ABSTRACT
Invasive aspergillosis is the most common invasive mold infection following a hematopoietic cell transplant. Widespread use of antifungal prophylaxis has led to the increasing incidence of cryptic Aspergillus species. Aspergillus calidoustus is one of those emerging species and is notorious for multidrug resistance to antifungals. Here, we report a case of disseminated A. calidoustus infection in a hematopoietic stem cell transplant recipient who was successfully treated with combination therapy that included a novel antifungal.
KEYWORDS: Aspergillus calidoustus, APX001, hematopoietic cell transplant
INTRODUCTION
Invasive aspergillosis (IA) is the most common invasive mold disease following hematopoietic cell transplantation (HCT) (1–3). Although invasive pulmonary aspergillosis is the most common manifestation, multiple organs can be involved. A. fumigatus is responsible for 56 to 68% of Aspergillus infections in HCT (2); however, with the introduction of mold-active triazole prophylaxis, the rate of cryptic Aspergillus species has increased in the last year (4–6). A. calidoustus is one of these emerging cryptic species that pose a significant clinical challenge in immunocompromised hosts, as the isolates are usually resistant to azoles, including voriconazole, which is the first-line treatment of IA (7), and therefore, the optimal antifungal therapy for this infection remains to be defined (5). Here, we report an immunocompromised patient with disseminated aspergillosis (pneumonia, brain abscess, endocarditis, and mycotic aneurysm) due to multidrug-resistant A. calidoustus who was successfully treated with combination therapy that included addition of a novel antifungal.
CASE PRESENTATION
The patient was a 40-year-old male with a past medical history of HCT for relapsed Hodgkin’s lymphoma 3 years prior to presentation, complicated by chronic graft-versus-host disease (GvHD) that required long-standing immunosuppression with ruxolitinib (5 mg twice a day), tacrolimus (1 mg twice a day), and prednisone (10 mg daily). He also had history of cytomegalovirus (CMV) reactivation treated with foscarnet. As part of routine screening, he was found to have an elevated serum galactomannan at 1.39 (reference value, <0.5; diagnosis day 0), despite being on prophylaxis with isavuconazole at 372 mg once daily. Computed tomography (CT) of the chest showed new patchy ground-glass opacities suspicious for fungal etiology (Fig. 1A). At this point, prophylaxis was switched to voriconazole at 6 mg/kg of body weight (400 mg) every 12 h for two doses and then 4 mg/kg (300 mg) every 12 h for treatment of probable invasive aspergillosis. On diagnosis day 39 (DD39), the voriconazole level remained subtherapeutic despite dose adjustments, serum Aspergillus galactomannan continued to increase to 3.3 (Fig. 1D), and repeat CT of the chest showed worsening multifocal infiltrates (Fig. 1A). Tacrolimus was discontinued on DD46. At this time the patient was admitted for bronchoscopy; bronchoalveolar lavage yielded Aspergillus galactomannan of 0.686, negative Aspergillus spp. PCR, and fungal cultures with no growth. CT of sinuses and facial bones obtained due to headache and to evaluate for fungal sinusitis showed findings suspicious for brain lesions. Magnetic resonance imaging (MRI) of the brain showed microhemorrhages and an irregular enhancing lesion in the left parietal lobe concerning for septic emboli/fungal cerebritis (Fig. 1B); a 2-dimensional echocardiograph was obtained with no findings of infective endocarditis. Micafungin at 100 mg intravenously (i.v.) daily and liposomal amphotericin B (5 mg/kg i.v. daily) were added to the regimen on DD52. The patient was discharged home on micafungin at 100 mg daily, voriconazole after increasing the dose to 550 mg every 12 h, and liposomal amphotericin B at 5 mg/kg three times weekly. We aimed for voriconazole levels of >3 μg/mL given significant fungal burden with central nervous system involvement. Due to subtherapeutic voriconazole level, elevated liver function tests, and persistently positive galactomannan (Fig. 1D), the patient was switched back to isavuconazole on DD80. Galactomannan levels nearly tripled from 2.06 on DD94 to 6.87 on DD102, so terbinafine at 500 mg twice daily was added and voriconazole resumed. Despite combination therapy, the patient continued to have a cough and increasing serum levels of Aspergillus galactomannan, reaching more than 10 (Fig. 1D). The immunosuppression regimen was tapered further by discontinuation of ruxolitinib on DD120. Next-generation sequencing of microbial cell-free DNA was obtained in peripheral blood on DD128, showing 3,528 DNA molecules per μL (MPM; reference value, <10) of Aspergillus calidoustus and 74 DNA MPM of Enterococcus faecalis, which was thought to be of no clinical significance. Given our previous experience in treating A. calidoustus with a regimen that included the synergistic combination of terbinafine and isavuconazole (7), voriconazole was switched again to isavuconazole at 372 mg once daily, along with terbinafine, liposomal amphotericin B, and micafungin. On DD131, the patient was admitted to the hospital due to shortness of breath, and a repeat 2-dimensional echocardiograph showed a 0.7- by 1.5-cm vegetation on the anterior leaflet of the mitral valve. Transesophageal echocardiography confirmed this finding with no evidence of abscess. Antibiotics for possible enterococcal endocarditis (ampicillin plus ceftriaxone) were added pending valve surgery, and the micafungin dose was increased to 150 mg daily.
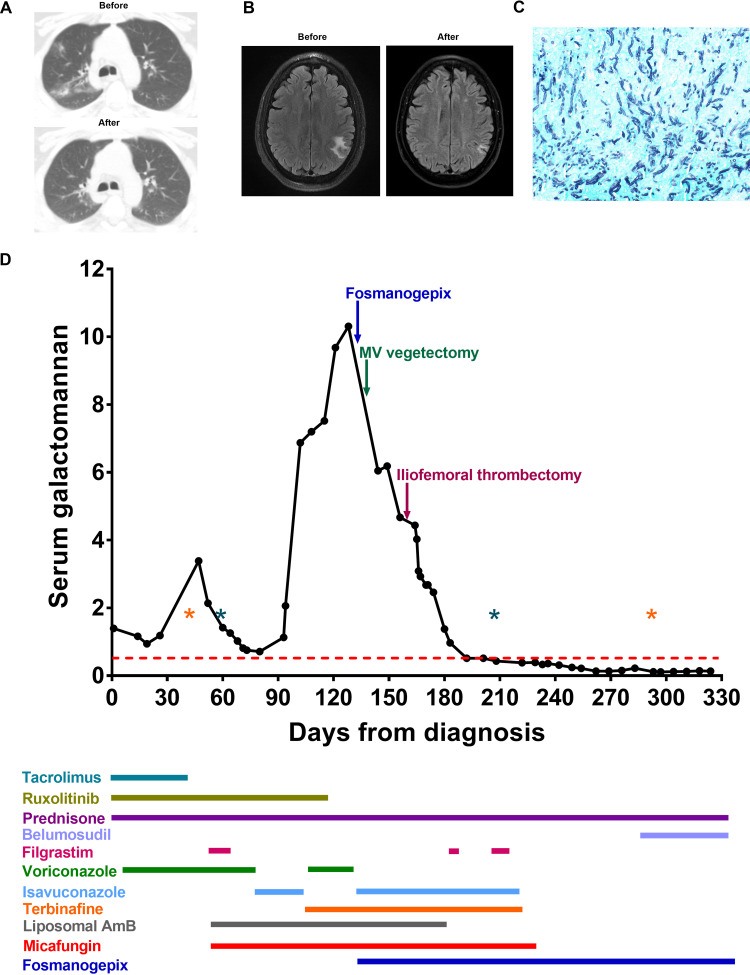
FIG 1.
Summary of case presentation. (A) Abnormal computed tomography of the chest obtained on days 35 and 318 after diagnosis. (B) Brain abscess with surrounding edema on magnetic resonance imaging on day 83 and radiological improvement on day 344 after diagnosis. (C) Grocott’s methenamine silver stain (GMS; ×400 magnification) highlights numerous septate hyphae with acute angle branching, consistent with Aspergillus morphology, in the excised femoral artery. (D) The galactomannan trend and timeline for antifungal therapy are shown. Each dot represents a serum galactomannan level for the corresponding day after diagnosis. The red dotted line represents a positive galactomannan index cutoff (0.5). Note the persistently positive galactomannan for months prior to the addition of fosmanogepix and surgical excision of infected vascular tissues. Asterisks represent episodes of clinically significant cytomegalovirus reactivation (orange) and neutropenia with an absolute neutrophil count below 1,000 cells/mm3 (green), both known risk factors for invasive aspergillosis. Immunosuppressive medications for chronic graft-versus-host disease and various antifungal therapy agents administered are shown in color-coded bars below the x axis; the length of each bar corresponds to the duration of therapy.
CHALLENGE QUESTION
In addition to surgical management, the addition of which of the following therapeutic options is more appropriate for this patient?
-
A.
Fosmanogepix
-
B.
Ibrexafungerp
-
C.
Rezafungin
-
D.
Opelconazole
TREATMENT AND OUTCOME
After obtaining informed consent, fosmanogepix (APX001) was added to the patient’s antifungal regimen under emergency investigational new drug (EIND) number 156107 on DD133. In view of endocarditis, the patient received loading with 1 g i.v. over 3 h every 12 h for two doses, followed by 600 mg over 3 h i.v. daily. The patient underwent mitral valve vegetectomy on DD138, and on DD140, after an uneventful postoperative course, he was switched to oral fosmanogepix at 400 mg twice a day. Mitral valve cultures grew A. calidoustus resistant to all triazoles (Table 1); ampicillin plus ceftriaxone was discontinued. After initiation of fosmanogepix and valve vegetectomy, serum galactomannan trended down by 4 points (from 10 to 6; Fig. 1D). The patient was continued on the same regimen of micafungin at 150 mg once daily, isavuconazole at 372 mg once daily, liposomal amphotericin B at 5 mg/kg three times weekly, fosmanogepix at 400 mg twice daily, and terbinafine at 500 mg twice daily with a further drop in serum Aspergillus galactomannan to 4.6 on DD156 (Fig. 1D). Ultrasound duplex of the right lower extremity was obtained due to worsening claudication, with evidence of nonocclusive thrombosis of the right common femoral artery and occlusive thrombosis of the right superficial femoral artery with recanalization. Suspicion of mycotic aneurysm was confirmed on DD157 during surgical exploration with findings of infected femoral artery with disintegration of the femoral wall and frank purulent material noted from the femoral bed; cultures from the excised common femoral artery thrombus and vessel wall did not grow any microorganisms, but histopathology showed focally necrotizing granulomatous vasculitis with giant cells and numerous septate hyphae with acute angle branching suggestive of Aspergillus species (Fig. 1C), which was confirmed by immunohistochemical stain. Serum galactomannan continued to trend down. Liposomal amphotericin B was discontinued on DD180 after completing 4 weeks postsurgery. Serum galactomannan became negative on DD208. After documenting two consecutive negative serum galactomannan levels obtained 2 weeks apart, micafungin was discontinued on DD229. The patient subsequently self-discontinued isavuconazole and terbinafine on DD236. From this point onward, patient was continued on fosmanogepix monotherapy. As of his last clinic follow-up, day DD324, the patient was tolerating fosmanogepix at 400 mg twice daily with no adverse effects, and his serum galactomannan levels remained negative (<0.5).
TABLE 1.
Antifungal susceptibility testing (includes single agents and combinations) for clinical isolate of Aspergillus calidoustus
| Antifungal | MIC (μg/mL) | MIC (μg/mL) |
|---|---|---|
| Single agents | ||
| Micafungin | 0.5 | |
| Posaconazole | >4 | |
| Voriconazole | >4 | |
| Isavuconazole | >4 | |
| Terbinafine | 0.5 | |
| Amphotericin (AMB) | 1 | |
| Fosmanogepix | ≤0.008 | |
| Synergy testing | ||
| AMB + micafungin | 1 | ≤0.03 |
| AMB + posaconazole | 1 | ≤0.03 |
| AMB + voriconazole | 1 | ≤0.03 |
| AMB + terbinafine | 1 | ≤0.015 |
| AMB + isavuconazole | 1 | ≤0.03 |
| Isavuconazole + terbinafine | ≤0.03 | 0.5 |
Our case illustrates the importance of serial monitoring of serum galactomannan to assess the response to therapy (8, 9). Antifungal resistance or infections caused by rare/cryptic Aspergillus species should be suspected if galactomannan level continues to increase despite antifungal therapy. This case also highlights the importance of the identification of Aspergillus at the species level given the changing epidemiology due to the widespread use of the antimold prophylaxis with emergence of resistant species and illustrates the role of in vitro susceptibility testing and synergy testing to guide therapy, although the correlation between in vitro MICs and in vivo efficacy of antifungals is difficult to assess (10). Rare/cryptic Aspergillus species are often azole-resistant, requiring combination therapy and/or novel antifungals. Ibrexafungerp, olorofim, and rezafungin have potent activity against A. calidoustus (11). Data on the efficacy and safety of ibrexafungerp for the treatment of mold infections are lacking; both ibrexafungerp and olorofim are currently being evaluated in phase 2 clinical trials for the treatment of invasive mold infections. As with other echinocandins, rezafungin has poor blood-brain barrier penetration, making it a suboptimal option for this patient with brain involvement. Likewise, opelconazole, a first-in-class inhaled antifungal, would not be an appropriate option for this patient given disseminated disease. In this case and our previous report, synergy testing justified the use of isavuconazole and terbinafine in combination; in vitro testing also confirmed a low MIC to fosmanogepix. Like for other novel agents aforementioned, clinical experience with fosmanogepix is limited. Fosmanogepix showed favorable responses in a case series of 12 patients with invasive Fusarium infection (https://eacademy.escmid.org/escmid/2021/eccmid-2021/332864/sanjeet.dadwal.fosmanogepix.treatment.for.fusarium.infections.in.patients.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D2%2Aspeaker%;3D447702%2Alabel%3D222), and it was well tolerated in phase I and phase II trials, with the majority of adverse events (more common was headache) being mild and transient (12). There are scarce data on tissue penetration, but fosmanogepix significantly improved survival and reduced the fungal burden in the brain and lungs in infected mice (13). To the best of our knowledge, this is the first clinical case reporting the safety and efficacy of this agent for management of disseminated resistant Aspergillus infection. In summary, tapering of immunosuppression, surgical debridement, and combination antifungal and/or novel antifungal therapies are critical components of the management of multidrug-resistant mold infections.
ACKNOWLEDGMENTS
We are indebted to Pfizer, Inc., for granting compassionate use of fosmanogepix for this patient.
We declare no competing interests. The study received no external funding.
REFERENCES
- 1.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) database. Clin Infect Dis 50:1091–1100. 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 2.Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, Pfaller M, Chang C, Webster K, Marr K. 2009. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of multicenter Prospective Antifungal Therapy (PATH) alliance registry. Clin Infect Dis 48:265–273. 10.1086/595846. [DOI] [PubMed] [Google Scholar]
- 3.Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, Apewokin S, Horn DL. 2012. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect 65:453–464. 10.1016/j.jinf.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Pavie J, Lacroix C, Hermoso DG, Robin M, Ferry C, Bergeron A, Feuilhade M, Dromer F, Gluckman E, Molina JM, Ribaud P. 2005. Breakthrough disseminated Aspergillus ustus infection in allogeneic hematopoietic stem cell transplant recipients receiving voriconazole or caspofungin prophylaxis. J Clin Microbiol 43:4902–4904. 10.1128/JCM.43.9.4902-4904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glampedakis E, Cassaing S, Fekkar A, Dannaoui E, Bougnoux M-E, Bretagne S, Neofytos D, Schreiber PW, Hennequin C, Morio F, Shadrivova O, Bongomin F, Fernández-Ruiz M, Bellanger AP, Arikan-Akdagli S, Erard V, Aigner M, Paolucci M, Khanna N, Charpentier E, Bonnal C, Brun S, Gabriel F, Riat A, Zbinden R, Le Pape P, Klimko N, Lewis RE, Richardson M, İnkaya AC, Coste AT, Bochud P-Y, Lamoth F. 2021. Invasive aspergillosis due to Aspergillus section Usti: a multicenter retrospective study. Clin Infect Dis 72:1379–1385. 10.1093/cid/ciaa230. [DOI] [PubMed] [Google Scholar]
- 6.Yamamuro R, Kimura M, Asano-Mori Y, Abe M, Nakamura S, Umeyama T, Yamagoe S, Miyazaki Y, Ogura S, Sakoh T, Mitsuki T, Yamaguchi K, Yuasa M, Kaji D, Kageyama K, Nishida A, Taya Y, Ishiwata K, Takagi S, Yamamoto H, Yamamoto G, Uchida N, Wake A, Taniguchi S, Araoka H. 2021. Clinical and microbiological characteristics of proven invasive aspergillosis due to rare/cryptic species in allogeneic hematopoietic stem cell transplant recipients. Antimicrob Agents Chemother 66:e01630-21. 10.1128/AAC.01630-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendoza MA, Anderson A, Morris MI, Lekakis L, Simkins J, Prado CE, Martinez OV, Komanduri KV, Camargo JF. 2020. Successful treatment of invasive fungal infection due to highly resistant Aspergillus calidoustus in an allogeneic hematopoietic cell transplant recipient. Mycopathologia 185:399–403. 10.1007/s11046-019-00423-x. [DOI] [PubMed] [Google Scholar]
- 8.Chai LYA, Kullberg B-J, Johnson EM, Teerenstra S, Khin LW, Vonk AG, Maertens J, Lortholary O, Donnelly PJ, Schlamm HT, Troke PF, Netea MG, Herbrecht R. 2012. Early serum galactomannan trend as a predictor of outcome of invasive aspergillosis. J Clin Microbiol 50:2330–2336. 10.1128/JCM.06513-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mercier T, Guldentops E, Lagrou K, Maertens J. 2018. Galactomannan, a surrogate marker for outcome in invasive aspergillosis: finally coming of age. Front Microbiol 9:661. 10.3389/fmicb.2018.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamoth F, Kontoyiannis DP. 2019. Therapeutic challenges of non-Aspergillus invasive mold infections in immunosuppressed patients. Antimicrob Agents Chemother 63:e01244-19. 10.1128/AAC.01244-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoenigl M, Sprute R, Egger M, Arastehfar A, Cornely OA, Krause R, Lass-Flörl C, Prattes J, Spec A, Thompson GR III, Wiederhold N, Jenks JD. 2021. The antifungal pipeline: fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs 81:1703–1729. 10.1007/s40265-021-01611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodges M, Ople E, Shaw K, Mansbach R, Van Marle S, van Hoogdalem E, Wedel P, Kramer W. 2017. First-in-human study to assess safety, tolerability and pharmacokinetics of APX001 administered by intravenous infusion to healthy subjects. Open Forum Infect Dis 4:S526. 10.1093/ofid/ofx163.1370. [DOI] [Google Scholar]
- 13.Gebremariam T, Alkhazraji S, Alqarihi A, Wiederhold NP, Shaw KJ, Patterson TF, Filler SG, Ibrahim AS. 2020. Fosmanogepix (APX001) is effective in the treatment of pulmonary murine mucormycosis due to Rhizopus arrhizus. Antimicrob Agents Chemother 64:e00178-20. 10.1128/AAC.00178-20. [DOI] [PMC free article] [PubMed] [Google Scholar]