ABSTRACT
Clinical studies have reported additive nephrotoxicity associated with the combination of vancomycin (VAN) and piperacillin-tazobactam (TZP). This study assessed differences in glomerular filtration rate (GFR) and urinary biomarkers between rats receiving VAN and those receiving VAN + TZP. Male Sprague-Dawley rats (n = 26) were randomized to receive 96 h of intravenous VAN at 150 mg/kg/day, intraperitoneal TZP at 1,400 mg/kg/day, or VAN + TZP. Kidney function was evaluated using fluorescein-isothiocyanate sinistrin and a transdermal sensor to estimate real-time glomerular filtration rate (GFR). Kidney injury was evaluated via urinary biomarkers, including kidney injury molecule-1 (KIM-1), clusterin, and osteopontin. Compared to a saline control, only rats in the VAN group showed significant declines in GFR by day 4 (−0.39 mL/min/100 g body weight; 95% confidence interval [CI], −0.68 to −0.10; P = 0.008). When the VAN + TZP and VAN alone treatment groups were compared, significantly higher urinary KIM-1 marginal linear predictions were observed in the VAN alone group on day 1 (18.4 ng; 95% CI, 1.4 to 35.3; P = 0.03), day 2 (27.4 ng; 95% CI, 10.4 to 44.3; P = 0.002), day 3 (18.8 ng; 95% CI, 1.9 to 35.8; P = 0.03), and day 4 (23.2 ng; 95% CI, 6.3 to 40.2; P = 0.007). KIM-1 was the urinary biomarker that most correlated with decreasing GFR on day 3 (Spearman’s rho, −0.45; P = 0.022) and day 4 (Spearman’s rho, −0.41; P = 0.036). Kidney function decline and increased KIM-1 were observed among rats that received VAN only but not those that received TZP or VAN + TZP. The addition of TZP to VAN does not worsen kidney function or injury in our translational rat model.
KEYWORDS: KIM-1, PK/PD, PK/TD, biomarker, nephrotoxicity, vancomycin
INTRODUCTION
Vancomycin (VAN) is a glycopeptide antibiotic that is a treatment of choice for many resistant Gram-positive infections. It remains the most frequently prescribed parenteral antibiotic in U.S. hospitals, and use has steadily increased over the past decade (1). Nephrotoxicity is a common adverse effect of VAN, with attributed rates of >10% (2–4). Piperacillin-tazobactam (TZP) is a beta-lactam/beta-lactamase inhibitor combination that is commonly coprescribed with vancomycin for empirical coverage of Gram-negative organisms (5, 6).
Recent clinical meta-analyses have found the combination of VAN and TZP to be associated with additive nephrotoxicity, as assessed by increased serum creatinine (SCr), compared to VAN alone (7–11). Luther et al. (9) found that the combination of VAN + TZP increased the odds of acute kidney injury (AKI) compared to VAN monotherapy (odds ratio [OR], 3.4; 95% confidence interval [CI], 2.57 to 4.50) or TZP monotherapy (OR, 2.7; 95% CI, 1.97 to 3.69). This difference in AKI also persisted when VAN + TZP was compared to VAN + cefepime or meropenem (OR, 2.68; 95% CI, 1.83 to 3.91) (9). More recently, Bellos et al. (10) recapitulated these findings in the largest meta-analysis of VAN + TZP nephrotoxicity to date. Their study included over 50,000 patients, and VAN + TZP was found to increase the odds of AKI, as assessed by SCr using the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, compared to VAN (OR, 2.05; 95% CI, 1.17 to 3.46), VAN + cefepime (OR, 1.80; 95% CI, 1.13 to 2.77), and VAN + meropenem (OR, 1.84; 95% CI, 1.02 to 3.10) (10). Despite these results from retrospective studies suggesting that VAN + TZP is associated with greater increases in SCr compared to VAN alone, it is still unclear whether this reflects overt nephrotoxicity. The underlying mechanism of additive nephrotoxicity associated with VAN + TZP remains unknown, and SCr is known to be a poor surrogate marker for both kidney function and damage (12).
Clinical study outcomes remain limited by mostly retrospective research design and use of SCr as the sole indicator of kidney injury and function. However, SCr is not a direct marker of kidney injury; instead, it is an imperfect surrogate for the glomerular filtration rate (GFR) (12–14), and changes in the secretion and/or reabsorption of creatinine can result in fluctuations in SCr that do not reflect a true loss of kidney function. An additional limitation of SCr is that during acute changes in kidney function, GFR can decline by as much as 50% before detectable rises in SCr occur (14). To address the limitations of using SCr as a surrogate for kidney function and injury, several translational animal studies have investigated the injury profile of VAN + TZP versus VAN using newer kidney biomarkers of injury (e.g., kidney injury molecule-1 [KIM-1], clusterin, and osteopontin [OPN]) and histopathology (15, 16). In these models, the results conflicted with retrospective human studies; additive nephrotoxicity associated with VAN + TZP was not observed in animal studies (15, 16). KIM-1 was identified as the most relevant urinary biomarker in animal studies for vancomycin-induced kidney injury, correlating with GFR changes and end-organ histopathologic damage at the proximal tubule (15, 17–19). While injury biomarkers and histopathology scores were not increased in rats receiving VAN + TZP versus VAN, the impact of TZP on glomerular function was not studied directly. Thus, the purpose of this study was to employ our translational rat model to directly assess kidney function differences between treatment groups by GFR and to correlate GFR to urinary injury biomarker expression in rats receiving VAN ± TZP, TZP alone, or control (saline).
RESULTS
Characteristics of animal cohort.
A total of 26 male Sprague-Dawley rats were studied, with the animal dosing group assignment shown in Table 1. One animal provided only terminal plasma samples due to occluded catheters; all other animals contributed complete data. One animal had missing GFR measurements on one experimental day due to sensor malfunction; all other animals contributed complete GFR data to the model. Mean weight change was not different between the VAN, TZP, VAN + TZP, and saline control groups (−1.39 g versus −2.27 g versus −1.59 g versus +5.50 g; P = 0.19).
TABLE 1.
Marginal differences versus saline as referent groupa
| Dosing day | VAN treatment group | TZP treatment group | VAN + TZP treatment group |
|---|---|---|---|
| Urine output difference (mL), mean (95% CI), P value | |||
| Baseline | 0.63 (−3.19 to 4.45), P = 0.747 | 2.85 (−1.12 to 6.82), P = 0.159 | 3.33 (−0.49 to 7.15), P = 0.088 |
| Day 1 | 4.09 (0.26 to 7.91), P = 0.036 | 1.40 (−2.57 to 5.37), P = 0.490 | 5.52 (1.69 to 9.34), P = 0.005 |
| Day 2 | 0.45 (−3.38 to 4.27), P = 0.820 | −1.98 (−5.95 to 1.99), P = 0.328 | 0.75 (−3.08 to 4.52), P = 0.703 |
| Day 3 | 0.49 (−3.33 to 4.32), P = 0.801 | 0.58 (−3.39 to 4.55), P = 0.773 | 3.19 (−0.63 to 7.02), P = 0.102 |
| Day 4 | 8.87 (5.05 to 12.7), P < 0.001 | −0.07 (−4.04 to 3.91), P = 0.974 | 6.83 (3.01 to 10.7), P < 0.001 |
| Urinary KIM-1 difference (ng), mean (95% CI), P value | |||
| Baseline | −1.24 (−18.9 to 16.4), P = 0.891 | −0.45 (−18.8 to 17.9), P = 0.961 | 1.22 (−16.4 to 18.9), P = 0.892 |
| Day 1 | 21.5 (3.83 to 39.2), P = 0.017 | −1.42 (−19.8 to 16.9), P = 0.879 | 3.14 (−14.5 to 20.8), P = 0.728 |
| Day 2 | 29.8 (12.2 to 47.5), P = 0.001 | −3.74 (−22.1 to 14.6), P = 0.689 | 2.47 (−15.2 to 20.1), P = 0.784 |
| Day 3 | 21.6 (3.91 to 39.2), P = 0.017 | −2.66 (−20.9 to 15.7), P = 0.776 | 2.74 (−14.9 to 20.4), P = 0.761 |
| Day 4 | 24.1 (6.41 to 41.7), P = 0.008 | −4.14 (−22.5 to 14.2), P = 0.658 | 0.83 (−16.8 to 18.5), P = 0.927 |
| Urinary clusterin difference (ng), mean (95% CI), P value | |||
| Baseline | 108 (−1,835 to 2,052), P = 0.913 | 507 (−1,511 to 2,524), P = 0.623 | 1,783 (−160 to 3,727), P = 0.072 |
| Day 1 | 1,563 (−381 to 3,507), P = 0.115 | 930 (−1,087 to 2,947), P = 0.366 | 2,870 (926 to 4,814), P = 0.004 |
| Day 2 | 2,030 (86 to 3,974), P = 0.041 | −597 (−2,614 to 1,420), P = 0.562 | 883 (−1,061 to 2,826), P = 0.374 |
| Day 3 | 1,760 (−184 to 3,704), P = 0.076 | 43 (−1,975 to 2,060), P = 0.967 | 1,843 (−101 to 3,787), P = 0.063 |
| Day 4 | 523 (−1,421 to 2,467), P = 0.598 | −228 (−2,246 to 1,789), P = 0.824 | 400 (−1,544 to 2,344), P = 0.687 |
| Urinary OPN difference (ng), mean (95% CI), P value | |||
| Baseline | 0.21 (−0.55 to 0.98), P = 0.586 | 0.17 (−0.62 to 0.97), P = 0.670 | 0.15 (−0.61 to 0.92), P = 0.695 |
| Day 1 | 0.47 (−0.29 to 1.23), P = 0.229 | 0.54 (−0.26 to 1.33), P = 0.186 | 0.47 (−0.29 to 1.23), P = 0.233 |
| Day 2 | 1.09 (0.33 to 1.86), P = 0.005 | 0.35 (−0.44 to 1.15), P = 0.383 | 0.51 (−0.25 to 1.28), P = 0.189 |
| Day 3 | 1.35 (0.58 to 2.11), P = 0.001 | 0.71 (−0.09 to 1.49), P = 0.082 | 0.89 (0.13 to 1.66), P = 0.022 |
| Day 4 | 1.73 (0.97 to 2.49), P < 0.001 | 0.49 (−0.29 to 1.29), P = 0.222 | 1.77 (1.01 to 2.53), P < 0.001 |
| GFR difference (mL/min/100 g body weight), mean (95% CI), P value | |||
| Baseline | 0.18 (−0.11 to 0.47), P = 0.234 | 0.18 (−0.12 to 0.48), P = 0.232 | 0.05 (−0.23 to 0.34), P = 0.712 |
| Day 1 | −0.16 (−0.46 to 0.14), P = 0.285 | 0.02 (−0.29 to 0.32), P = 0.922 | −0.16 (−0.44 to 0.13), P = 0.293 |
| Day 2 | −0.11 (−0.39 to 0.18), P = 0.474 | 0.0 (−0.30 to 0.30), P = 0.999 | 0.01 (−0.28 to 0.31), P = 0.938 |
| Day 3 | −0.01 (−0.30 to 0.28), P = 0.934 | 0.15 (−0.15 to 0.45), P = 0.339 | 0.17 (−0.12 to 0.46), P = 0.256 |
| Day 4 | −0.39 (−0.68 to −0.10), P = 0.008 | −0.13 (−0.43 to 0.17), P = 0.402 | −0.04 (−0.33 to 0.25), P = 0.794 |
CI, confidence interval; GFR, glomerular filtration rate; KIM-1, kidney injury molecule-1; OPN, osteopontin; TZP, piperacillin-tazobactam; VAN, vancomycin. Boldface indicates P value < 0.05.
GFR over time.
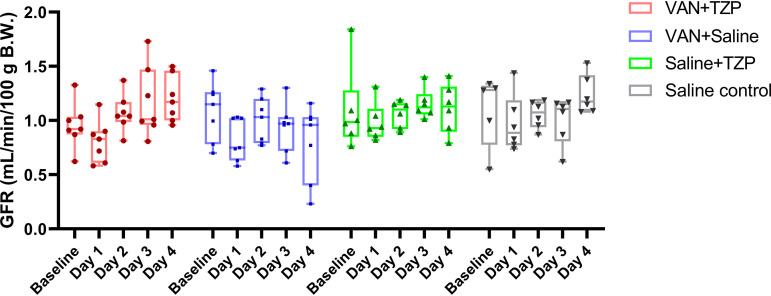
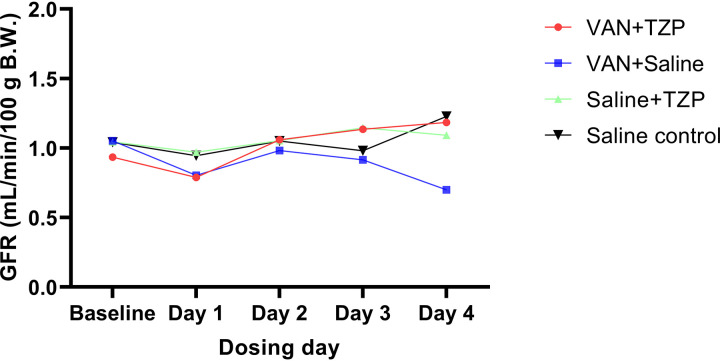
Baseline GFR was not different between the VAN, TZP, VAN + TZP, and saline control groups (with saline used as the referent group) (1.08 ± 0.27, 1.09 ± 0.38, 0.96 ± 0.21, and 0.91 ± 0.53 mL/min/100 g body weight; P = 0.756). Following administration of the first dose (day 1), all rats experienced a nonsignificant decline in GFR. Rats in the VAN group did not recover the GFR decrease and had progressive functional decline, whereas rats that received VAN + TZP, TZP, or saline control subsequently recovered their GFR over the remaining dosing days (Fig. 1). Compared to the saline control group, only rats that received VAN had a significant decline in GFR by day 4 (Fig. 2 and 0.39 mL/min/100 g body weight; 95% CI, −0.68 to −0.10; P = 0.008). In a direct comparison of the VAN + TZP and VAN alone groups, the same trend was observed; only rats that received VAN had a significant decline in GFR by day 4 (Fig. S4 and 0.35 mL/min/100 g body weight; 95% CI, −0.63 to −0.07; P = 0.013).
FIG 1.
Comparison of individual GFR measurements between treatment groups and across dosing days. The majority of the rats experienced a decline in glomerular filtration rate (GFR) after the first dose of study treatment was given on day 1. Rats in the vancomycin (VAN) + saline (blue) group showed progressive functional decline over the study days, while rats in all other groups recovered their GFR to baseline levels by day 4. Individual rats are depicted by each data point. B.W., body weight; TZP, piperacillin-tazobactam.
FIG 2.
Comparison of mean GFR measurements between treatment groups and across dosing days. When the saline control was used as the referent group, only rats that received VAN had a significant decline in GFR by day 4 (−0.39 mL/min/100 g body weight; 95% CI, −0.68 to −0.10; P = 0.008). Daily group mean GFR measurements are depicted by each data point.
Urine output and injury biomarkers.
Summary statistics for urine output and urinary biomarker differences from saline-treated animals as a referent group are listed in Table 1. Baseline urine output was not different among the treatment groups. Daily urine output was compared to baseline, and significant differences were seen on day 1 in rats that received VAN (mean difference, −6.9 mL; 95% CI, −11.7 to −2.2; P = 0.009) and on day 1 in the saline control group (mean difference, −3.5 mL; 95% CI, −5.7 to −1.3; P = 0.008).
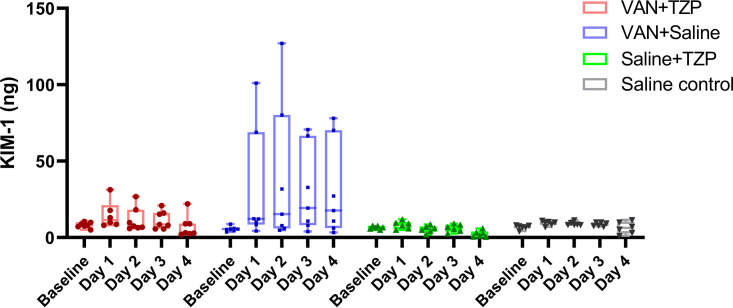
For analysis of urinary biomarkers, each of the treatment groups was compared to the saline control group. Rats in the VAN group had the highest urinary KIM-1, with marginal linear predictions significantly different after drug dosing on day 1 (Fig. 3) (21.5 ng; 95% CI, 3.8 to 39.2; P = 0.02), day 2 (29.8 ng; 95% CI, 12.2 to 47.5; P = 0.001), day 3 (21.6 ng; 95% CI, 3.9 to 39.2; P = 0.02), and day 4 (24.1 ng; 95% CI, 6.4 to 41.7; P = 0.008). In a direct comparison to VAN + TZP, rats that received VAN alone had significantly higher urinary KIM-1 after drug dosing on day 1 (18.4 ng; 95% CI, 1.4 to 35.3; P = 0.03), day 2 (27.4 ng; 95% CI, 10.4 to 44.3; P = 0.002), day 3 (18.8 ng; 95% CI, 1.9 to 35.8; P = 0.03), and day 4 (23.2 ng; 95% CI, 6.3 to 40.2; P = 0.007). Urinary clusterin marginal predictions were significantly higher among rats in the VAN group on day 2 (2,030 ng; 95% CI, 86 to 3,974; P = 0.04) of the study. Significantly elevated marginal predictions of urinary clusterin was also observed among rats in the VAN + TZP group on day 1 (2,869 ng; 95% CI, 926 to 4,814; P = 0.004). Urinary OPN marginal predictions were significantly higher among VAN group rats on day 2 (1.09 ng; 95% CI, 0.33 to 1.86; P = 0.005), day 3 (1.35 ng; 95% CI, 0.58 to 2.11; P = 0.001), and day 4 (1.73 ng; 95% CI, 0.97 to 2.49; P < 0.001). Rats in the VAN + TZP group also had significantly higher marginal predictions of urinary OPN on day 3 (0.89 ng; 95% CI, 0.13 to 1.66; P = 0.0 2) and day 4 (1.76 ng; 95% CI, 1.0 to 2.53; P < 0.001).
FIG 3.
Comparison of individual daily urinary kidney injury molecule-1 (KIM-1) measurements between treatment groups and across dosing days. Rats in the VAN group had the highest urinary KIM-1, with significant differences seen after drug dosing on day 1 (21.5 ng; 95% CI, 3.8 to 39.2; P = 0.02), day 2 (29.8 ng; 95% CI, 12.2 to 47.5; P = 0.001), day 3 (21.6 ng; 95% CI, 3.9 to 39.2; P = 0.02), and day 4 (24.1 ng; 95% CI, 6.4 to 41.7; P = 0.008). Individual rats are depicted by each data point.
Correlation between urinary biomarkers of injury and GFR.
Spearman’s rank correlation between urinary biomarkers and GFR readings are listed in Table 2. In the VAN group rats, urinary KIM-1 was significantly correlated with decreasing GFR on day 3 (Fig. 4) (Spearman’s rho, −0.45; P = 0.022) and day 4 (Spearman’s rho, −0.41; P = 0.036). Urinary clusterin was also negatively correlated with GFR on day 3 (Spearman’s rho, −0.42; P = 0.033). No significant correlations were observed between urinary OPN and GFR.
TABLE 2.
Summary of urinary biomarker correlations with glomerular filtration ratea
| Urinary biomarker | Day 0 (baseline) | Day 1 | Day 2 | Day 3 | Day 4 | |
|---|---|---|---|---|---|---|
| Kidney injury molecule-1 | Spearman’s rho | −0.35 | −0.29 | −0.26 | −0.45 | −0.41 |
| P value | 0.081 | 0.149 | 0.201 | 0.022 | 0.036 | |
| Clusterin | Spearman’s rho | −0.08 | −0.29 | −0.37 | −0.42 | −0.22 |
| P value | 0.685 | 0.166 | 0.065 | 0.033 | 0.272 | |
| Osteopontin | Spearman’s rho | −0.09 | 0.07 | −0.06 | 0.10 | −0.15 |
| P value | 0.657 | 0.752 | 0.778 | 0.622 | 0.461 |
Boldface indicates P value < 0.05.
FIG 4.
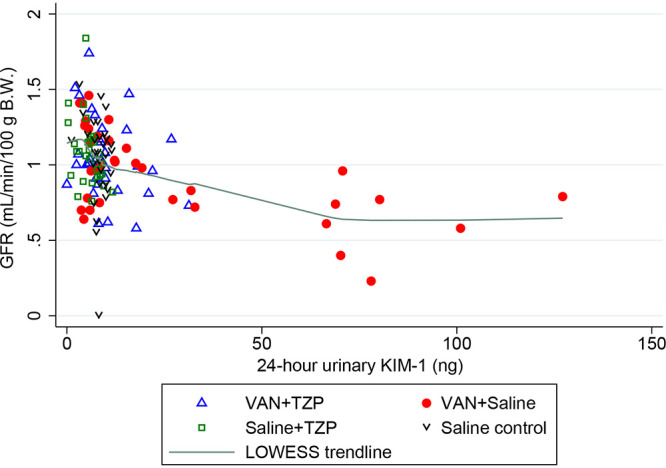
Spearman correlation of GFR with 24-h urinary KIM-1 in ng. Among rats that received VAN + saline, urinary KIM-1 was significantly correlated with decreasing GFR on day 3 (Spearman’s rho: −0.45, P = 0.022) and day 4 (Spearman’s rho: −0.41, P = 0.036). Individual rats are depicted by each data point and the green line depicts the locally weighted scatterplot smoothing (LOWESS) trendline.
Histopathological analysis.
Kidney histopathology demonstrated the highest Predictive Safety Testing Consortium (PSTC) scores in the VAN group (median, 2; interquartile range [IQR], 1), followed by the saline control group (median, 1.5; IQR, 1), TZP group (median, 1; IQR, 0), and the VAN + TZP group (median, 1; IQR, 0). The same trend was observed in tubular specific scores with the highest scores observed in the VAN group (median, 2; IQR, 1), followed by the saline control group (median, 1; IQR, 1), TZP group (median, 1; IQR, 0) and the VAN + TZP group (median, 1; IQR, 0). Degenerative renal tubular changes consisting of tubular epithelial necrosis or apoptosis were only observed among rats that received VAN alone. Other changes of tubular epithelial injury, cellular sloughing, and granular intratubular casts were consistently observed in kidneys from rats that received VAN alone. These indicators of tubular epithelial injury were observed less frequently and at lower severity in kidneys from rats that received VAN + TZP.
DISCUSSION
In this study, we found that VAN alone led to lower GFRs (as measured by fluorescein isothiocyanate-labeled sinistrin [FITC-sinistrin] clearance) after 4 days of drug dosing. The rise of urinary KIM-1, as a marker of proximal tubule injury, occurred as early as day 1 and correlated with the drop in GFR by day 3 among rats that received only VAN. Notably, similar trends in GFR and urinary KIM-1 expression were not observed in rats that received VAN + TZP; in fact, rats that received VAN + TZP recovered both GFR and urinary KIM-1 to baseline levels by day 4 of the experiment. Our results are concordant with previous preclinical studies for the injury biomarker KIM-1, in that renal injury was seen earlier and to a greater extent in the VAN alone group compared to the VAN + TZP group (15, 18, 19). Among the assessed urinary biomarkers, KIM-1 was most closely correlated with GFR over the course of the experiment. This is also consistent with our previous findings that higher urinary KIM-1 predicts worse histopathologic damage scores in the vancomycin-treated rat (15, 17). Clusterin and OPN are more general biomarkers of nonspecific tubule or glomerular damage, and both were less correlated with GFR changes in this study (17). These results and their concordance with previous animal studies provide further evidence that VAN + TZP is not associated with additive nephrotoxicity and may even be protective during the initial days of treatment.
To our knowledge, this is the first study to combine a transdermal monitoring device and intravenous fluorescent tracer for measurement of real-time GFR with renal injury biomarkers for vancomycin-induced kidney injury in a rat model. Similar to previous studies, we employed our well-established translational rat model to investigate allometrically scaled antibiotic doses and associated toxicity. Utilization of a transdermal monitoring device with an intravenous fluorescent tracer (fluorescein isothiocyanate-labeled sinistrin [FITC-sinistrin]) offers several important advantages over other approaches for estimating kidney function. First, in contrast to timed PK/PD studies in which sequential serum and/or urine samples must be collected, the transdermal device allows for continuous, noninvasive monitoring of the tracer (which is freely filtered and neither secreted nor reabsorbed by the kidneys in the case of FITC-sinistrin). Second, real-time estimation of GFR allows for the capture of early changes in renal function (i.e., days 1– to 3). Injury biomarkers are important; however, functional changes are arguably even more relevant for clinical translation. In humans, detectable rises in SCr are seen approximately 2–4 days after the initial renal injury (20, 21). Due to this lag time in SCr rise, early changes in renal function and/or damage may not be detected with conventional measures. Consequently, simultaneous capture of injury biomarkers and unbiased estimates of function allows for maximal translation to clinical utility. It is not expected that real-time functional measures of GFR will be available in routine clinical practice in the next few years; hence, understanding the relationship that defines the time course and magnitude of damage by using injury biomarkers will be important for predicting functional changes in clinical practice.
Several considerations for our study should be stated. First, because of technical difficulty, one animal did not have adequate GFR data collected on one study day. This animal was still included in our analysis because the statistical methodology that we utilized is flexible and does not require dropping the data (such as with analysis of variance [ANOVA] methods). The animal provided adequate GFR data on the other experimental days, in addition to serum and terminal kidney samples. No major interpretations change if this animal were in fact excluded from analyses (data not shown). Second, we measured 24-h urinary volumes, thereby capturing the total amounts of excreted biomarkers (versus obtaining spot concentrations). The findings do not drastically change when analyzed as 24-h concentrations (Fig. 4); however, this is a potential limitation of clinical studies in which total urine collections are logistically more difficult and less common. Future studies to enhance clinical translation may benefit from employing blood biomarkers where dilution and sample collection labor is less of a factor, compared to urinary biomarkers. Despite these concerns, it is notable that KIM-1 in the rat is a homologue of KIM-1b in humans. Human studies have corrected for urinary volume by standardizing to urinary creatinine (22); however, that is less necessary in well-controlled animal studies in which dilution can be fully calculated. Finally, while urinary KIM-1 in the rat has been linked to PK/PD predictions in humans, it is less clear what changes in rat GFR mean (23). While there is not a direct numeric translation for GFR, this study demonstrates proof of principle that GFR changes exist with VAN but do not with VAN + TZP at the administered doses. Minimally, one can conclude that TZP does not worsen kidney function or injury when added to VAN in the rat. Ultimately, these results may have clinical implications beyond the specific combination of VAN + TZP since specific renal injury biomarkers may be employed in monitoring other nephrotoxic medications. Further clarification of urinary biomarkers of renal injury is needed to elucidate specific biomarker elevations and expression patterns associated with various nephrotoxins, prior to realized functional kidney changes. In order to evaluate the nephrotoxic potential of longer courses of VAN + TZP, future studies should evaluate changes in renal function and injury associated with antibiotic exposure beyond 96 h. Although similar findings have been reported in a mouse model (16), further recapitulation of these findings in an alternative animal model will aid in validating our results.
In our translational rat model assessing kidney function, rats that received VAN had a significant decline in GFR by day 4, whereas no decline in kidney function was observed in other groups (including VAN + TZP). In conclusion, the addition of TZP to VAN does not worsen kidney function, as measured by GFR. These data are consistent with animal injury models and demonstrate that VAN + TZP is not associated with decreased glomerular function nor additive kidney injury, compared to VAN. Clinical studies that wish to assess the impact of TZP added to VAN should directly measure renal injury biomarkers and estimate GFR in an unbiased methodology that does not require serum creatinine.
MATERIALS AND METHODS
Experimental design and animals.
The experimental methods were similar to those we have previously reported for our employed translational rat model (15, 18, 19). All experiments were conducted at Midwestern University in Downers Grove, Illinois, in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (24) and were approved under the Institutional Animal Care and Use Committee protocol number 3151. In brief, male Sprague-Dawley rats (n = 26; age, approximately 8 to 10 weeks; mean weight, 274.8 g) were housed in a light- and temperature-controlled room (15, 18, 19) for the duration of the study and allowed free access to water and food (Fig. S1). All animals were placed in metabolic cages (Nalgene, Rochester, NY) for 24-h urine collection, starting prior to dosing (day 0), and were sampled every day for a period of 4 days.
The animals were assigned to one of four treatment groups in which they received either VAN 150 mg/kg/day intravenously over 2 min (n = 7), TZP 1,400 mg/kg/day intraperitoneal (n = 6), VAN + TZP (n = 7), or saline (n = 6) for 4 days (Fig. S2). VAN was given intravenously in the right jugular catheter as this has been shown to result in vancomycin-induced kidney injury in our rat model. TZP was given intraperitoneally to avoid precipitation with VAN (25). VAN 150 mg/kg/day was selected based on known nephrotoxic effect observed in previous studies (15, 19) and to approximate the human dose (25 mg/kg/day) allometrically scaled for the rat (i.e., 25 mg/kg × 6.2 [rat factor] = 155 mg/kg). TZP 1,400 mg/kg/day was selected to approximate typical human dosing (225 mg/kg/day), allometrically scaled for the rat (i.e., 225 mg/kg × 6.2 = 1,395 mg/kg). At baseline (prior to drug dosing) and following drug dosing on each day, FITC-sinistrin, 5 mg/100 g body weight was administered intravenously to quantify GFR as described below. Following completion of the dosing protocol, the rats were sacrificed and underwent nephrectomies.
Chemicals and reagents.
The rats were administered clinical grade vancomycin (lot number 167973; Fresenius Kabi, Lake Zurich, IL), piperacillin-tazobactam (lot number: 1PU19022; Apollo, Palm Beach Gardens, FL), and normal saline for injection (Hospira, Lake Forest, IL). VAN and TZP were prepared by weighing and dissolving the powder in normal saline to achieve final concentrations of 100 and 500 mg/mL, respectively. FITC-sinistrin was prepared by weighing and dissolving the powder in sterile 1× phosphate-buffered saline to achieve a final concentration of 40 mg/mL.
Blood, urine, and kidney sampling.
Double jugular vein catheters were surgically implanted 72 h prior to protocol initiation. Blood samples were drawn from the left catheter, while drug dosing occurred via the right catheter. Blood samples were obtained at prespecified time points (0 and 240 min). Each sample (0.2 mL/aliquot) was replaced with an equivalent volume of normal saline (NS) for maintenance of euvolemia. The blood samples were prepared as plasma with disodium EDTA salt dihydrate (lot number 19C1856562; Sigma-Aldrich Chemical Company, Milwaukee, WI) and centrifuged at 3,000 × g for 10 min (Thermo Fisher Scientific, Waltham, MA). Supernatants were collected and frozen at −80°C for batch analysis.
Urine samples were collected, and the volume was measured every 24 h starting from day 0. The urine samples were centrifuged at 400 × g for 5 min, and the resulting supernatant was collected, aliquoted, and stored at −80°C until batch analysis of renal biomarkers.
The rats underwent terminal nephrectomies once the dosing protocol was completed (Fig. S3). The left kidneys were formalin-fixed for batch histopathological analysis, and the right kidneys were flash frozen in liquid nitrogen and stored at −80°C.
GFR measurement.
A transdermal monitoring device and intravenous FITC-sinistrin was used to obtain real-time GFR readings. This methodology is an improvement over traditional markers of kidney function such as SCr and urine output. FITC-sinistrin is a biomarker for kidney function that shows comparable kinetics to gold-standard markers such as inulin, with superior handling and administration characteristics (26). In addition, the ability to estimate real-time GFR allows for the capture of much earlier changes in kidney function compared to creatinine (i.e., days 1 to 3). Transdermal sensors (MediBeacon GmBH, Mannheim, Germany) and FITC-sinistrin (lot number VE17200811; Fresenius Kabi, Hamburg, Germany) were used for GFR estimation. A small area on the dorsal side of each rat was depilated (Nair, Church & Dwight Co, Ewing, NJ) on day 0, 24 h prior to the start of the experimental protocol. FITC-sinistrin 5 mg/100 g body weight was administered daily as an intravenous push. Fluorescence was monitored continuously (one measurement every 8 s) via the transdermal sensor for 2 h at baseline on day 1 and 4 h after each daily dose (27). At the completion of the measurement period, the sensor was removed, and the data were transferred to a computer for analysis and storage. The data were analyzed using MB Studio software (MediBeacon GmBH, Mannheim, Germany). A three-compartment model with linear baseline correction was fit to determine FITC-sinistrin clearance vis-à-vis GFR.
Determination of urinary biomarkers of AKI.
The urine samples were batch analyzed to determine urinary concentrations of KIM-1, clusterin, and OPN. Microsphere-based Luminex xMAP technology was used for the determination of urinary protein biomarkers as previously described (17, 28). Urine samples were allowed to thaw at ambient room temperature, aliquoted into 96-well plates, and mixed with Milliplex MAP rat kidney toxicity magnetic bead panel 1 (EMD Millipore Corporation, Charles, MO). A separate standard curve was prepared and run with each assay plate per the manufacturer’s instructions. The results were analyzed, and urinary biomarker concentrations were determined using the manufacturer’s software, which utilizes flexible five-parameter (linear and logarithmic scale) curve-fitting models (Milliplex Analyst 5.1, VigeneTech, Carlisle, MA).
Histopathological analysis of kidneys.
Formalin-fixed kidneys were sent for histopathological analysis (IDEXX Bioanalytics, Columbia, MO). Blinded samples were prepared and scored according to the PSTC semiquantitative grading system. Scores range from 0 to 5, with 0 indicating no abnormality, 1 indicating minimal/very few/very small abnormalities, 2 indicating mild/slight/few/small abnormalities, 3 indicating moderate/moderate/number/moderate size abnormalities, 4 indicating marked/many/large abnormalities, and 5 indicating massive/extended number/extended size abnormalities noted. The worst overall score, representing total gross kidney damage, and the worst tubular scores were utilized in our analysis.
Statistical analysis.
A mixed-effect, restricted maximum likelihood estimation regression was used to compare urine output, mean weight loss, GFR, and urinary biomarkers among the treatment groups, with repeated measures occurring over days; measures were repeated at the level of the individual rat (Stata version 16.1, StataCorp LLC, College Station, TX). Spearman’s rank correlation coefficient with a Bonferroni correction was used to assess correlations between kidney injury (e.g., KIM-1, clusterin, and OPN) and function (e.g., GFR) by treatment day. All tests conducted were two-tailed, with an a priori level of statistical significance set at α = 0.05.
ACKNOWLEDGMENTS
The research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases under award number R21-AI149026 (to M.H.S. and G.P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
M.H.S. reports ongoing research contracts with Nevakar and SuperTrans Medical, as well as having filed U.S. patent 10688195B2. All other authors have no related conflicts of interest to declare.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Magill SS, O’Leary E, Ray SM, Kainer MA, Evans C, Bamberg WM, Johnston H, Janelle SJ, Oyewumi T, Lynfield R, Rainbow J, Warnke L, Nadle J, Thompson DL, Sharmin S, Pierce R, Zhang AY, Ocampo V, Maloney M, Greissman S, Wilson LE, Dumyati G, Edwards JR, Frank L, Godine D, Martin B, Parker E, Pasutti L, Friedman S, Jones A, Kosmicki T, Fisher J, Maslar A, Meek J, Melchreit R, Badrun F, Fiore A, Fridkin SK, Morabit SL, Perry LA, Perlmutter R, Vaeth E, Gross A, Harper J, Pattee B, Rahmathullah N, Baumbach J, Sievers M, Concannon C, Felsen C, Emerging Infections Program Hospital Prevalence Survey Team, et al. 2021. Antimicrobial use in US hospitals: comparison of results from emerging infections program prevalence surveys, 2015 and 2011. Clin Infect Dis 72:1784–1792. 10.1093/cid/ciaa373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J. 2012. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 54:621–629. 10.1093/cid/cir895. [DOI] [PubMed] [Google Scholar]
- 3.Aljefri DM, Avedissian SN, Rhodes NJ, Postelnick MJ, Nguyen K, Scheetz MH. 2019. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis 69:1881–1887. 10.1093/cid/ciz051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. 2009. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 49:507–514. 10.1086/600884. [DOI] [PubMed] [Google Scholar]
- 5.Sanders WE, Jr, Sanders CC. 1996. Piperacillin/tazobactam: a critical review of the evolving clinical literature. Clin Infect Dis 22:107–123. 10.1093/clinids/22.1.107. [DOI] [PubMed] [Google Scholar]
- 6.Perry CM, Markham A. 1999. Piperacillin/tazobactam: an updated review of its use in the treatment of bacterial infections. Drugs 57:805–843. 10.2165/00003495-199957050-00017. [DOI] [PubMed] [Google Scholar]
- 7.Giuliano CA, Patel CR, Kale-Pradhan PB. 2016. Is the combination of piperacillin-tazobactam and vancomycin associated with development of acute kidney injury?: a meta-analysis. Pharmacotherapy 36:1217–1228. 10.1002/phar.1851. [DOI] [PubMed] [Google Scholar]
- 8.Hammond DA, Smith MN, Li C, Hayes SM, Lusardi K, Bookstaver PB. 2016. Systematic review and metaanalysis of acute kidney injury associated with concomitant vancomycin and piperacillin/tazobactam. Clinid 64:666–674. 10.1093/cid/ciw811. [DOI] [PubMed] [Google Scholar]
- 9.Luther MK, Timbrook TT, Caffrey AR, Dosa D, Lodise TP, LaPlante KL. 2018. Vancomycin plus piperacillin-tazobactam and acute kidney injury in adults: a systematic review and meta-analysis. Crit Care Med 46:12–20. 10.1097/CCM.0000000000002769. [DOI] [PubMed] [Google Scholar]
- 10.Bellos I, Karageorgiou V, Pergialiotis V, Perrea DN. 2020. Acute kidney injury following the concurrent administration of antipseudomonal β-lactams and vancomycin: a network meta-analysis. Clin Microbiol Infect 26:696–705. 10.1016/j.cmi.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Blair M, Côté JM, Cotter A, Lynch B, Redahan L, Murray PT. 2021. Nephrotoxicity from vancomycin combined with piperacillin-tazobactam: a comprehensive review. Am J Nephrol 52:85–97. 10.1159/000513742. [DOI] [PubMed] [Google Scholar]
- 12.Avedissian SN, Pais GM, Liu J, Rhodes NJ, Scheetz MH. 2020. Piperacillin-tazobactam added to vancomycin increases risk for acute kidney injury: fact or fiction? Clin Infect Dis 71:426–432. 10.1093/cid/ciz1189. [DOI] [PubMed] [Google Scholar]
- 13.Vaidya VS, Ferguson MA, Bonventre JV. 2008. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol 48:463–493. 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duarte CG, Preuss HG. 1993. Assessment of renal function – glomerular and tubular. Clin Lab Med 13:33–52. 10.1016/S0272-2712(18)30459-1. [DOI] [PubMed] [Google Scholar]
- 15.Pais GM, Liu J, Avedissian SN, Hiner D, Xanthos T, Chalkias A, d’Aloja E, Locci E, Gilchrist A, Prozialeck WC, Rhodes NJ, Lodise TP, Fitzgerald JC, Downes KJ, Zuppa AF, Scheetz MH. 2020. Lack of synergistic nephrotoxicity between vancomycin and piperacillin/tazobactam in a rat model and a confirmatory cellular model. J Antimicrob Chemother 75:1228–1236. 10.1093/jac/dkz563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He M, Souza E, Matvekas A, Crass RL, Pai MP. 2021. Alteration in acute kidney injury potential with the combination of vancomycin and imipenem-cilastatin/relebactam or piperacillin/tazobactam in a preclinical model. Antimicrob Agents Chemother 65:e02141-20. 10.1128/AAC.02141-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pais GM, Avedissian SN, O’Donnell JN, Rhodes NJ, Lodise TP, Prozialeck WC, Lamar PC, Cluff C, Gulati A, Fitzgerald JC, Downes KJ, Zuppa AF, Scheetz MH. 2019. Comparative performance of urinary biomarkers for vancomycin-induced kidney injury according to timeline of injury. Antimicrob Agents Chemother 63:e00079-19. 10.1128/AAC.00079-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Donnell JN, Rhodes NJ, Lodise TP, Prozialeck WC, Miglis CM, Joshi MD, Venkatesan N, Pais G, Cluff C, Lamar PC, Briyal S, Day JZ, Gulati A, Scheetz MH. 2017. 24-hour pharmacokinetic relationships for vancomycin and novel urinary biomarkers of acute kidney injury. Antimicrob Agents Chemother 61:e00416-17. 10.1128/AAC.00416-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avedissian SN, Pais GM, O'Donnell JN, Lodise TP, Liu J, Prozialeck WC, Joshi MD, Lamar PC, Becher L, Gulati A, Hope W, Scheetz MH. 2019. Twenty-four hour pharmacokinetic relationships for intravenous vancomycin and novel urinary biomarkers of acute kidney injury in a rat model. J Antimicrob Chemother 74:2326–2334. 10.1093/jac/dkz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostermann M, Bellomo R, Burdmann EA, Doi K, Endre ZH, Goldstein SL, Kane-Gill SL, Liu KD, Prowle JR, Shaw AD, Srisawat N, Cheung M, Jadoul M, Winkelmayer WC, Kellum JA, Conference Participants. 2020. Controversies in acute kidney injury: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) conference. Kidney Int 98:294–309. 10.1016/j.kint.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delanaye P, Cavalier E, Pottel H. 2017. Serum creatinine: not so simple!. Nephron 136:302–308. 10.1159/000469669. [DOI] [PubMed] [Google Scholar]
- 22.Sabbisetti VS, Waikar SS, Antoine DJ, Smiles A, Wang C, Ravisankar A, Ito K, Sharma S, Ramadesikan S, Lee M, Briskin R, De Jager PL, Ngo TT, Radlinski M, Dear JW, Park KB, Betensky R, Krolewski AS, Bonventre JV. 2014. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol 25:2177–2186. 10.1681/ASN.2013070758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheetz MH, Pais GM, Lodise TP, Tong SY, Davis JS, O’Donnell JN, Liu J, Neely M, Prozialeck WC, Lamar PC, Rhodes NJ, Holland T, Avedissian SN. 2021. Of rats and men: a translational model to understand vancomycin pharmacokinetic/toxicodynamic relationships. Antimicrob Agents Chemother 65:e01060-21. 10.1128/AAC.01060-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
- 25.O’Donnell JN, Venkatesan N, Manek M, Rhodes NJ, Scheetz MH. 2016. Visual and absorbance analyses of admixtures containing vancomycin and piperacillin-tazobactam at commonly used concentrations. Am J Health Syst Pharm 73:241–246. 10.2146/ajhp150170. [DOI] [PubMed] [Google Scholar]
- 26.Pill J, Issaeva O, Woderer S, Sadick M, Kränzlin B, Fiedler F, Klötzer HM, Krämer U, Gretz N. 2006. Pharmacological profile and toxicity of fluorescein-labelled sinistrin, a novel marker for GFR measurements. Naunyn Schmiedebergs Arch Pharmacol 373:204–211. 10.1007/s00210-006-0067-0. [DOI] [PubMed] [Google Scholar]
- 27.Scarfe L, Schock-Kusch D, Ressel L, Friedemann J, Shulhevich Y, Murray P, Wilm B, de Caestecker M. 2018. Transdermal measurement of glomerular filtration rate in mice. J Vis Exp 58520. 10.3791/58520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhodes NJ, Prozialeck WC, Lodise TP, Venkatesan N, O’Donnell JN, Pais G, Cluff C, Lamar PC, Neely MN, Gulati A, Scheetz MH. 2016. Evaluation of vancomycin exposures associated with elevations in novel urinary biomarkers of acute kidney injury in vancomycin-treated rats. Antimicrob Agents Chemother 60:5742–5751. 10.1128/AAC.00591-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figures and table. Download aac.02132-21-s0001.pdf, PDF file, 0.3 MB (281.4KB, pdf)