ABSTRACT
Trimethoprim/sulfamethoxazole (TMP/SMZ) is considered the treatment of choice for infections caused by Stenotrophomonas maltophilia, but limited pharmacodynamic data are available to support current susceptibility breakpoints or guide optimal dosing. Time-kill studies using a TMP/SMZ concentration of 4/40 μg/mL were conducted to compare 4 S. maltophilia with 4 Escherichia coli isolates having the same MICs (0.25/4.75 to 4/76 μg/mL) in cation-adjusted Mueller-Hinton broth (CAMHB) and ISO-Sensitest broth (ISO broth). With the exception of the resistant isolates (4/76 μg/mL), which resulted in regrowth approaching the growth of the control, TMP/SMZ displayed significantly greater killing for E. coli than for S. maltophilia at each MIC. Against E. coli, the mean changes at 24 h were −4.49, −1.73, −1.59, and +1.83 log10 CFU for isolates with MICs of 0.25/4.75, 1/19, 2/39, and 4/74 μg/mL, respectively. The area under the concentration-time curve for the free, unbound fraction of the drug (fAUC)/MIC ratio required for stasis and 1-log10 and 2-log10 CFU reductions were 40.7, 59.5, and 86.3, respectively. In contrast, TMP/SMZ displayed no stasis or CFU reductions against any S. maltophilia isolate regardless of the MIC, and no pharmacodynamic thresholds were quantifiable. Observations were consistent in both CAMHB and ISO broth. These data add increasing evidence that current TMP/SMZ susceptibility breakpoints against S. maltophilia should be reassessed.
KEYWORDS: Gram negative, in vitro, pharmacodynamics, susceptibility breakpoint
TEXT
Stenotrophomonas maltophilia is a multidrug-resistant Gram-negative bacterium that is increasing in prevalence, particularly among critically ill and immunocompromised patients (1, 2). This pathogen can cause severe infections in the respiratory tract, bloodstream, and skin and skin structures, among various other body sites. Unfortunately, there are few antibiotic regimens that retain predictable microbiological activity against S. maltophilia, making treatment challenging. Among these antibiotics, trimethoprim/sulfamethoxazole (TMP/SMZ) is widely considered the drug of choice largely due to its high susceptibility rates (3, 4). The Clinical and Laboratory Standards Institute (CLSI) defines S. maltophilia as susceptible when TMP/SMZ MICs are ≤2/38 μg/mL (5). At this breakpoint, approximately 95% of S. maltophilia isolates worldwide are susceptible to TMP/SMZ (6). In contrast, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) defines susceptibility at MICs of ≤0.001 μg/mL, thereby making most S. maltophilia isolates, by this definition, fall into the intermediate category and require a higher dosage for treatment (7).
Clinical studies supporting the utilization of TMP/SMZ for the treatment of S. maltophilia infections are generally small, retrospective, single-center assessments and offer mixed results (8–13). Notably, no studies have evaluated outcome by TMP/SMZ MIC, and pharmacodynamic studies that characterize the exposure-response relationship for TMP/SMZ against S. maltophilia are not available. Such data could prove useful when reassessing susceptibility breakpoints and help to guide dosing regimen selection. Unfortunately, in vivo animal infection models are poorly translational for dihydrofolate reductase inhibitors since rodents have considerably high concentrations of thymidine in plasma compared with humans (14). This surplus permits the uptake of exogenous thymidine by bacteria and conversion into thymidylate by thiamine kinase, a known salvage pathway for DNA synthesis that antagonizes the in vitro activity of trimethoprim (15, 16). Notably, broth used for in vitro studies may also contain exogenous thymidine, which could influence the activity of TMP/SMZ against both Gram-positive and Gram-negative organisms (16, 17). Here, we employed time-kill studies to evaluate the exposure-response relationship of TMP/SMZ against 4 S. maltophilia clinical isolates compared with Escherichia coli isolates harboring the same MICs. E. coli was selected as a comparator since it is known to use exogenous thymidine to counteract TMP/SMZ activity (16), and resistance has been linked with clinical failure (18–20). This opportunity was also used to evaluate activity in standard cation-adjusted Mueller-Hinton broth (CAMHB) versus ISO-Sensitest broth (ISO broth), which may have differing thymidine concentrations.
RESULTS
Broth microdilution.
Modal TMP/SMZ broth microdilution MIC results ranged from 0.25/4.75 to 4/76 μg/mL (Table 1). MICs for each isolate were the same or within 1 MIC dilution between CAMHB and ISO broth. Two isolates (one S. maltophilia and one E. coli) tested 1 dilution lower in ISO broth than in CAMHB, while a single S. maltophilia isolate tested 1 dilution higher in ISO broth than in CAMHB. Since CAMHB is frequently used during in vitro pharmacodynamic studies, MICs derived from CAMHB were used as the reference for all comparisons.
TABLE 1.
Modal broth microdilution MICs for TMP/SMZ in cation-adjusted Mueller-Hinton broth and ISO-Sensitest broth against the 4 S. maltophilia and 4 E. coli isolatesa
| CAIRD ID | External ID | TMP/SMZ MIC (μg/mL) |
|
|---|---|---|---|
| CAMHB | ISO broth | ||
| S. maltophilia isolates | |||
| STM29 | 0.25/4.75 | 0.5/9.5 | |
| STMC43-11 | 1/19 | 1/19 | |
| STM106 | IHMA 2097696 | 2/38 | 1/19 |
| STMC42-15 | 4/76 | 4/76 | |
| E. coli isolates | |||
| EC25922 | ATCC 25922 | 0.25/4.75 | 0.25/4.75 |
| EC762 | CDC0019 | 1/19 | 1/19 |
| EC778 | IHMA 2249758 | 2/38 | 1/19 |
| EC780 | IHMA 2259927 | 4/76 | 4/76 |
TMP/SMZ, trimethoprim/sulfamethoxazole; CAMHB, cation-adjusted Mueller-Hinton broth; ISO broth, ISO-Sensitest broth.
Time-kill studies.
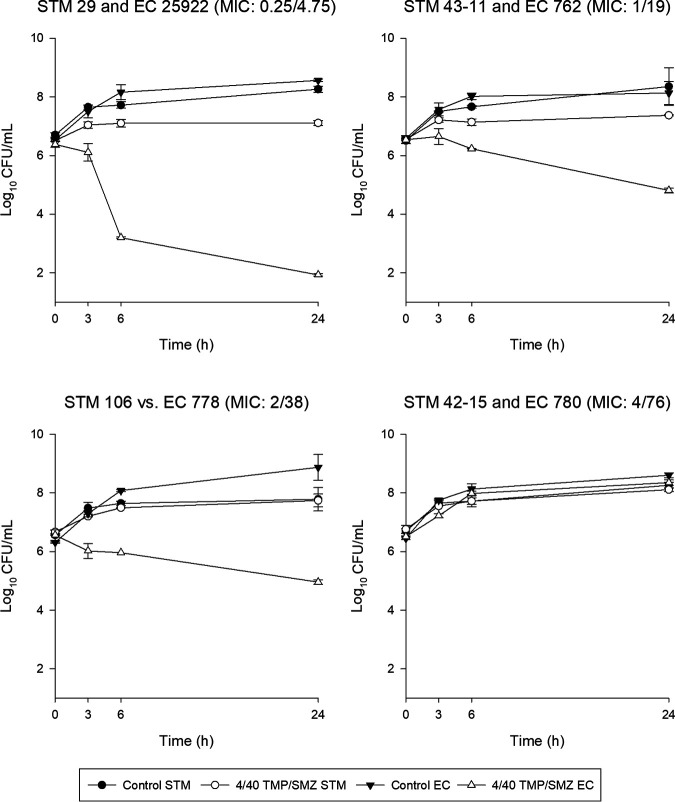
The average starting bacterial densities for S. maltophilia in CAMHB and ISO broth were 6.60 ± 0.11 and 6.59 ± 0.10 log10 CFU/mL, respectively. The mean starting bacterial densities for E. coli were 6.48 ± 0.11 log10 CFU/mL in CAMHB and 6.49 ± 0.16 log10 CFU/mL in ISO broth. Twenty-four-hour control bacterial densities increased robustly for S. maltophilia (CAMHB, 8.08 ± 0.39 log10 CFU/mL; ISO broth, 8.40 ± 0.35 log10 CFU/mL) and E. coli (CAMHB, 8.45 ± 0.38 log10 CFU/mL; ISO broth, 8.51 ± 0.30 log10 CFU/mL). After exposure to a concentration of TMP/SMZ of 4/40 μg/mL for 24 h, which is consistent with the average free steady-state concentration for 20 mg/kg of body weight daily (trimethoprim component, ∼100 μg · h/mL) in humans (21), none of the S. maltophilia isolates demonstrated greater than a static CFU reduction in CAMHB (Fig. 1). In contrast, E. coli isolates with MICs of <4/76 μg/mL achieved >1-log10 CFU/mL reductions. The results were nearly identical in ISO broth (data not shown). The nonsusceptible S. maltophilia and E. coli isolates regrew to control CFU in both media. Aside from the isolates with an MIC of 4/76 μg/mL, significant differences were observed between the S. maltophilia and E. coli isolates at all other MICs in both media (Table 2). Finally, no significant differences were seen between CAMHB and ISO broth for individual isolates with the exception of two observations (EC762 and EC780).
FIG 1.
In vitro time-kill growth curves of S. maltophilia and E. coli in CAMHB after exposure to TMP/SMZ at 4/40 μg/mL. Isolates are referenced to CAMHB MICs for comparison.
TABLE 2.
Comparison of 24-h CFU reductions following TMP/SMZ exposure in time-kill studies between S. maltophilia and E. coli at the same MIC in CAMHB and by broth for individual isolatesa
| Comparison | Mean change in log10 CFU/mL at 24 h ± SD | P valueb | |
|---|---|---|---|
| By isolate at the same MIC (TMP/SMZ MIC [μg/mL]) | S. maltophilia | E. coli | |
| CAMHB | |||
| STM29 vs EC25922 (0.25/4.75) | 0.60 ± 0.08 | −4.49 ± 0.06 | <0.001 |
| STMC43-11 vs EC762 (1/19) | 0.81 ± 0.16 | −1.73 ± 0.10 | 0.007 |
| STM106 vs EC778 (2/38) | 1.07 ± 0.16 | −1.59 ± 0.26 | 0.005 |
| STMC42-15 vs EC780 (4/76) | 1.36 ± 0.08 | 1.83 ± 0.07 | 0.016 |
| ISO brothc | |||
| STM29 vs EC25922 (0.25/4.75) | 0.49 ± 0.06 | −4.42 ± 0.65 | <0.001 |
| STMC43-11 vs EC762 (1/19) | 1.15 ± 0.06 | −2.93 ± 0.16 | 0.002 |
| STM106 vs EC778 (2/38) | 1.03 ± 0.18 | −1.46 ± 0.24 | 0.003 |
| STMC42-15 vs EC780 (4/76) | 1.52 ± 0.15 | 1.20 ± 0.11 | NS |
| By broth for individual isolates | CAMHB | ISO broth | |
| S. maltophilia | |||
| STM29 | 0.60 ± 0.08 | 0.49 ± 0.06 | NS |
| STMC43-11 | 0.81 ± 0.16 | 1.15 ± 0.06 | NS |
| STM106 | 1.07 ± 0.16 | 1.03 ± 0.18 | NS |
| STMC42-15 | 1.36 ± 0.08 | 1.52 ± 0.15 | NS |
| E. coli | |||
| EC25922 | −4.49 ± 0.06 | −4.42 ± 0.65 | NS |
| EC762 | −1.73 ± 0.10 | −2.93 ± 0.16 | 0.010 |
| EC778 | −1.59 ± 0.26 | −1.46 ± 0.24 | NS |
| EC780 | 1.83 ± 0.07 | 1.20 ± 0.11 | 0.022 |
TMP/SMZ, trimethoprim/sulfamethoxazole; CAMHB, cation-adjusted Mueller-Hinton broth; ISO broth, ISO-Sensitest broth; NS, not significant.
Comparisons were done by one-way ANOVA and the Holm-Sidak pairwise test for multiple comparisons.
Isolates referenced by their CAMHB MICs during comparison.
Pharmacodynamic analyses.
Final maximum-effect (Emax) model fits are shown in Fig. 2a for S. maltophilia and Fig. 2b for E. coli. Data from both CAMHB and ISO broth were analyzed together since no changes in parameters or thresholds were observed when analyzed separately (data not shown). Table 3 provides the Emax model parameters and area under the concentration-time curve for the free, unbound fraction of the drug (fAUC)/MIC exposures (of the trimethoprim component) required for stasis and 1-log10 and 2-log10 CFU reductions. Notably, values were quantifiable for only E. coli.
FIG 2.
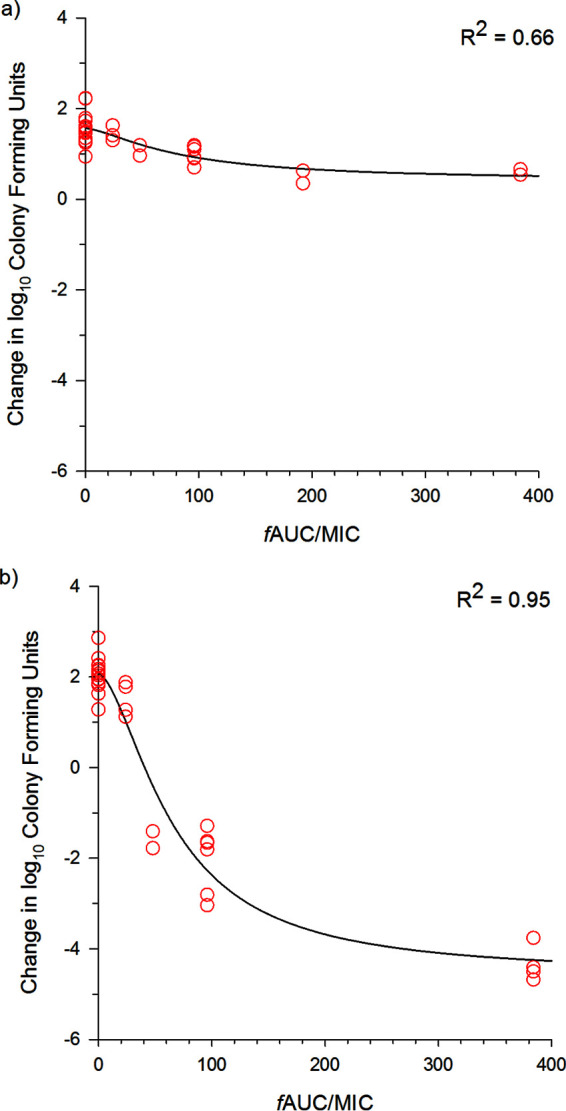
TMP/SMZ exposure-response Emax model fits based on the trimethoprim component for S. maltophilia (a) and E. coli (b). Individual data points are plotted by the modal MIC in the respective media.
TABLE 3.
Final Emax model parameters and fAUC/MIC ratios (of the trimethoprim component) required for stasis and 1-log10 and 2-log10 CFU reductions for S. maltophilia and E. colia
| Parameter | S. maltophilia | E. coli |
|---|---|---|
| Model parameters | ||
| Imax | 1.16 | 6.63 |
| IC50 | 83.5 | 65.8 |
| E0 | 1.57 | 2.05 |
| Gamma | 1.47 | 1.66 |
| PD threshold (fAUC/MIC ratio [of the trimethoprim component]) | ||
| Stasis | NC | 40.7 |
| 1-log10 CFU reduction | NC | 59.9 |
| 2-log10 CFU reduction | NC | 86.3 |
Imax, maximum effect (difference between the minimum and maximum observed CFU); IC50, inhibitory concentration required for 50% of the maximum effect; E0, maximum observed CFU; Gamma, slope constant; PD, pharmacodynamic; NC, not able to be calculated.
DISCUSSION
Attributed to its high susceptibility rate, TMP/SMZ is considered by many to be the antibiotic of choice for the treatment of infections caused by S. maltophilia (2). The CLSI and EUCAST define susceptibility breakpoints at ≤2/38 μg/mL and ≤0.001 μg/mL (trimethoprim component only), respectively, which results in fewer than ∼5% of S. maltophilia isolates being defined as resistant. In the United States, TMP/SMZ is not approved for the treatment of any Gram-negative infections outside the urinary tract (approved dose of 8 to 10 mg/kg/day divided into 2 to 4 equal doses daily); however, dosing regimens of 15 to 20 mg/kg daily are indicated for Pneumocystis jirovecii pneumonia (PJP) (22). In retrospective studies of TMP/SMZ for the treatment of S. maltophilia infection, dosing regimens vary from 8 to 10 mg/kg/day up to 15 to 20 mg/kg/day in individual patients. Unfortunately, no pharmacodynamic studies for TMP/SMZ are available to establish stasis and 1-log CFU reduction thresholds to support current susceptibility breakpoints or optimal dosing against S. maltophilia. Here, we performed time-kill studies against 4 S. maltophilia clinical isolates with increasing MICs, which offered an opportunity to compare exposure-response relationships with E. coli isolates having the same MICs as well as to compare the effects of different broth media for these in vitro studies.
The effects of thymidine concentrations on TMP/SMZ in vitro and in vivo activities against certain Gram-positive and Gram-negative bacteria are well established (16, 17, 23, 24). Furthermore, the CLSI requires broth quality control (QC) with Enterococcus faecalis ATCC 29212, an organism very sensitive to the antagonistic effects of exogenous thymidine, to confirm the suitability of media used during MIC studies (25). To our knowledge, no data are available to demonstrate or refute that S. maltophilia uses these exogenous nucleosides to escape TMP/SMZ activity. Given that both media passed all QCs, it was not surprising that TMP/SMZ MICs in CAMHB and ISO media for these S. maltophilia and E. coli isolates were within 1 dilution of each other, if not the same. Further studies are needed to understand if exogenous thymidine can be used by S. maltophilia. Additionally, 24-h time-kill CFU reductions were consistent for these isolates compared between broths (Table 2), with only two exceptions among E. coli isolates. EC762 showed a 1-log10-greater reduction in CFU at 24 h in ISO broth (−2.93 log10 CFU) than in CAMHB (−1.73 log10 CFU); notably, this isolate demonstrated the same MIC (1/19 μg/mL) in both media. EC780 also showed a significant difference in CFU at 24 h, but regrowth that approached the growth of the control isolates was observed in both media. Since most MIC and in vitro pharmacodynamic studies within our laboratory are conducted with CAMHB, we referenced the isolates by their MICs in this medium for comparisons and used the broth-specific MICs during pharmacodynamic analyses (i.e., fAUC/MIC ratio).
In CAMHB, the kill curves of S. maltophilia when exposed to average free steady-state concentrations of an aggressive TMP/SMZ 20-mg/kg daily dosage demonstrated little activity. Regardless of the TMP/SMZ MIC (0.25/4.75 to 4/76 μg/mL), 24-h CFU counts were +0.6 to +1.36 log10 CFU (Fig. 1). In contrast, TMP/SMZ demonstrated substantial killing at 24 h against the E. coli isolates, which was in agreement with their MICs. Mean changes in 24-h CFU were −4.49, −1.73, −1.59, and +1.83 log10 CFU for isolates with MICs of 0.25/4.75, 1/19, 2/39, and 4/74 μg/mL, respectively. All changes were significantly different from S. maltophilia at the same MIC (Table 2). Similar observations were made in ISO broth.
Previous literature corroborates the lack of in vitro single-agent trimethoprim/sulfamethoxazole activity against S. maltophilia despite defined susceptibility; however, none of these studies attempted to characterize any exposure-response relationship. Biagi and colleagues evaluated TMP/SMZ as monotherapy and in combination with cefiderocol against S. maltophilia in a time-kill model using CAMHB (26). Exposures of 4× MIC demonstrated stasis or regrowth at 24 h against the three susceptible isolates (TMP/SMZ MICs of ≤0.5 μg/mL). Of note, the addition of cefiderocol at 1/2 the MIC increased 24-h kill by 1 to 2 logs for these isolates. Time-kill studies using a fixed concentration of 2/38 μg/mL were also unable to identify CFU reductions against 12 S. maltophilia isolates with TMP/SMZ MICs of between 0.25/4.75 and ≥8/152 μg/mL (27). Using an in vitro chemostat pharmacodynamic model, Zelenitsky and colleagues simulated free-drug exposures of TMP/SMZ at 5 mg/kg (trimethoprim component) every 12 h (q12h) against 4 S. maltophilia isolates with MICs of 1/19 to 2/38 μg/mL (28). Similar to our time-kill experiments, stasis or regrowth was observed for all isolates.
Given the lack of any exposure-response analyses and the range of MICs for these S. maltophilia and E. coli isolates, Emax modeling using the composite curve of isolates was employed to determine the exposures required for stasis and 1-log10 and 2-log10 CFU reductions. For E. coli isolates, these thresholds were fAUC/MIC ratios (based on the trimethoprim component) of 40.7, 59.5, and 86.3, respectively. We assumed the fAUC/MIC ratio to be the pharmacodynamic parameter for TMP/SMZ, as have other studies targeting tuberculosis, melioidosis, Neisseria meningitidis infections, and enterococcal urinary tract infections (29–32). These studies reference an fAUC/MIC ratio of >25 as the threshold for the TMP component but admit that this target is arbitrary. Indeed, at least for E. coli, higher thresholds are required but should be achievable for most isolates currently defined as susceptible. In contrast, against these S. maltophilia isolates, we were unable to quantify the fAUC/MIC ratio required for any of these endpoints. Further pharmacodynamic studies are warranted to confirm that the fAUC/MIC ratio is the pharmacodynamic parameter best correlated with killing for TMP/SMZ as well as to conduct dose-ranging studies on individual isolates to determine if 1-log10 CFU reductions are achievable against S. maltophilia. For serious infections such as pneumonia and bloodstream infections, pharmacodynamic thresholds that achieve 1-log10 CFU reductions are preferred during dosage optimization and the selection of susceptibility breakpoints (33). It is therefore concerning that TMP/SMZ is unable to achieve such thresholds against this pathogen.
In summary, the exposure-response relationship was analyzed for TMP/SMZ against 4 S. maltophilia and E. coli isolates. Despite having the same MIC, these studies yielded discordant CFU reductions for susceptible S. maltophilia and E. coli isolates. TMP/SMZ fAUC/MIC thresholds for stasis and 1-log10 and 2-log10 CFU reductions were identified for E. coli. However, no such thresholds were observed against S. maltophilia, and further studies to define the TMP/SMZ pharmacodynamic target against this pathogen are needed. These data add increasing evidence that current TMP/SMZ susceptibility breakpoints against S. maltophilia should be reassessed.
MATERIALS AND METHODS
Antimicrobial agents and broth.
Trimethoprim (lot number 019M4019V, expiration date of October 2022) and sulfamethoxazole (lot number BCCB6035, expiration date of April 2022) analytical powders were purchased separately from Sigma-Aldrich (St. Louis, MO, USA). Stock solutions were prepared in line with CLSI document M100 (5). Cation-adjusted Mueller-Hinton broth (lot number 0286591, expiration date of April 2024; Becton, Dickinson and Company, Sparks, MD, USA) and ISO broth (lot number 3143645, expiration date of September 2025; Oxoid Ltd., Cheshire, UK) were acquired from approved vendors.
Isolates.
Eight clinical isolates (4 S. maltophilia and 4 E. coli) were included. Isolates were selected based on initial TMP/SMZ MICs in CAMHB and sourced from the CAIRD isolate repository, the American Type Culture Collection (ATCC), International Health Management Associates (IHMA), and the Centers for Disease Control and Prevention and Food and Drug Administration Antimicrobial Resistance Bank (CDC/FDA-ARB). Isolates were stored at −80°C and subcultured twice prior to experiments.
In vitro susceptibility.
MICs were determined in triplicate by broth microdilution to confirm previously reported TMP/SMZ MICs in CAMHB and to determine MICs in ISO broth. Briefly, trimethoprim/sulfamethoxazole trays (1:19) were prepared in each broth medium according to CLSI guidance with quality control testing (5, 25). Enterococcus faecalis ATCC 29212 and Pseudomonas aeruginosa ATCC 27853 were used as quality controls in both broth media.
Simulated TMP/SMZ exposures.
Time-kill studies were performed using the free-drug average steady-state concentration (fCss) for a 20-mg/kg daily TMP/SMZ dose (based on the trimethoprim component) from critically ill patients (21). The trimethoprim 24-h total drug area under the curve was ∼177 μg · h/mL and, when corrected by 44% protein binding (22) and divided by 24 h, was equal to an fCss of 4 μg/mL. When administered in the clinically available 1:5 ratio of TMP/SMZ, sulfamethoxazole total drug concentrations are 19 times higher than those of trimethoprim, but when corrected for sulfamethoxazole protein binding of 70%, the concentration ratio is 1:10; therefore, the sulfamethoxazole fCss was 40 μg/mL (or 4/40 μg/mL for the TMP/SMZ combination).
Time-kill studies.
Time-kill studies were conducted as described previously (34). All experiments were conducted in duplicate, and control experiments (no TMP/SMZ) were conducted simultaneously with treatment studies. CAMHB and ISO broth were inoculated with bacterial suspensions to achieve a starting inoculum of 106 CFU/mL. Final broth volumes were 10 mL. Broth was immediately incubated in a shaking water bath at 37°C. For each experiment, samples were taken at 0, 3, 6, and 24 h; serially diluted onto blood agar plates; and allowed to incubate overnight, and bacterial densities were enumerated (CFU per milliliter). The lowest accurately countable number was 5 × 101 CFU.
Pharmacodynamic and statistical analyses.
Trimethoprim exposure (fAUC/MIC ratio) was modeled using an Emax model (Hill equation) in Phoenix WinNonlin version 8.3 (Pharsight Corp., Mountain View, CA, USA) for each organism separately. Exposures predictive of stasis and 1-log10 and 2-log10 CFU reductions were calculated. Comparisons of 24-h CFU changes between isolates (S. maltophilia versus E. coli at the same MIC) and broth (CAMHB versus ISO broth for individual isolates) were made by one-way analysis of variance (ANOVA) with the Holm-Sidak test for multiple comparisons (Sigma Plot version 14; Systat Software, Inc., San Jose, CA).
ACKNOWLEDGMENTS
We thank Nicole DeRosa, Ceara Wettemann, Rebecca Stewart, Julio Rodriguez, and Jody Therieault for assistance with the conduct of these experiments.
This work was supported by the U.S. Food and Drug Administration (75F40120C00171).
We have no conflicts to disclose.
REFERENCES
- 1.Cai B, Tillotson G, Benjumea D, Callahan P, Echols R. 2020. The burden of bloodstream infections due to Stenotrophomonas maltophilia in the United States: a large, retrospective database study. Open Forum Infect Dis 7:ofaa141. 10.1093/ofid/ofaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooke JS. 2021. Advances in the microbiology of Stenotrophomonas maltophilia. Clin Microbiol Rev 34:e00030-19. 10.1128/CMR.00030-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livermore DM, Mushtaq S, Warner M, Woodford N. 2014. Comparative in vitro activity of sulfametrole/trimethoprim and sulfamethoxazole/trimethoprim and other agents against multiresistant Gram-negative bacteria. J Antimicrob Chemother 69:1050–1056. 10.1093/jac/dkt455. [DOI] [PubMed] [Google Scholar]
- 4.Brooke JS. 2012. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 25:2–41. 10.1128/CMR.00019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2021. Performance standards for antimicrobial susceptibility testing, 31st ed, CLSI supplement M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 6.Sader HS, Duncan LR, Arends SJR, Carvalhaes CG, Castanheira M. 2020. Antimicrobial activity of aztreonam-avibactam and comparator agents when tested against a large collection of contemporary Stenotrophomonas maltophilia isolates from medical centers worldwide. Antimicrob Agents Chemother 64:e01433-20. 10.1128/AAC.01433-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Committee on Antimicrobial Susceptibility Testing. 2021. Breakpoint tables for interpretation of MICs and zone diameters, version 11.0. http://www.eucast.org.
- 8.Hand E, Davis H, Kim T, Duhon B. 2016. Monotherapy with minocycline or trimethoprim/sulfamethoxazole for treatment of Stenotrophomonas maltophilia infections. J Antimicrob Chemother 71:1071–1075. 10.1093/jac/dkv456. [DOI] [PubMed] [Google Scholar]
- 9.Araoka H, Baba M, Okada C, Abe M, Kimura M, Yoneyama A. 2017. Evaluation of trimethoprim-sulfamethoxazole based combination therapy against Stenotrophomonas maltophilia: in vitro effects and clinical efficacy in cancer patients. Int J Infect Dis 58:18–21. 10.1016/j.ijid.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Ebara H, Hagiya H, Haruki Y, Kondo E, Otsuka F. 2017. Clinical characteristics of Stenotrophomonas maltophilia bacteremia: a regional report and a review of a Japanese case series. Intern Med 56:137–142. 10.2169/internalmedicine.56.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junco SJ, Bowman MC, Turner RB. 2021. Clinical outcomes of Stenotrophomonas maltophilia infection treated with trimethoprim/sulfamethoxazole, minocycline, or fluoroquinolone monotherapy. Int J Antimicrob Agents 58:106367. 10.1016/j.ijantimicag.2021.106367. [DOI] [PubMed] [Google Scholar]
- 12.Nys C, Cherabuddi K, Venugopalan V, Klinker KP. 2019. Clinical and microbiologic outcomes in patients with monomicrobial Stenotrophomonas maltophilia infections. Antimicrob Agents Chemother 63:e00788-19. 10.1128/AAC.00788-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamdi AM, Fida M, Abu Saleh OM, Beam E. 2020. Stenotrophomonas bacteremia antibiotic susceptibility and prognostic determinants: Mayo Clinic 10-year experience. Open Forum Infect Dis 7:ofaa008. 10.1093/ofid/ofaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nottebrock H, Then R. 1977. Thymidine concentrations in serum and urine of different animal species and man. Biochem Pharmacol 26:2175–2179. 10.1016/0006-2952(77)90271-4. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton-Miller JM. 1988. Reversal of activity of trimethoprim against gram-positive cocci by thymidine, thymine and ‘folates’. J Antimicrob Chemother 22:35–39. 10.1093/jac/22.1.35. [DOI] [PubMed] [Google Scholar]
- 16.Tokunaga T, Oka K, Takemoto A, Ohtsubo Y, Gotoh N, Nishino T. 1997. Efficacy of trimethoprim in murine experimental infection with a thymidine kinase-deficient mutant of Escherichia coli. Antimicrob Agents Chemother 41:1042–1045. 10.1128/AAC.41.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones C, Stevens DL, Ojo O. 1987. Effect of minimal amounts of thymidine on activity of trimethoprim-sulfamethoxazole against Staphylococcus epidermidis. Antimicrob Agents Chemother 31:144–147. 10.1128/AAC.31.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talan DA, Stamm WE, Hooton TM, Moran GJ, Burke T, Iravani A, Reuning-Scherer J, Church DA. 2000. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis in women: a randomized trial. JAMA 283:1583–1590. 10.1001/jama.283.12.1583. [DOI] [PubMed] [Google Scholar]
- 19.Brown PD, Freeman A, Foxman B. 2002. Prevalence and predictors of trimethoprim-sulfamethoxazole resistance among uropathogenic Escherichia coli isolates in Michigan. Clin Infect Dis 34:1061–1066. 10.1086/339491. [DOI] [PubMed] [Google Scholar]
- 20.Abrahamian FM, Krishnadasa A, Mower WR, Moran GJ, Coker JR, Talan DA. 2011. The association of antimicrobial resistance with cure and quality of life among women with acute uncomplicated cystitis. Infection 39:507–514. 10.1007/s15010-011-0163-z. [DOI] [PubMed] [Google Scholar]
- 21.Chin TW, Vandenbroucke A, Fong IW. 1995. Pharmacokinetics of trimethoprim-sulfamethoxazole in critically ill and non-critically ill AIDS patients. Antimicrob Agents Chemother 39:28–33. 10.1128/AAC.39.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Pharmaceuticals. 2017. Bactrim (trimethoprim/sulfamethoxazole) for injection, for intravenous use. Prescribing information. Sun Pharmaceuticals, Cranbury, NJ. [Google Scholar]
- 23.Barker J, Healing D, Hutchison JG. 1972. Characteristics of some co-trimoxazole-resistant Enterobacteriaceae from infected patients. J Clin Pathol 25:1086–1088. 10.1136/jcp.25.12.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanner EI, Bullin CH. 1974. Thymidine-dependent Escherichia coli infection and some associated laboratory problems. J Clin Pathol 27:565–568. 10.1136/jcp.27.7.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically (M07), 11th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 26.Biagi M, Vialichka A, Jurkovic M, Wu T, Shajee A, Lee M, Patel S, Mendes RE, Wenzler E. 2020. Activity of cefiderocol alone and in combination with levofloxacin, minocycline, polymyxin B, or trimethoprim-sulfamethoxazole against multidrug-resistant Stenotrophomonas maltophilia. Antimicrob Agents Chemother 64:e00559-20. 10.1128/AAC.00559-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei C, Ni W, Cai X, Zhao J, Cui J. 2016. Evaluation of trimethoprim/sulfamethoxazole (SXT), minocycline, tigecycline, moxifloxacin, and ceftazidime alone and in combinations for SXT-susceptible and SXT-resistant Stenotrophomonas maltophilia by in vitro time-kill experiments. PLoS One 11:e0152132. 10.1371/journal.pone.0152132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zelenitsky SA, Iacovides H, Ariano RE, Harding GK. 2005. Antibiotic combinations significantly more active than monotherapy in an in vitro infection model of Stenotrophomonas maltophilia. Diagn Microbiol Infect Dis 51:39–43. 10.1016/j.diagmicrobio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Cheng AC, McBryde ES, Wuthiekanun V, Chierakul W, Amornchai P, Day NP, White NJ, Peacock SJ. 2009. Dosing regimens of cotrimoxazole (trimethoprim-sulfamethoxazole) for melioidosis. Antimicrob Agents Chemother 53:4193–4199. 10.1128/AAC.01301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alsaad N, Dijkstra JA, Akkerman OW, de Lange WC, van Soolingen D, Kosterink JG, van der Werf TS, Alffenaar JW. 2016. Pharmacokinetic evaluation of sulfamethoxazole at 800 milligrams once daily in the treatment of tuberculosis. Antimicrob Agents Chemother 60:3942–3947. 10.1128/AAC.02175-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burgess DS, Frei CR, Lewis JS, II, Fiebelkorn KR, Jorgensen JH. 2007. The contribution of pharmacokinetic-pharmacodynamic modelling with Monte Carlo simulation to the development of susceptibility breakpoints for Neisseria meningitidis. Clin Microbiol Infect 13:33–39. 10.1111/j.1469-0691.2006.01617.x. [DOI] [PubMed] [Google Scholar]
- 32.Wisell KT, Kahlmeter G, Giske CG. 2008. Trimethoprim and enterococci in urinary tract infections: new perspectives on an old issue. J Antimicrob Chemother 62:35–40. 10.1093/jac/dkn147. [DOI] [PubMed] [Google Scholar]
- 33.Bulitta JB, Hope WW, Eakin AE, Guina T, Tam VH, Louie A, Drusano GL, Hoover JL. 2019. Generating robust and informative nonclinical in vitro and in vivo bacterial infection model efficacy data to support translation to humans. Antimicrob Agents Chemother 63:e02307-18. 10.1128/AAC.02307-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asempa TE, Nicolau DP, Kuti JL. 2019. In vitro activity of imipenem-relebactam alone or in combination with amikacin or colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother 63:e00997-19. 10.1128/AAC.00997-19. [DOI] [PMC free article] [PubMed] [Google Scholar]