ABSTRACT
Palmarumycin P3 (PP3) reduces fluconazole-induced MDR1 transcription to reverse azole resistance in clinical Candida strains. Here, we demonstrated that PP3 restores the susceptibility to several antifungal drugs for Candida albicans strains with gain-of-function mutations in the transcription factor Mrr1. In addition, PP3 inhibits the efflux of Mdr1 substrates by C. albicans strains harboring hyperactive MRR1 alleles. Molecular docking revealed that PP3 is a potential Mdr1 blocker that binds to the substrate binding pocket of Mdr1.
KEYWORDS: Candida albicans, azole resistance, Mdr1, Mrr1, palmarumycin P3
TEXT
Candida albicans is one of the most common causes of both superficial and systemic infections, with a mortality rate of ∼40% for the latter (1, 2). The limited success of current therapies in reducing the high mortality rate of invasive fungal infections is due in part to azole resistance (3). An important mechanism of azole resistance is upregulation of drug efflux pumps, including members of the ATP binding cassette (ABC) superfamily and major facilitator superfamily (MFS), which are involved in rapid drug extrusion (4). MFS transporters use the proton gradient across the cytoplasmic membrane to supply energy for transport. The MFS transporter Mdr1p, which is encoded by MDR1, exports an array of structurally diverse compounds, such as fluconazole (FLC), cerulenin, and brefeldin A (5, 6). In C. albicans, MDR1 overexpression is mainly the result of gain-of-function mutations in the transcription factor Mrr1 (7).
We previously demonstrated that the quinone derivative palmarumycin P3 (PP3) reduces the FLC-induced transcriptional expression of MDR1 in clinical C. albicans strains to reverse azole resistance (8). However, those strains did not harbor mutations in Mrr1 that constitutively induce the expression of MDR1 (9). In the present study, we found that PP3 also reverses azole resistance in C. albicans strains with gain-of-function mutations in Mrr1. In addition, PP3 inhibits the efflux of rhodamine-123 (Rh123) and increases intracellular accumulation of Nile red and a FLC analogue in C. albicans strain G5. Molecular docking indicated that PP3 acts as an Mdr1 blocker to prevent the expulsion of substrates, suggesting that PP3 directly inhibits the efflux pump Mdr1 in addition to regulating MDR1 expression induced by FLC.
PP3 and FLC have synergistic effects against C. albicans strains with gain-of-function mutations in Mrr1.
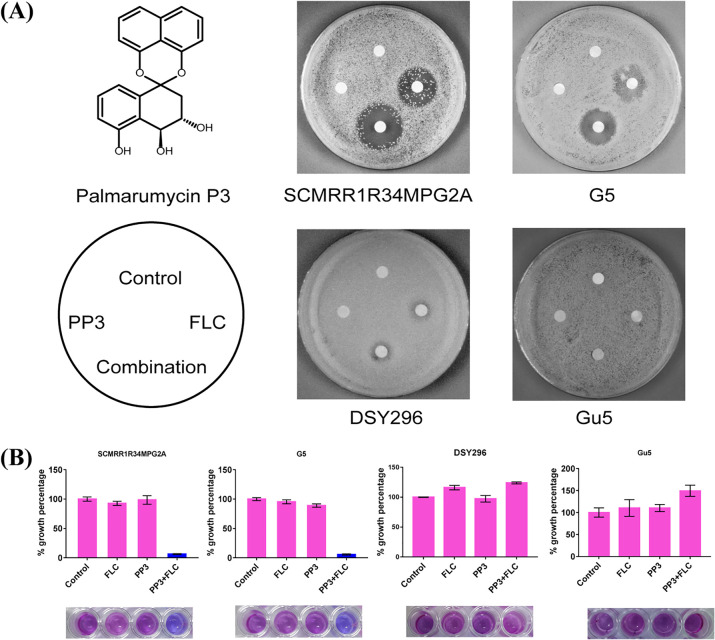
PP3 was previously shown to reverse azole resistance in C. albicans strains by reducing the transcription of FLC-induced MDR1 (8). Here, we used another group of C. albicans strains with gain-of-function mutations in Mrr1 and Tac1 to further explore the ability of PP3 to reverse azole drug resistance, namely, G5, an FLC-resistant clinical isolate from AIDS patient G with a gain-of-function G997V/G997V mutation in Mrr1 (10), SCMRR1R34MPG2A, an engineered C. albicans strain with a gain-of-function P683S/P683S mutation in Mrr1 (11), and Gu5 and DSY296, FLC-resistant clinical isolates with gain-of-function mutations in Tac1 (12, 13) (see the supplemental material). Analysis using the broth microdilution checkerboard method revealed synergistic activity of the combination of PP3 and FLC against the C. albicans strains with either type of gain-of-function mutation in Mrr1 but not in the strains with gain-of-function mutations in Tac1 (Table 1). The agar plate assay further confirmed that PP3 enhanced the antifungal action of FLC against both Mrr1 gain-of-function mutant strains (Fig. 1A). The alamarBlue assay showed that combined treatment with PP3 and FLC inhibited the growth of C. albicans strains SCMRR1R34MPG2A and G5 by more than 90%, whereas single treatment had minimal effects on C. albicans growth (Fig. 1B). Strain G5, with hyperactive Mrr1, was further treated with combinations of PP3 and other Mdr1 substrates, including cerulenin and brefeldin A, or other azole antifungal agents, including voriconazole (VRC) and posaconazole (PCZ). Synergistic effects of these combination treatments were also observed according to the fractional inhibitory concentration index (FICI) model (Table 2). These results suggest that PP3 is a specific azole resistance reversal agent for a variety of clinically derived strains with Mdr1-mediated resistance.
TABLE 1.
Susceptibility testing of PP3 alone and in combination with FLC against C. albicans strains by the checkerboard microdilution assay and drug interaction analysis according to the FICI model
| Strain | Characteristics | MIC80 (μg/ml) |
FICI | Interpretationa | |||
|---|---|---|---|---|---|---|---|
| Alone |
In combination |
||||||
| FLC | PP3 | FLC | PP3 | ||||
| G5 | FLC-resistant clinical isolate with MRR1G997V/MRR1G997V mutation | >256 | >128 | 16 | 16 | 0.1875 | SYN |
| SCMPG2A | Control strain for SCMRR1R34MPG2A | 1 | >128 | 0.5 | 16 | 0.625 | ADD |
| SCMRR1R34MPG2A | Engineered strain with MRR1P683S/MRR1P683S mutation | 16 | >128 | 1 | 16 | 0.1875 | SYN |
| Gu5 | FLC-resistant clinical isolate with TAC1G980E/TAC1G980E mutation | >256 | >128 | 128 | 64 | 1 | ADD |
| DSY296 | FLC-resistant clinical isolate with TAC1N977D/TAC1N977D mutation | 256 | >128 | 128 | 64 | 1 | ADD |
ADD, additive; SYN, synergistic.
FIG 1.
Synergistic effect of PP3 and FLC against Mrr1 mutant strains. (A) Growth inhibition evaluated using the disk diffusion assay. The indicated C. albicans strains (2 × 105 CFU) were plated on yeast extract-peptone-dextrose (YPD) agar plates, and cellulose disks impregnated with FLC and/or PP3 were placed on the plates. The following drug doses were used for each strain: 8 μg FLC and/or 16 μg PP3 for SCMRR1R34MPG2A, 32 μg FLC and/or 32 μg PP3 for G5, 32 μg FLC and/or 32 μg PP3 for DSY296, and 32 μg FLC and/or 32 μg PP3 for Gu5. Each plate was incubated at 30°C for 48 h for observation. (B) alamarBlue assay of the growth inhibitory effects of the indicated treatments against C. albicans. C. albicans cells (1 × 103 CFU/ml) were treated for 24 h with the indicated drugs at the following doses: 1 μg/ml FLC and/or 16 μg/ml PP3 for SCMRR1R34MPG2A, 16 μg/ml FLC and/or 16 μg/ml PP3 for G5, 32 μg/ml FLC and/or 16 μg/ml PP3 for DSY296, and 32 μg/ml FLC and/or 16 μg/ml PP3 for Gu5. Drug treatment was followed by staining with alamarBlue for 2 h in the dark, and the growth percentage was measured using a BioTek Synergy H1 microplate reader (excitation wavelength, 530 nm; emission wavelength, 590 nm).
TABLE 2.
Susceptibility testing of PP3 combined with Mdr1 substrates against C. albicans strain G5 by the checkerboard microdilution assay and drug interaction analysis according to the FICI model
| Substrate | MIC80 (μg/ml) |
FICI | Interpretationa | |||
|---|---|---|---|---|---|---|
| Alone |
In combination |
|||||
| Substrate | PP3 | Substrate | PP3 | |||
| Cerulenin | 32 | >128 | 8 | 16 | 0.375 | SYN |
| Brefeldin A | 128 | >128 | 32 | 16 | 0.375 | SYN |
| VRC | 0.25 | >128 | 0.0625 | 16 | 0.375 | SYN |
| PCZ | 0.25 | >128 | 0.0625 | 16 | 0.375 | SYN |
SYN, synergistic.
PP3 inhibits the activity of the efflux pump Mdr1.
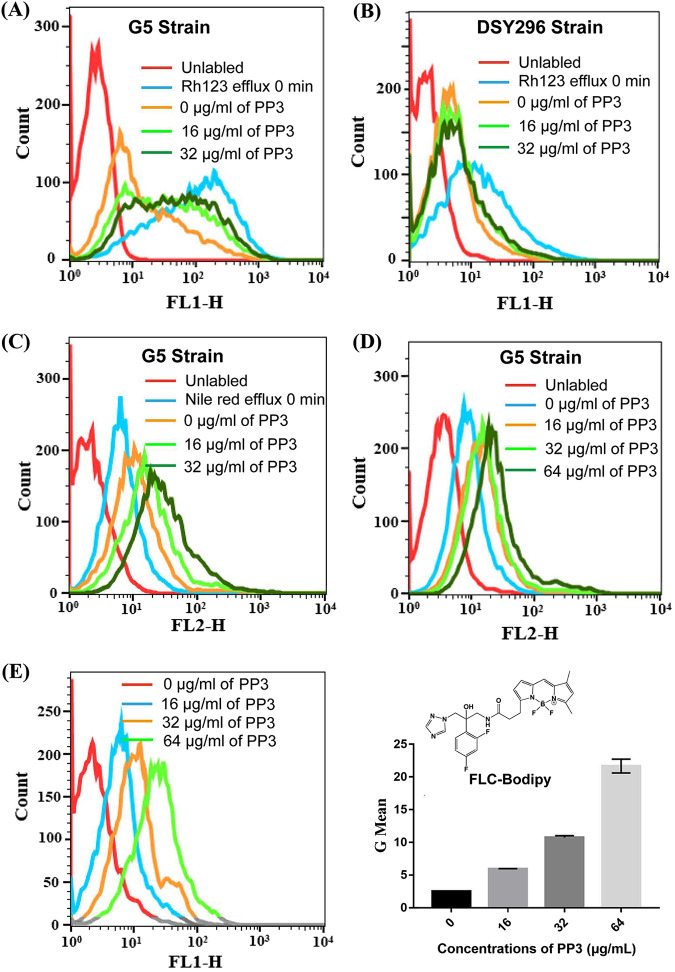
To confirm that the reversal of azole resistance by PP3 was mediated by inhibition of the efflux pump Mdr1, we measured the efflux activity of Mdr1. Flow cytometry revealed that 16 or 32 μg/ml PP3 inhibited the efflux of Rh123 by the hyperactive Mrr1 strain G5 (Fig. 2A). In contrast, PP3 treatment even at 32 μg/ml had only a minor effect on the efflux of Rh123 by the Tac1 mutant strain DSY296 (Fig. 2B). Efflux assays using Nile red, another commonly used efflux pump substrate with red fluorescence, showed that C. albicans G5 cells treated with PP3 accumulated more Nile red than untreated cells (Fig. 2C), suggesting that PP3 promotes the accumulation of Nile red by inhibiting the efflux activity of Mdr1. PP3 also induced the accumulation of Nile red in G5 cells, compared with cells treated with vehicle (Fig. 2D). Moreover, we synthesized fluorescein-labeled FLC (FLC-Bodipy) by attaching a Bodipy fluorescent group to the FLC skeleton according to a previously reported method (14). As expected, PP3 promoted the intracellular accumulation of FLC-Bodipy in C. albicans strain G5, as detected by flow cytometry (Fig. 2E), indicating that the activity of the efflux pump Mdr1 was inhibited by PP3.
FIG 2.
Effects of PP3 on efflux pump activity. (A and B) Rh123 efflux by the C. albicans strain G5 with hyperactive Mrr1 (A) and strain DSY296 with hyperactive Tac1 (B), assessed by flow cytometry. Energy-depleted cells were loaded with Rh123 (5 μM) for 30 min. After washing with PBS, the cells were treated with PP3 (0, 16, or 32 μg/ml) for 30 min. Efflux was initiated by adding glucose (40 mM) in all treatment groups. The fluorescence intensity of Rh123 was then monitored by flow cytometry. (C) Efflux of Nile red by C. albicans strain G5. Energy-depleted cells were loaded with Nile red (5 μM) for 30 min. After washing with PBS, the cells were treated with PP3 (0, 16, or 32 μg/ml) in PBS for 30 min, followed by the addition of glucose. The fluorescence intensity of Nile red was then monitored by flow cytometry. (D) Intracellular accumulation of Nile red by C. albicans strain G5. Cells cultured overnight were incubated with Nile red (5 μM) for 60 min in the presence of PP3 (0, 16, 32, or 64 μg/ml). The fluorescence intensity of Nile red was then monitored by flow cytometry. (E) Efflux activity assessed by measuring the accumulation of a fluorescent FLC analogue. Cells cultured overnight were treated with FLC-Bodipy (8 μg/ml) and PP3 (0, 16, 32, or 64 μg/ml) for 8 h. The fluorescence intensity of FLC-Bodipy was then monitored by flow cytometry (left). The flow cytometry data were analyzed using FlowJo, and the geometric mean value was obtained (right).
PP3 reduces the expression of MDR1 in Mrr1 mutant strains.
To distinguish whether PP3 inhibits the efflux activity of Mdr1 by reducing MDR1 expression, we performed quantitative real-time PCR (qPCR) to determine the expression level of MDR1 in C. albicans strains with hyperactive Mrr1 under PP3 treatment. Treatment with 16 or 32 μg/ml PP3 for 3 or 6 h decreased the mRNA level of MDR1 only slightly, compared with the untreated control (Fig. 3). An obvious reduction of MDR1 expression was observed after 12 h of treatment with PP3 (Fig. 3). However, PP3 inhibited Mdr1-mediated efflux after 30 min of treatment (Fig. 2A), implying that PP3 directly interacts with Mdr1.
FIG 3.
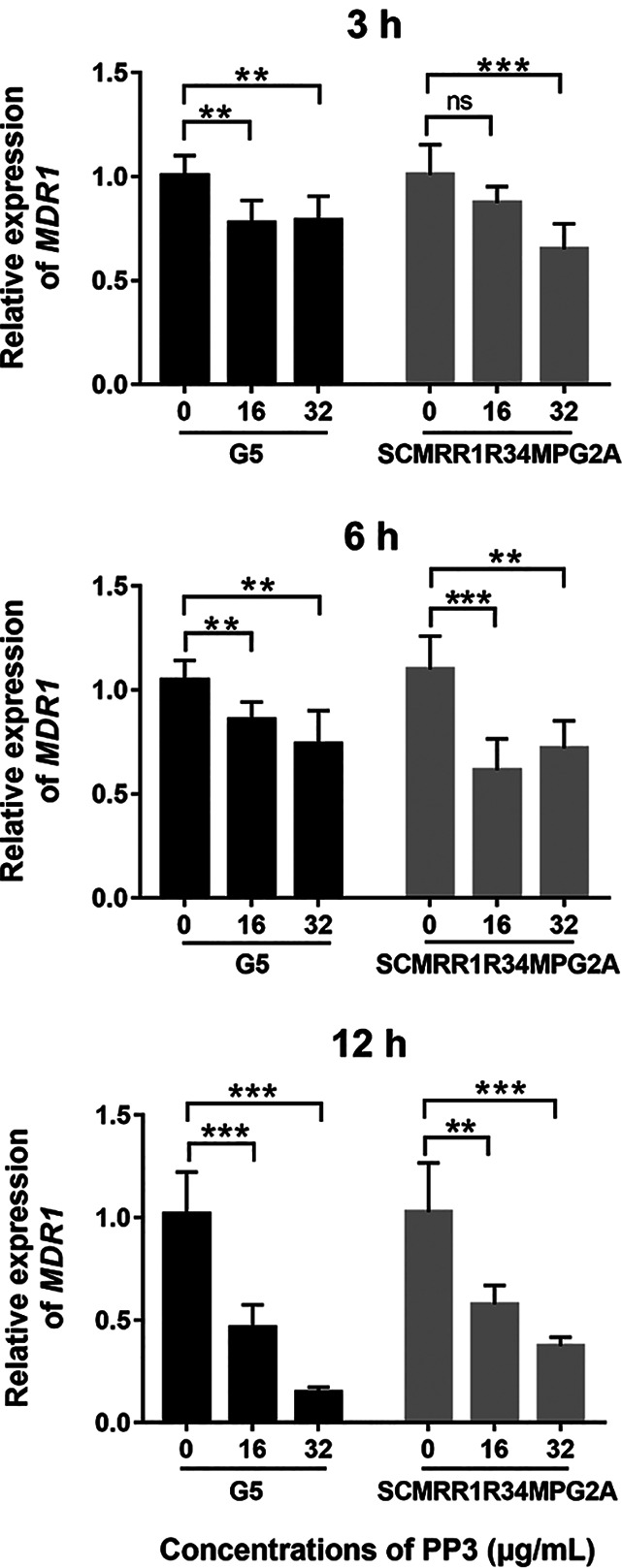
Transcript levels of MDR1 in response to PP3 treatment. C. albicans G5 or SCMRR1R34MPG2A cells were treated with PP3 for 3, 6, or 12 h at 30°C. The relative expression of the MDR1 genes was determined by qPCR and normalized to that of 18S RNA. The bars represent the means ± standard deviations. ns, nonsignificance; **, P < 0.01; ***, P < 0.001, significance in comparison with the control group.
Molecular docking analysis of the mode of binding of PP3 to Mdr1.
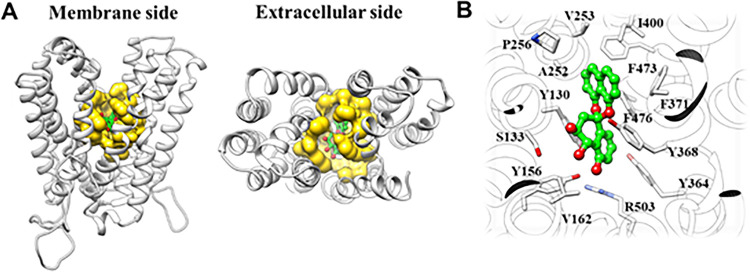
To characterize the direct interaction between Mdr1 and PP3 and the mode of binding, we first generated a homology model of C. albicans Mdr1 using the SWISS-MODEL server with the Alignment Mode algorithm (15). Based on the calculated global model quality estimate values, the known structure of monocarboxylate transporter 1 (PDB code 7CKR) was used as the template structure, and molecular docking was performed using AutoDock Vina (16, 17). PP3 bound in the previously reported binding pocket for Mdr1 substrates such as FLC, Nile red, cycloheximide, and anisomycin (Fig. 4), consistent with blocking of efflux function (18). The docking score for the interaction between PP3 and Mdr1 was −10.0 kcal/mol, suggesting a high confidence of docking.
FIG 4.
Binding mode of PP3 with Mdr1. (A) Predicted binding mode of PP3 with C. albicans Mdr1. The ligand binding pocket is illustrated as the yellow surface. (B) Interactions between PP3 and key residues in Mdr1.
Antifungal activity of FLC combined with PP3 in a Galleria mellonella infection model.
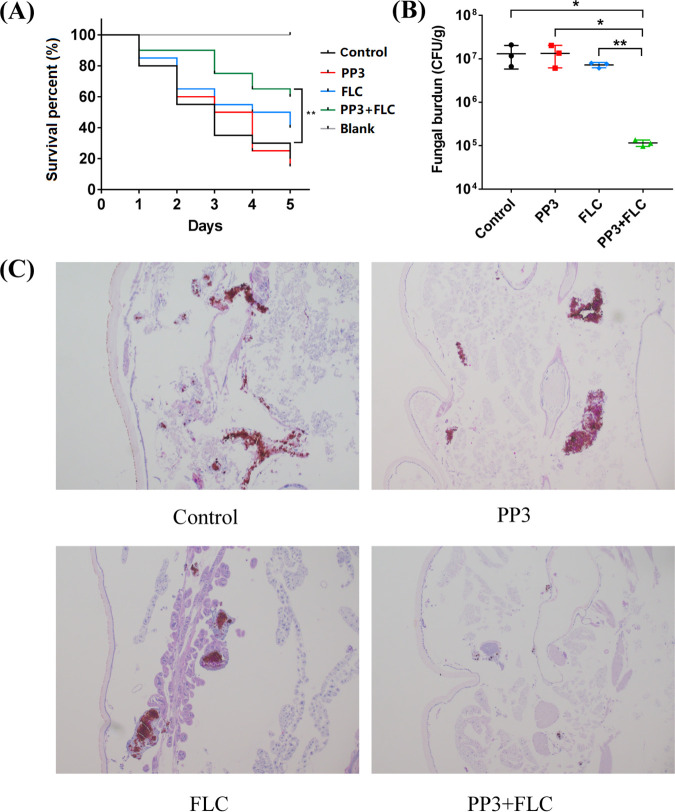
To assess the efficacy of the combination of FLC and PP3 in vivo, we used a G. mellonella infection model. The larvae were injected with ∼5 × 105 CFU of C. albicans G5 cells per larva via the last right proleg and were treated with drug after 2 h of infection. Treatment with PP3 (800 ng per larva) alone had a minimal effect on the survival of C. albicans-infected larvae, and individual treatment with FLC (800 ng per larva) only slightly improved the survival rate. In contrast, the combination treatment significantly increased the survival rate (Fig. 5A). In vivo efficacy was further confirmed by the results of fungal burden analysis. The combination of FLC and PP3 decreased the fungal burden by ∼100-fold in comparison with the control or treatment with PP3 alone and ∼62-fold in comparison with treatment with FLC alone (Fig. 5B). Histological examinations using periodic acid-Schiff (PAS) staining revealed a large number of fungal cells in the larvae treated with phosphate-buffered saline (PBS) or a single treatment, while fungal cells were rarely observed in the larvae treated with the combination of FLC and PP3 (Fig. 5C). The data presented above support the potential application of the combination of FLC and PP3 in the treatment of candidiasis caused by Candida strains with Mdr1-mediated azole resistance.
FIG 5.
In vivo efficacy of the PP3-FLC combination in the C. albicans-infected G. mellonella model. (A) Survival curves for C. albicans-infected G. mellonella larvae. Larvae were infected with C. albicans G5 (5 × 105 CFU/larva) and then received one of the following treatments: sterile PBS, PP3 (800 ng/larva), FLC (800 ng/larva), or their combination. The survival of infected larvae was monitored for 4 days (n = 20 per group). **, P < 0.01, compared with the control group. (B) Larval fungal burden after 4 days of infection (n = 3 per group). *, P < 0.05; **, P < 0.01, significant difference between the compared groups. (C) C. albicans colonization in infected larvae. Larvae were sacrificed 2 days after infection, fixed in paraformaldehyde, embedded in paraffin wax, sectioned longitudinally, and stained with PAS. The histopathology of infected G. mellonella larvae subjected to the different indicated treatments was examined by microscopy.
In conclusion, PP3 directly inhibits the efflux pump Mdr1 and regulates MDR1 expression to inhibit the efflux of Mdr1 substrates and reverse azole resistance. The mechanism by which PP3 reduces MDR1 expression remains unknown, and further investigations are warranted to determine how PP3 interferes with Mrr1-mediated activation of MDR1 expression.
ACKNOWLEDGMENTS
We are grateful to Joachim Morschhäuser of the University of Würzburg for kindly donating the C. albicans strains with mutations in Mrr1 and Tac1 used in this study.
This work was supported by the National Natural Science Foundation of China (grants 81773786, 81903674, 81903503, and 81630093), the Natural Science Fund for Excellent Young Scholars of Shandong Province of China (grant ZR2020YQ63), and the Young Scholars Program of Shandong University (grant 2017WLJH41).
M.Z. and W.C. designed the experiments and wrote the manuscript. M.S., M.Z., and J.L. performed microscopy, qPCR, flow cytometry, and animal experiments. F.X., J.S., and X.L. isolated the compound PP3. X.H. performed molecular docking. M.Z. and W.C. analyzed the data. M.S., M.Z., H.L., and W.C. prepared all figures. All authors reviewed the manuscript.
We declare no competing financial interests.
Footnotes
Supplemental material is available online only.
Contributor Information
Hongxiang Lou, Email: louhongxiang@sdu.edu.cn.
Wenqiang Chang, Email: changwenqiang@sdu.edu.cn.
REFERENCES
- 1.Dadar M, Tiwari R, Karthik K, Chakraborty S, Shahali Y, Dhama K. 2018. Candida albicans: biology, molecular characterization, pathogenicity, and advances in diagnosis and control: an update. Microb Pathog 117:128–138. 10.1016/j.micpath.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 2.Gunsalus KT, Tornberg-Belanger SN, Matthan NR, Lichtenstein AH, Kumamoto CA. 2016. Manipulation of host diet to reduce gastrointestinal colonization by the opportunistic pathogen Candida albicans. mSphere 1:e00020-15. 10.1128/mSphere.00020-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pristov KE, Ghannoum MA. 2019. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect 25:792–798. 10.1016/j.cmi.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 4.Prasad R, Banerjee A, Shah AH. 2017. Resistance to antifungal therapies. Essays Biochem 61:157–166. 10.1042/EBC20160067. [DOI] [PubMed] [Google Scholar]
- 5.Hiller D, Sanglard D, Morschhauser J. 2006. Overexpression of the MDR1 gene is sufficient to confer increased resistance to toxic compounds in Candida albicans. Antimicrob Agents Chemother 50:1365–1371. 10.1128/AAC.50.4.1365-1371.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasrija R, Banerjee D, Prasad R. 2007. Structure and function analysis of CaMdr1p, a major facilitator superfamily antifungal efflux transporter protein of Candida albicans: identification of amino acid residues critical for drug/H+ transport. Eukaryot Cell 6:443–453. 10.1128/EC.00315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morschhauser J, Barker KS, Liu TT, Bla BWJ, Homayouni R, Rogers PD. 2007. The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog 3:e164. 10.1371/journal.ppat.0030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie F, Chang W, Zhang M, Li Y, Li W, Shi H, Zheng S, Lou H. 2016. Quinone derivatives isolated from the endolichenic fungus Phialocephala fortinii are Mdr1 modulators that combat azole resistance in Candida albicans. Sci Rep 6:33687. 10.1038/srep33687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi H, Zhang Y, Zhang M, Chang W, Lou H. 2021. Molecular mechanisms of azole resistance in four clinical Candida albicans isolates. Microb Drug Resist 27:1641–1651. 10.1089/mdr.2020.0413. [DOI] [PubMed] [Google Scholar]
- 10.Franz R, Kelly SL, Lamb DC, Kelly DE, Ruhnke M, Morschhauser J. 1998. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob Agents Chemother 42:3065–3072. 10.1128/AAC.42.12.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schubert S, Barker KS, Znaidi S, Schneider S, Dierolf F, Dunkel N, Aid M, Boucher G, Rogers PD, Raymond M, Morschhauser J. 2011. Regulation of efflux pump expression and drug resistance by the transcription factors Mrr1, Upc2, and Cap1 in Candida albicans. Antimicrob Agents Chemother 55:2212–2223. 10.1128/AAC.01343-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popp C, Hampe I, Hertlein T, Ohlsen K, Rogers PD, Morschhäuser J. 2017. Competitive fitness of fluconazole-resistant clinical Candida albicans strains. Antimicrob Agents Chemother 61:e00584-17. 10.1128/AAC.00584-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coste A, Turner V, Ischer F, Morschhäuser J, Forche A, Selmecki A, Berman J, Bille J, Sanglard D. 2006. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics 172:2139–2156. 10.1534/genetics.105.054767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benhamou RI, Jaber QZ, Herzog IM, Roichman Y, Fridman M. 2018. Fluorescent tracking of the endoplasmic reticulum in live pathogenic fungal cells. ACS Chem Biol 13:3325–3332. 10.1021/acschembio.8b00782. [DOI] [PubMed] [Google Scholar]
- 15.Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T. 2018. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46:W296–W303. 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang N, Jiang X, Zhang S, Zhu A, Yuan Y, Xu H, Lei J, Yan C. 2021. Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell 184:370–383. 10.1016/j.cell.2020.11.043. [DOI] [PubMed] [Google Scholar]
- 17.Trott O, Olson AJ. 2010. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redhu AK, Banerjee A, Shah AH, Moreno A, Rawal MK, Nair R, Falson P, Prasad R. 2018. Molecular basis of substrate polyspecificity of the Candida albicans Mdr1p multidrug/H+ antiporter. J Mol Biol 430:682–694. 10.1016/j.jmb.2018.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental materials and methods. Download aac.02126-21-s0001.pdf, PDF file, 0.2 MB (243.6KB, pdf)