ABSTRACT
About 55% of U.S. Candida auris clinical cases were reported from New York and New Jersey from 2016 through 2020. Nearly all New York-New Jersey clinical isolates (99.8%) were fluconazole resistant, and 50% were amphotericin B resistant. Echinocandin resistance increased from 0% to 4% and pan-resistance increased from 0 to <1% for New York C. auris clinical isolates but not for New Jersey, highlighting the regional differences.
KEYWORDS: antifungals, Candida auris, minimum inhibitory concentration, epidemiological cutoff
INTRODUCTION
Candida auris is a multidrug-resistant fungal pathogen classified as an urgent public health threat by the Centers for Disease Control and Prevention (CDC) (1). First identified in the United States in 2013 and first detected in New York State in 2016 (2), C. auris was made nationally notifiable in 2018 (3). C. auris infection has caused severe illnesses among hospitalized patients and long-term care residents in the New York metropolitan area (4). As of December 31, 2020, of the 1,678 CDC tracked confirmed clinical cases in the United States, ∼55% were reported by New York and New Jersey (3). Additionally, approximately 10% of all colonized cases in New York developed symptomatic C. auris infections. A previous study which analyzed 51 clinical case-patients in New York reported that 45% died within 90 days of reporting, and 98% of all clinical isolates were fluconazole resistant (4). As of December 2020, major C. auris genotypes comprise South Asia (clade I), East Asia (clade II), Africa (clade III), and South America (clade IV) (5). Recently, a fifth clade was identified based on whole genome sequencing, from a patient in Iran without travel history (6). Clade I was the major genotype (>99%) among clinical isolates reported from New York and New Jersey.
The CDC has tentatively defined MIC breakpoints (CDC-BP) for fluconazole (FLC), amphotericin B (AMB), and echinocandins using values established for other pathogenic Candida species (7). In instances where no breakpoints have been established, epidemiological cutoff value (ECV) could be used to find upper limit of the wild type (UL-WT) values, which help to identify non-wild-type strains within a population regardless of their susceptibility (7, 8). ECVs may aid in an early detection of C. auris isolates with the possible acquired resistance (7). Although the criteria for both ECV estimation and breakpoint establishment include the determination of MIC/minimum effective concentration (MEC) distribution of the pathogens, the breakpoint is the preferred value for making clinical decisions (7). This study aims to summarize antifungal resistance trends for New York and New Jersey C. auris clinical isolates using the recommended CDC-BP criteria and ECVs as an additional information for those antifungals for which CDC-BP are not available.
Wadsworth Center Mycology Laboratory (WCML), New York State Department of Health serves as the Northeast Regional Laboratory in CDC’s Antibiotic Resistance Laboratory Network (AR Lab Network) for drug-resistant Candida spp. Suspected C. auris isolates from New York and New Jersey health care facilities were submitted to WCML and confirmed by Matrix Assisted Laser Desorption Ionization-time of flight mass spectrometry (MALDI-TOF MS) (9). Clinical isolates were defined as C. auris isolates from specimens that were collected from anatomical sites other than axilla-groin or nares, including blood, urine, and wounds. Genotyping of C. auris was performed by Sanger sequencing of the ribosomal genes (9). Antifungal susceptibility testing (AFST) was performed according to CLSI method M27-A3 for pathogenic yeasts (10). The MICs in μg/mL for the azoles and echinocandins were determined using the TREK frozen broth microdilution panel (catalog number CML2FCAN; Thermo Fisher Scientific, Marietta, OH, USA) (9, 11). MICs for AMB and 5-flucytosine (5-FC), were determined using Etest strips (AB Biodisk; bioMérieux, Solna, Sweden). AMB MICs of 1.5 μg/mL were rounded up to 2 μg/mL when determining susceptibility (12). MICs for 5-FC were only available from 2016 to September 2018 due to limited availability of 5-FC test strips in the United States. The UL-WT for posaconazole (POS), itraconazole (ITC), isavuconazole (ISA), voriconazole (VRC), and 5-FC were determined at the 97.5% estimation level using ECOFFinder (8). For New Jersey, there were <100 isolates tested against 5-FC, therefore, the UL-WT for 5-FC was not estimated. The isolates from New Jersey that were evaluated do not accurately represent the number of C. auris clinical cases in New Jersey, as only those isolates submitted to WCML for testing were included in this study. Statistical analyses, chi-squared and Fisher’s exact test, were performed using R statistical software (v4.1.0; R Core Team, Vienna, Austria).
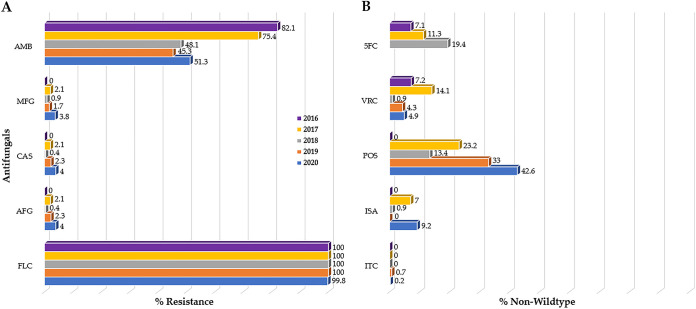
In total, 1,148 clinical C. auris isolates from 697 individuals in New York were evaluated: 28 from 2016, 141 from 2017, 231 from 2018, 300 from 2019, and 448 from 2020. Forty-five percent (512/1,148) of the isolates were recovered from the blood, followed by 23% (260/1,148) from urine, 13% (151/1,148) from respiratory tract, 12% (138/1,148) from other body sites and fluids, and 7% (87/1,148) from wounds (Fig. S1). Isolates with MIC values greater than or equal to the CDC tentative MIC breakpoint or estimated UL-WT were considered resistant or non-wild-type, respectively. All New York isolates were resistant to FLC (100%) during 2016 to 2019, while for 2020 resistance slightly dropped to 99.6% (446/448) (Table 1) with the modal MIC value at ≥256 μg/mL (Table S1). While isolates exhibited no resistance to echinocandins in 2016 (0/28), there was a steady increase in resistance isolates with 0.9% (2/231), 2.3% (7/300), and 4% (18/448) in 2018, 2019, and 2020, respectively (Fig. 1A). AMB resistance decreased significantly (P < 0.001) from 82.1% (23/28) in 2016 to 45.3% (136/300) in 2019 (Table 1) with the modal MIC value at the borderline, 1.5 μg/mL (Table S1). The percentage of isolates non-wild-type to 5-FC increased by year from 7.1% (2/28) in 2016 to 19.4% (31/160) in 2018. While the percentage of isolates non-wild-type to POS, ISA, and VRC fluctuated over time, a higher percentage was non-wild-type to POS (23.4% [33/141] in 2017; 13.4% [31/231] in 2018; 33% [99/300] in 2019; and 42.6% [191/448] in 2020) compared with the other azoles (Table 1 and Fig. 1B). Additionally, there was a noticeable increase in pan-resistance to three major classes of antifungals, from one isolate each in 2017 and 2018 to four isolates each in 2019 and 2020.
TABLE 1.
Resistance and non-wild-type antifungals pattern of New York C. auris clinical isolates from 2016 through 2020
| Year and total no. of isolates | 2016 (28) |
2017 (141) |
2018 (231) |
2019 (300) |
2020 (448) | P value | ||
|---|---|---|---|---|---|---|---|---|
| Antifungal | CDC BP | ECV UL-WT | % (n) resistance/non-wild-type | |||||
| FLC | ≥ 32 | - | 100% (28) | 100% (141) | 100% (231) | 100% (300) | 99.6% (446) | 0.6393 |
| ITC | - | 2 | 0 | 0 | 0 | 0.7% (2) | 0.2% (1) | 0.666 |
| ISA | - | 2 | 0 | 7.1% (10) | 0.9 (2) | 0 | 9.2% (41) | 4.42e−12 |
| POS | - | 0.5 | 0 | 23.4% (33) | 13.4% (31) | 33% (99) | 42.6% (191) | 2.2e−16 |
| VRC | - | 4 | 7.4% (2) | 14.2% (20) | 0.9% (2) | 4.3% (13) | 4.9% (22) | 3.94e-6 |
| AFG | ≥ 4 | - | 0 | 1.4% (2) | 0.4% (1) | 2.3% (7) | 4% (18) | 0.05707 |
| CAS | ≥ 2 | - | 0 | 1.4% (2) | 0.4% (1) | 2.3% (7) | 4% (18) | 0.05707 |
| MFG | ≥ 4 | - | 0 | 1.4% (2) | 0.9% (2) | 1.7% (5) | 3.8% (17) | 0.1549 |
| AMB | ≥ 2 | - | 82.1% (23) | 75.9% (107) | 48.1% (111) | 45.3% (136) | 51.3% (230) | 1.019e-9 |
| 5-FC | - | 0.125 | 7.1% (2) | 11.3% (16) | 19.4% (31)a | - | - | 0.0793 |
160 of 231 C. auris isolates were part of testing due to discontinuation of 5-FC Etest strips in 2018.
FIG 1.
Antifungal susceptibility pattern of C. auris clinical isolates from New York, 2016 to 2020. (A) Percent C. auris resistant isolates to antifungals for which CDC tentative MIC breakpoint (CDC-BP) are available. Most of the isolates were resistant to fluconazole (FLC) followed by amphotericin B (AMB), with a small number exhibiting resistance to echinocandins. (B) Percent C. auris non-wild-type isolates based on epidemiological cutoff values (ECV). A higher percentage of C. auris isolates were non-wild-type to posaconazole (POS) compared with the other azoles and 5-flucytosine (5-FC).
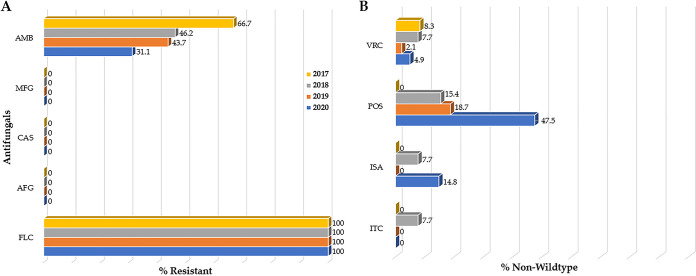
In total, 134 clinical C. auris isolates from 121 individuals in New Jersey were evaluated, including 12 from 2017, 13 from 2018, 48 from 2019, and 61 from 2020. Thirty-six percent (48/134) of the New Jersey C. auris isolates were recovered from urine, followed by 31% (41/134) from blood, 16% (22/134) from respiratory tract, 10% (13/134) from wound, and 7% (10/134) from other body sites and fluids (Fig. S2). All submitted New Jersey C. auris isolates were resistant to FLC and susceptible to the echinocandins (Table 2). The modal MIC values for FLC and AMB were ≥256 μg/mL and 1 μg/mL, respectively (Table S2). AMB resistance decreased from 66.7% (8/12) in 2017 to 31.1% (19/61) in 2020 (Fig. 2A). Like New York, a higher percentage of New Jersey C. auris isolates were non-wild-type to POS 15.4% (2/13) in 2018; 18.7% (9/48) in 2019; and 47.5% (29/61) in 2020 compared with the other azoles (Fig. 2B).
TABLE 2.
Resistance and non-wild-type antifungals pattern of New Jersey C. auris clinical isolates from 2017 through 2020
| Year and total no. of isolates | 2017 (12) |
2018 (13) |
2019 (48) |
2020 (61) | P value | ||
|---|---|---|---|---|---|---|---|
| Antifungal | CDC BP | ECV UL-WT | % (n) resistance/non-wild-type | ||||
| FLC | ≥ 32 | - | 100% (12) | 100% (13) | 100% (48) | 100% (61) | 1 |
| ITC | - | 2 | 0 | 7.7% (1) | 0 | 0 | 0.1852 |
| ISA | - | 2 | 0 | 7.7% (1) | 0 | 14.8% (9) | 0.01613 |
| POS | - | 0.5 | 0 | 15.4% (2) | 18.7% (9) | 47.5% (29) | 0.0002489 |
| VRC | - | 4 | 8.3% (1) | 7.7% (1) | 2.1% (1) | 4.9% (3) | 0.3546 |
| AFG | ≥ 4 | - | 0 | 0 | 0 | 0 | 1 |
| CAS | ≥ 2 | - | 0 | 0 | 0 | 0 | 1 |
| MFG | ≥ 4 | - | 0 | 0 | 0 | 0 | 1 |
| AMB | ≥ 2 | - | 66.7% (8) | 46.2% (6) | 43.7% (21) | 31.1% (19) | 0.1108 |
FIG 2.
Antifungal susceptibility pattern of C. auris clinical isolates from New Jersey, 2017 to 2020. (A) Percent C. auris resistant isolates to antifungals for which CDC tentative MIC breakpoint (CDC-BP) are available. All isolates were resistant to fluconazole (FLC) followed by amphotericin B (AMB), while none showed resistance to echinocandins. (B) Percent C. auris non-wild-type isolates based on epidemiological cutoff values (ECV). A higher percentage of C. auris isolates was non-wild-type to posaconazole (POS) compared with the other azoles.
Previous studies of C. auris clinical isolates in different parts of the world including New York, have reported FLC resistance, elevated MICs to other azoles and AMB, and low resistance to echinocandins (9, 13, 14). C. auris isolates in this study displayed high AMB resistance early in the outbreak which decreased during later years; however, the small sample size does not allow for any strong inference about the presence and evolution of AMB resistant C. auris early in the New York and New Jersey outbreak. There was a steady increase in echinocandins resistance observed between 2017 and 2020 in New York that was not observed in C. auris isolates from New Jersey. Pan-resistance, which was only observed in New York isolates, increased over time, but remained rare in 2020, 4 years into the New York outbreak (15). The precise mechanism(s) behind differences in C. auris echinocandin and pan-resistance between New York and New Jersey isolates is not clear from the present study. Previous studies in the U.S. including from New York documented C. auris echinocandin resistance in patient isolates after echinocandin therapy (15, 16). The observed resistance pattern might reflect intraregional differences in antifungal treatment practices, but further investigations are needed for confirmation (15). We noticed significant difference in the total submission of clinical C. auris isolates submitted from New York and New Jersey. Most likely this reflects a lag in submission from clinical laboratories in New Jersey. It is also likely that initial introduction and local spread were confined to New York health care facilities and surrounding metropolitan areas, including New Jersey, were affected subsequently.
Conclusions. This study highlights the antifungal resistance pattern of New York and New Jersey C. auris clinical isolates. Nearly all isolates were FLC resistant. Additionally, AMB resistance was high, but decreased significantly for New York in later years compared with earlier years of investigations. Echinocandin resistance was rare initially but increased for New York isolates in later years. Ten New York C. auris isolates from eight individuals were identified as pan-resistant.
ACKNOWLEDGMENTS
We thank Wadsworth Center (WC) Mycology Laboratory (ML) staff members for processing and AFST of C. auris clinical isolates. We thank clinical, public, and private laboratories in New York and New Jersey for assistance with sample submission to the WCML. We thank Drs. Elizabeth Berkow and Shawn Lockhart, the Centers for Disease Control and Prevention (CDC), for their continued support and guidance with the C. auris AFST. This work was supported partly by funds from the WC, the New York State Department of Health (NYSDOH), and the CDC grant number NU50CK000516. The contents of this manuscript are solely the authors’ responsibility and do not necessarily represent the official views of the NYSDOH or the CDC.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Anonymous. 2019. Antibiotic resistance threats in the United States, 2019. Centers for Disease Control and Prevention, Atlanta, GA. 10.15620/cdc:82532. [DOI] [Google Scholar]
- 2.Vallabhaneni S, Kallen A, Tsay S, Chow N, Welsh R, Kerins J, Kemble SK, Pacilli M, Black SR, Landon E. 2016. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus—United States, May 2013–August 2016. Morbidity and Mortality Wkly Report 65:1234–1237. 10.15585/mmwr.mm6544e1. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 2021. Tracking Candida auris, on Centers for Disease Control and Prevention. https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html. Accessed March 24, 2021.
- 4.Adams E, Quinn M, Tsay S, Poirot E, Chaturvedi S, Southwick K, Greenko J, Fernandez R, Kallen A, Vallabhaneni S, Haley V, Hutton B, Blog D, Lutterloh E, ZuckCer H. 2018. Candida auris in healthcare facilities, New York, USA, 2013–2017. Emerg Infect Dis 24:1816–1824. 10.3201/eid2410.180649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow NA, De Groot T, Badali H, Abastabar M, Chiller TM, Meis JF. 2019. Potential fifth clade of Candida auris, Iran, 2018. Emerging Infectious Diseases 25:1780–1781. 10.3201/eid2509.190686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anonymous. 2016. Principles and procedures for the development of epidemiological cutoff value for antifungal susceptibility testing, 1st edition ed, vol CSLI Guideline M57. Clinical and Laboratory Standard Institute, Wayne, Pennsylvania. [Google Scholar]
- 8.Espinel-Ingroff A, Turnidge J. 2016. The role of epidemiological cutoff values (ECVs/ECOFFs) in antifungal susceptibility testing and interpretation for uncommon yeasts and moulds. Rev Iberoam Micol 33:63–75. 10.1016/j.riam.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Y, O’Brien B, Leach L, Clarke A, Bates M, Adams E, Ostrowsky B, Quinn M, Dufort E, Southwick K, Erazo R, Haley VB, Bucher C, Chaturvedi V, Limberger RJ, Blog D, Lutterloh E, Chaturvedi S. 2020. Laboratory analysis of an outbreak of Candida auris in New York from 2016 to 2018: impact and lessons learned. J Clin Microbiol 58. 10.1128/JCM.01503-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anonymous. 2017. Reference method for broth dilution antifungal susceptibility testing of yeasts, 4th ed, vol CLSI document M27. Clinical and Laboratory Standards Institute, Wayne, Pennsylvania. [Google Scholar]
- 11.O’Brien B, Chaturvedi S, Chaturvedi V. 2020. In vitro evaluation of antifungal drug combinations against multidrug-resistant Candida auris isolates from New York Outbreak. Antimicrob Agents Chemother 64. 10.1128/AAC.02195-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon-Chung KJ, Bennett JA. 1992. Medical mycology. Lea & Febiger, Philadelphia, Pennsylvania. [Google Scholar]
- 13.Calvo B, Melo AS, Perozo-Mena A, Hernandez M, Francisco EC, Hagen F, Meis JF, Colombo AL. 2016. First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. J Infect 73:369–374. 10.1016/j.jinf.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Kathuria S, Singh PK, Sharma C, Prakash A, Masih A, Kumar A, Meis JF, Chowdhary A. 2015. Multidrug-resistant Candida auris misidentified as Candida haemulonii: characterization by matrix-assisted laser desorption ionization–time of flight mass spectrometry and DNA sequencing and its antifungal susceptibility profile variability by Vitek 2, CL. J Clin Microbiol 53:1823–1830. 10.1128/JCM.00367-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostrowsky B, Greenko J, Adams E, Quinn M, O’Brien B, Chaturvedi V, Berkow E, Vallabhaneni S, Forsberg K, Chaturvedi S. 2020. Candida auris isolates resistant to three classes of antifungal medications—New York, 2019. Morbidity and Mortality Wkly Report 69:6. 10.15585/mmwr.mm6901a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biagi MJ, Wiederhold NP, Gibas C, Wickes BL, Lozano V, Bleasdale SC, Danziger L. 2019. Development of high-level echinocandin resistance in a patient with recurrent Candida auris candidemia secondary to chronic Candiduria. Open Forum Infect Dis 6:ofz262. 10.1093/ofid/ofz262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2 and Fig. S1 and S2. Download aac.02242-21-s0001.pdf, PDF file, 0.2 MB (189.4KB, pdf)