ABSTRACT
The pyridoxal 5′-phosphate (PLP)-binding protein (PLPBP) plays an important role in vitamin B6 homeostasis. Loss of this protein in organisms such as Escherichia coli and humans disrupts the vitamin B6 pool and induces intracellular accumulation of pyridoxine 5′-phosphate (PNP), which is normally undetectable in wild-type cells. This accumulated PNP could affect diverse metabolic systems through the inhibition of some PLP-dependent enzymes. In this study, we investigated the as-yet-unclear mechanism of intracellular accumulation of PNP due to the loss of PLPBP protein encoded by yggS in E. coli. Genetic studies using several PLPBP-deficient strains of E. coli lacking a known enzyme(s) in the de novo or salvage pathways of vitamin B6, including pyridoxine (amine) 5′-phosphate oxidase (PNPO), PNP synthase, pyridoxal kinase, and pyridoxal reductase, demonstrated that neither the flux from the de novo pathway nor the salvage pathway solely contributed to the PNP accumulation caused by the PLPBP mutation. Studies of the strains lacking both PLPBP and PNPO suggested that PNP shares the same pool with PMP, and showed that PNP levels are impacted by PMP levels and vice versa. Here, we show that disruption of PLPBP perturbs PMP homeostasis, which may result in PNP accumulation in the PLPBP-deficient strains.
IMPORTANCE A PLP-binding protein (PLPBP) from the conserved COG0325 family has recently been recognized as a key player in vitamin B6 homeostasis in various organisms. Loss of PLPBP disrupts vitamin B6 homeostasis and perturbs diverse metabolisms, including amino acid and α-keto acid metabolism. Accumulation of PNP is a characteristic phenotype of PLPBP deficiency and is suggested to be a potential cause of the pleiotropic effects, but the mechanism of this accumulation has been poorly understood. In this study, we show that fluxes for PNP synthesis/metabolism are not responsible for the accumulation of PNP. Our results indicate that PLPBP is involved in the homeostasis of pyridoxamine 5′-phosphate, and that its disruption may lead to the accumulation of PNP in PLPBP deficiency.
KEYWORDS: pyridoxal 5'-phosphate, pyridoxine 5'-phosphate, pyridoxamine 5'-phosphate, vitamin B6, PLPBP, YggS
INTRODUCTION
Vitamin B6 is a generic term for six interconvertible vitamers: pyridoxal (PL), pyridoxine (PN), and pyridoxamine (PM); and their 5′-phosphorylated forms, pyridoxal 5′-phosphate (PLP), pyridoxine 5′-phosphate (PNP), and pyridoxamine 5′-phosphate (PMP) (Fig. 1). Among them, PLP serves as a cofactor for various enzymes associated with the metabolism of amino compounds, mostly amino acids (1, 2). PLP-dependent enzymes catalyze ubiquitous and versatile reactions, and ∼1.5% of all genes encode PLP-dependent enzymes in prokaryotes (3). PLP is essential for several enzymes that catalyze reactions in basic metabolism, but this molecule has an aldehyde group at C-4' which forms an undesired nonspecific adduct with amines and/or thiols, possibly causing toxicity (4). Intracellular PLP concentrations are tightly regulated by synthesis, catabolism, and/or (probably) transport by transcription and posttranscription mechanisms (4, 5). In mammals, disruption of PLP or B6 vitamer homeostasis causes severe neurological diseases, including vitamin B6 epilepsy. Several enzymes, including pyridoxine(amine) 5′-phosphate oxidase (PNPO), PL kinase (PLK), and PLP phosphatase (PLPP), have been identified as key players in B6 vitamin homeostasis (Fig. 1) (4–8).
FIG 1.
Biosynthesis and salvage pathways of vitamin B6. PLP is synthesized de novo through the DXP-dependent pathway (in E. coli and S. enterica) or the DXP-independent pathway (a representative organism is S. cerevisiae). PLP is also produced via the salvage pathway, which involves PLP phosphatase (PLPP), PL/PN/PM kinase (PLK), PNP/PMP oxidase (PNPO), and/or PL reductase (PLR). Known pathways are represented by solid arrows, and unknown and/or unidentified pathways are shown by broken arrows. Distribution of each enzyme in E. coli, S. enterica, S. cerevisiae, and humans is shown in boxes. Presence or absence of each enzyme is shown by colored or white boxes, respectively. Enzymes’ names in E. coli are shown in parentheses. Abbreviations: E, E. coli; S, S. enterica; Y, S. cerevisiae, H, H. sapiens; PL, pyridoxal; PN, pyridoxine; PM, pyridoxamine; PLP, pyridoxal 5′-phosphate; PNP, pyridoxine 5′-phosphate; PMP, pyridoxamine 5′-phosphate.
Recently, the PLPBP protein (COG0325), a PLP-binding protein highly conserved in various organisms from bacteria to mammals, has been recognized as a key player in vitamin B6 homeostasis (9–13). This protein exhibits a typical TIM-barrel structure and is structurally similar to bacterial alanine racemase and eukaryotic ornithine decarboxylase, which are representatives of fold-type III PLP-dependent enzymes (14, 15). No PLP-dependent enzymatic activities, including transamination, racemization, or decarboxylation of various d- and l-amino acids, have been described for PLPBP, and its biochemical function(s), except for the covalent binding of PLP via a Schiff base, remain to be elucidated (16). However, recent studies have revealed that this protein impacts the regulation of diverse metabolic pathways. In Escherichia coli, loss of the PLPBP protein (encoded by yggS) perturbs intracellular pools of amino acids, α-keto acids, coenzyme A, and vitamin B6, and is synthetically lethal with mutations in either glyA (encoding serine hydroxymethyltransferase) or serA (encoding D-3-phosphoglycerate dehydrogenase) (10–12, 16–19). In humans, mutations in the PLPBP gene (previously called PROSC) disrupt intracellular PLP homeostasis and cause vitamin B6-dependent epilepsy, with predicted dysfunction of several PLP-dependent enzymes, including aromatic l-amino acid decarboxylase and glycine cleavage enzyme (GCV) (9, 13, 20–22). A plpbp-deficient zebrafish exhibited changes in B6 vitamer levels, signs of impaired PLP-dependent enzyme activity, and epileptic phenotypes that could be suppressed by PN (13). A PLPBP-deficient cyanobacterium (pipY mutant) caused sensitivity to β-Cl-d-alanine and d-cycloserine, which are antibiotics targeting PLP-dependent enzymes (23). In Salmonella enterica, plpbp (yggS) deletion affected intracellular and extracellular levels of B6 vitamers, including accumulation of endogenous PNP in the cell and excretion of PLP in the growth medium (24).
Accumulation of intracellular PNP is a characteristic phenotype of PLPBP deficiency. Loss of PLPBP induces intracellular accumulation of PNP, which is normally undetectable in wild-type cells of various organisms, including E. coli, S. enterica, and humans (HEK293 cells) (10–13, 24). In E. coli, high levels of PNP are proposed to be the root cause of several phenotypes observed in the yggS-deficient strain, including disrupted intracellular pools of amino acids and α-keto acids, PN sensitivity, and synthetic lethality with a glyA or serA mutation (11, 12). PNP competes with PLP to inhibit the glycine cleavage (GCV) system, perturbs Thr/Ile/Val metabolism, and induces the accumulation of toxic metabolite Val under certain conditions (11, 12). Similar PNP-dependent inhibition of some PLP-dependent enzymes might occur in other organisms, since the intracellular concentration of PNP is comparable to that of PLP in organisms with PLPBP deficiency (10–13, 24). At present, there is little information to infer a mechanistic link between a yggS mutation and the disruption of vitamin B6 homeostasis with PNP accumulation. The purified E. coli PLPBP protein showed no reactivity to PNP (10). Furthermore, in E. coli deletion of yggS did not affect the expression level of pyridoxine (amine) 5′-phosphate oxidase (PNPO) (encoded by pdxH), the only known enzyme capable of metabolizing PNP (10). Consistently, our recent study with a yggS-deficient S. enterica strain, which expressed a heterologous PLP synthesis pathway and could not synthesize PNP, suggested that PNP accumulation is a downstream consequence rather than a direct effect of yggS deletion (24).
In this study, we investigated the mechanistic details of PNP accumulation, which are relevant to understanding the molecular function of PLPBP. We analyzed the effect of PLPBP deletion on the vitamin B6 pool using several E. coli strains which lacked some of the enzymes involved in vitamin B6 biosynthesis or metabolism, including PNPO, PNP synthase, pyridoxal kinase, and pyridoxal reductase; in addition, we showed that fluxes for PNP synthesis/metabolism are not responsible for the accumulation of PNP. This study provides evidence that PLPBP plays an essential role in the homeostasis of pyridoxamine 5′-phosphate (PMP), and that the disruption of PMP homeostasis is linked to PNP accumulation in PLPBP deficiency.
RESULTS
PNP accumulation in the plpbp mutant is independent of B6 synthesis or salvage.
Previous studies have shown that the accumulation of PNP is independent of its biosynthetic pathway and origin, as the loss of PLPBP induces the accumulation of PNP in E. coli, S. enterica, and humans (HEK293 cells) (10–13, 24). These organisms differ in their biosynthetic and metabolic pathways for vitamin B6. Both E. coli and S. enterica biosynthesize PLP by the deoxyxylulose 5-phosphate (DXP)-dependent pathway (25), whereas HEK293 cells take up exogenous vitamin B6 and convert it to PLP (Fig. 1). In addition, we found that a PLPBP-deficient Saccharomyces cerevisiae strain (YBL036C mutant) also accumulates detectable levels of PNP (Fig. 2, wild type: not detected, plpbp; 1.7 pmol/mg cells, P < 0.001). In S. cerevisiae, PLP is synthesized via a DXP-independent pathway by the action of a PLP synthase composed of Pdx1/Pdx2 (26, Fig. 1). An in vitro assay suggested that purified PLPBP from E. coli does not bind PNP (10). Similarly, we did not detect any phosphatase or oxidase activity against PNP with purified E. coli PLPBP (data not shown). PLPBP might have a regulatory role for one or more enzymes in the vitamin B6 metabolic pathway (salvage pathway) and whose disruption would be linked to PNP accumulation in the cells.
FIG 2.
Intracellular B6 pools of the wild-type and plpbp mutant S. cerevisiae. The wild-type (AE1103) and plpbp (YBL036C) mutant (AE1104) of S. cerevisiae BY4742 strains were grown in an SC medium containing uracil, in the absence or presence of 50 μM PM. Intracellular vitamin B6 profiles were analyzed by HPLC as described in Materials and Methods. Error bars depict standard deviation from the mean from three biological replicates. * denotes P < 0.05, ** denotes P < 0.01, and *** denotes P < 0.001 (determined by Student’s t test).
In E. coli, PNP is synthesized by the action of PNP synthase (PdxJ) and then oxidized to PLP by PNPO (encoded by pdxH) (25, 27, 28). PNP is also produced from PLP via a salvage pathway involving PLP phosphatase (PLPP, YbhA) (29), PL reductase (PLR, PdxI) (30), and PL kinase (PLK, PdxK) (31, 32) (Fig. 1). No enzyme responsible for the dephosphorylation of PNP has been identified. PNP is also formed from an exogenous B6 vitamer(s). E. coli is capable of taking up all three nonphosphorylated forms of B6 vitamers, but the mechanism of B6 transport in E. coli is not clear. E. coli lacks homologs of the PLP transporter recently reported in Actinobacillus (33) and of other B6 transporters described in eukaryotes (34).
To investigate the possibility that PLPBP affects the flux of the known pathway for vitamin B6, the PLPBP gene (yggS) was deleted from the background of pdxJ, pdxI, or pdxK strains, which were blocked in either the de novo pathway or the salvage pathway for PNP synthesis. Deletion of yggS in the pdxJ, pdxI, or pdxK backgrounds did not significantly affect the growth rate when strains were grown in M9-glucose medium (1 μM PL was supplemented for the pdxJ mutants) (Fig. 3A to D). These strains were grown to log phase, and endogenous levels of B6 vitamers were quantified by high-pressure liquid chromatography (HPLC). The HPLC data showed that the loss of PLPBP in any background disrupted the intracellular pool of B6 vitamers and resulted in the accumulation of PNP (Fig. 3E to H). Note that PNP accumulated in pdxJ yggS is expected to be of exogenous origin, while that in pdxI yggS and pdxK yggS is of endogenous origin. In total, these results indicate that differences in flux from either the de novo pathway or the salvage pathway are not solely responsible for PNP accumulation in carriers of the yggS mutation.
FIG 3.
Effect of yggS mutation under the pdxJ, pdxI, or pdxK background on the growth and B6 pool. Panels A through D show the growth of pdxJ (DM16637), pdxI (DM16795), or pdxK (DM16634) and their yggS mutants (pdxJ yggS [DM16605], pdxI yggS [DM16796], or pdxK yggS [DM16683]). Strains were grown in an M9-glucose medium. The pdxJ and pdxJ plpbp strains were cultivated in the presence of 1 μM PL. Panels E through H represent intracellular vitamin B6 profiles. Growth and HPLC data were obtained from three biological replicates as described in Materials and Methods. Error bars depict standard deviation from the mean. * denotes P < 0.05, ** denotes P < 0.01, and *** denotes P < 0.001 (determined by Student’s t test); ns, not significant.
pdxH yggS double mutant exhibits synthetic growth defect.
PNPO (encoded by pdxH) is the only known enzyme that is capable of metabolizing PNP in E. coli. To examine whether PLPBP affects the functionality of PNPO, a double mutant of E. coli with lesions in pdxH and yggS was constructed (pdxH yggS strain). Both of the pdxH and the pdxH yggS mutants grew in LB medium supplemented with 1 μM PL (data not shown). In contrast, the pdxH yggS double mutant failed to grow in an M9-glucose medium containing 1 μM PL (Fig. 4A). The impaired growth of the double mutant was completely restored when yggS was expressed in trans (Fig. 4A), confirming that this phenotype is caused by the absence of PLPBP. The phenotype in the absence of PNPO indicated that PLPBP has some PNPO-independent function.
FIG 4.
yggS mutation under the pdxH background increases PLP/(PL) requirement. The pdxH (DM16698), pdxH yggS (DM16735), and pdxH yggS harboring pUS plasmid (AE229) strains were grown in an M9-glucose medium containing (A) 1 μM PL or (B) 1 μM PL and 0.4% Casamino Acids. (C) Growth of the pdxH and pdxH yggS strains both harboring pBAD-pdxST plasmid (AE238 and AE239) in an M9-glucose medium supplemented 0.2% arabinose. (D) Growths of the pdxH yggS strain in an M9-glucose medium supplemented with various concentrations of exogenous PL (0, 1, 5, 10, or 50 μM PL). (E) Intracellular vitamin B6 pools of pdxH (DM16698) and pdxH yggS (DM16735) strains grown in an M9-glucose medium supplemented with 1 μM and 0.4% Casamino Acids. Growth and HPLC data were obtained from three biological replicates as described in Materials and Methods. Error bars depict standard deviation from the mean. *** denotes P < 0.001 (determined by Student’s t test); ns, not significant.
The conditional lethality of the pdxH yggS double mutant was unexpected since the mutant of S. enterica strain with the same genotype grew well in both a rich medium and a minimal synthetic medium supplemented with PL (24). Thus, we further investigated the reason for the lethality. Growth assays found that the growth of the double mutant was significantly restored with the addition of Casamino Acids (Fig. 4B). In a screening experiment with the genomic library of B. subtilis strain 168, we identified that a plasmid which contained a fragment encoding PLP synthase (encoded by pdxS and pdxT) allowed the growth of pdxH yggS mutant on M9-glucose medium containing 1 μM PL (data not shown). PLP synthase produces PLP in a single-step reaction from glyceraldehyde 3-phosphate, ribose 5-phosphate, and glutamine (35). Consistent with this result, a plasmid that encodes full-length PLP synthase (PdxS-PdxT) under the control of arabinose-inducible promoter restored the growth of the pdxH yggS mutant (Fig. 4C). In addition, the growth defect of the pdxH yggS mutant was also rescued with increased levels of exogenous PL (in the absence of Casamino Acids). When 50 μM PL was supplemented to the medium, the pdxH yggS mutant reached an optical density similar to that of the pdxH mutant grown with 1 μM PL (Fig. 4D). Together, these observations showed that loss of PLPBP increases the PLP(PL) requirement under a background of pdxH, and that this requirement is negated by Casamino Acids.
High levels of PNP cause lethality in the pdxH yggS double mutant.
To investigate the reason for the increased PLP(PL) requirement of the pdxH yggS mutant, we analyzed the intracellular B6 pools of pdxH and pdxH yggS strains grown in the presence of 1 μM PL and 0.4% Casamino Acids. The pdxH yggS strain had a pattern of B6 vitamers that was distinct from the parental strain, with elevated levels of PNP and decreased levels of PMP (pdxH yggS versus pdxH: PLP, 24 versus 32 pmol/mg cells [P = 0.1]; PNP, 45 versus 10 pmol/mg cells [P < 0.001] PMP, 17 versus 49 pmol/mg cells [P < 0.001]) (Fig. 4E). These HPLC data indicated that PLPBP has a PNPO-independent function that impacts PNP levels.
We have shown that PNP causes toxicity by inhibiting a certain PLP-dependent enzyme(s), such as the glycine cleavage system, and/or by perturbing amino acid, keto acid, and purine metabolisms in E. coli (11, 12). It was thus possible that the altered B6 pool, high PNP levels, and/or low PMP levels are responsible for the increased PLP(PL) requirement and growth defect of the pdxH yggS mutant.
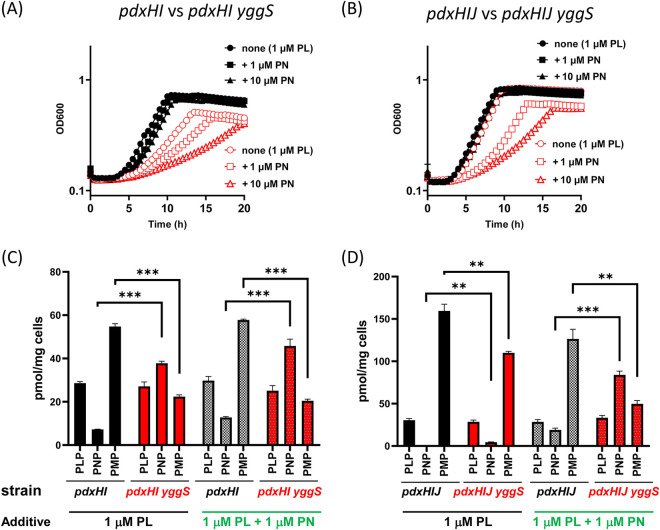
In the pdxH and pdxH yggS mutants, PNP is synthesized from two pathways: the de novo and the salvage pathways. E. coli possesses PL reductase, encoded by pdxI (which is absent in S. enterica), for the efficient conversion of exogenous PL to PN during growth (30) (Fig. 1). Two yggS mutants were constructed under the pdxH pdxI (pdxHI) and pdxH pdxI pdxJ (pdxHIJ) backgrounds to block the flux of PNP synthesis from the salvage pathway and from both pathways, respectively. Growth assay found that both of the yggS mutants grew well in an M9-glucose medium supplemented with 1 μM PL (without Casamino Acids), and the growth of the pdxHIJ yggS strain was indistinguishable from that of the parental strain (pdxHIJ) (Fig. 5A and B). This result, in combination with the fact that the S. enterica pdxH yggS strain originally lacking PdxI does not exhibit lethality, indicates that the formation of PNP from the flux via PdxI is responsible for the higher PLP(PL) requirement and growth defect of the E. coli pdxH yggS mutant.
FIG 5.
Formation of PNP causes growth defect and perturbs intracellular B6 pools in pdxHI yggS and pdxHIJ yggS mutants. (A, B) Disruption of pdxI restored growth defect of pdxH yggS. The pdxHI (black symbols) and pdxHI yggS (red symbols) strains (A) or pdxHIJ (black symbols) and pdxHIJ yggS strains (red symbols) (B) were grown in an M9-glucose medium supplemented with 1 μM PL and in the absence or presence of PN (1, 10 μM). (C, D) Intracellular vitamin B6 profiles of pdxHI and pdxHI yggS (C) or pdxHIJ and pdxHIJ yggS (D) strains cultured in M9-glucose medium containing 1 μM PL. PN was added at 1 μM. Growth and HPLC data were obtained from three biological replicates as described in Materials and Methods. Error bars depict standard deviation from the mean. ** denotes P < 0.01 and *** denotes P < 0.001 (determined by Student’s t test).
PNP-dependent limitation of PMP causes growth defect in the pdxH yggS mutant.
Growth assays found that the two yggS mutants (pdxHI yggS and pdxHIJ yggS) were sensitive to exogenous PN (Fig. 5A and B). In these strains, PdxK is the only enzyme responsible for PNP metabolism, but this enzyme has been shown to have no critical effect on PNP accumulation in the yggS mutant (Fig. 3H). This PN-sensitivity phenotype in the absence of PdxI indicated that the yggS mutants may have some differences in the dynamics following PNP synthesis rather than in the PNP synthesis process. To address this possibility, intracellular PNP concentrations of the four strains (pdxHI, pdxHI yggS, pdxHIJ, and pdxHIJ yggS) grown in the presence or absence of PN were determined. In the absence of PN, the pdxHI yggS mutant had significantly elevated levels of PNP and low levels of PMP compared to the parental strain and, interestingly, this B6 pool’s profile is similar to that observed in the pdxH yggS strain (see Fig. 5C and Fig. 4E). Exogenous PN elevated intracellular PNP concentration in the two strains, but it did not critically affect the B6 pool profile. In contrast, neither the pdxHIJ nor the pdxHIJ yggS strain accumulated PNP when grown in synthetic medium supplemented with PL, as expected (Fig. 5D). No significant difference was observed for the intracellular PLP concentrations in the two strains, but the PMP levels in the pdxHIJ yggS strain were approximately 70% of the parental strain (110 versus 159 pmol/mg cells, P = 0.006). In the presence of exogenous PN, a remarkable change in the B6 pool was observed in the pdxHIJ yggS strain, where the addition of PN significantly increased the PNP level and led to a decrease in the PMP level (Fig. 5D); this B6 pool profile was similar to that observed in the pdxH yggS and pdxHI yggS strains (Fig. 4E and 5C). In total, these results demonstrate that the loss of PLPBP reduces a strain’s ability to maintain PNP at low concentrations. The inverse correlation between PNP and PMP levels suggests that they share a common location. The accumulation of PNP leads to decreased PMP levels, probably by pushing away PMP from the pool they share.
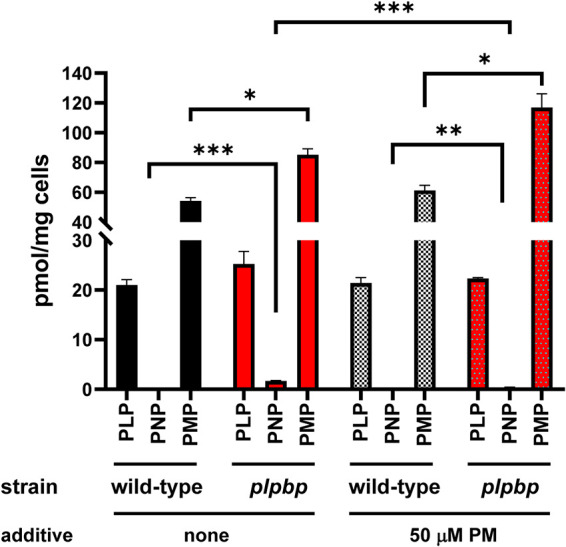
PMP is used as a cofactor for aminotransferases to donate an amino group to some keto acids, including α-ketoglutarate and pyruvate (1, 2). The decrease in PMP levels may cause growth defects in the pdxH yggS mutant (and the pdxHI yggS and pdxIJH yggS mutants) by disrupting amino acid and keto acid metabolism. The Casamino Acids requirement for the growth of the pdxH yggS mutant (Fig. 4B) may indicate that limitation of PMP in the reaction of aminotransferases is the cause of the growth defect, and that when amino acids are provided, they reduce the amount of PMP required. To examine this possibility, the growth of pdxH and pdxH yggS mutants was assessed in an M9-glucose medium supplemented with 10 μM pyridoxamine (PM). Interestingly, exogenous PM significantly restored the growth of the pdxH yggS mutant (Fig. 6A). These observations supported our hypothesis that PNP-dependent limitation of PMP causes the growth defect in the pdxH yggS mutant.
FIG 6.
Exogenous PM increases PMP concentration and eliminates growth defects and PNP accumulation in the pdxH yggS mutant. (A and B) Growth (A) and intracellular B6 vitamer profile (B) of pdxH (DM16698) and pdxH yggS (DM16735) grown in an M9-glucose medium supplemented with 10 μM PM. (C) Representative chromatograms obtained by HPLC analyses of B6 pools of wild-type (JE6653) and yggS (DM16862) strains grown in an M9-glucose medium in the absence or presence of 10 μM PM. (D) Comparison of the intracellular B6 vitamers in the yggS mutant (DM16862) grown in the absence or presence of 10 μM PM. Growth and HPLC data were obtained from three biological replicates as described in Materials and Methods. Error bars depict standard deviation from the mean. * denotes P < 0.05, and *** denotes P < 0.001 (determined by Student’s t test).
PLPBP functions in PMP homeostasis, the disruption of which may link to PNP accumulation.
To assess the effect of PM on intracellular B6 pools, we measured the B6 pools of pdxH and pdxH yggS mutants cultured in the presence of PM. High levels of intracellular PMP were detected in both the pdxH and pdxH yggS mutants (Fig. 6B), and the concentrations were ∼20-fold higher than those of mutants grown in the absence of PM (Fig. 4E, grown in the M9 medium containing PL and Casamino Acids). The recovery of growth with increasing PMP levels supports the conclusion that the growth defect in the pdxH yggS mutant is linked to decreased PMP levels. Interestingly, the PNP level in the pdxH yggS mutant was decreased to a level similar to that of the pdxH mutant (Fig. 6B). The bidirectional interaction between PNP and PMP pools in the pdxH yggS mutant confirmed that the two vitamers share the same pools and raised the possibility that the disruption of PMP homeostasis leads to PNP accumulation.
To assess the latter possibility, wild-type and yggS single-mutant strains were grown in the absence or presence of exogenous PM (50 μM), and their intracellular B6 pools were analyzed. The addition of PM did not affect the growth of either the wild-type or the yggS strains (data not shown). Although exogenous PM did not affect the intracellular pool of B6 vitamers in the wild-type strain, it markedly increased PMP concentration in the yggS mutant, with an approximately 10-fold increase in intracellular PMP compared to the wild type (Fig. 6C and D; PMP, 815 versus 98 pmol/mg cells, P < 0.001, yggS versus wild type). Importantly, the intracellular PNP concentration of the yggS mutant was decreased by ∼30% in the presence of exogenous PM (+PM, 25 pmol/mg cells versus w/o PM, 18 pmol/mg cells; P = 0.01). These data demonstrated that a change in PMP levels can affect PNP levels. It should be noted that exogenous PM also significantly decreased (∼80%) intracellular PNP levels in the PLPBP mutant of S. cerevisiae (+PM, 0.3 pmol/mg cells versus w/o PM, 1.7 pmol/mg cells; P < 0.001) (Fig. 2). The known metabolic pathway of PMP is conversion to PLP by transaminases and PNPO (Fig. 1). The fact that the pdxH yggS mutant accumulates more PMP compared to pdxH (Fig. 6B) may suggest that PLPBP plays a role in the transamination reaction. In total, these observations demonstrate that PLPBP has a critical role in PMP homeostasis, and suggest that disruption of PMP homeostasis may contribute to PNP accumulation in the PLPBP mutant.
DISCUSSION
A series of studies that analyzed the intracellular pool of B6 vitamers in PLPBP-deficient strains of E. coli, S. enterica, S. cerevisiae, zebrafish, and humans has revealed a conserved role of this protein in the homeostasis of the intracellular vitamin B6 pool (9–13, this study). Loss of PLPBP induces PNP accumulation in various organisms via unknown mechanisms, which leads to diverse metabolic perturbations (11, 12).
Our data on various E. coli strains lacking PLPBP clearly show that the known pathways and/or fluxes for PNP synthesis/metabolism (shown as green arrows in Fig. 7) do not critically impact PNP accumulation. The inverse correlation between PMP and PNP levels observed in the strains lacking PLPBP and PNPO indicates that these two vitamers exist in the same place. This location is probably found in some PLP-dependent enzymes, such as transaminases. Since PMP, unlike PLP, is not covalently attached to proteins and binds weakly to enzymes, PNP could easily enter the active site on a transaminase and expel PMP. It is also possible that PNP binds to PLP-dependent enzymes other than transaminases. Some E. coli PLP-enzymes, such as glycine decarboxylase, tryptophan synthase, and glutamate decarboxylase, have been shown to bind PNP more strongly than PMP (12, 36, 37). PNP in proteins is protected from further metabolisms, such as dephosphorylation. It is therefore possible that the accumulated PNP is found in proteins such as PLP-dependent enzymes.
FIG 7.
Proposed mechanism of PNP accumulation in the plpbp mutants. PNP is produced from the DXP-dependent pathway and/or the salvage pathway (green lines). PNP enters the active site of some PLP-dependent enzymes, including a transaminase(s) (red line). Unlike PLP, PMP is not covalently attached to proteins and is weakly bound to enzymes, so it can be expelled from the PLP-dependent enzymes by PNP. PNP in proteins is protected from metabolisms such as dephosphorylation. In the presence of PNPO (PdxH), free PMP is oxidized to PLP and replenished in the active center of PLP-dependent enzymes, eliminating PNP during these processes. In the absence of PNPO, this cycle does not occur, and free PMP undergoes dephosphorylation, resulting in a decrease in PMP. PLPBP may be involved in the supply of PLP and/or PMP to PLP-dependent enzymes, either through a direct or indirect mechanism (blue or orange boxed arrows). The accumulation of PNP in yggS-deficient strains may be due to a decrease in the efficiency of PLP and/or PMP production/regeneration, which prevents PNP from being expelled from the proteins.
A putative mechanism of PNP accumulation in the plpbp mutant is proposed in Fig. 7. We propose that PNP accumulation in an PLPBP-deficient mutant may be due to decreased efficiency of PLP and/or PMP production/regeneration, and/or reduced supply of PLP and/or PMP to PLP-dependent enzymes, which prevents PNP from being expelled from these proteins (blue and orange boxed arrows in Fig. 7). The higher PMP accumulation in the PLPBP mutant in the presence of PM (Fig. 6B and C) suggests a decreased efficiency of PMP-to-PLP conversion in the PLPBP mutant. A role of PLPBP in the cycling between PLP and PMP, as well as in α-keto acid metabolism, was also suggested in a recent study with S. enterica (24, 38).
It is currently unclear how PLPBP modulates the production/regeneration of PLP and/or PMP. If PLPBP is directly involved in this process, it may be acting as an enzyme, catalyzing the transamination of PLP with certain amino donors to produce the corresponding keto acid and PMP, and vice versa (Fig. 7, blue boxed arrow). We have examined whether E. coli PLPBP catalyzes transamination with proteinogenic amino acids by UV-vis spectra analyses under a single turnover condition (in the absence of keto acid), but no transaminase activity was detected under the condition examined (16). In addition, no transamination activity was observed with PMP and pyruvate, oxaloacetate, and/or ketoglutarate using apo-PLPBP from E. coli. PLPBP has solvent-exposed PLP and the putative active site structure is completely different from known PLP-dependent transaminases, which belong to the fold-type I or fold-type IV PLP-dependent enzyme family (14, 16). If PLPBP catalyzes transamination, there should be some unidentified mechanism and amino donors for this process. It is also possible that PLPBP is indirectly involved in the PLP/PMP recycling process; for example, by regulating some PLP-dependent transaminase(s). A recent biochemical study on human PLPBP, using chemical cross-linking paired with coimmunoprecipitation, did not observe an overrepresentation of PLP-enzymes and other enzymes in the salvage pathway among the PLPBP interactors (44). Therefore, it is also possible that PLPBP indirectly controls one or more PLP-binding enzymes, for example, by regulating PLP levels in enzymes (Fig. 7, orange boxed arrow).
In conclusion, this study shows that neither the flux from the de novo pathway nor the salvage pathway solely contributed to PNP accumulation caused by the PLPBP mutation. Our data suggest that PLPBP plays role in PMP homeostasis, the disruption of which may link to PNP accumulation. Although the molecular basis of PLPBP-mediated PMP homeostasis remains to be investigated, this study provides important insights into the molecular function of PLPBP proteins.
MATERIALS AND METHODS
Strains, media, and chemicals.
Plasmids and strains used in this study are listed in Table 1. All E. coli strains were derivatives of E. coli BW25113 (JE6653) (Table 1) and were grown either in an LB medium or in an M9 medium containing 0.2% glucose at 37°C and 160 rpm (16). When required, Casamino Acids (0.4%), antibiotics (ampicillin [100 μg/mL in LB, 20 μg/mL in M9], kanamycin [50 μg/mL in LB, 10 μg/mL in M9], chloramphenicol [30 μg/mL in LB, 10 μg/mL in M9]), and/or B6 vitamer (PL, PN, PM; 0.1 to 50 μM) were supplemented to the medium. For solid media, agar was added to a final concentration of 1.5% (wt/vol). S. cerevisiae BY4742, and the PLPBP (YBL036C)-deficient strains were grown in SC medium at 30°C with shaking. Q5 DNA polymerase and restriction enzymes were purchased from New England BioLabs. B6 vitamers were obtained from Tokyo Kasei. Synthetic oligonucleotides were purchased from Fasmac.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| Saccharomyces cerevisiae | ||
| AE1103 | BY4742 wild type | Laboratory collection |
| AE1104 | PLPBP(YBL036C)::Gen | Laboratory collection |
| E. coli | ||
| JE6653 (WT) | BW25113 wild type | Laboratory collection |
| AE1169 (WT/pBAD) | Wild type/pBAD-MycHisA (empty vector) | This study |
| AE1227 (WT/pdxST+) | Wild type/pBAD-pdxST | This study |
| DM16862 (yggS) | yggS::Km | 16 |
| DM16795 (pdxI) | pdxI::Cm | 30 |
| DM16796 (pdxI yggS) | pdxI::Cm yggS::Km | This study |
| DM16698 (pdxH) | pdxH | 30 |
| AE238 (pdxH/pdxST+) | pdxH/pBAD-pdxST | This study |
| DM16735 (pdxH plpbp) | pdxH yggS::Km | This study |
| AE229 (pdxH yggS/yggS+) | pdxH yggS::Km/pUS | This study |
| AE239 (pdxH yggS/pdxST+) | pdxH yggS/pBAD-pdxST | This study |
| DM16037 (pdxJ) | pdxJ | 30 |
| DM16665 (pdxJ yggS) | pdxJ yggS::Km | This study |
| DM16029 | pdxK::Km (JW2548-KC) | NBRP |
| DM16634 (pdxK) | pdxK | This study |
| DM16683 (pdxK yggS) | pdxK yggS::Km | This study |
| AE204 (pdxHI) | pdxH pdxI | This study |
| AE206 (pdxHI yggS) | pdxH pdxI yggS | This study |
| AE213 (pdxHIJ) | pdxH pdxI pdxJ::Km | This study |
| AE215 (pdxHIJ yggS) | pdxH pdxI pdxJ::Km yggS | This study |
| Plasmids | ||
| pAE248 (pBAD-pdxST) | pBAD-MycHisA expressing B. subtilis pdxS-pdxT | This study |
| pUS | pUC19 expressing E. coli yggS | 43 |
| pU0 | Control vector for pUS | 43 |
Construction of plasmids and strains.
Plasmid and strain constructions are listed in Table 1. Plasmid pBAD-pdxST was constructed by cloning pdxS-pdxT from B. subtilis at the NcoI and XhoI sites of pBAD/mycHisA plasmid (Invitrogen) using a restriction enzyme-independent cloning method (39). Primers used for the amplification of the pdxS-pdxT genes were as follows: pdxS-fw, 5′-AATAGGAGGAATTAACCATGGCTCAAACAGGTACTGAACG-3′ and pdxT-rv, 5′-TACCAGCTGCAGATCTCGAGCATATACAAGTGCCTTTTGCTTATATTCCTC-3′. The sequence of the inserted gene was confirmed using a BigDye Terminator Cycle Sequencing Kit v. 3.1 (Applied Biosystems).
The initial in-frame deletions of yggS, pdxH, or pdxI were introduced into a wild-type BW25113 strain as described previously (40) and then moved to the E. coli strains with relevant background by P1 transduction (41). The E. coli pdxJ::Km and pdxK::Km mutants (Keio collection) were obtained from the National BioResource Project (NIG, Japan) (40). When required, antibiotic markers were eliminated using pCP20 plasmid (42). Gene deletions were confirmed by colony PCR with primers annealing to the upstream/downstream regions flanking the target gene deletion locus.
Growth assay.
E. coli strains were precultured in 3 mL LB at 37°C overnight. Cells were washed twice with a phosphate buffer (0.6% Na2HPO4, 0.3% KH2PO4, 0.1% NH4Cl, 0.05% NaCl [pH 7.0]) before inoculation into an M9-glucose medium. Growth was monitored in a 96-well plate or in a glass test tube (16.5 mm in diameter by 165 mm in height), by measuring the optical density at 600 nm (OD650) using an Epoch2 plate reader (BioTek) or an OD-Monitor C&T apparatus (Taitec).
B6 vitamer pool analysis.
Cells were harvested at the log phase (OD650 = 0.4 to 0.8) and subjected to intracellular B6 pool analysis as described previously, with slight modification (11, 12). Briefly, cell pellets were treated with 5 volumes (vol/wt) of 0.8 M HClO4 (50 μL for 10 mg cells), resuspended, and incubated on ice for 15 min with occasional agitation. After the addition of 2.5 volumes of chilled 0.8 M K2CO3, the mixture was centrifuged, and the supernatant was applied to a 150 × 4.6 mm COSMOSIL PBr column (3-μm particle size, Nacalai Tesque). Mobile phase A (33 mM phosphoric acid and 8 mM 1-octanesulfonic acid [pH 2.2]) and mobile phase B (80% [vol/vol] acetonitrile) were used for the separation of the B6 vitamers with the following gradient program (time, % A/% B): 0 min, 100/0; 2 min, 98.5/1.5; 7 min, 85/15; 17 min, 75/25; 19 min, 37/63; and column equilibration with 100% A for 13 min. The total flow rate was 1 mL/min. A volume of 1 M potassium phosphate buffer containing 0.1% sodium bisulfite (pH 7.5, 0.3 mL/min) was used to enhance the fluorescence of PLP. The column was maintained at 28°C. Excitation and emission wavelengths were 328 nm and 393 nm, respectively.
Data availability.
The data are available on reasonable request from the corresponding author.
ACKNOWLEDGMENTS
This work was supported by the JSPS KAKENHI (17KK0153), the Asahi Glass Foundation, and the Institution for Fermentation, Osaka (IFO) to T.I.
We state that we have no conflicts of interest in presenting this work.
Contributor Information
Tomokazu Ito, Email: ito-t@agr.nagoya-u.ac.jp.
Michael Y. Galperin, NCBI, NLM, National Institutes of Health
REFERENCES
- 1.Eliot AC, Kirsch JF. 2004. Pyridoxal phosphate enzymes: mechanistic, structural, and evolutionary considerations. Annu Rev Biochem 73:383–415. 10.1146/annurev.biochem.73.011303.074021. [DOI] [PubMed] [Google Scholar]
- 2.Toney MD. 2011. Controlling reaction specificity in pyridoxal phosphate enzymes. Biochim Biophys Acta 1814:1407–1418. 10.1016/j.bbapap.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Percudani R, Peracchi A. 2003. A genomic overview of pyridoxal-phosphate-dependent enzymes. EMBO Rep 4:850–854. 10.1038/sj.embor.embor914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson MP, Plecko B, Mills PB, Clayton PT. 2019. Disorders affecting vitamin B6 metabolism. J Inherit Metab Dis 42:629–646. 10.1002/jimd.12060. [DOI] [PubMed] [Google Scholar]
- 5.di Salvo ML, Contestabile R, Safo MK. 2011. Vitamin B(6) salvage enzymes: mechanism, structure and regulation. Biochim Biophys Acta 1814: 1597-608. 10.1016/j.bbapap.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Mills PB, Surtees RA, Champion MP, Beesley C, Dalton N, Scambler PJ, Heales SJ, Briddon A, Scheimberg I, Hoffmann GF, Zschocke J, Clayton PT. 2005. Neonatal epileptic encephalopathy caused by mutations in the PNPO gene encoding pyridox(am)ine 5′-phosphate oxidase. Hum Mol Genet 14:1077-86. 10.1093/hmg/ddi120. [DOI] [PubMed] [Google Scholar]
- 7.Mills PB, Struys E, Jakobs C, Plecko B, Baxter P, Baumgartner M, Willemsen MA, Omran H, Tacke U, Uhlenberg B, Weschke B, Clayton PT, Mutations in antiquitin in individuals with pyridoxine-dependent seizures. 2006. Mutations in antiquitin in individuals with pyridoxine-dependent seizures. Nat Med 12:307–309. 10.1038/nm1366. [DOI] [PubMed] [Google Scholar]
- 8.Narisawa S, Wennberg C, Millan JL. 2001. Abnormal vitamin B6 metabolism in alkaline phosphatase knock-out mice causes multiple abnormalities, but not the impaired bone mineralization. J Pathol 193:125–133. . [DOI] [PubMed] [Google Scholar]
- 9.Darin N, Reid E, Prunetti L, Samuelsson L, Husain RA, Wilson M, El Yacoubi B, Footitt E, Chong WK, Wilson LC, Prunty H, Pope S, Heales S, Lascelles K, Champion M, Wassmer E, Veggiotti P, de Crécy-Lagard V, Mills PB, Clayton PT. 2016. Mutations in PROSC disrupt cellular pyridoxal phosphate homeostasis and cause vitamin-B6-dependent epilepsy. Am J Hum Genet 99:1325–1337. 10.1016/j.ajhg.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prunetti L, El Yacoubi B, Schiavon CR, Kirkpatrick E, Huang L, Bailly M, El Badawi-Sidhu M, Harrison K, Gregory JF, Fiehn O, Hanson AD, de Crécy-Lagard V. 2016. Evidence that COG0325 proteins are involved in PLP homeostasis. Microbiology (Reading) 162:694–706. 10.1099/mic.0.000255. [DOI] [PubMed] [Google Scholar]
- 11.Ito T, Yamamoto K, Hori R, Yamauchi A, Downs DM, Hemmi H, Yoshimura T. 2019. Conserved pyridoxal 5′-phosphate-binding protein YggS impacts amino acid metabolism through pyridoxine 5′-phosphate in Escherichia coli. Appl Environ Microbiol 85:e00430-19. 10.1128/AEM.00430-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito T, Hori R, Hemmi H, Downs DM, Yoshimura T. 2020. Inhibition of glycine cleavage system by pyridoxine 5′-phosphate causes synthetic lethality in glyA yggS and serA yggS in Escherichia coli. Mol Microbiol 113:270–284. 10.1111/mmi.14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnstone DL, Al-Shekaili HH, Tarailo-Graovac M, Wolf NI, Ivy AS, Demarest S, Roussel Y, Ciapaite J, van Roermund CWT, Kernohan KD, Kosuta C, Ban K, Ito Y, McBride S, Al-Thihli K, Abdelrahim RA, Koul R, Al Futaisi A, Haaxma CA, Olson H, Sigurdardottir LY, Arnold GL, Gerkes EH, Boon M, Heiner-Fokkema MR, Noble S, Bosma M, Jans J, Koolen DA, Kamsteeg E-J, Drögemöller B, Ross CJ, Majewski J, Cho MT, Begtrup A, Wasserman WW, Bui T, Brimble E, Violante S, Houten SM, Wevers RA, van Faassen M, Kema IP, Lepage N, Lines MA, Dyment DA, Wanders RJA, Verhoeven-Duif N, Ekker M, Boycott KM, et al. Care4Rare Canada Consortium. 2019. PLPHP deficiency: clinical, genetic, biochemical, and mechanistic insights. Brain 142:542–559. 10.1093/brain/awy346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eswaramoorthy S, Gerchman S, Graziano V, Kycia H, Studier FW, Swaminathan S. 2003. Structure of a yeast hypothetical protein selected by a structural genomics approach. Acta Crystallogr D Biol Crystallogr 59:127–135. 10.1107/s0907444902018012. [DOI] [PubMed] [Google Scholar]
- 15.Tremiño L, Forcada-Nadal A, Contreras A, Rubio V. 2017. Studies on cyanobacterial protein PipY shed light on structure, potential functions, and vitamin B6-dependent epilepsy. FEBS Lett 591:3431–3442. 10.1002/1873-3468.12841. [DOI] [PubMed] [Google Scholar]
- 16.Ito T, Iimori J, Takayama S, Moriyama A, Yamauchi A, Hemmi H, Yoshimura T. 2013. Conserved pyridoxal protein that regulates Ile and Val metabolism. J Bacteriol 195:5439–5449. 10.1128/JB.00593-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito T, Yamauchi A, Hemmi H, Yoshimura T. 2016. Ophthalmic acid accumulation in an Escherichia coli mutant lacking the conserved pyridoxal 5′-phosphate-binding protein YggS. J Biosci Bioeng 122:689–693. 10.1016/j.jbiosc.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Nichols RJ, Sen S, Choo YJ, Beltrao P, Zietek M, Chaba R, Lee S, Kazmierczak KM, Lee KJ, Wong A, Shales M, Lovett S, Winkler ME, Krogan NJ, Typas A, Gross CA. 2011. Phenotypic landscape of a bacterial cell. Cell 144:143–156. 10.1016/j.cell.2010.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Côté JP, French S, Gehrke SS, MacNair CR, Mangat CS, Bharat A, Brown ED. 2016. The genome-wide interaction network of nutrient stress genes in Escherichia coli. mBio 7:e01714-16. 10.1128/mBio.01714-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plecko B, Zweier M, Begemann A, Mathis D, Schmitt B, Striano P, Baethmann M, Vari MS, Beccaria F, Zara F, Crowther LM, Joset P, Sticht H, Papuc SM, Rauch A. 2017. Confirmation of mutations in PROSC as a novel cause of vitamin B6-dependent epilepsy. J Med Genet 54:809–814. 10.1136/jmedgenet-2017-104521. [DOI] [PubMed] [Google Scholar]
- 21.Tremiño L, Forcada-Nadal A, Rubio V. 2018. Insight into vitamin B6-dependent epilepsy due to PLPBP (previously PROSC) missense mutations. Hum Mutat 39:1002–1013. 10.1002/humu.23540. [DOI] [PubMed] [Google Scholar]
- 22.Shiraku H, Nakashima M, Takeshita S, Khoo CS, Haniffa M, Ch'ng GS, Takada K, Nakajima K, Ohta M, Okanishi T, Kanai S, Fujimoto A, Saitsu H, Matsumoto N, Kato M. 2018. PLPBP mutations cause variable phenotypes of developmental and epileptic encephalopathy. Epilepsia Open 3:495–502. 10.1002/epi4.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labella JI, Cantos R, Espinosa J, Forcada-Nadal A, Rubio V, Contreras A. 2017. PipY, a member of the conserved COG0325 family of PLP-binding proteins, expands the cyanobacterial nitrogen regulatory network. Front Microbiol 8:1244. 10.3389/fmicb.2017.01244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vu HN, Ito T, Downs DM. 2020. The role of YggS in vitamin B6 homeostasis in Salmonella enterica is informed by heterologous expression of yeast SNZ3. J Bacteriol 202:e00383-20. 10.1128/JB.00383-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laber B, Maurer W, Scharf S, Stepusin K, Schmidt FS. 1999. Vitamin B6 biosynthesis: formation of pyridoxine 5′-phosphate from 4-(phosphohydroxy)-L-threonine and 1-deoxy-D-xylulose-5-phosphate by PdxA and PdxJ protein. FEBS Lett 449:45–48. 10.1016/s0014-5793(99)00393-2. [DOI] [PubMed] [Google Scholar]
- 26.Neuwirth M, Strohmeier M, Windeisen V, Wallner S, Deller S, Rippe K, Sinning I, Macheroux P, Tews I. 2009. X-ray crystal structure of Saccharomyces cerevisiae Pdx1 provides insights into the oligomeric nature of PLP synthases. FEBS Lett 583:2179–2186. 10.1016/j.febslet.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Lam HM, Winkler ME. 1992. Characterization of the complex pdxH-tyrS operon of Escherichia coli K-12 and pleiotropic phenotypes caused by pdxH insertion mutations. J Bacteriol 174:6033–6045. 10.1128/jb.174.19.6033-6045.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao G, Winkler ME. 1995. Kinetic limitation and cellular amount of pyridoxine (pyridoxamine) 5′-phosphate oxidase of Escherichia coli K-12. J Bacteriol 177:883–891. 10.1128/jb.177.4.883-891.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugimoto R, Saito N, Shimada T, Tanaka K. 2018. Identification of YbhA as the pyridoxal 5′-phosphate (PLP) phosphatase in Escherichia coli: importance of PLP homeostasis on the bacterial growth. J Gen Appl Microbiol 63:362–368. 10.2323/jgam.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Ito T, Downs DM. 2020. Pyridoxal reductase, PdxI, is critical for salvage of pyridoxal in Escherichia coli. J Bacteriol 202:e00056-20. 10.1128/JB.00056-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Zhao G, Winkler ME. 1996. Identification of the pdxK gene that encodes pyridoxine (vitamin B6) kinase in Escherichia coli K-12. FEMS Microbiol Lett 141:89–95. 10.1111/j.1574-6968.1996.tb08368.x. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Tsui HC, Man TK, Winkler ME. 1998. Identification and function of the pdxY gene, which encodes a novel pyridoxal kinase involved in the salvage pathway of pyridoxal 5′-phosphate biosynthesis in Escherichia coli K-12. J Bacteriol 180:1814–1821. 10.1128/JB.180.7.1814-1821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan C, Zimmer A, Shah M, Huynh MS, Lai CC, Sit B, Hooda Y, Curran DM, Moraes TF. 2021. Actinobacillus utilizes a binding protein-dependent ABC transporter to acquire the active form of vitamin B6. J Biol Chem 297:101046. 10.1016/j.jbc.2021.101046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stolz J, Vielreicher M. 2003. Tpn1p, the plasma membrane vitamin B6 transporter of Saccharomyces cerevisiae. J Biol Chem 278:18990–18996. 10.1074/jbc.M300949200. [DOI] [PubMed] [Google Scholar]
- 35.Raschle T, Amrhein N, Fitzpatrick TB. 2005. On the two components of pyridoxal 5′-phosphate synthase from Bacillus subtilis. J Biol Chem 280:32291–32300. 10.1074/jbc.M501356200. [DOI] [PubMed] [Google Scholar]
- 36.Mechanik ML, Torchinsky YM, Florentiev VL, Karpeisky MY. 1971. Interaction of the apoenzyme of L-glutamate decarboxylase with pyridoxal phosphate analogues. FEBS Lett 13:177–180. 10.1016/0014-5793(71)80229-6. [DOI] [PubMed] [Google Scholar]
- 37.Tschopp J, Kirschner K. 1980. Subunit interactions of tryptophan synthase from Escherichia coli as revealed by binding studies with pyridoxal phosphate analogues. Biochemistry 19:4514–4521. 10.1021/bi00560a020. [DOI] [PubMed] [Google Scholar]
- 38.Vu HN, Downs DM. 2021. Loss of YggS (COG0325) impacts aspartate metabolism in Salmonella enterica. Mol Microbiol 116:1232–1240. 10.1111/mmi.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu C, Donovan WP, Shikapwashya-Hasser O, Ye X, Cole RH. 2014. Hot Fusion: an efficient method to clone multiple DNA fragments as well as inverted repeats without ligase. PLoS One 9:e115318. 10.1371/journal.pone.0115318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomason LC, Costantino N, Court DL. 2007. E. coli genome manipulation by P1 transduction. Curr Protoc Mol Bio 2007:1.17.1–1.17.8. 10.1002/0471142727.mb0117s79. [DOI] [PubMed] [Google Scholar]
- 42.Cherepanov PP, Wackernagel W. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9–14. 10.1016/0378-1119(95)00193-a. [DOI] [PubMed] [Google Scholar]
- 43.Ito T, Uozumi N, Nakamura T, Takayama S, Matsuda N, Aiba H, Hemmi H, Yoshimura T. 2009. The implication of YggT of Escherichia coli in osmotic regulation. Biosci Biotechnol Biochem 73:2698–2704. 10.1271/bbb.90558. [DOI] [PubMed] [Google Scholar]
- 44.Fux A, Sieber SA. 2020. Biochemical and proteomic studies of human pyridoxal 5'-phosphate-binding protein (PLPBP). ACS Chem Biol 15:254–261. 10.1021/acschembio.9b00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available on reasonable request from the corresponding author.