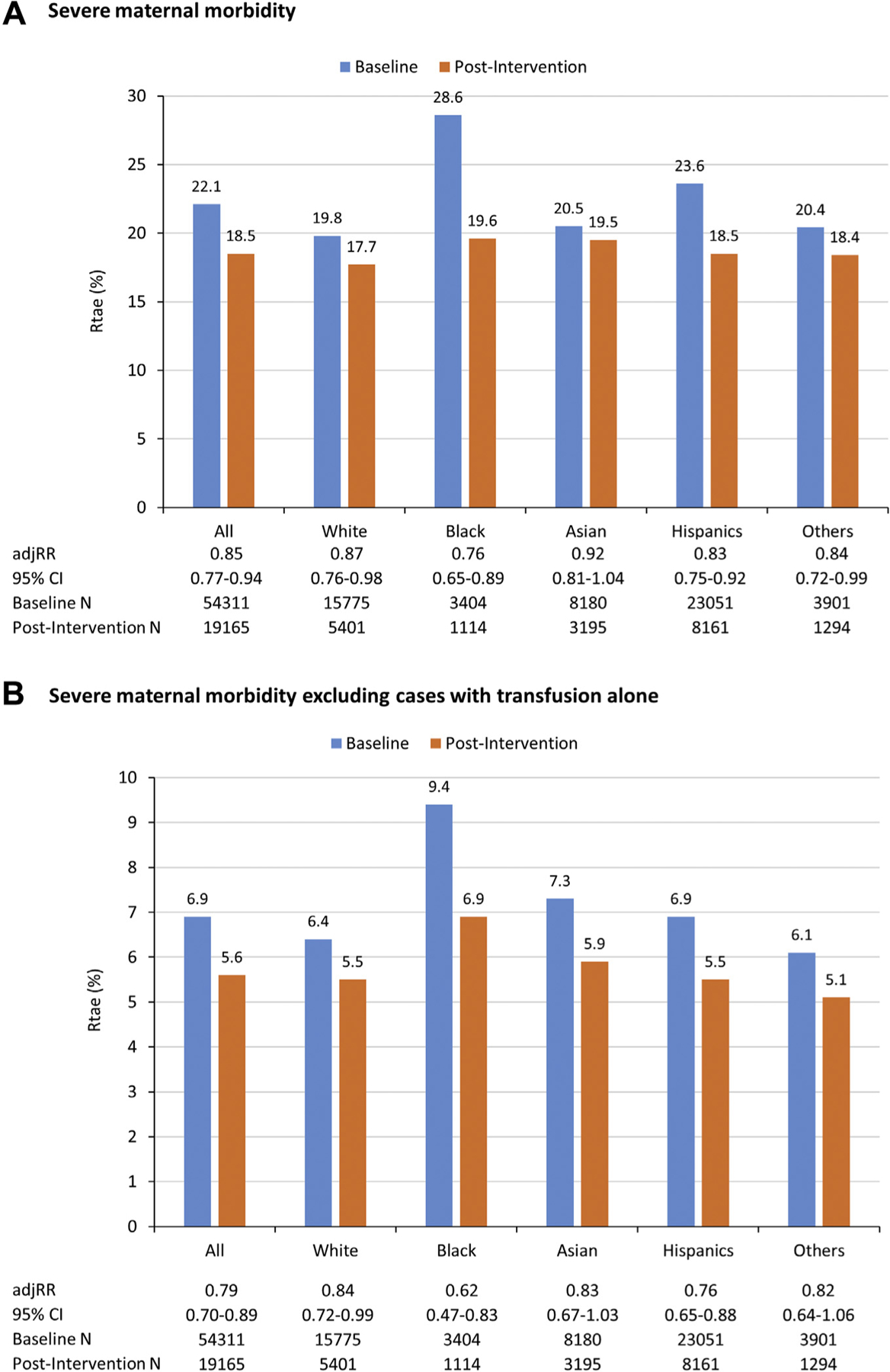
Rate of A, SMM and B, SMM excluding cases with transfusion alone, by race/ethnicity and study period. Poisson generalized estimating equation model included study period, race/ethnicity, the interaction term between study period and race/ethnicity, and all of the maternal sociodemographic and clinical factors listed in Table 4.
adjRR, adjusted relative risk; CI, confidence interval. N, Number of denominator (women with obstetric hemorrhage); SMM, severe maternal morbidity.
Risk adjustment models for comparing racial differences
We then assessed racial differences in the risk of SMM among women with obstetric hemorrhage, using white women as the reference. At baseline, black mothers were associated with a greater risk of SMM compared with white mothers (RR, 1.34; 95% CI, 1.26–1.42) (Table 4, model 1). The baseline racial difference was significant even after adjusting for all of the sociodemographic and clinical factors (RR, 1.22; 95% CI, 1.15–1.30) (Table 4, model 4). In the postintervention period, the black–white RR decreased to 1.22 in the unadjusted model, although it remained significant (95% CI, 1.05–1.40) (Table 4, model 1). However, once clinical factors and especially method of delivery (eg, cesarean) were added to the adjustment model, the racial difference were no longer significant (RR, 1.14; 95% CI, 0.98–1.32) (Table 4, model 3), and (RR, 1.07; 95% CI, 0.92–1.24) (Table 4, model 4). Among all the covariates, method of delivery influenced the black–white relative risk the most (Supplemental Tables 2 and 3).
TABLE 4.
Association between race/ethnicity and SMM, both total and excluding cases with transfusion alone, by study period
| Race/ethnicity | Total SMM | SMM excluding cases with transfusion alonea | ||
|---|---|---|---|---|
| Baseline | Postintervention | Baseline | Postintervention | |
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Model 1b: Unadjusted model | ||||
| White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 1.34 (1.27–1.42) | 1.22 (1.05–1.40) | 1.46 (1.30–1.65) | 1.22 (0.98–1.52) |
| Asian | 1.03 (0.95–1.11) | 1.13 (1.00–1.28) | 1.15 (1.05–1.25) | 1.09 (0.90–1.32) |
| Hispanics | 1.09 (1.03–1.15) | 1.10 (0.99–1.23) | 1.05 (0.95–1.15) | 0.96 (0.82–1.12) |
| Others | 1.08 (1.00–1.18) | 1.07 (0.92–1.25) | 1.02 (0.87–1.19) | 0.97 (0.75–1.27) |
| Model 2c: Adjusted for sociodemographic factors | ||||
| White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 1.32 (1.25–1.39) | 1.18 (1.02–1.37) | 1.47 (1.30–1.66) | 1.16 (0.91–1.49) |
| Asian | 1.02 (0.96–1.09) | 1.12 (0.98–1.26) | 1.11 (1.01–1.21) | 1.05 (0.86–1.27) |
| Hispanics | 1.07 (1.01–1.12) | 1.06 (0.95–1.18) | 1.04 (0.95–1.13) | 0.94 (0.80–1.12) |
| Others | 1.07 (0.98–1.17) | 1.06 (0.90–1.23) | 0.99 (0.84–1.16) | 0.96 (0.73–1.26) |
| Model 3d: Adjusted for sociodemographic and clinical factors (except for method of delivery) | ||||
| White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 1.29 (1.21–1.38) | 1.14 (0.98–1.32) | 1.43 (1.25–1.63) | 1.05 (0.81–1.37) |
| Asian | 1.03 (0.97–1.10) | 1.09 (0.97–1.23) | 1.09 (0.99–1.20) | 1.05 (0.86–1.27) |
| Hispanics | 1.09 (1.04–1.15) | 1.07 (0.97–1.17) | 1.05 (0.96–1.16) | 0.96 (0.83–1.13) |
| Others | 1.09 (1.00–1.19) | 1.04 (0.89–1.21) | 0.98 (0.83–1.16) | 0.94 (0.72–1.23) |
| Model 4e: Adjusted for sociodemographic, and clinical factors including method of delivery (fully adjusted model) | ||||
| White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 1.22 (1.15–1.30) | 1.07 (0.92e1.24) | 1.33 (1.16–1.52) | 0.99 (0.76–1.29) |
| Asian | 1.02 (0.96–1.09) | 1.09 (0.96e1.23) | 1.06 (0.96–1.18) | 1.05 (0.86–1.27) |
| Hispanics | 1.09 (1.04–1.15) | 1.06 (0.97e1.16) | 1.05 (0.95–1.16) | 0.96 (0.82–1.12) |
| Others | 1.07 (0.98–1.16) | 1.03 (0.89e1.21) | 0.95 (0.80–1.12) | 0.92 (0.70–1.22) |
CI, confidence interval; RR, relative risk; SMM, severe maternal morbidity.
Cases with transfusion alone were excluded from the numerator
Unadjusted model. Model included study period, race ethnicity, and the interaction term between study period and race ethnicity
Based on model 1, additionally included the following maternal sociodemographic factors: age at delivery, education levels, parity, and insurance status
Based on model 2, additionally included the following maternal clinical factors: prenatal visits, pre-pregnancy body mass index, multiple pregnancy, chronic hypertension, gestational diabetes, previous cesarean delivery, labor induction, and pre-term birth
Fully adjusted model. Based on model 3, additionally included method of delivery.
Results for SMM excluding transfusion-only cases were similar. In this group of women, the racial/ethnic inequality between black and white women for SMM decreased from baseline (RR, 1.46; 95% CI, 1.30–1.65) to the postintervention period (RR, 1.22; 95% CI, 0.98–1.52, (Table 3, model 1). After adjusting for all of the covariates, the significant black–white differences at baseline (RR, 1.33; 95% CI, 1.16–1.52) were attenuated in the postintervention period (RR, 0.99; 95% CI, 0.76–1.28, (Table 4, model 4). Again, results from the single-covariate model and sensitivity analysis suggested that the method of delivery was the most influential covariate (Supplemental Tables 4 and 5).
Sensitivity analyses
Because some of the SMM diagnoses may not be directly related to excessive bleeding, we performed additional sensitivity analysis restricting our analyses to SMM diagnoses that had the strongest relationship to hemorrhage, including transfusion; acute renal failure; adult respiratory distress; cardiac arrest; disseminated intravascular coagulation; acute heart failure; pulmonary edema; shock; hysterectomy; and ventilation. Almost 99% of SMM cases and almost 93% of SMM cases excluding transfusion alone were related to hemorrhage, and all results were nearly identical to those presented (Supplemental Tables 6 and 7).
Comment
Large variations in rates of SMM exist across hospitals in the United States.1 Both “within-hospital” and “between-hospital” disparities in SMM have been documented,13 and variations in the quality of care delivery have been shown to contribute to racial/ethnic disparities in SMM.28 Obstetric hemorrhage is an acute delivery event that accounts for approximately one half of SMM. Importantly, maternal deaths and severe complications from hemorrhage have been judged to have a high degree of preventability largely due to provider improvement opportunities.29 Therefore, reducing variations in clinical care processes by implementing standard protocols has the potential to both improve hemorrhage outcomes and concurrently reduce racial/ethnic disparities. Obstetric hemorrhage provides a useful model to explore the impact of QI efforts on reducing disparities in maternal outcomes.
Principal findings
In this cross-sectional study among women with obstetric hemorrhage in the 99 hospitals participated in the QI collaborative, we showed that SMM rate was reduced in every racial/ethnic group after the intervention. Of particular notice, the risk of SMM was no longer greater in black women compared with white women in the postintervention period after accounting for sociodemographic and clinical factors. The marked improvement in black rates of SMM from hemorrhage and the narrowing of black–white difference are important findings suggesting QI efforts can be effective in both improving maternal outcomes and reducing inequities in care delivery for a specific medical condition.
Clinical implications
Results from adjusted analyses indicated that the remaining racial inequalities in SMM could be largely explained by controlled sociodemographic and clinical factors with the method of delivery being the most influential factor. Cesarean delivery has been estimated to be associated with a larger proportion of SMM than was any other risk factor.30 Currently, black women have greater rates of cesarean delivery compared with women in other race/ethnic groups, but this difference only began in the 1990s.31 Reducing disparity in the cesarean rate, where possible, may allow further reduction in black–white difference in SMM from hemorrhage.
Blood transfusions are an important driver for SMM cases. The greater transfusion rate among black mothers may be partially related to greater rates of anemia among black women when presenting for delivery (2–6 times greater than white women).32–34 Anemia, in turn, increases the risk of transfusion, particularly in the setting of surgical delivery. Improved recognition and treatment of anemia before delivery may be another approach to reduce black–white disparity in SMM.
The subset of women with SMM excluding transfusion-only cases represents a group of women with potentially more severe diagnoses or procedures including hysterectomy, renal or respiratory failure. Among these women, a more pronounced narrowing of black–white difference was observed. Again, greater cesarean delivery rates among black women appeared to account for a large portion of the remaining difference in this group of women.
Research implications
QI has been suggested as an important approach to address racial/ethnic disparities.35 However, in practice, QI efforts have been shown to have a variable impact on racial/ethnic disparities in outcomes.20–23,36 There is limited experience examining the potential for QI to reduce obstetric disparities. Our study provides several insights to better understand the application of QI for successful reduction of disparities.
First, QI efforts may have variable effectiveness because the focus of the intervention does not sufficiently address the primary sources of racial/ethnic disparities. Social determinants, underlying risk factors, structural racism, lack of trust and respect from providers, and the quality of the care received all contribute to disparities in maternal outcomes.13 In addition, specific causes of mortality and morbidity may be more or less amenable to QI efforts. Evaluating the relative contribution of these factors to performance gaps among racial/ethnic groups is important in designing effective interventions. We suggest, similar to Wise,37 that QI efforts for reducing disparities are the most likely to be successful when they (1) target care-sensitive conditions (2) for an acute process (3) where poor access to highly effective treatment (4) is the dominant reason for the morbidity. Management of obstetric hemorrhage offers such an example. Case reviews of SMM due to hemorrhage have consistently identified provider and system-related factors as the dominant contributors to adverse outcomes.8,38 At the same time, implementation of systematic approaches to management of hemorrhage have been shown to reduce rates of SMM.18,39 For other conditions that are less acute, more closely tied to patient risk factors and chronic exposure to racism, solutions will need to be more broadly based. We suggest that eliminating racial disparities will require appropriate matching between the source of disparity, the causal pathway and the intervention(s).
Limitations and strengths
The limitations of our study must be viewed in light of its design. As we were looking for population effects, we relied on administrative data for outcomes. However, we took extra steps for validation of outcomes including case reviews and outlier checks. The risk of coding variation was minimized by following the same hospitals with the same racial mix over time. Although we included a broad range of maternal risk factors associated with SMM in the adjusted model, we may not have accounted for unmeasured confounders. In addition, even with our very large number of mothers, we may have limited sample size for detecting statistical interactions.
Our study has several strengths. Self-reported racial/ethnic data were obtained from birth certificates, which are generally regarded as the gold standard. Our intervention reached a large sample of women and included a broad range of hospital sizes and affiliations that collectively care for more than 250,000 births annually. We focused on a defined subset of women diagnosed with hemorrhage that allowed us to deploy a clearly defined intervention bundle with nationally recognized outcome measures.
Conclusion
Our findings point to opportunities for reduction in black–white disparity for the most common maternity complication, hemorrhage, by implementing national safety bundles for prevention and response to obstetric hemorrhage. In addition, our data suggest that efforts to decrease the greater rate of cesarean deliveries among black women may show added benefit. The greater rate of anemia at labor admission in black women also provides a prenatal care improvement opportunity that could impact transfusion rates. These clinical efforts should be in parallel with efforts to reverse bias and racism in the medical system by treating black women with respect and dignity, better understanding their circumstances, and listening to and acting on their concerns. All of these approaches are necessary to address the persistent disparities that we see in obstetric care.
Supplementary Material
AJOG at a Glance.
Why was the study conducted?
It was not known whether a large-scale quality improvement collaborative could reduce racial disparity in severe maternal complications following obstetric hemorrhage.
Key Findings
In this cross-sectional study that included 73,476 women with obstetric hemorrhage from 99 hospitals who participated in a hemorrhage quality improvement collaborative, the rate of severe maternal morbidity was reduced for all races. The black–white differences were no longer significant following case mix adjustment.
What does this add to what is known?
Maternal quality-improvement activities that focus on improving access to highly effective treatments have the potential to reduce racial disparities for care-sensitive acute hospital-focused morbidities such as hemorrhage.
Acknowledgments
We wish to acknowledge the 40 mentors and the many hospital teams who led this effort at the facility level.
The research in this publication was supported by funding from Merck, through its Merck for Mothers program, and is the sole responsibility of the authors. Merck for Mothers is known as MSD for Mothers outside the United States and Canada. The funder has no involvement in study design, collection, analysis, or interpretation of results. J.P.’s effort was supported in part by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD083368-01 and R01 HD08467-01, PI J.P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health. This funding source had no role in any of the following aspects of this study: design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
The authors report no conflict of interest.
Contributor Information
Elliott K. Main, Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, Stanford University School of Medicine, Stanford, CA; California Maternal Quality Care Collaborative, Stanford, CA.
Shen-Chih Chang, Division of Neonatal and Developmental Medicine, Department of Pediatrics, Stanford University School of Medicine, Stanford, CA; California Maternal Quality Care Collaborative, Stanford, CA.
Ravi Dhurjati, Division of Neonatal and Developmental Medicine, Department of Pediatrics, Stanford University School of Medicine, Stanford, CA; California Maternal Quality Care Collaborative, Stanford, CA; California Perinatal Quality Care Collaborative, Stanford, CA.
Valerie Cape, Division of Neonatal and Developmental Medicine, Department of Pediatrics, Stanford University School of Medicine, Stanford, CA; California Maternal Quality Care Collaborative, Stanford, CA.
Jochen Profit, Division of Neonatal and Developmental Medicine, Department of Pediatrics, Stanford University School of Medicine, Stanford, CA; California Perinatal Quality Care Collaborative, Stanford, CA.
Jeffrey B. Gould, Division of Neonatal and Developmental Medicine, Department of Pediatrics, Stanford University School of Medicine, Stanford, CA; California Maternal Quality Care Collaborative, Stanford, CA; California Perinatal Quality Care Collaborative, Stanford, CA.
References
- 1.Callaghan WM. Overview of maternal mortality in the United States. Semin Perinatol 2012;36:2–6. [DOI] [PubMed] [Google Scholar]
- 2.Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Race, ethnicity, and nativity differentials in pregnancy-related mortality in the United States: 1993–2006. Obstet Gynecol 2012;120:261–8. [DOI] [PubMed] [Google Scholar]
- 3.Grobman WA, Bailit JL, Rice MM, et al. Racial and ethnic disparities in maternal morbidity and obstetric care. Obstet Gynecol 2015;125:1460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Severe Maternal Morbidity Indicators and Corresponding ICD Codes during Delivery Hospitalizations. Available at: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/smm/severe-morbidity-ICD.htm. Accessed December 6, 2018.
- 5.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol 2012;120:1029–36. [DOI] [PubMed] [Google Scholar]
- 6.Creanga AA, Berg CJ, Ko JY, et al. Maternal mortality and morbidity in the United States: where are we now? J Womens Health (Larchmt) 2014;23:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creanga AA, Bateman BT, Kuklina EV, Callaghan WM. Racial and ethnic disparities in severe maternal morbidity: a multistate analysis, 2008–2010. Am J Obstet Gynecol 2014;210:435.e1–8. [DOI] [PubMed] [Google Scholar]
- 8.Berg CJ, Harper MA, Atkinson SM, et al. Preventability of pregnancy-related deaths: results of a state-wide review. Obstet Gynecol 2005;106:1228–34. [DOI] [PubMed] [Google Scholar]
- 9.Berg CJ, Mackay AP, Qin C, Callaghan WM. Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993–1997 and 2001–2005. Obstet Gynecol 2009;113:1075–81. [DOI] [PubMed] [Google Scholar]
- 10.Danel I, Berg C, Johnson CH, Atrash H. Magnitude of maternal morbidity during labor and delivery: United States, 1993–1997. Am J Public Health 2003;93:631–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geller SE, Cox SM, Callaghan WM, Berg CJ. Morbidity and mortality in pregnancy: laying the groundwork for safe motherhood. Womens Health Sssues 2006;16:176–88. [DOI] [PubMed] [Google Scholar]
- 12.Howell EA, Egorova NN, Balbierz A, Zeitlin J, Hebert PL. Site of delivery contribution to black-white severe maternal morbidity disparity. Am J Obstet Gynecol 2016;215:143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howell EA, Zeitlin J. Improving hospital quality to reduce disparities in severe maternal morbidity and mortality. Semin Perinatol 2017;41:266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Anala 2010;110:1368–73. [DOI] [PubMed] [Google Scholar]
- 15.Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994–2006. Am J Obstet Gynecol 2010;202:353.e1–6. [DOI] [PubMed] [Google Scholar]
- 16.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367:1066–74. [DOI] [PubMed] [Google Scholar]
- 17.Kramer MS, Berg C, Abenhaim H, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol 2013;209:449.e1–7. [DOI] [PubMed] [Google Scholar]
- 18.Main EK, Goffman D, Scavone BM, et al. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Obstet Gynecol 2015;126:155–62. [DOI] [PubMed] [Google Scholar]
- 19.Weinick RM, Hasnain-Wynia R. Quality improvement efforts under health reform: how to ensure that they help reduce disparities—not increase them. Health Aff (Millwood) 2011;30:1837–43. [DOI] [PubMed] [Google Scholar]
- 20.Arean PA, Ayalon L, Hunkeler E, et al. Improving depression care for older, minority patients in primary care. Med Care 2005;43:381–90. [DOI] [PubMed] [Google Scholar]
- 21.Sequist TD, Adams A, Zhang F, Ross-Degnan D, Ayanian JZ. Effect of quality improvement on racial disparities in diabetes care. Arch Intern Med 2006;166:675–81. [DOI] [PubMed] [Google Scholar]
- 22.Trivedi AN, Grebla RC, Wright SM, Washington DL. Despite improved quality of care in the Veterans Affairs health system, racial disparity persists for important clinical outcomes. Health Aff (Millwood) 2011;30:707–15. [DOI] [PubMed] [Google Scholar]
- 23.Trivedi AN, Zaslavsky AM, Schneider EC, Ayanian JZ. Trends in the quality of care and racial disparities in Medicare managed care. N Engl J Med 2005;353:692–700. [DOI] [PubMed] [Google Scholar]
- 24.Werner RM, Asch DA, Polsky D. Racial profiling: the unintended consequences of coronary artery bypass graft report cards. Circulation 2005;111:1257–63. [DOI] [PubMed] [Google Scholar]
- 25.Main EK, Cape V, Abreo A, et al. Reduction of severe maternal morbidity from hemorrhage using a state perinatal quality collaborative. Am J Obstet Gynecol 2017;216:298.e1–11. [DOI] [PubMed] [Google Scholar]
- 26.Main EK, Dhurjati R, Cape V, et al. Improving maternal safety at scale with the mentor model of collaborative improvement. Jt Comm J Qual Patient Saf 2018;44:250–9. [DOI] [PubMed] [Google Scholar]
- 27.Beate D Probabilistic record linkages for generating a comprehensive epidemiological research file on maternal and infant health. Available at: http://www.health-info-solutions.com/Probabilistic%20Record%20Linkages%20may%202002.pdf. Accessed September 1, 2018.
- 28.Howell EA, Egorova N, Balbierz A, Zeitlin J, Hebert PL. Black-white differences in severe maternal morbidity and site of care. Am J Obstet Gynecol 2016;214:122.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Main EK, McCain CL, Morton CH, Holtby S, Lawton ES. Pregnancy-related mortality in California: causes, characteristics, and improvement opportunities. Obstet Gynecol 2015;125:938–47. [DOI] [PubMed] [Google Scholar]
- 30.Leonard SA, Main EK, Carmichael SL. The contribution of maternal characteristics and cesarean delivery to an increasing trend of severe maternal morbidity. BMC Pregnancy Childbirth 2019;19:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taffel SM. Cesarean delivery in the United States, 1990. Vital and health statistics Series 21, Data on natality, marriage, and divorce. Hyattsville, MD: US Department of Health and Human Services; 1994:1–24. [PubMed] [Google Scholar]
- 32.Adebisi OY, Strayhorn G. Anemia in pregnancy and race in the United States: blacks at risk. Fam Med 2005;37:655–62. [PubMed] [Google Scholar]
- 33.Le CH. The prevalence of anemia and moderate-severe anemia in the US population (NHANES 2003–2012). PLoS One 2016;11:e0166635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohamed MA, Ahmad T, Macri C, Aly H. Racial disparities in maternal hemoglobin concentrations and pregnancy outcomes. J Perinat Med 2012;40:141–9. [DOI] [PubMed] [Google Scholar]
- 35.Aaron KF, Clancy CM. Improving quality and reducing disparities: toward a common pathway. JAMA 2003;289:1033–4. [DOI] [PubMed] [Google Scholar]
- 36.Sehgal AR. Impact of quality improvement efforts on race and sex disparities in hemodialysis. JAMA 2003;289:996–1000. [DOI] [PubMed] [Google Scholar]
- 37.Wise PH. The anatomy of a disparity in infant mortality. Ann Rev Public Health 2003;24:341–62. [DOI] [PubMed] [Google Scholar]
- 38.Geller SE, Rosenberg D, Cox SM, et al. The continuum of maternal morbidity and mortality: factors associated with severity. Am J Obstet Gynecol 2004;191:939–44. [DOI] [PubMed] [Google Scholar]
- 39.Shields LE, Wiesner S, Fulton J, Pelletreau B. Comprehensive maternal hemorrhage protocols reduce the use of blood products and improve patient safety. Am J Obstet Gynecol 2015;212:272–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


