ABSTRACT
Recently, remdesivir and molnupiravir were approved for treating COVID-19 caused by SARS-CoV-2 infection. However, little is known about the impact of these drugs on other viruses preexisted in COVID-19 patients. Here we report that remdesivir but not molnupiravir induced lytic reactivation of Kaposi’s sarcoma-associated herpesvirus (KSHV) and Epstein-Barr virus (EBV), two major oncogenic herpesviruses. Remdesivir induced mature virion production from latently infected cells. Mechanistic studies showed that remdesivir induced KSHV and EBV reactivation by regulating several intracellular signaling pathways.
KEYWORDS: KSHV, EBV, SARS-CoV-2, COVID-19, remdesivir, molnupiravir
INTRODUCTION
Since the outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at the end of 2019, the triggered COVID-19 pandemic has caused over five million deaths, according to the data released from the Johns Hopkins Coronavirus Resource Center (https://coronavirus.jhu.edu/), and serious social problems worldwide. Further still, increasing data show that SARS-CoV-2 infection is able to aggravate preexisting diseases, including cancer and other infectious diseases (1). Several cases of reactivation of human herpesviruses, such as Epstein-Barr virus (EBV), Herpes simplex viruses (HSV), human cytomegalovirus (HCMV), varicella zoster virus (VZV), and herpes zoster (HZ), among severe COVID-19 patients or COVID vaccinated personnel have been reported (2–6). Our previous data also showed that SARS-CoV-2 encoded proteins were able to induce KSHV reactivation in vitro, thereby promoting virus dissemination and initiation of oncogenesis (7). Therefore, coinfection of SARS-CoV-2 should be considered as a high-risk factor for those patients with these herpesvirus infections.
Recently, two antiviral drugs, remdesivir and molnupiravir (both targeting the viral RNA-dependent, RNA polymerase to interfere with viral replication), were authorized by the United States Food and Drug Administration (FDA) for COVID-19 treatment due to their clinical benefits (https://www.fda.gov/drugs). There are some other candidates with antiviral activities indicated for use in the treatment of COVID-19 patients, such as azithromycin, chloroquine diphosphate, hydroxychloroquine sulfate, and nafamostat mesylate (8, 9). Unexpectedly, our recent data indicated that several of them may affect Kaposi’s sarcoma-associated herpesvirus (KSHV) lytic reactivation, especially azithromycin and nafamostat mesylate, both of which significantly increased viral lytic gene expression and virion production via the activation of MAPK and NF-κb signaling pathways (7), respectively, raising a concern about using these drugs in COVID-19 patients who already have a preexisting herpesvirus infection. Therefore, it is meaningful to investigate the impact of anti-COVID-19 drugs on chronic viral infections.
KSHV and EBV represent two oncogenic gammaherpesviruses that may lead to several human tumors (10). Similar to other herpesviruses, they have an alternative life cycle, latent and lytic replication phases, both of which are essential for tumorigenesis (11). Compared with latency when only a limited number of viral genes are expressed, lytic reactivation permits the expression of the majority of viral genes, in a sequential fashion of immediate early, early, and late genes (12, 13). Increasing data report that lytic reactivation requires the involvement of several cellular signaling pathways, such as AMPK and STAT3. A previous study showed AMPK suppressed KSHV infection and replication, which was further supported by the observation that an AMPK inhibitor, compound C, augmented viral lytic gene expression and subsequent virion production (14). In contrast, knockdown or chemical inhibition of STAT3 resulted in KSHV lytic activation via suppression of KAP1 (15). Similarly, STAT3 inhibition was previously shown to induce EBV lytic activation in B lymphocytes (16).
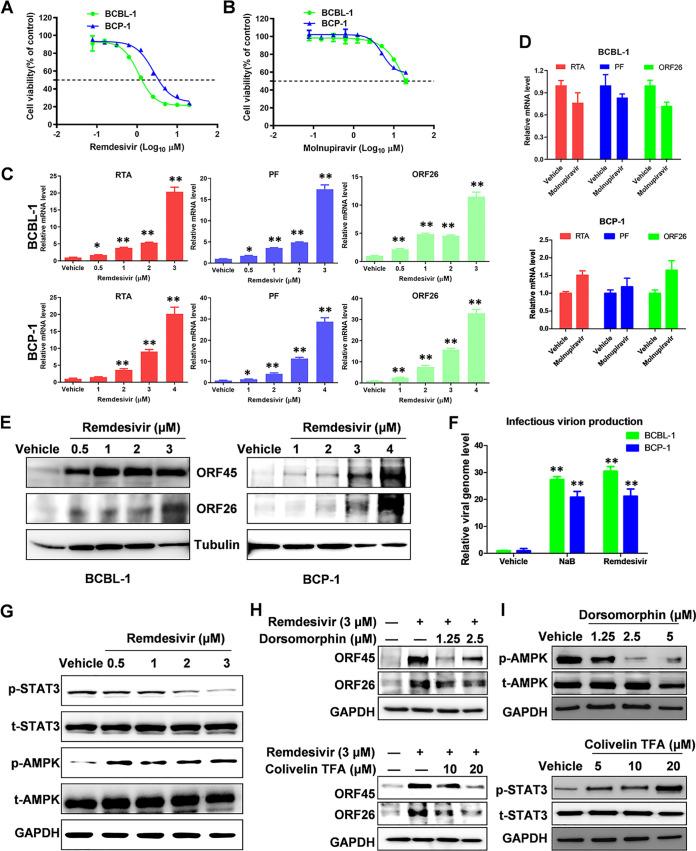
In this study, we sought to determine whether remdesivir and molnupiravir treatment affects lytic reactivation of KSHV and EBV. Initially, the cytotoxicity of remdesivir and molnupiravir against two KSHV-infected primary effusion lymphoma (PEL) cell lines, BCBL-1 and BCP-1, were evaluated at 72 h posttreatment by the WST-1 assay as described previously (17). The data indicated cytotoxic concentrations (CC50) of remdesivir for BCBL-1 and BCP-1 of 1.2 μM and 2.6 μM (Fig. 1A), respectively. In contrast, the CC50 of molnupiravir for these cells was around 20 μM (Fig. 1B). Next, qRT-PCR analysis showed remdesivir treatment significantly induced the expression of viral lytic genes, including RTA (immediate early gene), PF (early gene), and ORF26 (late gene), in a dose-dependent manner in both PEL cell lines (Fig. 1C). In contrast, molnupiravir treatment showed little change of viral lytic gene expression even at the dose of CC50 (Fig. 1D). We then confirmed remdesivir treatment increased the expression of ORF45 (immediate early gene) and ORF26 (late gene) at the protein level using the Western blot (WB) assay in both PEL cell lines (Fig. 1E). To measure the production of infectious virion, qPCR assay was used to test viral DNA levels extracted from HEK293 cells, which were infected by the supernatants from BCBL-1 and BCP-1 cells following incubation with each of the compounds. Our findings demonstrate remdesivir treatment effectively increased virion production to a similar level of sodium butyrate (NaB), a classical chemical inducer for KSHV reactivation (Fig. 1F).
FIG 1.
The effects of remdesivir and molnupiravir on KSHV lytic reactivation. (A–B) The cytotoxicity of remdesivir and molnupiravir on BCBL-1 and BCP-1 cells was examined at 72 h posttreatment using the WST-1 cell proliferation assays (Roche). (C–E) Cells were treated with a dose range of remdesivir or molnupiravir (20 μM), respectively, for 72 h, then the transcripts of representative lytic genes were quantified by using qRT-PCR. Protein expression was measured by using Western blot. (F) The supernatants from cells treated with remdesivir at CC50 concentration were collected to infect naive HEK293T cells, then viral genome levels were quantified by using qPCR with LANA specific primers. The sodium butyrate (NaB, 0.3 mM) was used as a positive control. The CC50 for each compound was calculated using GraphPad Prism 5.0. (G) BCBL-1 cells were exposed to a dose range of remdesivir for 72 h, then protein expression was measured by using Western blot. (H) Cells were treated with remdesivir (3 μM) in combination with dorsomorphin (an AMPK inhibitor) or colivelin TFA (a STAT3 activator), respectively, for 48 h, then protein expression was measured by using Western blot. (I) Cells were treated with dorsomorphin or colivelin TFA, respectively, for 48 h, then protein expression was measured by using Western blot. Error bars represent the SD for 3 independent experiments. *, P < 0.05; **, P < 0.01 (two-tailed Student's t test).
To investigate the underlying mechanisms, we examined the activities of several key intracellular signaling pathways associated with KSHV lytic replication in remdesivir-treated PEL cells by using the WB assay. Our results indicated that remdesivir treatment mainly increased AMPK phosphorylation while decreasing STAT3 phosphorylation in a dose-dependent fashion in BCBL-1 cells (Fig. 1G). Moreover, the addition of dorsomorphin (an AMPK inhibitor) or colivelin TFA (a STAT3 inducer) blocked remdesivir-induced expression of viral lytic proteins ORF45 and ORF26 (Fig. 1H), confirming the involvement of AMPK and STAT3 signaling in remdesivir-induced KSHV lytic reactivation. The impact of dorsomorphin and colivelin TFA on AMPK and STAT3 signaling activities, respectively, were validated by WB assay (Fig. 1I).
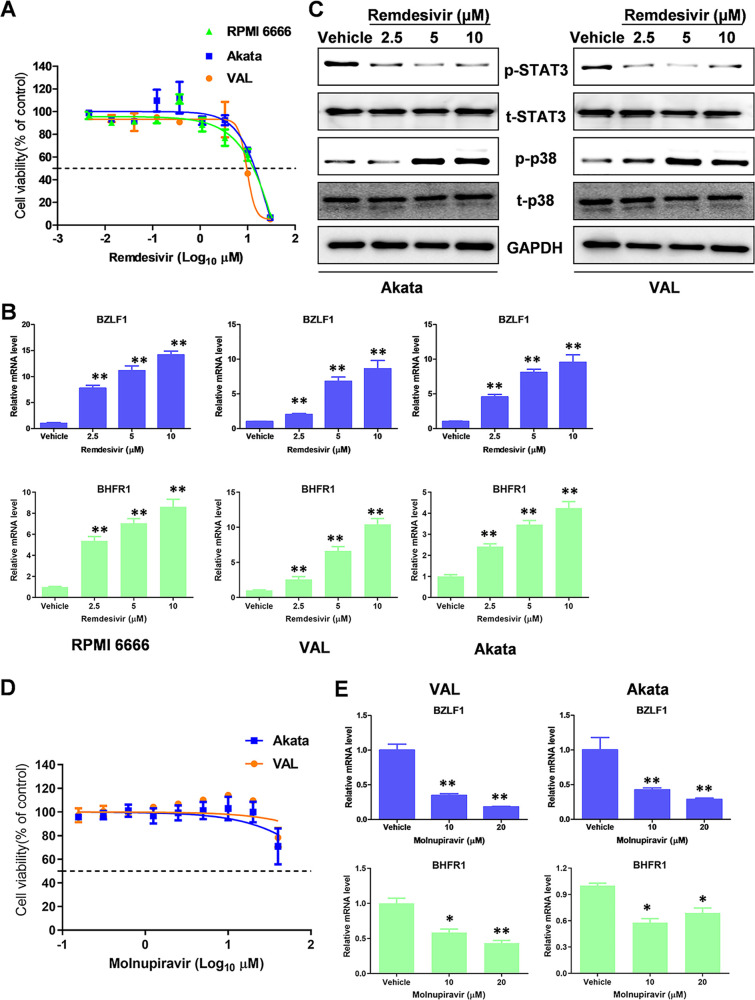
In addition, we examined the effects of remdesivir on EBV lytic reactivation in EBV+ lymphoma cells. Three different types of EBV+ lymphoma cell lines, RPMI 6666 (Hodgkin's lymphoma), Akata (Burkitt's lymphoma), and VAL (diffuse large B-cell lymphoma) were used as our model. We found that the CC50 of remdesivir for these EBV+ lymphoma cell lines was around 10 μM (Fig. 2A). Remdesivir treatment increased the expression of viral lytic genes, such as BZLF1 (immediate early gene) and BHFR1 (early gene), in all of three EBV+ lymphoma cell lines as quantified by qRT-PCR (Fig. 2B). The WB results indicated that remdesivir treatment mainly reduced STAT3 but increased p38 MAPK phosphorylation from EBV+ lymphoma cells (Fig. 2C), two signaling pathways that are associated with EBV reactivation (16, 18). These data indicate that remdesivir may also induce EBV lytic reactivation from latently infected cells. In contrast, molnupiravir treatment had limited cytotoxicity on EBV+ lymphoma cell lines (CC50 ≫ 20 μM, Fig. 2D). Interestingly, molnupiravir treatment significantly reduced EBV lytic gene expression from these lymphoma cells (Fig. 2E), although the mechanisms remain unknown.
FIG 2.
The effects of remdesivir and molnupiravir on EBV lytic reactivation. (A) The cytotoxicity of remdesivir on EBV+ lymphoma cell lines, RPMI 6666, Akata, and VAL was examined at 72 h posttreatment using the WST-1 cell proliferation assays. (B) The transcripts of representative lytic genes were quantified by using qRT-PCR. (C) The protein expression was measured by using Western blot. (D–E) Cells were treated with molnupiravir for 72 h, then the cytotoxicity and viral gene expression were measured as above. Error bars represent the SD for 3 independent experiments. *, P < 0.05; **, P < 0.01 (two-tailed Student's t test).
In summary, we evaluated the effects of two recently FDA-approved anti-COVID-19 drugs, remdesivir and molnupiravir, on lytic reactivation of human oncogenic herpesviruses. Although both drugs target SARS-CoV-2 RNA polymerase and interfere with viral replication, only remdesivir strongly induces KSHV and EBV lytic reactivation. These data suggest a potential risk of treating COVID-19 patients with preexisting oncogenic herpesvirus infection with remdesivir. Reactivation of these preexisting infections may increase viral pathogenesis and tumorigenesis, especially for immunocompromised or immunosuppressed patients that are already at an elevated risk of KSHV/EBV-associated malignancies. Therefore, continuous monitoring of viral loads and assessing risk of developing virus-associated malignancies are necessary for these patients with remdesivir treatment, even after they have fully recovered from COVID-19.
ACKNOWLEDGMENTS
This work was supported by NIH/NCI R01CA228166, the Arkansas Bioscience Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000. Funding source had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We declare that we have no conflicts of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Bennett S, Tafuro J, Mayer J, Darlington D, Wong CW, Muntean E-A, Wong N, Mallen C, Kwok CS. 2021. Clinical features and outcomes of adults with coronavirus disease 2019: a systematic review and pooled analysis of the literature. Int J Clin Pract 75:e13725. 10.1111/ijcp.13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonnet A, Engelmann I, Moreau AS, Garcia B, Six S, El Kalioubie A, Robriquet L, Hober D, Jourdain M. 2021. High incidence of Epstein-Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect Dis Now 51:296–299. 10.1016/j.idnow.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Balc’h P, Pinceaux K, Pronier C, Seguin P, Tadié J-M, Reizine F. 2020. Herpes simplex virus and cytomegalovirus reactivations among severe COVID-19 patients. Crit Care 24:530. 10.1186/s13054-020-03252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng F, Willis A, Kunjukunju N. 2021. Acute retinal necrosis from reactivation of varicella zoster virus following BNT162b2 mRNA COVID-19 vaccination. Ocul Immunol Inflamm 10.1080/09273948.2021.2001540. [DOI] [PubMed] [Google Scholar]
- 5.Iwai S, Takayama K, Sora D, Takeuchi M. 2021. A case of acute retinal necrosis associated with reactivation of varicella zoster virus after COVID-19 vaccination. Ocul Immunol Inflamm 10.1080/09273948.2021.2001541. [DOI] [PubMed] [Google Scholar]
- 6.Shah S, Baral B, Chamlagain R, Murarka H, Raj Adhikari Y, Sharma Paudel B. 2021. Reactivation of herpes zoster after vaccination with an inactivated vaccine: a case report from Nepal. Clin Case Rep 9:e05188. 10.1002/ccr3.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Dai L, Barrett L, James J, Plaisance-Bonstaff K, Post SR, Qin Z. 2021. SARS-CoV-2 proteins and anti-COVID-19 drugs induce lytic reactivation of an oncogenic virus. Commun Biol 4:682. 10.1038/s42003-021-02220-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautret P, Lagier JC, Honore S, Hoang VT, Colson P, Raoult D. 2021. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open label non-randomized clinical trial revisited. Int J Antimicrob Agents 57:106243. 10.1016/j.ijantimicag.2020.106243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann M, Schroeder S, Kleine-Weber H, Muller MA, Drosten C, Pohlmann S. 2020. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob Agents Chemother 64:e00754-20. 10.1128/AAC.00754-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V, WHO International Agency for Research on Cancer Monograph Working Group . 2009. A review of human carcinogens—Part B: biological agents. Lancet Oncology 10:321–322. 10.1016/S1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 11.Jha HC, Banerjee S, Robertson ES. 2016. The role of gammaherpesviruses in cancer pathogenesis. Pathogens 5:18. 10.3390/pathogens5010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenney SC, Mertz JE. 2014. Regulation of the latent-lytic switch in Epstein-Barr virus. Semin Cancer Biol 26:60–68. 10.1016/j.semcancer.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broussard G, Damania B. 2020. Regulation of KSHV latency and lytic reactivation. Viruses 12:1034. 10.3390/v12091034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng F, He ML, Jung JU, Lu C, Gao SJ. 2016. Suppression of Kaposi's sarcoma-associated herpesvirus infection and replication by 5 '-AMP-activated protein kinase. J Virol 90:6515–6525. 10.1128/JVI.00624-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King CA, Li XF, Barbachano-Guerrero A, Bhaduri-McIntosh S. 2015. STAT3 regulates lytic activation of Kaposi's sarcoma-associated herpesvirus. J Virol 89:11347–11355. 10.1128/JVI.02008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill ER, Koganti S, Zhi J, Megyola C, Freeman AF, Palendira U, Tangye SG, Farrell PJ, Bhaduri-McIntosh S. 2013. Signal transducer and activator of transcription 3 limits Epstein-Barr virus lytic activation in B lymphocytes. J Virol 87:11438–11446. 10.1128/JVI.01762-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai L, Trillo-Tinoco J, Bai A, Chen Y, Bielawski J, Del Valle L, Smith CD, Ochoa AC, Qin Z, Parsons C. 2015. Ceramides promote apoptosis for virus-infected lymphoma cells through induction of ceramide synthases and viral lytic gene expression. Oncotarget 6:24246–24260. 10.18632/oncotarget.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonnella R, Granato M, Farina A, Santarelli R, Faggioni A, Cirone M. 2015. PKC theta and p38 MAPK activate the EBV lytic cycle through autophagy induction. Biochim Biophys Acta 1853:1586–1595. 10.1016/j.bbamcr.2015.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.02395-21-s0001.pdf, PDF file, 0.2 MB (186.9KB, pdf)